Abstract
A series of hitherto unknown 3′-α-[1,2,3]-substituted triazolo-2′,3′-dideoxypyrimidine nucleoside analogues of the anti-HIV 3′-azido-3′-deoxythymidine (AZT) were synthesized through catalyzed alkyne-azide 1,3-dipolar cycloaddition (Huisgen reaction). Those 3′-[1,2,3]-triazolo analogues bearing an azido alkyl chain were evaluated for their anti-HIV activity against HIV-1 in primary human lymphocytes as well as for their cytotoxicity in different cells. None of them inhibit HIV replication (EC50 > 20 μM); two of them were converted to their triphosphate form to evaluate their HIV-RT inhibition.
Keywords: 3′-triazolo nucleosides, CuAAC reaction, “click” chemistry, antiviral activity
Pharmacomodulation has become central to drug discovery and has played a major role in the research of new treatments for viral infectious diseases. However, the discovery and process optimization of potential agents is often slow, expensive and often involves complex synthesis. The “click chemistry” proposed by Sharpless et al.[1] has emerged as a fast and efficient approach to simplify compound synthesis. The Huisgen 1,3-dipolar cycloaddition of azides and terminal alkynes is one of the best known and powerful click reactions.[2] This highly specific, irreversible, and chemo-selective reaction is also termed the copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC), which offers exclusively the 1,4-isomer if catalyzed by copper (I).[3] Recently, several research teams have also explored some experiments that afford the selective 1,5-regioisomer 1,2,3-triazol via the use of azides and 1-bromomagnesium acetylenes[4] or 1-trimethylsilyl acetylenes[5] and palladium[6] or ruthenium[7] as catalyst. The use of 1,3-dipolar cycloaddition has been recently reviewed by Amblard et al.[8] to enhance the discovery of new nucleoside analogues. Several teams have reported the synthesis and HIV evaluation of 3′-heterocyclic[9] 9 and 3′-C-branched-azido-chain[10] 10 substituted 3′-deoxythimidines, with no antiviral activity. Thus, as part of our drug discovery program on nucleosides containing a 1,2,3-triazolo moiety,[11] we report herein a full account of a new generation of 3′-substituted-2′,3′-dideoxy-nucleoside analogues bearing an azido-alkyl-chain functionalized-[1,2,3]-triazolo moiety at the 3′-position (Figure 1) through a CuAAC or trimethylsilyl-directed cyclo-addition reactions.
FIGURE 1.
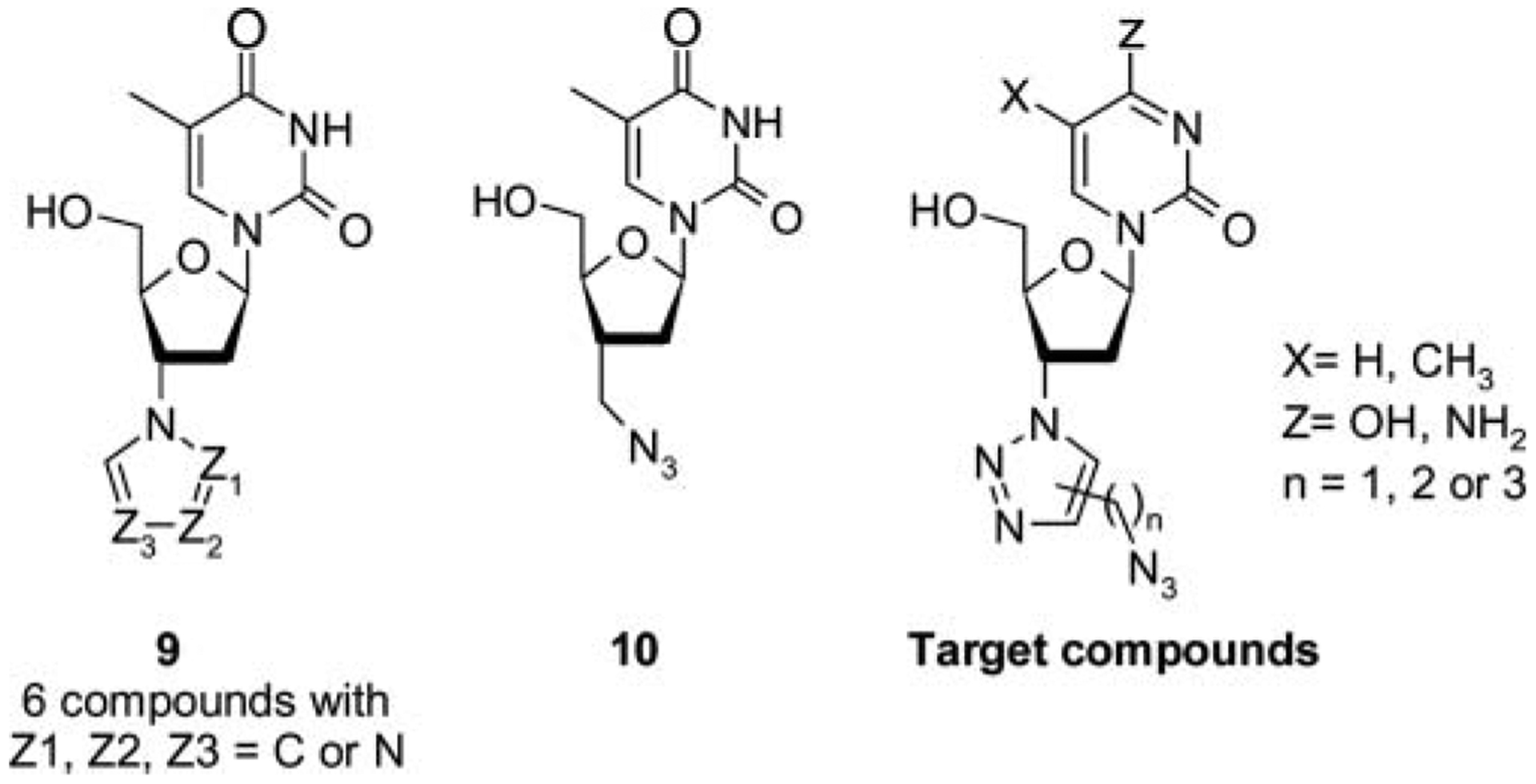
Some 3′-triazolo- or 3′-azidomethyl nucleosides and target compounds.
In this work, we tried to study the influence of the length of the azido-alkyl-chain functionalized heterocycle ring and its effect on the regioselectivity (1,4 and 1,5). All synthesized compounds were evaluated for their anti-HIV activity and their cytotoxicities.
The synthesis of 3′-substituted deoxynucleosides substituted at the 3′-position with an functionalized 1,2,3-triazol, is summarized in Scheme 1. Starting from the known 5′-benzoyl-AZT[12] or 5′-silylated-3′-azido-2′,3′-dideoxyuridine[13] and commercial alkynes, the 1,3-dipolar cyclo-addition was catalyzed by sodium ascorbate/CuSO4[14] in a 1/1 mixture water/tBuOH at room temperature, to afford the regioselectivity (1,4) 2 and 2′a,b,c with no contamination by the 1,5-regioisomer. Reaction of 1′ with N,O-bis(trimethylsilyl) acetamide in toluene was stirred at room temperature, then addition of 3-(trimethylsilyl)propargyl alcohol (110°C, 16 hours) gave the 1,4,5-trisubstituted-[1,2,3]-triazole. Cleavage of trimethylsilyl (TMS) group via with aqueous HF (48%) for 3 hours, provide the 1,5-substituted triazole 7′a in 26% yield. The regioselectivity of the ligation leading to 1,5 or 1,4-disubstitued-[1,2,3]-triazole moiety was confirmed by 1H, 13C NMR long range correlation spectra (HMBC). Mesylation of free hydroxyl group followed by treatment with NaN3 afforded azide derivatives 3, 3′a,b,c and 7′a, respectively.
SCHEME 1.
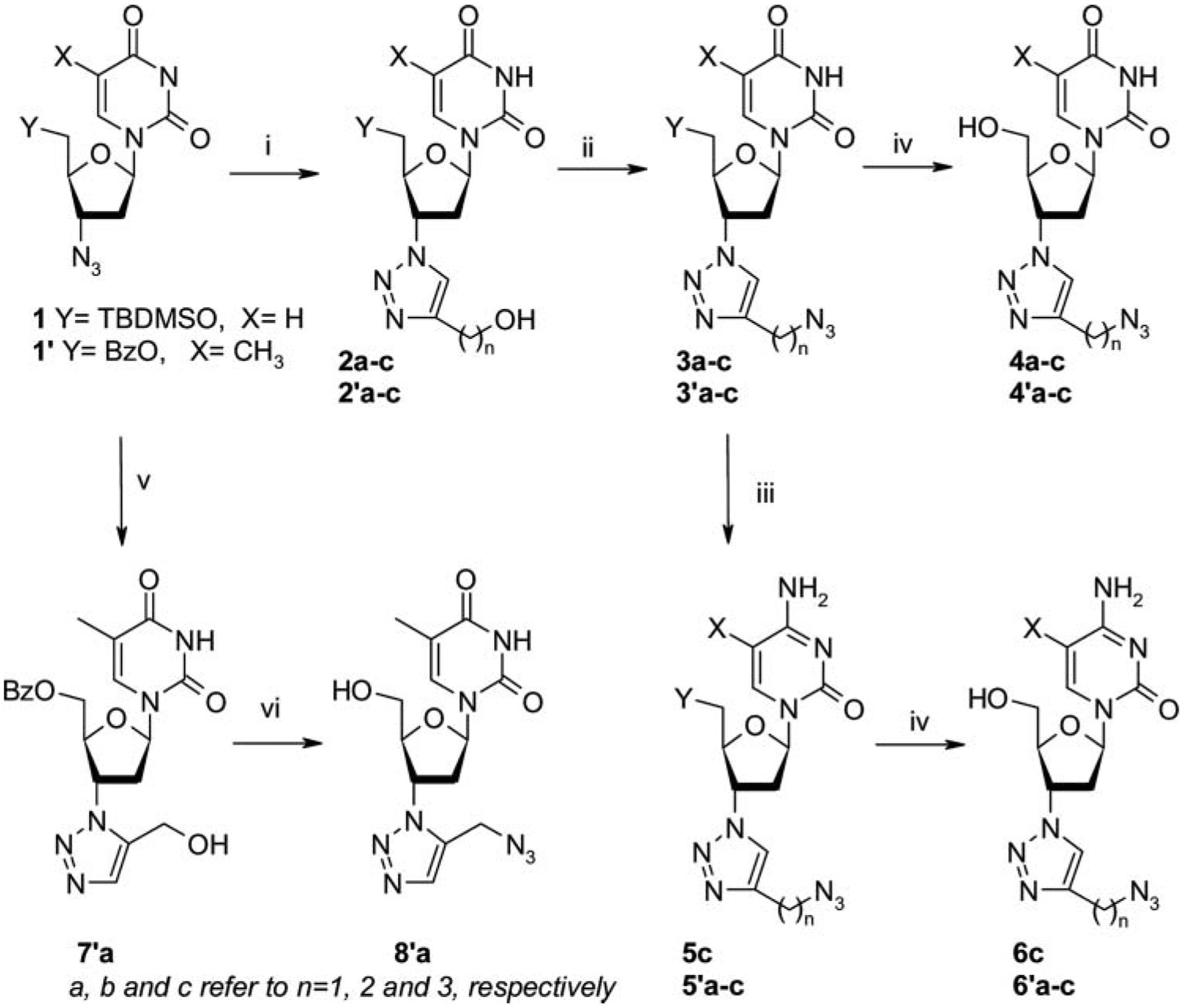
Reagents and conditions: (i) sodium ascorbate, CuSO4, alkyne, tert-Butanol/H2O; (ii) a) MsCl, Et3N, CH2Cl2, 0°C to room temperature, b) NaN3, DMF; (iii) a) 2,4,6-Triisopropylbenzenesulfonyl chloride, DMAP, Et3N, CH2Cl2, b) NH4OH; (iv) NH3/MeOH, 3°C for benzoyl group; TBAF/THF for silyl group; (v) a) BSA, 3-(trimethylsilyl)propargyl alcohol, toluene, 110°C, b) 48% HF(aq), 26% for two steps; (vi) a) MsCl, pyridine, room temperature, 2 hours, b) NaN3, DMF, 85°C, 3 hours, c) NH3/CH3OH, 49%.
Several methods have been reported for the conversion of uracil to cytosine analogues[15] and are based on (1) a first activation of the 4-carbonyl moiety either (mainly by heating with P2S5,[15a] by tris-(1,2,4-triazolyl)phosphate,[15b] as o-nitrophenol derivative,[15c] with SOCl2,[15d] or 2,4,6-triisopropylbenzensulfonic chloride[15e]) then (2) a subsequent treatment with ammonia, primary or secondary amine to give the desired N4-modified cytosine analogues. Thus, uridines 4c and 4′a-c, were reacted with 2,4,6-triisopropylbenzenesulfonyl chloride, Et3N, and DMAP in CH2Cl2, then treated by a solution of NH4OH. The desired cytidines 5c and 5-methylcytidines 5′a-c were isolated in good yields.
The standard deprotection of benzoyl group with NH3/MeOH and silyl group with a solution of TBAF/THF, led to the free 3′-substituted-2′,3′-dideoxynucleoside analogues[16–20] 4a, 4′a, 6c, 6′c, and 8′a.
All final compounds were screened against HIV-1 for their biological activity. The antiviral[21] assay was performed in peripheral blood mononuclear (PBM) cells and compared to AZT activity. None of the synthesized compounds showed significant activities and displayed toxic effects on un-infected PHA-stimulated primary human peripheral blood mononuclear (PBM), CEM (T-lymphoblastoid cell line), or Vero (African green monkey kidney) cells.[22]
In order to measure the inhibitory activity of those compounds on HIV-1 RT DNA polymerase, compounds 4′a and 8′a were converted to their corresponding triphosphates.[23] The HIV-1 RT inhibition mediated DNA polymerization on a DNA/DNA T/P is reported in Figure 2. In this experiment, 3′-azido-3′-deoxy-thymidine triphosphate (AZT-TP) was included as a control.[24,25] Unlike AZT-TP, no 3′-triazole-thymidine triphosphate analogues were incorporated into the nascent DNA chain by wildtype (WT) HIV-1 RT up to 50 μM. Accordingly, chain-termination and inhibition of HIV-1 RT were not observed. Those analogues do not inhibit the HIV-1 RT. The large 3′-triazole group may prevent proper positioning in the HIV-1 RT nucleotide binding site, thus preventing efficient incorporation.
FIGURE 2.
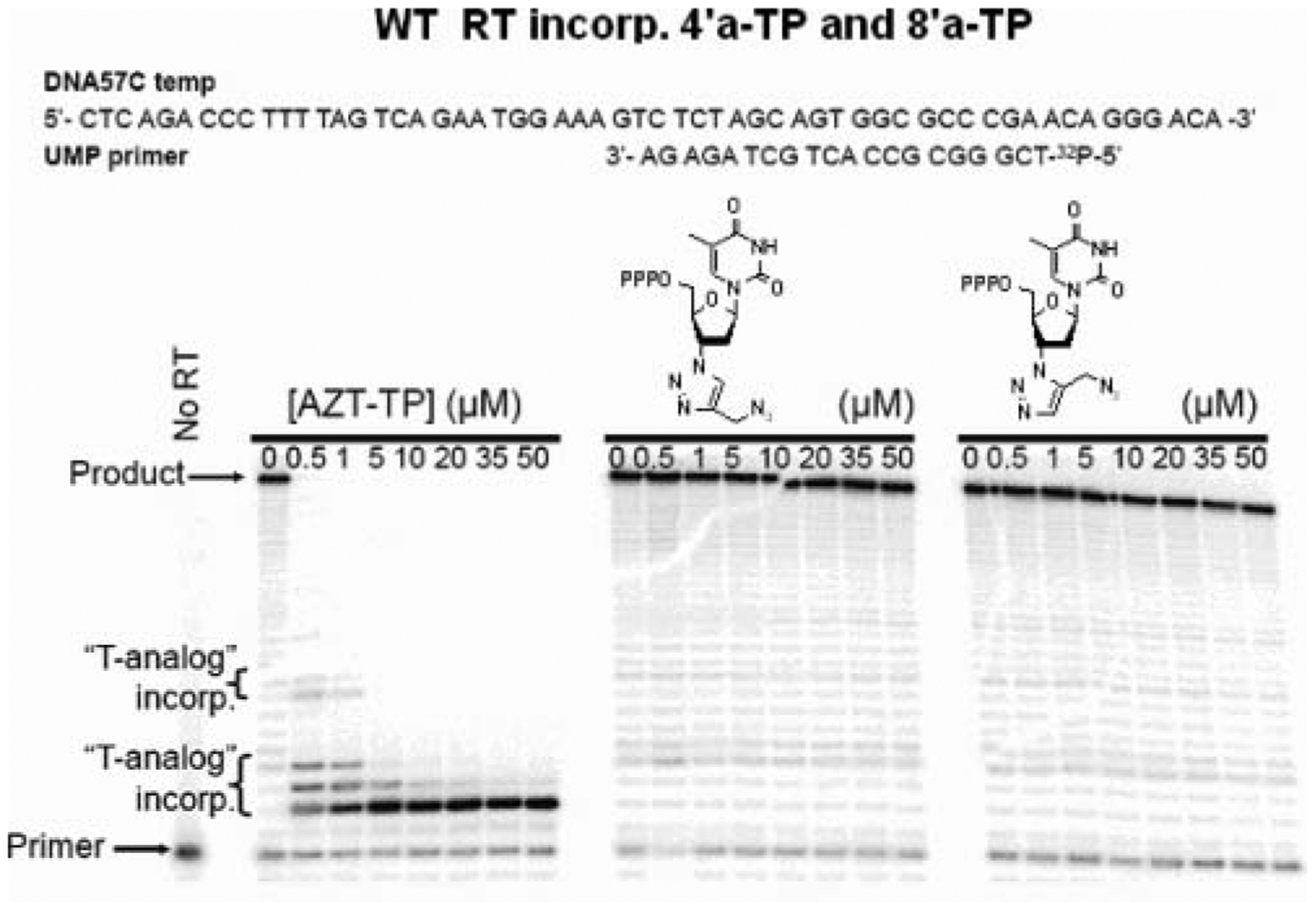
HIV-1 RT inhibition mediated DNA polymerization on a DNA/DNA T/P.
In conclusion, we have synthesized several hitherto unknown 3′-[1,2,3]-triazolo substituted-2′,3′-dideoxy-nucleosides via catalyzed regioselective azide-alkyne 1,3-dipolar Huisgen’s cycloaddition. Two compounds were converted to their triphosphate analogues and the HIV-1 RT inhibition mediated DNA polymerization on a DNA/DNA T/P realized. The synthesized compounds were evaluated in human PBM cells infected by HIV-1 and none of them showed significant activities.
Acknowledgments
This work was supported in part by NIH grants 5R37-AI-041980, 4R37-AI-025899, 5P30-AI-50409 (CFAR), and the Department of Veterans Affairs (to R. F. S.).
REFERENCES AND NOTES
- 1.a) Kolb HC; Sharpless KB The growing impact of click chemistry on drug discovery. Drug Discovery Today 2003, 8, 1128–1137 [DOI] [PubMed] [Google Scholar]; b) Kolb HC; Finn MG; Sharpless KB Click chemistry: diverse chemical function from a few good reaction. Angew. Chem. Int. Ed 2001, 40, 2004–2021. [DOI] [PubMed] [Google Scholar]
- 2.a) Huisgen R Kinetik Und Mechanismus 1.3-Dipolarer Cycloadditionen. Angew. Chem. Int. Ed, 1963, 75, 742–754 [Google Scholar]; b) Huisgen R Survey, mechanism In 1,3-Dipolar Cycloaddition Chemistry; Padwa A, Ed.; Wiley: New York, 1984, pp 1–176. [Google Scholar]
- 3.Wu P; Feldman AK; Nugent AK; Hawker CJ; Scheel A; Voit B; Pyun J; Frechet JM; Sharpless KB; Fokin VV Efficiency and fidelity in a click-chemistry route to triazole dendrimers by the copper(I)-catalyzed ligation of azides and alkynes. Angew. Chem., Int. Ed 2004, 43, 3928–3932. [DOI] [PubMed] [Google Scholar]
- 4.Krasinski A; Fokin VV; Sharpless KB Direct synthesis of 1,5-disubstituted-4-magnesio-1,2,3-triazoles, revisited. Org. Lett 2004, 6, 1237–1240. [DOI] [PubMed] [Google Scholar]
- 5.a) Coats SJ; Link JS; Gauthier D; Hlasta DJ Trimethylsilyl-directed 1,3-dipolar cycloaddition reactions in the solid-phase synthesis of 1,2,3-triazoles. Org. Lett 2005, 7, 1469–1472 [DOI] [PubMed] [Google Scholar]; b) Hlasta DJ; Ackerman JH Steric effects on the regioselectivity of an azide-alkyne dipolar cycloaddition reaction: the synthesis of human leukocyte elastase inhibitors. J. Org. Chem 1994, 59, 6184–6189. [Google Scholar]
- 6.Chuprakov S; Chernyack N; Dudnick AS; Gevorgyan V Direct Pd-catalyzed arylation of 1,2,3-triazoles. Org. Lett 2007, 9, 2333–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L; Chen X; Xue P; Sun HHY; Williams ID; Sharpless KB; Fokin VV; Jia G Ruthenium-catalyzed cycloaddition of alkynes and organic azides. J. Am. Chem. Soc 2005, 127, 15998–15999. [DOI] [PubMed] [Google Scholar]
- 8.Amblard F; Cho JH; Schinazi RF Cu(I)-catalyzed Huisgen azide–alkyne 1,3-dipolar cycloaddition reaction in nucleoside, nucleotide, and oligonucleotide chemistry. Chem. Rev 2009, 109, 4207–4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wingerinck P; Van Aerschot A; Janssen G; Claes P; Balzarini Y; De Clercq E; Herdewijn P Synthesis and antiviral activity of 3′-heterocyclic substituted 3′-deoxythymidines. J. Med. Chem 1990, 33, 868–873. [DOI] [PubMed] [Google Scholar]
- 10.a) Svansson L; Kvarnstroem I; Classon B; Samuelsson B Synthesis of Some 2′,3′-Dideoxy-3′-C-Fluoromethyl and 3′-C-Azidomethyl Nucleosides. Nucleosides Nucleotides Nucleic Acids 1992, 11, 1353–1366 [Google Scholar]; b) Lin T-S; Zhu J-L; Dutschman GE; Cheng Y-C; Prusoff WH Syntheses and biological evaluations of 3′-deoxy-3′-C-branched-chain-substituted nucleosides. J. Med. Chem 1993, 36, 353–362. [DOI] [PubMed] [Google Scholar]
- 11.a) Broggi J; Díez-González S; Petersen JL; Berteina-Raboin S; Nolan S; Agrofoglio LA Study of copper(I) catalysts for the synthesis of carbanucleosides via azide-alkyne 1,3-dipolar cycloaddition. Synthesis 2008, 141–148 [Google Scholar]; b) Pradère U; Roy V; McBrayer TR; Schinazi RF; Agrofoglio LA Preparation of ribavirin analogues by copper- and ruthenium-catalyzed azide-alkyne 1,3-dipolar cycloaddition. Tetrahedron 2008, 64, 9044–9051 [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Broggi J; Joubert N; Díez-González S; Berteina-Raboin S; Zevaco T; Nolan SP; Agrofoglio LA Synthesis of (±)-1,2,3-triazolo-3′-deoxy-4′-hydroxymethyl carbanucleosides via ‘click’ cycloaddition. Tetrahedron 2009, 65, 1162–1170 [Google Scholar]; d) Broggi J; Kumamoto H; Berteina-Raboin S; Nolan SP; Agrofoglio LA Click azide-alkyne cycloaddition for the synthesis of D-(−)-1,4-disubstituted triazolo-carbanucleosides. Eur. J. Org. Chem 2009, 12, 1880–1888. [Google Scholar]
- 12.CAS Registry Number 10606-78-0.
- 13.CAS Registry Number 120625-01-6.
- 14.Rostovtsev VV; Green LG; Fokin VV; Sharpless KBA A stepwise Huisgen Cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem., Int. Ed 2002, 41, 2596–2599. [DOI] [PubMed] [Google Scholar]
- 15.a) Fox JJ; van Praag D; Wempen I; Doerc IL; Cheong L; Knoll JE; Edidinoff ML; Bendick A; Brown GB Thiation of nucleosides. II. Synthesis of 5-methyl-2′-deoxycytidine and related pyrimidine nucleosides. J. Am. Chem. Soc 1959, 81, 178–187 [Google Scholar]; b) Sung WL Synthesis of 4-triazolopyrimidinone nucleotide and its application in synthesis of 5-methylcytosine-containing oligodeoxyribonucleotides. Nucleic Acids Res. 1981, 6, 6139–6151 [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Nyilas A; Chattopadhyaya J Synthesis of O2′-methyluridine, O2′-methylcytidine, N4,O2′-dimethylcytidine and N4,N4,O2′-trimethylcytidine from a common intermediate. Acta Chem. Scand. B 1986, 40, 826–830 [DOI] [PubMed] [Google Scholar]; d) Lin T-S; Mancini WR Synthesis and antineoplastic activity of 3′-azido and 3′-amino analogues of pyrimidine deoxyribonucleoside. J. Med. Chem 1983, 26, 544–548 [DOI] [PubMed] [Google Scholar]; e) Matsuda AY; Sasaki T; Ueda T Nucleosides and nucleotides. 95. Improved synthesis of 1-(2-azido-2-deoxy-.beta.-D-arabinofuranosyl)cytosine (Cytarazid) and -thymine. Inhibitory spectrum of Cytarazid on the growth of various human tumor cells in vitro. J. Med. Chem 1991, 34, 999–1002. [DOI] [PubMed] [Google Scholar]
- 16.Selected data for compound 4a: 1H NMR (400 MHz, CD3OD): 8.17 (s, 1H), 8.07 (d, J = 8.1 Hz, 1H), 6.47 (t, J = 6.4 Hz, 1H), 5.73 (d, J = 8.1 Hz, 1H), 5.43 (dt, J = 5.6 and 8.5 Hz, 1H), 4.49 (s, 2H), 4.39 (dt, J = 3.2 and 5.6 Hz, 1H), 3.89 (dd, J = 3.0 and 12.2 Hz, 1H), 3.77 (dd, J = 3.2 and 12.2 Hz, 1H), 2.95 (m, 1H), 2.76 (ddd, J = 6.0, 8.4 and 14.4 Hz, 1H). 13C NMR (400 MHz, CD3OD): 166.24, 152.18, 144.15, 142.65, 124.72, 102.82, 87.19, 86.62, 62.19, 61.26, 46.09, 39.26.
- 17.Selected data: compound 4′a: 1H NMR (400 MHz, CD3OD): 8.15 (s, 1H), 7.89 (d, J = 1.2 Hz, 1H), 6.45 (t, J = 6.4 Hz, 1H), 5.40 (m, 1H), 4.47 (s, 2H), 4.33 (m, 1H), 3.86 (dd, J = 3.2 and 12.4 Hz, 1H), 3.73 (dd, J = 3.6 and 12.4 Hz, 1H), 2.86 (m, 1H), 2.69 (m, 1H), 1.88 (d, J = 1.2 Hz, 3H). 13C NMR (400 MHz, CD3OD): 165.21, 151.10, 142.87, 137.06, 123.49, 110.48, 85.48, 85.16, 60.91, 59.95, 44.82, 37.82, 11.29.
- 18.Selected data for compound 6c: 1H NMR (400 MHz, CD3OD): 8.09 (d, J = 7.2 Hz, 1H), 7.93 (s, 1H), 6.41 (t, J = 6.4 Hz, 1H), 5.93 (d, J = 7.2 Hz, 1H), 5.35 (dt, J = 5.6 and 8.4 Hz, 1H), 4.39 (dt, J = 3.2 and 6.0 Hz, 1H), 3.89 (dd, J = 3.2 and 12.4 Hz, 1H), 3.76 (dd, J = 3.2 and 12.4 Hz, 1H), 3.37 (t, J = 6.8 Hz, 2H), 2.97 (m, 1H), 2.80 (t, J = 7.6 Hz, 2H), 2.65 (ddd, J = 5.6, 8.4 and 14.0 Hz, 1H), 1.95 (quin, J = 6.8 Hz, 2H). 13C NMR (400 MHz, CD3OD): 167.78, 158.20, 148.34, 142.82, 123.07, 96.12, 87.97, 86.55, 62.08, 60.78, 51.73, 39.90, 29.67, 23.48.
- 19.Selected data for compound 6′c: 1H NMR (400 MHz, CD3OD): 7.95 (d, J = 0.8 Hz, 1H), 7.93 (s, 1H), 6.43 (t, J = 6.0 Hz, 1H), 5.36 (dt, J = 6.0 and 8.8 Hz, 1H), 4.38 (dt, J = 3.2 and 6.0 Hz, 1H), 3.92 (dd, J = 2.8 and 12.4 Hz, 1H), 3.76 (dd, J = 3.2 and 12.4 Hz, 1H), 3.36 (t, J = 6.8 Hz, 2H), 2.95 (m, 1H), 2.80 (t, J = 7.2 Hz, 2H), 2.65 (ddd, J = 6.0, 8.8 and 14.0 Hz, 1H), 1.99 (d, J = 0.8 Hz, 3H), 1.94 (quin, J = 7.2 Hz, 2H). 13C NMR (400 MHz, CD3OD): 167.43, 158.26, 148.32, 140.21, 123.07, 104.46, 87.67, 86.46, 62.46, 60.69, 51.73, 39.82, 29.68, 23.49, 13.40.
- 20.Selected data for compound 8′a: 1H NMR (400 MHz, CD3OD): 7.90 (d, J = 1.2 Hz, 1H), 7.78 (s, 1H), 6.58 (t, J = 6.8 Hz, 1H), 5.30 (m, 1H), 4.66 (d, J = 3.6 Hz, 2H), 4.36 (q, J = 2.8 Hz, 1H), 3.85 (dd, J = 3.2 and 11.8 Hz, 1H), 3.74 (dd, J = 3.2 and 12.2 Hz, 1H), 2.79 (m, 1H), 2.66 (m, 1H), 1.90 (d, J = 1.2 Hz, 3H). 13C NMR (400 MHz, CD3OD): 184.63, 151.18, 137.05, 133.40, 133.10, 110.54, 85.97, 85.47, 61.32, 58.38, 41.81, 38.11, 11.29.
- 21.Schinazi RF; Sommadossi JP; Saalman V; Cannon DL; Xie M-W; Hart GC; Hahn EF Activities of 3′-azido-3′-deoxythymidine nucleotide dimers in primary lymphocytes infected with human immunodeficiency virus type 1. Antimicrob. Agents Chemother 1990, 34, 1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stuyver LJ; Lostia S; Adams M; Mathew J; Pai BS; Grier J; Tharnish PM; Choi Y; Chong Y; Choo H; Chu CK; Otto MJ; Schinazi RF Antiviral activities and cellular toxicities of modified 2′,3′-dideoxy-2′,3′-didehydrocytidine analogues. Antimicrob. Agents Chemother 2002, 46, 3854–3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burgess K; Cook D Syntheses of nucleoside triphosphates. Chem. Rev 2000, 100, 2047–2060. [DOI] [PubMed] [Google Scholar]
- 24.a) Sluis-Cremer N; Sheen C-W; Zelina S; Argoti Torres PS; Parikh UM; Mellors JW Molecular mechanism by which the K70E mutation in human immunodeficiency virus type 1 reverse transcriptase confers resistance to nucleoside reverse transcriptase inhibitors. Antimicrob. Agents Chemother 2007, 51, 48–53 [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Parikh UM; Zelina S; Sluis-Cremer N; Mellors JW Molecular mechanisms of bidirectional antagonism between K65R and thymidine analog mutations in HIV-1 reverse transcriptase. AIDS 2007, 21, 1405–1414 [DOI] [PubMed] [Google Scholar]; c) Sluis-Cremer N; Arion D; Parikh U; Koontz D; Schinazi RF; Mellors JW; Parniak MA Enzyme catalysis and regulation. J. Biol. Chem 2005, 280, 29047–29052. [DOI] [PubMed] [Google Scholar]
- 25. Wildtype (WT) HIV-1 (LAI) reverse transcriptase (RT) was purified as described previously. The protein concentration of the purified enzyme was determined spectrophotometrically at 280 nm using an extinction coefficient (ϵ280) of 260450 M−1 cm−1, and by Bradford protein assays (Sigma-Aldrich, St. Louis, MO, USA). AZT-TP was purchased from TriLink Biotechnologies, Inc (San Diego, CA, USA), dNTPs were acquired from GE Healthcare (Piscataway, NJ, USA), and [γ-32P] ATP was obtained from PerkinElmer Life Sciences (Boston, MA, USA). DNA oligonucleotides were synthesized by IDT (Coralville, IA, USA). The ability of 3′-triazole thymidine analogues to inhibit HIV-1 RT DNA synthesis was evaluated using a DNA/DNA template/primer (T/Ps). The sequences of the T/P substrate are provided in Figure 2, and the DNA primers were 5′-radiolabeled with [γ-32P]ATP as described previously.[3] Briefly, reactions were carried out in 50 mM Tris (pH 7.5), 50 mM KCl, 10 mM MgCl2 containing 20 nM T/P, 0.5 μM each dNTP and various concentrations of AZT-TP, RS-467a-TP, or RS-689-TP (0–50 μM). Reactions were initiated by the addition of 200 nM WT RT, incubated for 10 minutes at 37°C and then quenched using 20 μL of gel loading buffer (98% deionized formamide containing 1 mg/mL each of bromophenol blue and xylene cyanol). Samples were then denatured at 95°C for 10 minutes and polymerization products were separated from substrates by denaturing gel electrophoresis using 14% acrylamide containing 7 M urea. Gels were analyzed using phosphorimaging with a GS525 Molecular Imager and Quantity One Software (Biorad Laboratories, Inc., Hercules, CA, USA).


