Abstract
Objectives
Obesity is a risk factor for type 2 diabetes (T2D), but prospective data relating adiposity measures to incident prediabetes are scant.
Methods
The Pathobiology of Prediabetes in A Biracial Cohort study followed normoglycemic African Americans (AA) and European Americans (EA) with parental history of T2D for the primary outcome of incident prediabetes (impaired fasting glucose and/or impaired glucose tolerance) for 5.5 years. Serial assessments included anthropometry and body fat composition. We analyzed weight, body mass index (BMI), waist, total, and abdominal fat mass in relation to incident prediabetes risk.
Results
Of the 376 subjects enrolled (217 AA, 159 EA; mean age 44.2 years, BMI 31.4 kg/m2), 343 (192 AA, 151 EA) had evaluable follow-up data. A total of 101 (52 AA, 49 EA) developed prediabetes during follow-up. Progressors to prediabetes had a mean baseline weight of 90.0 ± 20.4 kg versus 82.9 ± 21.7 kg among nonprogressors (P = 0.0036). During 5.5 (mean 2.62) years of follow-up, the weight change among nonprogressors was 0.63 ± 6.11 kg compared with 2.54 ± 6.91 kg among progressors (ANOVA P = 0.0072). Progressors also showed greater increases in total fat (P = 0.0015) and trunk fat (P = 0.0005) mass than nonprogressors. Adjusted for age and sex, the significant predictors of incident prediabetes were BMI (P = 0.0013), waist (P < 0.0001), total fat (P = 0.0025), and trunk fat (P < 0.0001) mass.
Conclusions
Among obese free-living offspring of parents with T2D, long-term normoglycemic status was associated with a weight gain of ~0.2 kg/y, whereas progression to prediabetes was associated with a weight gain of ~1 kg/y.
Keywords: impaired fasting glucose, impaired glucose tolerance, ethnicity, adiposity, body fat, diabetes offspring
Obesity is a well-known risk factor for type 2 diabetes (T2D) [1-4]. Impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) both describe prediabetic states, representing an intermediate stage in the pathogenesis of T2D [5, 6]. There is increasing evidence that prediabetes is a toxic milieu for the initiation of microvascular and macrovascular complications [7, 8]. In the Paris Prospective Study, cardiovascular mortality was 2-fold higher in persons with IGT compared with those who had normal glucose tolerance (NGT) [9]. The risk factors for T2D include obesity, family history, low high-density lipoprotein cholesterol and high triglycerides, hypertension, and ethnicity, among others [10]. These same risk factors often are present in people with prediabetes. Prediabetes precedes the development of T2D by several years; in the Diabetes Prevention Program (DPP), progression from IGT to T2D occurred at an annual rate of ~10% [11]. Previous studies have identified numerous risk factors predictive of progression from prediabetes to T2D, including age, baseline fasting plasma glucose (FPG), 2-hour postchallenge plasma glucose (2hrPG), family history, weight, body mass index (BMI), insulin sensitivity, beta-cell function, and gene variants, among others [12-18].
Few studies have focused on the proximal stage of transition from normoglycemia to prediabetes (IFG or IGT). In the Baltimore Longitudinal Study on Aging (BLSA), a predominantly European American (EA) cohort of initially normoglycemic participants developed incident prediabetes (IFG and/or IGT) at an average rate of 6.2%/year during 10 years of follow-up [19]. The BLSA demonstrated different patterns of progression from normoglycemia to IFG, IGT, or combined IFG and IGT and identified male sex, obesity, insulin resistance, and impaired first-phase insulin secretion as significant factors associated with progression to prediabetes and T2D [19].
The Pathobiology of Prediabetes in A Biracial Cohort (POP-ABC) study enrolled initially normoglycemic African Americans (AA) and EA offspring of parents with T2D and followed them for 5.5 years, for the primary outcome of incident prediabetes [20-22]. The incidence rate of prediabetes was similar (~11%/y) among AA and EA participants in the POP-ABC study [22]. Of the POP-ABC participants who developed prediabetes, 44% reached that endpoint by the IFG criterion, 35% did so by the IGT criterion, and 20.6% had IFG + IGT. The proportion of subjects who converted to prediabetes by IFG (42.6% vs. 46%), IGT (34% vs. 36.2%), or IFG + IGT (20.0% vs. 21.3%) criteria was similar in AA versus EA participants [22].
Similar to the findings of the BLSA, the POP-ABC study identified male sex, older age, obesity, insulin resistance, impaired insulin secretion, and several additional baseline behavioral and biochemical variables as predictors of progression from normoglycemia to incident prediabetes [22-26].
The prediabetes state presents an opportunity for early interventions to prevent progression to T2D, as has been demonstrated by numerous studies [11, 27, 28]. The latter studies used lifestyle modification aimed at inducing a target amount of weight loss as a strategy for diabetes prevention [11, 27-29]. Unlike the well-documented predictors of progression from prediabetes to T2D [12-18], few studies have assessed the quantitative relationship between longitudinal changes in body weight and the risk of incident prediabetes. In 1 such study of Pima Indians with initial NGT, those who gained a mean of 5.6 kg in body weight over ~5 years progressed to IGT, whereas participants who maintained NGT status had a mean weight gain of 2.6 kg during the same period [3]. Thus, among Pima Indians, a mean weight gain of ~1 kg/y predicted the development of prediabetes, whereas a mean weight gain of ~0.5 kg/y appeared to protect individuals from experiencing glycemic progression [3]. Similar prospective studies on the quantitative relationship between weight trajectories and risk of incident prediabetes are scant for most populations besides the Pima.
The present report expands observations from the POP-ABC study by analyzing serial changes in body weight and adiposity measures in relation to the risk of progression from normoglycemia to prediabetes during longitudinal follow-up. Being a prospective, natural history study, no interventions to alter body weight or health behavior were offered to POP-ABC study participants. Thus, any trajectories in weight would reflect spontaneous changes that occur in free-living individuals. We reasoned that analysis of the relationship between such spontaneous trajectories in adiposity measures and the risk of prediabetes would be informative in a manner that extends previous observations derived from analysis of baseline data in a more diverse population.
Research Design and Methods
Participants
The design and methods, baseline characteristics, and early results for the POP-ABC study have been published [20-22]. In brief, eligibility criteria for enrollment included a history of T2D in 1 or both biological parents, ages 18 to 65 years, self-reported non- Hispanic white (EA) or non-Hispanic black (AA) race/ethnicity status, and normal FPG (<100 mg/dL [5.6 mmol/L]) and NGT (2hrPG < 140 mg/dL [7.8 mmol/L], as previously described [20-22]. Excluded from participation were individuals with a history of diabetes, those taking medications known to alter body weight or glucose metabolism (e.g., glucocorticoids, thiazide diuretics >25 mg/day, beta-blockers, any antidiabetes drug), and persons enrolled in behavioral weight loss programs or having a history of bariatric surgery [20-22]. Enrollment in the POP-ABC study began in September 2006 and ended in February 2010, and participants were followed until study close-out on March 31, 2012.
Procedures and measurements
Assessments
Participants arrived at the General Clinical Research Center after an overnight fast. Initial procedures consisted of a structured medical interview and a general physical examination and other prespecified assessments. Weight was measured in duplicate on a calibrated balance beam scale. Standing height was determined in duplicate with a standard stadiometer. The BMI was calculated as the weight in kilograms divided by the height in meters squared. Waist circumference was determined to the nearest 0.1 cm at the midpoint between the highest point of the iliac crest and the lowest costal margin in the mid-axillary line, using a Gulick II tape measure, as previously described [14-16]. Body composition was assessed using dual-energy x-ray absorptiometry (Hologic Discovery A80044A, Hologic Inc., Bedford, MA). Clinical examination and FPG measurements were performed quarterly, standard 75-g oral glucose tolerance tests (OGTT) annually, and other assessments were staggered during 5.5 years of follow-up.
Written instructions were provided to subjects before scheduled visits for OGTT and FPG testing. The instructions stipulated that participants consume their usual diet with adequate carbohydrates, refrain from strenuous exercise and alcohol consumption for 24 hours, and avoid smoking on the morning of the test. Venous blood specimens were obtained before (0 minutes) and at 30 minutes and 120 minutes after ingestion of 75 g flavored glucose (Trutol 75; Custom Laboratories, Baltimore, MD). Plasma glucose was measured with a glucose oxidase method (Yellow Spring Instruments Co., Inc., Yellow Spring, OH).
The University of Tennessee institutional review board approved the POP-ABC study protocol. Written informed consent was given by all participants before initiation of the study, which was conducted in accordance with the World Medical Association’s Declaration of Helsinki.
Definition of outcome
The primary outcome was occurrence of prediabetes, as defined by the American Diabetes Association criteria [10]. The participants who reached that endpoint (progressors) were those whose tests showed IFG (FPG 100-125 mg/dL [5.6-6.9 mmol/L]) and/or IGT (2hrPG 140-199 mg/dL [7.8-11.0 mmol/L]) during 5.5 years of follow-up (mean 2.62 years). Nonprogressors were participants who maintained normal FPG and normal 2hrPG [16]. A confirmatory test was performed within 6 weeks for each prediabetes endpoint occurrence observed during quarterly visits, using the standard 75-g OGTT as the method of confirmation. All endpoints were independently adjudicated by the Institutional Data and Safety Officer (Murray Heimberg, MD, PhD).
Statistical analysis
Data are reported as mean ± SD unless otherwise specified. Significance level was set as P < 0.05. The primary outcome is progression to prediabetes, and participants were dichotomized as progressors and nonprogressors. Serial weights were analyzed using repeated measures ANOVA. Linear and logistic regression models were used to analyze baseline and serial variables as predictors of incident prediabetes, after adjustments for age, sex, race/ethnicity, and baseline glucose. All statistical analyses were performed with the use of SAS statistical software, version 9.4 (SAS Institute, Cary, NC).
Results
Cohort characteristics
Of the 376 subjects enrolled in the POP-ABC study (217 AA, 159 EA; mean age 44.2 years, BMI 31.4 kg/m2), 343 offspring (192 AA, 151 EA) completed the main POP-ABC study with evaluable follow-up data [16]. Table 1 summarizes the baseline characteristics of the study subjects. The mean age was 44.2 ± 10.6 years; women constituted ~70% of the cohort. Compared with EA offspring, AA participants were 4 years younger and had higher values for weight and BMI, similar values for total and trunk fat mass, and lower values for FPG at enrollment (Table 1). As reported previously, during 5.5 years of follow-up (mean 2.62 years), 101 of the 343 POP-ABC study participants developed prediabetes (29.5%), 10 subjects developed T2D (2.91%), and 232 participants (67.6%) were nonprogressors [22].
Table 1.
Characteristics of Study Subjects at Enrollment
| Characteristic | European American | African American | P Value |
|---|---|---|---|
| Number | 151 | 192 | |
| Age (y) | 47 ± 10 | 43 ± 10 | 0.0007 |
| Pre-/postmenopause weight (kg) |
54/49 81 ± 21 |
95/45 88 ± 22 |
0.0147 0.004 |
| FPG (mg/dL) | 93.1 ± 6.4 | 91.0 ± 6.8 | 0.003 |
| 2hrPG (mg/dL) | 125 ± 24 | 124 ± 28 | 0.7203 |
| HbA1c (%) | 5.4 ± 0.4% | 5.7±0.5% | <0.001 |
| BMI (kg/m2) | 28.7 ± 6.7 | 31.3 ± 7.5 | 0.0008 |
| Waist circumference(cm) | 93 ± 15 | 96 ± 16 | 0.09 |
Values are means ± SD. To convert plasma glucose from mg/dL to mmol/L, divide by 18.
Abbreviations: 2hrPG, 2-hour postchallenge plasma glucose; BMI, body mass index; FPG, fasting plasma glucose.
Serial anthropometrics and incident prediabetes
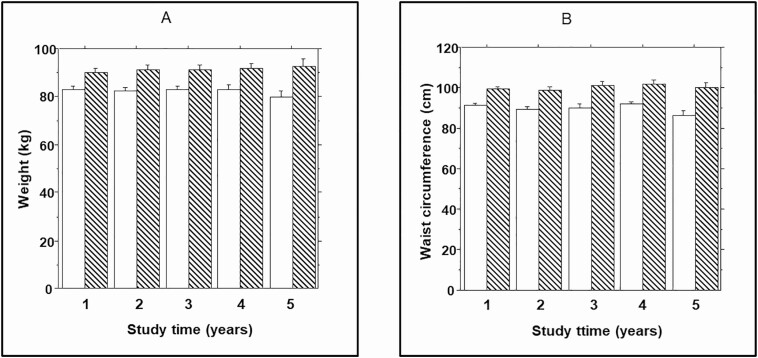
In the present report, we analyzed longitudinal data on adiposity measures (weight, waist size, and body fat) in relation to prediabetes outcome during a mean 2.62 years of follow-up. Compared with participants who maintained normoglycemic status from enrollment to end of study, those who progressed to prediabetes had a higher mean weight at baseline (90.0 ± 20.4 kg vs. 82.9 ± 21.7 kg, P = 0.0036). Analysis of serial weight data during follow-up indicated that the mean weight change among non-progressors was 0.63 ± 6.11 kg (median 1.28, interquartile range [IQR] 4.25 kg) compared with 2.54 ± 6.91 kg (median 2.25, IQR 6.95 kg) among progressors (repeated measures ANOVA P = 0.0072) (Fig. 1A). The mean weight change during follow-up among nonprogressors did not differ significantly by race/ethnicity (0.68 ± 5.23 kg in EA and 0.51 ± 6.83 kg in AA, P = 0.87) or gender (0.70 ± 6.03 kg in women and 0.41 ± 6.57 kg in men, P = 0.65). Also, there were no significant differences by race/ethnicity or sex in weight change among progressors, although values were numerically higher in AA versus EA participants (2.68 ± 7.23 kg vs. 1.57 ± 6.59 kg, P = 0.29) and in men versus women (3.59 ± 8.20 kg vs. 1.78 ± 5.82 kg, P = 0.36).
Figure 1.
(A) Body weight and (B) waist circumference in progressors (striped bars) to prediabetes and nonprogressors (open bars) at enrollment (year 1) and during annual follow-up visits. Repeated measures ANOVA P = 0.0072 (weight) and 0.0001 (waist) for comparisons between progressors and nonprogressors.
Waist circumference was higher at baseline (99.5 ± 12.5 cm vs. 90.5 ± 15.3 cm, P = 0.002) and increased by a greater amount in progressors (1.86 ± 5.80 cm; median 1.50, IQR 8.00 cm) compared with nonprogressors (1.10 ± 5.44 cm; median 1.00, IQR 7.12 cm) during follow-up (repeated measures ANOVA P = 0.0001) (Fig. 1B).
Serial body fat measures and incident prediabetes
In addition to clinical anthropometric data, serial data on body composition analysis were available for 220 participants (115 AA, 105 EA; ~70% female) with confirmed endpoints who completed 3 consecutive examinations with dual-energy x-ray absorptiometry during the first 3 years of the study. The baseline characteristics of this subgroup were similar to those of the full cohort (Table 2). During 5.5 years of follow-up (mean 2.62 years), 89 of the 220 participants (40.5%) developed either prediabetes (N = 81 [43 AA, 38 EA] or T2D (N = 8 [3 AA, 5 EA]), and 131 participants (59.5%) were nonprogressors.
Table 2.
Baseline Characteristics of the Body Fat Analysis Subgroup
| Characteristic | European American | African American | P Value |
|---|---|---|---|
| Number (women/men) | 105 (68/37) | 115 (83/32) | |
| Age (y) | 46.5 ± 10.5 | 42.5 ± 10.3 | 0.007 |
| FPG (mg/dL) | 93.5 ± 6.05 | 91.2 ± 7.26 | 0.003 |
| 2hrPG (mg/dL) | 125 ± 23.1 | 123 ± 29.2 | 0.76 |
| Weight (kg) | 80.4 ± 19.0 | 87.8 ± 18.9 | 0.005 |
| BMI (kg/m2) | 28.1 ± 5.75 | 30.9 ± 6.40 | 0.0009 |
| Waist circumference (cm) | 92.4 ± 14.9 | 95.7 ± 14.7 | 0.10 |
| Total fat mass (kg) | 27.8 ± 11.3 | 31.8 ± 12.8 | 0.10 |
| Trunk fat mass (kg) | 14.8 ± 7.01 | 15.4 ± 7.41 | 0.11 |
Abbreviations: 2hrPG, 2-hour postchallenge plasma glucose; BMI, body mass index; FPG, fasting plasma glucose.
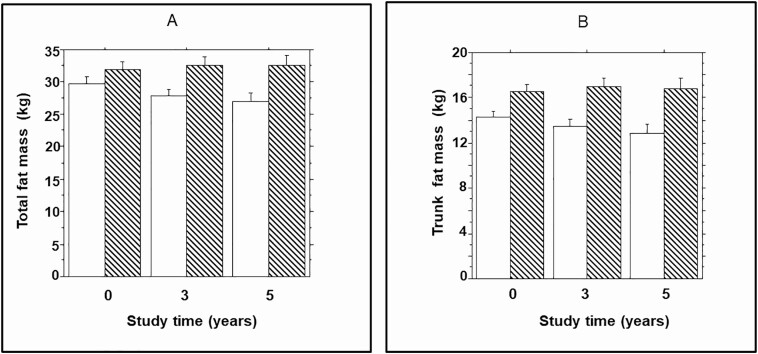
Compared with participants who maintained normoglycemic status from enrollment to end of study, those who progressed to prediabetes had higher total body fat mass at enrollment (32.3 ± 13.0 kg vs. 29.7 ± 14.0 kg, P = 0.002). Furthermore, the mean change in total fat mass during follow-up was higher among progressors to prediabetes (0.59 ± 3.90 kg; median 0.54, IQR 3.44 kg) compared with nonprogressors (-2.86 ± 4.34 kg; median 0.39, IQR 4.92 kg) during follow-up (repeated measures ANOVA P = 0.0015) (Fig. 2A). Trunk fat mass (a measure of visceral adiposity) also was higher at baseline (16.6 ± 6.80 kg vs. 14.3 ± 7.3 kg, P = 0.04) in progressors to prediabetes compared with nonprogressors. The trunk fat mass increased further in progressors (0.27 ± 2.47 kg; median 0.45, IQR 2.36 kg) compared with nonprogressors (-1.54 ± 2.59 kg; median 0.36, IQR 3.14 kg) during follow-up (repeated measures ANOVA P = 0.0005) (Fig. 1B).
Figure 2.
(A) Total fat mass and (B) trunk fat mass in progressors (striped bars) to prediabetes and nonprogressors (open bars)) at enrollment (year 1) and during year 3 and year 5 follow-up dual-energy x-ray absorptiometry assessments. Repeated measures ANOVA P = 0.0015 (total fat mass) and 0.0005 (trunk fat mass) for comparisons between progressors and nonprogressors.
The relationship between serial changes in weight, waist circumference, total fat mass and trunk fat mass, and the risk of incident prediabetes was consistent in AA and EA offspring (Table 3). Using linear regression models, serial changes in weight (P = 0.0013), BMI (P = 0.0013), waist circumference (P < 0.0001), total fat (P = 0.0025), and trunk fat mass (P < 0.0001) were significant predictors of progression to prediabetes. In logistic regression models, each 1-SD (19.2 kg) higher baseline weight predicted a 17.5% increase in the risk of progression from normoglycemia to prediabetes. Each 1-SD (15 cm) higher baseline waist circumference predicted a 5% increase in the risk of incident prediabetes. The longitudinal change in weight also predicted prediabetes risk: each 1-SD (6.12 kg) increase in weight from baseline during follow-up was associated with a 7.2% increased risk of incident prediabetes (Table 4).
Table 3.
Serial Adiposity Measures in Relation to Incident Prediabetes Among African American and European American Offspring of Parents With Type 2 Diabetes
| African American | European American | |||
|---|---|---|---|---|
| Repeated Measures | F Ratio | P Value | F Ratio | P Value |
| Weight (kg) | 5.20 | 0.027 | 4.51 | 0.040 |
| Waist circumference (cm) | 8.56 | 0.0071 | 11.6 | 0.0016 |
| Total fat mass (kg) | 5.27 | 0.025 | 6.45 | 0.014 |
| Trunk fat mass (kg) | 5.84 | 0.018 | 7.90 | 0.0070 |
F ratios and P values were derived from repeated-measures ANOVA.
Table 4.
Logistic Regression Predicting Progression to Prediabetesa
| Odds Ratio | 95% CI | P Value | |
|---|---|---|---|
| Baseline weight (per 1SD) | 1.175 | 1.004-1.376 | 0.04 |
| Change in weight (per 1SD) | 1.072 | 1.001-1.148 | 0.04 |
| Baseline waist (per 1SD) | 1.051 | 1.021-1.083 | 0.0009 |
a Adjusted for age, sex, race/ethnicity, baseline fasting, and 2-hour post-load glucose.
Abbreviation: CI, confidence interval.
As shown in Table 1, 149 of our female participants were premenopausal and 94 were postmenopausal. However, we found no association between menopausal status and the risk of incident prediabetes, before (odds ratio 1.527 [95% confidence interval, 0.858-2.716], P = 0.1497) and after (odds ratio 0.887 [95% confidence interval, 0.387-2.029], P = 0.7755) adjustment for age.
Discussion
Owing to the strong association between obesity and increased risk of T2D [1-4, 27], weight loss has been used as a strategy for preventing or delaying progression from prediabetes to T2D [11, 17, 28, 29]. Our present study focused on adiposity measures at an earlier stage, namely, during transition from normoglycemia to prediabetes. There is insufficient knowledge regarding the impact of weight trajectories on impaired glucose regulation during the proximal stages in the pathophysiology of dysglycemia. In 1 study, serial metabolic assessments for ~5 years in Pima Indians with baseline NGT status identified 17 subjects who progressed through IGT (prediabetes) to T2D and 31 subjects who maintained NGT status (nonprogressors) [3]. Among progressors, the transition from NGT to prediabetes was associated with a mean weight gain of 5.6 kg (~1 kg/y) compared with 2.6 kg (~0.5 kg/y) among nonprogressors [3]. The Pima Indian study also showed that increases in fat mass and fat-free mass accounted for the weight gain observed in the progressors [3].
Because data on the quantitative relationship between fatness and incident prediabetes risk are lacking for most populations other than Pima Indians, we analyzed measures of adiposity in our diverse POP-ABC study population. Among our cohort, comprised of obese free-living offspring of parents with T2D, long-term maintenance of normoglycemia was associated with a weight gain of ~0.2 kg/y, whereas progression to prediabetes was associated with a weight gain of ~1 kg/y. Furthermore, compared with participants who maintained normoglycemia, POP-ABC participants who developed incident prediabetes showed greater longitudinal increases in waist circumference, total body fat, and trunk fat mass during follow-up. Our POP-ABC study findings, obtained from a large cohort of AA and EA with parental history of T2D, are remarkably concordant with the previous report from a smaller sample of a less diverse population [3]. Both studies found that weight maintenance or modest loss was associated with sustained normoglycemia, whereas modest weight gain (~1 kg/y) was associated with increased risk of incident prediabetes among individuals at perhaps the highest known risks for T2D, namely, Pima Indians [30] and people with parental diabetes [31]. Along with the prior report [3] and data from diabetes prevention trials [11, 28, 29], our present findings strengthen the notion that relatively modest changes in body weight could have disproportionate effects on glycemic outcomes.
It is important to focus on the proximal stage of transition from normoglycemia to prediabetes, for several reasons. First, spontaneous remission of prediabetes is rare because most affected individual eventually progress to develop T2D [32, 33]. Second, prediabetes is a toxic state that is associated with the initiation of microvascular and macrovascular complications [7, 8]. Third, it is plausible that the interval between normoglycemia and prediabetes might be more amenable to simple interventions than more advanced stages of dysglycemia. For example, participants in the placebo arm of the DPP experienced progression from prediabetes to T2D at annual rate of ~10%, despite having gained no further weight during the study period [11, 17]. (The weight change from baseline to end of study was -0.1 kg, - 2.1, and -5.6 kg in the placebo, metformin, and lifestyle-intervention groups, respectively, in the DPP [17].) In contrast, our present findings and those of the Pima Indian study [3] showed that weight maintenance or modest weight loss was associated with protection from transition from NGT to prediabetes.
Thus, in the setting of established prediabetes, prevention of progression to T2D requires interventions to induce weight loss: in the DPP cohort, each 1-kg weight loss (~1% of enrollment weight) resulted in a 16% reduction in the risk of progression from prediabetes to T2D [17].
Remarkably, our present findings suggest that at the more proximal stage, during transition from normoglycemia to prediabetes, avoidance of weight gain (operationally more feasible than induction of weight loss), even on a background of obesity and strong genetic risk, might be protective of glycemic escalation. It must be noted, though, that the relationship between body weight and dysglycemia is rather complex and subject to several interacting factors, including genetic predisposition and beta cell function. For example, a study of Europeans subjects found that insulin sensitivity and beta cell glucose sensitivity predicted changes in glucose tolerance among initially NGT subjects independently of sex, age, and obesity [34]. Clearly, it would be valuable to determine the specific role of weight trajectories on incident prediabetes in a model that accounts for all known genetic, biochemical, and glucoregulatory factors (including insulin sensitivity and insulin secretion). Such a comprehensive analysis was beyond the scope of the present paper.
Although the risk factors for T2D have been well described [3, 4, 10-16], surprisingly few prospective studies have focused on risk factors for the more proximal stage of incident prediabetes [3, 19, 34]. The present study adds to the few prospective studies on weight change and incident prediabetes [3, 19, 34]. Another strength derives from our diverse cohort (comprising high-risk AA and Caucasian offspring of parents with T2D) that enabled observation in a broader group than the predominantly Caucasian or Pima Indian populations in previous studies [3, 19, 34]. Additional strengths of our study include the rigorous ascertainment of prediabetes outcomes and the inclusion of corroborative data from body fat measurements.
Our cohort was predominantly female, of whom 94 (38.6%) were postmenopausal. Increased risk of dysglycemia (including T2D and prediabetes) has been reported in postmenopausal women versus premenopausal women, likely because of changes in hormonal and metabolic homeostasis [35, 36]. However, the data remain somewhat controversial when findings from retrospective studies are compared with those from prospective studies [36, 37]. For example, after adjustment for age, no association was found between natural menopause or bilateral oophorectomy and the risk of progression from prediabetes to T2D among women followed prospectively in the DPP [37]. In the present study, we found no association between menopausal status and the risk of incident prediabetes, before or after adjustment for age.
One methodological weakness of our study is that the OGTT used for ascertaining prediabetes endpoint is known to have poor reproducibility [38]. However, that is an inherent weakness of most studies in the field that employed OGTT, and there is no a priori reason to expect that the methodological weakness would be restricted to any specific group of participants (e.g., progressors vs. nonprogressors). Another weakness is that, by design, our POP-ABC study excluded individuals without parental diabetes. Arguably, that would limit the generalizability of our findings to the general population.
Furthermore, being a natural history study, POP-ABC study participants were not offered any medication or lifestyle counseling and were studied in their free-living state. Thus, intervention studies would be needed to test the effects of avoidance of weight gain (vs. induction of weight loss) as a minimalist approach to preventing progression from normoglycemia to prediabetes.
In conclusion, among obese AA and EA offspring of parents with T2D, a modest weight gain of ~ 1 kg/y predicted progression to prediabetes, whereas weight maintenance was associated with preservation of long-term normoglycemic status. Our findings suggest that prevention of further weight gain among obese but normoglycemic individuals might be an effective strategy for decreasing the risk of impaired glucose regulation and incident prediabetes.
Acknowledgments
We are indebted to the participants who volunteered for this study and to the research staff at the General Clinical Research Center for their expert support during the execution of the study. We thank Murray Heimberg, MD, PhD, for his service as the Institutional Data and Safety Officer.
Financial Support: The POP-ABC study was supported by grant R01 DK067269 from the National Institutes of Health and grant 7-07-MN-13 from the American Diabetes Association, awarded to S.D.-J. The funding sources had no role in the design and execution of the POP-ABC study, or analysis and publication of the data obtained from the study.
Author Contributions: All authors materially participated in the research and article preparation and gave final approval for the version submitted. S.D.-J. conceived of and designed the study, analyzed data, wrote manuscript; N.A.A.H. drafted and revised manuscript; S.E. collected data, performed analysis, reviewed and revised the manuscript; and C.E. collected data, reviewed and revised the manuscript.
Glossary
Abbreviations
- 2hrPG
2-hour postchallenge plasma glucose
- AA
African American
- BLSA
Baltimore Longitudinal Study on Aging
- BMI
body mass index
- DPP
Diabetes Prevention Program
- EA
European American
- FPG
fasting plasma glucose
- IFG
impaired fasting glucose
- IGT
impaired glucose tolerance
- IQR
interquartile ratio
- NGT
normal glucose tolerance
- OGTT
oral glucose tolerance test
- POP-ABC
Pathobiology of Prediabetes in A Biracial Cohort
- T2D
type 2 diabetes
Additional Information
Disclosure Summary: The authors have no conflict of interest to disclose regarding the content of this manuscript.
Data Availability
The datasets generated during and analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed May 11, 2020.
- 2. Centers for Disease Control and Prevention. Adult obesity prevalence maps, 2018. https://www.cdc.gov/obesity/data/prevalence-maps.html. Accessed May 11, 2020.
- 3. Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999;104(6):787-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care. 1994;17(9):961-969. [DOI] [PubMed] [Google Scholar]
- 5. Genuth S, Alberti KG, Bennett P, et al. Expert Committee on the diagnosis and classification of Diabetes Mellitus2, the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003;26:3160-3167. [DOI] [PubMed] [Google Scholar]
- 6. Dagogo-Jack S, Santiago JV. Pathophysiology of type 2 diabetes and modes of action of therapeutic interventions. Arch Intern Med. 1997;157(16):1802-1817. [PubMed] [Google Scholar]
- 7. Brannick B, Wynn A, Dagogo-Jack S. Prediabetes as a toxic environment for the initiation of microvascular and macrovascular complications. Exp Biol Med (Maywood). 2016;241(12):1323-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abdul-Ghani MA, DeFronzo RA. Pathophysiology of prediabetes. Curr Diab Rep. 2009;9(3):193-199. [DOI] [PubMed] [Google Scholar]
- 9. Eschwege E, Richard JL, Thibult N, et al. Coronary heart disease mortality in relation with diabetes, blood glucose and plasma insulin levels. The Paris Prospective Study, ten years later. Horm Metab Res Suppl. 1985;15:41-46. [PubMed] [Google Scholar]
- 10. American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020;43(Suppl. 1):S14-S31. [DOI] [PubMed] [Google Scholar]
- 11. Knowler WC, Barrett-Connor E, Fowler SE, et al. ; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Edelstein SL, Knowler WC, Bain RP, et al. Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes. 1997;46(4):701-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Crandall J, Schade D, Ma Y, et al. ; Diabetes Prevention Program Research Group . The influence of age on the effects of lifestyle modification and metformin in prevention of diabetes. J Gerontol A Biol Sci Med Sci. 2006;61:1075-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hanley AJ, Wagenknecht LE, Norris JM, et al. Insulin resistance, beta cell dysfunction and visceral adiposity as predictors of incident diabetes: the Insulin Resistance Atherosclerosis Study (IRAS) Family study. Diabetologia. 2009;52(10):2079-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kitabchi AE, Temprosa M, Knowler WC, et al. ; Diabetes Prevention Program Research Group . Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: effects of lifestyle intervention and metformin. Diabetes. 2005;54(8):2404-2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bray GA, Jablonski KA, Fujimoto WY, et al. ; Diabetes Prevention Program Research Group . Relation of central adiposity and body mass index to the development of diabetes in the Diabetes Prevention Program. Am J Clin Nutr. 2008;87(5):1212-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hamman RF, Wing RR, Edelstein SL, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29(9):2102-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hivert MF, Jablonski KA, Perreault L, et al. ; DIAGRAM Consortium; Diabetes Prevention Program Research Group . Updated genetic score based on 34 confirmed type 2 diabetes loci is associated with diabetes incidence and regression to normoglycemia in the diabetes prevention program. Diabetes. 2011;60(4):1340-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meigs JB, Muller DC, Nathan DM, Blake DR, Andres R; Baltimore Longitudinal Study of Aging . The natural history of progression from normal glucose tolerance to type 2 diabetes in the Baltimore Longitudinal study of aging. Diabetes. 2003;52(6):1475-1484. [DOI] [PubMed] [Google Scholar]
- 20. Dagogo-Jack S, Edeoga C, Nyenwe E, Chapp-Jumbo E, Wan J. Pathobiology of Prediabetes in a Biracial Cohort (POP-ABC): design and methods. Ethn Dis. 2011;21(1):33-39. [PMC free article] [PubMed] [Google Scholar]
- 21. Dagogo-Jack S, Edeoga C, Ebenibo S, Chapp-Jumbo E; Pathobiology of Prediabetes in a Biracial Cohort (POP-ABC) Research Group . Pathobiology of Prediabetes in a Biracial Cohort (POP-ABC) study: baseline characteristics of enrolled subjects. J Clin Endocrinol Metab. 2013;98(1):120-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dagogo-Jack S, Edeoga C, Ebenibo S, Nyenwe E, Wan J; Pathobiology of Prediabetes in a Biracial Cohort (POP-ABC) Research Group . Lack of racial disparity in incident prediabetes and glycemic progression among black and white offspring of parents with type 2 diabetes: the pathobiology of prediabetes in a biracial cohort (POP-ABC) study. J Clin Endocrinol Metab. 2014;99(6):E1078-E1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boucher AB, Adesanya EA, Owei I, et al. Dietary habits and leisure-time physical activity in relation to adiposity, dyslipidemia, and incident dysglycemia in the pathobiology of prediabetes in a biracial cohort study. Metabolism. 2015;64(9):1060-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jiang Y, Owei I, Wan J, Ebenibo S, Dagogo-Jack S. Adiponectin levels predict prediabetes risk: the Pathobiology of Prediabetes in A Biracial Cohort (POP-ABC) study. BMJ Open Diabetes Res Care. 2016;4(1):e000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Owei I, Umekwe N, Provo C, Wan J, Dagogo-Jack S. Insulin-sensitive and insulin-resistant obese and non-obese phenotypes: role in prediction of incident pre-diabetes in a longitudinal biracial cohort. BMJ Open Diabetes Res Care. 2017;5(1):e000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Owei I, Umekwe U, Stentz F, Wan J, Dagogo-Jack S. Amino acid signature predictive of incident prediabetes: a case-control study nested Within the Longitudinal Pathobiology of Prediabetes in a Biracial Cohort Metabolism. Metabolism 2019;98:76-83. doi: 10.1016/j.metabol.2019.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nano J, Dhana K, Asllanaj E, et al. Trajectories of BMI before diagnosis of type 2 diabetes: the rotterdam study. Obesity (Silver Spring). 2020;28(6):1149-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20(4):537-544. [DOI] [PubMed] [Google Scholar]
- 29. Tuomilehto JLJ, Eriksson JG, Valle TT, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343-1392. [DOI] [PubMed] [Google Scholar]
- 30. Knowler WC, Pettitt DJ, Saad MF, Bennett PH. Diabetes mellitus in the Pima Indians: incidence, risk factors and pathogenesis. Diabetes Metab Rev. 1990;6(1):1-27. [DOI] [PubMed] [Google Scholar]
- 31. Moonesinghe R, Beckles GLA, Liu T, Khoury MJ. The contribution of family history to the burden of diagnosed diabetes, undiagnosed diabetes, and prediabetes in the United States: analysis of the National Health and Nutrition Examination Survey, 2009–2014. Genet Med. 2018;20(10):1159-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sallar A, Dagogo-Jack S. Regression from prediabetes to normal glucose regulation: state of the science. Exp Biol Med (Maywood) 2020;245(10):889-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gong Q, Zhang P, Wang J, et al. ; Da Qing Diabetes Prevention Study Group . Morbidity and mortality after lifestyle intervention for people with impaired glucose tolerance: 30-year results of the Da Qing Diabetes Prevention Outcome Study. Lancet Diabetes Endocrinol. 2019;7(6):452-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ferrannini E, Natali A, Muscelli E, et al. ; RISC Investigators . Natural history and physiological determinants of changes in glucose tolerance in a non-diabetic population: the RISC Study. Diabetologia. 2011;54(6):1507-1516. [DOI] [PubMed] [Google Scholar]
- 35. Szmuilowicz ED, Stuenkel CA, Seely EW. Influence of menopause on diabetes and diabetes risk. Nat Rev Endocrinol. 2009;5(10):553-558. [DOI] [PubMed] [Google Scholar]
- 36. Heianza Y, Arase Y, Kodama S, et al. Effect of postmenopausal status and age at menopause on type 2 diabetes and prediabetes in Japanese individuals: Toranomon Hospital Health Management Center Study 17 (TOPICS 17). Diabetes Care. 2013;36(12):4007-4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim C, Edelstein SL, Crandall JP, et al. ; Diabetes Prevention Program Research Group . Menopause and risk of diabetes in the Diabetes Prevention Program. Menopause. 2011;18(8):857-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Balion CM, Raina PS, Gerstein HC, et al. Reproducibility of impaired glucose tolerance (IGT) and impaired fasting glucose (IFG) classification: a systematic review. Clin Chem Lab Med. 2007;45(9):1180-1185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.