Abstract
Objectives
The purpose of this study was to establish age- and sex-specific pediatric reference intervals of serum potassium (K), sodium (Na), chlorine (Cl), calcium (Ca), magnesium (Mg), and phosphorus (P) using a direct sampling technique.
Methods
In accordance with the a priori approach, healthy individuals (n = 6,466, aged 1 month to <18 years) were enrolled from five regions in Jilin Province, China, and all analytes were performed in the center laboratory. Reference intervals were divided according to the regression tree and Harris and Boyd’s method, and then they were calculated by the nonparametric rank method. The dynamic changes of reference intervals were evaluated by the lambda-mu-sigma (LMS) method.
Results
Reference intervals of serum Na and Ca were divided into three age-specific partitions. The concentrations of K, Cl, and Mg remained stable with age. However, only dramatic sex-specific changes of P were shown in those 11 to less than 13 years old and 13 to less than 15 years old, with an earlier peak time in females than in males. The correlation between Na and Cl was the strongest among all serum electrolytes (r = 0.31).
Conclusions
Serum electrolyte reference intervals for children and adolescents were established by regression tree, z test, and the LMS method, which provide a more accurate interpretation for diagnosis and prognosis evaluation of clinical pediatric diseases.
Key Points.
Serum electrolyte reference intervals for children and adolescents are established by regression tree, Harris and Boyd’s method, and the lambda-mu-sigma method.
Medical professionals can help diagnose a disease based on a reference interval for a specific population.
Some biomarkers change dynamically during growth and development, so age and sex differences need to be considered when establishing reference intervals.
Reference intervals (RIs) are commonly defined as the 95% of values of a special laboratory test for a healthy reference population. In general, the lower reference limit (LRL) to the upper reference limit (URL) is equal to the 2.5th to 97.5th centiles of the test result distribution (except in some special cases).1 The RIs are considered a critical factor in clinical decision making for helping the diagnosis of disease and the analysis of laboratory examination results. Otherwise, the erroneous estimation of these ranges can lead to misdiagnosis, enormous expense, great psychological pressure, and unnecessary treatments. Currently, most clinical laboratories in China adopt RIs from manufacturer package inserts, textbooks, or literature.2 However, they may not be as robust as commonly believed. Many factors can contribute to changes in analyte concentrations, including age, sex, pubertal development, ethnicity, diurnal variation, and nutritional status. In fact, our research group has conducted some studies in the early stage, and the results confirm that age, sex, and statistical methods have a certain influence on the reference interval.3,4 Therefore, establishing accurate RIs for local laboratories is one of the most important factors for clinical diagnosis.
Electrolytes are of crucial importance for many physiologic functions. Potassium (K) plays a critical role in the maintenance of normal action potentials in muscles and nerve cells, the regulation of acid base balance, and assistance in cardiac muscle cell activity.5,6 Sodium (Na) is involved in maintaining the balance of fluid, acid base, and the osmolarity of plasma. Moreover, it also helps to transmit impulses in the nerve and muscle fibers to conduct muscle and nerve activity.6,7 Meanwhile, chlorine (Cl) serves numerous physiologic functions, such as the maintenance of osmotic pressure, the preservation of electrical neutrality, and the movement of water between fluid compartments.8 Furthermore, calcium (Ca) and phosphorus (P) are important components in bone metabolism indices. Ca plays a considerable role in muscle contraction and relaxation, certain enzyme activation, coagulation, and bonding with proteins to pass through capillary walls. P is involved in many biochemical processes, neuromuscular functions, and RBC functions.9 As for magnesium (Mg), its study mainly focuses on carbohydrate metabolism and protein synthesis. Maintaining balance of electrolytes in the body is essential to overall function and health. A slight imbalance needs to be reflected immediately through the RIs.
In China, RI standards of electrolytes for a healthy Chinese adult population were established in 2012.10 Due to the dynamic changes associated with development, physiology, nutrition, and diseases during growth, reliable and accurate RIs are particularly important for the pediatric population.11 However, clinical laboratories rarely establish pediatric RIs based on the local population, for the determination of pediatric RIs requires a tremendous amount of time and cost, as well as a sufficient number of healthy individuals (more than 120 in each subgroup).12 Although several national and international initiatives have established pediatric RIs based on healthy children and adolescents in Western countries,13 there are some diversities in population, sampling technique, and analytical procedure. Therefore, it is necessary to establish pediatric RIs suitable for the Chinese pediatric population.
Recently, some scholars from China have committed to establishing pediatric RIs in different regions.14,15 Yet, the partitions in those studies were determined by visually inspecting distribution, scatterplots for overall trends, Harris and Boyd’s method (z test), and other conventional statistical methods. Although these studies add some new information for the pediatric RIs, medical clinical significance also needs to be considered when the RIs are determined by statistical methods. In addition, large-sample studies using the pediatric population also need to investigate the partitioned methods in diverse ethnic groups or analysis systems. Therefore, appropriate statistical methods applied to the partition of RIs are indispensable and worthy of constant discussion.
The current study aimed to determine the age- and sex-specific pediatric RIs for serum electrolytes of K, Na, Cl, Ca, Mg, and P in five areas of Jilin Province. Direct sampling technique was used for recruitment of all healthy children and adolescents, and then a large-sample research was formed. The First Hospital of Jilin University was considered the central laboratory for sample testing. Furthermore, some statistical methods are proposed to be innovative. RIs were divided by regression tree and z test, with dynamic changes described by the lambda-mu-sigma (LMS) method. Moreover, the relationship among the electrolytes was also analyzed to describe the internal connections of each electrolyte.
Materials and Methods
Participants and Data Collection
Healthy children and adolescents from community centers, primary schools, middle schools, and high schools (aged 1 month to <18 years) were recruited from August 2017 to November 2018 in Changchun, Jilin, Yanbian Korean Autonomous Prefecture, Songyuan and Baishan, Jilin Province. The sample collection involved 12 schools and nine hospitals, and the areas were evenly distributed. Healthy individuals were enrolled according to the questionnaire responses, physical examination, and clinical laboratory examination. Recruitment posters were issued and all potential participants completed a questionnaire, including questions on chronic diseases, medication history, dietary and lifestyle habits, physical activity patterns, allergies, and a general question concerning subjective health. Body mass index (BMI) was calculated as the ratio of body weight to the square of body height (kg/m2). Participants were excluded from the study if they had a recent history of acute or chronic infection; digestive, kidney, metabolic, rheumatic, and thyroid diseases; any systemic disease; atherosclerosis and vascular disease; heart disease; malignant tumors; burns and muscle damage; weight and height exceeding more than 10% of the average for the same sex; hypertension (systolic blood pressure ≥140 mm Hg and diastolic blood pressure ≥90 mm Hg); use of prescribed medications (within 2 weeks); surgery (within 6 months); or donation of blood (within 6 months). They were also excluded if they met the following clinical laboratory criteria: positive for hepatitis B surface antigen, hepatitis C virus, or human immunodeficiency virus antibody; creatinine more than 97 μmol/L (male) or more than 73 μmol/L (female); creatine kinase more than 500.0 U/L; uric acid more than 475.0 μmol/L; albumin less than 35.0 g/L; glucose more than 7.0 mmol/L; C-reactive protein more than 10.0 mg/L; WBC count less than 3.0 × 109/L or more than 12.0 × 109/L; or hemoglobin less than 120 g/L (male) or less than 110 g/L (female). This study was approved by the ethics committee of all participating hospitals.
Sample Collection
The participants were instructed to maintain their normal diet and exercise level, as well as to fast for more than 8 hours prior to blood sample collection, while those younger than 3 years needed to fast 3 to 6 hours. Following the completion of written informed consent and a health questionnaire from the parents or guardians, 4 mL of venous blood was collected in plastic vacutainer tubes containing gel (Vacusiner SST; BD) by certified phlebotomists between 7:30 and 9:30 am. After a full clot for 30 minutes, blood samples were centrifuged at 1,200g for 10 minutes at room temperature. Except for the samples of hemolysis, lipidemia, and jaundice, all qualified samples were transported by cold chain trucks to ensure the activity of the analyte at 2°C to 8°C. Samples beyond Changchun had to be transported within 8 hours, while samples in Changchun required 2 hours. All assays needed to be performed within 1 hour after receipt in the central laboratory, the First Hospital of Jilin University.
Analytical Performance of the Methods
All serum electrolytes were analyzed by the Ortho VITORS 5600 automatic biochemical analyzer (Ortho-Clinical Diagnostic). Serum K, Na, and Cl were measured by the direct potentiometry method, and serum Ca, Mg, and P were measured using a method based on colorimetry. Samples analyses were performed only after quality control analysis within acceptable limits, and daily, weekly, and monthly preventative maintenance was performed as specified by the manufacturer. Technical details about the system performance analyses are specified in Table 1. Reagent kits, calibrators, and quality control materials were supported by Ortho-Clinical Diagnostics. The Department of Laboratory Medicine at the First Hospital of Jilin University was accredited according to ISO 15189 Medical Laboratories–Particular Requirements for Quality and Competence by the China National Accreditation Service for Conformity Assessment in 2012.
Table 1.
Analytical Performance of Ortho VITROS 5600 Chemistry Analyzer
| Analyte | Unit | Bias of Accuracy, % | Precision, % | Measuring Range | Reference Method | Reference Material | |||
|---|---|---|---|---|---|---|---|---|---|
| Low Level | High Level | ||||||||
| Within- Day | Between- Day | Within- Day | Between- Day | ||||||
| Potassium | mmol/L | 0.40 | 1.14 | 1.65 | 0.98 | 1.52 | 1.00-18.26 | Flame atomic emission spectroscopy | NIST SRM 918 |
| Sodium | mmol/L | 0.38 | 0.77 | 1.42 | 0.60 | 1.46 | 75.0-177.1 | Flame atomic emission spectroscopy | NIST SRM 919 |
| Chlorine | mmol/L | 0.44 | 0.93 | 2.03 | 0.60 | 1.83 | 72.3-122.0 | Coulometric- amperometric titration | NIST SRM 919 |
| Calcium | mg/dL | –0.40 | 0.59 | 2.02 | 0.62 | 1.45 | 4.97-36.39 | Atomic absorption | NIST SRM 915 |
| Magnesium | mg/dL | 1.70 | 1.20 | 2.75 | 0.70 | 1.96 | 0.19-9.99 | Flame atomic absorption | NIST SRM 929 |
| Phosphorus | mg/dL | 1.71 | 1.15 | 1.59 | 0.94 | 1.51 | 0.50-13.00 | Phosphomolybdate/p- semidine hydrochloride | NIST SRM 200 |
Statistical Analysis
Data were analyzed in accordance with our previous studies14 and the Clinical and Laboratory Standards Institute EP28-A3c guideline.16 Participants were stratified by sex and classified into 1-year intervals. For each group, outlier exclusion was performed using the Dixon method,17 and the normality distribution test was adopted by the one-sample Kolmogorov-Smirnov test. When the distribution of data was non-Gaussian, the Box-Cox method was necessary to convert the normal distribution. Then, the z test was used to judge the sex difference for those of the same age.18 Smoothed continuous centile curves were constructed by the LMS method using the maximum penalized likelihood estimation technique and determined with the Box-Cox transformation λ (L), median μ (M), and coefficient of variation σ (S). The centile 100α of y at t was given by C100α(t) = M(t)[1 + L(t)S(t)Zα]1/L(t),19 where Zα was the normal equivalent deviate of size α. This showed that if the L, M, and S curves were smooth, then so were the centile curves. The freedom of L, M, and S was gradually adjusted to fit by software, which visually presented the continuous centile curve values as continuous lines as a function of age. After sex was stratified, the regression tree algorithm (a subclass of classification and regression tree methods) for age, which was a continuous outcome variable, was applied in our analyses to find the best split point according to the minimum mean squared error (MSE). MSE was computed as follows: , where n was the number of subgroups, was observations, and was the one-step-ahead forecasts.20 Immediately, the z test was used to determine the significant difference of the mean value between the two subgroups divided by the regression tree. Afterward, age- and sex-specific RIs could be calculated according to the adjusted optimal segmentation points. If the sample size of partitions was more than 120, the RIs were calculated using the nonparametric rank method. For partitions with a sample size of more than 40 and less than 120, the robust method was applied. Ultimately, the 90% confidence intervals around the LRL and URL were calculated using percentile bootstrap estimates. Spearman rank correlation analysis, a nonparametric method, was performed to assess the relationships among electrolyte levels.
Statistical tests were performed with SPSS 22.0 (SPSS) and R 3.6.0 (R Core Team). All graphics were drawn with GraphPad Prism 7.0 software (GraphPad Software), and the parameters of continuous centile curves were estimated by LMS chartmaker Light 2.54 (Medical Research Council).
Results
Population Characteristics
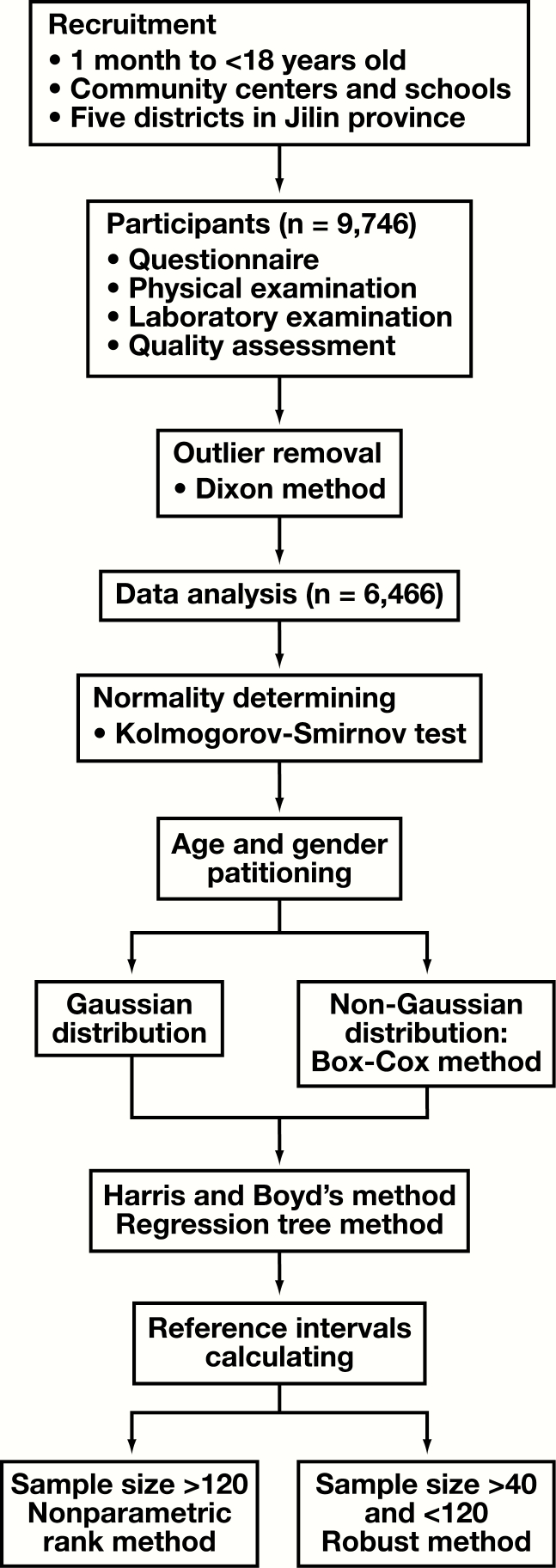
A total of 9,746 children and adolescents were recruited in the present study. After the application of exclusion criteria, 6,466 individuals with 3,207 males (aged 1 month to <18 years) and 3,259 females (aged 1 month to <18 years), with a male to female ratio of 1:1.02, were enrolled in our study. In addition, height and weight standardized growth charts helped determine the growth level and nutritional status of individual children. After comparing with Chinese national standards,21 the results show that although there are some differences in height and weight standardized growth charts, they mainly concentrated at the higher age of the weight standardized growth charts (Supplemental Figure 1; all supplemental materials can be found at American Journal of Clinical Pathology online). The overall gap is not significant, further indicating that the data in this study are representative. The protocol for data removal and RI establishment is shown in Figure 1.
Figure 1.
Protocol for establishment of reference intervals.
Age- and Sex-Specific RIs
RIs of serum K, Cl, and Mg were only one partition without sex and age differences. Due to the age difference, the RIs of serum Na and Ca were divided into three partitions. RIs of serum P were divided into six partitions according to age, and there were sex differences between children aged 11 to less than 13 years and 13 to less than 15 years. The low and high reference limits for each sex from 1 month to less than 18 years of age are shown in Table 2, and the suggested RIs are shown in Table 3.
Table 2.
Age-Specific and Sex-Specific 2.5th and 97.5th Percentiles for Electrolyte Levels
| Age | Sex | No. | Potassium, mmol/L | Sodium, mmol/L | Chlorine, mmol/L | Calcium, mg/dL | Magnesium, mg/dL | Phosphorus, mg/dL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2.5th | 50th | 97.5th | 2.5th | 50th | 97.5th | 2.5th | 50th | 97.5th | 2.5th | 50th | 97.5th | 2.5th | 50th | 97.5th | 2.5th | 50th | 97.5th | |||
| 1 mo to <1 y | M | 88 | 4.11 | 4.65 | 5.30 | 131.3 | 136.2 | 139.8 | 97.6 | 101.2 | 105.7 | 9.78 | 10.70 | 11.38 | 1.77 | 2.07 | 2.26 | 4.96 | 6.01 | 6.88 |
| F | 56 | 4.11 | 4.72 | 5.32 | 131.5 | 136.7 | 141.1 | 97.0 | 101.6 | 106.1 | 9.90 | 10.94 | 11.54 | 1.80 | 2.04 | 2.26 | 5.17 | 6.07 | 6.88 | |
| 1-<2 y | M | 128 | 3.79 | 4.36 | 5.15 | 131.4 | 136.8 | 140.5 | 96.7 | 100.8 | 105.7 | 9.54 | 10.62 | 11.30 | 1.68 | 2.02 | 2.28 | 4.52 | 5.67 | 6.75 |
| F | 135 | 3.81 | 4.49 | 5.17 | 131.8 | 136.5 | 140.6 | 96.6 | 101.9 | 105.9 | 9.54 | 10.66 | 11.26 | 1.73 | 1.97 | 2.24 | 4.52 | 5.64 | 6.60 | |
| 2-<3 y | M | 231 | 3.75 | 4.37 | 5.05 | 132.8 | 136.7 | 141.6 | 99.0 | 102.4 | 105.4 | 9.54 | 10.42 | 11.50 | 1.56 | 1.87 | 2.11 | 4.62 | 5.48 | 6.38 |
| F | 237 | 3.77 | 4.38 | 5.17 | 133.0 | 136.8 | 140.3 | 98.9 | 102.5 | 105.5 | 9.46 | 10.46 | 11.46 | 1.58 | 1.85 | 2.14 | 4.65 | 5.51 | 6.50 | |
| 3-<4 y | M | 191 | 3.79 | 4.32 | 5.23 | 133.0 | 137.3 | 143.1 | 97.8 | 102.5 | 106.6 | 9.66 | 10.42 | 11.38 | 1.58 | 1.85 | 2.14 | 4.65 | 5.42 | 6.57 |
| F | 193 | 3.76 | 4.38 | 5.21 | 133.7 | 137.8 | 142.7 | 98.3 | 102.7 | 106.6 | 9.50 | 10.46 | 11.14 | 1.56 | 1.82 | 2.09 | 4.55 | 5.48 | 6.41 | |
| 4-<5 y | M | 123 | 3.75 | 4.36 | 5.18 | 133.1 | 137.9 | 143.6 | 98.0 | 102.4 | 106.8 | 9.22 | 10.38 | 11.30 | 1.56 | 1.82 | 2.14 | 4.58 | 5.48 | 6.57 |
| F | 124 | 3.66 | 4.26 | 5.21 | 132.7 | 138.8 | 143.2 | 97.9 | 102.3 | 105.7 | 9.42 | 10.42 | 11.22 | 1.63 | 1.85 | 2.09 | 4.43 | 5.45 | 6.54 | |
| 5-<6 y | M | 297 | 3.77 | 4.39 | 5.28 | 133.4 | 137.8 | 143.0 | 97.6 | 101.8 | 106.0 | 9.26 | 10.30 | 11.22 | 1.53 | 1.85 | 2.09 | 4.49 | 5.58 | 6.75 |
| F | 317 | 3.86 | 4.42 | 5.30 | 133.7 | 138.0 | 142.7 | 97.6 | 101.8 | 105.9 | 9.34 | 10.42 | 11.18 | 1.56 | 1.82 | 2.11 | 4.49 | 5.61 | 6.72 | |
| 6-<7 y | M | 122 | 3.73 | 4.47 | 5.11 | 132.8 | 138.8 | 143.4 | 98.1 | 101.8 | 105.8 | 8.94 | 9.94 | 11.30 | 1.63 | 1.82 | 2.09 | 4.55 | 5.48 | 6.38 |
| F | 121 | 3.81 | 4.41 | 5.19 | 133.0 | 139.0 | 143.4 | 97.5 | 101.8 | 106.0 | 8.94 | 10.10 | 11.34 | 1.58 | 1.85 | 2.14 | 4.52 | 5.48 | 6.41 | |
| 7-<8 y | M | 123 | 3.64 | 4.38 | 5.16 | 132.8 | 138.6 | 143.4 | 97.1 | 101.9 | 106.4 | 8.94 | 10.18 | 11.02 | 1.63 | 1.90 | 2.19 | 4.37 | 5.42 | 6.29 |
| F | 120 | 3.68 | 4.3 | 5.10 | 132.8 | 138.3 | 142.8 | 97.4 | 101.8 | 106.4 | 8.94 | 10.26 | 11.10 | 1.60 | 1.92 | 2.19 | 4.27 | 5.39 | 6.13 | |
| 8-<9 y | M | 162 | 3.75 | 4.41 | 5.30 | 133.2 | 138.1 | 142.8 | 97.6 | 101.4 | 105.3 | 9.22 | 10.18 | 10.94 | 1.58 | 1.87 | 2.16 | 4.52 | 5.39 | 6.16 |
| F | 121 | 3.92 | 4.45 | 5.29 | 133.6 | 138.5 | 142.3 | 98.1 | 101.2 | 105.7 | 9.18 | 10.26 | 10.86 | 1.65 | 1.87 | 2.14 | 4.43 | 5.33 | 6.10 | |
| 9-<10 y | M | 224 | 3.84 | 4.47 | 5.24 | 134.7 | 139.3 | 143.5 | 97.6 | 101.8 | 106.0 | 9.18 | 10.14 | 10.98 | 1.63 | 1.85 | 2.14 | 4.58 | 5.36 | 6.23 |
| F | 222 | 3.83 | 4.36 | 5.17 | 135.0 | 139.4 | 143.7 | 97.5 | 102.1 | 105.8 | 9.10 | 10.26 | 11.02 | 1.60 | 1.87 | 2.14 | 4.62 | 5.42 | 6.38 | |
| 10-<11 y | M | 207 | 3.83 | 4.49 | 5.10 | 135.2 | 140.1 | 144.5 | 97.4 | 101.3 | 105.8 | 9.10 | 10.10 | 10.98 | 1.60 | 1.82 | 2.07 | 4.40 | 5.33 | 6.32 |
| F | 191 | 3.72 | 4.44 | 5.10 | 136.0 | 139.9 | 144.2 | 97.5 | 101.3 | 105.9 | 9.10 | 10.06 | 10.82 | 1.60 | 1.80 | 2.04 | 4.43 | 5.39 | 6.29 | |
| 11-<12 y | M | 192 | 3.83 | 4.50 | 5.22 | 136.5 | 140.4 | 144.8 | 97.7 | 101.7 | 105.5 | 9.30 | 10.22 | 11.06 | 1.58 | 1.85 | 2.11 | 4.65 | 5.70 | 6.54 |
| F | 244 | 3.76 | 4.36 | 5.16 | 135.6 | 139.7 | 144.3 | 97.1 | 101.7 | 105.4 | 9.18 | 10.14 | 10.90 | 1.60 | 1.85 | 2.11 | 4.03 | 5.27 | 6.54 | |
| 12-<13 y | M | 362 | 3.79 | 4.44 | 5.19 | 136.3 | 140.5 | 144.4 | 97.2 | 101.6 | 105.6 | 9.38 | 10.30 | 11.18 | 1.60 | 1.87 | 2.16 | 4.46 | 5.79 | 6.88 |
| F | 353 | 3.67 | 4.34 | 5.06 | 136.0 | 139.9 | 144.3 | 97.2 | 101.8 | 106.0 | 9.42 | 10.22 | 10.94 | 1.60 | 1.85 | 2.16 | 3.87 | 4.99 | 6.38 | |
| 13-<14 y | M | 250 | 3.68 | 4.42 | 5.25 | 136.6 | 140.8 | 144.5 | 97.1 | 101.4 | 105.1 | 9.06 | 10.30 | 10.94 | 1.60 | 1.90 | 2.19 | 4.06 | 5.48 | 6.81 |
| F | 257 | 3.66 | 4.28 | 5.25 | 135.9 | 139.7 | 144.2 | 98.3 | 101.7 | 105.3 | 9.14 | 10.18 | 10.98 | 1.58 | 1.85 | 2.14 | 3.87 | 4.83 | 6.10 | |
| 14-<15 y | M | 122 | 3.83 | 4.47 | 5.20 | 136.4 | 140.9 | 144.3 | 99.0 | 101.5 | 104.4 | 9.26 | 10.10 | 10.98 | 1.58 | 1.87 | 2.16 | 3.96 | 5.17 | 6.35 |
| F | 124 | 3.63 | 4.32 | 5.10 | 137.3 | 140.6 | 143.9 | 98.1 | 102.0 | 105.8 | 9.02 | 10.02 | 10.78 | 1.58 | 1.85 | 2.14 | 3.62 | 4.74 | 5.73 | |
| 15-<16 y | M | 132 | 3.66 | 4.37 | 5.26 | 134.6 | 140.2 | 144.3 | 97.5 | 100.9 | 104.4 | 8.94 | 10.18 | 10.70 | 1.65 | 1.94 | 2.24 | 3.87 | 4.74 | 5.82 |
| F | 198 | 3.77 | 4.35 | 5.14 | 134.8 | 139.2 | 144.0 | 98.0 | 102.0 | 105.5 | 9.06 | 10.10 | 10.86 | 1.56 | 1.90 | 2.21 | 3.93 | 4.62 | 5.45 | |
| 16-<17 y | M | 124 | 3.79 | 4.32 | 4.88 | 135.7 | 140.1 | 143.8 | 96.3 | 100.5 | 104.5 | 9.14 | 10.18 | 10.94 | 1.68 | 2.02 | 2.28 | 3.87 | 4.74 | 5.76 |
| F | 123 | 3.59 | 4.20 | 4.75 | 136.2 | 139.2 | 143.0 | 98.0 | 102.2 | 105.7 | 9.02 | 10.02 | 10.86 | 1.68 | 1.94 | 2.24 | 3.84 | 4.58 | 5.27 | |
| 17-<18 y | M | 129 | 3.65 | 4.18 | 4.66 | 134.5 | 139.5 | 144.2 | 95.6 | 100.9 | 105.7 | 9.66 | 10.42 | 11.14 | 1.58 | 1.85 | 2.21 | 3.53 | 4.00 | 5.08 |
| F | 123 | 3.55 | 4.15 | 4.77 | 134.1 | 139.6 | 143.9 | 96.1 | 101.4 | 106.0 | 9.38 | 10.26 | 10.98 | 1.58 | 1.87 | 2.24 | 3.56 | 4.09 | 5.11 |
Table 3.
Age-Specific and Sex-Specific Pediatric Reference Intervals for Electrolyte Levels
| Analyte | Unit | Age | Sex | No. | Lower Limit (95% Confidence Interval) | Upper Limit (95% Confidence Interval) |
|---|---|---|---|---|---|---|
| Potassium | mmol/L | 1 mo to <18 y | F + M | 6,466 | 3.76 (3.73-3.77) | 5.18 (5.16-5.19) |
| Sodium | mmol/L | 1 mo to <3 y | F + M | 875 | 131.9 (131.7-132.1) | 140.6 (140.4-141.2) |
| 3-<9 y | F + M | 2,014 | 133.3 (133.1-133.4) | 143.0 (142.8-143.1) | ||
| 9-<18 y | F + M | 3,577 | 135.2 (135.1-135.3) | 144.2 (144.1-144.3) | ||
| Chlorine | mmol/L | 1 mo to <18 y | F + M | 6,466 | 97.4 (97.3-97.5) | 105.7 (105.7-105.8) |
| Calcium | mg/dL | 1 mo to <2 y | F + M | 407 | 9.62 (9.54-9.66) | 11.38 (11.26-11.42) |
| 2-<6 y | F + M | 1,713 | 9.38 (9.34-9.42) | 11.30 (11.26-11.34) | ||
| 6-<18 y | F + M | 4,346 | 9.14 (9.10-9.18) | 10.98 (10.98-11.02) | ||
| Magnesium | mg/dL | 1 mo to <18 y | F + M | 6,466 | 1.58 (1.58-1.60) | 2.19 (2.19-2.19) |
| Phosphorus | mg/dL | 1 mo to <1 y | F + M | 144 | 5.11 (4.92-5.27) | 6.88 (6.85-6.88) |
| 1-<11 y | F + M | 3,589 | 4.52 (4.52-4.55) | 6.47 (6.44-6.50) | ||
| 11-<13 y | M | 554 | 4.52 (4.46-4.58) | 6.85 (6.81-6.88) | ||
| F | 597 | 3.90 (3.87-3.96) | 6.47 (6.38-6.50) | |||
| 13-<15 y | M | 372 | 4.03 (3.96-4.09) | 6.75 (6.69-6.85) | ||
| F | 381 | 3.75 (3.69-3.81) | 5.92 (5.82-6.13) | |||
| 15-<17 y | F + M | 577 | 3.87 (3.87-3.90) | 5.61 (5.48-5.76) | ||
| 17-<18 y | F + M | 252 | 3.56 (3.53-3.56) | 5.08 (5.02-5.11) |
Variation Tendency
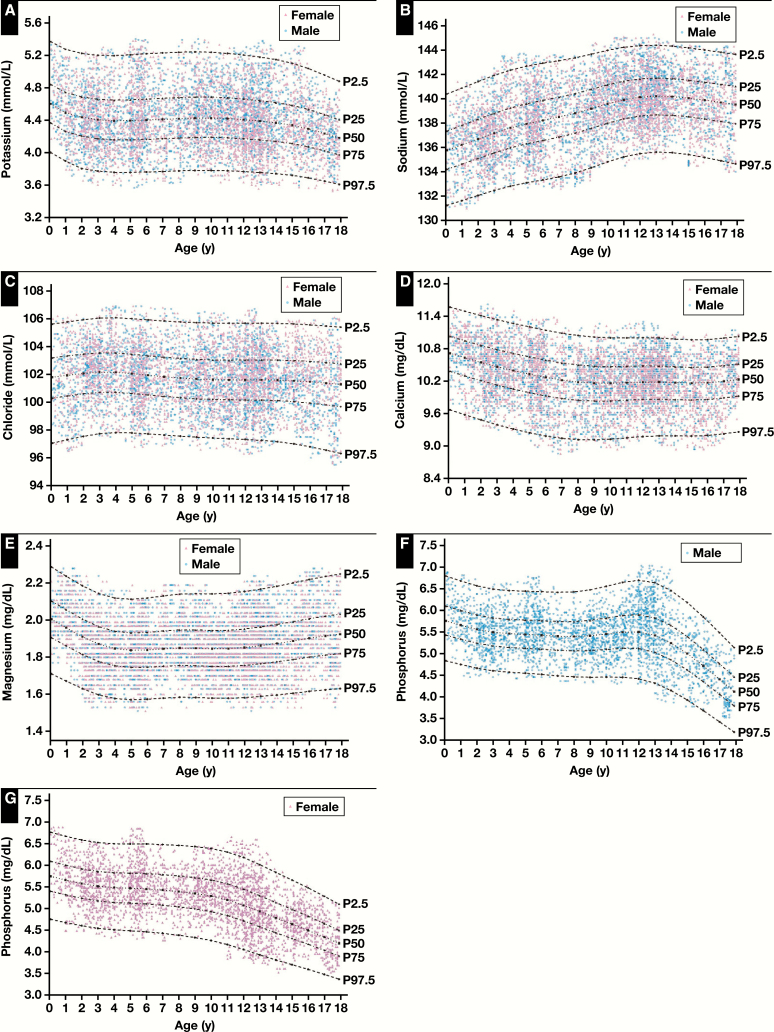
The continuous centile curves for the serum electrolyte results are shown in Figure 1. The trends of the serum electrolytes should be interpreted from the median lines (50th centile) of the charts, since it is the most stable and representative of physiology. Most analytes showed substantial age-specific dynamics, especially in the first several years of life and after the onset of puberty. Concentrations of Na increased slowly with age and gradually stabilized after 12 years old. The median levels of K and Cl remained relatively stable throughout the age range, with only one broad age partition. Mg and Ca concentrations also remained relatively consistent; after a slow decrease in the first 4 years for Mg and in the first 6 years for Ca, concentrations remained stable throughout the age range. P required five age partitions and additional sex partitions from 11 to less than 15 years old. Concentrations of P remained stable in the first 10 years, with females dropping rapidly thereafter, while males aged 11 to less than 13 years increased slightly and then dropped rapidly. Graphical representations of the RIs are provided in Figure 2A (K), Figure 2B (Na), Figure 2C (Cl), Figure 2D (Ca), Figure 2E (Mg), Figure 2F (P, male), and Figure 2G (P, female).
Figure 2.
Continuous reference intervals for serum potassium (A), sodium (B), chloride (C), calcium (D), magnesium (E), and phosphorus (F, G) according to age and sex. P stands for percentile. P2.5 presents as the 2.5th value of the group; P25, the 25th value of the group; P50, the 50th value of the group; P75, the 75th value of the group; and P97.5, the 97.5th value of the group.
Correlation Analysis
In total, correlations were assessed among six serum electrolytes using the Spearman rank correlation method. When age, sex, and BMI were added as additional variables, 36 total correlations were involved in the study. Of these, seven variables, with the exception of Mg and sex, significantly correlated with age (P < .05). BMI (r = 0.74) and Na (r = 0.42) positively correlated with age, while P (r = –0.43), Ca (r = –0.21), K (r = –0.10), and Cl (r = –0.10) decreased with age. The analysis also showed that P, K, Cl, and Mg were weakly associated with sex, and the maximum level of correlation coefficient was –0.14 for P. In addition, when all serum electrolytes were analyzed together, Mg did not correlate with Cl, and the maximum level of correlation coefficient was 0.31 between Na and Cl.
Discussion
The establishment of pediatric RIs faces a major challenge since the collection of blood samples is difficult to achieve. Therefore, when pediatric RIs are described, it is not surprising that the sample size is relatively small and RIs vary among laboratories. For the above reasons, many clinical laboratory accreditation organizations and licensing agencies require each laboratory to verify or establish RIs for each method. Furthermore, testing technology and the reference population change with alterations in environmental factors, nutritional factors, and immigration. Hence, experts suggest that the RI database should be updated every few years.22Table 4 lists the comparison between the current study and other RI studies on electrolytes in children and adolescents. Compared with Chinese adult standards, the LRL of K was slightly higher in children and adolescents than in adults. In addition, RIs of K, Na, and Cl were established using the Canadian Laboratory Initiative on Pediatric Reference Intervals (CALIPER) cohort for the first time in 2019.23 CALIPER showed a higher LRL and URL for K and a more significant downward trend for the URL. The differences may be due to the distribution of the sample over age and the disparity of the analysis systems and methods (dry chemistry method vs liquid chemistry method). However, compared with other studies,25,27 such differences were not substantial. In the present study, a slight increment of K was shown in the first year of life compared with other periods. This may be due to the reduction of K secretion by the principal cells of the cortical collecting tubule, which is contributed by the reduction of Na+/K+ ATPase activity. Furthermore, K secretion rates only approach adult levels after 6 weeks of life and are related to increased renal outer medullary potassium channels.28
Table 4.
Comparison of Reference Intervals for Electrolyte With Other Studiesa
| Analyte | Present Study Ortho VITRO 5600 |
Chinese Adult10 Multiplatform |
CALIPER23 Dimension EX |
CALIPER23 Siemens ADVIA XPT/1800 |
CALIPER33 Abbott ARCHITECT c8000 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Mb | F | Age | Mb | F | Age | Mb | F | Age | Mb | F | Age | Mb | F | |
| Potassium, mmol/L | 1 mo to <18 y | 3.76-5.18 | 20-79 y | 3.5-5.3 | 0-<1 y | 3.9-6.0 | 0-<1 y | 4.3-6.7 | |||||||
| 1-<19 y | 4.0-5.5 | 1-<19 y | 4.0-5.3 | ||||||||||||
| Sodium, mmol/L | 1 mo to <3 y | 131.9-140.6 | 20-79 y | 137-147 | 0-<19 y | 139-145 | 0-<19 y | 139-146 | |||||||
| 3-<9 y | 133.3-143.0 | ||||||||||||||
| 9-<18 y | 135.2-144.2 | ||||||||||||||
| Chloride, mmol/L | 1 mo to <18 y | 97.4-105.7 | 20-79 y | 99-110 | 0-<4 y | 102-111 | 0-<19 y | 104-109 | |||||||
| 4-<19 y | 102-108 | ||||||||||||||
| Calcium, mg/dL | 1 mo to <2 y | 9.62-11.38 | 20-79 y | 8.46-10.01 | 0-<2 y | 8.74-10.54 | 0-<2 y | 9.54-11.50 | 0-<1 y | 8.5-11.0 | |||||
| 2-<6 y | 9.38-11.30 | 2-<19 y | 8.54-9.74 | 2-<5 y | 9.50-10.78 | 1-<19 y | 9.2-10.5 | ||||||||
| 6-<18 y | 9.14-10.98 | 5-<19 y | 9.14-10.22 | ||||||||||||
| Magnesium, mg/dL | 1 mo to <18 y | 1.58-2.19 | 20-79 y | 1.82-2.48 | 0-14 d | 1.99-3.94 | |||||||||
| 15 d to <1 y | 1.97-3.09 | ||||||||||||||
| 1-<19 y | 2.09-2.84 | ||||||||||||||
| Phosphate, mg/dL | 1 mo to <1 y | 5.11-6.88 | 20-79 y | 2.63-4.68 | 0-<1 y | 4.31-7.31 | 0-<1 y | 4.21-7.71 | 0-14 d | 5.6-10.5 | |||||
| 1-<11 y | 4.52-6.47 | 1-<5 y | 4.86-6.47 | 1-<5 y | 4.40-6.16 | 15 d to <1 y | 4.8-8.4 | ||||||||
| 11-<13 y | 4.52-6.85 | 3.90-6.47 | 5-<13 y | 4.62-6.19 | 5-<13 y | 4.00-5.70 | 1-<5 y | 4.3-6.8 | |||||||
| 13-<15 y | 4.03-6.75 | 3.75-5.92 | 13-<16 y | 3.35-5.76 | 3.53-5.48 | 13-<16 y | 3.25-5.64 | 3.25-5.20 | 5-<13 y | 4.1-5.9 | |||||
| 15-<17 y | 3.87-5.61 | 16-<19 y | 2.88-5.08 | 16-<19 y | 2.69-4.86 | 13-<16 y | 3.2-5.5 | 3.5-6.2 | |||||||
| 17-<18 y | 3.56-5.08 | 16-<19 y | 2.9-5.0 | ||||||||||||
| Analytes | Present Study Ortho VITRO 5600 |
AACB25 Multiplatform |
CHMS26 Ortho VITRO 5600 |
||||||||||||
| Age | Mb | F | Age | Mb | F | Age | Mb | F | |||||||
| Potassium, mmol/L | 1 mo to <18 y | 3.76-5.18 | 0-<1 wk | 3.8-6.5 | 3-5 y | 3.9-4.6 | |||||||||
| 1-<26 wk | 4.2-6.7 | 6-79 y | 3.8-4.9 | ||||||||||||
| 26 wk to <2 y | 3.9-5.6 | ||||||||||||||
| 2-<18 y | 3.6-5.3 | ||||||||||||||
| Sodium, mmol/L | 1 mo to <3 y | 131.9-140.6 | 0-<1 wk | 132-147 | 3-5 y | 135-142 | |||||||||
| 3-<9 y | 133.3-143.0 | 1 wk to <18 y | 133-144 | 6-15 y | 136-143 | ||||||||||
| 9-<18 y | 135.2-144.2 | 16-49 y | 137-143 | 137-142 | |||||||||||
| 50-79 y | 136-143 | ||||||||||||||
| Chloride, mmol/L | 1 mo to <18 y | 97.4-105.7 | 0-<1 wk | 98-115 | 3-5 y | 100-107 | |||||||||
| 1 wk to <18 y | 97-110 | 6-11 y | 101-107 | ||||||||||||
| 12-29 y | 101-106 | 100-107 | |||||||||||||
| 30-79 y | 102-108 | ||||||||||||||
| Calcium, mg/dL | 1 mo to <2 y | 9.62-11.38 | 0-<1 wk | 7.41-11.22 | 3-5 y | 9.4-10.6 | |||||||||
| 2-<6 y | 9.38-11.30 | 1-<26 wk | 8.82-11.22 | 6-15 y | 9.3-10.5 | ||||||||||
| 6-<18 y | 9.14-10.98 | 26 wk to <2 y | 8.82-10.82 | 16-19 y | 9.2-10.4 | ||||||||||
| 2-<18 y | 8.82-10.62 | 20-39 y | 9.1-10.4 | 9.0-10.1 | |||||||||||
| 40-79 y | 9.0-10.2 | ||||||||||||||
| Magnesium, mg/dL | 1 mo to <18 y | 1.58-2.19 | 0-<1 wk | 1.46-2.43 | |||||||||||
| 1 wk to <18 y | 1.58-2.67 | ||||||||||||||
| Phosphate, mg/dL | 1 mo to <1 y | 5.11-6.88 | 0-<1 wk | 3.87-8.83 | 3-5 y | 4.4-6.0 | |||||||||
| 1-<11 y | 4.52-6.47 | 1-<4 wk | 4.65-8.52 | 6-10 y | 4.4-5.7 | ||||||||||
| 11-<13 y | 4.52-6.85 | 3.90-6.47 | 4-<26 wk | 4.49-7.74 | 11-15 y | 3.8-5.9 | 3.6-5.6 | ||||||||
| 13-<15 y | 4.03-6.75 | 3.75-5.92 | 26 wk to <1 y | 4.03-7.12 | 16-47 y | 2.9-4.7 | |||||||||
| 15-<17 y | 3.87-5.61 | 1-<4 y | 3.41-6.81 | 48-79 y | 2.8-4.7 | 3.1-4.8 | |||||||||
| 17-<18 y | 3.56-5.08 | 4-<15 y | 2.79-6.19 | ||||||||||||
| 15-<18 y | 2.48-5.73 | ||||||||||||||
| 18-<20 y | 2.32-5.11 |
AACB, Australasian Association of Clinical Biochemists; CHMS, Canadian Health Measures Survey.
aValues for males and females are presented as reference intervals.
bOne range indicates that there is no sex difference in this age group, and the reference intervals for male and female are the same.
Both the LRL and URL for Na were lower than the Chinese adult standard and CALIPER.23 Interestingly, three age partitions were required to reflect minor fluctuations in electrolyte concentrations in the current study, while four age partitions for ages 3 to 79 years were required from the Canadian Health Measures Survey.26 In addition, continuous centile curves in the present study enabled people to immediately appreciate the gradual rising trend of Na with age. The gradual rise during infancy may be related to the dynamic change in the glomerular filtration rate and antidiuretic hormone resistance. Beyond this period, the continued rise is a complex interplay of several physiologic changes.29
Cl and Mg concentrations remained relatively constant throughout childhood and adolescence, with no age- and sex-related variations. Both RIs were lower than the Chinese adult standard, and the URL was lower than the study from the Australasian Association of Clinical Biochemists (AACB).25 Of note, compared with a survey conducted on the Ortho VITROS 5600 analysis system, however, such differences in Mg were not shown, and the RIs in our research were confirmed.30 Unfortunately, the RIs for Mg in the CALIPER trial were nontransferable to the analysis system. Subsequently, the physiologic trend for Cl, which showed a downward trend with age, was similar to Australia’s.31 The subtle changes in childhood may be caused by the increase in bicarbonate.32 In addition, CALIPER suggests that RIs for serum Mg show a high level in healthy infants during the first 2 weeks after delivery and progressively drop to adult levels during the remainder of the first year of life to adult levels.33 Even though this phenomenon exists in the current study, age was not divided for the result of the z test. Therefore, combined RIs were suggested for the interpretation of Mg levels in both groups. The remarkably constant concentrations of the serum Mg depend on tight regulation and feedback mechanisms.24
Serum Ca RIs in CALIPER, analyzed on the Siemens ADVIA XPT/1800, were relatively different from ours.23 Compared with the Chinese adult standard, higher LRL and URL for Ca were shown. Furthermore, there were no sex differences, which differed from sex diversity between 1 and 20 years old using the dry chemistry method.30 Because the maternal supply abruptly discontinues at birth, and fetal Ca concentrations drop rapidly after delivery, rapid mineral accumulation must continue to support growth. However, the process is handicapped by impaired renal recovery due to the immaturity of renal tubules.34 For these reasons, RIs for serum Ca showed high levels for children younger than 2 years and slightly declined between 2 and less than 18 years.
The LRL and URL for P were slightly higher than the Chinese adult standard. However, such a phenomenon did not occur in the study by the AACB.25 Sex partitions between CALIPER and the current study were inconsistent, in which the former was 13 to less than 16 years old, while the latter was 11 to less than 15 years.35 The reason may be due to the different populations, methods of the estimation, equipment, and timing of sample collection. Similarly, despite the physiologic adaptations, the utilization of P outpaces the accumulation and retention efforts in the neonatal period. P shows substantial age- and sex-specific dynamics: serum P concentrations are physiologically higher in younger children and decrease steadily with age through late adolescence.34,36 In addition, the fall in P levels during adolescence is earlier in females than in males, mimicking the sex-related delay in skeletal growth.32 The reason for this pattern is unclear, but it may be related to all critical processes of bone growth and modeling in childhood, including growth plate development, bone matrix mineralization, and osteoblast maturation. Another explanation could be the correlation of fibroblast growth factor 23 and Klotho with age.37 At the age of 11 years in females and 12 years in males, the concentrations of P reach their peak. The phenomenon may be associated with the increase of bone density, with the peak of bone mineral deposition later in males than in females, and both sexes achieve maximal bone density after the pubertal growth spurt.28
The correlations of most analytes with age and sex were confirmed by the trend of continuous centile curves and the calculated value of the RIs. Although K (r = –0.10) and Cl (r = –0.10) correlated with age (P < .05), given the result of the z test and the weak correlation coefficient, one broad age partition was applied in the current study. Likewise, there were no sex partitions except P. CALIPER deem that the understanding of associations among biomarkers can provide future studies of potential confounding factors or particular variables that should be considered in test result interpretation for specific diseases.38 Although only Ca, Mg, and P in the electrolytes were included in their study,38 the results provided a new idea. Correlation analysis among electrolytes can reveal the intrinsic links and contribute to the joint diagnosis of the disease, which is worthy of further research.
The strengths of our study include the direct sampling technique, a larger sample size based on the pediatric population, and special statistical methods for the partition of RIs. Even though the indirect determination of laboratory-specific RIs using patients’ laboratory data has been proven useful by other studies,39,40 the truly “disease-free” status and health of reference individuals are hard to judge by the statistical procedure. Hence, the direct sampling technique, the a priori approach described in EP28-A3c, was used in the current study. The direct sampling technique permitted the use of defined inclusion and exclusion criteria and reduced variation due to preanalytical and analytical factors. In addition to this, partitioning with age presents a major challenge. Although the data are divided by age and sex as dictated by a firm statistical basis, the medical relevance to the different divisions and clinical differences must be taken into consideration. From the above reasons, RIs established here do reflect growth and development dynamic changes by quoting LMS, z test, and regression tree methods. Discrete age groups may lose some details of the physiologic variation of analytes with age. With this in mind, the continuous centile curves are used to complement age- and sex-specific RIs, as well as improve the presentation and interpretation of laboratory reference limits. Besides, classification and regression tree analysis divides the data by a relationship between predictors and response variables to find the best split point, which can avoid some artificial or arbitrary segmentation of reference values.
There are also some limitations. Reported values in this study are directly applicable only to the Chinese population and these analytical platforms. However, the transference of the published RIs according to the guideline is possible. Therefore, they might also be useful for any laboratory if they were transferred to different but similar methods or if they are validated using a local patient population. Unfortunately, the transference and validation were lacking in this study. In addition, data from the short phase after birth also should increase. The sample used in this study was serum, not heparinized plasma. Even though most electrolyte analytes are not significantly different in the above environments, some reports have found that K has a statistically significant difference and clinical significance between serum and heparinized plasma.41 Lower values for K concentrations have been confirmed in plasma according to previous studies, and this may be attributed to the prevention of clot formation with platelet rupture and potassium release.42 Considering the above reasons, some scholars believe that plasma is the preferred sample type for K. For other assays, serum samples are acceptable. Or, if necessary, separate RIs should be established for plasma.43 Further studies, which take these variables into account, will need to be undertaken.
In summary, age- and sex-specific pediatric RIs for six electrolytes were established for healthy Chinese children and adolescents in Jilin Province. This large study covered a wide age span from 1 month to less than 18 years and used a modern analytical chemistry platform with the dry chemical method. K, Cl, and Mg did not require partition. Only P required sex partition in children aged 11 to less than 15 years. In addition, the potential correlation between Na and Cl was also worthy to study. Age- and sex-specific pediatric RIs not only reflected marked changes and fluctuations in growth and development but also improved the accuracy of laboratory clinical diagnosis, making it an integral part of clinical decision making.
Supplementary Material
All phases of this study were supported in part by grants from the National Science Foundation of China (81501839, to Q.Z.) and the Scientific and Technological “13th Five-Year Plan” Project of Jilin Provincial Department of Education (JJKH20180214KJ, to Q.Z.). J.X. is also funded by Jilin Province Health and Technology Innovation Development Program (2017J071), the Jilin Science and Technology Development Program (20170623092TC-09, 20160101091JC, 20150414039GH, 20190304110YY), The First Hospital Translational Funding for Scientific & Technological Achievements (JDYYZH-1902002), and Norman Bethune Program of Jilin University (2012223).
References
- 1. Tahmasebi H, Higgins V, Fung AWS, et al. Pediatric reference intervals for biochemical markers: gaps and challenges, recent national initiatives and future perspectives. EJIFCC. 2017;28:43-63. [PMC free article] [PubMed] [Google Scholar]
- 2. Higgins V, Truong D, Woroch A, et al. CLSI-based transference and verification of CALIPER pediatric reference intervals for 29 Ortho VITROS 5600 chemistry assays. Clin Biochem. 2018;53:93-103. [DOI] [PubMed] [Google Scholar]
- 3. Guo W, Zhou Q, Jia Y, et al. Age- and sex-specific reference intervals for myocardial enzyme activity in healthy Chinese Han population aged 1∼<18 years. Biomed Res Int. 2019;2019:2018598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guo W, Zhou Q, Jia Y, et al. Division of myocardial enzyme reference intervals in population aged 1 to <18 years old based on Fisher’s optimal segmentation method. Comput Math Methods Med. 2020;2020:2013148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kear TM. Fluid and electrolyte management across the age continuum. Nephrol Nurs J. 2017;44:491-496. [PubMed] [Google Scholar]
- 6. McLafferty E, Johnstone C, Hendry C, et al. Fluid and electrolyte balance. Nurs Stand. 2014;28:42-49. [DOI] [PubMed] [Google Scholar]
- 7. Muhsin SA, Mount DB. Diagnosis and treatment of hypernatremia. Best Pract Res Clin Endocrinol Metab. 2016;30:189-203. [DOI] [PubMed] [Google Scholar]
- 8. Berend K, van Hulsteijn LH, Gans RO. Chloride: the queen of electrolytes? Eur J Intern Med. 2012;23:203-211. [DOI] [PubMed] [Google Scholar]
- 9. Song L. Calcium and bone metabolism indices. Adv Clin Chem. 2017;82:1-46. [DOI] [PubMed] [Google Scholar]
- 10. National Health Committee of the People’s Republic of China. Reference intervals for common clinical biochemistry tests. WS/T 404–2012. http://www.nhc.gov.cn/wjw/s9492/wsbz_4.shtml Accessed April 22, 2020. [Google Scholar]
- 11. Chan MK, Seiden-Long I, Aytekin M, et al. Canadian Laboratory Initiative on Pediatric Reference Interval Database (CALIPER): pediatric reference intervals for an integrated clinical chemistry and immunoassay analyzer, Abbott ARCHITECT ci8200. Clin Biochem. 2009;42:885-891. [DOI] [PubMed] [Google Scholar]
- 12. Lee HR, Shin S, Yoon JH, et al. Reference intervals of hematology and clinical chemistry analytes for 1-year-old Korean children. Ann Lab Med. 2016;36:481-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tahmasebi H, Trajcevski K, Higgins V, et al. Influence of ethnicity on population reference values for biochemical markers. Crit Rev Clin Lab Sci. 2018;55:359-375. [DOI] [PubMed] [Google Scholar]
- 14. Li X, Wang D, Yang C, et al. Establishment of age- and gender-specific pediatric reference intervals for liver function tests in healthy Han children. World J Pediatr. 2018;14:151-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu J, Dai Y, Lee Y, et al. Pediatric reference intervals of liver and renal function tests from birth to adolescence in Chinese children as performed on the Olympus AU5400. Clin Chim Acta. 2019;490:142-146. [DOI] [PubMed] [Google Scholar]
- 16. Horowitz G, Altaee S, Boyd J, et al. Establishing, and Verifying Reference Intervals in the Clinical Laboratory; Approved Guideline. Wayne, PA: Clinical and Laboratory Standards Institute; 2010:1-20. CLSI document EP28-A3c. [Google Scholar]
- 17. Liu J, Yuan E, Zhang Z, et al. Age- and sex-specific reference intervals for blood copper, zinc, calcium, magnesium, iron, lead, and cadmium in infants and children. Clin Biochem. 2012;45:416-419. [DOI] [PubMed] [Google Scholar]
- 18. Ichihara K, Boyd JC; IFCC Committee on Reference Intervals and Decision Limits (C-RIDL) An appraisal of statistical procedures used in derivation of reference intervals. Clin Chem Lab Med. 2010;48:1537-1551. [DOI] [PubMed] [Google Scholar]
- 19. Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med. 1992;11:1305-1319. [DOI] [PubMed] [Google Scholar]
- 20. Chester R, Khondoker M, Shepstone L, et al. Self-efficacy and risk of persistent shoulder pain: results of a classification and regression tree (CART) analysis. Br J Sports Med. 2019;53:825-834. [DOI] [PubMed] [Google Scholar]
- 21. Li H, Ji CY, Zong XN, et al. Height and weight standardized growth charts for Chinese children and adolescents aged 0 to 18 years. Zhonghua Er Ke Za Zhi. 2009;47:487-492. [PubMed] [Google Scholar]
- 22. Cho SM, Lee SG, Kim HS, et al. Establishing pediatric reference intervals for 13 biochemical analytes derived from normal subjects in a pediatric endocrinology clinic in Korea. Clin Biochem. 2014;47:268-271. [DOI] [PubMed] [Google Scholar]
- 23. Tahmasebi H, Higgins V, Woroch A, et al. Pediatric reference intervals for clinical chemistry assays on Siemens ADVIA XPT/1800 and Dimension EXL in the CALIPER cohort of healthy children and adolescents. Clin Chim Acta. 2019;490:88-97. [DOI] [PubMed] [Google Scholar]
- 24. Ghasemi A, Syedmoradi L, Zahediasl S, et al. Pediatric reference values for serum magnesium levels in Iranian subjects. Scand J Clin Lab Invest. 2010;70:415-420. [DOI] [PubMed] [Google Scholar]
- 25. Tate JR, Sikaris KA, Jones GR, et al. Harmonising adult and paediatric reference intervals in Australia and New Zealand: an evidence-based approach for establishing a first panel of chemistry analytes. Clin Biochem Rev. 2014;35:213-235. [PMC free article] [PubMed] [Google Scholar]
- 26. Adeli K, Higgins V, Nieuwesteeg M, et al. Biochemical marker reference values across pediatric, adult, and geriatric ages: establishment of robust pediatric and adult reference intervals on the basis of the Canadian Health Measures Survey. Clin Chem. 2015;61:1049-1062. [DOI] [PubMed] [Google Scholar]
- 27. Ridefelt P, Aldrimer M, Rödöö PO, et al. Population-based pediatric reference intervals for general clinical chemistry analytes on the Abbott Architect ci8200 instrument. Clin Chem Lab Med. 2012;50:845-851. [DOI] [PubMed] [Google Scholar]
- 28. Loh TP, Metz MP. Trends and physiology of common serum biochemistries in children aged 0-18 years. Pathology. 2015;47:452-461. [DOI] [PubMed] [Google Scholar]
- 29. Čukuranović R, Vlajković S. Age related anatomical and functional characteristics of human kidney. Organ. 2005;7:14. [Google Scholar]
- 30. Blasutig IM, Jung B, Kulasingam V, et al. Analytical evaluation of the VITROS 5600 integrated system in a pediatric setting and determination of pediatric reference intervals. Clin Biochem. 2010;43:1039-1044. [DOI] [PubMed] [Google Scholar]
- 31. Hoq M, Matthews S, Karlaftis V, et al. ; HAPPI Kids Study Team Reference values for 30 common biochemistry analytes across 5 different analyzers in neonates and children 30 days to 18 years of age. Clin Chem. 2019;65:1317-1326. [DOI] [PubMed] [Google Scholar]
- 32. Sikaris KA. Physiology and its importance for reference intervals. Clin Biochem Rev. 2014;35:3-14. [PMC free article] [PubMed] [Google Scholar]
- 33. Colantonio DA, Kyriakopoulou L, Chan MK, et al. Closing the gaps in pediatric laboratory reference intervals: a CALIPER database of 40 biochemical markers in a healthy and multiethnic population of children. Clin Chem. 2012;58:854-868. [DOI] [PubMed] [Google Scholar]
- 34. Loh TP, Antoniou G, Baghurst P, et al. Development of paediatric biochemistry centile charts as a complement to laboratory reference intervals. Pathology. 2014;46:336-343. [DOI] [PubMed] [Google Scholar]
- 35. Estey MP, Cohen AH, Colantonio DA, et al. CLSI-based transference of the CALIPER database of pediatric reference intervals from Abbott to Beckman, Ortho, Roche and Siemens Clinical Chemistry Assays: direct validation using reference samples from the CALIPER cohort. Clin Biochem. 2013;46:1197-1219. [DOI] [PubMed] [Google Scholar]
- 36. Zierk J, Arzideh F, Rechenauer T, et al. Age- and sex-specific dynamics in 22 hematologic and biochemical analytes from birth to adolescence. Clin Chem. 2015;61:964-973. [DOI] [PubMed] [Google Scholar]
- 37. Gkentzi D, Efthymiadou A, Kritikou D, et al. Fibroblast growth factor 23 and Klotho serum levels in healthy children. Bone. 2014;66:8-14. [DOI] [PubMed] [Google Scholar]
- 38. Higgins V, Hooshmand S, Adeli K. Principal component and correlation analysis of biochemical and endocrine markers in a healthy pediatric population (CALIPER). Clin Biochem. 2019;66:29-36. [DOI] [PubMed] [Google Scholar]
- 39. Milinković N, Ignjatović S, Zarković M, et al. Indirect estimation of reference intervals for thyroid parameters. Clin Lab. 2014;60:1083-1089. [DOI] [PubMed] [Google Scholar]
- 40. Zhu XT, Wang KJ, Zhou Q, et al. Establishing reference intervals of thyroid hormone based on a laboratory information system [in Chinese]. Zhonghua Nei Ke Za Zhi. 2020;59:129-133. [DOI] [PubMed] [Google Scholar]
- 41. Lum G, Gambino SR. A comparison of serum versus heparinized plasma for routine chemistry tests. Am J Clin Pathol. 1974;61:108-113. [DOI] [PubMed] [Google Scholar]
- 42. Doumas BT, Hause LL, Simuncak DM, et al. Differences between values for plasma and serum in tests performed in the Ektachem 700 XR Analyzer, and evaluation of “plasma separator tubes (PST).” Clin Chem. 1989;35:151-153. [PubMed] [Google Scholar]
- 43. Miles RR, Roberts RF, Putnam AR, et al. Comparison of serum and heparinized plasma samples for measurement of chemistry analytes. Clin Chem. 2004;50:1704-1706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.