Abstract
Objectives
Neuropsychiatric symptoms (NPS) are common among individuals with dementia of the Alzheimer’s type (DAT). We sought to characterize which NPS more purely relate to cognitive dysfunction in DAT, relative to other NPS.
Method
Demographic, neurocognitive, neuroimaging, and NPS data were mined from the Alzheimer’s Disease Neuroimaging Initiative database (n = 906). Using factor analysis, we analyzed the degree to which individual NPS were associated with DAT-associated cognitive dysfunction. We also employed item response theory to graphically depict the ability of individual NPS to index DAT-associated cognitive dysfunction across a continuum ranging from cognitively normal to mild DAT.
Results
Psychotic symptoms (hallucinations and delusions) were more strongly related to the continuum of DAT-associated cognitive dysfunction than other NPS, with the strength of the relationship peaking at high levels of disease severity. Psychotic symptoms also negatively correlated with brain volume and did not relate to the presence of vision problems. Aberrant motor behavior and apathy had relatively smaller associations with DAT-associated cognitive dysfunction, while other NPS showed minimal associations.
Discussion
Psychotic symptoms most strongly indexed DAT-associated cognitive dysfunction, whereas other NPS, such as depression and anxiety, were not as precisely related to the DAT-associated cognitive dysfunction.
Keywords: Alzheimer’s disease, Dementia, Mild cognitive impairment, Quantitative methods
From the earliest description of dementia of the Alzheimer’s type (DAT) in 1906, DAT has been characterized as a disease that, beyond progressive cognitive decline, is often marked by the development or exacerbation of neuropsychiatric symptoms (NPS; Hippius & Neundörfer, 2003). Late-life onset of NPS may reflect DAT pathology (Bassiony et al., 2000; Lee et al., 2012). Mood disturbances, physiological NPS (e.g., changes in eating and sleeping behavior, aberrant motor behavior), and psychotic symptoms have been shown to relate to DAT (Delrieu et al., 2015; Lee et al., 2012; Senanarong et al., 2004; Wilson, Gilley, Bennett, Beckett, & Evans, 2000). Other reports, however, have found no relation between certain NPS and DAT (e.g., Breitve et al., 2016), suggesting that NPS in old age may alternatively reflect factors that are not necessarily associated with DAT (e.g., comorbid medical conditions) or adjustment issues related to lifestyle changes that are common in older age (e.g., becoming a widow/er or changing living arrangements).
Whether a NPS is attributable to a neurodegenerative disease or some other cause (psychosocial or organic) is difficult to determine due to the multidimensional nature of changes related to aging. For example, older adults commonly experience symptoms of depression secondary to arthritis or diabetes (Foley, Ancoli-Israel, Britz, & Walsh, 2004). Hearing loss, similarly, was associated with feelings of depression and loneliness in older adults (Chen, 1994). Bereavement, which affects up to 45% of women and 15% of men over the age of 65 (Gullotta & Bloom, 2003), was related with changes in appetite and eating (Rosenbloom & Whittington, 1993) as well as sleep problems (Byrne & Raphael, 1997). An estimated 25% of cognitively normal (CN) older adults are affected by nonpsychotic NPS (Geda et al., 2008), while between 3% and 12% of CN older adults are believed to experience affective NPS (e.g., symptoms of depression, apathy, and irritability), suggesting that factors other than a neurodegenerative disease may be driving the genesis of these symptoms (Geda et al., 2008; Lyketsos et al., 2000).
The prevalence of hallucinations in DAT is estimated to be between 7% and 41%; similarly, the prevalence of delusions in DAT is between 34% and 55% (Ropacki & Jeste, 2005; Scarmeas et al., 2005; Wilson et al., 2000). Psychotic NPS were associated with greater cognitive impairment during the early stages of the disease (Weamer et al., 2009) and more rapid cognitive decline in DAT (Scarmeas et al., 2005; Wilkosz, Miyahara, Lopez, DeKosky, & Sweet, 2006; Wilson et al., 2000). Increased atrophy of the temporal and parietal lobes was shown to correspond with hallucinations and apathy across the clinical spectrum of DAT (Donovan et al., 2014). The existence of hallucinations and delusions in DAT is believed to signal a more clinically severe phenotype of DAT, though it is unclear whether this phenotype is neurobiologically distinct from DAT without psychotic symptoms (Murray, Kumar, DeMichele-Sweet, & Sweet, 2014; Reeves, Gould, Powell, & Howard, 2012). In individuals without a history of psychosis, substance abuse, or another neurodegenerative process (e.g., dementia with Lewy bodies [DLB]), there are few plausible etiologies of late-life psychotic NPS besides DAT.
Many NPS have been identified as potential indicators of DAT risk, progression, and severity (Bassiony et al., 2000; Breitve et al., 2016; Lee et al., 2012). Given the heterogeneity of the etiology of NPS in older age, there are theoretical reasons to hypothesize that certain NPS more specifically relate to DAT-associated cognitive dysfunction than others. For example, hallucinations and delusions may be more strongly related to the DAT continuum relative to other NPS. Determining which NPS relate most strongly to DAT, and at what level of disease severity the associations are strongest, will help clinicians and researchers more accurately in the differential diagnosis of DAT versus DLB and other forms of dementia. Previous research on NPS in DAT typically investigated how individual NPS corresponded to discrete diagnostic categories of cognitive dysfunction, ranging from CN to probable DAT (e.g., Lee et al., 2012; Mah, Binns, & Steffens, 2015). One recent investigation revealed that apathy was an indicator of prodromal DAT (Delrieu et al., 2015), and a second study showed that anxiety strongly predicted conversion from mild cognitive impairment (MCI) to DAT (Mah et al., 2015). These two studies, and many others, focused on how a single NPS related to categorical classifications of MCI and DAT.
Other studies have examined a greater breadth of NPS in DAT (Craig, Mirakhur, Hart, McIlroy, & Passmore, 2005). Craig and colleagues (2005), for example, found that the prevalence and persistence of NPS varied across each symptom in a 435-person cohort. To our knowledge, only a handful of studies have looked at NPS broadly as they pertain to clinical diagnosis, such as a study that used the National Alzheimer’s Coordinating Center Uniform Data Set (NACC) which suggested that “increasing dementia severity […] was related to […] more psychosis symptoms in AD, DLB, AD/DLB, and AD/VAD [patients]” (Johnson, Watts, Chapin, Anderson, & Burns, 2011). These investigations more comprehensively measured a larger constellation of NPS. Like other studies that examined a single NPS, however, the associations of NPS with DAT were based on categorical or ordinal diagnostic cutoffs, and the associations between NPS and the categorical or ordinal definition of DAT were typically captured with a single metric, such as a Pearson product-moment correlation coefficient, t test, or a logistic-based odd ratio (e.g., Delrieu et al., 2015).
The aforementioned studies are a fruitful starting point for investigating the relation between NPS and cognition in DAT. What is still needed, however, is an approach that quantifies the associations between individual NPS and DAT across the range of DAT severity, which should include individuals with DAT and MCI, as well as CN individuals. Such a study would facilitate robust comparisons between different NPS and their specific relations to the continuum of DAT-associated cognitive dysfunction. The present study extended the literature by deriving an empirical model that quantifies the magnitude of the relations between the 12 NPS assessed by the Neuropsychiatric Inventory Questionnaire (NPI-Q; Kaufer et al., 2000) and DAT-associated cognitive dysfunction. Here, we used factor analysis to establish a continuum of DAT-associated cognitive dysfunction by deriving a global factor score that is comprised of neuropsychological tests from the four cognitive domains that are affected by DAT over the course of the disease—memory, language, visuospatial abilities, and executive function. To validate our global cognitive factor as a reflective measurement of DAT-associated cognitive dysfunction, we then assessed whether our factor score correlated with brain volume (as measured by magnetic resonance imaging [MRI]) and performance on the Clinical Dementia Rating Scale Sum of Boxes (CDR-SB; O’Bryant et al., 2010), the Alzheimer’s Disease Assessment Scale 11 (standard) (ADAS 11; Llano, Laforet, & Devanarayan, 2011) and ADAS 13 (with two additional subtests), and the Mini-Mental State Examination (MMSE; Folstein, Folstein, & McHugh, 1975)—four widely accepted measures of cognitive dysfunction used in DAT clinical research. We then loaded individual NPS onto this global factor score of DAT-associated cognitive dysfunction to determine which NPS were most strongly related to the cognitive manifestation of the disease. Finally, we also used item response theory (IRT) to reveal how individual NPS measured on the NPI-Q indexed DAT-associated cognitive dysfunction across the clinical spectrum of the disease.
Drawing from previous research, we hypothesized that hallucinations and delusions would more precisely indicate the DAT continuum compared to other NPS, as hallucinations and delusions may reflect a more advanced form of DAT that has progressed from the temporal lobe into neocortical regions (Murray et al., 2014; Reeves et al., 2012). Given the influence of confounding factors on the etiology of other NPS in older adults, we hypothesized that all other NPS would be less related to the DAT continuum. Symptoms of anxiety and depression, for example, were expected to indicate DAT-related cognitive dysfunction at lower levels of disease severity relative to psychotic symptoms, as anxiety and depression symptoms are associated with conversion from MCI to DAT. We also hypothesized that symptoms of depression and anxiety would show relatively weak relations to DAT-associated cognitive dysfunction, as they can easily stem from other non-DAT etiologies. Additionally, we hypothesized that the existence of hallucinations and delusions in participants would not be related to conditions affecting vision and the eye.
Method
Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (see adni.loni.usc.edu for more information). The ADNI was launched in 2003 as a public–private partnership. The initial goal of ADNI was to recruit 800 participants but ADNI has been followed by two other initiatives, ADNI-GO and ADNI-2. To date, these three protocols have been used to recruit over 1,500 adults, ages 55 to 90 years, to participate in the research. The sample consists of older adults who are cognitively healthy, those with early or late MCI, and those with early AD. Demographic information and clinical data used for this study were downloaded from the ADNI data repository on May 28, 2014. Data for the current analyses come from individuals who had complete data for key cognitive and NPS variables, described below, at baseline assessment. Data analyses were approved by the Texas A&M University Institutional Review Board, and all authors were affiliated with Texas A&M when the data for this report were mined and analyzed.
Participants
The present study used baseline data from 906 participants (384 female, 42%) enrolled across all three ADNI phases; Table 1 provides a summary of demographic and cognitive test scores for the participants, broken down by clinical diagnosis.
Table 1.
Participant Demographics and Cognitive Test Data
| Clinical diagnosis | ||||
|---|---|---|---|---|
| Demographic | CN (n = 222) | eMCI (n = 125) | LMCI (n = 386) | DAT (n = 173) |
| Age in years (SD) | 75.85 (5.07) | 71.47 (7.68) | 74.82 (7.36) | 75.57 (7.33) |
| Years of education (SD) | 16.03 (2.86) | 15.83 (2.64) | 15.65 (2.94) | 14.74 (3.07) |
| Sex (% male) | 51.40% | 54.4% | 65.28% | 50.90% |
| Ethnicity (% non-Hispanic) | 98.60% | 93.60% | 96.40% | 98.80% |
| Race (% White) | 91.40% | 90.40% | 93.50% | 94.20% |
| APOE4 (% carriers) | 26.58% | 41.13% | 53.37% | 64.16% |
| CDR-SB (SD) | 0.03 (0.12) | 1.26 (0.69) | 1.60 (0.89) | 4.22 (1.65) |
| ADAS 11 (SD) | 6.16 (2.91) | 7.61 (3.15) | 11.42 (4.36) | 18.10 (6.01) |
| ADAS 13 (SD) | 9.43 (4.18) | 12.36 (5.30) | 18.56 (6.25) | 28.34 (7.33) |
| MMSE (SD) | 29.09 (1.00) | 28.30 (1.51) | 27.02 (1.79) | 23.42 (2.04) |
Note: CN = cognitively normal; eMCI = early mild cognitive impairment; LMCI = late mild cognitive impairment; DAT = dementia of the Alzheimer’s type; SD = standard of deviation; APOE4 = apolipoprotein E; CDR-SB = Clinical Dementia Rating Scale Sum of Boxes; ADAS 11 and ADAS 13 = Alzheimer’s Disease Assessment Scale (11 item and 13 item); MMSE = Mini-Mental State Examination.
Participants were an average of 74.76 years old (SD = 7.04), highly educated (M = 15.59, SD = 2.94 years), and the majority identified racially as White (n = 840, 93%). Other races include Black or African American (n = 42, 5%), Asian (n = 15, 2%), and American Indian or Alaskan Native (n = 2, 0%); six participants (1%) identified as more than one race, and one participant (0%) indicated unknown race. Twenty-one (2%) participants identified ethnicity as Hispanic or Latinx; 878 (97%) reported that they were not Hispanic or Latinx, and seven (1%) were unknown.
Baseline diagnoses represented a range of cognitive impairment: 222 participants (25%) were CN, 511 (56%) had MCI (early or late stage), and 173 (18%) had presumed DAT. We chose to include the CN participants so that we could model DAT along the continuum of normal cognitive function to DAT-associated cognitive impairment. In the ADNI, CN participants served as the controls and showed no signs of MCI or dementia. CN participants were cognitively normal, defined as a Clinical Dementia Rating Scale (CDR; Morris, 1997) score of 0, an MMSE score (Folstein, Folstein, & McHugh, 1975) between 24 and 30, and normal memory functioning defined as scoring above education-adjusted cutoffs on the Wechsler Memory Scale-Revised (WMS-R) Logical Memory II subscale (Wechsler, 1987). MCI participants had a subjective memory concern and significant amnestic dysfunction, defined as a CDR score of 0.5 plus an abnormal score on the WMS-R Logical Memory II subscale. Participants with MCI, however, had sufficiently preserved functional abilities and an MMSE score between 24 and 30, such that they did not meet criteria for DAT. Participants with DAT met National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association (NINCDS/ADRDA) criteria (McKhann et al., 1984), had an MMSE between 20 and 26, and a CDR of 0.5 or 1. Finally, participants were excluded from admission into the ADNI cohort if they had a history of significant neurologic disease (including multi-infarct dementia and subdural hematoma), as well as various psychiatric disorders like major depressive disorder, schizophrenia, and bipolar disorder.
A significant plurality of our sample had no NPS present (n = 421, 46.47%), whereas a slight majority (n = 485, 53.53%) had at least one NPS as documented by the NPI-Q. On average, participants had at least one NPS present out of the 12 assessed by the NPI-Q (M = 1.36, SD = 1.76). The most commonly reported baseline NPS within our sample was irritability/lability (n = 224, 24.72%), while the prevalence of other NPS, such as agitation/aggression (n = 144, 15.89%), depression/dysphoria (n = 168, 18.54%), anxiety (n = 155, 17.11%), apathy/indifference (n =139, 15.34%), and sleep disturbances (n = 140, 15.45%), was somewhat lower. Aberrant motor behavior (n = 52, 5.74%), changes in appetite (n = 85, 9.38%), elation/euphoria (n = 22, 2.43%), disinhibition (n = 72, 7.95%), delusions (n = 21, 2.32%), and hallucinations (n = 10, 1.10%) had relatively low prevalence. Table 2 shows the breakdown of NPI-Q symptom endorsement by clinical grouping.
Table 2.
Neuropsychiatric Inventory Questionnaire (NPI-Q) Symptom Endorsement by Clinical Groupings
| Clinical diagnosis | ||||
|---|---|---|---|---|
| NPI-Q symptom endorsed | CN | MCI | DAT | Total |
| Delusions | 0 | 6 | 15 | 21 |
| Hallucinations | 1 | 1 | 8 | 10 |
| Agitation/Aggression | 6 | 93 | 45 | 144 |
| Depression/Dysphoria | 11 | 102 | 55 | 168 |
| Anxiety | 8 | 92 | 55 | 155 |
| Elation/Euphoria | 0 | 14 | 8 | 22 |
| Apathy/Indifference | 3 | 77 | 59 | 139 |
| Disinhibition | 1 | 42 | 29 | 72 |
| Irritability/Lability | 15 | 149 | 60 | 224 |
| Aberrant Motor Behavior | 1 | 24 | 27 | 52 |
| Sleep | 20 | 79 | 41 | 140 |
| Appetite | 1 | 55 | 29 | 85 |
Note: CN = cognitively normal; MCI = mild cognitive impairment; DAT = dementia of the Alzheimer’s type.
Neuropsychological and Neuropsychiatric Measures
In the present study, we examined cognitive performance and the presence of NPS. The procedures used for each of these domains are briefly described here (see full description online at: adni.loni.usc.edu). In the ADNI sample, participants were assessed using neuropsychological tests to evaluate cognitive performance. Neuropsychological measures of memory, language, visuospatial abilities, and executive function reflect the breadth of cognitive decline that occurs in DAT, and are commonly used in clinical research to assess cognitive dysfunction. Here, we analyzed data from cognitive measures that capture each of these cognitive domains using the identical constellation of cognitive tests of memory, language, visuospatial abilities, and executive function from ADNI and methods defined in a previous paper from our lab that used the exact factor-analytical methods employed in this report (Balsis et al., 2018). The methods section from Balsis and colleagues (2018) is provided below:
To assess memory ability, we used data from the Alzheimer ‘s disease Assessment Scale – Cognition (ADAS-Cog; Mohs, Rosen, & Davis, 1983; Rosen, Mohs, & Davis, 1984) and Delayed Recall and Word Recognition subtests and Rey Auditory Verbal Learning Test (Rey, 1964), Immediate Recall, Delayed Recall, and Recognition. Each measure was placed into up to five equal bins; those scores were summed across tests. The range in the dataset for this variable was determined, and then values were placed into five equal bins (0, 1, 2, 3 and 4) using the unstandardized scores to represent our Memory domain via the interval binning function in SPSS (IBM Corporation, 2014), which creates equal-sized bins. To assess language ability, the same process was carried out for ADAS-Cog Naming (Rosen et al., 1984), Boston Naming Test (Kaplan, Goodglass, & Weintraub, 1983), and Category Fluency-Animals (adapted from the CERAD Verbal Fluency test; Morris et al., 1989). To assess visuo- spatial ability, we used the same procedure for ADAS-Cog Constructional Praxis (Rosen et al., 1984) and Clock Drawing Test (Goodglass & Kaplan, 1983) Command and Copy. Finally, to assess executive function, we used ADAS- Cog Number Cancellation (Rosen et al., 1984) and Trail Making Test A and B (Reitan, 1958; Reitan & Wolfson, 1985). These are the values that we parameterized to rep- resent the cognitive performance assessment. Additional measures used to verify the latent continuum and characterize the sample were the Mini Mental State Examination (Folstein, Folstein, & McHugh, 1975) and the CDR-SOB (O’Bryant et al., 2008). (p. 967)
The NPI-Q (Kaufer et al., 2000) was used to assess the presence of NPS. The NPI-Q (Kaufer et al., 2000) is an informant-report assessment of the presence of various NPS (e.g., depression, anxiety) for each participant. Symptoms are marked as absent or present (0 or 1). If the symptom is present, the clinician then asks follow-up questions to determine the severity. For the purpose of our study, we considered the presence or absence of the NPS as assessed by the baseline present/absent determination. Range restriction prevented analysis of severity levels, as few individuals endorsed the severity of a symptom as greater than “mild.” Neuroimaging data used in this report (MRI and fludeoxyglucose positron emission tomography [FDG-PET]) were gathered by ADNI researchers using previously described methods (Jack et al., 2010; Jagust et al., 2015). Brain imaging values were extracted and residualized for race, gender, intracranial volume, and age for the following bilateral regions of interest: hippocampus, entorhinal cortex, fusiform gyrus, middle temporal gyrus, whole brain volume, and whole brain FDG-PET.
Defining a Factor for “DAT-Associated Cognitive Dysfunction”
For the present analyses, we defined a continuum of DAT-associated cognitive dysfunction using factor analytic methods described previously (Balsis et al., 2018). To determine whether these four items were unidimensional enough for these analyses, we conducted an exploratory factor analysis (EFA) on the set of items. One indication of unidimensionality is that the set of items have a ratio of first to second eigenvalue that is greater than 3 to 1 (Embretson, Reise, & Reise, 2000). The ratio between the first and second eigenvalue was greater than 3 to 1. The first eigenvalue was 2.21, and the second eigenvalue was 0.73, which equals a ratio of 3.03 to 1. A second indication of unidimensionality is reflected in confirmatory factor analysis (CFA) fit indices. Hu and Bentler (1999) showed that hypothetical structural models are a relatively good fit to observed data when the Tucker–Lewis index (TLI; Tucker & Lewis, 1973) and comparative fit index (CFI; Bentler, 1990) values are close to .95 and the root mean-squared error of approximation (RMSEA; Steiger, 1980) is less than .06. Using these recommended cutoffs, we concluded that the data (TLI = .99, CFI = 1.00, RMSEA = .06) were robustly unidimensional. Together, these indicators were sufficiently unidimensional to proceed with bifactor and IRT analyses.
Assessing the Relations Between NPS and DAT-Associated Cognitive Dysfunction
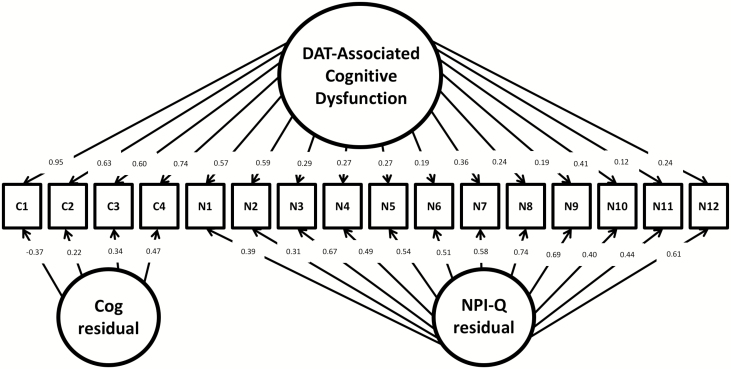
Using Mplus and the ADNI data, we generated a maximum likelihood bifactor model proposed by Reise, Morizot, & Hays (2007). The bifactor approach seen in Figure 1 represents a comprehensive model that allows for the four cognitive variables and the 12 NPI variables to define the factor of “DAT-associated cognitive dysfunction” while simultaneously accounting for residual variables within each subset of indicators.
Figure 1.
Bifactor model of dementia of the Alzheimer’s type (DAT)-associated cognitive dysfunction represented across cognitive and neuropsychiatric variables. Using a bifactor model, DAT-related cognitive dysfunction is modeled as a function of cognitive and neuropsychiatric variables. Factor loadings indicate each variable’s strength of relationship to the general factor (DAT-related cognitive dysfunction), as well as the cognitive or neuropsychiatric residual factors. Note: cog = cognitive; NPI-Q = Neuropsychiatric Inventory Questionnaire; C1 = memory cognitive factor; C2 = language cognitive factor; C3 = visuospatial cognitive factor; C4 = executive function cognitive factor; N1 = NPI-Q Delusions; N2 = NPI-Q Hallucations; N3 = NPI-Q Agitation/Agression; N4 = NPI-Q Depression/Dysphoria; N5 = NPI-Q Anxiety; N6 = NPI-Q Eltation/Euphoria; N7 = NPI-Q Apathy/Indifference; N8 = NPI-Q Disinhibition; N9 = NPI-Q Irritability/Lability; N10 = NPI-Q Motor Disturbance; N11 = NPI-Q Nighttime Behaviors; N12 = NPI-Q Appetite/Eating.
We additionally compared the bifactor model to an additional IRT analysis that linked the noncognitive indicators to a latent continuum of “DAT-associated cognitive dysfunction” via IRT-linking procedures using the IRT-LR-DIF software program (Thissen, 2001). IRT-LR-DIF is a statistical software package used to establish parameters for “anchor” items that define the latent continuum of interest and then test the extent to which individual “candidate” items index that latent continuum. Using this program, we established the latent continuum across the four cognitive markers described above using Samejima’s graded response model (Samejima, 1970). Parameters for each of the NPI-Q items were established separately using the 2 parameter logistic model (2PL), defining the strength and location of each NPS as it relates to the latent continuum. The formula for the 2PL model is below (with similar notation to that used in Baker, 2001):
| (1.1) |
In Formula 1.1, the a parameter represents the strength of the association between a variable and the latent continuum (θ) and is equivalent to the slope of the item characteristic curve at its inflection point. The b parameter is the θ value that corresponds to this inflection point. This function monotonically increases and reveals the probability that an item is endorsed at any given level of the latent continuum. This function is commonly called an item characteristic curve (Hambleton, Swaminathan, & Rogers, 1991).
We used Formula 1.1 and IRT-LR-DIF to establish a and b parameters for each of the NPI-Q NPS variables: Delusions, Hallucinations, Agitation/Aggression, Depression/Dysphoria, Anxiety, Elation/Euphoria, Apathy/Indifference, Disinhibition, Irritability/Lability, Aberrant Motor Behavior, Nighttime Behavior, and Appetite. We then graphed the functional relationships between each NPS and the latent continuum using a derivative of Formula 1.1, which provides a useful metric of information for visualizing both the strength of relationship between an item (as reflected by the a parameter) and the latent continuum as well as the relative strength of relationship across the latent continuum (as reflected by the b parameter). This derivated function is defined by the following formula (Baker, 2001):
| (1.2) |
Results
Relation Between NPS and the DAT Cognitive Dysfunction: Factor Analysis and IRT
The bifactor model showed excellent fit (RMSEA = .02; X2 (88, n = 906) = 116.29 (df = 88), p < .05; TLI = .98; CFI = .99). Among the cognitive variables, factor loadings with the main dimension (DAT-related cognitive dysfunction) were between .60 and .95, reflecting the main dimension indexing cognitive variance among participants. NPI-Q Hallucinations and Delusions had the highest loadings of NPI-Q factors at .57 and .59, respectively, reflecting a strong association between these two symptoms and DAT-associated cognitive dysfunction. Figure 1 provides a representation of the factor loadings of the NPS and the cognitive variables onto the continuum of DAT-associated cognitive dysfunction.
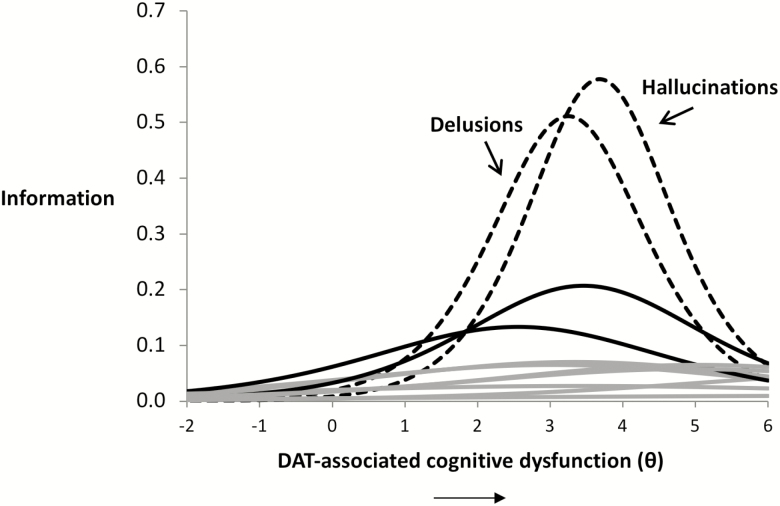
Results from the IRT analysis dovetailed nicely with the results from the bifactor model. Specifically, findings from the IRT analysis likewise indicated that NPS varied in the strength of their relationship to the latent continuum of DAT-associated cognitive dysfunction. The strength of the relations, captured in the variables’ a parameters, ranged from 0.20 to 1.52. Again, the two strongest indicators of the DAT continuum were Hallucinations (a = 1.52) and Delusions (a = 1.43). An analysis of the IRT information functions reveals the relative strength of the relations between these two symptoms and the continuum of DAT-associated cognitive dysfunction (Figure 2). The strength of association of Hallucinations and Delusions was greatest in the relatively high/more severe levels of DAT-associated cognitive dysfunction, as indicated by the variables’ b values (Hallucinations = 3.68, Delusions = 3.25).
Figure 2.
Item response theory (IRT) model of the relation between neuropsychiatric symptoms (NPS) and dementia of the Alzheimer’s type (DAT)-associated cognitive dysfunction. The ability of the 12 Neuropsychiatric Inventory Questionnaire (NPI-Q) NPS to index the continuum of DAT-related cognitive dysfunction is presented. The X-axis depicts the continuum of DAT-associated cognitive dysfunction, ranging from low to high levels of severity, representing the b parameter of the IRT function. The Y-axis denotes “information gained,” referring to the ability of an item (i.e., a NPS) to indicate the latent continuum, a derivative of the a parameter of the IRT function. Here, we see that NPI-Q Hallucinations and Delusions index DAT-related cognitive dysfunction more strongly than other symptoms from the NPI-Q, particularly so at greater levels of DAT-related cognitive dysfunction.
The other symptoms had relatively weak relationships with the latent continuum, as indicated by lower a parameters (Aberrant Motor Behavior = 0.91; Apathy/Indifference = 0.73; Agitation/Aggression = 0.53; Anxiety = 0.52; Depression/Dysphoria = 0.51; Disinhibition = 0.51; Appetite/Eating = 0.48; Elation/Euphoria = 0.46; Irritability/Lability = 0.33; Nighttime Behaviors = 0.20). Further inspection of Figure 2 reveals that relative to Hallucinations and Delusions, the other symptoms showed weaker relationships with the continuum of DAT-associated cognitive dysfunction. This is reflected in the relative height of these curves. Aberrant Motor Behavior (a = 0.91) and Apathy (a = 0.73) were mildly related to DAT-associated cognitive dysfunction at relatively stable (but low) levels across the continuum. The relations between Aberrant Motor Behavior and Apathy with the continuum are indicated in Figure 2 by the two solid black lines, with Aberrant Motor Behavior representing the higher curve. All other NPS related less strongly to the continuum of DAT-associated cognitive decline, and are represented collectively as solid gray lines in Figure 2. As these other symptoms were related weakly to the latent continuum, the b parameters for many of these items were largely uninformative and essentially uninterpretable because these items had virtually no indexing power of the latent continuum (as indicated by the low a parameters).
Assessing the External Validity of the Latent Continuum
To assess the external validity of our factor of DAT-associated cognitive dysfunction, we conducted Pearson product-moment correlations (Pearson’s r) between participants’ factor scores and scores on the CDR-SB (O’Bryant et al., 2008), Alzheimer’s Disease Assessment Scale 11 (standard) (ADAS 11; Llano et al., 2011) and ADAS 13 (with two additional subtests), and the MMSE—four widely accepted measures of cognitive dysfunction used in DAT clinical research. Our factor of “DAT-associated cognitive dysfunction” dovetailed well with participants’ scores on the CDR-SB (r = .61, p < .001), the ADAS 11 (r = .76, p < .001), the ADAS 13 (r = .81, p < .001), and the MMSE (r = −.64, p < .001).
We also conducted Pearson’s two-tailed correlational tests between participants’ ADNI neuroimaging data and participants’ factor scores to verify that our factor correlated well with the neurodegeneration seen in DAT. Results showed that factor scores correlated positively with ventricle size (r = .27, p < .001) and negatively with MRI volume in the hippocampus (r = −.45, p < .001), entorhinal cortex (r = −.46, p < .001), fusiform gyrus (r = −.40, p < .001), and middle temporal gyrus volume (r = −.45, p < .0001), as well as whole-brain FDG-PET (r = −.47, p < .001). Taken together, the correlations between our factor, commonly used clinical neuropsychological tests of global cognition, and brain variables further supported the idea that our factor was a valid measure of DAT-associated cognitive dysfunction.
Post Hoc Analyses
To assess whether the strength of NPI-Q Hallucinations as an index of DAT-associated cognitive dysfunction was due to a disease affecting the eye (e.g., glaucoma) instead of processes affecting the brain (e.g., DAT) we ran several post hoc phi correlations between patients’ visual problems (rated dichotomously as present or absent) and the endorsement of NPI-Q Hallucinations. There was no correlation between endorsement of “blurred vision (with no further explanation)” and endorsement of NPI-Q hallucinations (r = .01, p = .72). Similarly, there were no significant correlations between endorsement of having cataracts (r = −.01, p = .75), glaucoma (r = −.01, p = .86), or macular degeneration (r = −.01, p = .83) with the endorsement of NPI-Q hallucinations. Vision problems appeared to be unrelated to the hallucinations experienced by the participants.
Discussion
This study examined the relative contributions of the NPS assessed using the NPI-Q across a continuum of DAT-associated cognitive dysfunction in a large, well-defined sample of patients with DAT and MCI, as well as CN individuals. Findings supported our hypotheses, with hallucinations and delusions appearing to have the strongest relations to DAT-associated cognitive dysfunction, most saliently at the highest levels of disease severity in our sample (mild-to-moderate DAT). Most nonpsychotic NPS showed relatively weaker relations to the continuum of DAT-related cognitive dysfunction. Aberrant motor behavior and apathy, however, were moderately related with the continuum, while depression and anxiety were not.
According to these findings, symptoms of depression, anxiety, and many other NPS may not reflect DAT-related cognitive dysfunction as purely as hallucinations and delusions. We speculate that symptoms of depression and anxiety may not relate to DAT-associated cognitive dysfunction as strongly because the etiology of these symptoms can be influenced by a variety of factors beyond DAT, ranging from “a disturbed central metabolism of monoamines 5-hydroxytryptamine, noradrenaline and dopamine” (Gareri, De Fazio, & De Sarro, 2002) to “social and behavioral factors” (Durisko, Mulsant, & Andrews, 2015). Hallucinations and delusions in this type of controlled sample should relate more exclusively to DAT-associated neurological changes given the lack of history of psychosis or another neurodegenerative disease (e.g., DLB) among participants.
Given our results, we posit that hallucinations and delusions in DAT may reflect a relatively moderate stage of DAT pathology that affects neocortical areas, in addition to limbic regions. The occurrence of hallucinations in DAT, in particular, has several nonmutually exclusive theoretical explanations (El Haj, Gallouj, Dehon, Roche, & Larøi, 2018; El Haj, Jardri, Larøi, & Antoine, 2016; El Haj, Larøi, Gély-Nargeot, & Raffard, 2015; El Haj et al., 2017). Hallucinations in DAT may be driven by decreased inhibition of irrelevant memories and thoughts (El Haj et al., 2015, 2018). While, indeed, hallucinations and delusions are traditionally present early in the genesis of DLB (McKeith et al., 1996), the manifestation of hallucinations and delusions in the ADNI cohort, which excluded patients with a diagnosis of DLB, appears to occur in mild-to-moderate DAT. In a sample of DLB patients, one would expect the hallucination and delusion curves to be further to the left than those seen in Figure 2, as these symptoms often appear in earlier stages of DLB.
The limitations of this study should be acknowledged. Our analysis is cross-sectional, and future investigations should examine this issue longitudinally. DAT pathology is a progressive neurodegenerative disease, and thus longitudinal modeling is an important goal. The strength of our results also relies on the assumption that the ADNI cohort consists of individuals who represent the continuum of DAT-associated cognitive dysfunction. Given the subgroups recruited for and studied in the ADNI (CN, MCI, and probable DAT), as well as the rigorous protocol used by the ADNI to determine a participant’s diagnosis, this seems like a plausible assumption. Consistent with the ADNI protocol for participant exclusion, our sample has excluded individuals with previous histories of major neurological or psychiatric disorders (e.g., drug abuse, major depression, and schizophrenia). While, indeed, these exclusionary criteria strengthen the focus of the ADNI on DAT pathology, previous work with ADNI (Donovan et al., 2014) suggested excluding participants based on previous neurological and psychiatric history likely accounts for the discrepancy between the proportion of the current sample that are reported to experience hallucinations (1.10%) and delusions (2.32%) versus previous reports which suggested the baseline prevalence of hallucinations in DAT was between 7% and 41%, and between 34% and 55% for delusions (Ropacki & Jeste, 2005; Scarmeas et al., 2005; Wilson et al., 2000). The ADNI exclusion criteria also limit the generalization of our model to other patients with DAT (many of whom have comorbid pathologies). Nonetheless, we found a strong relation between psychotic symptoms and DAT-associated cognitive dysfunction which peaks in the mild-to-moderate stages of disease severity (the highest clinical severity level included in the ADNI). Future work will need to investigate how measuring psychotic symptoms during the diagnostic process of DAT in the mild-to-moderate stages adds value in discriminating DAT from other forms of dementia, such as DLB.
Similar to other clinical research data sets, the ADNI sample is ethnically homogenous, and as such our analyses need to be cross-validated in a more ethnically heterogeneous sample. This is especially important given the increased prevalence of DAT in Latinx and African American individuals (Mehta & Yeo, 2017). Thus, future studies could replicate our investigation in other robust databases that study DAT (e.g., the NACC). Additional work with ADNI and other data sets should also explore the role that apolipoprotein genetic status, psychotropic medication intake, and medical comorbidities may have on the presence of hallucinations and delusions across DAT.
The psychometric limitations of the NPI-Q must also be discussed when considering the current findings. On the NPI-Q hallucinations are characterized by a single item: “Does the patient have hallucinations such as false visions or voices? Does he or she seem to hear or see things that are not present?” (Kaufer et al., 2000). Similarly, delusions on the NPI-Q are accounted for by a single question: “Does the patient have false beliefs, such as thinking that others are stealing from him/her or planning to harm him/her in some way?” (Kaufer et al., 2000). By only assessing each symptom with one item, the complexity of psychotic symptoms in DAT cannot be fully represented. The current data additionally did not adequately allow for the exploration of how NPI-Q symptom severity relates to DAT-associated cognitive dysfunction, as most NPI-Q respondents answered “mild” to severity level. Additional psychometric concerns associated with the NPI-Q and its administration must be considered, such as respondent bias and interrater reliability. Nonetheless, the NPI-Q is currently one of the most widely used measures to assess NPS in clinical and research settings and is both a reliable and valid assessment of NPS in geriatric populations (McKeith et al., 1996). Given the wide use of the NPI-Q as a quick tool to screen for NPS in older adult patients, our findings provide further evidence of a psychotic phenotype of DAT (Weamer et al., 2009) that may occur at more advanced disease severity. Though more research is needed to understand the differences between DAT with and without psychosis, the use of lengthier measures supplemented with imaging and biomarkers (when available) is recommended to assist clinicians in differentiating between DAT with and without psychosis and possibly also between DAT with psychosis and DLB.
Similarly, the limitations of the neuropsychological variables that comprise our global factor of DAT-associated cognitive dysfunction must be discussed. No individual neurocognitive test is a pure assessment of DAT clinical severity. Though the individual neurocognitive tests that comprise our global factor of DAT-associated cognitive dysfunction are not perfectly pure representations of their respective cognitive domains, the tests we selected do capture nuanced parts of cognitive performance in various ways. For example, the immediate and delayed recall portions of the Rey Auditory Verbal Learning Test (Rey, 1964) can provide a distinction between immediate learning from encoding versus longer-term memory retrieval. Similarly, Trail Making Test A can measure visuomotor processing speed, while Trail Making Test B assesses cognitive flexibility and inhibition (Reitan, 1958; Reitan & Wolfson, 1985).
In summary, hallucinations and delusions seem to signal DAT-associated cognitive dysfunction more strongly than other NPS. The statistical approach used in the derivation of this model is a fruitful starting point for future investigation and provides a way by which we can understand more precisely how NPS and DAT cognitive dysfunction relate along the DAT disease continuum.
Funding
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd; Janssen Alzheimer Immunotherapy Research & Development, LLC; Johnson & Johnson Pharmaceutical Research & Development LLC; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada.
Acknowledgments
Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Conflict of Interest
The first and last authors are responsible for data analyses. The authors report no conflicts of interest.
References
- Baker F. B. (2001). The basics of item response theory Retrieved from http://ericae.net/irt/baker/software.htm
- Balsis S., Geraci L., Benge J., Lowe D. A., Choudhury T. K., Tirso R., … Doody R. S.; Alzheimer’s Disease Neuroimaging Initiative (2018). Statistical model of dynamic markers of the Alzheimer’s pathological cascade. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 73, 964–973. doi:10.1093/geronb/gbx156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassiony M. M., Steinberg M. S., Warren A., Rosenblatt A., Baker A. S., & Lyketsos C. G (2000). Delusions and hallucinations in Alzheimer’s disease: Prevalence and clinical correlates. International Journal of Geriatric Psychiatry, 15, 99–107. doi:10.1002/(SICI)1099-1166(200002)15:2<99::AID-GPS82>3.0.CO;2-5 [DOI] [PubMed] [Google Scholar]
- Bentler P. M. (1990). Comparative fit indexes in structural models. Psychological Bulletin, 107, 238–246. doi:10.1037//0033-2909.107.2.238 [DOI] [PubMed] [Google Scholar]
- Breitve M. H., Hynninen M. J., Brønnick K., Chwiszczuk L. J., Auestad B. H., Aarsland D., & Rongve A (2016). A longitudinal study of anxiety and cognitive decline in dementia with Lewy bodies and Alzheimer’s disease. Alzheimer’s Research & Therapy, 8, 3. doi:10.1186/s13195-016-0171-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. J., & Raphael B (1997). The psychological symptoms of conjugal bereavement in elderly men over the first 13 months. International Journal of Geriatric Psychiatry, 12, 241–251. doi:10.1002/(sici)1099-1166(199702)12:2<241::aid-gps590>3.0.co;2-0 [DOI] [PubMed] [Google Scholar]
- Chen H. L. (1994). Hearing in the elderly. Relation of hearing loss, loneliness, and self-esteem. Journal of Gerontological Nursing, 20, 22–28. doi:10.3928/0098-9134-19940601-07 [DOI] [PubMed] [Google Scholar]
- Craig D., Mirakhur A., Hart D. J., McIlroy S. P., & Passmore A. P (2005). A cross-sectional study of neuropsychiatric symptoms in 435 patients with Alzheimer’s disease. The American Journal of Geriatric Psychiatry, 13, 460–468. doi:10.1176/appi.ajgp.13.6.460 [DOI] [PubMed] [Google Scholar]
- Delrieu J., Desmidt T., Camus V., Sourdet S., Boutoleau-Bretonnière C., Mullin E., … Lebouvier T.; Alzheimer’s Disease Neuroimaging Initiative (2015). Apathy as a feature of prodromal Alzheimer’s disease: An FDG-PET ADNI study. International Journal of Geriatric Psychiatry, 30, 470–477. doi:10.1002/gps.4161 [DOI] [PubMed] [Google Scholar]
- Donovan N. J., Wadsworth L. P., Lorius N., Locascio J. J., Rentz D. M., Johnson K. A., … Marshall G. A.; Alzheimer’s Disease Neuroimaging Initiative (2014). Regional cortical thinning predicts worsening apathy and hallucinations across the Alzheimer disease spectrum. The American Journal of Geriatric Psychiatry, 22, 1168–1179. doi:10.1016/j.jagp.2013.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durisko Z., Mulsant B. H., & Andrews P. W (2015). An adaptationist perspective on the etiology of depression. Journal of Affective Disorders, 172, 315–323. doi:10.1016/j.jad.2014.09.032 [DOI] [PubMed] [Google Scholar]
- El Haj M., Gallouj K., Dehon H., Roche J., & Larøi F (2018). Hallucinations in Alzheimer’s disease: Failure to suppress irrelevant memories. Cognitive Neuropsychiatry, 23, 142–153. doi:10.1080/13546805.2018.1443062 [DOI] [PubMed] [Google Scholar]
- El Haj M., Jardri R., Larøi F., & Antoine P (2016). Hallucinations, loneliness, and social isolation in Alzheimer’s disease. Cognitive Neuropsychiatry, 21, 1–13. doi:10.1080/13546805.2015.1121139 [DOI] [PubMed] [Google Scholar]
- El Haj M., Larøi F., Gély-Nargeot M. C., & Raffard S (2015). Inhibitory deterioration may contribute to hallucinations in Alzheimer’s disease. Cognitive Neuropsychiatry, 20, 281–295. doi:10.1080/13546805.2015.1023392 [DOI] [PubMed] [Google Scholar]
- El Haj M., Roche J., Jardri R., Kapogiannis D., Gallouj K., & Antoine P (2017). Clinical and neurocognitive aspects of hallucinations in Alzheimer’s disease. Neuroscience and Biobehavioral Reviews, 83, 713–720. doi:10.1016/j.neubiorev.2017.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embretson S., Reise S., & Reise S. P (2000). Item response theory for psychologists. Mahwah, NJ: Lawrence Erlbaum Associates. Inc. [Google Scholar]
- Foley D., Ancoli-Israel S., Britz P., & Walsh J (2004). Sleep disturbances and chronic disease in older adults: Results of the 2003 National Sleep Foundation Sleep in America Survey. Journal of Psychosomatic Research, 56, 497–502. doi:10.1016/j.jpsychores.2004.02.010 [DOI] [PubMed] [Google Scholar]
- Folstein M. F., Folstein S. E., & McHugh P. R (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198. doi:10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Gareri P., De Fazio P., & De Sarro G (2002). Neuropharmacology of depression in aging and age-related diseases. Ageing Research Reviews, 1, 113–134. doi:10.1016/s0047-6374(01)00370-0 [DOI] [PubMed] [Google Scholar]
- Geda Y. E., Roberts R. O., Knopman D. S., Petersen R. C., Christianson T. J., Pankratz V. S., … Rocca W. A (2008). Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging: Population-based study. Archives of General Psychiatry, 65, 1193–1198. doi:10.1001/archpsyc.65.10.1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullotta T. P., & Bloom M (2003). Encyclopedia of primary prevention and health promotion. New York, NY: Springer Science & Business Media. [Google Scholar]
- Hambleton R. K., Swaminathan H., & Rogers H. J (1991). Fundamentals of item response theory (Vol. 2). Newbury Park, CA: Sage. [Google Scholar]
- Hippius H., & Neundörfer G (2003). The discovery of Alzheimer’s disease. Dialogues in Clinical Neuroscience, 5, 101–108. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3181715/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L., & Bentler P. M (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal, 6, 1–55. doi:10.1080/10705519909540118 [Google Scholar]
- Jack C. R. Jr., Bernstein M. A., Borowski B. J., Gunter J. L., Fox N. C., Thompson P. M., … Weiner M. W.; Alzheimer’s Disease Neuroimaging Initiative (2010). Update on the magnetic resonance imaging core of the Alzheimer’s disease neuroimaging initiative. Alzheimer’s & Dementia, 6, 212–220. doi:10.1016/j.jalz.2010.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust W. J., Landau S. M., Koeppe R. A., Reiman E. M., Chen K., Mathis C. A., … Wang A. Y (2015). The Alzheimer’s disease neuroimaging initiative 2 PET core: 2015. Alzheimer’s & Dementia, 11, 757–771. doi:10.1016/j.jalz.2015.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. K., Watts A. S., Chapin B. A., Anderson R., & Burns J. M (2011). Neuropsychiatric profiles in dementia. Alzheimer Disease and Associated Disorders, 25, 326–332. doi:10.1097/WAD.0b013e31820d89b6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufer D. I., Cummings J. L., Ketchel P., Smith V., MacMillan A., Shelley T., … DeKosky S. T (2000). Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. The Journal of Neuropsychiatry and Clinical Neurosciences, 12, 233–239. doi:10.1176/jnp.12.2.233 [DOI] [PubMed] [Google Scholar]
- Lee G. J., Lu P. H., Hua X., Lee S., Wu S., Nguyen K., … Thompson P. M.; Alzheimer’s Disease Neuroimaging Initiative (2012). Depressive symptoms in mild cognitive impairment predict greater atrophy in Alzheimer’s disease-related regions. Biological Psychiatry, 71, 814–821. doi:10.1016/j.biopsych.2011.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano D. A., Laforet G., & Devanarayan V.; Alzheimer’s Disease Neuroimaging Initiative (2011). Derivation of a new ADAS-cog composite using tree-based multivariate analysis: Prediction of conversion from mild cognitive impairment to Alzheimer disease. Alzheimer Disease and Associated Disorders, 25, 73–84. doi:10.1097/WAD.0b013e3181f5b8d8 [DOI] [PubMed] [Google Scholar]
- Lyketsos C. G., Steinberg M., Tschanz J. T., Norton M. C., Steffens D. C., & Breitner J. C (2000). Mental and behavioral disturbances in dementia: Findings from the Cache County Study on Memory in Aging. The American Journal of Psychiatry, 157, 708–714. doi:10.1176/appi.ajp.157.5.708 [DOI] [PubMed] [Google Scholar]
- Mah L., Binns M. A., & Steffens D. C.; Alzheimer’s Disease Neuroimaging Initiative (2015). Anxiety symptoms in amnestic mild cognitive impairment are associated with medial temporal atrophy and predict conversion to Alzheimer disease. The American Journal of Geriatric Psychiatry, 23, 466–476. doi:10.1016/j.jagp.2014.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeith I. G., Galasko D., Kosaka K., Perry E. K., Dickson D. W., Hansen L. A., … Perry R. H (1996). Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): Report of the consortium on DLB international workshop. Neurology, 47, 1113–1124. doi:10.1212/WNL.47.5.1113 [DOI] [PubMed] [Google Scholar]
- McKhann G., Drachman D., Folstein M., Katzman R., Price D., & Stadlan E. M (1984). Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology, 34, 939–944. doi:10.1212/WNL.34.7.939 [DOI] [PubMed] [Google Scholar]
- Mehta K. M., & Yeo G. W (2017). Systematic review of dementia prevalence and incidence in United States race/ethnic populations. Alzheimer’s & Dementia, 13, 72–83. doi:10.1016/j.jalz.2016.06.2360 [DOI] [PubMed] [Google Scholar]
- Morris J. C. (1997). Clinical dementia rating: A reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. International Psychogeriatrics, 9(S1), 173–176. doi:10.1017/S1041610297004870 [DOI] [PubMed] [Google Scholar]
- Murray P. S., Kumar S., Demichele-Sweet M. A., & Sweet R. A (2014). Psychosis in Alzheimer’s disease. Biological Psychiatry, 75, 542–552. doi:10.1016/j.biopsych.2013.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Bryant S. E., Lacritz L. H., Hall J., Waring S. C., Chan W., Khodr Z. G., … Cullum C. M (2010). Validation of the new interpretive guidelines for the clinical dementia rating scale sum of boxes score in the national Alzheimer’s coordinating center database. Archives of Neurology, 67, 746–749. doi:10.1001/archneurol.2010.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves S. J., Gould R. L., Powell J. F., & Howard R. J (2012). Origins of delusions in Alzheimer’s disease. Neuroscience and Biobehavioral Reviews, 36, 2274–2287. doi:10.1016/j.neubiorev.2012.08.001 [DOI] [PubMed] [Google Scholar]
- Reise S. P., Morizot J., & Hays R. D (2007). The role of the bifactor model in resolving dimensionality issues in health outcomes measures. Quality of Life Research, 16, 19–31. doi:10.1007/s11136-007-9183-7 [DOI] [PubMed] [Google Scholar]
- Reitan R. M. (1958). Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and Motor Skills, 8, 271–276. doi:10.2466/pms.1958.8.3.271 [Google Scholar]
- Reitan R., & Wolfson D (1985). The Halstead-Reiter neuropsychological test battery. Tucson, AZ: Neuropsychology Press. [Google Scholar]
- Rey A. (1964). L’examen clinique en psychologie [The clinical psychological examination]. Paris: Presses Universitaires de France. [Google Scholar]
- Ropacki S. A., & Jeste D. V (2005). Epidemiology of and risk factors for psychosis of Alzheimer’s disease: A review of 55 studies published from 1990 to 2003. The American Journal of Psychiatry, 162, 2022–2030. doi:10.1176/appi.ajp.162.11.2022 [DOI] [PubMed] [Google Scholar]
- Rosenbloom C. A., & Whittington F. J (1993). The effects of bereavement on eating behaviors and nutrient intakes in elderly widowed persons. Journal of Gerontology, 48, S223–S229. doi:10.1093/geronj/48.4.S223 [DOI] [PubMed] [Google Scholar]
- Samejima F. (1970). Estimation of latent ability using a response pattern of graded scores. Psychometrika, 35, 139–139. doi:10.1007/BF02290599 [Google Scholar]
- Scarmeas N., Brandt J., Albert M., Hadjigeorgiou G., Papadimitriou A., Dubois B., … Stern Y (2005). Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Archives of Neurology, 62, 1601–1608. doi:10.1001/archneur.62.10.1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senanarong V., Cummings J. L., Fairbanks L., Mega M., Masterman D. M., O’Connor S. M., & Strickland T. L (2004). Agitation in Alzheimer’s disease is a manifestation of frontal lobe dysfunction. Dementia and Geriatric Cognitive Disorders, 17, 14–20. doi:10.1159/000074080 [DOI] [PubMed] [Google Scholar]
- Steiger J. H. (1980). Statistically based tests for the number of common factors. In Paper presented at the annual meeting of the Psychometric Society, Iowa City, IA, May 1980.
- Thissen D. (2001). Software for the computation of the statistics involved in item response theory likelihood-ratio tests for differential item functioning. Chapel Hill: University of North Carolina at Chapel Hill. [Google Scholar]
- Tucker L. R., & Lewis C (1973). A reliability coefficient for maximum likelihood factor analysis. Psychometrika, 38, 1–10. doi:10.1007/BF02291170 [Google Scholar]
- Weamer E. A., Emanuel J. E., Varon D., Miyahara S., Wilkosz P. A., Lopez O. L., … Sweet R. A (2009). The relationship of excess cognitive impairment in MCI and early Alzheimer’s disease to the subsequent emergence of psychosis. International Psychogeriatrics, 21, 78–85. doi:10.1017/S1041610208007734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. (1987). WMS-R: Wechsler memory scale-revised. New York, NY: Psychological Corporation. [Google Scholar]
- Wilkosz P. A., Miyahara S., Lopez O. L., Dekosky S. T., & Sweet R. A (2006). Prediction of psychosis onset in Alzheimer disease: The role of cognitive impairment, depressive symptoms, and further evidence for psychosis subtypes. The American Journal of Geriatric Psychiatry, 14, 352–360. doi:10.1097/01.JGP.0000192500.25940.1b [DOI] [PubMed] [Google Scholar]
- Wilson R. S., Gilley D. W., Bennett D. A., Beckett L. A., & Evans D. A (2000). Hallucinations, delusions, and cognitive decline in Alzheimer’s disease. Journal of Neurology, Neurosurgery, and Psychiatry, 69, 172–177. doi:10.1136/jnnp.69.2.172 [DOI] [PMC free article] [PubMed] [Google Scholar]