Abstract
Logopenic progressive aphasia is a neurodegenerative syndrome characterized by sentence repetition and naming difficulties arising from left-lateralized temporoparietal atrophy. Clinical descriptions of logopenic progressive aphasia largely concentrate on profiling language deficits, however, accumulating evidence points to the presence of cognitive deficits even on tasks with minimal language demands. Although non-linguistic cognitive deficits in logopenic progressive aphasia are thought to scale with disease severity, patients at discrete stages of language dysfunction display overlapping cognitive profiles, suggesting individual-level variation in cognitive performance, independent of primary language dysfunction. To address this issue, we used principal component analysis to decompose the individual-level variation in cognitive performance in 43 well-characterized logopenic progressive aphasia patients who underwent multi-domain neuropsychological assessments and structural neuroimaging. The principal component analysis solution revealed the presence of two, statistically independent factors, providing stable and clinically intuitive explanations for the majority of variance in cognitive performance in the syndrome. Factor 1 reflected ‘speech production and verbal memory’ deficits which typify logopenic progressive aphasia. Systematic variations were also confirmed on a second, orthogonal factor mainly comprising visuospatial and executive processes. Adopting a case-comparison approach, we further demonstrate that pairs of patients with comparable Factor 1 scores, regardless of their severity, diverge considerably on visuo-executive test performance, underscoring the inter-individual variability in cognitive profiles in comparably ‘logopenic’ patients. Whole-brain voxel-based morphometry analyses revealed that speech production and verbal memory factor scores correlated with left middle frontal gyrus, while visuospatial and executive factor scores were associated with grey matter intensity of right-lateralized temporoparietal, middle frontal regions and their underlying white matter connectivity. Importantly, logopenic progressive aphasia patients with poorer visuospatial and executive factor scores demonstrated greater right-lateralized temporoparietal and frontal atrophy. Our findings demonstrate the inherent variation in cognitive performance at an individual- and group-level in logopenic progressive aphasia, suggesting the presence of a genuine co-occurring cognitive impairment that is statistically independent of language function and disease severity.
Keywords: primary progressive aphasia, principal component analysis, visuospatial functioning, executive functioning, language
Ramanan et al. found systematic inter-individual variation in language and non-linguistic general cognitive performance in patients with logopenic progressive aphasia, a patient group conceptualized as showing predominant naming and repetition deficits. These impairments were independent of disease severity and degree of aphasia and were associated with distinct, non-overlapping neural correlates.
Graphical Abstract
Graphical Abstract.
Introduction
Logopenic progressive aphasia (LPA) is a rare neurodegenerative brain disorder, the canonical features of which centre on language dysfunction, including slowing in spontaneous speech, phonological errors and paraphasias, sentence repetition, sentence comprehension and word-finding difficulties (Gorno-Tempini et al., 2008; Gorno-Tempini et al., 2011; Leyton et al., 2014). By contrast, grammatical and articulatory processing and semantic comprehension remain relatively spared in the early stages of the disease (Gorno-Tempini et al., 2008). The unique language profile of LPA is proposed to reflect a breakdown in lexical retrieval, phonological working memory and phonological processing, functions that together support sentence repetition, naming, spontaneous speech and working memory (Henry and Gorno-Tempini, 2010; Leyton et al., 2012). Neuroanatomically, the locus of atrophy in early stages of LPA is predominantly left-lateralized and centred on the left inferior parietal lobule, lateral temporal and perisylvian cortical regions surrounding the left superior/middle/inferior temporal gyrus (Gorno-Tempini et al., 2008; Rohrer et al., 2010; Leyton et al., 2012; Teichmann et al., 2013; Krishnan et al., 2016). Over time, however, LPA progresses to affect fronto-insular, medial parietal and temporal cortices, encroaching into right-hemisphere temporoparietal regions (Galantucci et al., 2011; Rogalski et al., 2011b; Rohrer et al., 2013; Brambati et al., 2015; Tu et al., 2015). At a pathological level, the majority of LPA patients (>90%) present with abnormal levels of cortical β-amyloid, characteristic of Alzheimer’s disease (Rabinovici et al., 2008; Leyton et al., 2011; Chare et al., 2014; Santos-Santos et al., 2018), although recent histopathological and biomarker evidence also points to the presence of non-Alzheimer pathologies in a minority of clinically diagnosed LPA patients (Mesulam et al., 2014; Bergeron et al., 2018).
While current classification criteria and clinical descriptions of LPA emphasize the fine-grained characterization of language dysfunction, mounting evidence points to co-occurring non-linguistic cognitive deficits in this syndrome. Notably, LPA patients have been reported to show impaired processing speed, sustained attention and working memory and dysexecutive profiles (Rohrer et al., 2012; Foxe et al., 2013; Magnin et al., 2013; Butts et al., 2015). Significant socioemotional dysfunction including loss of empathy and impaired emotion detection abilities has also been documented (Hazelton et al., 2017; Multani et al., 2017; Fittipaldi et al., 2019). Finally, LPA patients demonstrate significant verbal episodic and autobiographical memory difficulties (Butts et al., 2015; Casaletto et al., 2017; Win et al., 2017; Eikelboom et al., 2018; Ramanan et al., 2020a) comparable to that observed in typical Alzheimer’s disease (Ramanan et al., 2016; Ramanan et al., 2020a, b). While such deficits could manifest simply as a by-product of language and lexical retrieval difficulties in LPA, compromised performance on tasks with minimal language demands suggests otherwise. For example, LPA patients show significant impairments on non-verbal tasks of episodic memory (Ramanan et al., 2016, 2020b), spatial span (Foxe et al., 2013; Foxe et al., 2016), spatial orientation (Magnin et al., 2013) and visuospatial processing (Butts et al., 2015; Watson et al., 2018), all of which circumvent language demands. Moreover, impairments on non-verbal episodic memory and emotion processing in LPA have been shown to persist when disease severity and language dysfunction are statistically controlled for (Ramanan et al., 2016; Multani et al., 2017). Clinical and carer reports further corroborate these findings, with the majority of LPA patients presenting with visible extra-linguistic general cognitive difficulties (Owens et al., 2018). Further, changes in socioemotional, attention and memory functions in LPA are detectable 1–3 years prior to spousal recognition of frank expressive language difficulties in patients (Pozzebon et al., 2018). Together, these findings argue against language dysfunction as the sole mediator of general cognitive decline in LPA and suggest the presence of genuine co-occurring non-linguistic cognitive deficits.
Given the marked heterogeneity in test performance across cognitive domains and between individual cases in LPA, data-driven approaches hold considerable promise to refine our understanding of this syndrome, as they can simultaneously model systematic variations at a domain- and individual-level. Previous studies in LPA have employed cluster analysis techniques to identify endophenotypes or ‘clusters’ of LPA patients, based on their language performance. These clusters tend to vary primarily along with disease severity and degree of aphasia (Machulda et al., 2013; Leyton et al., 2015), and then by level of overall cognitive impairment (Owens et al., 2018). The clinical interpretability of these clusters, however, remains limited for two main reasons. First, endophenotypes of LPA identified purely on the basis of language performance tend to overlap significantly in terms of their overall cognitive performance. This suggests that classifying patients exclusively in terms of language dysfunction masks important variations in general cognitive performance in LPA. Second, when examined relative to other primary progressive aphasia syndromes in the context of language performance, LPA rarely emerges as an independent cluster, instead of mingling with other neurodegenerative disorders of language (Sajjadi et al., 2012; Maruta et al., 2015; Hoffman et al., 2017; Ingram et al., 2019). Together, these findings suggest that the current practice of identifying LPA endophenotypes on the basis of language disturbances alone, cannot adequately capture the multidimensional nature of cognitive impairments in this syndrome.
Here, we adopted the hypothesis that the multifaceted cognitive dysfunction in LPA reflects graded variations along multiple, continuous dimensions, rather than strictly defined categorical clusters. Graded approaches have been employed to great effect in the post-stroke aphasia literature, where patients present with variable combinations of expressive and receptive language impairments and co-occurring general cognitive deficits attributable to variations in the size and location of lesions (Kummerer et al., 2013; Butler et al., 2014; Mirman et al., 2015; Halai et al., 2017; Ramsey et al., 2017) and more recently in large-scale examinations of frontotemporal lobar degeneration-related syndromes (Murley et al., 2020) or variations in semantic dementia/temporal lobe variant of frontotemporal dementia (Ding et al., 2020). In particular, principal component analysis (PCA) has been used as a data-driven method to reveal statistically reliable, graded differences across individual cases, placing them relative to each other within the resultant multidimensional space and, in turn, relating these principal components, rather than individual test scores, to the pattern of the patients’ lesions/atrophy. PCA approaches have been used to ‘compress’ and extract weighted scores from multidimensional data (see e.g. Hoffman et al., 2017; Ramanan et al., 2017), aiding the determination of independence or inter-dependence between cognitive domains. In addition, emergent components from PCA can be used to place participants along a spectrum, enabling characterization of graded variations between participants across cognitive domains. Accordingly, the emergence of a single, weighted component from the PCA would allude to considerable within-group homogeneity, such that a group varies systematically along only one axis of a multidimensional space. In contrast, the emergence of multiple, statistically orthogonal factors confirms systematic, independent differences in multiple cognitive domains within a patient cohort.
To this end, we employed PCA to explore the neurocognitive architecture of language and general cognitive performance in a large well-characterized sample of LPA patients (N = 43). Our primary aims were to reveal the extent of graded variations in cognitive performance within the LPA syndrome, and to use the emergent components to characterize patient performance at the individual level. We predicted that marked cognitive heterogeneity would be evident, regardless of the severity of language impairments. Finally, we sought to establish the neural substrates of the graded variation in cognitive performance within the LPA syndrome, using voxel-based morphometry (VBM).
Materials and Methods
Below, we report how we determined our sample size, all data exclusions, all inclusion/exclusion criteria, whether inclusion/exclusion criteria were established prior to data analyses, all manipulations, and all measures in the study.
Participants
A total of 73 participants were recruited through FRONTIER, the frontotemporal dementia research group at the Brain and Mind Centre, The University of Sydney, Australia. Forty-three patients with a clinical diagnosis of LPA, presenting with early anomia, word-finding and sentence repetition difficulties, were included (Gorno-Tempini et al., 2011). Diagnoses were arrived at by consensus among a multidisciplinary team comprising a senior neurologist (J.R.H.), a clinical neuropsychologist and an occupational therapist, based on comprehensive clinical and neuropsychological examination along with structural neuroimaging. Disease severity for LPA patients was established using the clinician-indexed Frontotemporal Lobar Degeneration-modified Clinical Dementia Rating Sum of Boxes score (CDR-FTLD SoB; Knopman et al., 2008).
Thirty healthy control participants were selected through the research volunteer panel at Neuroscience Research Australia and local community clubs. Controls were matched to patient groups for sex, age and education and scored 0 on the CDR-FTLD SoB measure. Healthy controls scored 88 or above on the Addenbrooke’s Cognitive Examination—Revised (ACE-R: Mioshi et al., 2006) or its updated counterpart, the Addenbrooke’s Cognitive Examination—III (ACE-III: Hsieh et al., 2013) —both of which assess global cognitive functioning. Exclusion criteria for participants included a history of significant head injury, cerebrovascular disease, alcohol and drug abuse, other primary psychiatric, neurological or mood disorders and limited English proficiency.
All participants or their Person Responsible provided written informed consent in accordance with the Declaration of Helsinki. This study was approved by the South Eastern Sydney Local Healthy District and The University of New South Wales ethics committees.
General and targeted neuropsychological assessments
Participants underwent extensive neuropsychological testing. Global cognitive functioning was indexed using the ACE-R/ACE-III total score (Mioshi et al., 2006; Hsieh et al., 2013), which includes subtests of attention (max = 18), verbal memory (max = 26), verbal fluency (max = 14), language (max = 26), and visuospatial (max = 16) function. A subset of LPA patients (N = 23, ∼53% of the LPA sample) completed the ACE-III (Hsieh et al., 2013). For comparability, their ACE-III subtest scores were transformed to the equivalent ACE-R subtest scores (see So et al., 2018).
Targeted cognitive assessments of language, visuospatial function, memory and executive functioning were administered. Confrontation naming, single-word comprehension, single-word repetition and semantic association were assessed using the Sydney Language Battery (SYDBAT: Savage et al., 2013). Visuo-constructional abilities were assessed using the Copy score (max = 36) of the Rey-Osterrieth Complex Figure test (ROCF: Osterrieth, 1944), while the 3-min delayed recall (max = 36) of the ROCF was used to index nonverbal episodic memory. Auditory attention and working memory were measured using Digit Span Forward and Backward tests, respectively (Strauss et al., 2006). Finally, executive dysfunction was indexed via the Trail Making Test B-A time difference (TMT B-A: Reitan, 1958).
Statistical analyses
Statistical analyses of behavioural data were conducted using a combination of RStudio v3.3.0 (R Core Team, 2016) and MATLAB (The Mathworks Inc., Natick, MA, USA), described below and in Supplementary material.
Step 1: Characterizing group differences
Group differences in demographic, clinical and neuropsychological performance between LPA and Control groups were explored. For binomially distributed variables (i.e. sex), Chi-squared tests were used. For all continuous variables (i.e. demographic, clinical and neuropsychological test measures), normality of distribution was examined using the Shapiro–Wilk tests and box-and-whisker plots. Accordingly, t-tests or Wilcoxon–Mann–Whitney tests were respectively employed when data met or violated normality assumptions. Two-tailed Pearson’s correlations (r values) with false discovery rate correction for multiple comparisons (Benjamini and Hochberg, 1995) were used to examine associations between neuropsychological test performance and clinician-indexed disease severity (CDR-FTLD SoB) in the LPA group. For all analyses of group differences and correlations, an alpha of P ≤ 0.05 was employed.
Step 2: Tabulating and imputing missing data and standardizing scores
All subsequent statistical analyses were conducted in the LPA group. As PCA algorithms operate on standardized datasets with no missing variables, the frequency of missing neuropsychological data was first tabulated and plotted for subsequent imputation (Supplementary Fig. 1). Across all neuropsychological test measures, the LPA group had a total of 4.8% missing data with the majority of patients (17/43 LPA, i.e. 39.5% of LPA group) missing TMT B-A data (Supplementary Fig. 1). All available data were converted into percentages (detailed in Supplementary material), and this final dataset was used for imputation.
Missing data were imputed using a probabilistic PCA using k-fold cross-validation approach (with k = 4; detailed in Supplementary material). Briefly, this approach offers improved stability as compared to the list-wise exclusion of rows with missing data, while simultaneously guarding against overfitting of imputed data points (unlike imputation of group mean) (see Tipping and Bishop, 1999; Ilin and Raiko, 2010). The output was a ‘full’ dataset with no missing values.
Step 3: Identifying principal cognitive factors
The final ‘full’ standardized dataset was entered into an orthogonally rotated (varimax) PCA. Varimax rotation facilitates interpretations of PCA output by maximizing the dispersion of factor loadings between components, allowing for a little variance to be shared commonly between emergent components. In line with standard approaches (Jolliffe, 2002), factors with an eigenvalue of 1.0 and above were extracted. Each factor was given a label reflecting the majority of tests loading heavily (i.e. loadings > 0.5) on that factor.
It must be noted that factor names are simply shorthand labels that reflect the majority of cognitive tests loading onto that particular factor, and by no means reflect the entirety of cognitive processes that underpin performance on each test loading onto that particular factor. Individual patient scores on each factor were extracted and used as orthogonal covariates in subsequent neuroimaging analyses. In addition, we projected the lower bound of normality (i.e. −1.96 standard error of the mean) from the control data into the patients’ PCA space to facilitate behavioural interpretation of patient factor scores relative to control test performance (detailed in Supplementary material). Finally, associations among disease severity, disease duration and emergent factor scores were examined using two-tailed Pearson’s correlations.
Step 4: Computing deviations from expected cognitive performance
As PCA results are one-step removed from raw test scores, we used PCA factor scores to predict each patient’s ‘ideal’ test performance and compared their predicted and raw test neuropsychological performance (adopting the approach used in Lambon Ralph et al., 2003). This approach translates information from the PCA space back into readily comprehensible predicted test scores, allowing for direct and intuitive comparisons of expected and actual test performance between LPA patients.
Our PCA generated two orthogonal factors. Tests that loaded heavily on Factor 1 resembled measures on which LPA patients typically show early deficits (e.g. naming, repetition, verbal working memory and short-term memory). By contrast, tests that loaded heavily on the orthogonal factor (Factor 2) reflected measures on which performance is traditionally thought to be affected in later stages of LPA (e.g. visuospatial, executive and comprehension measures). We therefore treated each patient’s Factor 1 score as a simple metric of how ‘logopenic’ they are and used these scores to predict test performance on neuropsychological measures loading differentially on Factors 1 and 2. This comparison would demonstrate how comparably logopenic patients (with similar Factor 1 scores) diverge on test measures posited to be relatively preserved, until later stages of LPA.
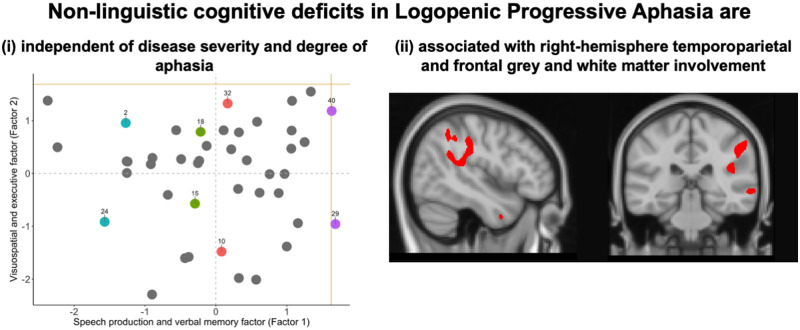
To do this, we first visually identified and selected four pairs of LPA patients (denoted using pairwise matching colours in Fig. 1). Each pair was carefully selected so that they (i) had comparable scores on Factor 1 but, (ii) diverged on Factor 2 scores and (iii) were sampled across varying Factor 1 scores to reflect the spread of distribution along the x-axis (see Lambon Ralph et al., 2003 for similar analyses). Following pair selection, we employed a series of linear regression analyses using Factor 1 scores to predict performance on select neuropsychological tasks that loaded heavily on Factor 1 (SYDBAT Naming and Repetition and Digit Span Forward) and Factor 2 (SYDBAT Comprehension and ROCF Copy and Delayed Recall). Each pair’s predicted scores were then visually compared to their raw neuropsychological test scores.
Figure 1.
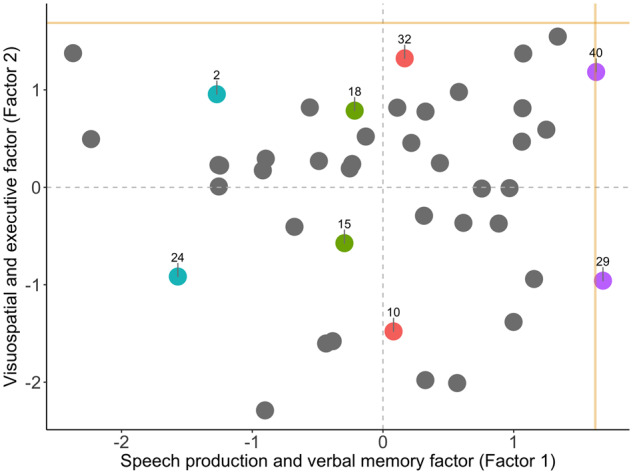
PCA results. Factor scores of LPA patients on the speech production and verbal memory factor (i.e. Factor 1) and visuospatial and executive factor (i.e. Factor 2) emerging from the varimax-rotated PCA. Coloured data points indicate individual patients who were examined in pairwise fashion in subsequent statistical analyses, with matching colours denoting patient pairs of interest. Gold lines indicate lower bound of normality (−1.96 standard error from the mean) as estimated from the Control group (calculation detailed in Supplementary material). LPA = logopenic progressive aphasia.
Image acquisition
Sixty-three participants (35 LPA and 28 Controls) underwent structural T1-weighted brain MRI using a 3 T Philips MRI scanner with standard quadrature head coil (eight channels). All 3D T1-weighted images were acquired using the following sequences: coronal acquisition, matrix 256 × 256 mm, 200 slices, 1 mm isotropic voxel resolution, echo time/repetition = 2.6/5.8 ms, flip angle α = 8°.
We used combined grey and white matter VBM to account for co-occurring cortical grey and subcortical white matter changes that are prototypical of neurodegenerative disease syndromes such as LPA (Brambati et al., 2015). Such a method has been employed in populations presenting with diffuse, co-occurring grey and white matter changes such as healthy ageing (Giorgio et al., 2010), post-stroke aphasia (Halai et al., 2017) and frontotemporal lobar degeneration syndromes (Lansdall et al., 2017; Murley et al., 2020). VBM analyses were conducted using Statistical Parametric Mapping software (SPM12: Wellcome Trust Centre for Neuroimaging, https://www.fil.ion.ucl.ac.uk/spm/software/spm12/, accessed 26 August 2020). Full details of the standard pre-processing pipeline are provided in Supplementary material.
VBM analyses
Whole-brain changes in grey and white matter intensity
Voxel-wise differences of grey and white matter intensity between LPA and Control groups were assessed using independent t-tests, with age and total intracranial volume included as nuisance variables. Clusters were extracted, corrected for Family-Wise Error at P < 0.01 with a cluster threshold of 100 contiguous voxels. Emergent clusters were subsequently binarized into a mask that was used to compute voxel-level variance in grey and white matter intensity (see below).
Variance in grey and white matter intensity across participants
VBM correlation analyses are entirely constrained by variations in voxel-level intensity and test performance. In the context of progressive diseases, this means that highly atrophic regions that subsequently have uniformly low voxel-level variance are unlikely to emerge in the correlation analyses as they are consistently affected across cases. These regions, nevertheless, could be critical to explaining the observed behavioural profile and therefore, it is important to interpret VBM results in the context of whole-brain voxel-level variance. To complement our atrophy analyses, we therefore computed voxel-level inter-subject variance maps of grey and white matter intensity for all participants. The resultant whole-brain images were further masked to consider only clusters emerging in our atrophy analyses. As before, age and total intracranial volume were regressed out as nuisance variables prior to extracting variance maps.
Grey and white matter intensity changes in patients stratified on factor scores
We further investigated whole-brain changes in grey and white matter intensity in patients with ‘low’ and ‘high’ factor scores. Patients were stratified into two folds on either end of a zero score on Factor 1 and Factor 2 each (see Supplementary Fig. 2). Stratifying on Factor 1 resulted in 15 patients with negative (low) and 20 patients with positive (high) scores while stratifying on Factor 2 resulted in 16 patients with negative (low) and 19 patients with positive (high) scores (Supplementary Fig. 2). Patients split on Factor 1 scores had comparable Factor 2 scores and vice versa (both P-values > 0.1). When compared to patients with higher Factor 1 scores, those with lower Factor 1 scores had greater disease severity (t = 2.52; P = 0.016), whilst the difference of disease duration was not statistically significant (t = 1.9; P = 0.065). In contrast, no significant group differences were noted on disease severity (t = 0.37; P = 0.70) and disease duration (t = −1.19; P = 0.24) between patients split on Factor 2 scores. Regression models with separate directional contrasts (i.e. independent t-tests) were used to assess differences in cortical grey matter and subcortical white matter intensities between LPA subgroups (i.e. high and low scorers) on each Factor score, with age and total intracranial volume included as nuisance variables. Clusters were extracted at P < 0.001, uncorrected, with a cluster threshold of 100 contiguous voxels.
Correlations with PCA-generated factor scores
Finally, correlation analyses within the LPA group (N = 35) were employed to examine associations between whole-brain grey and white matter intensity and PCA-generated factor scores. A correlation-only statistical model was implemented for additional statistical power, using t-contrasts to measure associations between grey and white matter intensity and PCA-generated factor scores. Age and total intracranial volume were included as nuisance covariates in the analyses. Anatomical locations of statistical significance were overlaid on the Montreal Neurological Institute (MNI) standard brain with maximum co-ordinates provided in MNI stereotaxic space. Clusters were extracted using a threshold of P < 0.001 uncorrected for multiple comparisons with a cluster threshold of 100 contiguous voxels.
Data availability
The ethical requirement to ensure patient confidentiality precludes public archiving of our data. Researchers who would like to access the raw data should contact the corresponding authors, who will liaise with the ethics committee that approved the study, and accordingly, as much data that are required to reproduce the results will be released to the individual researcher. The code used for this project has been made available for review on the Open Science Framework website (https://osf.io/bn534/). No part of the study procedures or analyses were preregistered prior to the research being undertaken.
RESULTS
Demographic, clinical and neuropsychological test performance
Demographic, clinical and neuropsychological scores are presented in Table 1. No significant group differences emerged for sex distribution, age and education (all P-values > 0.1). LPA patients performed significantly worse than controls on measures of global cognition, as well as targeted neuropsychological assessments of episodic memory, semantic naming and comprehension, single-word repetition, visuo-constructional abilities and executive function (all P-values < 0.0001; see Table 1). Carers of LPA patients reported significant changes in behaviour and memory on the CBI-R relative to Controls (both P-values < 0.0001). These profiles are in keeping with previous descriptions of the LPA cognitive profile (Magnin et al., 2013; Butts et al., 2015; Ramanan et al., 2016; Watson et al., 2018).
Table 1.
Demographic, clinical and general neuropsychological assessment performance for all groups
| LPA | Control | Group effect | |
|---|---|---|---|
| N | 43 | 30 | |
| Sex (M:F) | 19:24 | 14:16 | 2 < 0.001; P > 0.1 |
| Age (years) | 70.5 (7.9) | 72.6 (2.8) | t = 1.57; P = 0.12 |
| Education (years) | 12.2 (3.2) | 13.2 (2.0) | t = 1.6; P = 0.11 |
| Disease duration (years) | 2.7 (2.0) | ||
| Disease severity (CDR-FTLD SoB) | 5.2 (3.5) | ||
| CBI-R total (%) | 33.8 (22.8) | 4.3 (4.8) | W = 59.5; P < 0.0001 |
| CBI-R memory (%) | 11.8 (6.2) | 1.9 (2.6) | W = 77.5; P < 0.0001 |
| ACE-R total (100)a | 61.0 (15.4) | 95.0 (3.3) | W = 1286; P < 0.0001 |
| Neuropsychological tests | |||
| ACE-R attention total (18) | 12.4 (3.3) | 17.7 (.5) | W = 1258; P < 0.0001 |
| ACE-R memory total (26) | 13.8 (5.8) | 24.1 (1.7) | W = 1229.5; P < 0.0001 |
| ACE-R fluency total (14) | 4.5 (2.8) | 12.2 (1.5) | W = 1281.5; P < 0.0001 |
| ACE-R language total (26) | 17.6 (5.3) | 25.2 (.9) | W = 1202.5; P < 0.0001 |
| ACE-R visuospatial total (16) | 6.5 (6.1) | 15.6 (.8) | W = 1224; P < 0.0001 |
| SYDBAT naming (30) | 15.4 (6.9) | 26.6 (2.4) | W = 1095.5; P < 0.0001 |
| SYDBAT comprehension (30) | 26.1 (2.5) | 29.0 (1.5) | W = 924; P < 0.0001 |
| SYDBAT repetition (30) | 25.6 (5.5) | 29.8 (.5) | W = 923.5; P < 0.0001 |
| SYDBAT semantic (30) | 25.3 (3.2) | 28.0 (1.5) | W = 844; P < 0.0001 |
| Digit span forward (16) | 6.5 (2.5) | 11.2 (2.1) | W = 1083; P < 0.0001 |
| Digit span backward (16) | 3.6 (2.0) | 8.2 (2.4) | t = 8.3; P < 0.0001 |
| ROCF copy (36) | 24.6 (8.9) | 32.8 (3.1) | W = 859; P < 0.0001 |
| ROCF delayed recall (36) | 8.8 (4.9) | 17.5 (4.9) | W = 870; P < 0.0001 |
| TMT B-A time difference (s) | 165.1 (152.6) | 42.6 (20.6) | W = 45; P < 0.0001 |
Notes. Maximum test scores reported in brackets; For all groups, mean and standard deviation reported; 2 = Chi-square value; based on the Shapiro–Wilk test outputs, t-test (t-value) employed when data met normality assumptions or Wilcoxon–Mann–Whitney test (W-value) employed when data violated normality assumptions; For all statistical comparisons, P-values bolded if P < 0.05.
23/43 (53%) LPA patients had ACE-III scores which were converted into ACE-R scores (see Methods section).
ACE-R = Addenbrooke’s Cognitive Examination – Revised; CBI-R = Cambridge Behavioural Inventory – Revised; CDR-FTLD SoB = Clinical Dementia Rating – Frontotemporal Lobar Degeneration Sum of Boxes; LPA = logopenic progressive aphasia; ROCF = Rey-Osterrieth Complex Figure; SYDBAT = Sydney Language Battery; TMT B-A = Trail Making Test parts B – A.
Correlations between disease severity and neuropsychological test performance
LPA Digit Span Forward performance correlated with disease severity scores on the CDR-FTLD SoB (r = −0.39; P = 0.010). No other significant correlations emerged between neuropsychological test performance and disease severity in LPA (all P-values ≥ 0.059; see Supplementary Table 1).
Identifying principal cognitive factors
Factors and individual test loadings from the varimax-rotated PCA output are displayed in Table 2, while factor loadings for all LPA patients are displayed in Fig. 1 and Supplementary Table 2. The sample size was considered adequate for the analysis (Kaiser–Meyer–Olkin statistic = 0.63). The PCA solution revealed two independent, orthogonal factors that together accounted for 56.4% of the total variance (Factor 1 = 41.8% and Factor 2 = 14.6% of total variance) in LPA cognitive performance. The extraction of a three or four component solution, by contrast, aided little additional explanatory power (Factor 3 = 9.4% and Factor 4 = 7.6%) and only served to split the measures loading on Factor 2 into further independent principal components. We, therefore, chose the two-factor solution for its stability, explanatory power and clinical intuitiveness in explaining LPA cognitive performance.
Table 2.
Factor loadings for neuropsychological test measures on the omnibus varimax-rotated PCA
| Neuropsychological tests | Factor 1 | Factor 2 |
|---|---|---|
| Speech production and verbal memory factor | Visuospatial and executive factor | |
| ACE-R language total | 0.849 | 0.114 |
| Digit span forward | 0.801 | 0.053 |
| SYDBAT repetition | 0.788 | 0.036 |
| SYDBAT naming | 0.687 | 0.200 |
| ACE-R memory total | 0.662 | 0.290 |
| Digit SPAN BACKWARD | 0.604 | 0.405 |
| ROCF copy | 0.111 | 0.918 |
| SYDBAT semantic association | 0.196 | 0.801 |
| SYDBAT comprehension | 0.056 | 0.782 |
| TMT B-A time difference | 0.146 | 0.727 |
| ROCF delayed recall | 0.380 | 0.660 |
| ACE-R attention total | 0.444 | 0.582 |
| ACE-R visuospatial total | 0.437 | 0.322 |
| ACE-R fluency total | 0.364 | 0.283 |
Notes. Tests that load heavily (loadings > 0.5) on each factor are indicated in bold. Scores for only LPA patients were entered into the PCA.
LPA = logopenic progressive aphasia; ACE-R = Addenbrooke’s Cognitive Examination – Revised; SYDBAT = Sydney Language Battery; ROCF = Rey-Osterrieth Complex Figure; TMT B-A = Trail Making Test parts B-A.
Factor 1 loaded heavily on tests of verbal memory (ACE-R Memory Total), phonological working memory (Digit Span Forward and Backward, SYDBAT Repetition), naming (ACE-R Language Total, SYDBAT Naming) and repetition (SYDBAT Repetition and Digit Span Forward and Backward) (Table 2). Together, these tests index cognitive and language processes that are canonically impaired in LPA; therefore, we labelled this factor the ‘speech production and verbal memory factor’.
Our PCA analyses further suggested the presence of an orthogonal set of variations on a second factor. Factor 2 mainly loaded on measures of executive (Trails Time Difference), attention (ACE-R Attention Total) and visuospatial (ROCF Copy and Delayed Recall) abilities. In addition, the SYDBAT Comprehension subtest performance also loaded onto this factor. For brevity, we refer to this factor as the ‘visuospatial and executive factor’. Importantly, patients with both high and low Factor 1 scores exhibited uniform variation on Factor 2 scores and this variation was noted both proximally and distally from the lower bound of normal control performance (Fig. 1). Together, these findings suggest that Factor 2 is not solely accounted by the emergence of additional impairments with disease severity but instead reflects systematic variations on visuospatial and executive performance in LPA patients.
In summary, our PCA pointed to the existence of two orthogonal sets of variations in neuropsychological performance in LPA. While the first factor resembles the classic language profile of LPA, the uniform distribution of scores on Factor 2 suggests a co-occurring primary disruption of visuospatial and executive processes in this syndrome.
Associations between factor scores, disease severity and disease duration
No significant correlations were found between disease severity (CDR-FTLD SoB) and scores on the speech production and verbal memory factor (Factor 1; r = −0.25; P = 0.1) or visuospatial and executive factor (Factor 2; r = −0.16; P > 0.1) (Supplementary Fig. 3). In contrast, there was a significant correlation between disease duration and the speech production and verbal memory factor (Factor 1; r = −0.53; P = 0.0002), but not with the visuospatial and executive factor (Factor 2; r = 0.13; P > 0.1) (Supplementary Fig. 4). The lack of strong and statistically significant associations, especially on Factor 2, supports our PCA findings of systematic variations on visuospatial and executive test performance, regardless of the disease severity or disease duration of LPA patients.
Comparably logopenic cases diverge on visuospatial and executive performance
In a second step, we aimed to demonstrate how patients who present as ‘comparably logopenic’ can show divergent visuospatial and executive performance. For this, we first chose LPA patient pairs with comparable Factor 1 scores (i.e. coloured pairs in Fig. 1). We used their Factor 1 scores to predict neuropsychological performance on selected measures loading differentially on Factors 1 and 2. These predicted scores were then compared to their actual raw neuropsychological performance (Figs 2 and 3).
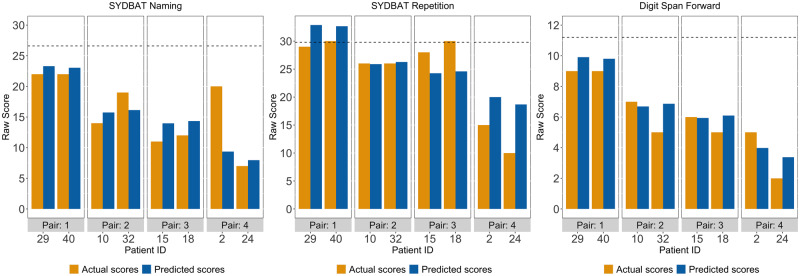
Figure 2.
Predicted and actual scores for LPA patient pairs on three example tests loading on the speech production and verbal memory factor (i.e. Factor 1) from the varimax-rotated PCA. Dotted lines for each test indicate actual Control mean. LPA = logopenic progressive aphasia; SYDBAT = Sydney Language Battery.
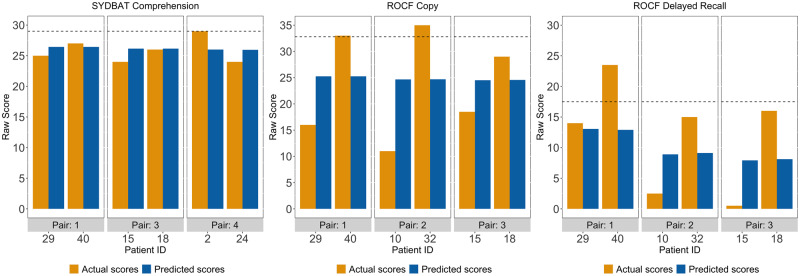
For tests loading on the speech production and verbal memory factor (Factor 1), predicted and actual scores were nearly similar across all patient pairs (except for pair 4 on SYDBAT Naming) (Fig. 2). This pattern confirmed our prediction as comparably ‘logopenic’ patients should display near-identical performance on cognitive tasks that are prototypically affected in the LPA syndrome. By contrast, patients displaying comparable ‘logopenic’ presentations (on Factor 1) diverged considerably in terms of predicted and actual scores on visuo-executive measures (Factor 2: ROCF Copy and Delayed Recall) (Fig. 3). At an individual level, these findings support the view that while two LPA patients can manifest with comparable severity of ‘logopenic’ symptoms, considerable heterogeneity exists in terms of co-occurring visuospatial and executive impairment in this syndrome.
Figure 3.
Predicted and actual scores for LPA patient pairs on three example tests loading on visuospatial and executive factor (i.e. Factor 2) from the varimax-rotated PCA. Only three pairs presented as one patient from one of the excluded pairs was missing data on the SYDBAT Comprehension or the ROCF measures. Dotted lines for each test indicate actual Control mean. LPA = logopenic progressive aphasia; ROCF = Rey-Osterrieth Complex Figure; SYDBAT = Sydney Language Battery.
VBM results
Group differences in grey and white matter intensity
Group differences in grey and white matter intensity are presented in Supplementary Table 3 and Fig. 4A. Relative to Controls, the LPA group displayed significant reductions in grey and white matter intensity predominantly in temporo-parietal regions including bilateral superior/middle/inferior temporal gyri (left > right) and bilateral angular and supramarginal gyri (left > right) and underlying white matter bundles, namely the inferior longitudinal and inferior fronto-occipital fasciculi. This cluster extended medially through the underlying white matter into posterior/middle cingulate cortices (left > right) and subcortically into bilateral hippocampi (across the longitudinal axis) and parahippocampal gyri through the cingulum bundle, further into the bilateral thalami, amygdalae (all left > right) and the underlying anterior thalamic radiation (Fig. 4A). Relative to Controls, the LPA group further demonstrated reduced grey and white matter intensity in frontal regions such as bilateral insular and superior/middle frontal cortices (both left > right) and underlying white matter connections from the superior longitudinal fasciculus, extending to the right orbitofrontal cortex and its underlying white matter connections into the bilateral temporal poles through the uncinate fasciculus (Fig. 4A). These patterns of atrophy are in line with previous descriptions of cortical grey matter and subcortical white matter damage in LPA (Gorno-Tempini et al., 2004; Galantucci et al., 2011; Rohrer et al., 2013; Rogalski et al., 2014; Tu et al., 2015).
Figure 4.
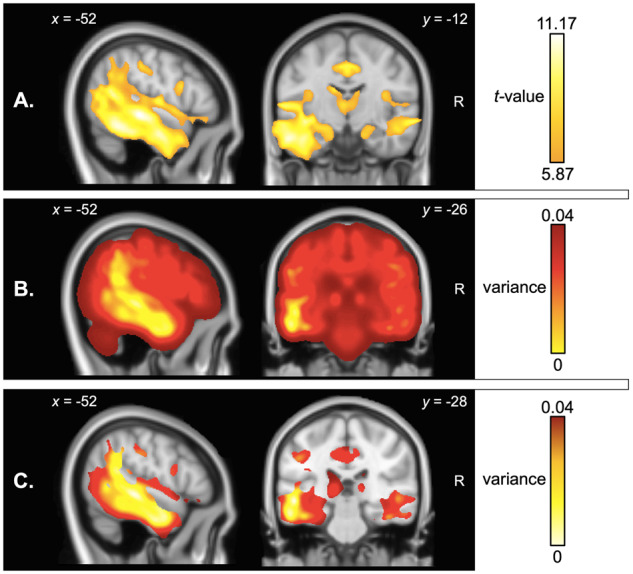
VBM analyses of whole-brain atrophy. Panels indicate (A) regions of significant grey and white matter intensity reduction in LPA compared to Controls, (B) voxel-wise variance in grey and white matter intensity in LPA compared to Controls and (C) voxel-wise variance in regions of peak atrophy (computed within a mask of regions emerging from the atrophy analysis in A. Coloured voxels in A indicate regions that emerged significant in the VBM analyses at P < 0.01 corrected for Family-Wise Error with a cluster threshold of 100 contiguous voxels. Age and total intracranial volume were included as covariates in all analyses. Clusters are overlaid on the MNI standard brain with x and y co-ordinates reported in MNI standard space. LPA = logopenic progressive aphasia; R = right.
Mapping voxel-wise variance in grey and white matter intensity
Visual inspection of variance maps revealed that variance in whole-brain grey and white matter intensity was lowest in left perisylvian regions, typically affected in the earliest stages of LPA (Fig. 4B). Examining variance within regions of peak atrophy revealed that the area of lowest variance was centred on the left superior/middle temporal gyrus extending into the left temporoparietal junction and inferior parietal cortex; regions that together demonstrated maximal atrophy (i.e. lowest grey and white matter intensity) in LPA (Fig. 4C). By contrast, regions located at the ‘edges’ of the atrophy clusters and beyond demonstrated maximal variance.
Grey and white matter intensity changes in patients stratified on factor scores
Group differences in grey and white matter intensity are presented in Supplementary Table 4 and Supplementary Fig. 5. No significant results emerged for contrasts comparing high and low scores on the speech production and verbal memory factor (Factor 1). In contrast, direct comparison of LPA subgroups revealed that compared to cases with higher visuospatial and executive factor scores (Factor 2), patients with lower visuospatial and executive factor scores demonstrated greater grey and white matter intensity reduction in predominantly right temporoparietal regions including angular gyrus and supramarginal gyri connecting to superior/middle temporal gyri through the subcortical component of the middle/inferior longitudinal fasciculus. This cluster extended medially towards the right precuneus, posterior cingulate and occipital cortices. This cluster further extended rostrally towards right frontal regions such as middle/inferior frontal gyrus and middle cingulate gyrus through the subcortical cingulum bundle and superior longitudinal fasciculus tract, and subcortically towards the right parahippocampal regions and fusiform gyrus. Additionally, a relatively smaller cluster centred around the left angular gyrus, precuneus and underlying superior/inferior longitudinal fasciculus bundles was noted. No significant results emerged for the reverse contrast (Supplementary Table 4).
Neural correlates of principal cognitive factors
Associations between grey and white matter intensity and factor scores in the LPA group are displayed in Fig. 5 and Table 3.
Figure 5.
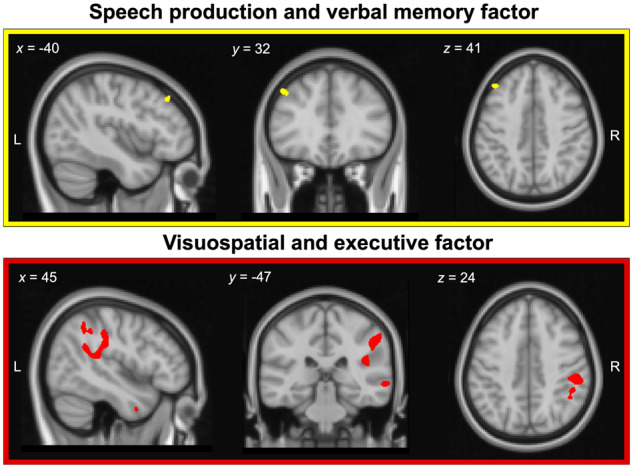
Regions of grey and white matter intensity that uniquely correlate with factor scores on the speech production and verbal memory factor (i.e. Factor 1; upper panel) and visuospatial and executive factor (i.e. Factor 2; lower panel) in LPA patients. Both factors were derived from varimax-rotated PCA of neuropsychological test performance in the LPA group. Coloured voxels indicate regions that emerged significant in the VBM analyses at a threshold of P < 0.001 uncorrected for multiple comparisons with a cluster threshold of 100 contiguous voxels. All clusters reported at t = 4.09 for speech production and verbal memory factor and t ≥ 3.6 for visuospatial and executive factor. Age and total intracranial volume were included as covariates in the analyses. Clusters are overlaid on the MNI standard brain with x, y and z co-ordinates reported in MNI standard space. L = left; LPA = logopenic progressive aphasia; PCA = principal component analysis; R = right.
Table 3.
VBM results showing regions of grey and white matter intensity that correlate with PCA-generated Factor 1 and Factor 2 scores in the LPA group
| Regions | Side | Number of voxels | Peak MNI co-ordinates |
t-value | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Speech production and verbal memory factor (Factor 1) | ||||||
| Middle frontal gyrus | Left | 146 | −43 | 33 | 42 | 4.09 |
| Visuospatial and executive factor (Factor 2) | ||||||
| Supramarginal gyrus and angular gyrus, extending into the superior parietal and insular cortices through the superior longitudinal fasciculus, and into superior/middle temporal gyrus through the inferior longitudinal fasciculus | Right | 3694 | 51 | −29 | 42 | 5.14 |
| Supramarginal and angular gyrus | Right | 820 | 48 | −50 | 48 | 4.32 |
| Superior/middle temporal gyrus and underlying middle/inferior longitudinal fasciculus components | Right | 760 | 56 | −55 | 8 | 4.4 |
| Middle/inferior temporal gyrus and underlying inferior longitudinal fasciculus component | Right | 510 | 58 | −48 | −21 | 3.85 |
| Middle temporal gyrus and underlying inferior longitudinal fasciculus component | Right | 388 | 64 | −34 | −3 | 4.42 |
| Precentral gyrus connecting to middle/inferior frontal gyrus through superior longitudinal fasciculus | Right | 337 | 40 | −8 | 53 | 3.9 |
| Middle/inferior temporal gyrus extending into temporal pole through underlying inferior longitudinal fasciculus component | Right | 202 | 42 | 3 | −28 | 3.6 |
| Postcentral gyrus and supramarginal gyrus | Right | 188 | 61 | −16 | 22 | 3.83 |
| Fusiform gyrus extending towards lingual gyrus, parahippocampal cortex and cerebellum | Right | 175 | 25 | −59 | −11 | 3.69 |
Note. MRI data were available for 35 LPA patients. Clusters presented above emerged as significant in the VBM analyses at a threshold of P < 0.001 uncorrected with a cluster threshold of 100 contiguous voxels. Age and total intracranial volume were included as covariates in the analyses.
LPA = logopenic progressive aphasia; MNI = Montreal Neurological Institute.
Speech production and verbal memory factor (Factor 1)
In the overall LPA group, speech production and verbal memory factor scores were found to correlate with grey matter intensity of the left middle frontal gyrus (Table 3, Fig. 5, upper panel).
Visuospatial and executive factor (Factor 2)
Visuospatial and executive factor scores in LPA correlated with grey and white matter intensity in right lateral parietal (supramarginal gyrus, angular gyrus) and medial parietal (precentral and postcentral gyri), right lateral temporal regions (superior/middle/inferior temporal gyri) and the right middle frontal gyrus. Additionally, a small cluster in the ventral temporal cortex (fusiform, lingual and parahippocampal gyrus) extending into the right cerebellar cortex was noted. Changes in white matter intensity of the right superior longitudinal fasciculus (connecting frontoparietal cortices) and right middle/inferior longitudinal fasciculus (connecting temporoparietal cortices) were further found to correlate with visuospatial and executive factor scores (Table 3, Fig. 5, lower panel).
In summary, both factors were found to correlate with distinct neural regions, with the speech production and verbal memory factor scores (Factor 1) correlating with grey matter intensity of the middle frontal gyrus, and the visuospatial and executive factor scores (Factor 2) correlating with largely right-sided temporoparietal and frontal regions and their underlying white matter connections. Importantly, the regions to emerge as significant in our covariate analyses (Fig. 5) are not the areas of maximal atrophy in LPA (Fig. 4A) but rather those with greater variance in grey and white matter intensity (Fig. 4B and C) which flank the areas of maximal atrophy.
DISCUSSION
This study demonstrates that the presence of visuospatial and executive deficits in LPA, beyond core language disturbance, does not reflect advancing disease severity. Instead, these deficits in LPA form their own independent cognitive dimension with discrete neuroanatomical bases and are reliably present even in the early stages of LPA. In more detail, the PCA identified two emergent factors capturing the heterogeneity of the LPA cognitive profile. The first factor reflected the expressive language and phonological working memory impairments that are not only diagnostic of LPA (Gorno-Tempini et al., 2008) but hold discriminative ability in differentiating LPA from other primary progressive aphasia syndromes (Gorno-Tempini et al., 2011). Our findings mesh well with previous studies employing other data-driven approaches such as two-step and hierarchical clustering analyses in LPA (Machulda et al., 2013; Leyton et al., 2015; Owens et al., 2018) and confirm that verbal working memory, repetition and naming difficulties typify the language profile of this syndrome.
Importantly, however, our PCA approach revealed a second, orthogonal factor comprising non-verbal episodic memory, visuo-constructional, attentional and executive processing, as well as receptive language and comprehension measures. This visuospatial and executive factor was independent of expressive language difficulties in LPA, running counter to the view that ‘general cognitive’ impairment in LPA reflects little more than the language demands of neuropsychological measures (Machulda et al., 2013; Owens et al., 2018). In fact, if the emergence of Factor 2 brought into question the effects of disease severity on test performance, we would hypothetically expect two key patterns to emerge in our data. First, the PCA would produce a single factor loading on all tests, indicating the overarching operation of disease severity on cognitive performance. As this was not the case, we would then expect individual LPA patients to ‘drop off’ towards a negative Factor 2 score, as their language impairments increased on Factor 1. In contrast, we found that performance deficits on this second, independent factor were pervasive across the entire LPA cohort, regardless of the severity of their language impairments. Again, this finding is not easily accommodated by previous proposals that global cognitive decline in LPA is a product of advancing disease severity (Funayama et al., 2013; Machulda et al., 2013; Owens et al., 2018). Rather, our findings indicate the presence of a genuine co-occurring global cognitive impairment, spanning multiple domains, that is independent of language function and disease severity. This view is in keeping with recent findings of marked nonverbal memory and emotion processing disturbances, even after accounting for expressive language impairments and disease severity in LPA (Ramanan et al., 2016; Multani et al., 2017). More generally, these results add to the view that subtypes of Alzheimer’s disease reflect graded rather than absolute variations presumably reflecting individual differences in the exact distribution of Alzheimer’s pathology (c.f., Lambon Ralph et al., 2003).
At an individual-level, systematic variations on the visuospatial and executive factor, regardless of patient performance on the language factor, underline at the graded nature of the changes across patients. Adopting a case-comparison approach, we demonstrated that two LPA patients with comparable expressive language impairment (determined on Factor 1) diverge considerably on their visuo-executive performance. Importantly, this pattern was present even when comparing pairs of LPA patients with mild, moderate or severe language difficulties, suggesting attention, executive and visuospatial deficits are core features of the LPA syndrome. From a clinical standpoint, our findings align well with previous descriptions of single cases of LPA presenting with ‘atypical’ symptoms. For example, single cases of LPA have been described to present with a marked breakdown in attentional processing manifesting in hemi-spatial neglect (Zilli and Heilman, 2016). Similarly, individuals with LPA have been described as presenting with profound and co-occurring visuospatial disturbances notable in judging distances and reach-to-grasp difficulties (Fitzpatrick et al., 2019). Importantly, these ‘atypical’ symptoms emerged in the context of otherwise language deficits and atrophy profiles typical of LPA (Zilli and Heilman, 2016; Fitzpatrick et al., 2019). Our case-comparison findings indicate that marked individual-level variability in non-linguistic cognitive performance is a key feature of LPA and suggest caution in excluding cases who present with such early co-occurring deficits.
We next explored associations between factor scores and cortical and subcortical brain changes in LPA. Performance on the speech production and verbal memory factor was found to correlate with grey and white matter changes of the left middle frontal gyrus. This region is a key frontal node of the language and executive processing networks, with well-described roles in supporting fluency in expressive language (Abrahams et al., 2003; Rogalski et al., 2011a) and working memory (Whitwell et al., 2015b). In particular, middle frontal, along with neighbouring prefrontal cortical regions are posited to play a role in maintaining information within working memory (D'Esposito and Postle, 1999). Disrupted functional connectivity of the middle frontal gyrus with prefrontal, lateral and medial parietal regions has been linked to working memory impairments in LPA (Whitwell et al., 2015b), with cortical thickness of this region further associated with reduced verbal fluency (as measured by mean length of utterance during story telling) in patients with primary progressive aphasia (Rogalski et al., 2011a). Although not typical of the early LPA atrophy pattern, middle frontal gyrus atrophy has been described previously in the syndrome (Rohrer et al., 2010; Phillips et al., 2019) and tends to become more salient as atrophy progresses along the left sylvian fissure into fronto-insular regions (Rohrer et al., 2013). It is possible, therefore, that this middle frontal region shows greater inter-participant variance and thus greater sensitivity to detect associations in the VBM correlation analyses. This is in contrast to the left temporoparietal cortices which are atrophied early and consistently in LPA patients, and thus, resultantly, have low atrophy variance across the group. Future explorations of the temporal unfolding of cortical atrophy patterns and their inter-participant variance, in relation to the cognitive profiles outlined here will be important.
Turning our attention to Factor 2, performance on the visuospatial and executive factor was found to correlate with grey and white matter intensity of right-lateralized temporoparietal and prefrontal regions, including precentral, inferior parietal, lateral temporal, inferior frontal and insular cortices. Moreover, LPA patients with poorer scores on the visuospatial and executive factor tended to demonstrate greater right-hemisphere temporoparietal and prefrontal involvement. Right-lateralized regions such as precentral gyrus and superior/inferior parietal regions are typically proposed to regulate goal-directed and stimulus-driven attentional abilities (Corbetta and Shulman, 2002), while middle/inferior frontal regions have been noted to aid in executive processing by regulating control and inhibitory functions (Aron et al., 2004; Sridharan et al., 2008), respectively. More generally, right-hemisphere frontoparietal regions also form key nodes of the multiple demand network of the brain—a neurocognitive system exerting cognitive control and enabling flexibility towards successful performance across diverse cognitive domains (Cole et al., 2013; Camilleri et al., 2018; Marek and Dosenbach, 2018). Accordingly, primary dysfunction of right-parietal regions, such as that noted in hemispatial neglect, results in multiple cognitive dysfunctions spanning attention, episodic memory and executive control (see e.g. Lee et al., 2008), presumably by disrupting shared underlying cognitive control and flexibility computations. Such a pattern has also been noted in LPA, wherein the presence of right-hemisphere frontal and temporoparietal atrophy reliably signals the emergence of attentional, executive and general cognitive impairments in the syndrome (Machulda et al., 2013). Similarly, although impairment in single-word comprehension currently forms an exclusion criterion for the diagnosis of LPA (Gorno-Tempini et al., 2011), recent studies incorporating in vivo confirmation of underlying Alzheimer’s pathology revealed marked single-word comprehension difficulties in LPA (Leyton et al., 2015; Louwersheimer et al., 2016). In fact, LPA patients with single-word comprehension impairment tend to demonstrate greater atrophy to right-lateralized temporal regions, centred on the fusiform and inferior/middle temporal cortices (Faria et al., 2014; Leyton et al., 2015). We speculate that encroachment of atrophy into right temporoparietal and prefrontal grey/white matter may predict the onset of visuospatial and executive performance impairments in LPA; however, longitudinal studies will be crucial to test this proposal.
The current findings must be interpreted in the context of certain caveats. First, the majority of our LPA patients have not yet come to autopsy, precluding confirmation of underlying Alzheimer versus non-Alzheimer pathology in our cohort. Nevertheless, we rigorously applied the diagnostic criteria of LPA (Gorno-Tempini et al., 2011) to ensure the exclusion of other primary progressive aphasia syndromes presenting with primary semantic processing or grammatical impairments. Studies employing PCA approaches necessarily rely upon the nature of data fed into the model. Given that this was a retrospective study, we were constrained by the cognitive measures available to us, however, we included detailed standardized measures of multiple cognitive domains, leading to findings that, in the context of the existing literature, make intuitive sense. Given emerging evidence of behavioural and neuropsychiatric changes in LPA (e.g. increased reports of anxiety; Magnin et al., 2013), future studies will benefit from exploring if behavioural and functional changes in LPA occur independently of language impairment in the syndrome or co-occur with the visuospatial and executive factor identified here. Of further importance is the need to establish associations between cognitive factors and underlying pathological markers in LPA, given extant evidence for distinct patterns of cognitive performance and lateralized deposition of underlying pathology in LPA patients with underlying Alzheimer versus non-Alzheimer pathology (Mesulam et al., 2008; Whitwell et al., 2015a; Giannini et al., 2017). Finally, we reported our VBM results at an uncorrected threshold of P < 0.001, however, this threshold is far more conservative than traditional multiple comparison approaches such as false discovery rate and is increasingly used when exploring links between cognition and neurodegeneration (Whitwell et al., 2010; Sheelakumari et al., 2019).
Despite these limitations, our findings hold important clinical implications relevant to the diagnosis and characterization of LPA. Identification of heterogeneity in cognitive function in LPA underscores the need for comprehensive neuropsychological workup beyond language in primary progressive aphasia. By limiting their primary focus to language impairments, clinicians will underestimate the presence and severity of visuospatial and executive impairments in LPA, potentially leading to increased functional disturbances and carer burden. We further speculate that the emergence of visuospatial and executive impairments in LPA can be thought of as converse to atypical variants of Alzheimer’s disease such as posterior cortical atrophy. Although described as a syndrome with preponderant visual disturbances due to early right-sided parietal atrophy, posterior cortical atrophy patients gradually demonstrate increasing language and verbal working memory dysfunction (Crutch et al., 2013; Trotta et al., 2019). This would suggest the existence of a possible continuum between these syndromes, with LPA unfolding to resemble posterior cortical atrophy later in the disease course (Fitzpatrick et al., 2019). More generally, these collective results might imply that typical Alzheimer’s disease and its multiple atypical subtypes might all be reconceptualized in terms of graded variations within a single multiple dimensional space (Lambon Ralph et al., 2003). Future studies replicating our findings in a larger cohort of LPA patients, as well as directly comparing the cognitive, behavioural and neural trajectories of these syndromes over time will be critical to address this question.
In conclusion, we provide new insights into the syndrome of LPA, by revealing a fundamental impairment of visuospatial and executive processes, independent of the characteristic language difficulties in this syndrome. This visuospatial and executive impairment varies systematically across LPA patients, irrespective of disease severity and correlates with right-lateralized temporoparietal and frontal regions. Our findings reveal the inherent complexity of the LPA syndrome in terms of cognitive profiles and neural atrophy patterns and suggest that reconceptualization of the LPA syndrome and its relationship to typical and atypical variants of Alzheimer’s disease is warranted.
Supplementary material
Supplementary material is available at Brain Communications online.
Supplementary Material
Acknowledgements
The authors are grateful to the patients and families for their continued support of our research. The authors wish to acknowledge the Sydney Informatics Hub funded by The University of Sydney for providing access to High Performance Computing (HPC) facilities.
Funding
This work was supported in part by funding to Forefront, a collaborative research group specialized to the study of frontotemporal dementia and motor neurone disease, from the National Health and Medical Research Council (NHMRC) of Australia program grant (APP1037746) and the Australian Research Council (ARC) Centre of Excellence in Cognition and its Disorders Memory Program (CE110001021). Siddharth Ramanan is supported by a Faculty of Science Ph.D. Research Scholarship from The University of Sydney. Olivier Piguet is supported by an NHMRC Senior Research Fellowship (APP1103258). Muireann Irish is supported by an ARC Future Fellowship (FT160100096) and an ARC Discovery Project (DP180101548). Matthew A. Lambon Ralph is supported by a UKRI-MRC Programme Grant (MR/R023883/1) and an ERC Advanced grant (GAP: 670428 - BRAIN2MIND_NEUROCOMP).
Competing interests
The authors report no competing interests.
Glossary
- ACE-R/ACE-III =
Addenbrooke’s Cognitive Examination – Revised/III
- CBI-R =
Cambridge Behavioural Inventory – Revised
- CDR-FTLD SoB =
Clinical Dementia Rating Frontotemporal Lobar Degeneration Sum of Boxes
- LPA =
logopenic progressive aphasia
- MNI =
Montreal Neurological Institute
- PCA =
principal component analysis
- ROCF =
Rey-Osterrieth Complex Figure
- SPM =
statistical parametric mapping
- SYDBAT =
Sydney Language Battery
- TMT B-A =
Trail Making Test time difference on parts B-A
- VBM =
voxel-based morphometry
References
- Abrahams S, Goldstein LH, Simmons A, Brammer MJ, Williams SC, Giampietro VP, et al. Functional magnetic resonance imaging of verbal fluency and confrontation naming using compressed image acquisition to permit overt responses. Hum Brain Mapp 2003; 20: 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci 2004; 8: 170–7. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate - a practical and powerful approach to multiple testing. J R Stat Soc B 1995; 57: 289–300. [Google Scholar]
- Bergeron D, Gorno-Tempini ML, Rabinovici GD, Santos-Santos MA, Seeley W, Miller BL, et al. Prevalence of amyloid-beta pathology in distinct variants of primary progressive aphasia. Ann Neurol 2018; 84: 729–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambati SM, Amici S, Racine CA, Neuhaus J, Miller Z, Ogar J, et al. Longitudinal gray matter contraction in three variants of primary progressive aphasia: a tenser-based morphometry study. Neuroimage Clin 2015; 8: 345–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler RA, Lambon Ralph MA, Woollams AM. Capturing multidimensionality in stroke aphasia: mapping principal behavioural components to neural structures. Brain 2014; 137: 3248–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts AM, Machulda MM, Duffy JR, Strand EA, Whitwell JL, Josephs KA. Neuropsychological profiles differ among the three variants of primary progressive aphasia. J Int Neuropsychol Soc 2015; 21: 429–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri JA, Muller VI, Fox P, Laird AR, Hoffstaedter F, Kalenscher T, et al. Definition and characterization of an extended multiple-demand network. Neuroimage 2018; 165: 138–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaletto KB, Marx G, Dutt S, Neuhaus J, Saloner R, Kritikos L, et al. Is “Learning” episodic memory? Distinct cognitive and neuroanatomic correlates of immediate recall during learning trials in neurologically normal aging and neurodegenerative cohorts. Neuropsychologia 2017; 102: 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chare L, Hodges JR, Leyton CE, McGinley C, Tan RH, Kril JJ, et al. New criteria for frontotemporal dementia syndromes: clinical and pathological diagnostic implications. J Neurol Neurosurg Psychiatry 2014; 85: 865–70. [DOI] [PubMed] [Google Scholar]
- Cole MW, Reynolds JR, Power JD, Repovs G, Anticevic A, Braver TS. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci 2013; 16: 1348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 2002; 3: 201–15. [DOI] [PubMed] [Google Scholar]
- Crutch SJ, Lehmann M, Warren JD, Rohrer JD. The language profile of posterior cortical atrophy. J Neurol Neurosurg Psychiatry 2013; 84: 460–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR. The dependence of span and delayed-response performance on prefrontal cortex. Neuropsychologia 1999; 37: 1303–15. [DOI] [PubMed] [Google Scholar]
- Ding J, Chen K, Liu H, Huang L, Chen Y, Lv Y, et al. A unified neurocognitive model of semantics language social behaviour and face recognition in semantic dementia. Nat Commun 2020; 11: 2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikelboom WS, Janssen N, Jiskoot LC, van den Berg E, Roelofs A, Kessels RPC. Episodic and working memory function in primary progressive aphasia: a meta-analysis. Neurosci Biobehav Rev 2018; 243–54. [DOI] [PubMed] [Google Scholar]
- Faria AV, Sebastian R, Newhart M, Mori S, Hillis AE. Longitudinal imaging and deterioration in word comprehension in primary progressive aphasia: potential clinical significance. Aphasiology 2014; 28: 948–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fittipaldi S, Ibanez A, Baez S, Manes F, Sedeno L, Garcia AM. More than words: Social cognition across variants of primary progressive aphasia. Neurosci Biobehav R 2019; 100: 263–84. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick D, Blanco-Campal A, Kyne L. A case of overlap posterior cortical atrophy and logopenic variant primary progressive aphasia. Neurologist 2019; 24: 62–5. [DOI] [PubMed] [Google Scholar]
- Foxe D, Irish M, Hodges JR, Piguet O. Verbal and visuospatial span in logopenic progressive aphasia and Alzheimer's disease. J Int Neuropsychol Soc 2013; 19: 247–53. [DOI] [PubMed] [Google Scholar]
- Foxe D, Leyton CE, Hodges JR, Burrell JR, Irish M, Piguet O. The neural correlates of auditory and visuospatial span in logopenic progressive aphasia and Alzheimer's disease. Cortex 2016; 83: 39–50. [DOI] [PubMed] [Google Scholar]
- Funayama M, Nakagawa Y, Yamaya Y, Yoshino F, Mimura M, Kato M. Progression of logopenic variant primary progressive aphasia to apraxia and semantic memory deficits. BMC Neurol 2013; 13: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galantucci S, Tartaglia MC, Wilson SM, Henry ML, Filippi M, Agosta F, et al. White matter damage in primary progressive aphasias: a diffusion tensor tractography study. Brain 2011; 134: 3011–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannini LAA, Irwin DJ, McMillan CT, Ash S, Rascovsky K, Wolk DA, et al. Clinical marker for Alzheimer disease pathology in logopenic primary progressive aphasia. Neurology 2017; 88: 2276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio A, Santelli L, Tomassini V, Bosnell R, Smith S, De Stefano N, et al. Age-related changes in grey and white matter structure throughout adulthood. Neuroimage 2010; 51: 943–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Brambati SM, Ginex V, Ogar J, Dronkers NF, Marcone A, et al. The logopenic/phonological variant of primary progressive aphasia. Neurology 2008; 71: 1227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol 2004; 55: 335–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology 2011; 76: 1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halai AD, Woollams AM, Lambon Ralph MA. Using principal component analysis to capture individual differences within a unified neuropsychological model of chronic post-stroke aphasia: revealing the unique neural correlates of speech fluency, phonology and semantics. Cortex 2017; 86: 275–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelton JL, Irish M, Hodges JR, Piguet O, Kumfor F. Cognitive and affective empathy disruption in non-fluent primary progressive aphasia syndromes. Brain Impair 2017; 18: 117–29. [Google Scholar]
- Henry ML, Gorno-Tempini ML. The logopenic variant of primary progressive aphasia. Curr Opin Neurol 2010; 23: 633–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P, Sajjadi SA, Patterson K, Nestor PJ. Data-driven classification of patients with primary progressive aphasia. Brain Lang 2017; 174: 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh S, Schubert S, Hoon C, Mioshi E, Hodges JR. Validation of the Addenbrooke's cognitive examination III in frontotemporal dementia and Alzheimer's disease. Dement Geriatr Cogn Disord 2013; 36: 242–50. [DOI] [PubMed] [Google Scholar]
- Ilin A, Raiko T. Practical approaches to principal component analysis in the presence of missing values. J Mach Learn Res 2010; 11: 1957–2000. [Google Scholar]
- Ingram RU, Halai AD, Pobric G, Sajjadi S, Patterson K, Lambon Ralph MA, Graded, multi-dimensional intragroup and intergroup variations in primary progressive aphasia and post-stroke aphasia. bioRxiv 2019: 2019.12.29.882068. [DOI] [PMC free article] [PubMed]
- Jolliffe IT. Principal component analysis. 2nd edn New York, USA: Springer, 2002. [Google Scholar]
- Knopman DS, Kramer JH, Boeve BF, Caselli RJ, Graff-Radford NR, Mendez MF, et al. Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain 2008; 131: 2957–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan K, Machulda MM, Whitwell JL, Butts AM, Duffy JR, Strand EA, et al. Varying degrees of temporoparietal hypometabolism on FDG-PET reveal amyloid-positive logopenic primary progressive aphasia is not a homogeneous clinical entity. J Alzheimers Dis 2016; 55: 1019–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummerer D, Hartwigsen G, Kellmeyer P, Glauche V, Mader I, Kloppel S, et al. Damage to ventral and dorsal language pathways in acute aphasia. Brain 2013; 136: 619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph MA, Patterson K, Graham N, Dawson K, Hodges JR. Homogeneity and heterogeneity in mild cognitive impairment and Alzheimer's disease: a cross-sectional and longitudinal study of 55 cases. Brain 2003; 126: 2350–62. [DOI] [PubMed] [Google Scholar]
- Lansdall CJ, Coyle-Gilchrist ITS, Jones PS, Vazquez Rodriguez P, Wilcox A, Wehmann E, et al. Apathy and impulsivity in frontotemporal lobar degeneration syndromes. Brain 2017; 140: 1792–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BH, Kim EJ, Ku BD, Choi KM, Seo SW, Kim GM, et al. Cognitive impairments in patients with hemispatial neglect from acute right hemisphere stroke. Cogn Behav Neurol 2008; 21: 73–6. [DOI] [PubMed] [Google Scholar]
- Leyton CE, Ballard KJ, Piguet O, Hodges JR. Phonologic errors as a clinical marker of the logopenic variant of PPA. Neurology 2014; 82: 1620–7. [DOI] [PubMed] [Google Scholar]
- Leyton CE, Hodges JR, McLean CA, Kril JJ, Piguet O, Ballard KJ. Is the logopenic-variant of primary progressive aphasia a unitary disorder? Cortex 2015; 67: 122–33. [DOI] [PubMed] [Google Scholar]
- Leyton CE, Piguet O, Savage S, Burrell J, Hodges JR. The neural basis of logopenic progressive aphasia. J Alzheimers Dis 2012; 32: 1051–9. [DOI] [PubMed] [Google Scholar]
- Leyton CE, Villemagne VL, Savage S, Pike KE, Ballard KJ, Piguet O, et al. Subtypes of progressive aphasia: application of the International Consensus Criteria and validation using beta-amyloid imaging. Brain 2011; 134: 3030–43. [DOI] [PubMed] [Google Scholar]
- Louwersheimer E, Keulen MA, Steenwijk MD, Wattjes MP, Jiskoot LC, Vrenken H, et al. Heterogeneous language profiles in patients with primary progressive aphasia due to Alzheimer's disease. J Alzheimers Dis 2016; 51: 581–90. [DOI] [PubMed] [Google Scholar]
- Machulda MM, Whitwell JL, Duffy JR, Strand EA, Dean PM, Senjem ML, et al. Identification of an atypical variant of logopenic progressive aphasia. Brain and Language 2013; 127: 139–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnin E, Chopard G, Ferreira S, Sylvestre G, Dariel E, Ryff I, et al. Initial neuropsychological profile of a series of 20 patients with logopenic variant of primary progressive aphasia. JAD 2013; 36: 799–808. [DOI] [PubMed] [Google Scholar]
- Marek S, Dosenbach NUF. The frontoparietal network: function, electrophysiology, and importance of individual precision mapping. Dialogues Clin Neurosci 2018; 20: 133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruta C, Pereira T, Madeira SC, De Mendonca A, Guerreiro M. Classification of primary progressive aphasia: do unsupervised data mining methods support a logopenic variant? Amyotroph Lateral Scler Frontotemporal Degener 2015; 16: 147–59. [DOI] [PubMed] [Google Scholar]
- Mesulam M, Wicklund A, Johnson N, Rogalski E, Leger GC, Rademaker A, et al. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol 2008; 63: 709–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Weintraub S, Rogalski EJ, Wieneke C, Geula C, Bigio EH. Asymmetry and heterogeneity of Alzheimer's and frontotemporal pathology in primary progressive aphasia. Brain 2014; 137: 1176–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mioshi E, Dawson K, Mitchell J, Arnold R, Hodges JR. The Addenbrooke's Cognitive Examination Revised (ACE-R): a brief cognitive test battery for dementia screening. Int J Geriat Psychiatry 2006; 21: 1078–85. [DOI] [PubMed] [Google Scholar]
- Mirman D, Chen Q, Zhang Y, Wang Z, Faseyitan OK, Coslett HB, et al. Neural organization of spoken language revealed by lesion-symptom mapping. Nat Commun 2015; 6: 6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multani N, Galantucci S, Wilson SM, Shany-Ur T, Poorzand P, Growdon ME, et al. Emotion detection deficits and changes in personality traits linked to loss of white matter integrity in primary progressive aphasia. Neuroimage Clin 2017; 16: 447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murley AG, Coyle-Gilchrist I, Rouse MA, Jones PS, Li W, Wiggins J, et al. Redefining the multidimensional clinical phenotypes of frontotemporal lobar degeneration syndromes. Brain 2020; 143: 1555–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterrieth P. Filetest de copie d'une figure complex: contribution a l'etude de la perception et de la memoire [The test of copying a complex figure: a contribution to the study of perception and memory]. Archives de Psychologie 1944; 30: 286–356. [Google Scholar]
- Owens TE, Machulda MM, Duffy JR, Strand EA, Clark HM, Boland S, et al. Patterns of neuropsychological dysfunction and cortical volume changes in logopenic aphasia. J Alzheimers Dis 2018; 66: 1015–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JS, Da Re F, Irwin DJ, McMillan CT, Vaishnavi SN, Xie SX, et al. Longitudinal progression of grey matter atrophy in non-amnestic Alzheimer's disease. Brain 2019; 142: 1701–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzebon M, Douglas J, Ames D. Spousal recollections of early signs of primary progressive aphasia. Int J Lang Comm Dis 2018; 53: 282–93. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: a language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing, 2016. [Google Scholar]
- Rabinovici GD, Jagust WJ, Furst AJ, Ogar JM, Racine CA, Mormino EC, et al. Abeta amyloid and glucose metabolism in three variants of primary progressive aphasia. Ann Neurol 2008; 64: 388–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanan S, de Souza LC, Moreau N, Sarazin M, Teixeira AL, Allen Z, et al. Determinants of theory of mind performance in Alzheimer's disease: a data-mining study. Cortex 2017; 88: 8–18. [DOI] [PubMed] [Google Scholar]
- Ramanan S, Flanagan E, Leyton CE, Villemagne VL, Rowe CC, Hodges JR, et al. Non-verbal episodic memory deficits in primary progressive aphasias are highly predictive of underlying amyloid pathology. J Alzheimers Dis 2016; 51: 367–76. [DOI] [PubMed] [Google Scholar]
- Ramanan S, Foxe D, El-Omar H, Ahmed RM, Hodges JR, Piguet O, et al. Evidence for a pervasive autobiographical memory impairment in logopenic progressive aphasia: clinical and neural correlates. medRxiv 2020. a. [DOI] [PubMed]
- Ramanan S, Marstaller L, Hodges JR, Piguet O, Irish M. Understanding the neural basis of episodic amnesia in logopenic progressive aphasia: A multimodal neuroimaging study. Cortex 2020. b; 125: 272–87. [DOI] [PubMed] [Google Scholar]
- Ramsey LE, Siegel JS, Lang CE, Strube M, Shulman GL, Corbetta M. Behavioural clusters and predictors of performance during recovery from stroke. Nat Hum Behav 2017; 1. doi: 10.1038/s41562-016-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills 1958; 8: 271–6. [Google Scholar]
- Rogalski E, Cobia D, Harrison TM, Wieneke C, Thompson CK, Weintraub S, et al. Anatomy of language impairments in primary progressive aphasia. J Neurosci 2011. a; 31: 3344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski E, Cobia D, Harrison TM, Wieneke C, Weintraub S, Mesulam MM. Progression of language decline and cortical atrophy in subtypes of primary progressive aphasia. Neurology 2011. b; 76: 1804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski E, Cobia D, Martersteck A, Rademaker A, Wieneke C, Weintraub S, et al. Asymmetry of cortical decline in subtypes of primary progressive aphasia. Neurology 2014; 83: 1184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Caso F, Mahoney C, Henry M, Rosen HJ, Rabinovici G, et al. Patterns of longitudinal brain atrophy in the logopenic variant of primary progressive aphasia. Brain Lang 2013; 127: 121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Ridgway GR, Crutch SJ, Hailstone J, Goll JC, Clarkson MJ, et al. Progressive logopenic/phonological aphasia: erosion of the language network. Neuroimage 2010; 49: 984–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Rossor MN, Warren JD. Alzheimer's pathology in primary progressive aphasia. Neurobiol Aging 2012; 33: 744–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjadi SA, Patterson K, Arnold RJ, Watson PC, Nestor PJ. Primary progressive aphasia: a tale of two syndromes and the rest. Neurology 2012; 78: 1670–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Santos MA, Rabinovici GD, Iaccarino L, Ayakta N, Tammewar G, Lobach I, et al. Rates of amyloid imaging positivity in patients with primary progressive aphasia. JAMA Neurol 2018; 75: 342–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage S, Hsieh S, Leslie F, Foxe D, Piguet O, Hodges JR. Distinguishing subtypes in primary progressive aphasia: application of the Sydney language battery. Dement Geriatr Cogn Disord 2013; 35: 208–18. [DOI] [PubMed] [Google Scholar]
- Sheelakumari R, Bineesh C, Varghese T, Kesavadas C, Verghese J, Mathuranath PS. Neuroanatomical correlates of apathy and disinhibition in behavioural variant frontotemporal dementia. Brain Imaging Behav 2019. doi: 10.1007/s11682-019-00150-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So M, Foxe D, Kumfor F, Murray C, Hsieh S, Savage G, et al. Addenbrooke's cognitive examination III: psychometric characteristics and relations to functional ability in dementia. J Int Neuropsychol Soc 2018; 24: 854–63. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA 2008; 105: 12569–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E, Sherman EMS, Spreen O, A compendium of neuropsychological tests: administration, norms, and commentary. 3rd edn. Oxford: Oxford University Press, 2006. [Google Scholar]
- Teichmann M, Kas A, Boutet C, Ferrieux S, Nogues M, Samri D, et al. Deciphering logopenic primary progressive aphasia: a clinical, imaging and biomarker investigation. Brain 2013; 136: 3474–88. [DOI] [PubMed] [Google Scholar]
- Tipping ME, Bishop CM. Probabilistic principal component analysis. J R Stat Soc B 1999; 61: 611–22. [Google Scholar]
- Trotta L, Lamoureux D, Bartolomeo P, Migliaccio R. Working memory in posterior cortical atrophy. Neurol Sci 2019; 40: 1713–6. [DOI] [PubMed] [Google Scholar]
- Tu S, Leyton CE, Hodges JR, Piguet O, Hornberger M. Divergent longitudinal propagation of white matter degradation in logopenic and semantic variants of primary progressive aphasia. J Alzheimers Dis 2015; 49: 853–61. [DOI] [PubMed] [Google Scholar]
- Watson CL, Possin K, Allen IE, Hubbard HI, Meyer M, Welch AE, et al. Visuospatial functioning in the primary progressive aphasias. J Int Neuropsychol Soc 2018; 24: 259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Duffy JR, Strand EA, Machulda MM, Senjem ML, Schwarz CG, et al. Clinical and neuroimaging biomarkers of amyloid-negative logopenic primary progressive aphasia. Brain Lang 2015. a; 142: 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Jack CR, Jr, Boeve BF, Parisi JE, Ahlskog JE, Drubach DA, et al. Imaging correlates of pathology in corticobasal syndrome. Neurology 2010; 75: 1879–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Jones DT, Duffy JR, Strand EA, Machulda MM, Przybelski SA, et al. Working memory and language network dysfunctions in logopenic aphasia: a task-free fMRI comparison with Alzheimer's dementia. Neurobiol Aging 2015. b; 36: 1245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win KT, Pluta J, Yushkevich P, Irwin DJ, McMillan CT, Rascovsky K, et al. Neural correlates of verbal episodic memory and lexical retrieval in logopenic variant primary progressive aphasia. Front Neurosci 2017; 11. doi: 10.3389/fnins.2017.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilli EM, Heilman KM. Spatial neglect in a patient with logopenic progressive aphasia. Neurocase 2016; 22: 30–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The ethical requirement to ensure patient confidentiality precludes public archiving of our data. Researchers who would like to access the raw data should contact the corresponding authors, who will liaise with the ethics committee that approved the study, and accordingly, as much data that are required to reproduce the results will be released to the individual researcher. The code used for this project has been made available for review on the Open Science Framework website (https://osf.io/bn534/). No part of the study procedures or analyses were preregistered prior to the research being undertaken.