Abstract
Objectives
To describe the demographics and evaluate the clinical outcomes of hypoxic coronavirus disease-2019 (COVID-19) patients treated with different immunomodulatory (IM) drugs in a resource-limited setting.
Materials and methods
We conducted a retrospective cohort study of these patients admitted to our hospital between March 22 and May 31, 2020. Data were abstracted from multiple electronic data sources or patient charts to provide information on patient characteristics, clinical, laboratory variables, and outcomes.
Results
A total of 134 patients met the inclusion criteria and were followed up till June 7, 2020. The median age of the patients was 55.6 years (range 20–89 years) and 68% were men. At least one comorbidity was seen in 72% of the patients with diabetes (44%) and hypertension (46%) being the most common. At triage, fever (82%), shortness of breath (77%), and cough (61%) were the most common presenting symptoms. A PaO2/FiO2 ratio less than 300 was seen in 60%, and 4.5% required invasive mechanical ventilation within 72 hours of hospital admission. Five immunomodulatory agents (hydroxychloroquine, methylprednisolone, colchicine, etoricoxib, and tocilizumab) were administered in different combinations. Overall, in-hospital mortality was 26.9%, and 32% required mechanical ventilation. Around 69% of patients were discharged home. Five variables (SpO2, PaO2/FiO2 ratio, leucocytosis, lymphopenia, and creatinine) on admission were found to be significant in the patients who died.
Conclusion
Our study provides the characteristics and outcomes of hypoxic COVID-19 patients treated with IM drugs in varied combination. Five independent variables were strong predictors of mortality.
How to cite this article
Mahale N, Rajhans P, Godavarthy P, Narasimhan VL, Oak G, Marreddy S, et al. A Retrospective Observational Study of Hypoxic COVID-19 Patients Treated with Immunomodulatory Drugs in a Tertiary Care Hospital. Indian J Crit Care Med 2020;24(11):1020–1027.
Keywords: Acute respiratory distress syndrome, Coronavirus disease-2019, Immunomodulatory drugs, Resource-limited settings
Introduction
India reported the first case of coronavirus disease 2019 (COVID-19) on January 30, 2020.1 The World Health Organization (WHO) declared COVID-19 as pandemic on March 11, 2020, and our hospital admitted the first COVID patient on March 22. Though the exact pathogenesis of severe acute respiratory syndrome-coronavirus-2 (SARS CoV-2) is unknown, various hypotheses have proposed cytokine storm or hyperinflammatory syndrome as probable causes for rapid worsening of the disease. To date, there is no evidence that any potential therapy improves outcomes in patients with COVID-192–4 and various antiviral and immunomodulatory (IM) drugs have been repurposed for the treatment of COVID-19.
Currently, there is no published data about baseline characteristics, clinical features, and outcomes from India. Considering the impact the disease has on the public health especially in a resource-limited country like ours, we conducted a retrospective cohort study of the demographics and outcomes of hypoxic COVID-19 patients admitted to our hospital who were treated with various IM agents.
The pharmacology and rationale for use of these IM agents in COVID-19 are depicted in Table 1.
Table 1.
| Drug class | Clinical experience | Mechanism | Rationale for use in COVID-19 | Dosage | Adverse effects |
|---|---|---|---|---|---|
| Colchicine-alkaloid Antigout agent |
GRECCO-19 trial17 | Exerts anti-inflammatory and IM effects by inhibition of tubulin polymerization causing disruption of cytoskeletal functions and inhibition of nucleotide oligomerization domain (NOD)-like receptors protein 3 (NLRP3) inflammasome assembly | Might diminish or ameliorate the COVID-19 inflammatory storm associated with severe forms of the disease by suppressing proinflammatory cytokines and chemokines 2 | 0.5 mg/day for 1 week | Common: Diarrhea, vomiting |
| Inhibit the synthesis of TNF–alpha, IL-6, monocyte migration, and the secretion of matrix metalloproteinase-9 | Serious: Myelosuppression | ||||
| Methylprednisolone Synthetic corticosteroid |
Fadel et al.15 | Binds to and activates specific nuclear receptors, resulting in altered gene expression and inhibition of proinflammatory cytokine production | With potent anti-inflammatory and antifibrotic properties, low doses of corticosteroids may prevent an extended cytokine response and may accelerate resolution of pulmonary and systemic inflammation in pneumonia | 1 mg/kg in two divided doses for 5 days |
|
| Hydroxychloroquine | Borba et al.24 | Impairs the terminal glycosylation of the angiotensin-converting enzyme 2 (ACE2) receptor, which is the binding site for the envelope spike glycoprotein and has been shown to inhibit endolysosome function | As inhibitors of heme polymerase, they are also believed to have additional antiviral activity via alkalinization of the phagolysosome, which inhibits the pH-dependent steps of viral replication | 400 mg Day 1 | QT interval prolongation |
| Alkylated 4-amino-quinolines | Chorin et al.25 | 200 mg × 5 days | Cardiomyopathy | ||
| Anti-malarial | Hypoglycemia | ||||
| Tocilizumab Humanized monoclonal antibody directed against IL-6 receptor |
Klopfenstein et al.14 Guaraldi et al.15 | Humanized monoclonal antibody targeting both forms of the IL-6 receptor (membrane-bound and soluble) | Blocking the IL-6 pathway might reduce the vigorous inflammatory response reducing the cytokine storm | 8 mg/kg (up to a maximum of 800 mg per dose), with an interval of 12 hours |
|
| Etoricoxib Selective COX-2 inhibitor |
Sander et al.31 | COX-2 inhibition directly responsible for downstream production of PGE2 | Prostaglandin E2 as a modulator of viral Infections | 90 mg OD for 5 days |
|
Materials and Methods
Study Overview
The study was conducted by the Department of Critical Care Medicine in a tertiary care hospital located in Pune, India. The study was approved by the institutional ethics committee (IHR_2020_APR_NM_361) and due to the nature of the retrospective chart review, the need for informed consent from individual patients was waived.
Criteria for Patient Selection
All patients who presented with symptoms of fever, cough, breathlessness, myalgia or fatigue, and travel history were tested as per the government policy7 and admitted to the hospital. Patients who tested positive by RT-PCR for COVID-19, shortness of breath at rest or with exercise (6-minute walk test), and room air saturation less than 94% requiring oxygen less than 4 L/minute were admitted to the monitored isolation ward while those requiring oxygen support more than 4 L/minute were admitted to the intensive care unit (ICU). Five IM drugs, namely tocilizumab,14,15 colchicine,17 hydroxychloroquine,24,25 methylprednisolone,18 and etoricoxib31 were prescribed in various combinations to these hypoxic patients as per the discretion of the treating physician. The patients were grouped into seven mutually exclusive groups for analysis with a maximum of three drugs in each group for ease in collection of data. Ceftriaxone and azithromycin were given to majority of our patients for the first 3–5 days. Patients were discharged from the ICU and hospital based on a predecided government policy.7
Laboratory Confirmation
Confirmation of COVID-19 was done through real-time reverse transcriptase-polymerase chain reaction (RT-PCR) at National Institute of Virology, Pune, on nasopharyngeal and oropharyngeal samples collected by trained staff as per the government policy.
Inclusion Criteria
COVID RT-PCR-positive patients aged more than 18 years who required oxygen therapy within 72 hours of their hospital admission.
Exclusion Criteria
Patients who were already on steroids or immunosuppressant drugs for any other clinical condition.
Imminent death within 24 hours of hospital admission (more than two organ failures on admission).
Data Source
Clinical data pertaining to admitted patients meeting the inclusion criteria were collected between March 22 and May 31, 2020, inclusive of those dates, and the clinical outcomes were monitored till June 7, 2020, the final date of follow-up. Demographics, clinical and laboratory data on admission and the subsequent trends, mode of respiratory support (invasive mechanical ventilation, noninvasive mechanical ventilation, oxygen mask), fraction of inspired oxygen (FiO2), arterial partial pressure of oxygen (PaO2), PaO2/FiO2 ratio, and IM agents administered were collected by a team of two senior registrars from the electronic medical records and were entered into a computerized database. The collected data were analyzed and interpreted by two independent intensivists. The clinical team provided clarification on missing or redundant data.
Data Analysis
Radiological assessment included analysis of chest radiographs and bedside ultrasonography (USG). Laboratory tests were performed as per the clinical needs of the patients at discretion of treating physicians. Electrocardiogram (ECG) and chest radiographs were analyzed by the registrars and confirmed by intensivists. Patients with a baseline QT interval (QTc) more than 500 ms were not administered hydroxychloroquine (HCQ). Data pertaining to coexisting conditions were ascertained from documents/history.
All the authors have checked for the correctness of the data and have reviewed the manuscript and vouch for the correctness, accuracy, and completeness of the data and for the adherence of the study to the protocol submitted.
Study Definitions
The date of disease onset was defined as the day when the symptoms of fever, cough, breathlessness, myalgia, or fatigue were noticed.
Patients were grouped into mild, moderate, and severe acute respiratory distress syndrome (ARDS) based on calculation of PaO2/FiO2 ratios8 (Table 2).
Table 2.
PaO2/FiO2 ratio
| P/F ratio | Category |
|---|---|
| >300 | Normal |
| ≤300 (mild) | Mild |
| ≤200 (moderate) | Moderate |
| ≤100 (severe) | Severe |
QT prolongation was considered if QTc was more than 470 ms in males and 450 ms in females.9 Lymphocytopenia was defined as the absolute lymphocyte count of less than 1000/mm3.10
Study Outcomes
In-hospital mortality, requirement for mechanical ventilation, and discharge or present status of the patients as of June 7, 2020, were recorded separately and also presented on a predefined ordinal scale (Table 3). Length of stay in hospital and ICU were also determined.
Table 3.
Ordinal scale
| 1 | Discharge to home |
| 2 | Hospitalized not requiring oxygen but ongoing care for COVID-related or other medical conditions |
| 3 | Hospitalized requiring oxygen |
| 4 | Hospitalized requiring noninvasive ventilation or high-flow oxygen devices |
| 5 | Hospitalized on invasive mechanical ventilation |
| 6 | Death |
Statistical Analysis
Statistical analysis was carried out with the help of IBM SPSS statistics for Windows, version 23, (IBM Corp., Armonk, NY, USA). The data were analyzed using descriptive statistics. No statistical sample size calculation was performed a priori. Continuous variables were presented as median and interquartile range (IQR), and categorical variables were expressed as counts and percentages. The Chi2 test was used for categorical variables as appropriate. The multivariate regression analysis was carried out to identify independent variables as predictors. All statistical tests were two-tailed, and statistical significance was defined as p < 0.05. No imputation was made for missing data. As our study population was not derived from random selection, the analysis was not adjusted for multiple comparisons and given the possibility of type I error, all statistics are deemed to be descriptive only.
Results
Baseline Characteristics on Admission
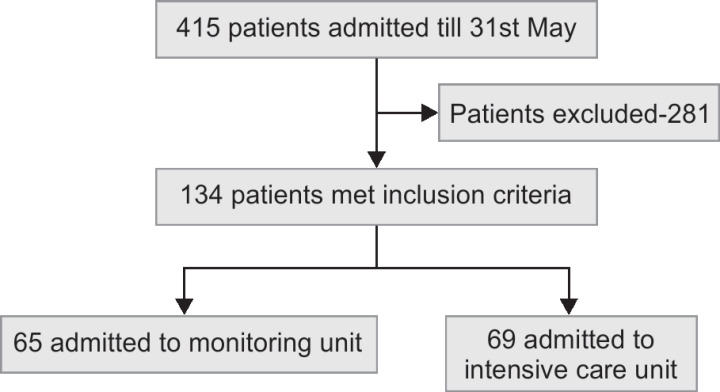
During the study period, a total of 415 confirmed COVID-19 patients were admitted to our hospital. Based on the clinical condition and oxygen requirements, 134 patients who met the inclusion criteria were admitted to monitoring unit or ICU while the rest to the isolation wards.
The median age of the patients in our study was 55.6 years (range 20–89 years) and 68% were men. Fever was the presenting symptom in 82.1% of patients while shortness of breath (77.2%), cough (61.9%), myalgia/fatigue (28.4%), gastrointestinal symptoms (13.4%), and sore throat (7.5%) were the other presenting complaints. Approximately 72% of our study population had at least one comorbidity; diabetes (44%) and hypertensions (46%) were the most common comorbidities observed (Table 4B).
Table 4.
Baseline characteristics on admission
| A. Demographics | ||
|---|---|---|
| Characteristics | N | % |
| Men | 91 | 67.9 |
| Age in years, median | 55.6 years | (Range 20–89 years) |
| B. Coexisting conditions | ||
|---|---|---|
| Comorbidity | N | % |
| Diabetes mellitus | 59 | 44 |
| Hypertension | 62 | 46 |
| Ischemic heart disease | 19 | 19 |
| Obesity | 16 | 16 |
| Chronic obstructive lung disease/interstitial lung disease | 9 | 9 |
| Chronic kidney disease | 4 | 4 |
| Cancer | 1 | 1 |
| C. Laboratory | ||
|---|---|---|
| Parameters | N | Median |
| White blood cell count/mm3 | 132 | 7420 (4945–10,810) |
| Creatinine in mg% | 130 | 0.99 ( 0.78–1.58) |
| Nucleotide oligomerization domain (NOD)-like receptor (NLR) | 134 | 5.93 (3.2–9.8) |
| IL-6 pg/mL | 24 | 62.8 (18.5–100.5) |
| C-reactive protein mg/L | 105 | 118 (56.3–181) |
| Ferritin ng/mL | 57 | 384.4 (135.4–936.4) |
| D-dimer ng/mL | 70 | 1015.3 (524.5–1527.2) |
| D. Chest X-ray (n = 133)* | ||
|---|---|---|
| Finding | N | % |
| Normal | 19 | |
| Zone 1–4 | 104 | 78.2 |
| Zone 1–4, lobar pneumonia | 10 | 7 |
| E. USG Chest (n = 46) | ||
|---|---|---|
| Normal | 5 | 10.9 |
| B lines | 29 | 63 |
| B lines + subpleural consolidation | 11 | 23.9 |
| Subpleural consolidation | 1 | 2.2 |
| F. Proportion of patients with P/F ratios (n = 116) | ||
|---|---|---|
| P/F ratio category | Patients on admission n (%) | Patients with worst P/F ratio n (%) |
| ≤300 (mild) | 42 (31.3) | 30 (22.4) |
| ≤200 (moderate) | 36 (26.9) | 22 (16.4) |
| ≤100 (severe) | 18 (13.4) | 48 (35.8) |
| ≥300 (normal) | 20 (14.9) | 16 (11.9) |
| G. Respiratory assist devices used within 72 hours of admission | ||
|---|---|---|
| Respiratory assist device | n | (%) |
| Nasal prongs | 58 | 43.3 |
| O2 mask | 15 | 11.2 |
| Nonrebreathing mask | 40 | 29.8 |
| High-flow nasal oxygen | 15 | 11.2 |
| Invasive mechanical ventilation | 6 | 4.5 |
Zones on chest X-ray: Zone 1, apical zone-above the clavicle; Zone 2, between the clavicle and cardiac silhouette; Zone 3, midzone: level of hilar structures; Zone 4, bases
Lymphocytopenia was seen in 51.1% and thrombocytopenia in 13.6% of patients. Inflammatory markers interleukin 6, ferritin, and D-dimer were done in selected patients as per the discretion of the treating physician. The number of patients tested and median values are depicted in Table 4C.
About 86% of the chest radiographs were abnormal with 78.2% showing involvement of zones 1–4, while 7.5% had involvement of zones 1–4 and lobar pneumonia. Bedside lung ultrasound reports were available for 46 patients; 63% had B lines and 23.9% had both B lines and subpleural consolidation (Table 4D and E).
About 60% of patients had a PaO2/FiO2 ratio less than 300 on admission. The PaO2/FiO2 ratio ≤150 was seen in 50% of the patients with comorbidities as compared to 31.6% of patients without comorbidities [(p = 0.040, OR 1.2 (95% CI 1.0–1.5)] (Table 4F).
Respiratory devices required by these patients (within 72 hours of admission) have been depicted in the Table 4G.
Immunomodulatory Drugs
Distribution of IM drugs used (Table 5A): The IM drugs were given to patients in various combinations as per the discretion of the treating physicians. Twenty-three patients could not be placed into any mutually exclusive groups. Hydroxychloroquine (n = 119) and low-dose methylprednisolone (n = 116) were given to majority of the patients.
Outcome (Table 5B): Higher percentage of mortality and ventilator requirements were seen in patient group IV (HCQ+ MPS+ tocilizumab) while patients in group V (only HCQ) had the lowest.
IM drugs and hospital stay (Table 5C): The mean ICU and hospital stay was longer for patients in group IV (HCQ + MP + tocilizumab) as compared to other groups.
Interval between symptom onset and initiation of IM drugs (Table 5D): Out of 94 patients who were given IM drugs within 5 days of symptom onset, 63.8% were discharged home and 28.7% died. While 77.5% out of 40 patients who were given IM drugs after 5 days were discharged home and 22.5% died.
Table 5.
Immunomodulatory drugs
| A. Distribution of IM drugs used (n = 134) | |||
|---|---|---|---|
| Group | IM drug group | N | % |
| I | HCQ + MP | 42 | 31.3 |
| II | HCQ + MP + colchicine | 39 | 29.1 |
| III | HCQ + MP + etoricoxib | 4 | 3.0 |
| IV | HCQ + MP + tocilizumab | 5 | 3.7 |
| V | HCQ | 12 | 9.0 |
| VI | MP* | 9 | 6.7 |
| VII | Others** | 23 | 17.2 |
| Total | 134 | 100 | |
| B. Outcome | ||||||
|---|---|---|---|---|---|---|
| Mortality | Requirement of mechanical ventilation | |||||
| Group | Drug | Given | Died | % | Req. vent | % |
| I | HCQ + MP | 42 | 10 | 23.8 | 12 | 28.6 |
| II | HCQ + MP + colchicine | 39 | 11 | 28.2 | 15 | 38.5 |
| III | HCQ + MP + etoricoxib | 4 | 0 | 0 | 0 | 0 |
| IV | HCQ + MP + tocilizumab | 5 | 2 | 40 | 2 | 40 |
| V | HCQ | 12 | 2 | 16.7 | 1 | 8.3 |
| VI | MP* | 9 | 3 | 33.3 | 3 | 33.3 |
| VII | Others** | 23 | 8 | 34.8 | 7 | 30.4 |
| C. Analysis of IM drugs distribution vs mean ICU and hospital stay | |||||
|---|---|---|---|---|---|
| Distribution of IM drugs | Mean ICU stay (n = 65) | Mean hospital stay (n = 134) | |||
| Group | IM drug | n | Mean | n | Mean |
| I | HCQ + MP | 16 | 3.50 | 42 | 10.67 |
| II | HCQ + MP + colchicine | 31 | 6.39 | 39 | 12.77 |
| III | HCQ + MP + etoricoxib | 0 | 0 | 4 | 11.50 |
| IV | HCQ + MP + tocilizumab | 2 | 15.00 | 5 | 15.60 |
| V | HCQ | 4 | 3.25 | 12 | 10.17 |
| VI | MP* | 4 | 2.50 | 9 | 9.00 |
| VII | Others** | 12 | 6.92 | 23 | 14.52 |
| D. Interval between symptom onset to IM drug initiation | ||
|---|---|---|
| Parameter | Interval <5 days (n = 94) | Interval ≥5 days (n = 40) |
| Ordinal scale | ||
| Well and discharged home | 60 (63.8) | 31 (77.5) |
| Hospitalized, not requiring O2 | 2 (2.1) | 0 (0) |
| Hospitalized, requiring NIV or high-flow O2 devices | 1 (1.1) | 0 (0) |
| Hospitalized, requiring invasive mechanical ventilation | 4 (4.3) | 0 (0) |
| Death | 27 (28.7) | 9 (22.5) |
HCQ, hydroxychloroquine; ICU, intensive care unit; MP, methylprednisolone; NIV, noninvasive ventilation
Low dose
Contains drug combinations not present in any of the predefined groups
Mortality Statistics
The mean age of patients who died was 58.8 ± 12.0 years with 61% being men and 80.5% had more than one associated comorbid condition (Flowchart 1).
Flowchart 1.
Patient inclusion and disposition on admission
The proportion of patients with hypertension was significantly higher among the patients who died (Table 6).
Table 6.
Mortality statistics
| Parameter | Died n (%) |
|---|---|
| Coexisting conditions | |
| Diabetes mellitus | 17 (47.2) |
| Hypertension | 23 (63.9) |
| Ischemic heart disease | 6 (16.7) |
| Obesity | 7 (19.4) |
| Respiratory parameters | |
| P/F ratio <200 | 34 (94.4) |
| Invasive ventilation | 36 (100%) |
| Laboratory parameters | |
| Normal lymphocyte count | 12 (33.3) |
| Highest s. creatinine >1.2 mg% | 28 (77.8) |
| Thrombocytopenia | 5 (13.9) |
| Total | 36 (100) |
A multivariate regression analysis was carried out based on the available independent variables from clinical and laboratory data, to identify factors predicting mortality. A model was constructed using significant predictors from both groups together (Table 7).
Table 7.
Significant predictors
| Multivariate regression analysis | |||||
|---|---|---|---|---|---|
| Model | Unstandardized coefficients | Standardized coefficients | t | p value | |
| B | Std. error | Beta | |||
| (Constant) | −0.092 | 0.205 | −0.450 | 0.654 | |
| Highest S. creatinine, mg% | 0.315 | 0.068 | 0.342 | 4.628 | 0.000 |
| SpO2 <80% | 0.278 | 0.080 | 0.269 | 3.455 | 0.001 |
| PF ratio <200 | 0.164 | 0.072 | 0.179 | 2.272 | 0.025 |
| Absolute lymphocyte count <1,000/mm3 | 0.163 | 0.065 | 0.181 | 2.518 | 0.013 |
| WBC more than 12,000/mm3 | 0.195 | 0.087 | 0.171 | 2.247 | 0.027 |
Dependent variable: mortality
It was found that SpO2 <80%, respiratory rate >22/minute.
PaO2/FiO2 <200, white blood cell count >12,000/mm3, absolute lymphocyte count <1000/mm3, and the highest serum creatinine >1.2 mg% were significant predictors.
The overall model fit was R2 = 46.8%.
Outcomes and Adverse Events
None of the 134 patients were lost to follow-up during the study. A primary endpoint (discharge or death) occurred in 95.6% of patients. About 68.7% of patients were discharged home, while 26.9% died and rest were still at hospital undergoing various stages of treatment till the time of analysis of data. The cause of death is depicted in Table 8B. About 50% of patients had hyperglycemia, 20.1% had QTc prolongation, and secondary bacterial infection was seen in 13.4% of patients while 40.3% patients had no known adverse events documented during the course of study (Table 8C).
Table 8.
Outcome data
| A. Outcome ordinal scale (at the time of going to analysis) | ||
|---|---|---|
| Scale order | Ordinal scale description | Patients n (%) |
| 1 | Well and discharged home | 91 (68.7) |
| 2 | Hospitalized, not requiring O2 | 2 (1.5) |
| 3 | Hospitalized, requiring NIV or high-flow O2 devices | 1 (0.7) |
| 4 | Hospitalized, requiring invasive mechanical ventilation | 4 (3) |
| 5 | Death | 36 (26.9) |
| B. Causes of death (n = 36) | |
|---|---|
| Cause of death | n (%) |
| Respiratory | 19 (52.8) |
| Multiorgan failure + sepsis | 15 (41.7) |
| Cardiac | 2 (5.6) |
| 34 (94.4%) died in the intensive care unit | |
| C. Adverse events (n = 134) | |
|---|---|
| Adverse event | n (%) |
| High sugars | 67 (50) |
| QTC prolongation | 27 (20.1) |
| Acute coronary syndrome | 5 (3.7) |
| Secondary bacterial infection | 18 (13.4) |
| Shock | 20 (14.9) |
| None | 54 (40.3) |
Discussion
This retrospective study would be to our knowledge the first study in India and among other resource-limited countries that presents a wide spectrum of descriptive and analytical data of hypoxic COVID-19 patients treated with a varied combination of IM drugs. During the course of our study, we noted that five variables among the clinical and laboratory parameters were found to be significant predictors of mortality. No single IM drug or in combination with others was associated with outcome.
Immunomodulation remains to be the mainstay of treatment based on our previous experiences with SARS and H1N1 pandemics in the absence of any effective direct antiviral drug therapy.11,12 Siddiqi et al.13 proposed a three-stage classification of COVID-19 illness and suggested starting of anti-inflammatory therapies such as steroids from stage IIB when hypoxia develops. Majority of our patients received hydroxychloroquine and methyl prednisolone. Owing to the nonuniformity in groups of IM drugs administered and lack of control groups, only descriptive analysis was possible in our study. However, we noted a longer ICU/hospital stay, higher percentage of mortality, and ventilator requirements in patients receiving a combination of tocilizumab along with HCQ and methyl prednisolone. This is contrary to the results from case control studies by Klopfenstein et al.14 and Guaraldi et al.15 that showed significantly less percentage of mortality in the tocilizumab group. The observations of our study may be explained by the fact that tocilizumab was administered to a very small proportion of patients. On the other hand, the length of the hospital/ICU stay in our patients is comparable to study by Tariq Kewan et al.,16 which showed longer duration of hospital stay in the tocilizumab group.
In a study conducted by Spyridon G. Deftereos et al.,17 no statistically significant outcomes were observed in participants who received colchicine along with HCQ, azithromycin, and tocilizumab.
Two recent studies by Fadel et al.18 and Fernández-Cruz et al.19 reported a beneficial effect on mortality in patients treated with steroids in early phase where the interval between symptom onset to IM drug initiation was a median value of 8 and 10 days, respectively. Most of our patients were initiated with IM drugs before 5 days of onset of illness, which is much earlier when compared to the above studies.
More than half of our patients were admitted to the ICU. About 32% (n = 43) of our patients required mechanical ventilation during the hospital stay. This percentage is higher when compared to the earliest statistics from Wuhan, China20 (16%) and lower when compared to Lombardy, Italy21 (88%) and fairly comparable to other studies from Wuhan, China (30%).22
Considering the fact that 96% of our patients had attained the primary endpoints, the mortality rate in our study was very low, which is similar to most of the statistics from New York,23 Lombardy,21 and Wuhan.20 Our patients were much younger and the proportion of hypertensives was significantly higher in those who died. In-hospital mortality rate of 26.9% and a higher mortality (83.7%) in patients who were mechanically ventilated were comparable to results from different regions of the world. It is difficult to compare mortality rates between studies because the outcomes can be affected by healthcare systems, resources, patient demographics, and prevalence of comorbidities. Mortality rates might be higher in studies conducted over long-term or at a different epidemiological stage.
Among adverse events, our incidence of QTc prolongation (20%) was higher than other studies where HCQ was used. This could be because of our threshold of QT prolongation being >450 ms in males and 470 ms in females as compared to other studies and also the fact that most of our patients were coadministered azithromycin.
A study by Borba et al.24 in patients suffering from SARI COVID-19, looking at high-dose vs. low-dose HCQ therapy found QT prolongation in 18% of high-dose compared to 11% of low-dose group pointing toward dose-related toxicity. Chorin et al.25 showed that in 84 patients treated with hydroxychloroquine and azithromycin, QTc prolonged maximally from baseline in 11% of patients, representing the high-risk group for arrhythmia.
The incidence of hyperglycemia (50%) and clinically significant secondary bacterial infections requiring escalation of antibiotics was comparable with other studies.26
Recent studies27–31 have reported an association between age, high WBC counts, and absolute neutrophil value with low lymphocyte count (neutrophil–lymphocyte ratio) and worse outcomes.
In our study, we found that the following five variables (SpO2 <80%, RR >22/minute, PaO2/FiO2 < 200, WBC >12,000/mm3, ALC <1000/mm3, sr. creatinine > 1.2 mg%) were found to be significant predictors of mortality in our patients. Even though these variables have not been validated as a scoring system, the need of the time is to develop a scoring system based on ubiquitous clinical findings and laboratory biomarkers for early triage and disposition especially in resource-limited settings.
Limitations
Our study has several limitations. First, this was a retrospective observational study with all its inherited biases. Second, the data were collected from electronic medical health record database, thereby precluding detailed information about the patients demographics and baseline medications. Third, the nonuniformity in the distribution of the IM agents, limited investigations being done because of cost constraints, high heterogeneity observed, due to the participants’ inclusion criteria as well as by the studies design making the data redundant for comparative analysis. Fourth, as the follow-up time of our study was relatively short compared to the course of the disease, it could change the outcome variables studied.
Strengths
Our study would be one of its kind comparing groups of IM drugs and their outcomes in resource-limited settings. It also provides a wide spectrum of demographic data of critical COVID-19 patients from our country and their outcomes in a tertiary level hospital in India.
Implications for Future Research
Larger trials with a more robust study design, randomized control trials comparing the different IM agents in regards to important clinical outcome variables. Construction and validation of outcome predictor scores based on easily available clinical and laboratory variables to guide the clinicians for better allocation of the scarce medical resources especially in resource-limited settings.
Conclusion
In our study of critically ill hypoxic COVID-19 patients, five different IM agents were used in varied combinations. The requirement of mechanical ventilation was seen in 32%, in-hospital mortality rate was 26.9%, and a higher mortality of 83.7% among the mechanically ventilated patients. Five variables among the clinical and laboratory parameters were found to be significant predictors of mortality.
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Andrews M, Areekal B, Rajesh K, Krishnan J, Suryakala R, Krishnan B, et al. First confirmed case of COVID-19 infection in India: a case report. Indian J Med Res. 2020;151(5):490–492. doi: 10.4103/ijmr.IJMR_2131_20. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Int Med. 2020;180(7):934. doi: 10.1001/jamainternmed.2020.0994. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respirat Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tufan A, Avanoğlu Güler A, Matucci-Cerinic M. COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turk J Med Sci. 2020;50(SI-1:):620–632. doi: 10.3906/sag-2004-168. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salvi R, Patankar P. Emerging pharmacotherapies for COVID-19. Biomed Pharmacother. 2020;128:110267. doi: 10.1016/j.biopha.2020.110267. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang I, Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J Intensive Care. 2020;8(1):36. doi: 10.1186/s40560-020-00453-4. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alijotas-Reig J, Esteve-Valverde E, Belizna C, et al. Immunomodulatory therapy for the management of severe COVID-19. Beyond the anti-viral therapy: a comprehensive review. Autoimmun Rev. 2020;19(7):102569. doi: 10.1016/j.autrev.2020.102569. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323(16):1574. doi: 10.1001/jama.2020.5394. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.[Internet]. Mohfw.gov.in. 2020. https://www.mohfw.gov.in/pdf/ICMRrevisedtestingstrategyforCOVID.pdf https://www.mohfw.gov.in/pdf/ICMRrevisedtestingstrategyforCOVID.pdf [cited 13 July 2020]. Available from:
- 12.ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. DOI: [DOI] [PubMed] [Google Scholar]
- 13.Siddiqi H, Mehra M. COVID-19 illness in native and immunosuppressed states: a clinical–therapeutic staging proposal. J Heart Lung Transplantat. 2020;39(5):405–407. doi: 10.1016/j.healun.2020.03.012. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klopfenstein T, Zayet S, Lohse A, Balblanc JC, Badie J, Royer PY, et al. Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients. Médecine et Maladies Infectieuses. 2020;(5) doi: 10.1016/j.medmal.2020.05.001. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guaraldi G, Meschiari M, Cozzi-Lepri A, Milic J, Tonelli R, Menozzi M, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. The lancet. Rheumatology. 2020;(8) doi: 10.1016/S2665-9913(20)30173-9. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kewan T, Covut F, Al–Jaghbeer MJ, Rose L, Gopalakrishna KV, Akbik B. Tocilizumab for treatment of patients with severe COVID–19: a retrospective cohort study. E Clin Med. 2020:100418. doi: 10.1016/j.eclinm.2020.100418. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deftereos SG, Giannopoulos G, Vrachatis DA, Siasos GD, Giotaki SG, Gargalianos P, et al. Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019. JAMA Network Open. 2020;3(6):e2013136. doi: 10.1001/jamanetworkopen.2020.13136. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fadel R, Morrison AR, Vahia A, Smith ZR, Chaudhry Z, Bhargava P, et al. Henry Ford COVID-19 Management Task Force, Oxford University Press for the Infectious Diseases Society of America; 2020. [Google Scholar]
- 19.Fernández Cruz A, Ruiz-Antorán B, Muñoz Gómez A, Sancho López A, Mills Sánchez P, Centeno-Soto GA, et al. Impact of glucocorticoid treatment in SARS-CoV-2 infection mortality: a retrospective controlled cohort study. Antimicrob Agents Chemother. 2020;(9) doi: 10.1128/aac.01168-20. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sander W, O'Neill H, Pohl C. Prostaglandin E2 as a modulator of viral infections. Front Physiol. 2017:8. doi: 10.3389/fphys.2017.00089. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vetter V. Clues or miscues? Circulation. 2007;115(20):2595–2598. doi: 10.1161/CIRCULATIONAHA.107.700195. DOI: [DOI] [PubMed] [Google Scholar]
- 22.Felsenstein S, Herbert J, McNamara P, Hedrich C. COVID-19: immunology and treatment options. Clin Immunol. 2020;215:108448. doi: 10.1016/j.clim.2020.108448. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borba MGS, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, Brito M, et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. JAMA Network Open. 2020;3(4):e208857. doi: 10.1001/jamanetworkopen.2020.8857. DOI: [DOI] [PubMed] [Google Scholar]
- 25.Chorin E, Dai M, Shulman E, Wadhwani L, Bar-Cohen R, Barbhaiya C, et al. The QT interval in patients with COVID-19 treated with hydroxychloroquine and azithromycin. Nat Med. 2020;26(6):808–809. doi: 10.1038/s41591-020-0888-2. DOI: [DOI] [PubMed] [Google Scholar]
- 26.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. 2020;323(20):2052. doi: 10.1001/jama.2020.6775. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Aller Clin Immunol. 2020;146(1):110–118. doi: 10.1016/j.jaci.2020.04.006. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang C, Qin L, Li K, Wang Q, Zhao Y, Xu B, et al. A novel scoring system for prediction of disease severity in COVID-19. Front Cell Infect Microbiol. 2020;10:318. doi: 10.3389/fcimb.2020.00318. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gong J, Ou J, Qiu X, Jie Y, Chen Y, Yuan L, et al. A tool for early prediction of severe coronavirus disease 2019 (COVID-19): a multicenter study using the risk nomogram in Wuhan and Guangdong. China Clin Infect Dis. 2020;(15):ciaa443. doi: 10.1093/cid/ciaa443. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L, He W, Yu X, Hu D, Bao M, Liu H, et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020;80(6):639–645. doi: 10.1016/j.jinf.2020.03.01. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang A, Liu J, Tao W, Li H. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol. 2020;84:106504. doi: 10.1016/j.intimp.2020.106504. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]