Abstract
Homoeostatic metaplasticity is a neuroprotective physiological feature that counterbalances Hebbian forms of plasticity to prevent network destabilization and hyperexcitability. Recent animal models highlight dysfunctional homoeostatic metaplasticity in the pathogenesis of Alzheimer’s disease. However, the association between homoeostatic metaplasticity and cognitive status has not been systematically characterized in either demented or non-demented human populations, and the potential value of homoeostatic metaplasticity as an early biomarker of cognitive impairment has not been explored in humans. Here, we report that, through pre-conditioning the synaptic activity prior to non-invasive brain stimulation, the association between homoeostatic metaplasticity and cognitive status could be established in a population of non-demented human subjects (older adults across cognitive spectrums; all within the non-demented range). All participants (n = 40; age range, 65–74, 47.5% female) underwent a standardized neuropsychological battery, magnetic resonance imaging and a transcranial magnetic stimulation protocol. Specifically, we sampled motor-evoked potentials with an input/output curve immediately before and after repetitive transcranial magnetic stimulation to assess neural plasticity with two experimental paradigms: one with voluntary muscle contraction (i.e. modulated synaptic activity history) to deliberately introduce homoeostatic interference, and one without to serve as a control condition. From comparing neuroplastic responses across these experimental paradigms and across cohorts grouped by cognitive status, we found that (i) homoeostatic metaplasticity is diminished in our cohort of cognitively impaired older adults and (ii) this neuroprotective feature remains intact in cognitively normal participants. This novel finding suggests that (i) future studies should expand their scope beyond just Hebbian forms of plasticity that are traditionally assessed when using non-invasive brain stimulation to investigate cognitive ageing and (ii) the potential value of homoeostatic metaplasticity in serving as a biomarker for cognitive impairment should be further explored.
Keywords: dementia, plasticity, cognitive ageing, TMS, mild cognitive impairment
Neural plasticity was assessed in healthy older adults with TMS. Upon neuropsychological evaluation, participants were stratified using validated cutoffs for cognitive dysfunction. Sundman et al. observed divergent neural plasticity profiles, particularly when stimulation was preceded by synaptic pre-conditioning. This suggests that mechanisms of metaplasticity may be associated with cognitive decline.
Graphical Abstract
Graphical Abstract.
Introduction
A conceptual shift has occurred in the field of Alzheimer’s disease research to recognize the disease along a wider spectrum, with greater consideration for pre-clinical neuropathological alterations that precede the manifestation of clinical symptoms (Dubois et al., 2016). This pathophysiological onset of disease can precede the clinical syndrome of amnestic dementia by decades (Caselli and Reiman, 2013). In order to develop targeted interventions for disease modification and prevention, there is an urgent need for enhanced characterization of the neurophysiological features at this pre-clinical stage. One potential surrogate marker is altered neural plasticity.
Neural plasticity can be broadly defined as the brain’s ability to reorganize in the presence of external or internal stimuli in order to adapt to a lifetime of experiences in our capricious environments. Importantly, the nervous system’s ability to reorganize represents a critical neurobiological underpinning of learning and memory (Lynch, 2004). Long-term potentiation (LTP), famously conceptualized by Hebb as neurons that fire together, wire together, is a well-studied mechanism of neural plasticity that results in synapse-specific strengthening. Long-term potentiation, however, carries high potential for network destabilization since it is a positive feedback loop that must be tightly regulated to prevent runaway excitability (Abbott and Nelson, 2000). This can be achieved through the phenomena of homoeostatic metaplasticity, referred to as the plasticity of synaptic plasticity, which dynamically refines synaptic scaling in an activity-dependent manner (Abraham and Bear, 1996). In the presence of prolonged neural activity, for example, homoeostatic metaplasticity spawns a compensatory synaptic downscaling to make subsequent LTP less favorable (Jang and Chung, 2016). This homoeostatic synaptic downscaling simultaneously promotes long-term depression, which, as the inverse of LTP, is the process of synapse-specific weakening in an activity-dependent manner. Thus, the stable, yet flexible neural function that enables complex human behavior depends on the dynamic interplay between mechanisms of homoeostatic and Hebbian synaptic plasticity (Turrigiano et al., 1998; Tononi and Cirelli, 2014; Fernandes and Carvalho, 2016). Long-term potentiation deficits in early Alzheimer’s disease has long been appreciated (Rowan et al., 2003), but there is accumulating evidence, implicating diminished homoeostatic metaplasticity in the genesis of the pathophysiological cascade (Jang and Chung, 2016; Frere and Slutsky, 2018).
Non-invasive brain stimulation technologies, like transcranial magnetic stimulation (TMS), can be used to characterize changes in neural plasticity in healthy aging and in neurological disease (Freitas et al., 2013). To do so, TMS is applied to the motor cortex in which single-pulse measures of cortical excitability (motor-evoked potentials; MEPs) are interleaved with a repetitive TMS (rTMS) paradigm. The degree of change in the amplitude of MEPs induced by the patterned rTMS is representative of neural reorganization and, thus, is inferred as an index of neural plasticity (Bestmann and Krakauer, 2015; Rossini et al., 2015). Depending on the frequency of patterned stimuli employed, rTMS paradigms can induce either long-term depression or LTP-like neural plasticity (Ziemann et al., 2008). Despite well-documented inter-individual variability, a recent meta-analysis reports that the intermittent theta burst stimulation (iTBS) variant of rTMS robustly increases cortical excitability, which is evidenced by a relative increase in the amplitude of MEPs and thought to reflect LTP-like effects (Huang et al., 2005; Wischnewski and Schutter, 2015). Though it is impossible to directly demonstrate a link between the effects of rTMS and synaptic plasticity in the human brain, several lines of converging evidence in support of this inference have been reviewed elsewhere (Hoogendam et al., 2010).
Though alterations to the primary motor cortex (M1) are generally not representative of cognitive function, TMS-M1 studies afford outsized utility for the characterization of neurophysiological features of cognitive aging relative to other stimulation sites. Notably, TMS-M1 studies among populations with Alzheimer’s disease and mild cognitive impairment suggest that M1 is not exempt from pathophysiological changes that underpin cognitive decline in hallmark brain regions of dementia. Recent systemic reviews highlight a rich body of literature in this clinical population that points to both baseline hyperexcitability and impaired motor cortical plasticity in response to rTMS (Freitas et al., 2011; Bunse et al., 2014). A deeper examination of TMS-M1 studies in Alzheimer’s disease indicates that deficits in LTP-like plasticity are observed in both newly diagnosed and late-stage patients (Koch et al., 2012; Nardone et al., 2014; Di Lorenzo et al., 2016; Motta et al., 2018). Furthermore, this body of research also suggests that LTP-like deficits are correlated with both the severity of clinical symptoms and the biomarkers like CSF-Tau (Di Lorenzo et al., 2016; Koch et al., 2017). Among patients diagnosed with mild cognitive impairment, findings pertaining to LTP-like plasticity are less consistent (Trebbastoni et al., 2015; Lahr et al., 2016). Thus, even earlier along the spectrum of disease (i.e. pre-clinical), measures of Hebbian-like plasticity may lack the sensitivity to characterize neurophysiological features of pre-clinical Alzheimer’s disease.
When adding different synaptic activation pre-conditions to the experimental design, TMS paradigms can actually be used to probe homoeostatic metaplasticity. Briefly, this can be achieved either by exogenous or by endogenous experimental modulation of synaptic activation history prior to TMS application. The former can be achieved by combining multiple rTMS exposures in short succession to ‘prime’ (i.e. exogenously modulate the synaptic activation history) with the first bout of rTMS prior to the subsequent ‘test’ application of rTMS (Müller et al., 2007; Murakami et al., 2012). Such studies report that increasing the preceding activation history by priming the stimulation site with iTBS precludes the otherwise faciliatory effects for subsequent exposure to iTBS, which is indicative of homoeostatic metaplasticity (i.e. activity-dependent synaptic down-scaling following the iTBS priming). Alternatively, the synaptic activation history can also be modulated endogenously by instructing participants to voluntarily contract the muscle that corresponds with the targeted stimulation site, which, in turn, alters the synaptic activation history of the targeted cortical tissue (Vallence and Ridding, 2014; Müller-Dahlhaus and Ziemann, 2015). There are numerous reports in healthy younger adults, demonstrating that voluntary contraction of the targeted hand muscle prior to TBS introduces homoeostatic interference that prevents the induction of Hebbian-like neural plasticity (Gentner et al., 2008; Iezzi et al., 2008; Goldsworthy et al., 2014).
In this study, we sought to investigate this neurophysiological phenomenon of homoeostatic metaplasticity in older adults across the cognitive spectrum within the non-demented range. Specifically, in addition to assessing the LTP-like response to iTBS in the conventional fashion, we introduced an experimental paradigm that artificially elevated the preceding synaptic activation history of the targeted cortical tissue to introduce homoeostatic interference. Furthermore, in the scope of cognitive aging, it was our objective to investigate this phenomenon in association with cognitive status in a population of healthy older adults. With this approach, we could investigate how the iTBS effect is influenced by (i) cognitive status, (ii) the experimental paradigm (i.e. Hebbian versus Homoeostatic TMS paradigm) and (iii) the interactions therein. To the best of our knowledge, this is the first report to characterize the integrity of the dynamic interplay between homoeostatic metaplasticity and Hebbian plasticity in older adults presenting with cognitive impairment.
Materials and methods
Participants
Forty community dwelling, healthy older adults (mean ± standard deviation: 69.2 ± 2.9 years, age range: 65–74 years, 19 women) enrolled in this study. No participants had a clinical diagnosis for either mild cognitive impairment or Alzheimer’s disease. Exclusion criteria also included: (i) diagnosis of any clinical neuropsychiatric condition, (ii) medications and/or recreational drugs potentially affecting the central nervous system and (iii) contraindications to TMS/magnetic resonance imaging (MRI) that would adversely influence the safety profile. All experimental procedures were approved by the University of Arizona Institutional Review Board. Informed written consent was obtained in accordance with the Declaration of Helsinki.
Experimental design
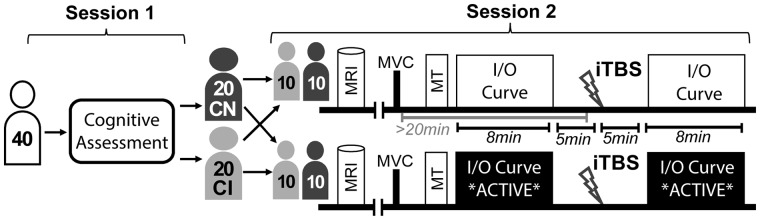
Each participant attended two study visits (Fig. 1). During session 1, participants completed a comprehensive neuropsychological battery. As detailed below, groups were counterbalanced to include 20 classified as cognitively normal (CN) and 20 classified as cognitively impaired (CI).
Figure 1.
Experimental design. Participants were stratified by cognitive status and randomly assigned to participate in one of the two experimental paradigms. Thus, there were four experimental cohorts, each with 10 participants. CN: cognitively normal; CI: cognitively impaired; MRI: magnetic resonance imaging; MVC: maximum voluntary contraction; MT: motor threshold; I/O: input/output; iTBS: intermittent theta burst stimulation
Session 2 was composed of both MRI and TMS. Transcranial magnetic stimulation was applied to the primary motor cortex. A single-pulse protocol was used to assess the features of cortical excitability both immediately before and after the application of iTBS. During single-pulse protocols, MEPs were measured with surface electromyography (EMG). There were two distinct experimental TMS paradigms and each participant was randomly assigned to one of the two paradigms:
The ‘active’ condition, during which participants were instructed to maintain a steady voluntary muscle contraction corresponding with 10–20% maximal force for the entirety of the 8-min sampling window. This paradigm modulated synaptic activation history of the targeted cortical tissue to probe homoeostatic metaplasticity.
The ‘rest’ condition during which participants were instructed to keep the muscles corresponding with the targeted stimulation site relaxed prior to the application of iTBS. In the ‘rest condition’, care was taken to make sure that the targeted muscle was relaxed for >20 min prior to the iTBS application to avoid potential homoeostatic interference. As a control condition, this paradigm probed Hebbian forms of plasticity.
Thus, as shown in Fig. 1, there were four subgroups: ‘Rest—Cog Normal, Rest—Cog Impaired, Active—Cog Normal, Active—Cog Impaired’. These subgroups were counterbalanced for age, sex and education (Table 1).
Table 1.
Participant characteristics
| Rest CN; n = 10 | Active CN; n = 10 | Rest CI; n = 10 | Active CI; n = 10 | Group comparisons | |
|---|---|---|---|---|---|
| Age (years) | 69.3 ± 2.6 | 67.9 ±2.9 | 68.8 ± 2.6 | 70.8 ± 2.1 | P > 0.1 |
| Sex | 7m, 3f | 5m, 5f | 5m, 5f | 4m, 6f | P > 0.1 |
| Education | 17.2 ± 1.9 | 16.6 ± 1.9 | 16.4 ± 2.3 | 16.8 ± 2.5 | P > 0.1 |
| Resting MT (%MSO) | 50.7 ± 6.5 | 52.9 ± 10.2 | 51.6 ± 6.7 | 50.7 ± 6.5 | P > 0.1 |
| Active MT (%MSO) | 43.8 ± 4.6 | 45.8 ± 7.7 | 46.4 ± 5.9 | 47.8 ± 10.2 | P > 0.1 |
| Montreal Cognitive Assessment (MoCA) | 28.2 ± 1.3a | 28.1 ± 2.6b | 26.4 ± 1.5a | 24.9 ± 2.1b | P < 0.005 |
| Average UDSNB-3 Normalized Score | 0.22 ± 0.3a | 0.31 ± 0.5b | −0.32 ± 0.4a | −0.64 ± 0.4b | P < 0.001 |
This table presents participant characteristics across the four experimental cohorts. To assess differences across the four groups, a one-way ANOVA was performed across the four cohorts. There was no significant difference among demographic characteristics, but as expected, there is a significant difference in the measures of cognition. MoCA is a general measure of cognition often used to screen cognitive deficits. The other measure included is a composite score of the UDSNB-3, which is an average of all adjusted Z-scores in the tasks comprising this battery.
Pairwise comparisons were performed with FLSD at P < 0.05.
Abbreviations: CN: Cognitively normal, CI: cognitively impaired, MSO: maximum stimulator output; UDSNB-3: Uniform Data Set Neuropsychological Battery, version 3.0.
Neuropsychological assessment
Cognitive functions were assessed using the National Alzheimer’s Coordinating Center Uniform Data Set Neuropsychological Battery, Version 3 (UDSNB-3; Weintraub et al., 2018). The UDSNB-3 was developed by The Neuropsychology Work Group of the National Institute of Aging Clinical Task Force, and it provides a valuable non-proprietary resource for conducting research on cognitive aging and dementia. The battery comprises the Montreal Cognitive Assessment (Nasreddine et al., 2005), Craft Story (Craft et al., 1996), Digit Span (Wechsler, 1987), Semantic and Verbal Fluency (Besser et al., 2018), Trail-making Test Parts A and B (Reitan and Wolfson, 1985), Benson Complex Figure Test (Possin et al., 2011) and the Multilingual Naming Task (Ivanova et al., 2013).
The standardized nature of the UDSNB-3 enables unique tools, such as the interactive web-based normative calculator that accounts for demographic characteristics (Shirk et al., 2011). Raw scores for each participant were entered into this web-based normative calculator to generate Z-scores that were adjusted for age, sex and education. Using these normalized scores, participants were classified as cognitively impaired according to the so-called ‘comprehensive criteria’ (Jak et al., 2009; Bondi et al., 2014). More specifically, individuals were classified as cognitively impaired if they scored >1 SD below adjusted normative data on either (i) two tasks within the same domain or (ii) three tasks across all domains (Bondi et al., 2014). In support of this strategy, a recent empirical analysis determined this comprehensive criteria to be more accurate than conventional approaches that classify individuals with cognitive impairment on the basis of a single impaired score (Jak et al., 2016).
Electromyography
Electromyography data were collected from the abductor pollicis brevis (APB) muscle, which was identified via manual palpation. Three electrodes were placed in a belly–tendon montage, with the grounding electrode placed on the ulnar tuberosity. The participants were seated upright in a comfortable position, with their arms resting on a supportive pillow. The EMG signal was amplified using a gain of 5000 with a bandpass filter between 30 and 1000 Hz, and a line filter (notch, 60 Hz). Electromyography signal was acquired through analog-to-digital converter (CED 1401) and Spike2 software (version 7.20). The EMG signal was subsequently post-processed using MATLAB to measure MEP response, which was defined as the difference between the maximum and the minimum amplitudes in the window from 20 to 50 ms after each TMS trigger.
Prior to the TMS protocols, the participants’ maximum voluntary contraction (MVC) was measured by instructing participants to pinch their thumb and pinky, and to exert maximal force for ∼5 s.
Transcranial magnetic stimulation
First, the participant’s structural T1 brain scan was uploaded into a 3D Neuronavigation platform and carefully co-registered to landmarks on the participant’s head using an infrared-based frameless stereotactic system (Polaris System, Localite Version 3.0.41). To identify the stimulation target corresponding with the APB muscle, the motor ‘hand knob’ area was visually identified on the left pre-central gyrus for each participant’s anatomical MRI (Yousry et al., 1997). The stimulation target was subsequently refined based on observed MEPs from the APB. The optimal motor hotspot was recorded within the neuronavigation system, which enabled precise coil positioning throughout the duration of the TMS session. The coil handle was oriented roughly perpendicular to the central sulcus to ensure optimal activation of the cortico-motor neural cells (Rossini et al., 2015).
Transcranial magnetic stimulation was delivered with MagPro ×100 (MagVenture Ltd.) equipment. All TMS procedures were completed by the same practitioner to avoid inter-investigator variability (Cueva et al., 2016) and the stimulation parameters conformed to the current guidelines from the International Federation of Clinical Neurophysiology (IFCN) to ensure both the safe application of TMS and the reliable acquisition of single-pulse measures of cortical excitability (Rossi et al., 2009; Rossini et al., 2015). Single-pulse TMS was applied with the ‘CB60’ figure-of-eight coil and iTBS was applied with the ‘Cool-B65 A/P’ coil. For both the procedures, biphasic pulses were delivered with the current flowing in AP-PA (Anterior–Posterior and Posterior–Anterior) directions.
Both resting and active motor thresholds (MT) were estimated with an adaptive method using a freeware program (TMS Motor Threshold Assessment Tool 2.0; http://www.clinicalresearcher.org/software.htm) that employs maximum-likelihood parameter estimation by sequential testing strategy without the need for a priori information. During the active MT acquisition, participants were instructed to maintain a steady muscle contraction in the targeted APB muscle at ∼10–20% of their previously determined MVC. Real-time visual feedback of the root mean square EMG signal was provided for participants to maintain steady muscle contraction. In addition to having physiological relevance (i.e. indicative of neuronal excitability), MTs are used to calibrate the intensity of subsequent TMS protocols.
Intermittent theta burst stimulation
Using the 3D NeuroNavigation platform, the coil was precisely positioned over the previously recorded motor hotspot. The iTBS protocol comprised 600 pulses at 80% of the active MT. The stimuli were patterned in three-pulse bursts at 50 Hz, which repeated at a frequency of 5 Hz, and were delivered in intermittent trains, each lasting 2 s with a 6.9-s inter-train interval for a total duration of 178 s.
Single-pulse TMS protocol
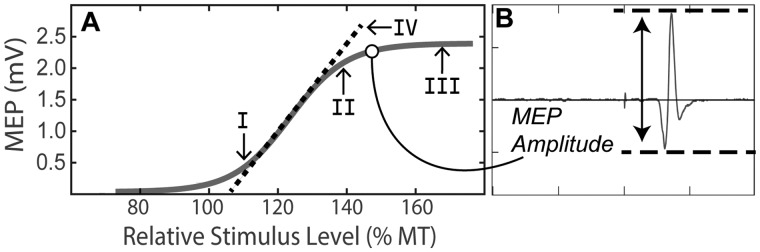
To assess cortical excitability before and after iTBS, we obtained an input-output (I/O) curve to assess the characteristics of MEP as a function of variable TMS intensities. The I/O curve is composed of a flat region at sub-threshold intensities where no MEP is elicited, a steep rise as MEP amplitudes linearly increase with increasing stimulator intensity, and a maximal plateau where MEP amplitude is saturated (Fig. 2) (Goetz et al., 2014). The sigmoidal relationship between the TMS intensity and the MEP amplitude was fit with the Boltzmann equation to evaluate specific parameters of the fitted curve (e.g. slope). The I/O curve was chosen rather than sampling at just a single TMS intensity because it provides a more comprehensive assessment of cortical–spinal excitability (Houdayer et al., 2008; Burke and Pierrot-Deseilligny, 2010; Goetz et al., 2014). Although it presents many advantages, relatively few studies have utilized I/O curves to measure changes in cortical excitability induced by rTMS (Houdayer et al., 2008; Burke and Pierrot-Deseilligny, 2010; Goetz et al., 2014; Vallence et al., 2015).
Figure 2.
Input–output curve. (A) A repetitive I/O curve during the rest paradigm. We examined the neuroplastic response at four distinct components of the curve: (I)110% MT, (II)140% MT, (III) 165% MT and (IV) the calculate slope of the fitted curve. (B) An example of MEP waveform. In this example, the TMS intensity was 145% MT and the peak-to-peak amplitude was determined to be ∼2100 uV. For each curve, 64 such MEPs were plotted with amplitude as a function of TMS intensity. The sigmoidal curve is fit with the Boltzmann equation
As shown in Fig. 1, the duration of the I/O curve protocol was ∼8 min. Upon the completion of the baseline I/O curve, there was a ∼5-min interval before the onset of the iTBS protocol to switch coils and prepare equipment. Following the iTBS protocol, there was another ∼5-min interval prior to the sampling of the post-iTBS I/O curve. Each I/O curve comprised 16 TMS intensity levels (80%, 90–155% with 5% increments, and 165% MT). Data from four MEP trials were acquired at each TMS intensity level, yielding a total of 64 MEPs. The TMS intensity is expressed as a percentage relative to the previously determined MT. The order of the TMS pulses was pseudo-randomized to minimize the potential hysteresis effect and to account for potential fatigue in the active condition (Möller et al., 2009). The inter-stimulus interval was pseudo-randomly jittered (6000–9000 ms) to avoid train effects.
During the active I/O acquisition, real-time visual feedback was provided for participants to maintain steady muscle contraction at 10–20% MVC in the APB muscle. The IFCN guidance for TMS delivered during the active condition calls for ‘slight tonic contraction of the target muscle at approximately 20% of MVC’ (Rossini et al., 2015). We further consulted previous literature to stay in line with this ∼20% of MVC guidance, while also trying to strike a balance between sufficient modulation of cortical activation history and limiting potential fatigue during the prolonged I/O acquisition. To address the former, Goldsworthy and colleagues (2014) reported that 10% of MVC was sufficient to pre-condition TBS response. To address the latter, we consulted studies with active I/O conditions that employed contractions in the 10–20% MVC range (Talelli et al., 2008; Van Den Bos et al., 2017).
MEP analysis
Motor-evoked potentials were quantitatively assessed by measuring peak-to-peak amplitudes of the MEP waveform acquired with EMG. The mean MEP amplitude at each of the 16 intensity levels was calculated for each curve and these mean MEP values were used in the subsequent group analysis. To scrub out non-physiological variance in line noise for sub-threshold intensities with no response detected in the REST condition, an artificial floor of 35 uV was selected (i.e. all values <35 uV were replaced with 35 uV).
Consistent with the literature, the neuroplastic response was assessed as the relative change in MEP amplitude induced by iTBS (i.e. degree of neural reorganization). This effect was captured by normalizing each participant’s post-iTBS MEP values to their respective baseline values for each sampled TMS intensity level. These normalized values reflect the raw difference in MEP amplitude (MEPDELTA = MEPPost-iTBS – MEPPre-iTBS). Thus, any positive grand means are indicative of an increase in cortical excitability, which is a proxy for LTP-like neural plasticity. This normalization procedure was selected because it resulted in values resembling a normal distribution, as opposed to an indexing approach which did not. Furthermore, this normalization procedure is more robust than % change MEP in cases of potential ceiling effect for recordings with higher baseline levels of cortical excitability.
Similar to previous research, we sought to leverage the full utility of the I/O curve by quantifying iTBS-induced neuroplasticity along different components of the curve (Vallence et al., 2015). This is advantageous because different physiological mechanisms are principally involved at different stimulus intensities. This insight comes from the data collected from human participants with electrodes implanted in their cervical spinal cords, from which epidural recordings of descending volleys of activity in the cortical spinal tract can be obtained. Briefly, there are D-waves, which reflect direct activation of pyramidal cell axons that descend from the cortex, and I-waves, which reflect indirect activation of these pyramidal neurons via trans-synaptic transmission from directly activated cortical interneurons (Di Lazzaro et al., 2012; Legatt et al., 2016). Motor-evoked potentials represent the aggregate sum of these descending cortical volleys (Bestmann and Krakauer, 2015). Accordingly, we analyzed the neuroplastic response to iTBS across each of the following four components of the I/O curve (Fig. 2):
110% MT: A recent empirical study investigating differential effects iTBS across the I/O curve components reported that iTBS effects are most pronounced at 110% of MT (Fig. 2, I) (Goldsworthy et al., 2016).
140% MT: This component was selected because, according to guidance from the IFCN, there is predominant contribution of late I-waves in the generation of MEPs at this TMS intensity level (Fig. 2, II) (Groppa et al., 2012). Notably, iTBS aftereffects principally involve these late I-waves.
165% MT: This value is the highest stimulus intensity included in our experimental design, and it represents the maximal plateau of the I/O curve (Fig. 2, III). At maximal intensities, there is typically less I-wave involvement as the pyramidal cell axons are directly stimulated resulting in the ‘D-wave’ (Di Lazzaro et al., 2004).
I/O Slope: This value reflects the rise of the fitted I/O curve (Fig. 2, IV), which was modelled using the Boltzmann’s equation. The slope parameter is believed to reflect the corticospinal pathway’s recruitment gain and/or trans-synaptic synchronization of cortical interneurons (Devanne et al., 1997; Ridding and Rothwell, 1997). If larger pools of neurons are excited in a less synchronous manner, phase cancellation could prevent the expected rise in the I/O curve and result in a reduced slope of the fitted curve (Pitcher et al., 2003).
Statistical analysis
There has been an increasingly salient debate surrounding reproducibility spanning virtually all domains of biomedical sciences, including neuropsychological research (Ioannidis, 2005; Amrhein et al., 2017; Szucs and Ioannidis, 2017; Fanelli, 2018). One of the issues at the centre of this debate is the heavy reliance on null hypothesis significant testing (NHST), i.e., P-values (Wasserstein and Lazar, 2016). Consequently, many have argued that Bayesian analysis should be integrated into ongoing research (Dienes and Mclatchie, 2018; Shrout and Rodgers, 2018), which has several key benefits worth highlighting:
Bayesian parameter estimation provides richer information when examining group differences (Kruschke, 2013).
Bayesian approaches may be particularly useful in the studies that might be underpowered with small sample sizes (McNeish, 2016; Melinscak and Montesano, 2016).
The customizable models offer more flexibility without many of the assumptions and constraints of NHST (Kruschke, 2010).
The posterior distribution of parameter estimates does not change when additional comparisons are made and hence there is no need to correct for multiple comparisons (Kruschke, 2010).
Herein, we report the results from NHST and Bayesian analyses in parallel to assess neuroplastic responses in each of the I/O curve components described above.
Null hypothesis significant testing
Baseline cortical excitability profiles were assessed for differences according to cognitive status. Resting and active MTs were examined independently, using separate one-way ANOVAs with GROUP (Cog Normal, Cog Impaired) as a between-group factor for each measure.
To analyse the iTBS response, traditional NHST analyses of raw (non-normalized) log-transformed MEP values comprised two separate three-way mixed ANOVAs for each of the two experimental paradigms (Active & Rest). Each three-way ANOVA included GROUP (Cog Normal, Cog Impaired) as a between-group factor, and INTENSITY (16 levels) and TIME (Pre-iTBS, Post-iTBS) as within-group factors. Since this analysis was performed on raw data not normalized to baseline values, we did not include PARADIGM as a between-group factor because raw MEPs are inherently different depending on the state of muscle contraction.
To further examine interactions across cognitive status and experimental paradigm, two-way ANOVAs with GROUP (Cog Normal, Cog Impaired) and PARADIGM (Active, Rest) as between-group factors for MEPDELTA at each of the four I/O curve components identified a priori. This analysis provided an examination of GROUP × PARADIGM interaction in the response to iTBS. Where appropriate, Greenhouse–Geisser corrections were applied to correct for non-sphericity of the data (uncorrected Degrees of Freedom are presented). Where appropriate, in the post-hoc analysis of these ANOVAs, we employed Fischer’s least significant difference (FLSD) value to identify significant differences across pairwise comparisons at each intensity. The FLSD was previously shown to provide the optimal balance of reducing both Type I and Type II errors to promote correct decisions (Carmer and Swanson, 1973). Risk of false discovery was further mitigated with our Bayesian analysis.
Bayesian analysis
In addition to the standard ANOVA analyses, we fitted a Bayesian generalized linear model using the brms package (Bürkner, 2017). In all Bayesian analyses, non-committal priors were used, which implies that the prior has minimal influence on the parameter estimates, and even a modest amount of data will overwhelm prior assumptions (Kruschke, 2013). To reduce the influence of outliers, the Bayesian models assumed Student’s T-distributions with heavier tails to account for large variance present in the normalized MEP data (Wang and Blei, 2018). The model was fit using an adaptive Hamiltonian Monte–Carlo Markov–Chain algorithm implemented in Stan (Carpenter et al., 2017). We ran four Markov chains simultaneously; each for 5000 iterations with the first 2500 of iterations discarded as warm-up samples to adaptively tune the Monte–Carlo Markov–Chain sampler. Convergence of chains was inspected visually and quantitatively ( statistic was confirmed to be ∼1.00 for all parameters).
In short, the Monte–Carlo Markov–Chain estimates regression coefficients through exploration of the parameter space to produce a posterior distribution of plausible parameter estimates. This posterior distribution indicates the relative probability of every possible parameter value for each coefficient, given the observed data. Importantly, such Bayesian analysis does not rely on P-values (Kruschke, 2010). Instead, the computed posterior distributions are reported using credibility intervals, which capture 89% of the posterior distribution’s probability mass. If the 89% density intervals for parameter estimates for two groups do not overlap, we can infer that there is a credible difference between the groups. As reported elsewhere, 89% is deemed to be a stable interval for this analysis, though it remains somewhat arbitrary (Makowski et al., 2019). To further aid interpretation, we also report the probability of direction, which is the proportion of the probability mass for each coefficient estimate that is above/below zero (reported as a range of 0.5–1.0). The higher this proportion is, the more likely that the particular parameter represents a true increase or decrease depending on the specified direction of probability. A proportion of 0.5 indicates that the parameter is equally likely to represent an increase or a decrease (Craddock et al., 2019; Makowski et al., 2019).
All statistical analysis was performed in R, version 3.6.0 (R Core Team, 2019). Unless otherwise stated, all data are shown as mean ± standard error of the mean. The criterion for NHST statistical significance was P < 0.05.
Data availability
Data supporting the findings of this study is available from the corresponding authors, upon reasonable request.
Results
Cognitive status is not associated with baseline cortical excitability profiles
Using separate one-way ANOVAs, we compared baseline cortical excitability profiles according to cognitive status. No differences in MTs were observed between cognitively normal and cognitively impaired participants for either active (F(1,36) = 1.0, P = 0.32) or resting (F(1,36) = 0.1, P = 0.83) paradigm. The intensity of iTBS was determined by the MT of individual participants and, accordingly, there was no difference in iTBS intensity across the four experimental cohorts (F(3,36) = 2.0, P = 0.13). The mean intensity used for iTBS was 37 ± 5% MSO.
In addition to MTs, we also assessed the amplitude of baseline MEPs for group differences. A two-way ANOVA with GROUP and PARADIGM as factors revealed a significant main effect of PARADIGM on baseline MEP amplitude (F(1,36) = 14.99, P = 0.00044). Given the background voluntary contraction, the active paradigm participants had significantly elevated baseline amplitude relative to those in in the rest paradigm. For cognitive groups, there was a slight increase in baseline MEP for cognitively normal participants, but this was not statistically significant (F(1,36) = 3.61, P = 0.066) (Fig. 3). Finally, there was no evidence of a GROUP×PARADIGM interaction for baseline MEP amplitudes (F(1,36) = 0.25, P > 0.62).
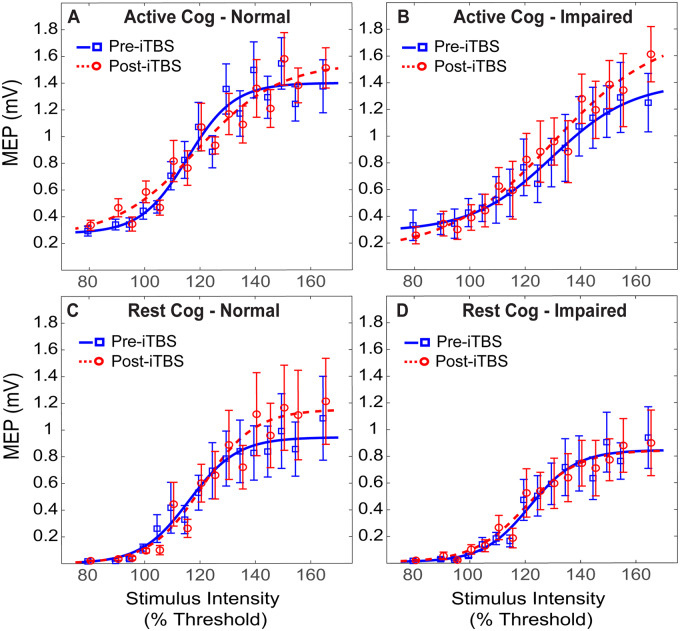
Figure 3.
Raw MEPs Pre- and Post-iTBS. The averaged pre- and post-iTBS I/O curves are plotted for each cohort. The mean MEPs for each intensity are plotted at both time points, and the sigmoidal I/O curve is fit with the Boltzmann equation. Blue denotes baseline cortical excitability profiles pre-iTBS and red denotes post-iTBS cortical excitability profiles. The difference between the two reflects response to iTBS, in which an LTP-like effect is observed when post-iTBS MEP amplitudes (red) are greater than baseline MEP values (blue)
Significant group differences for iTBS response present only in the active paradigm
In the active paradigm, the three-way ANOVA revealed a significant main effect of intensity (F(15, 270) = 119, P < 0.001), and a significant INTENSITY × TIME × GROUP interaction effect (F(15,270) = 3.1, P = 0.00014). There was a borderline group effect for cognitive status (F(1,18) = 3.8, P = 0.067), but no main effect of time (F(1,18) = 1.9, P = 0.18). Post-hoc analysis of the interaction effect revealed that there was an increase in MEP facilitation following iTBS only in cognitively impaired group. This interaction was facilitated by differential responses to iTBS according to cognitive status, which varied across levels of TMS intensity (Fig. 3A and B).
Conversely, in the rest paradigm, the analysis revealed only a significant main effect of intensity (F(15,270) = 109, P < 0.001), but no significant main effects of time (F(1,18) = 0.87, P = 0.36) and group (F(1,18) = 0.75, P = 0.39), nor any significant interaction effects (Fig. 3C and D).
Multiple IO curve components suggest diminished metaplasticity in cognitively impaired cohort
At 110% MT, the two-way ANOVA (GROUP × PARADIGM) performed on normalized MEP values revealed no significant main effect of group (F(1,36) = 0.33, P = 0.57), paradigm (F(1,36) = 0.96, P = 0.33), nor a GROUP × PARADIGM interaction (F(1,36) = 0.21, P = 0.65) (Fig. 4A). The Bayesian GLMM findings support this NHST analysis, but also provide richer information about the iTBS responses (Table 2). The 89% credibility intervals for the mean parameter estimate of all four groups overlap, implying there was no credible probability of group differences. Additionally, however, this model further suggested that there was a small, yet robust, increase in MEP only in the Active: Cog Impaired cohort as this was the only parameter where the vast majority of posterior samples for the coefficient fell above zero (P(β > 0) = 0.98).
Figure 4.
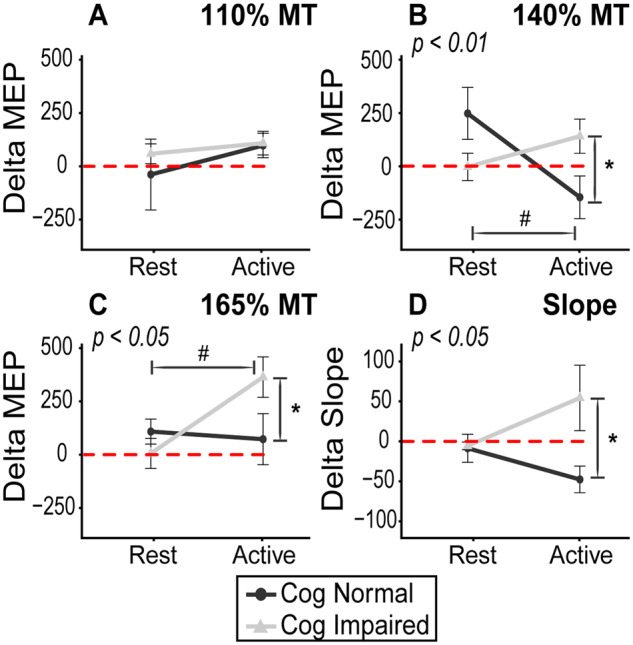
Interaction plots on normalized MEP data. The interaction effect of cognitive status and experimental paradigm on normalized MEP values are shown. Values > 0 represent an excitatory (LTP-like) iTBS effect and, and values < 0 reflect an inhibitory (long-term depression-like) response. This plot illustrates the divergent responses to iTBS by cognition and paradigm. There is a greater response to iTBS in the active paradigm in the cognitively impaired participants, but the opposite is true among cognitively normal participants. This is most evident 140% MT (B) where there is predominant late I-wave contribution, which is known to underly iTBS effects. The P-values depicted in subtitles indicate interaction effect result from two-way ANOVA (GROUP × PARADIGM). As indicated by FLSD, * denotes pairwise significant differences in group responses (within paradigm) and # denotes pairwise significant differences between conditions (within group)
Table 2.
Bayesian analysis of neuroplastic response
| Median parameter estimate | 89% Credible interval | Probability of direction | ||
|---|---|---|---|---|
| I. 110% | ||||
| Active: Cog normal | 70 | [−26, 164] | (β > 0 = 0.88) | |
| Active: Cog impaired | 90 | [22, 161] | Increase (β > 0 = 0.98) | |
| Rest: Cog normal | 45 | [−25, 128] | (β > 0 = 0.84) | |
| Rest: Cog impaired | 12 | [−43, 88] | (β > 0 = 0.64) | |
| II. 140% | ||||
| Active: Cog normal | −133 | [−286, 21.8]a | Decrease (β < 0 = 0.92) | |
| Active: Cog impaired | 127 | [−8.11, 273] | Increase (β > 0 = 0.93) | |
| Rest: Cog normal | 199 | [45.6, 347]a | Increase (β > 0 = 0.98) | |
| Rest: Cog impaired | −7.4 | [−147, 123] | (β < 0 = 0.54) | |
| III. 165% | ||||
| Active: Cog normal | 65 | [−89.6, 212]b | (β > 0 = 0.75) | |
| Active: Cog impaired | 361 | [223, 504]b | Increase (β > 0 = 1.00) | |
| Rest: Cog normal | 101 | [−34, 242] | (β > 0 = 0.88) | |
| Rest: Cog impaired | 15.5 | [−134, 146] | (β > 0 = 0.57) | |
| IV. Slope | ||||
| Active: Cog normal | −30 | [−50, −10]c,d | Decrease (β < 0 = 0.99) | |
| Active: Cog impaired | 4 | [−7, 15]c | (β > 0 = 0.71) | |
| Rest: Cog normal | 4 | [−5, 15]d | (β > 0 = 0.77) | |
| Rest: Cog impaired | −5 | [−15, 6] | (β < 0 = 0.77) |
This table presents findings from separate Bayesian GLMs performed at different components of the I/O curve. We report the (i) median parameter estimate from each respective posterior distribution, (ii) the 89% density intervals of the posterior distributions and (iii) probability of parameter direction. If the 89% credibility intervals do not overlap, we can infer there is a credible group difference, as denoted with a, b, c, d. The probability of direction represents the probability mass of the posterior distribution that falls above or below zero, representing an increase or decrease, respectively, in MEP values. Values of 0.5 indicate 50% chance of increase or decrease, whereas value of 1.0 indicates 100% probability of a specified direction. The direction corresponds with the sign on the median parameter estimate.
At 140% MT, the two-way ANOVA revealed a significant GROUP × PARADIGM interaction (F(1,36) = 8.2, P = 0.007), but no main effect of group (F(1,36) = 0.04, P = 0.85) or paradigm (F(1,36) = 1.76, P = 0.19). Underlying this interaction, FLSD post-hoc analysis revealed that the MEP response for cognitively normal individuals in the active paradigm was significantly blunted when compared to (i) cognitively normal peers in rest paradigm (P = 0.0054) and (ii) cognitively impaired peers in the same paradigm (P = 0.038) (Fig. 4B). This evidence suggests that homoeostatic metaplasticity is intact among cognitively normal participants, but notably absent in the cognitively impaired cohort. The Bayesian GLMM supported this finding. The most notable finding from this supplementary analysis at 140% MT was the striking difference in parameter estimates for the iTBS response in the active paradigm between the cognitively normal and the cognitively impaired individuals (Table 2 and Fig. 5). In this paradigm designed to introduce homoeostatic interference, the median coefficient estimate for the effect of iTBS was negative in cognitively normal individuals (β = −133; indicative of a decrease in MEP) with >90% of posterior samples being negative (P(β < 0) = 0.92; indicative of 92% probability that MEP will decrease), but positive in the cognitively impaired individuals (β = 127) with >90% of posterior samples falling above 0 (P(β > 0) = 0.93; indicative that an increase in MEP is 93% probable). Alternatively, in the rest paradigm, there was only a probable direction of effect in the cognitively normal individuals (increased MEP; P(β > 0) = 0.98), but not in cognitively impaired individuals (P(β > 0) = 0.54). Examination of credibility intervals indicates the presence of homoeostatic interference only in the cognitively normal individuals (Table 2).
Figure 5.
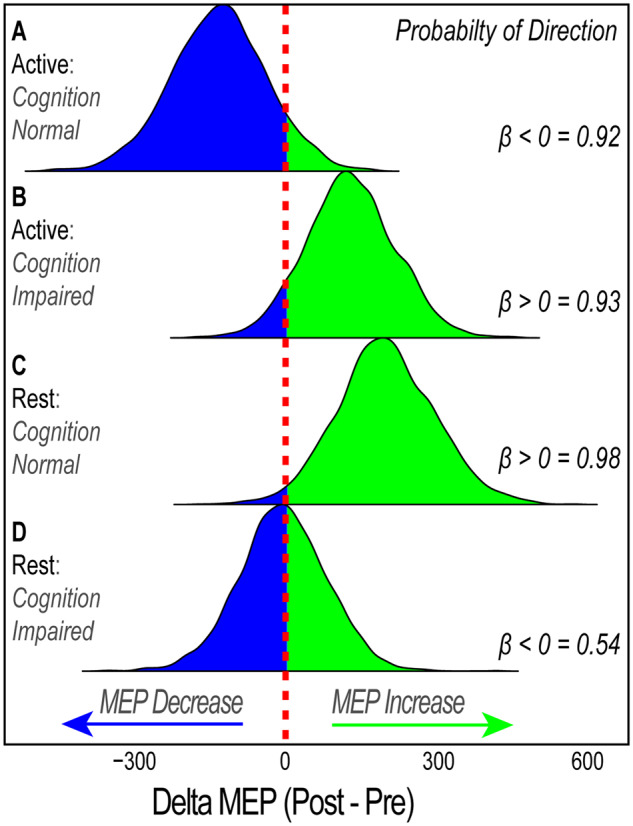
Posterior distribution for possible parameter values at 140% of MT. Derived from the Bayesian analysis of normalized MEP values at 140% MT, this figure shows the probability distribution of possible parameter values for each experimental cohort. The probability of direction value represents the probability mass of the posterior distribution that falls above or below zero, representing a predicted increase or decrease, respectively, in MEP values. Values of 0.5 indicate 50% chance of increase or decrease, whereas value of 1.0 indicates 100% probability of a specified direction. This figure shows that iTBS results in: (A) 92% probability that MEP will decrease in cognitively normal individuals when preceding synaptic activation history is high (apparent homoeostatic interference), (B) 93% probability that MEP will increase cognitively impaired individuals when preceding synaptic activation history is high (diminished homoeostatic metaplasticity), (C) 98% MEP will increase in cognitively normal individuals when preceding synaptic activation history is unaltered (strong LTP-like response), (D) ∼50% probability that MEP will either increase or decrease (weaker LTP-like response) in cognitively impaired individuals when preceding synaptic activation history is unaltered
At 165% MT, the maximal intensity tested, there was a significant interaction effect of GROUP x PARADIGM (F(1,36) = 4.9, P = 0.034), but no main effect of group (F(1,36) = 1.124, P = 0.30) or paradigm (F(1,36) = 3.28, P = 0.08). Post-hoc analysis with FLSD indicated (i) significant difference between the rest and the active experimental paradigms in the cognitively impaired cohort (P = 0.007), and (ii) significant group difference in iTBS responses between cognitively normal and cognitively impaired individuals only in the active paradigm (P = 0.03) (Fig. 4C). The Bayesian GLMM mirrored these findings with distinct credibility intervals for the parameter estimates across the same two pairwise comparisons reported above in the NHST analysis (Table 2). The Bayesian analysis further suggested that this effect was driven by a large excitatory response to iTBS among cognitively impaired individuals in the active paradigm with 100% of the posterior samples of this cohort’s parameter estimate falling above 0 (P(β > 0) = 1.00).
When examining the slope of the fitted I/O curve, a two-way ANOVA revealed a significant main effect for group (F(1,36) = 4.81, P = 0.03) and a significant interaction effect of GROUP × PARADIGM (F(1,36) = 4.27, P = 0.046), but no main effect of paradigm (F(1,36) = 0.19, P = 0.67). When examining change in slope, post-hoc analysis revealed significant group difference between cognitively normal and cognitively impaired individuals in the active paradigm (P = 0.03), with the slope increasing only in the cognitively impaired cohort (Fig. 4D). The Bayesian GLMM provided additional support for this finding, with distinct credibility intervals for the coefficients estimates for iTBS response in the active paradigm across cognitive status. Additionally, the credibility intervals were distinct among cognitively normal individuals across the active and rest paradigms. This effect appeared to be primarily driven by a decrease in I/O curve slope among cognitively normal participants with >99% of values in this posterior distribution falling below 0 (P(β < 0) = 0.99).
Discussion
Homoeostatic metaplasticity is associated with cognitive status
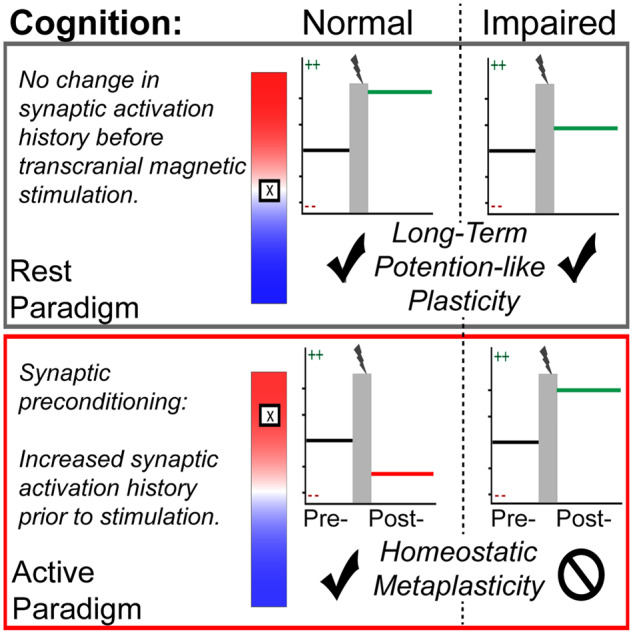
This study is the first to demonstrate that compromised homoeostatic metaplasticity may be a discernable feature of cognitive decline in older adults. Recall that homoeostatic metaplasticity dynamically refines synaptic scaling in an activity-dependent manner. In other words, when the immediate activation history of the synapse is high, this phenomenon is a protective physiological feature that triggers synaptic down-scaling to prevent runaway excitability (Abraham and Bear, 1996). Previous studies have probed this neurophysiological phenomenon in healthy young adults with TMS. Specifically, they report that experimentally modulating the synaptic activation history with targeted voluntary muscle activity prior to the rTMS application introduces homoeostatic interference that blunts the induction of LTP/long-term depression-like neural plasticity (Gentner et al., 2008; Iezzi et al., 2008; Goldsworthy et al., 2014).
Our findings from cognitively normal older adults (mean age, 67.9 ± 2.9 years) are consistent with the literature. This homoeostatic interference can be readily observed among cognitively normal individuals in the active paradigm (Figs. 3A, 4 and 5A). This indicates that, among cognitively normal older adults, artificially increasing the synaptic activation history triggers what can be conceptualized as a ‘homoeostatic harness’, which blunts the typical LTP-like response to iTBS.
Most notably, however, despite identical experimental procedures, this homoeostatic harness appears to be relatively absent among older adults presenting with cognitive impairment. Our Bayesian analysis indicates that, at all I/O curve levels assessed, there is >90% probability of an excitatory response to iTBS in this cohort of cognitively impaired individuals. This novel finding is visually evident as well (Figs. 3B, 4 and 5B), with consistent MEP facilitation across different components on the I/O curve in this experimental cohort, which, at times, exceeds the LTP-like response observed in other experimental cohorts in the control paradigm (Rest) without synaptic pre-conditioning.
When examining the interplay between homoeostatic and Hebbian mechanisms of neural plasticity across components of the I/O curve, the discriminant features associated with cognitive impairment appear to be most pronounced when the MEP response is probed at intensities with predominant late I-wave activity. Particularly, the interaction is nicely portrayed when examining MEPs elicited at 140% MT (Figs. 4B and 5), which the IFCN guidelines indicate that it is associated with maximal I-wave involvement (Groppa et al., 2012). This is notable, given prior evidence from epidural recordings that the neuroplastic response to iTBS is primarily mediated by the trans-synaptic activity of these late I-waves (Huang et al., 2011). Another advantage of utilizing the I/O curve rather than sampling at a single fixed intensity is that we can evaluate the overall shape of the motor response across variable intensities by examining the slope of the fitted curve. The typically steep gain in motor response depends on both the integrity of neuronal fibers in the descending corticospinal tract and the synchronous trans-synaptic activity that generates late I-waves (Devanne et al., 1997; Potter-Baker et al., 2016). Given the I-wave involvement in the neural plastic response to rTMS, the slope of the I/O curve is a reasonable outcome measure when assessing iTBS-induced plasticity (Devanne et al., 1997; Gangitano et al., 2002; Goldsworthy et al., 2016). In this study, the slope of the I/O curve appears to be a discernible indicator for homoeostatic metaplasticity. Specifically, in cognitively normal older adults, the homoeostatic ‘harness’ appears to reverse the polarity of the iTBS effect by decreasing the I/O curve slope, but there is still an observed increase in I/O curve slope in the cognitively impaired participants in the active paradigm.
This finding of discriminant homoeostatic metaplasticity is further evidenced by examining the interactions between cognitive status and experimental paradigm. Given our experimental design, in addition to observing group differences in iTBS response between cognitively normal and cognitively impaired individuals following the muscle contraction in the active paradigm, we are able to add an additional layer of evidence by comparing the responses across experimental paradigms within a given cognitive status (Fig. 4). The findings presented above are robust because they are consistent across both NHST and Bayesian analyses.
No evidence of discriminant LTP-like plasticity or altered cortical excitability
As observed from data collected in our rest paradigm, which is our control condition where there is no attempt to introduce homoeostatic interference, we report no strong evidence that LTP-like responses are associated with cognitive status in this study among non-demented older adults. The Bayesian analysis suggests a stronger probability of facilitation in cognitively normal adults at each of the four I/O curve components that we examined, but there was no credible difference in these LTP-like responses. Though absent in this study population, this feature is consistently reported in the literature among participants with Alzheimer’s disease (Koch et al., 2012; Nardone et al., 2014; Di Lorenzo et al., 2016; Motta et al., 2018).
Additionally, we report no evidence of hyperexcitability (Table 1) in participants with cognitive impairment. Hyperexcitability, as evidenced by a decrease in MT, is typically a robust finding in the TMS literature among participants diagnosed with Alzheimer’s disease (Freitas et al., 2011). From pharmacological trials, this is shown to be a reliable indicator of membrane excitability that is mediated by voltage-gated Na+ channels (Paulus et al., 2008). Together, this suggests that dysfunctional homoeostatic metaplasticity may precede LTP-like deficits and hyperexcitability in the context of cognitive aging and dementia.
Homoeostatic metaplasticity and Alzheimer’s disease pathophysiology
Numerous animal models directly implicate aberrant homoeostatic metaplasticity in the earliest phases Alzheimer’s disease (Megill et al., 2015; Gilbert et al., 2016; Li et al., 2017; Trillaud-Doppia and Boehm, 2018). Complex behavior requires a delicate balance between plasticity and network stability. Thus, it is likely that dysfunctional homoeostatic metaplasticity may directly contribute to cognitive dysfunction in early Alzheimer’s disease (Abraham and Robins, 2005; Styr and Slutsky, 2018). Though the behavioral implications are worthy of future discussion, we will focus this discussion on the pathophysiological links between aberrant homoeostatic metaplasticity and other pathological hallmarks of Alzheimer’s disease.
Failed homoeostatic control of synaptic strength could lead to a pathological level of excitability and even to the induction of epileptic seizures (Abraham, 2008). This is notable, given the numerous lines of converging evidence indicative of neuronal hyperexcitability in Alzheimer’s disease (Frere and Slutsky, 2018; Styr and Slutsky, 2018). In extreme cases, this may manifest with a higher risk of seizure in Alzheimer’s disease, some of which may be clinically silent (Amatniek et al., 2006; Lam et al., 2017). In other cases, this neuronal hyperexcitability may more insidiously contribute to the pathophysiological cascade of disease (Hynd et al., 2004). Importantly, this neuronal hyperexcitability is also thought to be present in the early stages of Alzheimer’s disease (Stargardt et al., 2015). Is it possible that deficits in homoeostatic metaplasticity precede, and perhaps even contribute to, this more well-documented pathological feature of Alzheimer’s disease? Some authors have previously suggested that this might be the case (Jang and Chung, 2016).
In this study, we offer evidence, suggesting this to be the case, but future research is required to follow-up on these exploratory results.
Limitations
There remains considerable inter-individual variability that we are not able to fully account for. Given our relatively small sample size, it is possible that there were more participants genetically pre-disposed to be non-responders to iTBS in our cohort. Future studies can more adequately account for this by collecting genetic data to assess critical genotypes (e.g. BDNF) (Cheeran et al., 2008). Similarly, to further reduce the influence of inter-individual variability, future work may be improved by collecting data for each of the two experimental paradigms from the same cohort of individuals in a cross-over design with an adequate wash-out period.
There are several other methodological features of this study that are worthy of further consideration. First, as it is conventional, our I/O curves were sampled at fixed intensity levels (80–165%). For some participants, 165% does not appear to reflect full saturation of MEP values to form an upper plateau and, thus, may not represent a true maximum. We are currently developing an adaptive protocol that dynamically changes intensity levels to ensure that the entire gain of the motor system is captured. Second, as an intrinsic feature of our study design, the MEPs themselves were sampled under different conditions (Active versus Rest). A background tonic voluntary contraction during MEP sampling is known to increase I-wave involvement (Devanne et al., 1997; Di Lazzaro et al., 2004). Future studies may want to consider sampling MEPs in a consistent fashion (e.g. only during rest), and then separately introduce voluntary background contraction to modulate synaptic activation history. Finally, this study endogenously modulated synaptic activation history prior to stimulation with targeted voluntary muscle contractions. Although this is a well-recognized approach to modulate homoeostatic metaplasticity (Vallence and Ridding, 2014; Müller-Dahlhaus and Ziemann, 2015), implementing methodology to exogenously prime the stimulation site may be more reliable to ensure more consistent synaptic pre-conditioning across all participants.
Conclusion
As a novel finding, we report the detection of dysfunctional homoeostatic metaplasticity in non-demented older adults presenting with cognitive impairment. Notably, this was observed in the absence of other neurophysiological features like impaired LTP-like plasticity and hyper-excitability, which are both consistently reported in the Alzheimer’s disease TMS literature. Given that dysfunctional homoeostatic metaplasticity is an emerging feature in the genesis of Alzheimer’s disease’s pathophysiological cascade, this represents a potential surrogate marker for the pre-clinical phase of the disease.
Acknowledgement
The authors thank Marc Lindley for his support in the preliminary pilot studies and Jacob Green for his contribution to literature reviews.
Funding
This work was supported by the National Institutes of Health R01 AG062543 (PI: Y.-H. C.), National Institutes of Health P30 AG019610 Arizona Alzheimer’s Consortium Pilot Study Program (Pilot Project PI: Y.-H. C.), the National Institutes of Health P30 AG019610 Arizona Alzheimer’s Consortium Pilot Study Program (Pilot Project PI: R.C.W., Co-I: Y.-H.C.), the BIO5 Team Scholars Award (PI: Y.-H. C.) and the Department of Defense through the National Defense Science & Engineering Graduate Fellowship (NDSEG) Program (M.H.S.).
Competing interests
The authors report no competing interests.
Glossary
- APB
abductor pollicis brevis
- CI
cognitively impaired
- CN
cognitively normal
- EMG
: electromyography
- FLSD
Fischer’s least significant difference
- I/O
input/output
- iTBS
intermittent theta-burst stimulation
- LTP
long-term potentiation
- MEP
motor-evoked potential
- MT
motor threshold
- MVC
maximum voluntary contraction
- NHST
null hypothesis significance testing
- MVC
repetitive TMS
- TMS
transcranial magnetic stimulation
References
- Abbott LF, Nelson SB. Synaptic plasticity: taming the beast. Nat Neurosci 2000; 3: 1178–83. [DOI] [PubMed] [Google Scholar]
- Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci 2008; 9: 387–99. [DOI] [PubMed] [Google Scholar]
- Abraham WC, Bear MF. Metaplasticity : plasticity of synaptic. Trends Neurosci 1996; 19: 126–30. [DOI] [PubMed] [Google Scholar]
- Abraham WC, Robins A. Memory retention—the synaptic stability versus plasticity dilemma. Trends Neurosci 2005; 28: 73–8. [DOI] [PubMed] [Google Scholar]
- Amatniek JC, Hauser WA, DelCastillo-Castaneda C, Jacobs DM, Marder K, Bell K, et al. Incidence and predictors of seizures in patients with Alzheimer’s disease. Epilepsia 2006; 47: 867–72. [DOI] [PubMed] [Google Scholar]
- Amrhein V, Korner-Nievergelt F, Roth T. The earth is flat (p > 0.05): significance thresholds and the crisis of unreplicable research. PeerJ 2017; 5: e3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besser L, Kukull W, Knopman DS, Chui H, Galasko D, Weintraub S, et al. Version 3 of the National Alzheimer’s Coordinating Center’s Uniform Data Set. Alzheimer Dis Assoc Disord 2018; 32: 351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestmann S, Krakauer JW. The uses and interpretations of the motor-evoked potential for understanding behaviour. Exp Brain Res 2015; 233: 679–89. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Edmonds EC, Jak AJ, Clark LR, Delano-Wood L, McDonald CR, for the Alzheimer’s Disease Neuroimaging Initiative, et al. Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. J Alzheimer Dis 2014; 42: 275–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunse T, Wobrock T, Strube W, Padberg F, Palm U, Falkai P, et al. Motor cortical excitability assessed by transcranial magnetic stimulation in psychiatric disorders: a systematic review. Brain Stimul 2014; 7: 158–69. [DOI] [PubMed] [Google Scholar]
- Burke D, Pierrot-Deseilligny E. Caveats when studying motor cortex excitability and the cortical control of movement using transcranial magnetic stimulation. Clin Neurophysiol 2010; 121: 121–3. [DOI] [PubMed] [Google Scholar]
- Bürkner PC. brms: an R package for Bayesian multilevel models using Stan. J Stat Softw 2017; 80: 1–21. [Google Scholar]
- Carmer SG, Swanson MR. An evaluation of ten pairwise multiple comparison procedures by Monte Carlo Methods. J Am Stat Assoc 1973; 68: 66–74. [Google Scholar]
- Carpenter B, Gelman A, Hoffman MD, Lee D, Goodrich B, Betancourt M, et al. Stan: a probabilistic programming language. J Stat Softw 2017; 76: 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli RJ, Reiman EM. Characterizing the preclinical stages of Alzheimer’s disease and the prospect of presymptomatic intervention. J Alzheimer Dis 2013; 33: S405–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeran B, Talelli P, Mori F, Koch G, Suppa A, Edwards M, et al. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J Physiol 2008; 586: 5717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock M, Klepousniotou E, El-Deredy W, Poliakoff E, Lloyd D. Transcranial alternating current stimulation at 10 Hz modulates response bias in the Somatic Signal Detection Task. Int J Psychophysiol 2019; 135: 106–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft S, Newcomer J, Kanne S, Dagogo-Jack S, Cryer P, Sheline Y, et al. Memory improvement following induced hyperinsulinemia in Alzheimer’s disease. Neurobiol Aging 1996; 17: 123–30. [DOI] [PubMed] [Google Scholar]
- Cueva AS, Galhardoni R, Cury RG, Parravano DC, Correa G, Araujo H, et al. Normative data of cortical excitability measurements obtained by transcranial magnetic stimulation in healthy subjects. Neurophysiol Clin Neurophysiol 2016; 46: 43–51. [DOI] [PubMed] [Google Scholar]
- Devanne H, Lavoie BA, Capaday C. Input-output properties and gain changes in the human corticospinal pathway. Exp Brain Res 1997; 114: 329–38. [DOI] [PubMed] [Google Scholar]
- Dienes Z, Mclatchie N. Four reasons to prefer Bayesian analyses over significance testing. Psychon Bull Rev 2018; 25: 207–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B, Hampel H, Feldman HH, Scheltens P, Aisen P, Andrieu S, Proceedings of the Meeting of the International Working Group (IWG) and the American Alzheimer’s Association on “The Preclinical State of AD”; July 23, 2015; Washington DC, USA, et al. Preclinical Alzheimer’s disease: definition, natural history, and diagnostic criteria. Alzheimer Dement 2016; 12: 292–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanelli D. Opinion: Is science really facing a reproducibility crisis, and do we need it to? Proc Natl Acad Sci USA 2018; 115: 2628–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes D, Carvalho AL. Mechanisms of homeostatic plasticity in the excitatory synapse. J Neurochem 2016; 139: 973–96. [DOI] [PubMed] [Google Scholar]
- Freitas C, Farzan F, Pascual-Leone A. Assessing brain plasticity across the lifespan with transcranial magnetic stimulation: why, how, and what is the ultimate goal? Front Neurosci 2013; 7: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas C, Mondragón-Llorca H, Pascual-Leone A. Noninvasive brain stimulation in Alzheimer’s disease: systematic review and perspectives for the future. Exp Gerontol 2011; 46: 611–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frere S, Slutsky I. Alzheimer’s Disease: from firing instability to homeostasis network collapse. Neuron 2018; 97: 32–58. [DOI] [PubMed] [Google Scholar]
- Gangitano M, Valero-Cabré A, Tormos JM, Mottaghy FM, Romero JR, Pascual-Leone Á. Modulation of input-output curves by low and high frequency repetitive transcranial magnetic stimulation of the motor cortex. Clin Neurophysiol 2002; 113: 1249–57. [DOI] [PubMed] [Google Scholar]
- Gentner R, Wankerl K, Reinsberger C, Zeller D, Classen J. Depression of human corticospinal excitability induced by magnetic theta-burst stimulation: evidence of rapid polarity-reversing metaplasticity. Cereb Cortex 2008; 18: 2046–53. [DOI] [PubMed] [Google Scholar]
- Gilbert J, Shu S, Yang X, Lu Y, Zhu L-Q, Man H-Y. β-Amyloid triggers aberrant over-scaling of homeostatic synaptic plasticity. Acta Neuropathol Commun 2016; 4: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz SM, Luber B, Lisanby SH, Peterchev AV. A novel model incorporating two variability sources for describing motor evoked potentials. Brain Stimul 2014; 7: 541–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsworthy MR, Müller-Dahlhaus F, Ridding MC, Ziemann U. Inter-subject variability of LTD-like plasticity in human motor cortex: a matter of preceding motor activation. Brain Stimul 2014; 7: 864–70. [DOI] [PubMed] [Google Scholar]
- Goldsworthy MR, Vallence AM, Hodyl NA, Semmler JG, Pitcher JB, Ridding MC. Probing changes in corticospinal excitability following theta burst stimulation of the human primary motor cortex. Clin Neurophysiol 2016; 127: 740–7. [DOI] [PubMed] [Google Scholar]
- Groppa S, Oliviero A, Eisen A, Quartarone A, Cohen L, Mall V, et al. A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol 2012; 123: 858–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogendam JM, Ramakers GMJ, Di Lazzaro V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul 2010; 3: 95–118. [DOI] [PubMed] [Google Scholar]
- Houdayer E, Degardin A, Cassim F, Bocquillon P, Derambure P, Devanne H. The effects of low- and high-frequency repetitive TMS on the input/output properties of the human corticospinal pathway. Exp Brain Res 2008; 187: 207–17. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron 2005; 45: 201–6. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Rothwell JC, Chen RS, Lu CS, Chuang WL. The theoretical model of theta burst form of repetitive transcranial magnetic stimulation. Clin Neurophysiol 2011; 122: 1011–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynd MR, Scott HL, Dodd PR. Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer’s disease. Neurochem Int 2004; 45: 583–95. [DOI] [PubMed] [Google Scholar]
- Iezzi E, Conte A, Suppa A, Agostino R, Dinapoli L, Scontrini A, et al. Phasic voluntary movements reverse the aftereffects of subsequent theta-burst stimulation in humans. J Neurophysiol 2008; 100: 2070–6. [DOI] [PubMed] [Google Scholar]
- Ioannidis JPA. Why most published research findings are false. PLoS Med 2005; 2: e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova I, Salmon DP, Gollan TH. The multilingual naming test in Alzheimer’s Disease: clues to the origin of naming impairments. J Int Neuropsychol Soc 2013; 19: 272–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jak AJ, Bondi MW, Delano-Wood L, Wierenga C, Corey-Bloom J, Salmon DP, et al. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am J Geriatr Psychiatry 2009; 17: 368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jak AJ, Preis SR, Beiser AS, Seshadri S, Wolf PA, Bondi MW, et al. Neuropsychological Criteria for Mild Cognitive Impairment and Dementia Risk in the Framingham Heart Study. J Int Neuropsychol Soc 2016; 22: 937–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang SS, Chung HJ. Emerging link between Alzheimer’s disease and homeostatic synaptic plasticity. Neural Plast 2016; 2016: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Di Lorenzo F, Bonnì S, Ponzo V, Caltagirone C, Martorana A. Impaired LTP-but not LTD-like cortical plasticity in Alzheimer’s disease patients. J Alzheimer Dis 2012; 31: 593–9. [DOI] [PubMed] [Google Scholar]
- Koch G, Di Lorenzo F, Loizzo S, Motta C, Travaglione S, Baiula M, et al. CSF tau is associated with impaired cortical plasticity, cognitive decline and astrocyte survival only in APOE4-positive Alzheimer’s disease. Sci Rep 2017; 7: 13728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruschke JK. Bayesian estimation supersedes the t-test. J Exp Psychol 2013; 142: 573–603. [DOI] [PubMed] [Google Scholar]
- Kruschke JK. What to believe: Bayesian methods for data analysis. Trends Cogn Sci 2010; 14: 293–300. [DOI] [PubMed] [Google Scholar]
- Lahr J, Peter J, Minkova L, Lauer E, Reis J, Heimbach B, et al. No difference in paired associative stimulation induced cortical neuroplasticity between patients with mild cognitive impairment and elderly controls. Clin Neurophysiol 2016; 127: 1254–60. [DOI] [PubMed] [Google Scholar]
- Lam AD, Deck G, Goldman A, Eskandar EN, Noebels J, Cole AJ. Silent hippocampal seizures and spikes identified by foramen ovale electrodes in Alzheimer’s disease. Nat Med 2017; 23: 678–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Mazzone P, et al. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol 2004; 115: 255–66. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Profice P, Ranieri F, Capone F, Dileone M, Oliviero A, et al. I-wave origin and modulation. Brain Stimul 2012; 5: 512–25. [DOI] [PubMed] [Google Scholar]
- Legatt AD, Emerson RG, Epstein CM, MacDonald DB, Deletis V, Bravo RJ, et al. ACNS guideline: transcranial electrical stimulation motor evoked potential monitoring. J Clin Neurophysiol 2016; 33: 42–50. [DOI] [PubMed] [Google Scholar]
- Li Q, Navakkode S, Rothkegel M, Soong TW, Sajikumar S, Korte M. Metaplasticity mechanisms restore plasticity and associativity in an animal model of Alzheimer’s disease. Proc Natl Acad Sci USA 2017; 114: 5527–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lorenzo F, Ponzo V, Bonnì S, Motta C, Negrão Serra PC, Bozzali M, et al. Long-term potentiation-like cortical plasticity is disrupted in Alzheimer’s disease patients independently from age of onset. Ann Neurol 2016; 80: 202–10. [DOI] [PubMed] [Google Scholar]
- Lynch MA. Long-term potentiation and memory. Physiol Rev 2004; 84: 87–136. [DOI] [PubMed] [Google Scholar]
- Makowski D, Ben-Shachar MS, Lüdecke D. bayestestR: describing effects and their uncertainty, existence and significance within the Bayesian framework. J Open Source Softw 2019; 4: 1541–9. [Google Scholar]
- McNeish D. On using Bayesian methods to address small sample problems. Struct Equ Model 2016; 23: 750–73. [Google Scholar]
- Megill A, Tran T, Eldred K, Lee NJ, Wong PC, Hoe H-S, et al. Defective age-dependent metaplasticity in a mouse model of Alzheimer’s Disease. J Neurosci 2015; 35: 11346–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melinscak F, Montesano L. Beyond p-values in the evaluation of brain–computer interfaces: a Bayesian estimation approach. J Neurosci Methods 2016; 270: 30–45. [DOI] [PubMed] [Google Scholar]
- Möller C, Arai N, Lücke J, Ziemann U. Hysteresis effects on the input-output curve of motor evoked potentials. Clin Neurophysiol 2009; 120: 1003–8. [DOI] [PubMed] [Google Scholar]
- Motta C, Di Lorenzo F, Ponzo V, Pellicciari MC, Bonnì S, Picazio S, et al. Transcranial magnetic stimulation predicts cognitive decline in patients with Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2018; 89: 1237–42. [DOI] [PubMed] [Google Scholar]
- Müller JFM, Orekhov Y, Liu Y, Ziemann U. Homeostatic plasticity in human motor cortex demonstrated by two consecutive sessions of paired associative stimulation. Eur J Neurosci 2007; 25: 3461–8. [DOI] [PubMed] [Google Scholar]
- Müller-Dahlhaus F, Ziemann U. Metaplasticity in human cortex. Neuroscientist 2015; 21: 185–202. [DOI] [PubMed] [Google Scholar]
- Murakami T, Müller-Dahlhaus F, Lu M-K, Ziemann U. Homeostatic metaplasticity of corticospinal excitatory and intracortical inhibitory neural circuits in human motor cortex. J Physiol 2012; 590: 5765–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardone R, Tezzon F, Höller Y, Golaszewski S, Trinka E, Brigo F. Transcranial magnetic stimulation (TMS)/repetitive TMS in mild cognitive impairment and Alzheimer’s disease. Acta Neurol Scand 2014; 129: 351–66. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bã©Dirian V, Charbonneau S, Whitehead V, Collin I, et al. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53: 695–9. [DOI] [PubMed] [Google Scholar]
- Paulus W, Classen J, Cohen LG, Large CH, Di Lazzaro V, Nitsche M, et al. State of the art: pharmacologic effects on cortical excitability measures tested by transcranial magnetic stimulation. Brain Stimul 2008; 1: 151–63. [DOI] [PubMed] [Google Scholar]
- Pitcher JB, Ogston KM, Miles TS. Age and sex differences in human motor cortex input-output characteristics. J Physiol 2003; 546: 605–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possin KL, Laluz VR, Alcantar OZ, Miller BL, Kramer JH. Distinct neuroanatomical substrates and cognitive mechanisms of figure copy performance in Alzheimer’s disease and behavioral variant frontotemporal dementia. Neuropsychologia 2011; 49: 43–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter-Baker KA, Varnerin NM, Cunningham DA, Roelle SM, Sankarasubramanian V, Bonnett CE, et al. Influence of corticospinal tracts from higher order motor cortices on recruitment curve properties in stroke. Front Neurosci 2016; 10: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team R. A language and environment for statistical computing [Internet]. R Found Stat Comput. 2019. Available from: http://www.r-project.org/
- Reitan RM, Wolfson D, The Halstead-Reitan neuropsychological test battery: Therapy and clinical interpretation. Tucson, AZ: Neurospychological Press; 1985. [Google Scholar]
- Ridding M, Rothwell JC. Stimulus/response curves as a method of measuring motor cortical excitability in man Pre ischaemia During ischaemia. Electroencephalogr Clin Neurophysiol 1997; 105: 340–4. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A, Avanzini G, Bestmann S, et al. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 2009; 120: 2008–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application: an updated report from an I.F.C.N. Committee. Clin Neurophysiol 2015; 126: 1071–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan MJ, Klyubin I, Cullen WK, Anwyl R. Synaptic plasticity in animal models of early Alzheimer’s disease. Phil Trans R Soc Lond B 2003; 358: 821–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirk SD, Mitchell MB, Shaughnessy LW, Sherman JC, Locascio JJ, Weintraub S, et al. A web-based normative calculator for the uniform data set (UDS) neuropsychological test battery. Alzheimers Res Ther 2011; 3: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout PE, Rodgers JL. Psychology, science, and knowledge construction: broadening perspectives from the replication crisis. Annu Rev Psychol 2018; 69: 487–510. [DOI] [PubMed] [Google Scholar]
- Stargardt A, Swaab DF, Bossers K. The storm before the quiet: neuronal hyperactivity and Aβ in the presymptomatic stages of Alzheimer’s disease. Neurobiol Aging 2015; 36: 1–11. [DOI] [PubMed] [Google Scholar]
- Styr B, Slutsky I. Imbalance between firing homeostasis and synaptic plasticity drives early-phase Alzheimer’s disease. Nat Neurosci 2018; 21: 463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szucs D, Ioannidis JPA. Empirical assessment of published effect sizes and power in the recent cognitive neuroscience and psychology literature. PLoS Biol 2017; 15: e2000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talelli P Waddingham W Ewas A Rothwell JC Ward NS.. The effect of age on task-related modulation of interhemispheric balance. Exp Brain Res 2008; 186: 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron 2014; 81: 12–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trebbastoni A, Pichiorri F, D’Antonio F, Campanelli A, Onesti E, Ceccanti M, et al. Altered cortical synaptic plasticity in response to 5-Hz repetitive transcranial magnetic stimulation as a new electrophysiological finding in amnestic mild cognitive impairment converting to Alzheimer’s Disease: results from a 4-year. Prospective Cohort S. Front Aging Neurosci 2015; 7: 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trillaud-Doppia E, Boehm J. The amyloid precursor protein intracellular domain is an effector molecule of metaplasticity. Biol Psychiatry 2018; 83: 406–15. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature 1998; 391: 892–6. [DOI] [PubMed] [Google Scholar]
- Vallence A-M, Goldsworthy MR, Hodyl NA, Semmler JG, Pitcher JB, Ridding MC. Inter- and intra-subject variability of motor cortex plasticity following continuous theta-burst stimulation. Neuroscience 2015; 304: 266–78. [DOI] [PubMed] [Google Scholar]
- Vallence A-M, Ridding MC. Non-invasive induction of plasticity in the human cortex: uses and limitations. Cortex 2014; 58: 261–71. [DOI] [PubMed] [Google Scholar]
- Van Den Bos MAJ Geevasinga N Menon P Burke D Kiernan MC Vucic S.. Physiological processes influencing motor-evoked potential duration with voluntary contraction. J Neurophysiol 2017; 117: 1156–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Blei DM. A general method for robust Bayesian modeling. Bayesian Anal 2018; 13: 1159–87. [Google Scholar]
- Wasserstein RL, Lazar NA. The ASA statement on p-values: context, process, and purpose. Am Stat 2016; 70: 129–33. [Google Scholar]
- Wechsler D, Wechsler Memory Scale—Revised: manual. San Antonio, Texas: The Psychological Corporation; 1987. [Google Scholar]
- Weintraub S, Besser L, Dodge HH, Teylan M, Ferris S, Goldstein FC, et al. Version 3 of the Alzheimer Disease Centers’ Neuropsychological Test Battery in the Uniform Data Set (UDS). Alzheimer Dis Assoc Disord 2018; 32: 10–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischnewski M, Schutter DJLG. Efficacy and time course of theta burst stimulation in healthy humans. Brain Stimul 2015; 8: 685–92. [DOI] [PubMed] [Google Scholar]
- Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, et al. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain 1997; 120: 141–57. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Paulus W, Nitsche MA, Pascual-Leone A, Byblow WD, Berardelli A, et al. Consensus: motor cortex plasticity protocols. Brain Stimul 2008; 1: 164–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the findings of this study is available from the corresponding authors, upon reasonable request.