Abstract
Toll-like receptors (TLRs) play an important role in the innate immune system, and recently, they have been shown to be involved in the regulation of blood pressure. The incidence of hypertension is higher in men, and it increases in postmenopausal women. In fact, premenopausal women are protected from cardiovascular disease compared with age-matched men, and it is well established that this protective effect is lost with menopause. However, the molecular mechanisms underlying this protection in women are unknown. Whether or not it could be related to differential activation of the innate immune system remains to be elucidated. This review focuses on (1) the differences between men and women in TLR activation and (2) whether TLR activation may influence the regulation of blood pressure in a sex-dependent manner.
Keywords: TLR, sex difference, blood pressure, hypertension
INTRODUCTION
Toll-like receptors (TLRs) participate in innate immunity by recognizing pathogen-associated molecular patterns and endogenous damage-associated molecular patterns (DAMPs). Antigen-presenting cells, such as monocytes/macrophages, dendritic cells (DCs), and B cells, are the cell populations with the highest expression of TLRs to recognize ligands in the periphery.1 Eleven types of TLRs (TLR1–TLR11) have been identified in humans. TLRs can be either localized on the cell surface or alternatively, some of the TLRs exist in intracellular compartments, localized in the endosome, lysosome, endolysosome, and endoplasmic reticulum (ER).2
The TLRs expressed in the cell surface display 2 major domains: an intracellular portion that is called the Toll/IL-1 receptor (TIR) domain and an extracellular portion that contains leucine-rich repeats. After ligand-induced dimerization of the ectodomains of the TIR, a signal transduction pathway is initiated. Furthermore, the TIR domain of TLRs recruits downstream adaptor molecules, including MyD88 (myeloid differentiation primary response gene 88), TIRAP/Mal (TIR-domain–containing adaptor/MyD88 adaptor–like), TICAM1/TRIF (TIR-domain–containing adaptor molecule 1/TIR-domain–containing adaptor-inducing interferon β), and TRAM (TRIF-related adaptor molecule).3 After the recruitment of distinct adaptor molecules, downstream signaling can be separated into 2 different pathways: MyD88-dependent and MyD88-independent signaling pathways or the so-called TRIF-dependent pathway. MyD88 is bound by all TLRs, except TLR3. Each signaling pathway results in the activation of inflammatory gene transcription factors [such as interferon-regulatory factors (IRFs), nuclear factor кB (NF-кB), and activator protein 1], thereby initiating an inflammatory response.2,3
Intracellular TLRs (TLR3, TLR7, and TLR9) are activated only after acidification of endolysosomal compartments. In fact, receptors must traffic from the ER through the Golgi apparatus to take up residence in the endolysosomes before stimulation. The release of TLRs from the ER involves the posttranslational modification N-glycosylation, Ca+2 stores, PRAT4A (also known as Cnpy3), and UNC93B1 (mediates the translocation of intracellular TLRs from the ER to the endolysosomes where they encounter and respond to their respective ligands). Furthermore, TLR7 and TLR9 are cleaved at endosomal compartments, which is essential for interaction with MyD88 and subsequent signaling. Cleavage of TLR3 has not been observed.4–9 See review10 for more details.
Hypertension is an important risk factor for cardiovascular disease (CVD) mortality. Between 2011 and 2014, the prevalence of hypertension among US adults was 45.6% (95% confidence interval, 43.6%–47.6%) using the new blood pressure (BP) thresholds from the 2017 American College of Cardiology/AHA guidelines versus 31.9% (95% confidence interval, 30.1%–33.7%) as predicted by the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.11,12
Interestingly, the stimulation and activation of TLRs with host-derived molecules have given rise to a possible association between TLRs and CVD.13 Hypertension development has been associated with the infiltration of immune cells in the kidneys, the vasculature, and the heart.14–21 Furthermore, prevalence, absolute BP values, and molecular mechanisms have shown sex differences in this disease.22 In fact, sex differences in BP levels have also been demonstrated in experimental models of hypertension, including spontaneously hypertensive rats (SHRs),23–26 Dahl salt–sensitive rats,27,28 deoxycorticosterone acetate-salt,29 angiotensin II (ANG II) administration,30–33 l-NAME–treated rats,34 and others.
This review will briefly summarize sex differences in hypertension, with a focus on an examination of the evidence for sex differences in the activation of TLRs and explore whether this may play a role in the sexual dimorphism observed in BP regulation.
SEX DIFFERENCES IN HYPERTENSION
The current review focuses primarily on biological factors that differentiate male and female subjects. The term “sex” refers to the different biological and physiological characteristics of men and women, such as reproductive organs, chromosomes, and hormones, whereas “gender” refers to the socially constructed characteristics of women and men—such as norms, roles, and relationships of and between groups of women and men.35 Consequently, the word “sex” will be used during this review.
Several mechanisms have been described to contribute to hypertension and the resultant end-organ dysfunction in consequence of high BP. Sex-related differences have been consistently described in most of these mechanisms as observed in data provided from both clinical and experimental studies.1,11,14–16
The prevalence of higher BP values in men relative to women starts at a young age, around the twenties, and this trend persists into the fifth–sixth decades of life. BP measurements collected from an observational cohort study comprising more than 20,000 subjects demonstrated that compared with men, women had lower values of systolic, diastolic, and mean arterial pressure at early ages (25 years old; 9, 6, and 7 mm Hg, respectively).36
Studies have shown that premenopausal women display a cardiovascular protection from hypertension compared with men at the same age, and after which, the prevalence becomes higher in women.11,37 Indeed, in later life, hypertension is more prevalent in women than men.11,22,36,38–44 Interestingly, the increases in the prevalence of hypertension among women begin in the same period when menopause is established, suggesting that sex hormones play a role on the BP regulation, a fact that is supported by numerous studies.45–49
Despite the amount of unquestionable evidence supporting sex differences in hypertension, no recommendations for sex-specific treatment strategies have been proposed, except the ones related to hypertensive disorders during pregnancy.50 In fact, a large study including more than 9000 subjects suggested that more intensive lowering of systolic BP to ~120 mm Hg, compared with standard practice of targeting ~140 mm Hg, promotes heterogenic impacts on cardiovascular and renal outcomes, comparing men and women.51 Therefore, studies like this may turn our attention to the necessity to better evaluate BP mechanisms that are differently modulated between sexes.
Animal experimental models have been used as strategies to evaluate different mechanisms contributing to sex-associated differences in BP.52 In fact, several mechanisms have been shown to differently regulate BP between males and females, including sex steroids, the renin–angiotensin system, the endothelin system, the sympathetic nervous system, arachidonic acid metabolites, the nitric oxide system, oxidative stress, obesity and metabolic syndrome, and the contribution of the immune system.52 Some of these systems are briefly described below, and they will be further discussed during this review.
The renin–angiotensin system plays a major role in BP regulation, and sex differences have been demonstrated with several components of this system.52 Wild-type male mice infused with a low dose of ANG II developed hypertension, whereas female mice did not (increase of BP around 30 vs. 7 mm Hg, respectively). Furthermore, gonadectomy and administration of an androgen receptor antagonist abolished ANG II–induced hypertension in male mice. By contrast, ovariectomy, estrogen receptor blockade, and estrogen receptor knockout increase the pressor effects of ANG II in female mice.30
Rats infused with ANG II displayed a significant augmentation in BP in both males and females. However, males displayed a greater increase in the number of renal proinflammatory Th17 and IL-17+ T cells than females. Furthermore, hypertension in male SHRs is more dependent on increases of oxidative stress elements than in female SHRs.53
SHRs display elevated levels of both ANG II and endothelin 1 (ET-1). Compared with female SHRs, male SHRs display accelerated progression of renal injury, blunted pressure–natriuresis relationship, and higher BP, with a mean difference of 22 mm Hg between the sexes.54–56 In fact, sex differences were demonstrated in the urinary excretion of prostanoids, subproducts from the arachidonic acid metabolism through COX activation, substances that are known to have antihypertensive and natriuretic effects. Conversely, female SHRs have enhanced prostaglandin E2 and thromboxane A2, and this effect is increased by orchidectomy but unchanged by ovariectomy (ovx), suggesting that PGE production is dependent on testosterone levels. In addition, orchidectomy abrogated the sexual dimorphism in BP and protein.54
Lower BP values in female SHRs are similar to BP values in castrated male SHRs, indicating a central role for male sex hormones in mediating the sex difference in BP in SHRs. In addition, androgen receptor antagonism attenuates hypertension in male SHRs. Interestingly, treatment with testosterone in ovx females presents similar BP measurements to that in gonad-intact males.55 By contrast, ovx does not change BP in female SHRs, although ovx female Dahl salt–sensitive rats showed augmented BP on normal salt diet and further increased on high salt diet compared with controls.57 Therefore, the contribution of sex hormones to BP control is complex, and more studies are needed to fully understand how male versus female sex hormones regulate BP.
Sex differences have also been shown to occur in the ET-1 system.58 Males display higher ET-1 levels, as well as a higher expression of ETA and ETB receptors in vascular smooth muscle cells (VSMCs), resulting in greater vascular contractility and remodelation. Alternatively, females display increased levels of ETB receptor expression in the endothelium, favoring the production of vasodilatory mediators, and therefore a lower BP.58 Of importance, ET-1 elicits higher inflammatory and oxidative stress activation in males, compared with females, which as discussed below could be central to sex differences in BP control.
The immune system is another sex-dependent target that is differently activated during hypertension.59,60 The kidney is important for the control of BP, and experimental hypertension has been shown through renal immune cell infiltration. In fact, kidneys of female SHRs have more T regulatory cells (Tregs) and IL-10+ T cells in the kidney, whereas kidneys from male SHRs have more Th17 cells, suggesting a sex difference in the renal T-cell profile in hypertensive rats.34,61,62 In fact, it was demonstrated that premenopausal females are protected from T-cell–mediated hypertension compared with male mice.63 Moreover, systolic BP responses to ANG II are similar between premenopausal and postmenopausal mice (Δ12 mm Hg); however, adoptive transfer of T cells significantly increased systolic BP in postmenopausal females (Δ28 mm Hg).63 Interestingly, male Rag−/− mice (lacking T and B cells) receiving adoptive T cells of males displayed increased BP after infusion of ANG II compared with female Rag−/− mice (Δ37.7 vs. Δ13.7 mm Hg, respectively).64 In the same way, peak mean arterial pressure was 13 mm Hg higher after adoptive T cells transfer of male versus female followed ANG II infusion.65 In addition, male animals display increased CD4+ cells and Th17 cells, whereas female animals have more CD8+ cells and Tregs in hypertension models, and adoptive transfer of T cells from female mice to male Rag−/− mice (lacking T and B cells) abrogated Ang II–induced increase in BP.22,61,64 Not all T cells are prohypertensive. Indeed, Tregs are antihypertensive, and females seem to be dependent on Tregs to maintain their lower BP relative to males. Depletion of Tregs in premenopausal mice induced a significant increase in BP. In fact, female rats are protected against deoxycorticosterone acetate-salt–induced hypertension through Tregs.66 Taking together these data, a differential activation of the adaptive immune system between the sexes in hypertension is well established.
Therefore, the mechanism resulting in differential BP regulation between the sexes is complex and may be differently activated according to their age. Sexual dimorphisms in several pathways involved in the pathophysiology of hypertension have been described, and among then, the immune system may be central in mediating sex differences in BP control because activation of the renin–angiotensin system and the endothelin system have both been shown to induce an immune response.
The Relationship Between the TLRs and Hypertension
Hypertension, for many reasons, is considered a low-grade inflammatory disease. The relationship between the adaptive system and hypertension has been well established. However, the evidences linking the innate immune system and hypertension are still under investigation. Several pathogen-associated molecular patterns and DAMPs are recognized by TLRs, initiating an immune response. TLR activation leads to overproduction of cytokines and chemokines, among other signaling molecules. Although TLRs are primarily associated with innate immunity, they simultaneously contribute to a variety of clinical disorders, including CVD, where hypertension is the major risk factor. Of importance, the incidence of polymorphisms in genes that participate in TLR pathways may also contribute to CVD development.67–70
TLR4 and Hypertension
TLR4 expression is enhanced in hypertension, suggesting a link with the increased BP. Studies have shown augmented TLR4 expression of mRNA and the protein level in mesenteric resistance arteries,71 VSMCs, and aortas from male SHRs72 compared with controls (WKY/Wistar).
In addition, l-NAME and ANG-II–induced hypertension models conducted in rats also showed enhanced TLR4 expression in the heart, VSMCs, and aorta.72–74 Inhibition of TLR4 using specific anti-TLR4 antibodies led to decreased BP and contractility improvement in resistance arteries from male SHRs,71,72 suggesting that TLR4 and the innate immune system play a role in hypertension. In addition, TLR4-deficient mice and rats treated with anti-TLR4 antibody have a blunted effect on the BP levels induced by ANG-II infusion.75,76
After TLR4 is activated, its own expression is increased and a possible mechanism by which TLR activation can be controlled is through the reduction of its expression. In fact, anti-TLR4 treatment decreased TLR4 protein expression in mesenteric resistance arteries in adult SHRs.71 Moreover, TLR4 activation is a MyD88-dependent pathway that culminates in the activation of NF-кB, which mediates the transcription of COX-2 and proinflammatory cytokine genes.77–79 Anti-TLR4 treatment decreased the expression of MyD88 and NF-кB (p65) in mesenteric resistance arteries from SHRs.80 Moreover, TLR4 antagonist (CLI-095) reduced p65 nuclear expression in VSMCs from and ANG II-induced hypertensive mice.74 TLR4 inhibition also attenuates the activation of inflammatory markers such as IL-6 serum levels and COX-2 protein expression in mesenteric arteries from SHRs.71
In the same way, TLR4-deficient mice displayed reduced TNF-α and IL-6 in hypertensive kidneys.75 Interestingly, endogenous TLR4 ligand heparin sulfate aggravated TNF-α and IL-6 mRNA response in cardiac tissue, which was significantly pronounced in SHRs.73 The inhibition as well as the absence of TLR4 is also capable in preventing ROS formation in various tissues, in response to hypertension.72,75,76 Because proinflammatory cytokines and ROS formation are all linked to the development and exacerbation of hypertension, decreasing TLR4 inhibition will likely act to lower BP through multiple mechanisms.
TLR9 and Hypertension
DAMPs can activate TLRs, initiating an inflammatory response in hypertension. Mitochondrial DNA (mtDNA), a DAMP that is recognized by TLR9, is elevated in the circulation of SHRs, and it is suggested to be associated with increased BP.81 In fact, increased BP and endothelial dysfunction were observed in normotensive rats treated with a specific agonist for TLR9, CpG oligonucleotide (ODN2395), and administration of inhibitory oligo dinucleotide for TLR9 (ODN2088) lowered systolic BP in SHRs.81 In addition, patients with essential arterial hypertension display increased CpG-rich cell-free DNA.82
Besides the canonical-inflammatory TLR9 signaling through MyD88, a newly established noncanonical stress tolerance TLR9 signaling pathway was demonstrated where on stimulation, sarcoplasmic reticulum/ER Ca2+ ATPase pump 2 (SERCA2) is reduced leading to activation of cell survival protein 5’ AMP-activated protein kinase.83 Acute in vitro incubation of mesenteric resistance arteries with ODN2395 resulted in augmented TLR9-induced inflammatory signaling (increased MyD88 and TRAF6 expression) and noncanonical TLR9-induced signaling (increased SERCA2 and phosphorylated AMPKαThr172 expression). Interestingly, the presence of ODN2088 inhibited the augmented protein expression.81
Furthermore, it has been demonstrated that SHRs treated with chloroquine, a lysosomotropic agent able to disrupt endosomal TLRs—including TLR9, show improved nitric oxide bioavailability as well as decreased BP, generation of reactive oxygen species, circulating T cells, and vascular infiltrating immune cells in SHRs.84,85 Of importance, mesenteric resistance arteries from young SHRs displayed decreased MyD88 and TRAF6 protein expression after chloroquine treatment, leading to lower NF-кB transcription, once its phosphorylation was reduced.84 Moreover, treatment with hydroxychloroquine in patients with lupus lowers the prevalence of thromboembolic events86 and reduced hypertension in mice with systemic lupus erythematosus.87 Although little is known regarding the role of TLR9 in BP control in female experimental models of hypertension, activation of TLR9 by CpG oligonucleotides during gestation also induces maternal hypertension and increased contractility in mesenteric resistance arteries of pregnant rats.88
TLR2, TLR3, and TLR7 and Hypertension TLR2
Inflammation of blood vessel plays a critical role during the initiation and maintenance of vascular diseases. In addition, vascular injury (through endothelial dysfunction) represents a link between cardiovascular risk factors and the increased incidence of hypertension. A study showed that vascular injury induced by cuff placement around the femoral artery in nontransgenic littermates results in increased TLR2 expression and mRNA expression of the proinflammatory cytokines TNF-α, IL-1β, and IL-6, as well as increased ROS production and neointimal proliferation through a TLR2-mediated signaling pathway. In fact, TLR2 knockout mice display blunted increases in vascular injury–mediated cytokine expression, ROS, and neointimal hyperplasia, suggesting that endogenous TLR2 activation might play a central role in the regulation of vascular inflammation.89 High-density lipoprotein (HDL) is considered antiatherogenic and is known to prevent endothelial dysfunction. Abnormal HDL isolated from adult and children patients with chronic kidney disease injected into mice significantly increased their BP. Furthermore, human aortic endothelial cells incubated with HDL from chronic kidney disease strongly inhibited NO production. Of importance, selective inhibition or genetic deficiency of TLR2 almost completely abrogated the adverse effects of modified HDL on endothelial NO bioavailability, endothelial repair, and BP.90 Moreover, in a model of ischemia/reperfusion, TLR2 contributes to coronary endothelial dysfunction, inducing endothelial injury due to augmented neutrophil-induced oxidative stress formation.91 Interestingly, distal and proximal tubules in kidneys of rats infused with ANG II displayed increased TLR2 mRNA and protein expression as well as TNF-α and the number of infiltrated and matured DCs, which were abolished by treatment with losartan.92
TLR3
Preeclampsia, a pregnancy-specific hypertensive syndrome, may result from overactivation of the maternal immune system and is characterized by endothelial dysfunction and excessive inflammation. It was demonstrated that pregnant rats treated with a TLR3 agonist (Poly I:C) displayed preeclampsia-like symptoms, including elevated systolic BPs, increased urinary protein excretion, and malformed pups/litter, which are exacerbated by the absence of interleukin 10.93,94 Interestingly, the TLR3 pathway is required for ANG II–induced hypertension; TLR3-deficient (Tlr3−/−) mice do not display increased systolic BP with ANG II infusion. Moreover, cardiac hypertrophy and increases in IL-6 and TNF-α gene expression in the kidney and heart caused by ANG II infusion were attenuated in Tlr3−/− mice.95 Otherwise, TLR3 activation improved mechanical and hypercholesterolemia-induced arterial injury. In fact, systemic administration of Poly I:C reduced neointima formation in an arterial injury model involving the placement of a perivascular collar, and this protection was lost in Tlr3−/− mice. In addition, deficiency of TLR3 accelerated the onset of atherosclerosis in hypercholesterolemic ApoE−/− mice, demonstrating a protective role of TLR3 signaling in the vessel wall.96
TLR7
MicroRNAs (miRNAs) are short (18–22 nt), single-stranded, noncoding RNAs involved in the regulation of physiological and pathological processes. Specifically, miR-17, miR-21, miR-34a, miR-92a, miR-126, miR-145, miR-146a, and miR-150 have been linked with the development of CVD. In fact, a study with normotensive and hypertensive middle-aged adults (with both men and women; female subjects were at least 1 year postmenopausal) showed increased circulating expression of miR-34a in hypertensive individuals, whereas miR-21, miR-146a, and miR-126 were lower. Moreover, these miRNAs were related with BP.97 Of importance, ssRNAs are ligands for TLR7. TLR activation leads to adaptive immune system activation, and inflammation through increased lymphocytes contributes to the development of hypertension. In this regard, transgenic mice that overexpress TLR7 have a massive expansion of inflammatory DCs and increased activation of B and T cells, which triggers autoimmunity.98
Therefore, evidence currently available supports the notion that TLRs, in particular TLR4 and TLR9, play a role in the BP control and could contribute to the development of arterial hypertension. However, in hypertension, it is unclear whether TLR4 and TLR9 are differently modulated in males compared with females. The lack of mechanistic data comparing expression, activity, and availability of endogenous ligands with TLRs between the sexes needs to be better investigated based on the well-accepted central role for the immune system in modulating both the development of hypertension and sex differences in hypertension. Table 1 summarizes the role of TLRs in hypertension.
TABLE 1.
Role of TLRs in Hypertension
| Observation | Local | Characteristics | References |
|---|---|---|---|
| Increased expression or activity of TLR | |||
| TLR2 | Femoral artery | Vascular injury but basal BP levels | 89 |
| TLR2 | Kidney | ANG-II | 92 |
| TLR3 | Placenta | Preeclampsia | 93 and 94 |
| TLR4 | MRA | SHR | 71 |
| TLR4 | Cardiac | l-NAME | 73 |
| TLR4 | CSMC | SHR | 76 |
| TLR4 | VSMCs | SHR/ANG-II | 72 |
| TLR4 | Aorta | SHR/ANG-II | 72 and 74 |
| Increased expression or activity of the TLR ligand | |||
| miR-34a (TLR7) | Plasma | Patients with essential arterial hypertension | 97 |
| mtDNA (TLR9) | Serum | SHR | 81 |
| CpG-rich cell-free DNA (TLR9) | Serum | Patients with essential arterial hypertension | 82 |
| Genetic or therapeutic interventions targeting to TLRs resulting in reduced blood pressure | |||
| TLR2-deficient mice | Coronary artery | Cardiac ischemia/reperfusion (basal BP levels) | 91 |
| TLR3-deficient mice | Heart and kidneys | ANG-II | 95 |
| Chloroquine (endosomal TLRs) | MRA | SHR | 84 and 85 |
| Hydroxychloroquine (endosomal TLRs) | Systemic lupus erythematosus | Thromboembolic events | 86 |
| Hydroxychloroquine (endosomal TLRs) | Aorta and kidney | Systemic lupus erythematosus mice | 87 |
| Anti-TLR4 | MRA and aorta | SHR | 71 and 72 |
| TLR4-deficient mice | Kidney | ANG-II | 75 |
CSMC, cavernosal smooth muscle cells; l-NAME, N-nitro-l-arginine methyl ester; MRA, mesenteric resistance artery.
SEX DIFFERENCES IN ACTIVATION/EXPRESSION OF TLRs
A sex bias in TLR activation has already been shown. In fact, women exhibit increased cellular-mediated and humoral-mediated immune responses and a higher risk of autoimmune disease compared with men.1,99–102
TLR4 RNA expression was evaluated in the postmortem fresh-frozen cerebellum sample from children who experienced inflammation before death. Inflammation, in this case, included exacerbated infection, asthma, asphyxia, inflammatory tumors, or that were treated with antibiotics or nonsteroidal anti-inflammatory drugs around death. TLR variants 1, 3, and 4 were augmented in cerebellums from children categorized with inflammatory disease. However, TLR4 variant 1 and variant 4, but not variant 3, were associated with sex and age.103 The limitation in this study was the experimental size of the sample.
A clinical study demonstrated that TLRs expressed in peripheral blood mononuclear cells (PBMCs) are differently activated between sexes. In healthy women, TLR4, TLR3, and TLR7 stimulated with lipopolysaccharide, Poly I:C, and loxoribine, respectively, displayed increased TLR7 ligand–induced type 1 interferon (IFN) responses compared with men.104,105 However, TLR3 and TLR4 activations, evaluated by IL-6 production after their stimulation, were not different between the sexes.105
Yet, PBMCs from men produced less IFN-α after TLR7 stimulation, as well as greater amounts of interleukin (IL)-10 in response to TLR8 and TLR9 ligands.106,107 By contrast, it was demonstrated that men presented higher levels of inflammatory cytokines compared with women after stimulating whole blood with various TLR ligands.
TLR expression in PBMCs seems to be selectively modulated by sex hormones. Estradiol stimulation increased RNA and protein levels of TLR8 in PBMCs from healthy premenopausal females, compared with age-matched males. On the other hand, incubation with testosterone had no effect in either sex.108
PBMCs from healthy women exposed to TLR1–8 stimulation display variations in IL-1β, IL-6, IL-8, and TNF-α production during different phases of the menstrual cycle, as demonstrated by in vitro experiments.109 After stimulation, all TLRs evaluated displayed a different pattern of cytokine release across the menstrual cycle, except TLR3 and TLR7. Interestingly, use of hormonal contraception was associated with a reduced capacity for circulating plasmacytoid DCs (pDCs) to produce IFN-α and TNF-α in response to TLR9 stimulation in the mucosa of the lower female genital tract.110 Moreover, there was a demonstrated association between the use of hormonal contraception and activation of migrating genital tract–derived DCs after stimulation with LPS, PAM3, and R848 (TLR4, TLR2, and TLR7/8, respectively).111
Sex hormones have been shown to modulate the immune system during pregnancy. On TLR7/8 stimulation, PBMCs from first-trimester pregnant women displayed decreased IFN-α production, compared with nonpregnant controls. By contrast, the production of TNF-α is increased after TLR4 stimulation in the beginning of pregnancy, followed by a significant decrease during pregnancy. In addition, TLR7-induced IFN-α production by pDCs is augmented, whereas TLR4-induced TNF-α production by monocytes is lower during pregnancy.112
Incubation with estradiol generated augmented TLR7 and TLR9 expression in pDCs from postmenopausal women, with markedly increased IFN-α production.113 Moreover, stimulation of pDCs by a TLR7 agonist also results in increased IFN production104,114 and higher mRNA expression of all IFN-α subtypes in pDCs extracted from women,115 even before puberty. However, puberty itself is associated with increased production of type 1 IFN,116 which could be explained by TLR7 localization into X chromosome, and the differential TLR expression between men and women.104 In fact, the presence of two X chromosomes may be responsible for the greater production of IFN-α in pDCs after TLR7 stimulation.116
Data from experimental models also evaluated TLRs in PBMCs. A greater mRNA expression of TLR2, TLR3, and TLR4 was shown in female peritoneal leukocytes of mice and rats compared with male mice and rats, as well as higher TLR2 and TLR4 expression in macrophages. By contrast, ovarian removal led to significantly reduced leukocyte mRNA expression of TLRs and protein expression of TLR2 and TLR4 on female resident macrophages.117 TLRs play an important role in the immune control of viruses, including mouse cytomegalovirus. A sex difference was demonstrated to cytomegalovirus infection, where female animals showed decreased expression of TLR9 accompanied by a lower activation of the innate immune system, compared with males.118 On the other hand, activation of TLR2 and TLR4 in splenic lymphocytes from mice with myocarditis induced by coxsackievirus B3 infection led to decreased expression of FoxP3+ regulatory T cells in male mice. The suppression of Treg cells by TLR signaling in males but not females suggests a correlation with increased myocarditis susceptibility in males.119
In accordance with human data, mouse pDCs have been demonstrated to be positively regulated by estradiol, where humanized mice present increased TLR7–mediated responses to human pDCs in female host mice relative to males. In agreement, exogenous and endogenous estrogen promoted enhanced TNF-α production by mouse pDCs after TLR7/TLR9 stimulation through the estrogen receptor, whereas inhibition and genetic ablation of estrogen receptors in DC lineage blunted the TLR7-mediated IFN response.1,113,120,121
It has been shown that macrophages from male mice generate a higher mRNA expression to TLR4 and its coreceptor CD14, as well as higher levels of IL-1β, following in vivo LPS exposition.122 By contrast, macrophages from mice treated with testosterone, generated in the absence of androgen, elicit lower TLR4 expression and activation on stimulation. In addition, in vivo removal of endogenous testosterone (orchiectomy) led to higher TLR4 cell surface expression and increased susceptibility to endotoxic shock.123
Nonetheless, data may follow a different regulatory pathway in different tissues because the pattern of TLR expression does not change during preovulation, menstruation, and implantation in the human fallopian tube epithelial cell line.124 In fact, the expression of TLR1–6 in the fallopian tube epithelial cells was demonstrated and was not altered by incubation with different concentrations of estradiol and progesterone. However, the simultaneous combination of both hormones was able to change TLR1, TLR4, TLR5, and TLR6 RNA expression.124 In addition, these hormones, in combination, suppressed the production of IL-6 induced by Poly I:C, a TLR3 agonist, suggesting that TLR3 function is somehow mediated by different concentrations of sex hormones, resulting in an inhibitory effect on the TLR3-induced pathway.125
Conversely, expression of IFN- or NF-кB-inducible genes (IFN-α/β or TNF-α) in the spleen and hypothalamus of rats by acute TLR3 activation through Poly I:C did not show sex differences, with the exception of IFN-α expression 8 hours after the activation, exclusively in the spleen from female rats.126
TLR7/8 encoded by the X chromosome is expressed in the X-sperm but not the Y-sperm. Activation of TLR7/8 selectively suppressed the mobility of X-sperm but not the Y-sperm without changing sperm viability or acrosome formation. Interestingly, in vitro fertilization using the ligand-selected high-mobility sperm resulted in 83% male pups, whereas activation of TLR7/8 resulted in slow mobility sperm and 81% female pups, suggesting that TLR7/8 are specific receptors that differentially impact the functions of X-sperm but not Y-sperm.127
THE ROLE OF THE TLRs IN SEX DIFFERENCES IN BP
Despite several studies demonstrating differences in BP control between males and females, there are no studies to date that have directly addressed the potential interaction between sex, TLRs, and hypertension.
Cellular necrosis after cell death or tissue injury results in the release of DAMPs (for instance, mtDNA, histone and S100 proteins, and heat shock proteins), which are recognized by TLRs leading to their activation.128–131 The susceptibility of cells to undergo cell death has been demonstrated to have sex differences.132–135 In fact, a recent study demonstrated that mtDNA released in hypertensive rats differently modulates vascular responses in males and females. In this work, male SHRs displayed increased mtDNA, proinflammatory cytokines, and higher contractility to phenylephrine in the aorta incubated with mtDNA, which was attenuated by TLR9 inhibitor. On the other hand, female SHRs did not show changes in the contractile response, however, displayed decreased proinflammatory cytokines and prevented oxidative stress.136
Consistent with TLR-sex difference, genetic differential expression of TLRs is observed in the kidney and mesenteric arteries from SHRs. TLR3, TLR5, TLR6, and TLR7 mRNAs are more highly expressed in the renal cortex of male versus female SHRs. TLR6 mRNA is also more highly expressed in the mesenteric arterial bed from male versus female SHRs. Conversely, TLR4 mRNA is more highly expressed in the mesenteric arterial bed of female versus male SHRs.137
Consistent with animal models, a cardiovascular cohort study at Framingham showed that platelets from women display increased TLR mRNA expression compared with men, and it is associated with different cardiovascular risk factors. In women, TLR1, TLR3, TLR6, and TLR7 were associated with body mass index, and TLR5, TLR7, and TLR10 were associated with the total cholesterol to HDL ratio. In men, TLR1, TLR2, and TLR3 were associated with the lipid profile and TLR8 with hypertension treatment. Similarly, TLR expression in men was more commonly associated with circulating inflammatory markers (TNFR1 and ICAM1), whereas in women TLR expression was associated with P-selectin levels.138
In addition, polymorphism TLR6 Ser249Pro, a single-nucleotide polymorphism, when observed in hypertensive women, resulted in lower left ventricular hypertrophy and reduced proinflammatory response. Conversely, hypertensive men carrying the TLR6 variant do not show echocardiographic features.139 Indeed, TLR4 knockout mice developed less-severe left ventricular dysfunction,140 and blockade of MyD88 attenuated load-induced cardiac growth.141 In the same way, an investigation of 2774 subjects reported that hyporesponsive TLR4 polymorphisms (Asp299Gly and Thr399Ile; single-nucleotide polymorphism [SNP]) were associated with an increased risk of myocardial infarction in men, but not women,142 further supporting sex-specific effects of TLR on cardiovascular function.
In pregnant women, hormonal changes lead to differences in response to TLR activation and BP regulation. Preeclampsia (characterized by high BP and proteinuria) shows irregularities in the immune system that could be linked to the immunological changes related to placental environments.143 Trophoblast surface as well as placentas from preeclamptic women overexpress TLR2, TLR4, TLR3, TLR7, TLR8, and TLR9 when compared with healthy pregnancies.144–146
Similar to data in human, in pregnant mice, intraperitoneal injections of TLR3, TLR7, and TLR8 agonists led to a significant increase in protein expression and mRNA for TLR3/7/8 in placenta and an increase in systolic BP at gestational day 17 compared with vehicle-treated controls.146 TLR3 and TLR9 in rodents, using Poly I:C and synthetic CpG oligonucleotide (respectively), led to the development of the characteristic markers of preeclampsia, including hypertension, endothelial dysfunction, decreased fetal weight, and proteinuria.88,93,94,147–150
These observations led to the inference that activation of TLRs is somehow sensitive to sex hormones and this could be one of the mechanisms related to sex differences in the BP control. However, it is imperative to make clear that most of the current available data on this field are limited, if immunity from these individuals is evaluated only by a single time point observations, because the innate immunity is a result of previous immunogenic expositions.
CONCLUSIONS
The present review summarizes recent studies that suggest a link between TLRs and hypertension. It is well known that the BP control in men and women is different, and exciting evidence demonstrates that expression/activation of TLRs are sex specific. However, the sex differences in TLR activation mediating hypertension remain to be elucidated. Figure 1 and Table 2 summarize sex differences and TLRs in hypertension.
FIGURE 1.
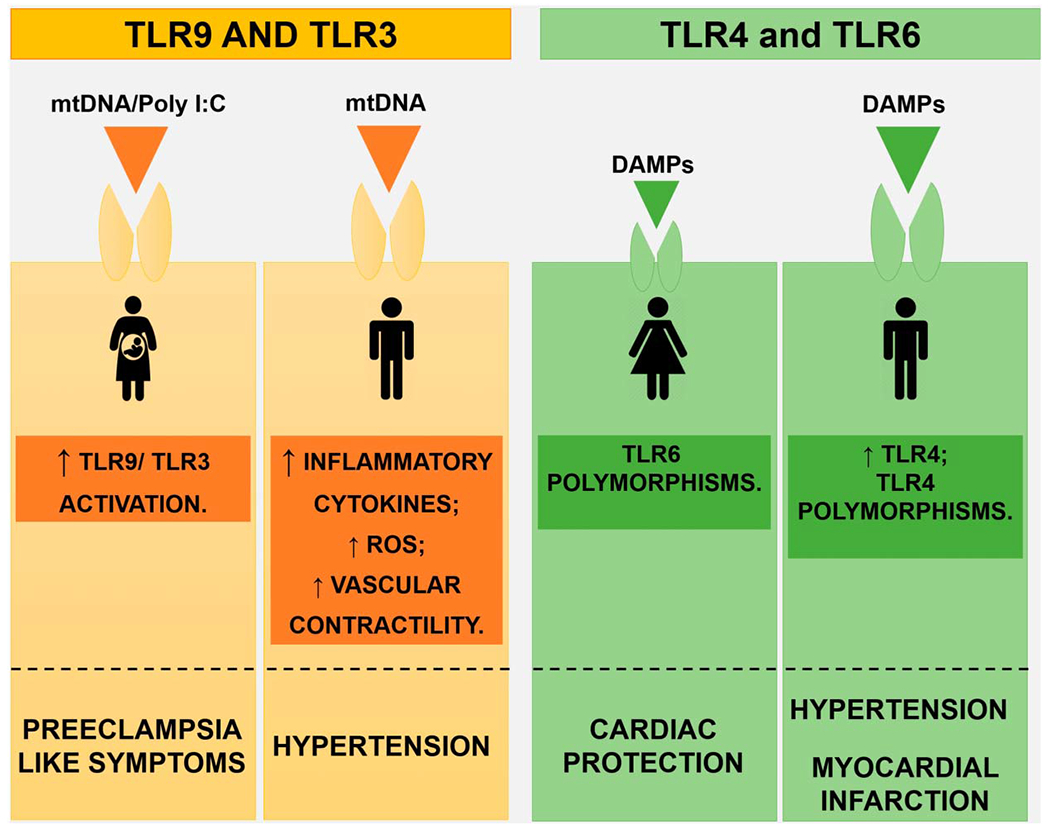
Sex differences in TLRs during hypertension: TLRs are differently modulated and expressed between the sexes, during hypertension. In fact, male SHRs display increased mtDNA, augmented TLR9 activation, and augmented expression of TLR4, compared with female SHRs, features that favor hypertension. When TLR9 and TLR3 are overactivated during pregnancy, preeclamptic-like symptoms are observed. In females, polymorphisms at TLR6 increase cardiac protection, whereas polymorphisms in TLR4 favor infarction in males. ROS, reactive oxygen species.
TABLE 2.
Sex Differences and TLRs in Hypertension
| Observation | Local | Hypertension Classification | Role on Hypertension | References |
|---|---|---|---|---|
| Female | ||||
| Increased expression or activity of TLR | ||||
| TLR3/TLR5/TLR6 | Renal cortex | SHR | Immune response | 137 |
| TLR4/TLR6 | MRA | SHR | Immune response | 137 |
| TLR1/TLR3/TLR6/TLR7 | Platelets | Basal BP levels | Associated cardiovascular risk | 138 |
| TLR5/TLR7/TLR10 | Platelets | Basal BP levels | factors with body mass index Associated cardiovascular risk factors with the total cholesterol to HDL ratio | 138 |
| Increased expression or activity of the TLR ligand | ||||
| mtDNA (TLR9) | Aorta | SHR | Decreased proinflammatory cytokines and prevented oxidative stress | 136 |
| Polymorphism | ||||
| TLR6—Ser249Pro | Serum/heart | Hypertension | Lower LV and reduced proinflammatory response | 139 |
| TLR4—Asp299Gly and Thr399Ile | Serum | Basal BP levels | No association with myocardial infarction | 142 |
| Male | ||||
| Increased expression or activity of TLR | ||||
| TLR7 | Renal cortex | SHR | Immune response | 137 |
| TLR1/TLR2/TLR3 | Platelets | Basal BP levels | Associated cardiovascular risk factors with lipid treatment | 138 |
| TLR8 | Platelets | Basal BP levels | Associated cardiovascular risk factors with hypertensive treatment | 138 |
| Increased expression or activity of the TLR ligand | ||||
| mtDNA (TLR9) | Aorta | SHR | Increased proinflammatory cytokines and higher contractility | 136 |
| Polymorphism | ||||
| TLR6—Ser249Pro | Serum/heart | Hypertension | No changes in echocardiographic features | 139 |
| TLR4—Asp299Gly and Thr399Ile | Serum | Basal BP levels | Increased risk of myocardial infarction | 142 |
| TLR4 knockout mice | Heart | Cardiac hypertrophy | Less-severe LV dysfunction | 140 and 141 |
| Pregnancy | ||||
| Increased expression or activity of TLR | ||||
| TLR2/TLR4/TLR3/TLR7/TLR8/TLR9 | Trophoblast and placenta surface | Women with preeclampsia | Classical markers of preeclampsia | 144–146 |
| Increased expression or activity of the TLR ligand | ||||
| Poly I:C/R-837/CLO97 (TLR3/TLR7/TLR8) | Placenta | Preeclampsia (animal model) | Classical markers of preeclampsia: Impaired relaxation, elevated systolic blood pressures, increased urinary protein concentrations, and malformed pups/litter | 93 and 146 |
| CpG oligonucleotide (TLR9) | MRA | Preeclampsia (animal model) | Classical markers of preeclampsia: Increased systolic blood pressure and resistance artery contraction and decreased fetal weight | 88 and 150 |
LV, left ventricle; MRA, mesenteric resistance artery.
Acknowledgments
Supported by the National Institutes of Health (NIH, PO1 HL-13604 to R.C.W.), Fundação de Amparo à Pesquisa do Estado de Mato Grosso (FAPEMAT, 0324552/2018 to F.R.G.), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, 305823/2015-9 to F.R.G.), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, 88881.190484/2018-01 to V.D.J.—scholarship), and Fundaçío de Amparo à Pesquisa do Estado de Goiás (FAPEG)-CAPES (to V.D.J. 201710267001159).
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Jiang W, Gilkeson G. Sex Differences in monocytes and TLR4 associated immune responses; implications for systemic lupus erythematosus (SLE). J Immunother Appl. 2014;23:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parizadeh SM, Ghandehari M, Heydari-majd M, et al. Toll-like receptors signaling pathways as a potential therapeutic target in cardiovascular disease. Curr Pharm Des. 2018;24:1887–1898. [DOI] [PubMed] [Google Scholar]
- 3.Sharma S, Garg I, Ashraf MZ. TLR signalling and association of TLR polymorphism with cardiovascular diseases. Vascul Pharmacol 2016;87:30–37. [DOI] [PubMed] [Google Scholar]
- 4.Chockalingam A, Brooks JC, Cameron JL, et al. TLR9 traffics through the Golgi complex to localize to endolysosomes and respond to CpG DNA. Immunol Cell Biol. 2009;87:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Häcker H, Mischak H, Miethke T, et al. CpG-DNA-specific activation of antigen-presenting cells requires stress kinase activity and is preceded by non-specific endocytosis and endosomal maturation. EMBO J. 1998;17:6230–6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leifer CA, Kennedy MN, Mazzoni A, et al. TLR9 is localized in the endoplasmic reticulum prior to stimulation. J Immunol. 2004;173:1179–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park B, Brinkmann MM, Spooner E, et al. Proteolytic cleavage in an endolysosomal compartment is required for Toll-like receptor 9 activation. Nat Immunol. 2008;9:1407–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pelka K, Bertheloot D, Reimer E, et al. The chaperone UNC93B1 regulates toll-like receptor stability independent of endosomal TLR transport. Immunity. 2018;48:911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Y, Liu B, Dai J, et al. Heat shock protein gp96 is a master chaperone for toll-like receptors and is important in the innate function of macrophages. Immunity. 2007;26:215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blasius AL, Beutler B. Intracellular toll-like receptors. Immunity. 2010;32:305–315. [DOI] [PubMed] [Google Scholar]
- 11.Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139:56–66. [DOI] [PubMed] [Google Scholar]
- 12.Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141:139–596. [DOI] [PubMed] [Google Scholar]
- 13.Vallejo JG. Role of Toll-like receptors in cardiovascular diseases. Clin Sci. 2011;121:1–10. [DOI] [PubMed] [Google Scholar]
- 14.Harrison DG. The immune system in hypertension. Trans Am Clin Climatol Assoc. 2014;125:130–140. [PMC free article] [PubMed] [Google Scholar]
- 15.Singh MV, Chapleau MW, Harwani SC, et al. The immune system and hypertension. Immunol Res. 2014;59:243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rudemiller N, Lund H, Jacob HJ, et al. CD247 modulates blood pressure by altering T lymphocyte infiltration in the kidney. Hypertension. 2014;63:559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodríguez-Iturbe B, Franco M, Tapia E, et al. Renal inflammation, autoimmunity and salt-sensitive hypertension. Clin Exp Pharmacol Physiol. 2012;39:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matrougui K, Zakaria AE, Kassan M, et al. Natural regulatory T cells control coronary arteriolar endothelial dysfunction in hypertensive mice. Am J Pathol. 2011;178:434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guzik TJ, Hoch NE, Brown KA, et al. Role of the T cell in the genesis of angiotensin II-induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crowley SD, Song YS, Lin EE, et al. Lymphocyte responses exacerbate angiotensin II-dependent hypertension. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1089–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kvakan H, Kleinewietfeld M, Qadri F, et al. Regulatory T cells ameliorate angiotensin II-induced cardiac damage. Circulation. 2009;119:2904–2912. [DOI] [PubMed] [Google Scholar]
- 22.Crislip GR, Sullivan JC. T-cell involvement in sex differences in blood pressure control. Clin Sci. 2016;130:773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ganten U, Schroder G, Witt M, et al. Sexual dimorphism of blood pressure in spontaneously hypertensive rats: effects of anti-androgen treatment. J Hypertens. 1989;7:721–726. [PubMed] [Google Scholar]
- 24.Masubuchi Y, Kumai T, Uematsu A, et al. Gonadectomy-induced reduction of blood pressure in adult spontaneously hypertensive rats. Acta Endocrinol. 1982;101:154–160. [DOI] [PubMed] [Google Scholar]
- 25.Iams SG, Mcmurthy JP, Wexler BC. Aldosterone, deoxycorticosterone, corticosterone, and prolactin changes during the lifespan of chronically and spontaneously hypertensive rats. Endocrinology. 1979;104:1357–1363. [DOI] [PubMed] [Google Scholar]
- 26.Chen YF, Meng QC. Sexual dimorphism of blood pressure in spontaneously hypertensive rats is androgen dependent. Life Sci. 1991;48:85–96. [DOI] [PubMed] [Google Scholar]
- 27.Crofton JT, Ota M, Share L. Role of vasopressin, the renin-angiotensin system and sex in Dahl salt-sensitive hypertension. J Hypertens. 1993;11:1031–1038. [DOI] [PubMed] [Google Scholar]
- 28.Rowland NE, Fregly MJ. Role of gonadal hormones in hypertension in the Dahl salt-sensitive rat. Clin Exp Hypertens A. 1992;14:367–375. [DOI] [PubMed] [Google Scholar]
- 29.Ouchi Y, Share L, Crofton JT, et al. Sex difference in the development of deoxycorticosterone-salt hypertension in the rat. Hypertension. 1987;9:172–177. [DOI] [PubMed] [Google Scholar]
- 30.Xue B, Johnson AK, Hay M. Sex differences in angiotensin II-induced hypertension. Braz J Med Biol Res. 2007;40:727–734. [DOI] [PubMed] [Google Scholar]
- 31.Chappell MC, Marshall AC, Alzayadneh EM, et al. Update on the angiotensin converting enzyme sex differences, and intracellular pathways. Front Endocrinol (Lausanne). 2014;4:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sullivan JC. Sex and the renin-angiotensin system: inequality between the sexes in response to RAS stimulation and inhibition. Am J Physiol Regul Integr Comp Physiol. 2008;294:1220–1226. [DOI] [PubMed] [Google Scholar]
- 33.Viegas VU, Liu ZZ, Nikitina T, et al. Angiotensin II type 2 receptor mediates sex differences in mice renal interlobar arteries response to angiotensin II. J Hypertens. 2012;30:1791–1798. [DOI] [PubMed] [Google Scholar]
- 34.Brinson KN, Elmarakby AA, Tipton AJ, et al. Female SHR have greater blood pressure sensitivity and renal T cell infiltration following chronic NOS inhibition than males. Am J Physiol Regul Integr Comp Physiol. 2013;305:701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization. Gender Mainstreaming for Health Managers: A Practical Approach. 2011:1–138. [Google Scholar]
- 36.Cheng S, Xanthakis V, Sullivan LM, et al. Blood pressure tracking over the adult life course: patterns and correlates in the Framingham heart study. Hypertension. 2012;60:1393–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beale AL, Kaye DM, Marques FZ. The role of the gut microbiome in sex differences in arterial pressure. Biol Sex Differ. 2019;10:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hermida RC, Ayala DE, Mojón A, et al. Differences between men and women in ambulatory blood pressure thresholds for diagnosis of hypertension based on cardiovascular outcomes. Chronobiol Int. 2013;30:221–232. [DOI] [PubMed] [Google Scholar]
- 39.Burt VL, Whelton P, Roccella EJ, et al. Prevalence of hypertension in the US adult population. Hypertension. 1995;3:305–313. [DOI] [PubMed] [Google Scholar]
- 40.Vriz O, Lu H, Visentin P, et al. Gender differences in the relationship between left ventricular size and ambulatory blood pressure in borderline hypertension: the HARVEST Study. Eur Heart J. 1997;18:664–670. [DOI] [PubMed] [Google Scholar]
- 41.Hermida RC, Ayala DE, Fernández JR, et al. Modeling the circadian variability of ambulatorily monitored blood pressure by multiple-component analysis. Chronobiol Int. 2002;19:461–481. [DOI] [PubMed] [Google Scholar]
- 42.Boynton RE, Todd RL. Blood pressure readings of 75,258 university students. Arch Intern Med. 1947;80:454–462. [DOI] [PubMed] [Google Scholar]
- 43.Cutler JA, Sorlie PD, Wolz M, et al. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988-1994 and 1999-2004. Hypertension. 2008;52:818–827. [DOI] [PubMed] [Google Scholar]
- 44.Cornoni-Huntley J, LaCroix AZ, Havlik RJ. Race and sex differentials in the impact of hypertension in the United States: The National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Arch Intern Med. 1989;149:780–788. [PubMed] [Google Scholar]
- 45.Costa-Hong VA, Muela HCS, Macedo TA, et al. Gender differences of aortic wave reflection and influence of menopause on central blood pressure in patients with arterial hypertension. BMC Cardiovasc Disord. 2018;18:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith JR, Koepp KE, Berg JD, et al. Influence of sex, menstrual cycle, and menopause status on the exercise pressor reflex. Med Sci Sports Exerc. 2019;51:874–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peinado AB, Harvey RE, Hart EC, et al. Neural control of blood pressure in women: differences according to age. Clin Auton Res. 2017;27:157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alsumali A, Mekary RA, Seeger J, et al. Blood pressure and neuropsychological test performance in healthy postmenopausal women. Maturitas. 2016;88:25–31. [DOI] [PubMed] [Google Scholar]
- 49.Begum A, Nessa A, Saki SA. Study on blood pressure changes in postmenopausal women. Mymensingh Med J. 2019;28:274–277. [PubMed] [Google Scholar]
- 50.Delles C, Currie G. Sex differences in hypertension and other cardiovascular diseases. J Hypertens. 2018;36:768–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Foy CG, Lovato LC, Vitolins MZ, et al. Gender, blood pressure, and cardiovascular and renal outcomes in adults with hypertension from the Systolic Blood Pressure Intervention Trial. J Hypertens. 2018;36:904–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reckelhoff JF. Sex differences in the regulation of blood pressure. In: Sex-Specific Analysis of Cardiovascular Function. Springer international; 2018:139–151. [Google Scholar]
- 53.Bhatia K, Elmarakby AA, El-remessey A, et al. Oxidative stress contributes to sex differences in angiotensin II-mediated hypertension in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2012;302:274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sullivan JC, Sasser JM, Pollock DM, et al. Sexual dimorphism in renal production of prostanoids in spontaneously hypertensive rats. Hypertension. 2005;45:406–411. [DOI] [PubMed] [Google Scholar]
- 55.Reckelhoff JF, Zhang H, Srivastava K. Gender differences in development of hypertension in spontaneously hypertensive rats: role of the renin-angiotensin system. Hypertension. 2000;35:480–483. [DOI] [PubMed] [Google Scholar]
- 56.Reckelhoff JF, Zhang H, Granger JP. Testosterone exacerbates hypertension and reduces pressure-natriuresis in male spontaneously hypertensive rats. Hypertension. 1998;31:435–439. [DOI] [PubMed] [Google Scholar]
- 57.Brinson KN, Rafikova O, Sullivan JC. Female sex hormones protect against salt-sensitive hypertension but not essential hypertension. Am J Physiol Regul Integr Comp Physiol. 2014;307:149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gohar EY, Giachini FR, Pollock DM, et al. Role of the endothelin system in sexual dimorphism in cardiovascular and renal diseases. Life Sci. 2016;159:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramirez LA, Sullivan JC. Sex differences in hypertension: where we have been and where we are going. Am J Hypertens. 2018;31:1247–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sylvester MA, Brooks HL. Sex-specific mechanisms in inflammation and hypertension. Curr Hypertens Rep. 2019;21:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zimmerman MA, Baban B, Tipton AJ, et al. Chronic ang II Infusion induces sex-specific increases in renal T cells in Sprague Dawley rats. Am JPhysiol Ren Physiol. 2014;308:706–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tipton AJ, Baban B, Sullivan JC. Female spontaneously hypertensive rats have greater renal anti-inflammatory T lymphocyte infiltration than males. Am J Physiol Regul Integr Comp Physiol. 2012;303:359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pollow DP, Uhlorn JA, Sylvester MA, et al. Menopause and FOXP3+ Treg cell depletion eliminate female protection against T cell-mediated angiotensin II hypertension. Am J Physiol Hear Circ Physiol. 2019;317: H415–H423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pollow DP, Uhrlaub J, Romero-Aleshire M, et al. Sex differences in T-lymphocyte tissue infiltration and development of angiotensin II hypertension. Hypertension. 2014;64:384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ji H, Zheng W, Li X, et al. Sex-specific T-cell regulation of angiotensin II-dependent hypertension. Hypertension. 2014;64:573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Belanger KM, Crislip GR, Gillis EE, et al. Greater T regulatory cells in females attenuate DOCA- salt—induced increases in blood pressure versus males. Hypertension. 2020;75:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arbour NC, Lorenz E, Schutte BC, et al. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet. 2000;25:187–191. [DOI] [PubMed] [Google Scholar]
- 68.Kiechl S, Lorenz E, Reindl M, et al. Toll-like receptor 4 polymorphisms and atherogenesis. N Engl J Med. 2002;347:185–192. [DOI] [PubMed] [Google Scholar]
- 69.Boekholdt SM, Agema WRP, Peters RJG, et al. Variants of toll-like receptor 4 modify the efficacy of statin therapy and the risk of cardiovascular events. Circulation. 2003;107:2416–2421. [DOI] [PubMed] [Google Scholar]
- 70.Child NJA, Yang IA, Pulletz MCK, et al. Polymorphisms in Toll-like receptor 4 and the systemic inflammatory response syndrome. Biochem Soc Trans. 2003;31:652–653. [DOI] [PubMed] [Google Scholar]
- 71.Bomfim GF, dos Santos RA, Oliveira MA, et al. Toll like receptor 4 contributes to blood pressure regulation and vascular contraction in spontaneously hypertensive rat. Clin Sci. 2014;122:535–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Batista PR, Palacios R, Martin A, et al. Toll-like receptor 4 upregulation by angiotensin II contributes to hypertension and vascular dysfunction through reactive oxygen species production. PLoS One. 2014;9:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eissler R, Schmaderer C, Rusai K, et al. Hypertension augments cardiac Toll-like receptor 4 expression and activity. Hypertens Res. 2011;34:551–558. [DOI] [PubMed] [Google Scholar]
- 74.Hernanz R, Martínez-Revelles S, Palacios R, et al. Toll-like receptor 4 contributes to vascular remodelling and endothelial dysfunction in angiotensin II-induced hypertension. Br J Pharmacol. 2015;172:3159–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pushpakumar S, Ren L, Kundu S, et al. Toll-like receptor 4 deficiency reduces oxidative stress and macrophage mediated inflammation in hypertensive kidney. Sci Rep. 2017;7:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nunes KP, Bomfim GF, Toque HA, et al. Toll-like receptor 4 (TLR4) impairs nitric oxide contributing to angiotensin II-induced cavernosal dysfunction. Life Sci. 2017;191:219–226. [DOI] [PubMed] [Google Scholar]
- 77.Bannerman DD, Erwert RD, Winn RK, et al. TIRAP mediates endotoxin-induced NF-кB activation and apoptosis in endothelial cells. Biochem Biophys Res Commun. 2002;295:157–162. [DOI] [PubMed] [Google Scholar]
- 78.Bannerman DD, Tupper JC, Erwert RD, et al. Divergence of bacterial lipopolysaccharide pro-apoptotic signaling downstream of IRAK-1. J Biol Chem. 2002;277:8048–8053. [DOI] [PubMed] [Google Scholar]
- 79.Kim SH, Chu HJ, Kang DH, et al. NF-kappa B binding activity and cyclooxygenase-2 expression in persistent CCl(4)-treated rat liver injury. J Korean Med Sci. 2002;17:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bomfim GF, Echem C, Martins CB, et al. Toll-like receptor 4 inhibition reduces vascular inflammation in spontaneously hypertensive rats. Life Sci. 2015;122:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McCarthy CG, Wenceslau CF, Goulopoulou S, et al. Circulating mitochondrial DNA and Toll-like receptor 9 are associated with vascular dysfunction in spontaneously hypertensive rats. Cardiovasc Res. 2015;107:119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Veiko NN, Konoroe P, Douin-Echinard V, et al. Toll-like receptors 2-deficient mice are protected against postischemic coronary endothelial dysfunction. Arterioscly Ontogenesis Biomed Khim. 2010;56:686–699.21395071 [Google Scholar]
- 83.Shintani Y, Drexler HC, Kioka H, et al. Toll-like receptor 9 protects non-immune cells from stress by modulating mitochondrial ATP synthesis through the inhibition of SERCA2. EMBO Rep. 2014;15:438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mccarthy CG, Wenceslau CF, Goulopoulou S, et al. Chloroquine suppresses the development of hypertension in spontaneously hypertensive rats. Am J Hypertens. 2017;30:173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mccarthy CG, Wenceslau CF, Goulopoulou S, et al. Autoimmune therapeutic chloroquine lowers blood pressure and improves endothelial function in spontaneously hypertensive rats. Pharmacol Res. 2016;113:384–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wallace DJ. Does hydroxychloroquine sulfate prevent clot formation in systemic lupus erythematosus? Arthritis Arthritis Rheum. 1987;30:1435–1436. [DOI] [PubMed] [Google Scholar]
- 87.Gómez-guzmán M, Jiménez R, Romero M, et al. Chronic hydroxychloroquine improves endothelial dysfunction and protects kidney in a mouse model of systemic lupus erythematosus. Hypertension. 2014;64:330–337. [DOI] [PubMed] [Google Scholar]
- 88.Goulopoulou S, Wenceslau CF, McCarthy CG, et al. Exposure to stimulatory CpG oligonucleotides during gestation induces maternal hypertension and excess vasoconstriction in pregnant rats. Am J Physiol Circ Physiol. 2016;310:1015–1025. [DOI] [PubMed] [Google Scholar]
- 89.Shishido T, Nozaki N, Takahashi H, et al. Central role of endogenous Toll-like receptor-2 activation in regulating inflammation, reactive oxygen species production, and subsequent neointimal formation after vascular injury. Biochem Biophys Res Commun. 2006;345:1446–1453. [DOI] [PubMed] [Google Scholar]
- 90.Speer T, Rohrer L, Blyszczuk P, et al. Abnormal high-density lipoprotein induces endothelial dysfunction via activation of toll-like receptor-2. Immunity. 2013;38:754–768. [DOI] [PubMed] [Google Scholar]
- 91.Favre J, Musette P, Douin-Echinard V, et al. Toll-like receptors 2-deficient mice are protected against postischemic coronary endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2007;27:1064–1071. [DOI] [PubMed] [Google Scholar]
- 92.Ahn KO, Lim SW, Li C, et al. Influence of angiotensin II on expression of toll-like receptor 2 and maturation of dendritic cells in chronic cyclosporine nephropathy. Transplantation. 2007;83:938–947. [DOI] [PubMed] [Google Scholar]
- 93.Tinsley JH, Chiasson VL, Mahajan A, et al. Toll-like receptor 3 activation during pregnancy elicits preeclampsia-like symptoms in rats. Am J Hypertens. 2009;22:1314–1319. [DOI] [PubMed] [Google Scholar]
- 94.Chatterjee P, Chiasson VL, Kopriva SE, et al. Interleukin 10 deficiency exacerbates toll-like receptor 3-induced preeclampsia-like symptoms in mice. Hypertension. 2011;58:489–96. [DOI] [PubMed] [Google Scholar]
- 95.Singh XMV, Cicha MZ, Nunez S, et al. Angiotensin II-induced hypertension and cardiac hypertrophy are differentially mediated by TLR3-and TLR4-dependent pathways. Am J Physiol Hear Circ Physiol. 2019; 316:H1027–H1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cole JE, Navin TJ, Cross AJ, et al. Unexpected protective role for Toll-like receptor 3 in the arterial wall. Proc Natl Acad Sci U S A. 2011;108:2372–2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hijmans JG, DIehl KJ, Bammert TD, et al. Association between hypertension and circulating vascular-related microRNAs. J Hum Hypertens. 2018;32:440–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Deane JA, Pisitkun P, Barrett RS, et al. Control of TLR7 expression is essential to restrict autoimmunity and dendritic cell expansion. Control. 2007;27:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Orton SM, Herrera BM, Yee IM, et al. Sex ratio of multiple sclerosis in Canada: a longitudinal study. Lancet Neurol. 2006;5:932–936. [DOI] [PubMed] [Google Scholar]
- 100.Butterworth M, McClellan B, Aklansmith M. Influence of sex on immunoglobulin levels. Nature. 1967;214:1224–1225. [DOI] [PubMed] [Google Scholar]
- 101.Eaton WW, Rose NR, Kalaydjian A, et al. Epidemiology of autoimmune diseases in Denmark. J Autoimmun. 2007;29:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Feldman CH, Hiraki LT, Liu J, et al. Epidemiology and sociodemographics of systemic lupus erythematosus and lupus nephritis among US adults with Medicaid coverage, 2000-2004. Arthritis Rheum. 2013;65:753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wright CL, Hoffman JH, McCarthy MM. Evidence that inflammation promotes estradiol synthesis in human cerebellum during early childhood. Transl Psychiatry. 2019;9:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Berghöfer B, Frommer T, Haley G, et al. TLR7 ligands induce higher IFN-α production in females. J Immunol. 2006;177:2088–2096. [DOI] [PubMed] [Google Scholar]
- 105.Khan N, Summers CW, Helbert MR, et al. Effects of age, gender, and immunosuppressive agents on in vivo toll-like receptor pathway responses. Hum Immunol. 2010;71:372–376. [DOI] [PubMed] [Google Scholar]
- 106.Torcia MG, Nencioni L, Clemente AM, et al. Sex differences in the response to viral infections: TLR8 and TLR9 ligand stimulation induce higher IL10 production in males. PLoS One. 2012;7:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Taneja V Sex hormones determine immune response. Front Immunol. 2018;9:1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Young NA, Wu LC, Burd CJ, et al. Estrogen modulation of endosome-associated toll-like receptor 8: an IFNα-independent mechanism of sex-bias in systemic lupus erythematosus. Clin Immunol. 2015;151:66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dennison U, McKernan DP, Scully P, et al. Menstrual cycle influences toll-like receptor responses. Neuroimmunomodulation. 2012;19:171–179. [DOI] [PubMed] [Google Scholar]
- 110.Michel KG, Huijbregts RPH, Gleason JL, et al. Effect of hormonal contraception on the function of plasmacytoid dendritic cells and distribution of immune cell populations in the female reproductive tract. J Acquir Immune Defic Syndr. 2015;68:511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shey MS, Maharaj N, Archary D, et al. Modulation of female genital tract-derived dendritic cell migration and activation in response to inflammatory cytokines and toll-like receptor agonists. PLoS One. 2016;11:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maria S, Niklaas C, Hendrik S, et al. Innate immune responses to toll-like receptor stimulation are altered during the course of pregnancy. J Reprod Immunol. 2018;128:30–37. [DOI] [PubMed] [Google Scholar]
- 113.Seillet C, Laffont S, Tre F, et al. The TLR-mediated response of plasmacytoid dendritic cells is positively regulated by estradiol in vivo through cell-intrinsic estrogen receptor α signaling. Immunobiology. 2012;119:454–464. [DOI] [PubMed] [Google Scholar]
- 114.Meier A, Chang JJ, Chan ES, et al. Sex differences in the TLR-mediated response of pDCs to HIV-1 are associated with higher immune activation in infected women. Nat Med. 2009;15:955–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ziegler SM, Beisel C, Sutter K, et al. Human pDCs display sex-specific differences in type I interferon subtypes and interferon α/β receptor expression. Eur J Immunol. 2017;47:251–256. [DOI] [PubMed] [Google Scholar]
- 116.Webb K, Peckham H, Radziszewska A, et al. Sex and pubertal differences in the type 1 interferon pathway associate with both X chromosome number and serum sex hormone concentration. Front Immunol. 2019;9:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Scotland RS, Stables MJ, Madalli S, et al. Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice. Blood. 2011;118:5918–5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Traub S, Demaria O, Chasson L, et al. Sex bias in susceptibility to MCMV infection: implication of TLR9. PLoS One. 2012;7:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Roberts BJ, Moussawi M, Huber SA. Sex differences in TLR2 and TLR4 expression and their effect on coxsackievirus-induced autoimmune myocarditis. Exp Mol Pathol. 2013;94:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Seillet C, Rouquié N, Foulon E, et al. Estradiol promotes functional responses in inflammatory and steady-state dendritic cells through differential requirement for activation function-1 of estrogen receptor α. J Immunol. 2013;190:5459–5470. [DOI] [PubMed] [Google Scholar]
- 121.Laffont S, Rouquié N, Azar P, et al. X-chromosome complement and estrogen receptor signaling independently contribute to the enhanced TLR7-mediated IFN-α production of plasmacytoid dendritic cells from women. J Immunol. 2014;193:5444–5452. [DOI] [PubMed] [Google Scholar]
- 122.Marriott I, Bost KL, Huet-hudson YM. Sexual dimorphism in expression of receptors for bacterial lipopolysaccharides in murine macrophages: a possible mechanism for gender-based differences in endotoxic shock susceptibility. J Reprod Immunol. 2006;71:12–27. [DOI] [PubMed] [Google Scholar]
- 123.Rettew JA, Huet-hudson YM, Marriott I. Testosterone reduces macrophage expression in the mouse of Toll-like receptor 4, a trigger for inflammation and innate immunity macrophage-like cell line culture. Biol Reprod. 2008;78:432–437. [DOI] [PubMed] [Google Scholar]
- 124.Zandieh Z, Amjadi F, Ashrafi M, et al. The effect of estradiol and progesterone on toll like receptor gene expression in a human fallopian tube epithelial cell line. Cell J. 2016;17:678–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zandieh Z, Amjadi F, Vakilian H, et al. Sex hormones alter the response of Toll-like receptor 3 to its specific ligand in fallopian tube epithelial cells. Clin Exp Reprod Med. 2018;45:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Flannery LE, Henry RJ, Kerr DM, et al. FAAH, but not MAGL, inhibition modulates acute TLR3-induced neuroimmune signaling in the rat, independent of sex. J Neurosci Res. 2018;96:989–1001. [DOI] [PubMed] [Google Scholar]
- 127.Umehara T, Tsujita N, Id MS. Activation of Toll-like receptor 7/8 encoded by the X chromosome alters sperm motility and provides a novel simple technology for sexing sperm. PLoS Biol. 2019;17:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schaefer L Complexity of danger: the diverse nature of damage-associated molecular patterns. J Biol Chem. 2014;289:35237–35245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Maeda A, Fadeel B. Mitochondria released by cells undergoing TNF-α-induced necroptosis act as danger signals. Cell Death Dis. 2014;5:e1312–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang Q, Raoof M, Chen Y, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol. 2001;167:2887–2894. [DOI] [PubMed] [Google Scholar]
- 132.Roh JS, Sohn DH. Damage-associated molecular patterns in inflammatory diseases. Immune Netw. 2018;18:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fairbanks SL, Young JM, Nelson JW, et al. Mechanism of the sex difference in neuronal ischemic cell death. Neurosci. 2012;6:183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Spritzer MD, Panning AW, Engelman SM, et al. Seasonal and sex differences in cell proliferation, neurogenesis, and cell death within the dentate gyrus of adult wild-caught meadow voles. Neuroscience. 2017;360:155–165. [DOI] [PubMed] [Google Scholar]
- 135.Jordan JJ, Chhim S, Margulies CM, et al. ALKBH7 drives a tissue and sex-specific necrotic cell death response following alkylation-induced damage. Cell Death Dis. 2017;8:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Echem C, Costa da TJ, Oliveira V, et al. Mitochondrial DNA: a new driver for sex differences in spontaneous hypertension. Pharmacol Res. 2019;144:142–150. [DOI] [PubMed] [Google Scholar]
- 137.Tipton AJ, Sullivan JC. Sex and gender differences in T cells in hypertension. Clin Ther. 2014;36:1882–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Koupenova M, Mick E, Mikhalev E, et al. Sex differences in platelet toll-like receptors and their association with cardiovascular risk factors. Arter Thromb Vasc Biol. 2015;35:1030–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sales ML, Schreiber R, Ferreira-sae MCS, et al. Toll-like receptor 6 Ser249Pro polymorphism is associated with lower left ventricular wall thickness and inflammatory response in hypertensive women. Am J Hypertens. 2009;23:649–654. [DOI] [PubMed] [Google Scholar]
- 140.Ha T, Li Y, Hua F, et al. Reduced cardiac hypertrophy in toll-like receptor 4-deficient mice following pressure overload. Cardiovasc Res. 2005;68:224–234. [DOI] [PubMed] [Google Scholar]
- 141.Ha T, Hua F, Li Y, et al. Blockade of MyD88 attenuates cardiac hypertrophy and decreases cardiac myocyte apoptosis in pressure overload-induced cardiac hypertrophy in vivo. Am J Physiol Hear Circ Physiol. 2019;290:985–994. [DOI] [PubMed] [Google Scholar]
- 142.Edfeldt K, Bennet AM, Eriksson P, et al. Association of hyporesponsive toll-like receptor 4 variants with risk of myocardial infarction. Eur Heart J. 2004;25:1447–1453. [DOI] [PubMed] [Google Scholar]
- 143.Afkham A, Eghbal-Fard S, Heydarlou H, et al. Toll-like receptors signaling network in pre-eclampsia: an updated review. J Cell Physiol. 2018;234:2229–2240. [DOI] [PubMed] [Google Scholar]
- 144.Kim YM, Romero R, Oh Y, et al. Toll-like receptor 4: a potential link between “danger signals,” the innate immune system, and preeclampsia? Am J Obstet Gynecol. 2005;193:1–8. [DOI] [PubMed] [Google Scholar]
- 145.Pineda A, Verdin-ter SL, Camacho A, et al. Expression of toll-like receptor TLR-2 , TLR-3 , TLR-4 and TLR-9 is increased in placentas from patients with preeclampsia. Arch Med Res. 2011;42:382–391. [DOI] [PubMed] [Google Scholar]
- 146.Chatterjee P, Weaver LE, Doersch KM, et al. Placental toll-like receptor 3 and toll-like receptor 7/8 activation contributes to preeclampsia in humans and mice. PLoS One. 2012;7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chatterjee P, Chiasson VL, Seerangan G, et al. Cotreatment with interleukin 4 and interleukin 10 modulates immune cells and prevents hypertension in pregnant mice. Am J Hypertens. 2015;28:135–142. [DOI] [PubMed] [Google Scholar]
- 148.Chatterjee P, Chiasson VL, Pinzur L, et al. Human placenta-derived stromal cells decrease inflammation, placental injury and blood pressure in hypertensive pregnant mice. Clin Sci. 2016;130:513–523. [DOI] [PubMed] [Google Scholar]
- 149.Chatterjee P, Chiasson VL, Seerangan G, et al. Depletion of MHC class II invariant chain peptide or γ–δ T-cells ameliorates experimental preeclampsia. Clin Sci. 2017;131:2047–2058. [DOI] [PubMed] [Google Scholar]
- 150.Goulopoulou S, Matsumoto T, Bomfim GF, et al. Preeclampsia, Toll-like receptor 9 activation: a novel mechanism linking placenta-derived mitochondrial DNA and vascular dysfunction in preeclampsia. Clin Sci. 2012;123:429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]


