Abstract
Purpose:
Myelodysplastic syndromes (MDS) are a class of clonal neoplastic disorders of largely unknown etiology, and published data remain inconclusive regarding the association between lifetime alcohol consumption and MDS risk. In these analyses, data from a population-based case-control study were used to investigate this association.
Methods:
Eligible cases of MDS were identified through the Minnesota Cancer Reporting System; controls were matched by sex and age-decile. A central review process was used to confirm MDS diagnosis and classify subtypes. Unconditional and polytomous logistic regression were used to calculate odds ratios (OR) and 95% confidence intervals (CI). Kaplan-Meier curves were used to compare survival by category of lifetime alcohol consumption.
Results:
In total, 398 cases of MDS and 698 controls were included. Alcohol consumption at 23–30, 31–49, and 50–65 years of age, recent consumption 1 year before diagnosis/interview, and lifetime consumption were not found to be significantly associated with MDS in males (OR range 0.63–0.99) or females (OR range 0.58–1.70). Analysis by MDS subtype further suggested there was not a significant association between recent alcohol consumption and odds of disease by subtype (OR range 0.39–1.13). Lifetime alcohol consumption was not significantly associated with survival after diagnosis of MDS
Conclusions:
Previously reported associations between alcohol consumption and MDS risk were inconsistent. Results from our analyses by sex and disease subtype do not support alcohol as a significant contributor to risk for MDS.
Keywords: Alcohol, myelodysplastic syndromes, epidemiology, case-control
Introduction
Ineffective hematopoiesis is the hallmark of myelodysplastic syndromes (MDS), a heterogeneous group of rare blood disorders [1, 2]. MDS are classified as clonal neoplasms of the myeloid stem cells, and they are further categorized into disease subtypes based on the degree of atypical proliferation and differentiation of the myeloid cells [2]. Changes in cell morphology are the result of acquired mutations over time [2], and nearly a third of individuals with MDS will later progress to acute myeloid leukemia (AML) [2, 3]. Incidence rates for MDS are estimated to be 4.8 per 100,000 people in the United States [4], although these reported estimates are expected to be lower than actual incidence rates due to underdiagnosis and underreporting [5–7].
The World Health Organization (WHO) re-classified MDS as a malignant neoplasm in 2000 [8]. Thus, population-based incidence data were not available prior to 2001 [5, 6] and etiology data are limited due to a lack of studies. Previous epidemiological studies conducted have identified few consistent factors. Prior chemotherapy and cancer treatments are well-documented risk factors for disease [9–12], in addition to occupational and environmental exposures to benzene, organic solvents, and agricultural pesticides and fertilizers [13–17]. Lifestyle risk factors such as tobacco use [2, 14, 16–20] and obesity [21] have also been implicated in MDS risk. It is suggested that these factors only partially explain the risk for MDS, with additional factors yet to be discovered [16, 22].
Prior studies have linked chronic alcohol consumption to hematopoietic disorders and changes in cell morphology during typical hematopoiesis [23]. A Finnish study reviewed 144 general hospital patients undergoing bone marrow examinations and peripheral blood counts. Their findings demonstrated morphological changes in cell lines associated with erythropoiesis, including macrocytosis, thrombocytopenia, and other cytopenias. Although the biological mechanism behind these changes is not entirely understood, they are the likely result of the hemotoxic effects of alcohol and its metabolites [23, 24].
To date, the evidence for an association between alcohol consumption and risk of MDS has been inconclusive. Early investigations by Ido et al. and Brown et al. reported positive associations between disease and exposure, although not all associations reached statistical significance [19, 25]. Over a decade later, a number of other case-control studies reported decreased odds ratios (OR) amongst light drinkers compared to abstainers [5, 16, 26], but a meta-analysis published in 2010 suggested there were no significant associations overall [18]. The limited sample sizes in previous studies and the variation in alcohol assessment have made it difficult to generalize data and accurately quantify the association between alcohol consumption and MDS.
In order to address the lack of conclusive evidence, we used data from a population-based case-control study of MDS in Minnesota to further evaluate the association between lifetime alcohol consumption and risk for MDS. Thorough analysis of alcohol consumption at different life stages has not been assessed in prior studies of MDS to date, thus providing a novel approach to investigating this association.
Methods
Human subjects research:
The Institutional Review Boards for each participating institution approved this study, which included the University of Minnesota, the Mayo Clinic, the Minnesota Department of Health, and area hospitals. Informed consent was obtained from all participants.
Case recruitment:
Cases were identified through the population-based Minnesota Cancer Reporting System (MCRS) with a diagnosis date between April 1, 2010 and October 31, 2014. Cases were eligible for the study if they had a diagnosis of MDS based on the following ICD-O-3 codes: 9980, 9982–9987, 9989 [8]. English and Spanish-speaking residents of Minnesota between 20 and 85 years of age were eligible for the study.
Following enrollment in the study, central review of pathology reports and diagnostic bone marrow specimens was completed by two pathologists, one cytogeneticist, and one medical oncologist to confirm diagnosis of MDS and classify by subtype using the 2008 WHO classification scheme [27]. In total, 398 cases with confirmed MDS were included in these analyses. MDS subgroups used for analyses included: refractory anemia (RA), refractory neutropenia (RN), and refractory thrombocytopenia (RT); refractory anemia with ring sideroblasts (RARS); refractory cytopenia with multilineage dysplasia (RCMD); refractory anemia with excess blasts, types 1 (RAEB-1) and 2 (RAEB-2); MDS with an isolated deletion of 5q (MDS del(5q)); therapy-related MDS (t-MDS); and other types of MDS, which contained cases of unclassifiable and not-otherwise-specified MDS (MDS other). Fifty-nine percent of patients referred to the study completed the interview. Questionnaire completion rates were slightly higher in males compared with females (p=0.06), but we did not observe differences in participating vs. non-participating cases when we compared the groups by MDS subtype (p=0.15), rural vs. urban residence (p=0.68) or age at diagnosis (median age = 75 years vs. 73 years, respectively; p=0.34). The median time from diagnosis to questionnaire completion was 140 days (range 7–1333 days).
Control recruitment:
The Minnesota State driver’s license/identification card list was used to identify and contact controls during the same study timeframe as cases (July 2010–July 2014). Controls were eligible for enrollment if they were Minnesota residents, between the ages of 20 to 85, understood either English or Spanish, and had no history of myeloid neoplasia. Eligible controls were frequency matched to cases by sex and age-decile. A total of 698 controls were recruited (49% response rate). Participating and non-participating controls did not differ with respect to sex (p=0.94), residence in a rural vs. urban location (p=0.65), or BMI (p=0.84). Participating controls were older than non-participating controls (median = 71 years vs. 67 years, respectively, p=0.03). The median time from first contact to questionnaire completion date was 39 days (2–1130 days).
Exposure assessment:
A self-administered risk factor questionnaire was provided to cases and controls to collect exposure data. The questionnaire included self-reported information on demographics, lifestyle, medical history, and occupational and environmental history.
Alcohol use over the lifetime was categorized based on sex and the 2015–2020 Dietary Guidelines from the U.S. Department of Health and Human Services and the U.S. Department of Agriculture [28]. Alcohol consumption was classified as any type of alcohol, including beer, wine, or liquors. In total, five categories were used to classify alcohol consumption for each sex. Consumption in males was classified into the following groups: abstainers or non-drinkers (none), occasional drinkers (<1 per month to 1–3 per month), light drinkers (1–2 per week to 3–6 per week), moderate drinkers (1–2 per day), and heavy drinkers (3+ per day). Females were grouped into similar categories: abstainers or non-drinkers (none), occasional drinkers (<1 per month to 1–3 per month), light drinkers (1–2 per week), moderate drinkers (3–6 per week to 1–2 per day), and heavy drinkers (3+ per day). For both sexes, moderate and heavy drinkers were combined into one group due to small sample sizes. Alcohol use was assessed at 23–30, 31–49, and 50–65 years of age, and 1 year prior to MDS diagnosis for cases or 1 year prior to the study interview for controls. Additionally, a composite score of lifetime alcohol consumption was estimated using methods previously described by Kunzmann et al [29]. Briefly, alcohol consumption was first normalized by amount and frequency (drinks per week) and then estimated as a weighted average of weekly consumption, which was then multiplied by the number of years in each age category. The resulting value for average annual consumption over the lifetime was then categorized into groups as described above for males and females.
Due to the matched study design, analyses were adjusted for age group. Other potential confounders included race and ethnicity (non-Hispanic white [NHW], other); education level (≤high school graduate, some post high school education, college graduate); annual household income (≤$40,000, $40,000-$80,000, >$80,000); personal history of cancer, excluding skin cancer (yes, no); smoking status (never, former, current smoker); benzene exposure (yes, no); chemotherapy exposure (yes, no); radiation exposure (yes, no); and BMI category (normal weight, 18.5–24.9 kg/m2; overweight, 25–29.9 kg/m2; obese, ≥30 kg/m2). Individuals that were underweight (<18.5 kg/m2) 2 years prior to diagnosis for cases or 2 years prior to questionnaire completion for controls were excluded.
Statistical analysis:
The associations between MDS and alcohol consumption, selected study population characteristics, and potential effect modifiers and confounders were evaluated using unconditional or polytomous logistic regression, as appropriate. Crude and adjusted ORs are reported with 95% confidence intervals (CI) and two-sided p-values. All analyses were stratified by either sex or disease subtype, and they were adjusted by continuous age or continuous age and sex to control for matching. Crude and adjusted odds ratios were not calculated for groups with fewer than 5 participants.
Potential confounders included previously reported risk factors or variables that were significantly correlated with recent alcohol consumption, MDS, or both. Furthermore, covariates that changed the crude and adjusted associations between lifetime alcohol consumption and MDS by more than 10% were considered for the final model. Independent variables were individually added to the regression model until all potential confounders were included; likelihood ratios and c-statistics were compared. The final unconditional logistic regression model, which included potential confounders, was used to evaluate the association between lifetime alcohol use and MDS by sex; polytomous logistic regression was used to evaluate the association between lifetime alcohol use and MDS by subtype with controls as the reference category.
Kaplan-Meier survival curves were used to compare survival curves across categories of lifetime alcohol consumption. A log-rank p-value was used to test for significant differences across groups.
Statistical analyses were performed using SAS Version 9.4 (SAS Institute Inc., Cary, NC, USA)
Results
We included 255 male and 143 female cases and 438 male and 260 female controls that met all inclusion criteria (Table 1). The median age of male cases and controls was 73 and 70.5 years, respectively. The median age for their female counterparts was 72.5 years for both cases and controls. The majority of participants reported their race as NHW (cases 98%; controls 97%); therefore, further adjustment by race was not included in final regression models. The majority of participants also reported drinking alcohol. For cases, 89% of males and 85% of females reported drinking alcohol. For controls, 90% of males and 86% of females reported drinking alcohol. For both sexes, there were no significant differences in education level, household income, personal history of cancer, radiation exposure, or BMI category between cases and controls. A significant positive association with former smoking and case-status was only observed in females, while a significant positive association with prior benzene exposure and case-status was only observed in males. Additionally, a significant positive association between prior chemotherapy exposure and case-status was observed in both males (OR 2.65, 95% CI 1.30–5.41) and females (OR 3.36, 95% CI 1.31–8.63).
Table 1.
Selected demographics and characteristics of study population by sex
| Males | Females | |||||||
|---|---|---|---|---|---|---|---|---|
| Cases, n (%) | Controls, n (%) | OR (95% CI)a | p value | Cases, n (%) | Controls, n (%) | OR (95% CI)a | p value | |
| n | 255 | 438 | 143 | 260 | ||||
| Age (years) | ||||||||
| < 50 | 6 (3) | 7 (2) | - | - | 4 (3) | 9 (3) | - | - |
| 50–59 | 23 (9) | 37 (8) | 15 (11) | 69 (27) | ||||
| 60–69 | 72 (28) | 163 (37) | 35 (24) | 38 (15) | ||||
| 70–79 | 92 (36) | 173 (40) | 59 (41) | 112 (43) | ||||
| ≥ 80 | 62 (24) | 58 (13) | 30 (21) | 32 (12) | ||||
| Median | 73 | 70.5 | 72.5 | 72.5 | ||||
| Education level | ||||||||
| ≤ High school graduate | 88 (35) | 127 (29) | Ref | 0.67 | 57 (40) | 76 (29) | Ref | 0.30 |
| Some post high school | 68 (27) | 131 (30) | 0.91 (0.58, 1.41) | 41 (29) | 95 (37) | 0.68 (0.39, 1.20) | ||
| College graduate | 98 (38) | 179 (41) | 1.11 (0.70, 1.75) | 44 (31) | 88 (34) | 1.01 (0.57, 1.80) | ||
| Household income | ||||||||
| ≤ $40,000 | 98 (39) | 131 (31) | Ref | 0.24 | 73 (53) | 112 (45) | Ref | 0.12 |
| $40,000 – 80,000 | 90 (36) | 168 (39) | 0.70 (0.46, 1.06) | 52 (38) | 83 (33) | 1.21 (0.72, 2.04) | ||
| > $80,000 | 62 (25) | 127 (30) | 0.76 (0.46, 1.25) | 13 (9) | 56 (22) | 0.56 (0.26, 1.20) | ||
| Personal history of cancer | ||||||||
| No | 180 (71) | 355 (81) | Ref | 0.85 | 100 (70) | 217 (83) | Ref | 0.81 |
| Yes | 75 (29) | 83 (19) | 0.95 (0.58, 1.57) | 43 (30) | 43 (17) | 1.10 (0.51, 2.38) | ||
| Smoking status | ||||||||
| Never smoker | 96 (38) | 190 (43) | Ref | 0.26 | 65 (46) | 151 (58) | Ref | 0.04 |
| Former smoker | 140 (55) | 205 (47) | 1.33 (0.92, 1.92) | 62 (43) | 84 (33) | 1.93 (1.16, 3.23) | ||
| Current smoker | 18 (7) | 42 (10) | 0.99 (0.52, 1.90) | 16 (11) | 24 (9) | 1.66 (0.74, 3.74) | ||
| Benzene exposure | ||||||||
| No | 199 (78) | 367 (84) | Ref. | 0.02 | 134 (94) | 251 (96) | Ref | 0.23 |
| Yes | 56 (22) | 71 (16) | 1.68 (1.10, 2.55) | 8 (5.6) | 9 (3.5) | 1.88 (0.67, 5.27) | ||
| Chemotherapy exposure | ||||||||
| No | 218 (85) | 417 (95) | Ref | 0.008 | 114 (80) | 241 (93) | Ref | 0.01 |
| Yes | 37 (15) | 21 (4.8) | 2.65 (1.30, 5.41) | 29 (20) | 19 (7.3) | 3.36 (1.31, 8.63) | ||
| Radiation exposure | ||||||||
| No | 220 (86) | 413 (94) | Ref | 0.19 | 122 (85) | 239 (92) | Ref | 0.51 |
| Yes | 35 (14) | 25 (5.7) | 1.59 (0.80, 3.16) | 21 (15) | 21 (8.1) | 0.73 (0.28, 1.89) | ||
| BMI categoryb | ||||||||
| normal weight, 18.5–24.9 kg/m2 | 58 (23) | 84 (19) | Ref | 0.52 | 46 (32) | 86 (33) | Ref | 0.85 |
| overweight, 25–29.9 kg/m2 | 105 (41) | 203 (46) | 0.78 (0.50, 1.20) | 46 (32) | 90 (35) | 0.94 (0.54, 1.66) | ||
| obese, ≥30 kg/m2 | 91 (36) | 151 (34) | 0.83 (0.53, 1.31) | 49 (35) | 79 (31) | 1.10 (0.63, 1.94) |
Abbreviations: Confidence interval (CI); Odds ratio (OR); Reference level (Ref)
Columns may not sum to totals due to missing data; matching variables included age-decile and sex; ORs not calculated for age group.
Adjusted for age (continuous), all other variables included in table, and lifetime alcohol consumption
Excludes 1 male and 3 females who were underweight; based on weight reported 2 years prior to diagnosis for cases and 2 years prior to questionnaire completion for controls
The distribution of alcohol use as well as the crude and adjusted associations between alcohol and MDS risk by sex are depicted in Table 2. Estimates using male and female data combined are also included. Alcohol consumption was modeled independently at different time points throughout adulthood. Reports of alcohol consumption at different ages were significantly correlated within individuals across all age categories (p<0.0001). In the crude analysis, alcohol consumption was not statistically significantly associated with MDS in any age group evaluated. In females, no significant associations were observed in the adjusted models for alcohol consumption in any age group, for recent alcohol consumption, or for lifetime alcohol consumption. In males, adjusted ORs were at or slightly below the null for all categories of alcohol consumption in all age groups. A significant negative association between lifetime alcohol consumption and MDS risk was observed for light drinkers (OR 0.63, 95%CI 0.40–0.99). The negative association with light alcohol consumption was also observed in the adjusted analyses of males and females combined (OR 0.71, 95% CI 0.50–0.99). No other significant associations were observed in the combined analysis.
Table 2.
Association between lifetime alcohol consumption and risk of MDS by sex
| Males | Females | Combined | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases, n (%) | Controls, n (%) | Crude OR (95%CI) | Adjusted OR (95% CI)a | Cases, n (%) | Controls, n (%) | Crude OR (95%CI) | Adjusted OR (95% CI)a | Crude OR (95%CI) | Adjusted OR (95% CI)a | |
| Alcohol consumption at 23–30 years | ||||||||||
| Abstainer | 33 (13) | 57 (13) | 0.85 (0.50, 1.45) | 0.81 (0.47, 1.41) | 31 (22) | 55 (21) | 1.21 (0.70, 2.09) | 1.43 (0.79, 2.59) | 1.01 (0.70, 1.49) | 1.07 (0.72, 1.60) |
| Occasional | 67 (26) | 99 (23) | Ref | Ref | 57 (40) | 124 (48) | Ref | Ref | Ref | Ref |
| Light | 113 (45) | 207 (47) | 0.81 (0.55, 1.20) | 0.73 (0.48, 1.10) | 27 (19) | 36 (14) | 1.74 (0.96, 3.16) | 1.51 (0.71, 2.89) | 1.05 (0.77, 1.42) | 0.95 (0.69, 1.31) |
| Moderate/Heavy | 40 (16) | 73 (17) | 0.87 (0.53, 1.43) | 0.71 (0.41, 1.22) | 27 (19) | 44 (17) | 1.49 (0.82, 2.69) | 1.46 (0.77, 2.79) | 1.11 (0.76, 1.63) | 0.96 (0.64, 1.45) |
| Alcohol consumption at 31–49 years | ||||||||||
| Abstainer | 40 (16) | 71 (16) | 0.89 (0.53, 1.47) | 0.92 (0.55, 1.57) | 28 (20) | 56 (22) | 0.90 (0.52, 1.57) | 1.07 (0.59, 1.95) | 0.90 (0.62, 1.31) | 1.00 (0.68, 1.48) |
| Occasional | 61 (24) | 99 (23) | Ref | Ref | 63 (44) | 117 (45) | Ref | Ref | Ref | Ref |
| Light | 99 (40) | 187 (43) | 0.83 (0.55, 1.25) | 0.80 (0.52, 1.21) | 21 (15) | 42 (16) | 0.89 (0.48, 1.64) | 0.91 (0.47, 1.80) | 0.88 (0.64, 1.20) | 0.85 (0.61, 1.19) |
| Moderate/Heavy | 52 (20) | 79 (18) | 1.04 (0.65, 1.68) | 0.92 (0.56, 1.53) | 29 (21) | 44 (17) | 1.20 (0.68, 2.11) | 1.21 (0.65, 2.45) | 1.11 (0.78, 1.60) | 1.03 (0.70, 1.51) |
| Alcohol consumption at 50–65 years | ||||||||||
| Abstainer | 62 (25) | 100 (23) | 1.08 (0.69, 1.70) | 0.99 (0.62, 1.59) | 44 (32) | 65 (26) | 1.33 (0.81, 2.20) | 1.56 (0.91, 2.66) | 1.18 (0.85, 1.66) | 1.21 (0.85, 1.71) |
| Occasional | 61 (25) | 106 (25) | Ref | Ref | 59 (42) | 119 (47) | Ref | Ref | Ref | Ref |
| Light | 73 (29) | 145 (33) | 0.86 (0.56, 1.32) | 0.80 (0.51, 1.24) | 15 (11) | 31 (12) | 0.88 (0.43, 1.79) | 0.88 (0.41, 1.88) | 0.90 (0.64, 1.27) | 0.86 (0.60, 1.23) |
| Moderate/Heavy | 52 (21) | 82 (19) | 1.09 (0.68, 1.75) | 0.99 (0.60, 1.63) | 22 (15) | 38 (15) | 1.21 (0.65, 2.25) | 1.14 (0.57, 2.24) | 1.15 (0.80, 1.67) | 1.08 (0.73, 1.60) |
| Alcohol consumption within 1 year | ||||||||||
| Abstainer | 46 (18) | 79 (18) | 0.92 (0.58, 1.46) | 0.94 (0.58, 1.52) | 40 (28) | 77 (30) | 0.87 (0.53, 1.44) | 1.08 (0.63, 1.87) | 0.90 (0.64, 1.26) | 0.99 (0.69, 1.41) |
| Occasional | 78 (31) | 125 (29) | Ref | Ref | 63 (44) | 110 (42) | Ref | Ref | Ref | Ref |
| Light | 102 (40) | 178 (41) | 0.99 (0.68, 1.44) | 0.89 (0.60, 1.33) | 25 (18) | 31 (12) | 1.58 (0.85, 2.95) | 1.70 (0.87, 3.34) | 1.09 (0.80, 1.48) | 1.01 (0.72, 1.39) |
| Moderate/Heavy | 27 (11) | 54 (12) | 0.89 (0.51, 1.54) | 0.80 (0.45, 1.44) | 14 (10) | 42 (16) | 0.73 (0.36, 1.48) | 0.58 (0.27, 1.23) | 0.83 (0.54, 1.28) | 0.71 (0.45, 1.12) |
| Lifetime Alcohol Consumption | ||||||||||
| Abstainer | 29 (11) | 44 (10) | 0.88 (0.48, 1.60) | 0.82 (0.44, 1.53) | 24 (17) | 37 (14) | 1.08 (0.59, 2.00) | 1.34 (0.69, 2.60) | 0.99 (0.65, 1.51) | 1.04 (0.67, 1.63) |
| Occasional | 52 (20) | 71 (16) | Ref | Ref | 59 (42) | 103 (40) | Ref | Ref | Ref | Ref |
| Light | 111 (44) | 221 (50) | 0.70 (0.46, 1.08) | 0.63 (0.40, 0.99) | 27 (19) | 57 (22) | 0.84 (0.48, 1.48) | 0.73 (0.39, 1.36) | 0.79 (0.57, 1.08) | 0.71 (0.50, 0.99) |
| Moderate/Heavy | 62 (24) | 102 (23) | 0.85 (0.53, 1.37) | 0.74 (0.44, 1.24) | 32 (23) | 63 (24) | 0.92 (0.53, 1.58) | 0.82 (0.44, 1.49) | 0.91 (0.64, 1.30) | 0.79 (0.54, 1.16) |
Abbreviations: Confidence interval (CI); Myelodysplastic syndromes (MDS); Odds ratio (OR); Reference level (Ref)
Columns may not sum to totals due to missing data; matching variables included age-decile and sex
Adjusted for benzene exposure, BMI group, household income, radiation exposure, smoking status, and age (continuous)
We stratified MDS cases by WHO subtype to determine if alcohol was associated with any particular subtype of MDS (Table 3). We collapsed similar MDS subtypes together to increase sample sizes in the groups (RA/RN/RT and RARS; RCMD; RAEB1 and RAEB2; MDS del(5q); and t-MDS). For MDS del(5q), a significant negative association between lifetime alcohol consumption and MDS risk was observed for light drinkers (OR 0.32, 95% CI 0.11–0.86). All other adjusted models comparing each subtype to the control group suggested no significant associations between lifetime alcohol consumption and MDS risk.
Table 3.
Association between recent alcohol consumption and risk of MDS by subtype
| RA/RN/RT/RARS | RCMD | RAEB-1/RAEB-2 | MDS del(5q) | t-MDS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases, n(%) | Adjusted OR (95% CI)a | Cases, n(%) | Adjusted OR (95% CI)a | Cases, n(%) | Adjusted OR (95% CI)a | Cases, n(%) | Adjusted OR (95% CI)a | Cases, n(%) | Adjusted OR (95% CI)a | |
| n (%) | 72 (18) | 106 (27) | 128 (32) | 27 (7) | 40 (10) | |||||
| Lifetime Alcohol consumption | ||||||||||
| Abstainer | 8 (22) | 0.70 (0.28, 1.75) | 16 (15) | 1.14 (0.55, 2.34) | 17 (13) | 1.01 (0.53, 1.95) | 4 (15) | 1.08 (0.32, 3.72) | 4 (10) | 0.60 (0.16, 2.30) |
| Occasional | 19 (26) | Ref | 26 (25) | Ref | 37 (29) | Ref | 12 (44) | Ref | 12 (30) | Ref |
| Light | 25 (35) | 0.87 (0.44, 1.73) | 39 (37) | 0.73 (0.41, 1.30) | 46 (36) | 0.67 (0.40, 1.12) | 6 (22) | 0.39 (0.13, 1.19) | 14 (35) | 0.64 (0.25, 1.64) |
| Moderate/Heavy | 19 (26) | 1.13 (0.53, 2.40) | 25 (24) | 0.87 (0.46, 1.64) | 28 (22) | 0.68 (0.38, 1.22) | 5 (19) | 0.45 (0.14, 1.45) | 9 (22) | 0.80 (0.28, 2.29) |
Abbreviations: Confidence interval (CI); Myelodysplastic syndromes (MDS); MDS with deletion of 5q (MDS del(5q)); Odds ratio (OR); Reference (Ref); Refractory anemia (RA); Refractory anemia with excess blasts, types 1 (RAEB-1) and 2 (RAEB-2); Refractory anemia with ring sideroblasts (RARS); Refractory cytopenia with multilineage dysplasia (RCMD); Refractory neutropenia (RN); Refractory thrombocytopenia (RT); therapy-related MDS (t-MDS)
Columns may not sum to totals due to missing data for 2 cases, 2 controls, and excluded subgroups; MDS unclassifiable (n=21) and not-otherwise-specified (n=4) excluded from analyses; Matching variables included age-decile and sex;
Adjusted for benzene exposure, BMI group, household income, radiation exposure, smoking status, age (continuous), and sex
Median time from diagnosis to death or last follow-up was 888 days (range 58 – 3479 days) in the cases overall. We did not observe statistically significant differences in survival by category of lifetime alcohol consumption overall or in analyses stratified by sex (Figure 1).
Figure 1.
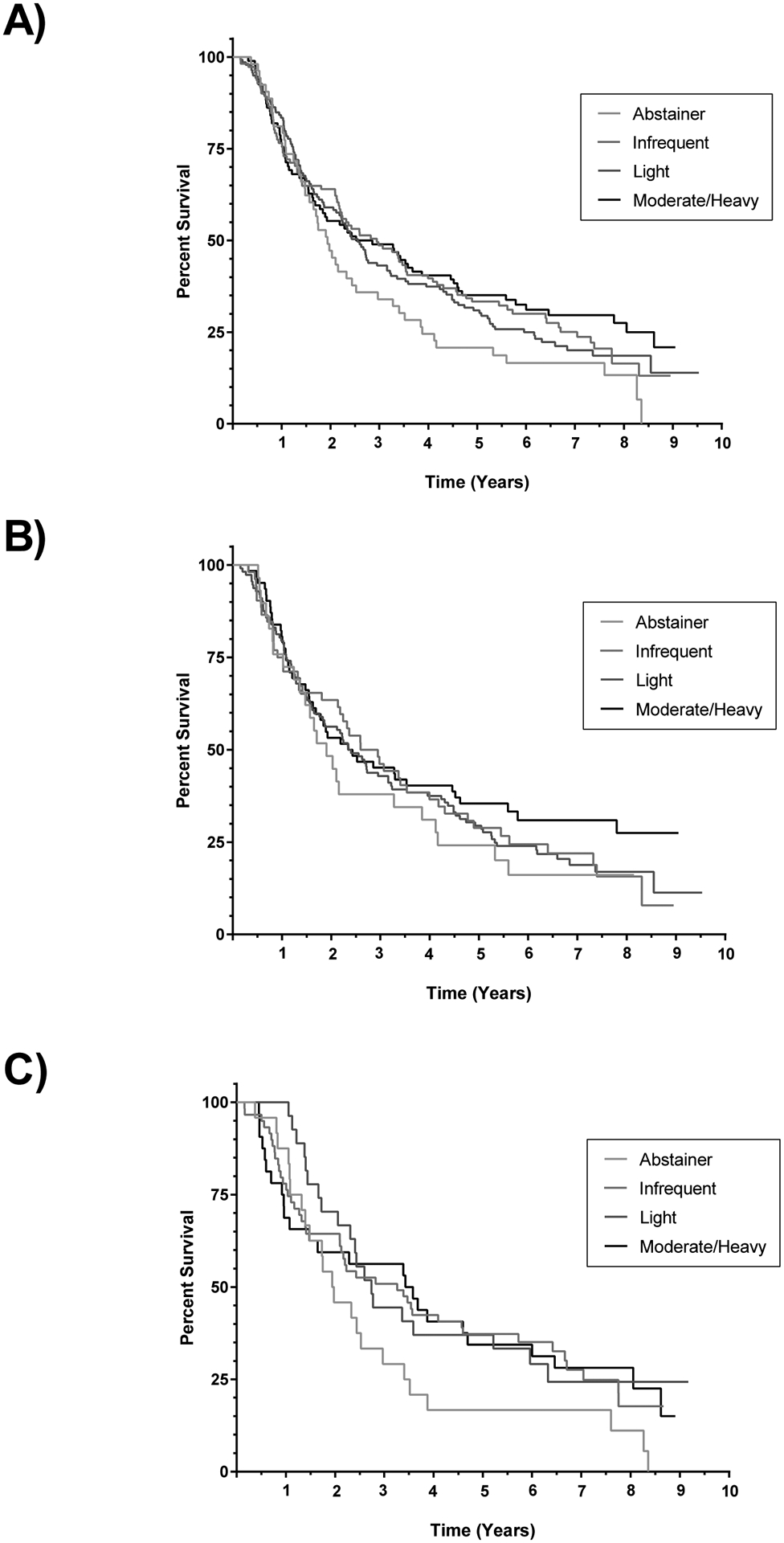
Kaplan-Meier survival curves comparing survival by category of lifetime alcohol consumption. + denotes censored. A) Comparison of survival in males and females combined. Log-rank p-value = 0.19. B) Comparison of survival in males. Log-rank p-value = 0.53. C) Comparison of survival in females. Log-rank p-value = 0.24.
Discussion
The association between lifetime alcohol consumption and MDS has not been previously assessed in a large-scale population-based study. Etiology of these rare blood disorders remains largely unknown due to disease heterogeneity and the limited number of studies that have been conducted. Previous studies assessing the relationship between alcohol and disease have reported inconsistent findings, suggesting harmful, protective, or inconclusive results. Additionally, prior studies of MDS risk and alcohol have solely relied on recent consumption data. The data reported here are the first investigation of lifetime alcohol use throughout early, middle, and late-adulthood. We did not find any evidence that alcohol is associated with MDS.
Our results suggesting that there is not a significant association between alcohol exposure and risk of MDS are consistent with other U.S. studies. A 2005 case-control study by Strom et al. reported no significant associations between drinkers (beer and liquor only) and non-drinkers. These non-significant ORs were observed when analyzed collectively (OR 0.77, 95% CI 0.52–1.13) and stratified by sex (male OR 0.82, 95% CI 0.51–1.30; female OR 0.58, 95% CI 0.27–1.27). Potential protective effects were observed in a wine drinker group (wine alone or in combination with beer and liquor) when compared to never drinkers [16]. Four years later, Ma et al. reported non-significant hazard ratios (HR range 0.38–1.19, p=0.83) comparing never drinkers to drinkers classified by tertile [30]. In the analyses presented here, dose response trends were inconsistent between males and females. Following stratification by sex and categorical alcohol consumption, adjusted regression models were limited by small sample sizes, particularly amongst females reporting moderate to heavy drinking. Therefore, there may be insufficient power to detect a true effect and we can’t rule out modest associations between alcohol consumption and MDS. In analyses where male and female data were combined to increase sample size, no meaningful associations were observed, further suggesting no significant associations between alcohol consumption and MDS risk.
Few previous studies have evaluated associations by MDS subtype due to small sample sizes. Analyses of subtype-specific risk factors could provide useful information for these heterogeneous disorders and would be especially relevant if modifiable risk factors were identified for higher risk subtypes. The association between lifetime alcohol consumption and risk for MDS by subtype was assessed here. We observed significant negative associations between light lifetime alcohol consumption in men (OR=0.63, 95% CI 0.40–0.99) and for the MDS del(5q) subtype (OR 0.32, 95% CI 0.11–0.86), but no other significant findings were observed among other subtypes or alcohol consumption groups and it is possible that these associations were due to chance due to the many comparisons we have made. We feel that small sample sizes in each subgroup limited our ability to draw conclusions. The only other study to evaluate associations by subtype to date reported an inverse association between wine consumption and RA/RARS (OR 0.52, 95% CI 0.29–0.95) and RAEB/RAEBT (OR 0.52, 95%CI 0.32–0.84) [16]. Antioxidants have been proposed as a possible mechanism for the protective association between wine and cancer [16, 31]. Our data do not distinguish between different types of alcohol; therefore, we were unable to evaluate associations specifically for wine consumption in our analysis.
The biological mechanisms and effects of ethanol on hematopoiesis are still under investigation, although possible links between hematologic disorders and alcohol use have been reported [23, 24]. In particular, multiple types of cytopenia, including anemia and thrombocytopenia, have been associated with chronic heavy alcohol consumption. Reactive compounds in alcohol cause hematoxicity, suppression of hematopoiesis, and cell damage, thus suggesting a potential connection to MDS and other blood disorders [23, 24].
Our large population-based study of MDS in Minnesota demonstrated many strengths in case/control ascertainment, data collection, and analysis. Cases were identified and approached shortly after diagnosis (median 140 days [range 7–1333 days]), and no proxy-interviews were conducted; rapid case ascertainment was utilized to minimize potential survival bias. Following case identification, controls were also selected from the population irrespective of exposure status.
Despite these strengths, there are also potential weaknesses that could limit our findings. Selection bias for both cases and controls is possible, especially in light of the low response rates in both case and control groups (59% and 49%, respectively). We were able to compare limited information between participants and non-participants, and data suggest that participants were likely to be slightly older than non-participants. Additionally, our analyses relied on self-reported data for many potential risk factors, and it is important to consider potential recall bias, under-reporting of alcohol consumption, and abstainer bias. To limit potential abstainer bias, or bias towards protective associations, occasional drinkers were used as the primary reference group for all analyses in this study [32]. Furthermore, unmeasured confounders could have contributed to the associations reported here, and the large majority of NHW participants limit the generalizability of the data. Finally, our data do not distinguish between different types of alcohol, limiting our ability to compare the impact of different types of alcohol.
These data contribute to the emerging literature surrounding alcohol as a risk factor for MDS. Our findings show no significant associations between lifetime or recent alcohol consumption and risk for MDS by sex and no significant association between lifetime alcohol consumption and risk for MDS by disease subtype. Further, they provide additional evidence to support previous studies that suggest alcohol is not a significant contributor to disease risk. Although some biological mechanisms demonstrate the toxicity of alcohol and a link to defective hematopoiesis, these data do not support a strong association between exposure and disease. Additional large-scale population studies of MDS are needed to further assess potential risk factors for disease, as much remains unknown.
Funding
This work was supported by the National Institutes of Health (JNP: R01CA142714).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Arber DA, Orazi A, Hasserjian R, et al. (2016) The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127:2391–2405. 10.1182/blood-2016-03-643544 [DOI] [PubMed] [Google Scholar]
- 2.Tefferi A, Vardiman JW (2009) Myelodysplastic syndromes. N Engl J Med 361:1872–1885. 10.1056/NEJMra0902908 [DOI] [PubMed] [Google Scholar]
- 3.Disperati P, Ichim CV, Tkachuk D, et al. (2006) Progression of myelodysplasia to acute lymphoblastic leukaemia: implications for disease biology. Leuk Res 30:233–239. 10.1016/j.leukres.2005.06.011 [DOI] [PubMed] [Google Scholar]
- 4.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) (2017) SEER Cancer Statistics Review 1975–2014. Table 30.1-Myelodysplastic Syndromes Counts, Percent, Age-Adjusted Rates by Subtype. 18 SEER Geographic Areas 2010–2014. National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, Bethesda, MD [Google Scholar]
- 5.Ma X (2012) Epidemiology of myelodysplastic syndromes. Am J Med 125:S2–5. 10.1016/j.amjmed.2012.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rollison DE, Howlader N, Smith MT, et al. (2008) Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001–2004, using data from the NAACCR and SEER programs. Blood 112:45–52. 10.1182/blood-2008-01-134858 [DOI] [PubMed] [Google Scholar]
- 7.Cogle CR (2015) Incidence and Burden of the Myelodysplastic Syndromes. Curr Hematol Malig Rep 10:272–281. 10.1007/s11899-015-0269-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fritz A, Percy C, Jack A, et al. (2000) International Classification of Diseases for Oncology, 3rd ed. World Health Organization [Google Scholar]
- 9.McLaughlin P, Estey E, Glassman A, et al. (2005) Myelodysplasia and acute myeloid leukemia following therapy for indolent lymphoma with fludarabine, mitoxantrone, and dexamethasone (FND) plus rituximab and interferon alpha. Blood 105:4573–4575. 10.1182/blood-2004-08-3035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrison VA, Rai KR, Peterson BL, et al. (2002) Therapy-related myeloid leukemias are observed in patients with chronic lymphocytic leukemia after treatment with fludarabine and chlorambucil: results of an intergroup study, cancer and leukemia group B 9011. J Clin Oncol Off J Am Soc Clin Oncol 20:3878–3884. 10.1200/JCO.2002.08.128 [DOI] [PubMed] [Google Scholar]
- 11.Pedersen-Bjergaard J, Larsen SO (1982) Incidence of acute nonlymphocytic leukemia, preleukemia, and acute myeloproliferative syndrome up to 10 years after treatment of Hodgkin’s disease. N Engl J Med 307:965–971. 10.1056/NEJM198210143071601 [DOI] [PubMed] [Google Scholar]
- 12.Pedersen-Bjergaard J, Daugaard G, Hansen SW, et al. (1991) Increased risk of myelodysplasia and leukaemia after etoposide, cisplatin, and bleomycin for germ-cell tumours. Lancet Lond Engl 338:359–363. 10.1016/0140-6736(91)90490-g [DOI] [PubMed] [Google Scholar]
- 13.Nagata C, Shimizu H, Hirashima K, et al. (1999) Hair dye use and occupational exposure to organic solvents as risk factors for myelodysplastic syndrome. Leuk Res 23:57–62 [DOI] [PubMed] [Google Scholar]
- 14.Nisse C, Lorthois C, Dorp V, et al. (1995) Exposure to occupational and environmental factors in myelodysplastic syndromes. Preliminary results of a case-control study. Leukemia 9:693–699 [PubMed] [Google Scholar]
- 15.Rigolin GM, Cuneo A, Roberti MG, et al. (1998) Exposure to myelotoxic agents and myelodysplasia: case-control study and correlation with clinicobiological findings. Br J Haematol 103:189–197 [DOI] [PubMed] [Google Scholar]
- 16.Strom SS, Gu Y, Gruschkus SK, et al. (2005) Risk factors of myelodysplastic syndromes: a case-control study. Leukemia 19:1912–1918. 10.1038/sj.leu.2403945 [DOI] [PubMed] [Google Scholar]
- 17.West RR, Stafford DA, Farrow A, Jacobs A (1995) Occupational and environmental exposures and myelodysplasia: a case-control study. Leuk Res 19:127–139 [DOI] [PubMed] [Google Scholar]
- 18.Du Y, Fryzek J, Sekeres MA, Taioli E (2010) Smoking and alcohol intake as risk factors for myelodysplastic syndromes (MDS). Leuk Res 34:1–5. 10.1016/j.leukres.2009.08.006 [DOI] [PubMed] [Google Scholar]
- 19.Ido M, Nagata C, Kawakami N, et al. (1996) A case-control study of myelodysplastic syndromes among Japanese men and women. Leuk Res 20:727–731 [DOI] [PubMed] [Google Scholar]
- 20.Pasqualetti P, Festuccia V, Acitelli P, et al. (1997) Tobacco smoking and risk of haematological malignancies in adults: a case-control study. Br J Haematol 97:659–662 [DOI] [PubMed] [Google Scholar]
- 21.Poynter JN, Richardson M, Blair CK, et al. (2016) Obesity over the life course and risk of acute myeloid leukemia and myelodysplastic syndromes. Cancer Epidemiol 40:134–140. 10.1016/j.canep.2015.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adès L, Itzykson R, Fenaux P (2014) Myelodysplastic syndromes. Lancet Lond Engl 383:2239–2252. 10.1016/S0140-6736(13)61901-7 [DOI] [PubMed] [Google Scholar]
- 23.Latvala J, Parkkila S, Niemelä O (2004) Excess alcohol consumption is common in patients with cytopenia: studies in blood and bone marrow cells. Alcohol Clin Exp Res 28:619–624. 10.1097/01.alc.0000122766.54544.3b [DOI] [PubMed] [Google Scholar]
- 24.Smith C, Gasparetto M, Jordan C, et al. (2015) The effects of alcohol and aldehyde dehydrogenases on disorders of hematopoiesis. Adv Exp Med Biol 815:349–359. 10.1007/978-3-319-09614-8_20 [DOI] [PubMed] [Google Scholar]
- 25.Brown LM, Gibson R, Burmeister LF, et al. (1992) Alcohol consumption and risk of leukemia, non-Hodgkin’s lymphoma, and multiple myeloma. Leuk Res 16:979–984. 10.1016/0145-2126(92)90077-k [DOI] [PubMed] [Google Scholar]
- 26.Liu P, Holman CDJ, Jin J, Zhang M (2016) Alcohol consumption and risk of myelodysplastic syndromes: a case-control study. Cancer Causes Control CCC 27:209–216. 10.1007/s10552-015-0698-x [DOI] [PubMed] [Google Scholar]
- 27.Vardiman JW, Thiele J, Arber DA, et al. (2009) The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 114:937–951. 10.1182/blood-2009-03-209262 [DOI] [PubMed] [Google Scholar]
- 28.U.S. Department of Health and Human Services and U.S. Department of Agriculture (2015) 2015–2020 Dietary Guidelines for Americans [Google Scholar]
- 29.Kunzmann AT, Coleman HG, Huang W-Y, Berndt SI (2018) The association of lifetime alcohol use with mortality and cancer risk in older adults: A cohort study. PLoS Med 15:e1002585 10.1371/journal.pmed.1002585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma X, Lim U, Park Y, et al. (2009) Obesity, lifestyle factors, and risk of myelodysplastic syndromes in a large US cohort. Am J Epidemiol 169:1492–1499. 10.1093/aje/kwp074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jang M, Cai L, Udeani GO, et al. (1997) Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275:218–220. 10.1126/science.275.5297.218 [DOI] [PubMed] [Google Scholar]
- 32.Stockwell T, Zhao J, Greenfield T, et al. (2016) Estimating under- and over-reporting of drinking in national surveys of alcohol consumption: identification of consistent biases across four English-speaking countries. Addict Abingdon Engl 111:1203–1213. 10.1111/add.13373 [DOI] [PMC free article] [PubMed] [Google Scholar]


