Abstract
BACKGROUND
Our aims were to assess whether arterial stiffness is associated with a higher risk for kidney dysfunction among persons without chronic kidney disease (CKD).
METHODS
We analyzed data from the national health checkup system in Japan; for our analyses, we selected records of individuals who completed assessments of cardio-ankle vascular index (CAVI) and kidney function from 2005 to 2016. We excluded participants who had CKD at baseline, defined as the presence of proteinuria or estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2. We compared 2 groups of CAVI measurements—the highest quartile (≧8.1) and the combined lower 3 quartiles (<8.1). We used Cox proportional hazards models to assess associations between these 2 groups and subsequent CKD events, proteinuria, eGFR <60 ml/min/1.73 m2, and rapid eGFR decline (greater than or equal to −3 ml/min/1.73 m2 per year).
RESULTS
The mean age of the 24,297 included participants was 46.2 years, and 60% were female. Over a mean follow-up of 3.1 years, 1,435 CKD events occurred. In a multivariable analysis, the hazard ratios with 95% confidence intervals (CIs) for the highest vs. combined lower quartiles of CAVI measurements were 1.3 (1.1, 1.5) for CKD events, 1.3 (0.96, 1.62) for proteinuria, 1.4 (1.1, 1.7) for eGFR <60 ml/min/1.73 m2, and the odds ratio with 95% CI was 1.3 (1.1, 1.4) for rapid eGFR decline.
CONCLUSIONS
Persons with CAVI measurements ≧8.1 had a higher risk for CKD events compared with their counterparts with CAVI measurements <8.1. Greater arterial stiffness among adults without CKD may be associated with kidney dysfunction.
Keywords: arterial stiffness, blood pressure, cardio-ankle vascular index, chronic kidney disease, hypertension
The kidneys filter a high volume of blood but have low vascular resistance to flow. Consequently, the kidneys are vulnerable to barotrauma when they are subjected to excessive pulsatile flow arising from aortic stiffness.1–3 Associations of aortic stiffness with chronic kidney disease (CKD), including albuminuria and low estimated glomerular filtration rate (eGFR), have been reported.4–11 However, prior studies recruited high-risk populations (e.g., participants with hypertension, diabetes, or a history of cardiovascular disease (CVD)) and individuals with CKD.5–10 The noted comorbidities themselves might lead to both arterial stiffness and decline in kidney function over time. Furthermore, arterial stiffness typically was estimated from pulse wave velocity (PWV),4–11 a measure that is influenced by blood pressure (BP) levels at the time PWV is obtained.
Stiffness parameter β is a measure of arterial stiffness. Stiffness parameter β may be less likely than PWV to be influenced by BP levels at the time measurements are taken.12 Stiffness parameter β has been used to develop a measure of arterial stiffness that is available in clinical practice—the cardio-ankle vascular index (CAVI).13–16 CAVI has been shown to be associated with a higher risk for incident CVD events, independent of cardiovascular risk factors.17,18 However, no studies have assessed the association between CAVI and incident CKD events. If CAVI measurements are associated with CKD risk, this would suggest that CAVI could be used to identify individuals at high risk for CKD events and who may benefit from nonpharmacological and pharmacological preventive interventions.
Using a database of participants who participated in the national health checkup program in Japan,19 we determined whether CAVI measurements among those without CKD are associated with kidney outcomes in later life.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request. In Japan, the Industrial Safety and Health Law requires employers to provide annual health checkups to their employees. The current prospective longitudinal study was conducted within this context by the Japan Health Promotion Foundation (see Supplemental Material online). For the current analyses, we selected records of individuals who enrolled in the study between October 2005 and March 2015 and (i) completed concurrent assessments of CAVI and covariates during their baseline visit and (ii) had kidney function assessments on at least 2 occasions (i.e., at baseline and from at least 1 follow-up visit after the baseline visit and before March 2016). These selection criteria yielded a final data set of records from 25,653 participants for our analyses. Our analysis excluded participants under 18 years of age (since written informed consent was not obtained from these participants’ parents), and participants who had CKD at the baseline examination. Specific examples for this approach are shown in Supplementary Figure S1 online. All participants provided written informed consent at each study visit, and the Institutional Review Boards of the Kawasaki Medical School approved the protocol.
BP and other measurements
Trained medical staff measured BP 2 times from the brachial artery in each participant’s right arm at 1-minute intervals after the participant had been sitting in a quiet room for 5 minutes. A standard mercury sphygmomanometer was used for measurements obtained before June 2013, and a validated automatic oscillometric device (HBP-1300, OMRON, Kyoto, Japan)20 was used after July 2013. A designation of hypertension was applied to persons having an average systolic BP ≥140 mm Hg or diastolic BP ≥90 mm Hg, persons using antihypertensive medication, or persons with a self-reported history of hypertension.21
Other data were collected using standardized protocols (see the Data Supplement online) and included height; weight; smoking; medication use; self-reported history of hypertension, diabetes, or CVD; and fasting laboratory values. History of hypertension, diabetes, or CVD was assessed for each participant using a standardized questionnaire (i.e., “Have you ever been diagnosed as having hypertension, diabetes, or CVD?”). Questionnaires regarding the use of BP- or glucose-lowering medication were also administered. Serum creatinine and spot urine specimens were collected on at least 2 occasions for each participant. Serum creatinine was assayed using an enzymatic method. eGFR was derived using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation modified by a Japanese coefficient (see Data Supplement online).22 Urinalysis by the dipstick method was performed on spot urine specimens collected in the early morning after overnight fasting. Urine dipstick results were interpreted by the medical staff in each local medical institution and recorded as (−), (±), (1+), (2+), and (3+). The policy of the Japanese Committee for Clinical Laboratory Standards (http://jccls.org/) is that all urine dipstick tests should be manufactured so that a urine dipstick result of 1+ will correspond to a urinary protein level of 30 mg/dl. We defined proteinuria as 1+ or more.
CAVI measurement
CAVI was measured at baseline after a few minutes of rest in a supine position using the cuff-oscillometric method (Vasera-1500; Fukuda Denshi, Tokyo, Japan).19 Cuffs were attached to the brachia and ankles, and pulse volume waveforms at 4 extremities were simultaneously recorded using a plethysmographic sensor connected to the cuffs. Measurements were recorded for 10 seconds under compression of 50 mm Hg. BP at the right arm and ankle was then measured by the cuff-oscillometric method, which was repeated to measure BP at the left arm and ankle. CAVI was calculated using the following formula,15 where Ps = SBP, Pd = DBP, ΔP = pulse pressure, ρ = blood density, and PWV = pulse wave velocity:
P s and Pd were obtained when CAVI measurements were obtained. PWV was calculated using an estimated path length from the origin of the aorta to the tibial artery at the ankle and the arrival time of the pulse waveform generated by cardiac contraction at the tibial artery.
Kidney outcomes
eGFR and proteinuria measurements were obtained at 2 or more clinic visits (mean (SD), 3.5 (2.0) visits) for each participant over a median (interquartile range) follow-up duration of 2.0 visits (1.0–5.0). CKD was defined as the presence of proteinuria or eGFR <60 ml/min/1.73 m2. Average annual change of eGFR was calculated by fitting linear regression using all eGFR measurements for each participant. We defined rapid eGFR decline as greater than or equal to −3 ml/min/1.73 m2 per year, which has been used previously as a cutoff that reflects 3× higher decreasing speed than that is expected for normal aging.23 The primary outcome was CKD events. Proteinuria, eGFR <60 ml/min/1.73 m2, annual change of eGFR during follow-up (as a continuous variable), and rapid eGFR decline, separately, were examined as secondary outcomes.
Statistical analyses
Descriptive statistics are presented as means and SDs, and proportions as appropriate. We calculated the incidence rate of CKD events for participants, overall, and in quartiles of CAVI measurements. The cumulative incidence of CKD events for the quartile was calculated using the Kaplan–Meier method. For analyses assessing CKD events, proteinuria, eGFR <60 ml/min/1.73 m2, or rapid eGFR decline as outcomes, we used Cox proportional hazards models. We estimated the hazard ratios (HRs) and 95% confidence intervals (CIs) for each kidney outcome associated with quartiles of CAVI measurements, as well as with CAVI measurements as a continuous variable. To minimize the effects of BP on CAVI measurements, in a secondary analysis, we calculated CAVI measurements using the formula suggested by Spronck et al.24 The proportionality assumption for the Cox analyses was confirmed via the inclusion of a time-by-CAVI interaction. The baseline exam date was defined as the time origin for time-to-event analysis. Follow-up time was censored on the date the event was ascertained. Participants who did not have events were censored at the last contact with the participant on or before March 2016. HRs were calculated in 2 levels of adjustment. A first model included adjustment for age, sex, and clinical and behavioral characteristics at the baseline exam (body mass index, smoking status, total cholesterol, high-density lipoprotein cholesterol, eGFR, history of diabetes, and history of CVD at baseline). In second model, we further adjusted for mean arterial pressure and prevalent hypertension at baseline. We tested for heterogeneity in the association between CAVI measurements and outcomes by prevalent hypertension and by eGFR (above or below 90 ml/min/1.73 m2) at baseline with the inclusion of multiplicative interaction terms. Stratified analyses were considered when a statistically significant interaction was present (P value <0.05). For analyses assessing differences in average annual eGFR change among the quartiles of CAVI measurements, as well as with CAVI measurements as a continuous variable, we used linear mixed models with 3 levels of adjustment, as described above. We provided betas (95% CIs) for differences in change of eGFR (ml/min/1.73 m2 per year) for CAVI measurements, interval, and the interaction term (i.e., CAVI measurement × interval), respectively. Based upon the individual term (i.e., CAVI measurement at baseline), we assessed the associations between CAVI measurement at baseline and eGFR during follow-up. Based upon the interaction between CAVI measurement and the time interval from baseline to follow-up, we calculated the additional decline in annual change during follow-up associated with a 1-SD increase in the CAVI measurement.
We conducted 4 sensitivity analyses. First, we adjusted for fasting glucose values, which were available from 15,234 participants. Second, in an analysis for CKD events, we adjusted for systolic BP instead of mean arterial pressure at baseline. Third, in an analysis for CKD events, we excluded participants taking antihypertensive medication at baseline. Fourth, in an analysis for annual eGFR change, we further adjusted for the span of time during which eGFR measurements were obtained and used to calculate eGFR change in each participant.
All statistical analyses were performed with SAS version 9.4 software (SAS Institute, Cary, NC). Statistical significance was defined as a P value <0.05 using 2-sided tests.
RESULTS
Of the 25,653 participants enrolled in the current analysis, we excluded 2 who were under 18 years of age, and 1,354 who had CKD at the baseline examination. This yielded a final analytical sample size of 24,297 participants. Among these, the mean (SD) age was 46.2 (13.0) years, 59.5% were women, and 18.8% were defined as having hypertension at baseline (Table 1). Participants in the highest vs. lower quartiles of CAVI measurements were older and had lower eGFR at baseline. The prevalence of hypertension and history of diabetes and CVD was higher among the highest vs. lower quartiles of CAVI measurements. CAVI measurements and mean arterial pressure were correlated (Pearson’s correlation = 0.39). The distribution of CAVI measurements and the thresholds used for categorizing participants into quartiles are shown in Supplementary Figure S2 online.
Table 1.
Characteristics of participants at baseline (n = 24,297)
| Total | 1st quartile (n = 5,290) | 2nd quartile (n = 6,010) | 3rd quartile (n = 6,285) | 4th quartile (n = 6,712) | |
|---|---|---|---|---|---|
| Age, years | 46.2 ± 13.0 | 33.5 ± 7.7 | 40.7 ± 8.4 | 47.7 ± 9.1 | 59.7 ± 9.4 |
| Women, n (%) | 14,461 (59.5) | 3,185 (60.2) | 3,619 (60.2) | 3,836 (61.0) | 3,821 (56.9) |
| Body mass index, kg/m2 | 22.4 ± 3.4 | 22.3 ± 3.8 | 22.3 ± 3.5 | 22.3 ± 3.2 | 22.5 ± 3.1 |
| Current smokers, n (%) | 4,974 (20.5) | 1,379 (26.1) | 1,322 (22.0) | 1,197 (19.1) | 1,076 (16.0) |
| Total cholesterol, mg/dl | 212.5 ± 37.5 | 196.1 ± 35.2 | 208.4 ± 35.6 | 218.8 ± 37.3 | 223.5 ± 35.9 |
| HDL cholesterol, mg/dl | 70.1 ± 18.8 | 68.7 ± 17.7 | 70.6 ± 18.8 | 71.7 ± 19.6 | 69.2 ± 19.0 |
| Hypertension, % | 4,565 (18.8) | 209 (4.0) | 518 (8.6) | 1,060 (16.9) | 2,778 (41.4) |
| History of diabetes, % | 439 (1.8) | 19 (0.4) | 31 (0.5) | 73 (1.2) | 316 (4.7) |
| History of CVD, % | 575 (2.4) | 53 (1.0) | 80 (1.3) | 108 (1.7) | 334 (5.0) |
| Antihypertensives, % | 1,255 (5.2) | 24 (0.5) | 72 (1.2) | 208 (3.3) | 951 (14.2) |
| Antidiabetic drugs, % | 218 (0.9) | 10 (0.2) | 11 (0.2) | 24 (0.4) | 173 (2.6) |
| SBP, mm Hg | 123.5 ± 15.5 | 116.7 ± 12.5 | 119.6 ± 13.4 | 123.3 ± 14.4 | 132.4 ± 16.3 |
| DBP, mm Hg | 72.5 ± 11.1 | 67.3 ± 9.5 | 70.4 ± 10.4 | 73.3 ± 10.8 | 77.5 ± 10.7 |
| MAP, mm Hg | 89.5 ± 11.8 | 83.8 ± 9.8 | 86.8 ± 10.7 | 90.0 ± 11.3 | 95.8 ± 11.6 |
| eGFR, ml/min/1.73 m2 | 87.4 ± 9.9 | 95.6 ± 7.9 | 90.8 ± 8.0 | 86.1 ± 8.0 | 79.2 ± 7.4 |
| CAVI, unit | 7.5 ± 1.0 | 6.3 ± 0.4 | 7.1 ± 0.2 | 7.7 ± 0.2 | 8.8 ± 0.6 |
Data are expressed as means ± SDs or counts (percentages). Abbreviations: CAVI, cardio-ankle vascular index; CVD, cardiovascular disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; MAP, mean arterial pressure; SBP, systolic blood pressure.
Associations of CAVI measurements with CKD events
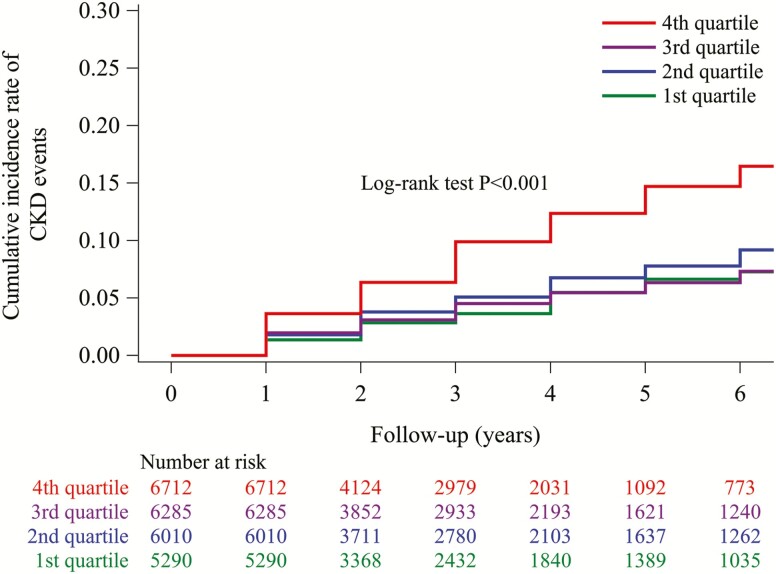
During a mean (interquartile range) follow-up of 3.1 (1.0, 4.0) years, 1,435 CKD events occurred. The cumulative incidence of CKD events was highest in the fourth quartile group (31.94 per 1,000 person-years) among the 4 groups (Figure 1). Since there was no difference in the cumulative incidence of CKD events among the third (14.90 per 1,000 person-years), second (16.90 per 1,000 person-years), and first (12.98 per 1,000 person-years) quartile groups, the 3 groups were merged and defined as the referent group. In an adjusted Cox model, the highest vs. lower quartiles of CAVI measurements (CAVI measurements ≥8.1 vs. <8.1) were associated with a higher risk for CKD events (HR, 1.34; 95% CI, 1.16, 1.54: Table 2, upper panel). After adjustment for mean arterial pressure and prevalent hypertension, the HR (95% CI) for CKD events was 1.28 (95% CI, 1.11, 1.48) for the highest vs. lower quartiles of CAVI measurements. The adjusted odds ratio (95% CI) for rapid eGFR decline was 1.25 (95% CI, 1.11, 1.41) for the highest vs. lower quartiles of CAVI measurements (Table 2, lower panel). When CAVI measurements were used as a continuous variable, the odds ratio (95% CI) associated with each SD increase was 1.23 (1.16, 1.32) for rapid eGFR decline.
Figure 1.
Cumulative incidence of CKD events for each CAVI group. The cumulative probability of CKD events by CAVI groups was calculated using the Kaplan–Meier method. The log-rank test was used to calculate the P value, which was <0.001. Participants were categorized into the 1st quartile group (CAVI ≤6.7 unit); the 2nd quartile group (CAVI 6.8–7.3 unit); the 3rd quartile group (CAVI 7.4–8.0 unit); or the 4th quartile group (CAVI ≥8.1 unit). Abbreviations: CAVI, cardio-ankle vascular index; CKD, chronic kidney disease.
Table 2.
Association of CAVI measurements wit incident CKD events and with rapid eGFR decline (n = 24,297)
| The highest vs. lower quartiles of CAVI measurements | Per 1 SD higher CAVI measurement | ||
|---|---|---|---|
| 1st–3rd quartiles (n = 17,585) | 4th quartile (n = 6,712) | ||
| CAVI range, units | <8.1 | ≥8.1 | +0.99 unit |
| Incident CKD events | |||
| Events/incidence rate (95% CI) | 838/15.02 (14.04, 16.06) | 597/31.94 (29.51, 34.56) | |
| Hazard ratio (95% confidence interval) | |||
| Model 1 | 1 (ref) | 1.34 (1.16, 1.54) | 1.19 (1.10, 1.29) |
| Model 2 | 1 (ref) | 1.28 (1.11, 1.48) | 1.15 (1.06, 1.25) |
| Rapid eGFR decline | |||
| Events/prevalence rate (95% CI) | 2,203/12.53 (12.05, 13.03) | 824/12.28 (11.51, 13.08) | |
| Odds ratio (95% confidence interval) | |||
| Model 1 | 1 (ref) | 1.25 (1.11, 1.40) | 1.22 (1.14, 1.30) |
| Model 2 | 1 (ref) | 1.25 (1.11, 1.41) | 1.23 (1.16, 1.32) |
The incidence rate is per 1,000 person-years. Adjusted HRs (95% CIs) for incident CKD events for the highest vs. lower quartiles of CAVI measurements and for 1-SD higher CAVI measurements are shown in the upper part of the table. The lower part of the table shows adjusted odds ratios (95% CIs) for rapid eGFR decline (−3 ml/min/1.73 m2 per year) for the highest vs. lower quartiles of CAVI measurements and for a 1-SD increase in CAVI. Model 1 includes adjustment for age, sex, body mass index, smoking status, total cholesterol, HDL cholesterol, eGFR, prevalent diabetes, and prevalent CVD. Model 2 includes the variables in Model 1, mean arterial pressure, and prevalent hypertension. eGFR is included only in the model for analyses reported in the lower part of the table. Abbreviations: CAVI, cardio-ankle vascular index; CI, confidence interval; CKD, chronic kidney disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; HR, hazard ratio.
CAVI measurements suggested by Spronck et al.24 were highly correlated with CAVI measurements used in the current analyses (Pearson’s correlation = 0.94) and yielded similar results (Supplementary Table S1 online). The cumulative incidence of eGFR <60 ml/min/1.73 m2, but not of proteinuria, was higher among the group with CAVI measurements ≥8.1 vs. <8.1 (Supplementary Figures S3 and S4 online). When proteinuria or eGFR <60 ml/min/1.73 m2 was assessed as outcomes separately, the associations with CAVI measurements were significant (Supplementary Table S2 online). There was no evidence of interaction between CAVI measurements, assessed as the highest vs. lower quartiles, and prevalent hypertension and baseline eGFR above or below 90 ml/min/1.73 m2 (all P > 0.20) in association with CKD events. When CAVI measurements were used as a continuous variable, the HRs (95% CI) associated with each SD higher level of CAVI measurements (+0.99 unit) were 1.15 (1.06, 1.25) for CKD events (Supplementary Table S1 online), 1.14 (0.99, 1.31) for proteinuria, and 1.21 (1.09, 1.36) for eGFR<60 ml/min/1.73 m2 (Supplementary Table S2 online).
Associations of CAVI measurements with change in eGFR during follow-up
Overall, eGFR declined by a mean of −0.89 (95% CI, −0.92 to −0.86) ml/min/1.73 m2 per year, and 3,027 participants had rapid eGFR decline during follow-up. The highest vs. lower quartiles of CAVI measurements were associated with greater decline in annual eGFR in an adjusted model (beta, −0.034; 95% CI, −0.060, −0.008 ml/min/1.73 m2 per year), and the association was present after we adjusted for mean arterial pressure and prevalent hypertension (Table 3). There was evidence of interaction between CAVI measurement, assessed as the highest vs. lower quartiles, and prevalent hypertension in association with eGFR change (P < 0.001). There was no evidence of interaction between CAVI measurements, assessed as the highest vs. lower quartiles, and baseline eGFR above or below 90 ml/min/1.73 m2 (all P > 0.20) in association with eGFR change (P = 0.20). In stratified analyses by hypertension at baseline, the adjusted beta (95% CI) for differences in eGFR change between the highest vs. lower quartiles of CAVI measurement was 0.028 (95% CI, −0.004, 0.060 ml/min/1.73 m2 per year) in participants without hypertension and −0.129 (95% CI, −0.184, −0.074 ml/min/1.73 m2 per year) in participants with hypertension (Supplementary Table S3 online). When CAVI measurements were used as a continuous variable, the adjusted beta (95% CI) for differences in eGFR change associated with each SD higher level of CAVI measurements was 0.031 (95% CI, 0.017, 0.045 ml/min/1.73 m2 per year) in participants without hypertension and −0.069 (95% CI, −0.097, −0.040 ml/min/1.73 m2 per year) in participants with hypertension (Supplementary Table S3 online).
Table 3.
Associations between CAVI measurements at baseline and eGFR change during follow-up (n = 24,297)
| The highest vs. lower quartiles of CAVI measurements | Per 1 SD higher CAVI measurement | ||
|---|---|---|---|
| 1st–3rd quartiles (n = 17,585) | 4th quartile (n = 6,712) | ||
| CAVI range, units | <8.1 | ≥8.1 | +0.99 unit |
| Annual change in eGFR | Beta (95% confidence interval) | ||
| Model 1 | |||
| CAVI | 0 (ref) | −0.038 (−0.251, 0.176) | −0.260 (−0.379, −0.141) |
| Interval | — | −0.783 (−0.795, −0.771) | −0.781 (−0.871, −0.691) |
| CAVI × interval | 0 (ref) | −0.034 (−0.060, −0.008) | −0.001 (−0.013, 0.011) |
| Model 2 | |||
| CAVI | 0 (ref) | −0.070 (−0.286, 0.146) | −0.295 (−0.416, −0.174) |
| Interval | — | −0.783 (−0.796, −0.771) | −0.781 (−0.871, −0.691) |
| CAVI × interval | 0 (ref) | −0.034 (−0.060, −0.008) | −0.001 (−0.013, 0.011) |
Adjusted betas (95% CIs) for differences in annual change in eGFR (ml/min/1.73 m2 per year) for the highest vs. lower quartiles of CAVI measurements and for 1-SD higher CAVI measurements are shown. We included interaction terms for CAVI measurement and the time interval from baseline to follow-up. Model 1includes adjustment for age, sex, body mass index, smoking status, total cholesterol, HDL cholesterol, prevalent diabetes, eGFR, and prevalent CVD. Model 2 includes the variables in Model 1, mean arterial pressure, and prevalent hypertension. Abbreviations: CAVI, cardio-ankle vascular index; CI, confidence interval; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein.
Sensitivity analyses
Fasting glucose values are available in 15,234 participants (63% of the entire population). Results were similar when we adjusted for fasting glucose (Supplementary Tables S4 and S5 online). In an analysis for CKD events, results were similar when we adjusted for systolic BP instead of mean arterial pressure (Supplementary Table S6 online) and when we excluded participants taking antihypertensive medication (Supplementary Table S7 online). In an analysis for annual eGFR change, results were similar when we adjusted for the span of time during which eGFR measurements were obtained for calculating eGFR change (Supplementary Table S8 online).
DISCUSSION
In this nationwide study that enrolled Japanese persons without CKD at baseline, those with CAVI measurements ≥8.1 had a higher risk for CKD events compared with their counterparts with CAVI measurements <8.1, independently of cardiovascular risk factors including BP. Participants with CAVI measurements ≥8.1 had a greater decline in annual eGFR compared with their counterparts with CAVI measurements <8.1. The association was stronger in participants with hypertension compared with those without hypertension.
Prior studies using PWV have shown that arterial stiffness is associated with kidney dysfunction among adults with CKD.5–10 CKD itself might lead to both arterial stiffness and decline in kidney function over time. The current study extends the previous knowledge by demonstrating the association of arterial stiffness with decline in kidney function over time among hypertensive adults without CKD. Moreover, we assessed CAVI measurements as a measure of arterial stiffness. CAVI also reflects the state of smooth muscle contraction rather than changes in BP,25 and thus it is influenced by vascular tone independently of BP levels. Therefore, the association between arterial stiffness and kidney dysfunction in our study might be less confounded by BP compared with associations reported when arterial stiffness was estimated from PWV.
There was no evidence of interaction between CAVI measurements and prevalent hypertension in association with CKD events. However, there was evidence of interaction between CAVI measurements and prevalent hypertension in association with eGFR change. Among participants with or without hypertension, higher CAVI measurement at baseline was associated with lower eGFR during follow-up. Higher CAVI measurement at baseline was associated with greater eGFR decline among participants with hypertension, but not among those without hypertension. This suggests that hypertension may accelerate vascular aging by causing mechanical injury to the vasculature and/or through concomitant inflammatory and oxidative stress, which ultimately contribute to greater declines in eGFR.26
CAVI measurement thresholds associated with subclinical CVD and CVD events remain to be determined. Persons with CAVI measurements ≥8.0 had a higher likelihood of coronary artery disease compared with their counterparts with CAVI measurements <8.0.26 Among persons with diabetes, CAVI measurements ≥9.0 vs. <9.0 were associated with a higher risk for CVD events.27 Consequently, the Physiological Diagnosis Criteria for Vascular Failure Committee in Japan recommended a threshold of <8.0 for defining normal CAVI measurements.28 The current study suggested that CAVI measurements ≥8.1 were associated with kidney dysfunction. However, since the effect size of CAVI measurements on kidney function was relatively modest in the current study, our results require further testing in an independent cohort to determine whether the assessment of CAVI in clinical practice improves detection and subsequent medical management of persons at higher risk for CKD events.
Strengths of this study include the well-characterized, nationwide sample of Japanese adults and the use of standardized data collection protocols. This study has several limitations. Possible residual confounding, including physical activity and dietary sodium intake, may affect associations between CAVI measurements and CKD events.29–31 Definition of CKD based on KDIGO guideline criteria requires 2 measurement opportunities which are at least 3 months apart. However, in this study, we defined CKD as the presence of proteinuria or eGFR <60 ml/min/1.73 m2 at a single occasion, which might not fully reflect a person’s kidney function. The mean eGFR at baseline was lower in the highest vs. lower quartiles of CAVI measurements. Thus, more participants with eGFR closer to 60 ml/min/1.73 m2 at baseline might be included in the highest vs. lower quartile groups, which may contribute to higher risk for CKD events in the highest vs. lower quartiles of CAVI measurements. However, eGFR declined in the highest vs. lower quartile groups more sharply on an annual basis and more rapidly over a longer time span. In the current study, carotid–femoral PWV and brachial–ankle PWV were not obtained. Therefore, it remains to be determined whether there is a difference in the associations between kidney outcomes and CAVI measurements vs. PWV measurements. Further studies are required to assess the optimal assessment of arterial stiffness to identify individuals at high risk for CKD events. Some classes of antihypertensive drugs (e.g., renin–angiotensin–aldosterone inhibitors) can affect CAVI and renal function,32,33 and the use of these medications may be a confounder due to their possible effects on CAVI and renal function. In the current study, classes of antihypertensive drug data were not available. However, results were similar when we excluded participants taking antihypertensive medication.
Our results may not be generalizable to other racial and ethnic groups (e.g., Whites, African Americans, and Hispanics).
This nationwide study of Japanese adults suggested that persons without CKD who also had CAVI measurements ≥8.1 had a higher likelihood of kidney dysfunction in later life compared with their counterparts with CAVI measurements <8.1. Greater arterial stiffness among adults without CKD may be associated with kidney dysfunction.
Supplementary Material
FUNDING
This work was supported by Grant-in-Aid for Prevention of lifestyle-related diseases from the Japan Health Promotion Foundation (2018-2019; no. 3001).
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1. O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension 2005; 46:200–204. [DOI] [PubMed] [Google Scholar]
- 2. Ito S, Nagasawa T, Abe M, Mori T. Strain vessel hypothesis: a viewpoint for linkage of albuminuria and cerebro-cardiovascular risk. Hypertens Res 2009; 32:115–121. [DOI] [PubMed] [Google Scholar]
- 3. Hashimoto J, Ito S. Central pulse pressure and aortic stiffness determine renal hemodynamics: pathophysiological implication for microalbuminuria in hypertension. Hypertension 2011; 58:839–846. [DOI] [PubMed] [Google Scholar]
- 4. Sedaghat S, Mattace-Raso FU, Hoorn EJ, Uitterlinden AG, Hofman A, Ikram MA, Franco OH, Dehghan A. Arterial stiffness and decline in kidney function. Clin J Am Soc Nephrol 2015; 10:2190–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Briet M, Bozec E, Laurent S, Fassot C, London GM, Jacquot C, Froissart M, Houillier P, Boutouyrie P. Arterial stiffness and enlargement in mild-to-moderate chronic kidney disease. Kidney Int 2006; 69:350–357. [DOI] [PubMed] [Google Scholar]
- 6. Wang MC, Tsai WC, Chen JY, Huang JJ. Stepwise increase in arterial stiffness corresponding with the stages of chronic kidney disease. Am J Kidney Dis 2005; 45:494–501. [DOI] [PubMed] [Google Scholar]
- 7. Upadhyay A, Hwang SJ, Mitchell GF, Vasan RS, Vita JA, Stantchev PI, Meigs JB, Larson MG, Levy D, Benjamin EJ, Fox CS. Arterial stiffness in mild-to-moderate CKD. J Am Soc Nephrol 2009; 20:2044–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim CS, Kim HY, Kang YU, Choi JS, Bae EH, Ma SK, Kim SW. Association of pulse wave velocity and pulse pressure with decline in kidney function. J Clin Hypertens (Greenwich) 2014; 16:372–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ford ML, Tomlinson LA, Chapman TP, Rajkumar C, Holt SG. Aortic stiffness is independently associated with rate of renal function decline in chronic kidney disease stages 3 and 4. Hypertension 2010; 55:1110–1115. [DOI] [PubMed] [Google Scholar]
- 10. Townsend RR, Anderson AH, Chirinos JA, Feldman HI, Grunwald JE, Nessel L, Roy J, Weir MR, Wright JT Jr, Bansal N, Hsu CY; CRIC Study Investigators . Association of pulse wave velocity with chronic kidney disease progression and mortality: findings from the CRIC Study (Chronic Renal Insufficiency Cohort). Hypertension 2018; 71:1101–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tomiyama H, Tanaka H, Hashimoto H, Matsumoto C, Odaira M, Yamada J, Yoshida M, Shiina K, Nagata M, Yamashina A. Arterial stiffness and declines in individuals with normal renal function/early chronic kidney disease. Atherosclerosis 2010; 212:345–350. [DOI] [PubMed] [Google Scholar]
- 12. Hayashi K. Experimental approaches on measuring the mechanical properties and constitutive laws of arterial walls. J Biomech Eng 1993; 115:481–488. [DOI] [PubMed] [Google Scholar]
- 13. Shirai K, Utino J, Otsuka K, Takata M. A novel blood pressure-independent arterial wall stiffness parameter; cardio-ankle vascular index (CAVI). J Atheroscler Thromb 2006; 13:101–107. [DOI] [PubMed] [Google Scholar]
- 14. Hayashi K, Yamamoto T, Takahara A, Shirai K. Clinical assessment of arterial stiffness with cardio-ankle vascular index: theory and applications. J Hypertens 2015; 33:1742–1757; discussion 1757. [DOI] [PubMed] [Google Scholar]
- 15. Shirai K, Song M, Suzuki J, Kurosu T, Oyama T, Nagayama D, Miyashita Y, Yamamura S, Takahashi M. Contradictory effects of β1- and α1-aderenergic receptor blockers on cardio-ankle vascular stiffness index (CAVI)—CAVI independent of blood pressure. J Atheroscler Thromb 2011; 18:49–55. [DOI] [PubMed] [Google Scholar]
- 16. Wohlfahrt P, Krajčoviechová A, Seidlerová J, Mayer O, Bruthans J, Filipovský J, Laurent S, Cífková R. Arterial stiffness parameters: how do they differ? Atherosclerosis 2013; 231:359–364. [DOI] [PubMed] [Google Scholar]
- 17. Satoh-Asahara N, Kotani K, Yamakage H, Yamada T, Araki R, Okajima T, Adachi M, Oishi M, Shimatsu A; Japan Obesity and Metabolic Syndrome Study (JOMS) Group . Cardio-ankle vascular index predicts for the incidence of cardiovascular events in obese patients: a multicenter prospective cohort study (Japan Obesity and Metabolic Syndrome Study: JOMS). Atherosclerosis 2015; 242:461–468. [DOI] [PubMed] [Google Scholar]
- 18. Matsushita K, Ding N, Kim ED, Budoff M, Chirinos JA, Fernhall B, Hamburg NM, Kario K, Miyoshi T, Tanaka H, Townsend R. Cardio-ankle vascular index and cardiovascular disease: systematic review and meta-analysis of prospective and cross-sectional studies. J Clin Hypertens (Greenwich) 2019; 21:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kario K, Kanegae H, Oikawa T, Suzuki K. Hypertension is predicted by both large and small artery disease. Hypertension 2019; 73:75–83. [DOI] [PubMed] [Google Scholar]
- 20. Meng L, Zhao D, Pan Y, Ding W, Wei Q, Li H, Gao P, Mi J. Validation of Omron HBP-1300 professional blood pressure monitor based on auscultation in children and adults. BMC Cardiovasc Disord 2016; 16:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ishimitsu T, Ito M, Ito S, Itoh H, Iwao H, Kai H, Kario K, Kashihara N, Kawano Y, Kim-Mitsuyama S, Kimura G, Kohara K, Komuro I, Kumagai H, Matsuura H, Miura K, Morishita R, Naruse M, Node K, Ohya Y, Rakugi H, Saito I, Saitoh S, Shimada K, Shimosawa T, Suzuki H, Tamura K, Tanahashi N, Tsuchihashi T, Uchiyama M, Ueda S, Umemura S; Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension . The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res 2014; 37:253–390. [DOI] [PubMed] [Google Scholar]
- 22. Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S. Modification of the CKD epidemiology collaboration (CKD-EPI) equation for Japanese: accuracy and use for population estimates. Am J Kidney Dis 2010; 56:32–38. [DOI] [PubMed] [Google Scholar]
- 23. Rifkin DE, Shlipak MG, Katz R, Fried LF, Siscovick D, Chonchol M, Newman AB, Sarnak MJ. Rapid kidney function decline and mortality risk in older adults. Arch Intern Med 2008; 168:2212–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spronck B, Avolio AP, Tan I, Butlin M, Reesink KD, Delhaas T. Arterial stiffness index beta and cardio-ankle vascular index inherently depend on blood pressure but can be readily corrected. J Hypertens 2017; 35:98–104. [DOI] [PubMed] [Google Scholar]
- 25. Kim B, Takada K, Oka S, Misaki T. Influence of blood pressure on cardio-ankle vascular index (CAVI) examined based on percentage change during general anesthesia. Hypertens Res 2011; 34:779–783. [DOI] [PubMed] [Google Scholar]
- 26. Guzik TJ, Touyz RM. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension 2017; 70:660–667. [DOI] [PubMed] [Google Scholar]
- 27. Chung SL, Yang CC, Chen CC, Hsu YC, Lei MH. Coronary artery calcium score compared with cardio-ankle vascular index in the prediction of cardiovascular events in asymptomatic patients with type 2 diabetes. J Atheroscler Thromb 2015; 22:1255–1265. [DOI] [PubMed] [Google Scholar]
- 28. Tanaka A, Tomiyama H, Maruhashi T, Matsuzawa Y, Miyoshi T, Kabutoya T, Kario K, Sugiyama S, Munakata M, Ito H, Ueda S, Vlachopoulos C, Higashi Y, Inoue T, Node K; Physiological Diagnosis Criteria for Vascular Failure Committee . Physiological diagnostic criteria for vascular failure. Hypertension 2018; 72:1060–1071. [DOI] [PubMed] [Google Scholar]
- 29. Kirkman DL, Ramick MG, Muth BJ, Stock JM, Pohlig RT, Townsend RR, Edwards DG. Effects of aerobic exercise on vascular function in nondialysis chronic kidney disease: a randomized controlled trial. Am J Physiol Renal Physiol 2019; 316:F898–F905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kimoto E, Shoji T, Shinohara K, Hatsuda S, Mori K, Fukumoto S, Koyama H, Emoto M, Okuno Y, Nishizawa Y. Regional arterial stiffness in patients with type 2 diabetes and chronic kidney disease. J Am Soc Nephrol 2006; 17:2245–2252. [DOI] [PubMed] [Google Scholar]
- 31. Wright JA, Cavanaugh KL. Dietary sodium in chronic kidney disease: a comprehensive approach. Semin Dial 2010; 23:415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brewster UC, Perazella MA. The renin-angiotensin-aldosterone system and the kidney: effects on kidney disease. Am J Med 2004; 116:263–272. [DOI] [PubMed] [Google Scholar]
- 33. Kinouchi K, Ichihara A, Sakoda M, Kurauchi-Mito A, Murohashi-Bokuda K, Itoh H. Effects of telmisartan on arterial stiffness assessed by the cardio-ankle vascular index in hypertensive patients. Kidney Blood Press Res 2010; 33:304–312. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.