Abstract
In the last decade, animal studies highlighted the sensitivity of hearing function to lack of specific cochlear dopamine receptors, while several studies on humans reported association between hearing loss and Parkinson’s disease, partially recovered after levodopa administration in de novo patients. Taken together, these observations suggest investigating the possible use of cochlear function outcome variables, particularly, otoacoustic emissions, as sensitive biomarkers of Parkinson’s disease. Any lateralization of hearing dysfunction correlated with Parkinson’s disease lateralization would (i) further confirm their association and (ii) provide a disease-specific differential outcome variable. Differential indicators are particularly useful for diagnostic purposes, because their effectiveness is not limited by physiological inter-subject fluctuations of the outcome variable. Recent advances in the acquisition and analysis techniques of otoacoustic emissions suggest using them for evaluating differential cochlear damage in the two ears. In this study, we quantitatively evaluated hearing function in a population of subjects with Parkinson’s disease, to investigate the occurrence of hearing loss, and, particularly, whether hearing dysfunction shows lateralization correlated with motor symptoms. Pure tone audiometry and distortion product otoacoustic emissions were used as outcome variables in 80 patients (mean age 65 ± 9 years) and 41 controls (mean age 64 ± 10 years). An advanced customized acquisition and analysis system was developed and used for otoacoustic testing, which guarantees response stability independent of probe insertion depth, and has the sensitivity necessary to accurately assess the low levels of otoacoustic response typical of elderly subjects. To our knowledge, this is the first study introducing the distinction between ipsilateral and contralateral ear, with respect to the body side more affected by Parkinson’s disease motor symptoms. Significant asymmetry was found in the auditory function, as both otoacoustic responses and audiometric hearing levels were worse in the ipsilateral ear. Significantly worse hearing function was also observed in patients with Parkinson’s disease compared to controls, confirming previous studies. Several pathophysiological mechanisms may be hypothesized to explain asymmetric cochlear damage in Parkinson's disease, including the impairment of dopamine release and the involvement of extra-dopaminergic circuits, with the cholinergic pathway as a likely candidate. The observed asymmetry in the audiological response of patients with Parkinson’s disease suggests that lateralization of hearing dysfunction could represent a specific non-motor signature of the disease. The possible diagnostic use of cochlear dysfunction asymmetry as a specific biomarker of Parkinson’s disease deserves further investigation, needing a more precise quantitative assessment, which would require a larger sample size.
Keywords: Parkinson’s disease, hearing loss, otoacoustic emissions
Hearing function was evaluated in 80 subjects with Parkinson’s disease, by means of pure tone audiometry and distortion product otoacoustic emissions. Significant auditory asymmetry was found, as parameters were worse on the body side more affected by motor symptoms. Also, patients showed worse auditory function when compared to controls.
Graphical Abstract
Graphical Abstract.
Introduction
Parkinson’s disease is a multi-system neurodegenerative disorder clinically characterized by a combination of motor and non-motor features (Lang and Lozano, 1998; Braak and Braak, 2000), contributing to the definition of different clinical phenotypes. The interest in classifying the entire Parkinson’s disease symptom complex, also with respect to patient quality of life (Chaudhuri et al., 2007; Martinez-Martin et al., 2009), has been growing recently (Barone et al., 2009; Chaudhuri and Schapira, 2009; Berg et al., 2015; Titova et al., 2017). However, in this framework, the possible involvement of sensory systems has been scarcely investigated, although earlier studies have reported the impairment of visual and auditory systems in patients with Parkinson's disease (Pisani et al., 2015; Vitale et al., 2016; Weil et al., 2016).
In the field of hearing research, alterations in patients with Parkinson's disease were observed in different frequency ranges, although the association with disease features has not been fully defined (Gawel et al., 1981; Fradis et al., 1988; Vitale et al., 2012; Pisani et al., 2015; Folmer et al., 2017; Shetty et al., 2019). Recent studies have also demonstrated impairment in central auditory processing (Folmer et al., 2017) and reduction of distortion-product otoacoustic emission (DPOAE) levels (Pisani et al., 2015), which reflect cochlear outer hair cell function.
It is well known that, at first, Parkinson's disease motor symptoms appear unilaterally, and remain asymmetric as the disease progresses (Postuma et al., 2015, 2018; Heinrichs-Graham et al., 2017). The purpose of this study was to evaluate auditory function and test the occurrence of an asymmetric impairment, which could represent a new lateralized feature of Parkinson's disease. To this aim, we evaluated hearing levels (HLs) and outer hair cell function, by means of pure tone audiometry (PTA) and DPOAE, in a large cohort of patients with Parkinson's disease, compared to a population of age- and sex-matched controls. Within the Parkinson's disease group, we compared the response of the two ears of each subject to assess, with a robust paired statistical test, whether hearing asymmetry was correlated with the lateralization of motor symptoms. If so, it could be proposed as a specific Parkinson's disease biomarker for diagnostic purposes. The possible association between auditory results and other clinical variables was also investigated.
Materials and methods
Patient selection and study design
In this observational study, we recruited out-patients admitted at the Parkinson’s Disease Center of the University of Rome ‘Tor Vergata’. Eligible patients had a diagnosis of idiopathic Parkinson's disease, according to Movement Disorder Society Clinical Diagnostic Criteria (Postuma et al., 2015, 2018). Patients were also required to meet the following inclusion criteria: (i) to have no history of hereditary hearing loss, acute noise trauma, past head injury, previous ear surgery or other otological/labyrinthine disorders; (ii) to have no concomitant neurological diseases except Parkinson's disease; (iii) to have no concomitant psychiatric diseases or dementia (Mini Mental State Examination >24/30); (iv) to be in complete agreement with the study design. Exclusion criteria were the following: systemic and/or inflammatory chronic diseases with known influence on hearing function, including diabetes, hypertension and/or history of hypertensive crisis, peripheral vasculopathy; concomitant or previous use of potentially ototoxic drugs; evidence of anamnestic factors interfering with auditory function, including chronic exposure to noise and work activity with high auditory risk.
Parkinson’s disease severity and progression were scored by Hoehn and Yahr scale; patients’ motor disability was quantified by the Motor examination section of the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale Part III (from 0 to 128). Considering the observational nature of this study, patients maintained their regular treatment depending on their needs and current clinical practice.
At the time of enrollment, all patients who met the entry criteria underwent neurological evaluation, including Movement Disorder Society-Unified Parkinson’s Disease Rating Scale Part III and Hoehn and Yahr staging. Following the study criteria, a medical history interview was conducted considering all potential external factors causing auditory dysfunction to eliminate any confounding factors. Once neurological examination was completed, all patients underwent audiological testing, performed by an expert otolaryngologist, who was not blinded to the Parkinsonian/control status, but was blinded to which motor side was most affected, except for those cases in which such asymmetry was evident.
A control group, whose average age and sex composition matched those of the Parkinson's disease group, consisting, when available, of co-inhabitants or age-matched relatives of patients (partners or siblings), was also enrolled. The control subjects were required to meet the same entry criteria as the patients, and they underwent the same anamnestic interview and audiological examination.
A total of 98 patients and 52 control subjects underwent enrollment in the study. The sample size was determined starting from the results of a previous study (Pisani et al., 2015), in which an equivalent diagnostic apparatus was used for measuring audiometric thresholds and DPOAE levels. In that study, 11 subjects were sufficient to achieve a high confidence level in the statistical comparison between cases and controls over a wide frequency range, in the presence of significant systematic differences between cases and controls. Based on the size of the standard errors of the measurements reported in that study, we increased sample size by a factor of four (to N = 40), a sensitivity with a 95% confidence level for differences of ∼5 dB for audiometric HL and 2 dB for DPOAE level could be achieved, a threshold which represents the intrinsic limits associated with the test–retest reproducibility of the two diagnostic techniques. Data analysis was performed by an Author blinded to the study groups.
All subjects were tested once within the time interval March 2018–June 2019.
Standard protocol approvals, registrations and patient consents
All participants gave written informed consent after receiving an extensive disclosure of study purposes, according to the Declaration of Helsinki. The local ethics committee at University of Rome ‘Tor Vergata’ approved the procedures (protocol no. 7/18, 7 February 2018).
Audiological testing
After otoscopic examination, PTA and DPOAEs were tested in an audiometric booth, in the same session. PTA thresholds were measured in each ear, at 11 standard audiometric frequencies ranging from 0.125 to 8 kHz, evaluating the threshold down to −5 dB HL with a 5 dB accuracy.
DPOAEs are low-level sounds generated in the organ of Corti, measured in the external ear canal without requiring patient cooperation (Probst et al., 1991). DPOAEs arise from the nonlinearity of the cochlear response, stimulated at two nearby frequencies, f1 and f2, producing tones called intermodulation distortion products, the most intense at a frequency of 2f1 and f2. In this study, we used stimulus levels (L1, L2)=(65, 55) dB forward pressure level, with a frequency ratio between f2 and f1 equal to 1.22. DPOAEs are unambiguously interpreted as signals generated in the cochlea, because the cochlear amplifier is the only nonlinear system involved in their generation (Shera and Guinan, 1999). DPOAE sensitivity to sensorineural hearing loss is well-established (Gorga et al., 1993; Sisto et al., 2007). Although DPOAEs are recorded at a frequency of 2f1 and f2, the outcome variable is clinically associated with frequency f2, because the generation region of the 2f1 and f2 DPOAE is near the cochlear place whose characteristic frequency is f2; thus, the outer hair cell-driven cochlear amplifier is actually tested at that frequency. The intra-cochlear wave generated near the f2 tonotopic region also propagates forward to the fDP resonant place, where it undergoes resonant amplification and partial backward reflection. This reflected wave constitutes a second DPOAE component, with different phase behaviour and diagnostic meaning, and the interference between the two components generates a characteristic oscillating spectral pattern, known as the DPOAE fine-structure.
In conventional commercial acquisition systems, correlation between DPOAE levels and HLs is limited by test–retest reproducibility and large inter-subject fluctuations of DPOAE levels in subjects with the same audiometric thresholds. Advanced DPOAE acquisition and analysis techniques, which are not implemented in commercial clinical instruments, significantly help to limit these uncertainties. In this study, DPOAE spectra were recorded with high frequency-resolution and were time–frequency filtered (Moleti et al., 2012) to unmix the distortion and reflection components, based on their different phase-gradient delay. Focusing on the unmixed distortion component minimizes the uncertainty typical of DPOAE measurements associated with fine-structure amplitude fluctuations due to interference between the two components. This approach also improves the signal-to-noise ratio (SNR) by up to 15 dB with respect to that of mixed DPOAE spectra, because most of the noise is removed by the filtering procedure (Moleti et al., 2012). Ear-canal calibration of forward pressure and otoacoustic emission (OAE) signal was also performed, which improves the reproducibility of the response, regardless of either probe insertion depth or individual acoustic impedance of the ear canal (Charaziak and Shera, 2017). High response reproducibility and very low noise floor allowed us to measure with high sensitivity the DPOAE response also in elderly and/or hearing-impaired patients, whose typical DPOAE levels fall often below the noise floor of commercial instruments. The unmixed distortion component in four half-octave bands was used as the DPOAE outcome variable. We remark that the half-octave levels reported in this study represent the total energy within each band, so they cannot be directly compared with the levels of the high frequency-resolution spectra. A data selection rule was applied after DPOAE component unmixing and before statistical analysis, resulting in inclusion in the study only of data for which either the noise was below a given threshold, or the SNR was higher than 3 dB, to guarantee that noise contribution to the estimated DPOAE component level was not significant.
Statistical analysis
All the statistical analyses were performed using the statistical software SPSS (version 25, IBM, USA) and R (version 3.5.3, R Foundation for Statistical Computing, Vienna, Austria). A significance criterion P < 0.05 was conventionally adopted.
Multivariate mixed effect models
Multivariate mixed-effect linear regression models were fitted to the data. These models are particularly useful in the case of repeated measurements on the same subject. Both fixed and random effect variables were accounted for. In particular, the subject was treated as a random variable and the non-independence of the observations on the same subject at different frequencies was taken into account. All DPOAE levels were treated as a unique variable, using a four-level factor to represent the half-octave frequency bands. The same data organization was used in the case of PTA, in which an 11-level factor was introduced to identify the frequency band. A two-level factor, ‘diagnosis’ was introduced to compare patients with Parkinson's disease to control subjects. An additional two-level factor was introduced and named ‘laterality’, distinguishing ipsilateral and contralateral ears with respect to the patients’ side more affected by Parkinson’s disease motor symptoms.
Parkinson's disease patients versus controls
To compare patients with Parkinson's disease to control subjects, an ANOVA test for repeated measurements was used. The frequency band and the ear were treated as within-subject factors (with four and two levels, respectively). The between-subjects factor, disease, is a two-level factor, distinguishing patients from controls. Sex was included as a between-subjects two-level factor, and age was included as a covariate factor. A complete factorial model was studied.
In such a comparison between two distinct populations, the sensitivity of the results mainly depends on the test–retest and inter-subject fluctuations of the diagnostic technique. At low signal levels, typical of elderly and/or impaired subjects, the advanced DPOAE technique used in this study helps to reduce these uncertainties; however, PTA may still prove more sensitive, because the typical change in DPOAE levels (in dB) due to sensorineural hearing loss is ∼50% of the audiometric change in cross-section studies (Gorga et al., 1993; Sisto et al., 2007).
Laterality
To investigate laterality effects on test frequency, in the case of both DPOAE (four frequency bands) and PTA (11 frequencies), we included age and ear in the following model:
| (1) |
along with an analogous model in which the outcome variable was PTA HL.
To test the significance of the model (1), we used two approaches: one containing the fixed effect variable under investigation (i.e. laterality) and one not containing it, compared by an ANOVA test. As paired comparisons between the ears of the same patients were performed, the presence of confounders was not relevant for this test.
Statistical association with clinical variables
Multivariate mixed effect models were also fitted to test the statistical significance of a set of clinical variables. Models like the one described by Equation (1) were fitted, in which the fixed effect variables were: levodopa equivalent daily dose (LEDD), disease duration and staging, motor impairment score. All of these variables are listed in Table 1.
Table 1.
Average clinical and demographic data of participants enrolled in the study
| Patients (n = 80) | Controls (n = 41) | |
|---|---|---|
| Age (years) | 65 ± 9 | 64 ± 10 |
| Gender | 43 M; 37 F | 24 M; 17 F |
| Most affected motor side | 34 L; 46 R | |
| Disease duration (months) | 67.4 ± 54.4 | |
| H&Y stage | 1.91 ± 0.62 | |
| MDS-UPDRS III | 22.3 ± 10.5 | |
| Clinical phenotype |
Tremor dominant: 18 Akynetic/rigid: 33 Mixed: 29 |
|
| LEDD (mg) | 466 ± 248 | |
| Dopamine agonist therapy | Y: 42; N: 38 |
Most affected motor side (L = Left; R = Right); dopamine agonist therapy (Y = Yes, N = No). Data are reported as mean ± standard deviation.
H&Y, Hoehn and Yahr stage; LEDD, levodopa equivalent daily dose (in milligrams); MDS-UPDRS III, Movement Disorder Society-Unified Parkinson’s Disease Rating Scale Part III.
Handling confounding factors
To avoid any confounder from the Parkinson's disease versus control comparison, the control group was matched to the Parkinson's disease group, by selecting co-inhabitants of approximately the same age.
Data availability statement
Data related to this study are available from Parkinson’s Disease Center, Department of Systems Medicine, University of Rome ‘Tor Vergata’, by written request, in accordance with the data-protection legislation in Europe (General Data Protection Regulation). Persons interested in obtaining access to the data should contact the corresponding Author.
Results
Subjects
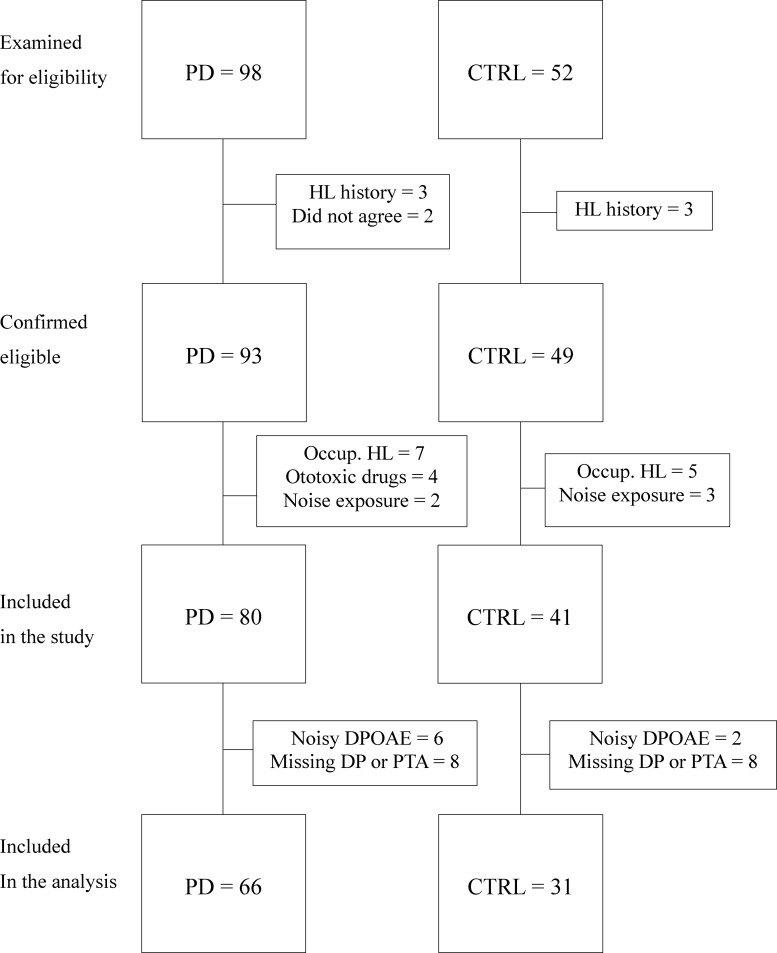
A total of 98 out-patients with Parkinson's disease and 52 consecutive controls were screened. Of those, five patients did not meet the inclusion criteria. More specifically, three patients had a history of hearing loss due to trauma or previous infection and two patients did not agree with the study design. Of the controls, three did not meet entry criteria, reporting a history of hearing loss. A total of 93 patients and 49 controls were confirmed as eligible for the study. Furthermore, 13 patients were excluded after the anamnestic interview because four reported hearing loss due to ototoxic drugs, two reported chronic noise exposure and seven revealed previous hearing impairment due to occupational exposure. Eight controls were also excluded after the anamnestic interview, five of them revealing hearing impairment due to occupational exposure and three reporting noise exposure.
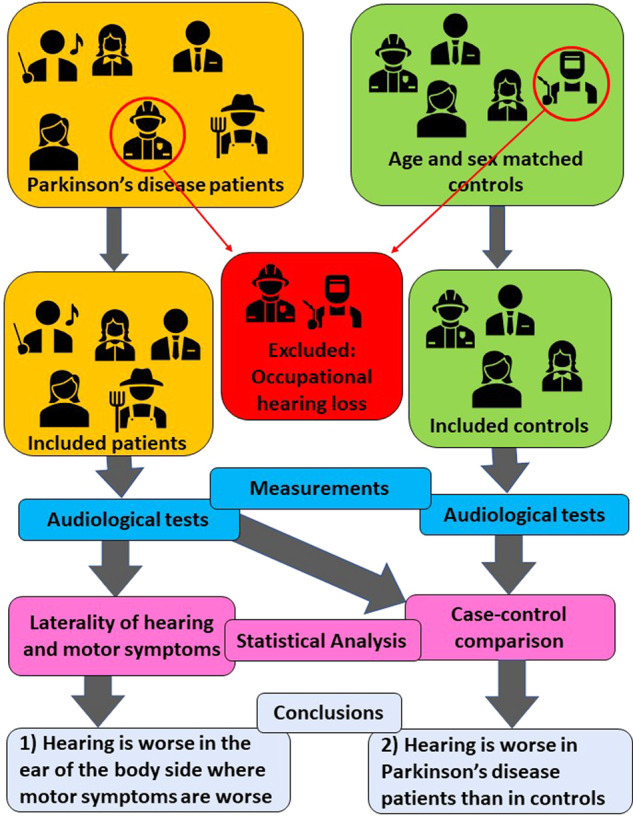
Thus, 80 patients with Parkinson's disease (43 males, 37 females; mean age 65 ± 9 years) and 41 controls (24 males, 17 females; mean age 64 ± 10 years) were included in the study. As the data analysis protocol required audiometric and DPOAE data from both ears of each subject, eight patients and eight controls were further excluded from the analysis because, for technical or logistic reasons (independent of their HL), it was not possible to record both DPOAE and PTA responses from both ears. The DPOAE noise rejection criterion further reduced the number of analysed subjects to 66 patients with Parkinson's disease (33 males, 33 females; mean age 64 ± 9 years) and 31 controls (15 males, 16 females; mean age 62 ± 10 years). The selection introduced no bias, meaning that the average clinical scores of the selected patients were fully consistent with those of the unselected population. The entire subject selection process is summarized in the flowchart of Fig. 1. Patients were classified according to age, sex, disease duration, staging, motor impairment (Movement Disorder Society-Unified Parkinson’s Disease Rating Scale Part III score), laterality as revealing the most affected side (right or left), therapy considering LEDD, as shown in Table 1.
Figure 1.
Study subject selection process. Flowchart depicting the subject selection process.
Audiological results overview
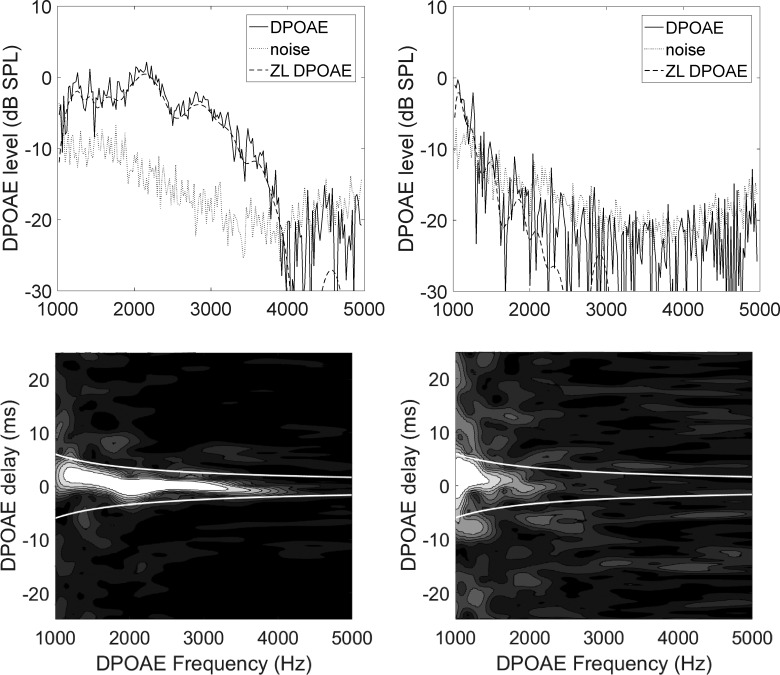
Typical DPOAE spectra and time–frequency representations are shown in Fig. 2 for an ear with normal hearing (HL ≤20 dB) and for an ear with hearing-impairment (HL between 25 and 70 dB, increasing with frequency), from our subject sample. In this case, the ear with normal hearing belongs to a control and the impaired ear to a patient with Parkinson's disease, but the opposite case may also occur in our sample. The two white lines in the bottom panels, in which the intensity plot (spectrogram) is normalized to its maximal value, delimit the short-latency filtering region to which the DPOAE distortion component (ZL) belongs. In that region, a zero-latency component is still visible even in spectral ranges (here, between 1.5 and 2.5 kHz) in which the overall SNR would be insufficient, while noise appears as randomly distributed intensity modulation in the same panels. System distortion for our testing apparatus was always below the noise floor, estimated as the off-band response level during acquisition.
Figure 2.
OAE spectra and spectrograms. DPOAE spectra (top) before and after (ZL) t–f filtering and relative time–frequency representations (bottom) for a normal-hearing ear (left) and a hearing-impaired ear (right). The frequency displayed is the 2f1–f2 distortion product frequency, which is ≈0.7f2, where f2 is the clinically relevant frequency. In the time–frequency plots, the zero-latency component is visible as a bright band parallel to the frequency axis. In the noisy case of the hearing-impaired ear, a zero-delay component is still visible also in the region between 1.5 and 2.5 kHz, despite SNR <1 for the unfiltered spectrum (top).
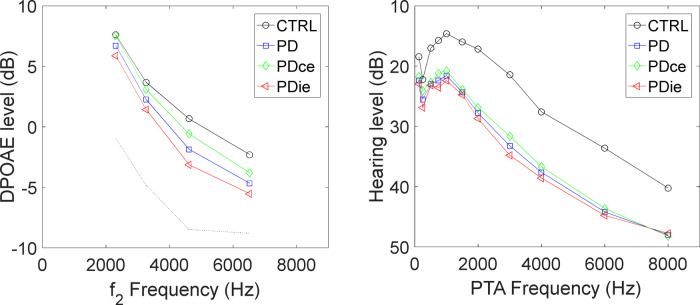
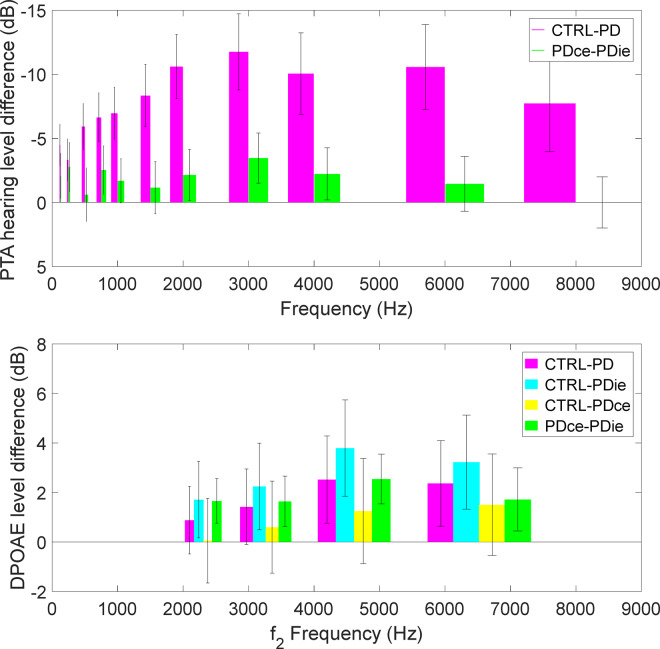
Figure 3 shows average DPOAE response and PTA HL as functions of frequency, in four groups of ears, the control ears, both ears of the patients with Parkinson’s disease, and the ipsilateral and contralateral ears, corresponding to the side more affected by motor symptoms. In Fig. 3 (left) only ZL DPOAE data and the corresponding estimated noise levels are plotted. Both PTA and DPOAE qualitatively show worse response for the ears of patients with Parkinson's disease, and, particularly, for the ipsilateral ears, with respect to controls. Due to the very wide age range of the two groups (∼40 years), a large fraction of the PTA and DPOAE response variability within the two groups is due to ageing. For this reason, it is very important to include age as a covariate factor in the following statistical analysis. The average differences between patients with Parkinson’s disease and controls are shown in more detail in Fig. 4 for DPOAE level and audiometric HL, the error bars representing the standard error of the plotted difference. Note that the standard errors of the difference between ipsilateral and contralateral are smaller than the other errors, because in this case a paired difference between the response of ears of the same subject (and its variance across the population) is considered. There was no significant systematic difference between the noise floor levels of ipsilateral and contralateral ears.
Figure 3.
DPOAE levels and PTA HLs in study subjects versus controls. Left: average zero-latency DPOAE levels, plotted against f2 frequency, in four half-octave frequency bands, for the control group (CTRL), for the Parkinson’s disease patient ears and for the two subgroups of ipsilateral and contralateral ears. Standard errors, not shown for clarity, are ∼1 dB for all groups. Right: average PTA HLs in 11 frequency bands for the control group (CTRL), for the Parkinson’s disease patient ears and for the two subgroups of ipsilateral and contralateral ears. Standard errors are ∼3 dB.
Figure 4.
Differences in average hearing and DPOAE levels between study patients and controls. Differences between the average levels measured in patients with Parkinson’s disease, ipsilateral and contralateral ears with respect to the control group (CTRL), as regards PTA thresholds (top) and DPOAE level, plotted against f2 frequency (bottom). The difference between the ipsilateral and contralateral ears is also explicitly shown. The response of ipsilateral ears is systematically worse by roughly 2 dB, for both diagnostic techniques. Error bars represent standard errors.
Statistical analysis
Parkinson's disease versus control comparison
In the case of PTA levels, the ANOVA test for repeated measures, including age as a covariate factor, yielded a significant result (P = 0.013) when all patient ears were compared to control ears.
With respect to DPOAE levels, the ANOVA test for repeated measurements on the complete study design (i.e. four frequency bands and two ears from the same subject) did not return significant results when all Parkinson's disease ears were compared to control ears, including age as a covariate factor. The complete factorial model yielded no significant result, also comparing the control ears to ipsilateral only. A planned comparison was performed between the ipsilateral ears and the controls in the third and the fourth half-octave bands (centered, respectively, at f2 = 4.6 and 6.5 kHz). The result of a one-tail Student’s t-test was P = 0.027 and P = 0.045, in the third and fourth band, respectively. The larger size of the laterality effect in the higher frequency bands is visible in Fig. 3, where the average DPOAE are shown for ipsilateral, contralateral and for the control ears.
Laterality effects in patients with Parkinson’s disease
The comparison between the model containing the fixed-effect ‘laterality’ and the reduced model, which does not contain it, is statistically significant for the DPOAE level (P < 0.00001). The regression coefficient for the linear mixed effect model is β = 1.99 (SE = 0.53, t-value = 3.78, P = 0.0002). This means that, on average, the DPOAE response of the ipsilateral ear was lower by ∼2 dB than the one of the contralateral ear of the same patient. To confirm this result, we checked that the factor ‘ear’ in the model of Equation (1) was not statistically significant, meaning that no significant difference between the left and right ear was present in the data of the patients with Parkinson’s disease. The difference between the left and right ear was tested also in the control group, yielding again no significant difference. Therefore, a laterality effect of this size may be considered as a specific feature of patients with Parkinson’s disease, correlated only with the side affected by their motor symptoms and not with any left–right asymmetry.
The same comparison between the models with and without the fixed-effect ‘laterality’ is statistically significant also for the PTA threshold (P = 0.05). The regression coefficient for the linear mixed effect model is β = −1.90 (SE = 0.67, t-value = −2.83, P = 0.046).
Statistical association with clinical variables
In patients with Parkinson’s disease, no association was demonstrated in our study, at a statistically significant level, between the audiological data (PTA and DPOAE) and the other clinical variables, i.e. disease duration and staging, motor impairment score, LEDD.
Discussion
The main result of this study is that, in patients with Parkinson’s disease, the DPOAE and PTA responses are significantly worse in the ear ipsilateral to the side manifesting worse motor symptoms. In more specific terms, hearing dysfunction in patients with Parkinson’s disease seems to parallel the asymmetry of patients’ motor impairment. In the laterality study, the uncertainty of the DPOAE technique with respect to variability of the OAE response in subjects with the same hearing loss is much less relevant, because one performs a paired comparison between the two ears of the same subject, tested in the same measurement session. Interestingly, as shown in Fig. 4, DPOAEs and PTA show the largest differences between ipsilateral and contralateral side in the same frequency range, i.e. above 2 kHz. It may also be worth mentioning that the slope of the PTA regression is almost twice the one of the DPOAE regression, in agreement with previous studies on hearing-impaired subjects (Gorga et al., 1993; Sisto et al., 2007) that reported a DPOAE level decrease in order 0.5 dB for 1 dB increase in PTA threshold.
The measured size of the DPOAE laterality effect reported in this study (1.9 dB average level difference) may be conveniently compared to the size of another differential measurement, the DPOAE level suppression caused by contralateral stimulation, which is typically of order 1 dB, and is currently used to evaluate the effectiveness of the medial olivocochlear efferent system. Despite its small size, the diagnostic power of the contralateral stimulation effect is related to its differential nature, because the difference is measured between the response of the same ear in the presence and absence of the contralateral noise suppressor. Therefore, the well-known large inter-subject variability of the OAE response among subjects with the same HL plays no confounding role. The observed DPOAE asymmetry between the ipsi- and contralateral ears tested at the same time with the same instrument under identical environmental conditions shares the same advantage, as a promising biomarker of Parkinson's disease.
The difference in audiometric thresholds between patients with Parkinson's disease and controls, by means of PTA testing, is in agreement with previous clinical results (Vitale et al., 2012, 2016; Shetty et al., 2019, Scarpa et al., 2020). Our results extend previous observations by tackling a large cohort of patients. In our study, PTA impairment seems to be present along multiple frequency bands, while in previous studies the extent of frequency involvement varied, possibly due to different sample sizes, experimental settings and patients’ clinical and demographic characteristics (Vitale et al., 2012, 2016; Pisani et al., 2015; Shetty et al., 2019).
In this study, we were not able to demonstrate significant correlations between hearing loss and a series of clinical variables, such as disease stage, motor disability, disease duration and ongoing treatment. These results agree with previous PTA studies reporting no correlation between hearing damage and motor symptoms or response to medical treatment in patients with Parkinson’s disease; however, the same studies found stage-dependent high-frequency hearing loss in patients with Parkinson’s disease, at odds with the present results (Vitale et al., 2012, 2016; Scarpa et al., 2020). Thus, whether hearing impairment represents a constant feature along the course of Parkinson’s disease still remains an object of debate.
Motor symptoms in Parkinson’s disease appear unilaterally, and, as a rule, they remain worse on the initially affected side as the disease progresses (Postuma et al., 2015, 2018; Heinrichs-Graham et al., 2017). However, little is known about whether the same asymmetric behaviour may occur in the spectrum of Parkinson’s disease non-motor symptoms, especially considering bilateral sensory systems. In previous studies, non-motor features of Parkinson’s disease showed no particular lateralization in their spectrum, likely because they seem to be related to a widespread brain disorder involving different neurotransmitter circuitries (Chaudhuri and Schapira, 2009; Chaudhuri et al., 2011). Of course, exceptions include Parkinson’s disease behavioural and emotional deficits, which involve brain areas presenting with physiological functional lateralization (Eitan et al., 2013). For instance, vocal emotion recognition was significantly worse among patients with left-sided motor symptoms, and, coherently, FDG-PET documented the impairment of right brain regions (Stirnimann et al., 2018).
In Parkinson’s disease, motor dysfunction is directly related to the progressive loss of nigrostriatal dopaminergic neurons (Dickson et al., 2009); yet, apart from the nigrostriatal pathway, dopamine acts as a neurotransmitter in several functional systems, including the auditory system. The main dopaminergic neural population related to hearing resides in the lateral olivocochlear bundle, which reaches the afferent auditory nerve, forming axodendritic synapses under the inner hair cells (Lendvai et al., 2011; Maison et al., 2012). Moreover, cochlear expression and localization of all five dopamine receptor subtypes has been demonstrated in rodents (Inoue et al., 2006; Maison et al., 2012). It appears that lateral olivocochlear bundle function is to modulate auditory nerve discharges, by facilitating or decreasing sound transmission, according to the presence of harmful inputs (e.g. noise); in this scenario, dopaminergic activation seems to have a net inhibitory effect, as a depletion in dopamine levels has been shown to decrease auditory function, possibly due to an increase in excitotoxicity (Ruel et al., 2001; Niu and Canlon, 2006). Such dopaminergic signaling seems to be mediated by both D1-like and D2-like receptor activation, with opposing effects: experiments with selective agonists and antagonists led to modifications in endocochlear potentials and auditory nerve activity (Ruel et al., 2001; Niu and Canlon, 2006; Garrett et al., 2011), whereas mice carrying targeted deletions of receptor subtypes showed different degrees of vulnerability to acoustic injury (Maison et al., 2012). In particular, Maison et al. (2012) compared wild-type mice to mice with targeted deletion of the dopamine receptors D1, D2, D4 and D5. A complete battery of audiological tests was performed to better understand the role of dopamine in cochlear activity. In the D2 knockout mice, lower DPOAE and auditory brainstem response levels and increased DPOAE and auditory brainstem response thresholds (both indicating lower hearing sensitivity) were measured with respect to wild-type mice, particularly in the high-frequency range. Considering that the sensitivity range of the mouse cochlea is shifted to higher frequencies with respect to humans, these results coming from an experimental animal model are remarkably compatible with results found in this study, in which patients with Parkinson’s disease show worse audiometric threshold and lower DPOAE response in the high-frequency range.
Although it is conceivable that cochlear dysfunction in Parkinson’s disease may be due to the impairment of dopaminergic transmission of either D1 or D2 receptor-linked processes, the significant asymmetry of such dysfunction should not be exclusively ascribed to local dopaminergic imbalance. Indeed, the lack of significant correlations between asymmetrical cochlear dysfunction and clinical parameters, such as disease stage and LEDD, might support the occurrence of non-dopaminergic mechanisms. However, proving that the deterioration of cochlear function is related to the disease stage and/or to the medication dose can be challenging: for example, the titration of pharmacological treatment might exert an impact per se, acting as a confounding factor: indeed, LEDD increases as the disease stage increases, and it has been shown to modulate cochlear impairment. As concerns the effect of therapy on patients’ cochlear function, a preliminary study (Pisani et al., 2015) on a small sample of 11 de novo patients showed improvement in DPOAE levels after one to three months of levodopa treatment; these data are not really at odds with our results, since no confounder related to the correlation between dose and stage was present in that short-term challenge with fixed doses of levodopa. Further studies involving much larger cohorts of patients and/or a prospective design are needed to define such aspect, focusing on the comparison between patients with similar disease staging but different LEDD.
Alternatively, we could hypothesize that the asymmetry in cochlear function observed in Parkinson’s disease may also involve other neurotransmitter circuitries. Given the fact that the more severe cochlear dysfunction is ipsilateral to patients’ more affected motor side, we cannot exclude that it may represent the expression of a greater degeneration in contralateral brainstem structures, involving cholinergic neurons, which may influence different efferent pathways. The olivocochlear bundle is divided into the medial olivocochlear, prevalently cholinergic and lateral, prevalently dopaminergic, systems. In animal studies, both systems have been shown to contain crossed and uncrossed fibres, while the proportion of crossed versus uncrossed axons in humans is unknown (Guinan, 2018). Medial olivocochlear neurons have myelinated axons that synapse on outer hair cells by releasing acetylcholine, and receive input from both cochlear nuclei, situated in the brainstem (Brown, 2014; Guinan, 2018). The exact biological function of medial olivocochlear activation is still debated, as it has been associated with an increase in SNR during detection of target signals, or to protection from acoustic trauma in several animal models (Fuente, 2015; Delano and Elgoyhen, 2016). It is also interesting to note that projections from higher structures, such as the auditory cortex or midbrain, may act on efferent function, as auditory cortex microstimulation modulated the amplitude of cochlear responses such as OAEs (Perrot et al., 2006; Dragicevic et al., 2015). In this light, Mellott et al. (2014) demonstrated the existence of projections from cholinergic cells in the superior olivary complex to the cochlear nucleus in guinea pigs. In the same study, a substantial number of cholinergic cells projecting to the cochlear nucleus was also found in the pedunculopontine tegmental nucleus, which is one of the main extra-dopaminergic structures postulated to be involved in Parkinson’s disease pathophysiology (Pahapill and Lozano, 2000; Stefani et al., 2007). Previous studies documented that pedunculopontine tegmental nucleus receives inhibitory projections from basal ganglia and projects to the pontomedullary reticular structures (Pahapill and Lozano, 2000). It has been suggested that, in Parkinson’s disease, the descending inhibitory output from basal ganglia may be overactive, thus reducing pedunculopontine tegmental nucleus excitation (Takakusaki et al., 2003). Although highly speculative, an involvement of pedunculopontine tegmental nucleus in midbrain circuitries negatively affecting olivocochlear function may play a role in the asymmetry of auditory dysfunction reported in this study.
The consistent correlation between DPOAE measurements and PTA results in our dataset confirms previous studies highlighting DPOAEs as an important diagnostic tool. From a technical standpoint, we demonstrate how advanced acquisition and analysis techniques may overcome some limitations of conventional OAE-based diagnostics, yielding high-quality data also in elderly patients, despite their typically low signal level. Our results suggest asymmetric auditory dysfunction as a possible new Parkinson-specific non-motor biomarker, measurable with high accuracy, thanks to its differential nature, even at the early stages of the disease.
Study limitations
This study suffers from some limitations, as the otolaryngologist performing audiological testing was not blinded to the disease status, but was blinded to which motor side was most affected, except for those cases in which such asymmetry was evident. Also, advanced DPOAE acquisition and analysis techniques are still not widely used in clinical settings, so few facilities may be able to effectively implement this added value. However, studies like this one could help to generate interest for such technical advancements among clinicians, especially as regards the possibility of getting clinically relevant information also in subjects with low signal levels. Alternative explanations to our study findings should also be considered, such as an asymmetry in middle ear muscle reflex, which could be investigated by performing additional audiological tests. It will be helpful for future studies to focus on such issues, to overcome these limitations.
Acknowledgements
We are thankful to prof. A. Magrini for having kindly provided access to the audiometric acoustically insulated booth of the Department of Biomedicine and Prevention of the University of Rome ‘Tor Vergata’, where all audiometric and otoacoustic tests have been performed.
Funding
This study was supported by ‘Istituto Nazionale Assicurazione Infortuni sul Lavoro (INAIL)’ BRiC 2016 grant ID17.
Competing interests
Dr Renata Sisto, Dr Andrea Viziano, Dr Alessandro Stefani, Dr Arturo Moleti, Dr Rocco Cerroni, Dr Claudio Liguori, Dr Elena Garasto and Dr Mariangela Pierantozzi report no conflict of interest.
Glossary
- DPOAE =
distortion-product otoacoustic emissions
- HL =
hearing level
- LEDD =
levodopa equivalent daily dose
- OAE =
otoacoustic emission
- PTA =
pure tone audiometry
- SNR =
signal-to-noise ratio
Contributor Information
Renata Sisto, INAIL Research, Department of Occupational and Environmental Medicine, Epidemiology and Hygiene, 00078 Monte Porzio Catone (Rome), Italy.
Andrea Viziano, Department of Physics, University of Rome ‘Tor Vergata’, 00133 Rome, Italy; Department of Clinical Sciences and Translational Medicine, University of Rome ‘Tor Vergata’, 00133 Rome, Italy.
Alessandro Stefani, Department of Systems Medicine, Parkinson’s Disease Center, University of Rome ‘Tor Vergata’, 00133 Rome, Italy.
Arturo Moleti, Department of Physics, University of Rome ‘Tor Vergata’, 00133 Rome, Italy.
Rocco Cerroni, Department of Systems Medicine, Parkinson’s Disease Center, University of Rome ‘Tor Vergata’, 00133 Rome, Italy.
Claudio Liguori, Department of Systems Medicine, Parkinson’s Disease Center, University of Rome ‘Tor Vergata’, 00133 Rome, Italy.
Elena Garasto, Department of Systems Medicine, Parkinson’s Disease Center, University of Rome ‘Tor Vergata’, 00133 Rome, Italy.
Mariangela Pierantozzi, Department of Systems Medicine, Parkinson’s Disease Center, University of Rome ‘Tor Vergata’, 00133 Rome, Italy.
References
- Barone P, Antonini A, Colosimo C, Marconi R, Morgante L, Avarello TP, et al. The PRIAMO study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord 2009; 24: 1641–9. [DOI] [PubMed] [Google Scholar]
- Berg D, Postuma RB, Adler CH, Bloem BR, Chan P, Dubois B, et al. MDS research criteria for prodromal Parkinson’s disease. Mov Disord 2015; 30: 1600–11. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Pathoanatomy of Parkinson’s disease. J Neurol 2000; 247 (Suppl 2): II3–10. [DOI] [PubMed] [Google Scholar]
- Brown MC. Single-unit labeling of medial olivocochlear neurons: the cochlear frequency map for efferent axons. J Neurophysiol 2014; 111: 2177–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charaziak KK, Shera CA. Compensating for ear-canal acoustics when measuring otoacoustic emissions. J Acoust Soc Am 2017; 141: 515–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri KR, Martinez-Martin P, Brown RG, Sethi K, Stocchi F, Odin P, et al. The metric properties of a novel non-motor symptoms scale for Parkinson’s disease: results from an international pilot study. Mov Disord 2007; 22: 1901–11. [DOI] [PubMed] [Google Scholar]
- Chaudhuri KR, Odin P, Antonini A, Martinez-Martin P. Parkinson’s disease: the non-motor issues. Parkinsonism Relat Disord 2011; 17: 717–23. [DOI] [PubMed] [Google Scholar]
- Chaudhuri KR, Schapira AHV. Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol 2009; 8: 464–74. [DOI] [PubMed] [Google Scholar]
- Delano PH, Elgoyhen AB. Editorial: auditory efferent system: new insights from cortex to cochlea. Front Syst Neurosci 2016; 10: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW, Braak H, Duda JE, Duyckaerts C, Gasser T, Halliday GM, et al. Neuropathological assessment of Parkinson’s disease: refining the diagnostic criteria. Lancet Neurol 2009; 8: 1150–7. [DOI] [PubMed] [Google Scholar]
- Dragicevic CD, Aedo C, León A, Bowen M, Jara N, Terreros G, et al. The olivocochlear reflex strength and cochlear sensitivity are independently modulated by auditory cortex microstimulation. JARO 2015; 16: 223–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitan R, Shamir RR, Linetsky E, Rosenbluh O, Moshel S, Ben-Hur T, et al. Asymmetric right/left encoding of emotions in the human subthalamic nucleus. Front Syst Neurosci 2013; 7: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folmer RL, Vachhani JJ, Theodoroff SM, Ellinger R, Riggins A. Auditory processing abilities of Parkinson’s disease patients. Biomed Res Int 2017; 2017: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradis M, Samet A, Ben-David J, Podoshin L, Sharf B, Wajsbort J, et al. Brainstem auditory evoked potentials to different stimulus rates in parkinsonian patients. Eur Neurol 1988; 28: 181–6. [DOI] [PubMed] [Google Scholar]
- Fuente A. The olivocochlear system and protection from acoustic trauma: a mini literature review. Front Syst Neurosci 2015; 9: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett AR, Robertson D, Sellick PM, Mulders WH. The actions of dopamine receptors in the guinea pig cochlea. Audiol Neurotol 2011; 16: 145–57. [DOI] [PubMed] [Google Scholar]
- Gawel MJ, Das P, Vincent S, Rose FC. Visual and auditory evoked responses in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry 1981; 44: 227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorga MP, Neely ST, Bergman BM, Beauchaine KL, Kaminski JR, Peters J, et al. A comparison of transient-evoked and distortion product otoacoustic emissions in normal-hearing and hearing-impaired subjects. J Acoust Soc Am 1993; 94: 2639–48. [DOI] [PubMed] [Google Scholar]
- Guinan JJ. Olivocochlear efferents: their action, effects, measurement and uses, and the impact of the new conception of cochlear mechanical responses. Hear Res 2018; 362: 38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Santamaria PM, Gendelman HE, Wilson TW. The cortical signature of symptom laterality in Parkinson’s disease. Neuroimage Clin 2017; 14: 433–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Matsubara A, Maruya S-I, Yamamoto Y, Namba A, Sasaki A, et al. Localization of dopamine receptor subtypes in the rat spiral ganglion. Neurosci Lett 2006; 399: 226–9. [DOI] [PubMed] [Google Scholar]
- Lang AE, Lozano AM. Parkinson’s disease. First of two parts. N Engl J Med 1998; 339: 1044–53. [DOI] [PubMed] [Google Scholar]
- Lendvai B, Halmos GB, Polony G, Kapocsi J, Horváth T, Aller M, et al. Chemical neuroprotection in the cochlea: the modulation of dopamine release from lateral olivocochlear efferents. Neurochem Int 2011; 59: 150–8. [DOI] [PubMed] [Google Scholar]
- Maison SF, Liu XP, Eatock RA, Sibley DR, Grandy DK, Liberman MC. Dopaminergic signaling in the cochlea: receptor expression patterns and deletion phenotypes. J Neurosci 2012; 32: 344–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Martin P, Rodriguez-Blazquez C, Abe K, Bhattacharyya KB, Bloem BR, Carod-Artal FJ, et al. International study on the psychometric attributes of the non-motor symptoms scale in Parkinson disease. Neurology 2009; 73: 1584–91. [DOI] [PubMed] [Google Scholar]
- Mellott JG, Bickford ME, Schofield BR. Descending projections from auditory cortex to excitatory and inhibitory cells in the nucleus of the brachium of the inferior colliculus. Front Syst Neurosci 2014; 8: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moleti A, Longo F, Sisto R. Time–frequency domain filtering of evoked otoacoustic emissions. J Acoust Soc Am 2012; 132: 2455–67. [DOI] [PubMed] [Google Scholar]
- Niu X, Canlon B. The signal transduction pathway for the dopamine D1 receptor in the guinea-pig cochlea. Neuroscience 2006; 137: 981–90. [DOI] [PubMed] [Google Scholar]
- Pahapill PA, Lozano AM. The pedunculopontine nucleus and Parkinson’s disease. Brain J Neurol 2000; 123: 1767–83. [DOI] [PubMed] [Google Scholar]
- Perrot X, Ryvlin P, Isnard J, Guénot M, Catenoix H, Fischer C, et al. Evidence for corticofugal modulation of peripheral auditory activity in humans. Cereb Cortex 2006; 16: 941–8. [DOI] [PubMed] [Google Scholar]
- Pisani V, Sisto R, Moleti A, Di Mauro R, Pisani A, Brusa L, et al. An investigation of hearing impairment in de-novo Parkinson’s disease patients: a preliminary study. Parinsonismk Relat Disord 2015; 21: 987–91. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 2015; 30: 1591–601. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Poewe W, Litvan I, Lewis S, Lang AE, Halliday G, et al. Validation of the MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 2018; 33: 1601–8. [DOI] [PubMed] [Google Scholar]
- Probst R, Lonsbury-Martin BL, Martin GK. A review of otoacoustic emissions. J Acoust Soc Am 1991; 89: 2027–67. [DOI] [PubMed] [Google Scholar]
- Ruel J, Nouvian R, D'Aldin CG, Pujol R, Eybalin M, Puel J-L. Dopamine inhibition of auditory nerve activity in the adult mammalian cochlea. Eur J Neurosci 2001; 14: 977–86. [DOI] [PubMed] [Google Scholar]
- Scarpa A, Cassandro C, Vitale C, Ralli M, Policastro A, Barone P, et al. A comparison of auditory and vestibular dysfunction in Parkinson's disease and Multiple System Atrophy. Parkinsonism Relat Disord 2020; 71: 51–7. [DOI] [PubMed] [Google Scholar]
- Shera CA, Guinan JJJ. Evoked otoacoustic emissions arise by two fundamentally different mechanisms: a taxonomy for mammalian OAEs. J Acoust Soc Am 1999; 105: 782–98. [DOI] [PubMed] [Google Scholar]
- Shetty K, Krishnan S, Thulaseedharan JV, Mohan M, Kishore A. Asymptomatic hearing impairment frequently occurs in early-onset Parkinson’s disease. JMD 2019; 12: 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisto R, Chelotti S, Moriconi L, Pellegrini S, Citroni A, Monechi V, et al. Otoacoustic emission sensitivity to low levels of noise-induced hearing loss. J Acoust Soc Am 2007; 122: 387–401. [DOI] [PubMed] [Google Scholar]
- Stefani A, Lozano AM, Peppe A, Stanzione P, Galati S, Tropepi D, et al. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson’s disease. Brain J Neurol 2007; 130: 1596–607. [DOI] [PubMed] [Google Scholar]
- Stirnimann N, N'Diaye K, Jeune FL, Houvenaghel J-F, Robert G, Drapier S, et al. Hemispheric specialization of the basal ganglia during vocal emotion decoding: evidence from asymmetric Parkinson’s disease and (18)FDG PET. Neuropsychologia 2018; 119: 1–11. [DOI] [PubMed] [Google Scholar]
- Takakusaki K, Habaguchi T, Ohtinata-Sugimoto J, Saitoh K, Sakamoto T. Basal ganglia efferents to the brainstem centers controlling postural muscle tone and locomotion: a new concept for understanding motor disorders in basal ganglia dysfunction. Neuroscience 2003; 119: 293–308. [DOI] [PubMed] [Google Scholar]
- Titova N, Qamar MA, Chaudhuri KR. The nonmotor features of Parkinson’s disease. Int Rev Neurobiol 2017; 132: 33–54. [DOI] [PubMed] [Google Scholar]
- Vitale C, Marcelli V, Abate T, Pianese A, Allocca R, Moccia M, et al. Speech discrimination is impaired in parkinsonian patients: expanding the audiologic findings of Parkinson’s disease. Parkinsonism Relat Disord 2016; 22: S138–43. [DOI] [PubMed] [Google Scholar]
- Vitale C, Marcelli V, Allocca R, Santangelo G, Riccardi P, Erro R, et al. Hearing impairment in Parkinson’s disease: expanding the nonmotor phenotype. Mov Disord 2012; 27: 1530–5. [DOI] [PubMed] [Google Scholar]
- Weil RS, Schrag AE, Warren JD, Crutch SJ, Lees AJ, Morris HR. Visual dysfunction in Parkinson’s disease. Brain J Neurol 2016; 139: 2827–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data related to this study are available from Parkinson’s Disease Center, Department of Systems Medicine, University of Rome ‘Tor Vergata’, by written request, in accordance with the data-protection legislation in Europe (General Data Protection Regulation). Persons interested in obtaining access to the data should contact the corresponding Author.