Abstract
While the general effect of CO2 enrichment on photosynthesis, stomatal conductance, N content, and yield has been documented, there is still some uncertainty as to whether there are interactive effects between CO2 enrichment and other factors, such as temperature, geographical location, water availability, and cultivar. In addition, the metabolic coordination between leaves and grains, which is crucial for crop responsiveness to elevated CO2, has never been examined closely. Here, we address these two aspects by multi-level analyses of data from several free-air CO2 enrichment experiments conducted in five different countries. There was little effect of elevated CO2 on yield (except in the USA), likely due to photosynthetic capacity acclimation, as reflected by protein profiles. In addition, there was a significant decrease in leaf amino acids (threonine) and macroelements (e.g. K) at elevated CO2, while other elements, such as Mg or S, increased. Despite the non-significant effect of CO2 enrichment on yield, grains appeared to be significantly depleted in N (as expected), but also in threonine, the S-containing amino acid methionine, and Mg. Overall, our results suggest a strong detrimental effect of CO2 enrichment on nutrient availability and remobilization from leaves to grains.
Keywords: Climate change, ree-air CO2 enrichment (FACE), multiple locations, N/C metabolism, physiology, varieties, wheat
A study of wheat grown at five FACE facilities around the world shows a low effect of CO2 on grain yield, and reveals relevant modifications in N and Mg metabolism.
Introduction
Wheat represents 27% of global grain production (FAOSTAT, 2018: http://www.fao.org/faostat/en/#home) and, along with corn and rice, is a major component of the human diet because of its high nutritional value: it is a source of carbohydrates, macroelements and micro (trace) elements, protein, and free amino acids. Bread wheat (Triticum aestivum L.) constitutes up to 95% of global wheat production, while durum wheat (Triticum turgidum ssp. durum) makes up the remainder (He et al., 2013). While bread wheat is cultivated in all parts of the world (Reynolds et al., 2012), durum wheat is mostly grown in the Mediterranean region (Oliveira et al., 2012). Like other crops, wheat cultivation will face serious challenges in the coming decades, with a need to increase production despite challenging environmental conditions caused by climate change. Doubling production by 2050 to meet the anticipated demand is expected to be difficult, since yield would have to increase by 2.4% per year globally whereas the actual rate of increase is only 1.3% per year and, furthermore, ~39% of wheat-cultivating areas have shown no increase in yield in a decade (Ray et al., 2012). Among important questions that must be answered to understand how wheat yield responds to changing climatic conditions is the potential impact of rising atmospheric CO2.
Atmospheric CO2 has progressively increased since the beginning of the industrial revolution, and the CO2 mole fraction is anticipated to double (the predicted mole fraction ranges between 730 and 1020 ppm) by 2100 (Meehl et al., 2007). Since photosynthetic activity in C3 plants is CO2-limited, it was predicted that the increase in atmospheric CO2 would stimulate photosynthesis and thus plant growth (Bowes, 1993). However, crop response to elevated CO2 varies widely, depending on growth (cultivation) conditions and inter- and intra-specific variability. While a CO2-driven increase in yield is generally observed, there is substantial variation, with sometimes no yield gain at all (Ainsworth and Long, 2005; Högy and Fangmeier, 2008). This effect mostly comes from photosynthetic acclimation to high CO2, which in turn disfavours grain production. In wheat, cultivation under free-air atmospheric CO2 enrichment (FACE) leads, on average, to a yield enhancement of only 10% (Kimball, 2010, 2016). Photosynthesis acclimates to elevated CO2 via lower average stomatal conductance (gc), lower photosynthetic capacity (maximum carboxylation rate Vcmax), and/or lower ribulose 1,5-bisphosphate regeneration ability (Miglietta et al., 1996; Adam et al., 2000; Rogers and Humphries, 2000; Wechsung et al., 2000; Zhang et al., 2009). Of course, wheat response to elevated CO2 also depends on water and N supply. In general, elevated CO2 leads to a higher relative increase in wheat grain yield under water-restricted conditions (up to 20%) compared with rain-fed plants (up to 10%), although yield absolute values are, as expected, lower under water restriction (Kimball et al., 1995; Hunsaker et al., 1996; Tubiello et al., 1999). In addition, the extent to which yield is stimulated by elevated CO2 under low water input depends on the sowing date and whether additional irrigation is implemented under control (well-watered) conditions (O’Leary et al., 2015). Unsurprisingly, minimal effects of elevated CO2 on photosynthesis and yield are observed under N-limited conditions (Li et al., 2000; Grant et al., 2001; Kimball et al., 2001a; Pacholski et al., 2015; Walker et al., 2017; Manderscheid et al., 2018). Furthermore, elevated CO2 triggers developmental changes that are detrimental to yield. In particular, wheat cultivated under FACE has a lower shoot-to-root ratio (Wechsung et al., 1995; Pacholski et al., 2015) and perhaps an increased root exudation rate (thereby increasing organic matter deposition in soil and thus soil respiration) (Pendall et al., 2001) and accelerated flag leaf senescence (Zhu et al., 2009). By contrast, elevated CO2 causes an increase in tiller number (Yang et al., 2007a, b) and has a marginal effect on transpiration, making plants slightly more water-efficient, especially under high N supply (Hunsaker et al., 1996; Erbs et al., 2009; O’Leary et al., 2015; Kimball, 2016; Manderscheid et al., 2018).
In addition to such quantitative changes in crop production and photosynthesis, elevated CO2 leads to modifications in leaf biochemical composition and grain quality. Under elevated CO2, wheat leaves invest more C in cellulose and flavonoids (Akin et al., 1995; Peñuelas et al., 2000) and are generally less N-rich, with a higher C/N ratio (Zhu et al., 2009; Aranjuelo et al., 2015) and lower amounts of proteins (or transcripts encoding them) involved in photosynthesis (Calvin cycle enzymes) (Nie et al., 1995; Adam et al., 2000; Zhang et al., 2009; Pandey et al., 2017). Proteomics conducted on leaves sampled during anthesis in wheat cultivated in a CO2-enriched greenhouse (at 700 µmol mol−1 CO2) have shown that the Rubisco content did not increase, while there was a slightly higher content of Rubisco activase (Aranjuelo et al., 2015). When grown under FACE, leaves have been found to contain less Rubisco and more mitochondrial malate dehydrogenase (NAD-dependent), suggesting a change in respiratory metabolism (respiratory CO2 loss) (Pandey et al., 2017). The detrimental effect of CO2 on the photosynthetic machinery of flag leaves is accompanied by an increase of ear photosynthesis and a faster decline in flag leaf N due to earlier senescence (Sicher and Bunce, 1998; Zhu et al., 2009). Taken as a whole, elevated CO2 has a major effect on leaf N metabolism, which in turn impacts on remobilization and thus grain filling during maturation.
Unsurprisingly therefore, wheat grains (and flour produced from them) produced at elevated CO2 have a generally lower nutritional quality, with lower N (and S) content, less protein, and more starch and fibres (Kimball et al., 2001a, b; Högy et al., 2013; Wroblewitz et al., 2013). We have recently shown that when wheat plants are grown in the greenhouse at elevated CO2 under ample N supply, grains are less N-rich and do not have the same kinetics of gliadin and glutenin accumulation (Soba et al., 2019). In this case, it has been suggested that this effect did not come from deficient N remobilization in leaves but rather a N dilution effect, whereby the quantity (or size) of grains increased while the total available N for remobilization did not change. In addition, under FACE conditions, an increase in the glutenin-to-gliadin ratio has been found (Pandey et al., 2017), as well as a general decrease in all storage proteins except for globulins and albumins (Wieser et al., 2008, but see Verrillo et al., 2017, where globulins also decreased). Metabolic analyses have demonstrated that at elevated CO2, grains contain more secondary metabolites (shikimate and quinate) and free hexoses (fructose and glucose) but less sucrose and alanine, regardless of developmental stage. At maturity, grains are generally depleted in free amino acids, except for the branched-chain amino acids valine, leucine, and isoleucine, which are significantly more abundant (Wroblewitz et al., 2013; Soba et al., 2019). Furthermore, it has been suggested that the metabolism of lysine recycling changes and favours the saccharopine pathway over pipecolate (Soba et al., 2019). It is also worth noting that elevated CO2 causes a decline in the content of several microelements in wheat grains, such as Fe and Zn (Wroblewitz et al., 2013; Pandey et al., 2017; Beleggia et al., 2018), further impacting on nutritional quality.
Nevertheless, the effect of elevated CO2 likely depends not only on the cultivation conditions (water and N supply, time of sowing, and temperature), but also on soil properties and the species and cultivar of wheat. For example, wheat lines of different ploidy do not respond similarly to elevated CO2 in FACE experiments, with hexaploid bread wheat being the most responsive in terms of yield (but the least in terms of photosynthesis) (Uprety et al., 2009). There is also substantial variability between cultivars, from no response at all to significant response to CO2, with little correlation between yield and photosynthesis, but significant correlation with N content and specific leaf area (Thilakarathne et al., 2013, 2015) or transpiration efficiency (Tausz-Posch et al., 2013b; but see also Bourgault et al., 2013). The impact of elevated CO2 on both yield and elemental contents has been found to depend on the cultivar (Fernando et al., 2014; Fares et al., 2016; Houshmandfar et al., 2016; Beleggia et al., 2018). Variability in the response to elevated CO2 between wheat cultivars likely results from differences in N and chlorophyll content, transpiration efficiency, or tillering capacity (Tausz-Posch et al., 2013a; Veres et al., 2017). Modelling has also suggested that variation in the response of wheat lines to elevated CO2 can result from differences in development and allocation (sink/source relationships) (Wolf et al., 2002). In addition, it is probable that the response to elevated CO2 may vary when soil properties change. This aspect is less documented but, in principle, the spatial variability of wheat yield in the field under ambient CO2 has been shown to be related to soil P or organic C content, soil structure, or the proportion of carbonates (Miller et al., 1988; Bhatti et al., 1991; Moulin et al., 1994). Since in wheat there is a relationship between root deposition stimulated by elevated CO2, root-driven acidification, and cation mobility (Cheng et al., 2010), the effect of elevated CO2 could be modulated by soil Na, Ca, and K content and pH.
Taken as a whole, whenever there are differences among growth conditions and cultivars, it is difficult to understand the physiological mechanisms underlying the response to elevated CO2. In fact, it could well be that FACE experiments conducted separately in different countries with different cultivars are not comparable, not only because soil and climate conditions vary but also because the response to CO2 is cultivar-specific. To tackle this issue, we conducted a targeted metabolic analysis of samples collected during FACE experiments (at 550 µmol mol−1 CO2) conducted in different countries (USA, Australia, Germany, China, and Italy) with different species (T. aestivum and T. turgidum ssp. durum) and cultivars (Table 1; Supplementary Fig. S1). We carried out elemental analyses (macroelements and some microelements), quantitation of amino acids and sugars, and targeted protein analysis in leaves and grains. Our objective was to assess whether a significant effect of elevated CO2 could be observed in both leaves and grains across countries and wheat lines and, by doing so, to look at the potential effect of leaf-to-grain nutrient remobilization on CO2 responsiveness. Our data could also be exploited further by exploring the potential relationship between yield (treated as a quantitative response variable) and grain biochemical composition. Our working hypothesis was that (i) detrimental effects of elevated CO2 on photosynthesis and N assimilation were associated with significant changes in protein and amino acid composition in leaves, and thus (ii) the amino acid composition of grains was affected, reflecting alteration of both the provision and recycling of nitrogenous compounds; and (iii) there might be a relationship between grain metabolic features and yield.
Table 1.
Growth conditions associated with the CO2 enrichment experiments in this study
| Site general information | Climatic conditions during growth | Fertilization | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Country | Location | Cultivation time window | Species and cultivars | Sowing month | Ptot (mm) | Tav min (°C) | Tav max (°C) | Cumulated d∙°C | Nitrogen (kg N ha−1) | Elemental N-P2O5-K2O |
| USA | Beltsville | 16/10/15–7/6/16 | T. durum Milan, PRL | October | 141 | 4.2 | 25.9 | 981 | 250 | 10-10-10 |
| Italy | Fiorenzuola | 9/11/15-11/7/16 | T. aestivum Janz, Kite; T. durum Claudio, Simeto | November | 347 | 10.3 | 27.8 | 1774 | 183 | 15-15-15 |
| Germany | Hohenheim | 5/4/16-1/8/16 | T. durum Miradoux, Duramant | April | 271 | 2.6 | 24.8 | 1679 | 202 | 10-5-5 |
| China | Beijing | 6/10/15–20/6/16 | T. durum Norin, Triumph | October | 307 | 10.9 | 30.8 | 1843 | 200 | 20-16-9 |
| Australia | Horsham | 1/06/16–21/12/16 | T. aestivum Janz, Kite | June | 334 | 9.0 | 23.1 | 2394 | 50 | 10-1-10 |
Soil mineral conditions are further documented in Supplementary Fig. S1. Cumulated d∙°C, cumulated temperature during cultivation period (in days∙°C); Ptot, total precipitation; Tav min, average minimum daily temperature; Tav max, average maximum daily temperature.
Materials and methods
Plant material and experimental design
The experiment was conducted with durum wheat (cultivars Miradoux, Duramant, Claudio, and Simeto) and bread wheat (cultivars Janz, Kite, Norin, Triumph, Milan, and PRL). The study involved five FACE facilities located in Stuttgart-Hohenheim (Germany), Fiorenzuola (Italy), Changping-Beijing (China), Horsham (Australia), and Beltsville (USA), in which plants were exposed to ambient (400 ppm) and elevated (550 ppm) CO2 conditions. FACE facilities included three to five rings (for each CO2 concentration condition) with a diameter of 12–14 m each or (in Stuttgart-Hohenheim) 2×2 m square plots. In all cases, CO2 enrichment was performed from sunrise to sunset (in Australia and Italy) or 24 h a day (in China, Germany, and the USA) and throughout the entire growing season. The principle of operation and the performance of the FACE systems used here have been described previously (Mollah et al., 2009; Fangmeier et al., 2016; Fares et al., 2016). When plants reached the stem elongation (Z31) and anthesis half-way (Z65) stages (BBCH), sampling and gas-exchange analyses were carried out in the last expanded leaves (Z31) or flag leaves (Z65). Collected leaf samples were immediately frozen in liquid N2 and stored at –80 °C for later analyses. Finally, at maturity stage (Z90), grain samples were collected for agronomic and metabolic analyses.
Gas exchange determinations
Analyses were carried out in healthy expanded flag leaves grown under ambient or elevated CO2 conditions. The light-saturated rate of CO2 assimilation (Asat), stomatal conductance (gs), and intercellular CO2 concentration (ci) were estimated at a photosynthetic photon flux density of 1500–1600 µmol m−2 s−1 using equations developed by von Caemmerer and Farquhar (1981). The vapour pressure deficit was 1.5 kPa. CO2 response curves were constructed by a series of measurements whereby photosynthesis was determined first under standard conditions (400 µmol mol−1), then at low CO2, then back to standard conditions, and then at high CO2: overall, the sequence of CO2 concentrations in the reference channel was 400, 200, 100, 50, 200, 400, 600, 750, and 950 µmol mol−1. Estimations of the maximum carboxylation velocity of Rubisco (Vcmax) and the maximum electron transport rate contributing to ribulose 1,5-bisphosphate regeneration (Jmax) (measurements made in Germany) were made using the method of Harley et al. (1992).
Mineral composition
C and N concentration (%) analyses were determined using an elemental analyzer (EA, Carlo Erba Strumentazione, Milan, Italy). Micronutrient and macronutrient concentrations were determined by inductively coupled plasma/optical emission spectrometry (ICP/OES, iCAP 6500 Duo, Thermo Fisher Scientific, Waltham, MA, USA).
C isotope discrimination
Carbon isotope composition was determined using an elemental analyzer (EA1108; Carlo Erba Strumentazione, Milan, Italy) coupled to an isotope ratio mass spectrometer (Delta C; Finnigan MAT, Bremen, Germany). Values were expressed as delta values δ 13C=(Rsample/Rstandard)–1 and expressed in ‰, where Rstandard is the 13C-to-12C ratio of the international standard (V-PDB). The carbon isotope discrimination (Δ) was calculated as follows:
| (1) |
where δ 13Cair (‰) is the isotope ratio in atmospheric CO2. The addition of (industrial) CO2 in the course of the FACE experiments was such that δ 13Cair was lower than the natural 13C abundance in the atmosphere (near –8‰). The δ 13C value of the added CO2 was known (for the experiment in Germany) or calculated from mass balance, taking advantage of the known contribution of added CO2 to total CO2: c=(550–400)/550=27.2%. In fact, if the isotope fractionation under ambient CO2 and under FACE conditions was the same, then the proportion of C coming from the added CO2 could be calculated as c=(δ OM,amb–δ* OM,FACE)/(δ CO2,amb–δ CO2,added), where OM refers to organic matter and amb refers to ambient conditions, and δ 13C values are abbreviated to δ to simplify the notation. δ* OM,FACE is the δ 13C value of organic matter under FACE conditions if the fractionation had not changed compared with ambient conditions. In practice, the isotope fractionation could have varied, and thus (neglecting denominators): δ* OM,FACE =δ OM,FACE+Δ FACE–Δ amb, where Δ FACE and Δ amb are the net photosynthetic fractionation under FACE and ambient conditions, respectively. These equations can be combined to give c=(δ OM,amb–δ OM,FACE)/(δ CO2,amb–δ CO2,added)–(Δ FACE–Δ amb)/(δ CO2,amb–δ CO2,added). The right term is relatively small since added industrial CO2 was naturally 13C depleted and thus δ CO2,amb–δ CO2,added is much larger than Δ FACE–Δ amb. That is, by neglecting the right term, δ CO2,added can be estimated (and therefore Δ FACE with equation 1) by imposing c=0.272. Note that such an approximation was not critical, since even under the assumption (Δ FACE–Δ amb)/(δ CO2,amb–δ CO2,added) represented 0.10 (i.e. 40% error in c); this would ultimately cause an error of only 1‰ in Δ FACE.
Abscisic acid content
Extraction, purification, and quantification of abscisic acid (ABA) were carried out as described by Torres et al. (2018), using a high-resolution mass spectrometry (HPLC-ESI-HRMS) system, with some modifications: freeze-dried material (15 mg) was used instead of frozen powdered material (0.1 g), and the residue obtained after final evaporation (SpeedVac) was redissolved in 0.25 ml methanol instead of 0.5 ml.
Soluble sugar and starch content
Sucrose, glucose, and fructose content were determined using a Beckman P/ACE5500 capillary electrophoresis system (Beckman Instruments, Fullerton, CA, USA), following the method of Cabrerizo et al. (2001). Starch content in the pellet was determined according to Ethier and Livingston (2004).
Amino acid content
Amino acids were derivatized at 22–25 °C for 12–16 h with 1 mM fluorescein isothiocyanate dissolved in 20 mM acetone/borate, pH 10. The content of single amino acids was determined by capillary electrophoresis in a Beckman-Coulter PA-800 system.
Protein content
Samples were previously quantified by microBCA analysis (Pierce) and similar amounts (5 µg per sample) were individually dissolved in 8 M urea, 25 mM ammonium bicarbonate, reduced with DTT, and alkylated with iodoacetamide, according to a method described by López-Ferrer et al. (2004). Digested samples were diluted with 0.2% trifluoroacetic acid in water and subjected to multiple reaction monitoring analysis using a 1D Plus nanoLC Ultra system (Eksigent, Dublin, CA, USA) interfaced to a Sciex 5500 QTRAP triple quadrupole mass spectrometer (Sciex, Framingham, MA, USA) equipped with a nano-electrospray ionization source and controlled by Analyst v.1.5.2. software (ABSciex). Trypsin-digested samples were loaded online on a C18 PepMap 300 µm internal diameter × 5 mm trapping column (5 µm, 100 Å, Thermo Scientific) and separated using a BioSphere C18 75 µm internal diameter × 150 mm capillary column (3 µm, 120 Å, Nanoseparations). A list of 84 transitions (usually 3–4 per peptide, with a preference toward higher-mass y series ions), corresponding to 21 unique peptides selected for 10 different proteins, was monitored. Skyline software determined automatically the collision energy values for the candidate peptides according to MacLean et al. (2010).
Statistical analysis
To explore the effect of CO2, univariate statistics were conducted with a two-way ANOVA, with one factor representing ‘conditions’ (time of sampling, country, and species/cultivar) while the second factor was CO2. It was not possible to carry out a three-way analysis because not all species/cultivars were represented in all countries. Thus, here, the two-way ANOVA was constructed with the factor ‘conditions’ having 12 possible qualitative values (combinations time–country–cultivar) and the factor ‘CO2’ having two values (ambient or elevated). Unless otherwise stated, statistical significance was accepted when P<0.05. Multivariate statistics were carried out using orthogonal projection on latent structure (OPLS; with Simca®, Umetrics), using CO2 as the predicted Y variable (while ‘conditions’ are embedded into orthogonal dimensions), and metabolic features (elemental contents, metabolites, and proteins) as predicting X variables. Before running the OPLS, a principal component analysis was conducted to check the presence of outliers (samples outside the Hotelling’s ellipse). The performance of the OPLS was assessed using the correlation coefficient between predicted and observed Y (R2), the cross-validated correlation coefficient (Q2), the Q2 intercept of the permutation test (which was checked to be negative), and the P-value of testing the OPLS model against a random-error model (i.e. average ±error) via a χ 2 test (this P-value is referred to as PCV-ANOVA). Univariate and multivariate analyses were combined using –log(P-value) (univariate) plotted against the OPLS loading (pcorr) in a volcano plot. To explore the relationship between yield and grain composition, we conducted (i) an OPLS using yield as the predicted Y variable (with the same parameters of performance as described above) and (ii) a univariate analysis by linear regression (done in R). Linear regression was done without and with variable elimination. Without variable elimination, each variable was correlated with yield separately and the associated P-value is reported. To carry out variable elimination, we used a multiple linear regression. It was carried out via sampling subsets (regsubsets) of eight variables (304 iterations) and a correlation plot was generated to select the eight most correlated variables overall. Then, a multiple linear regression model (lm) comprising only these eight variables was generated and the P-value for each variable was calculated.
Results
Photosynthetic parameters
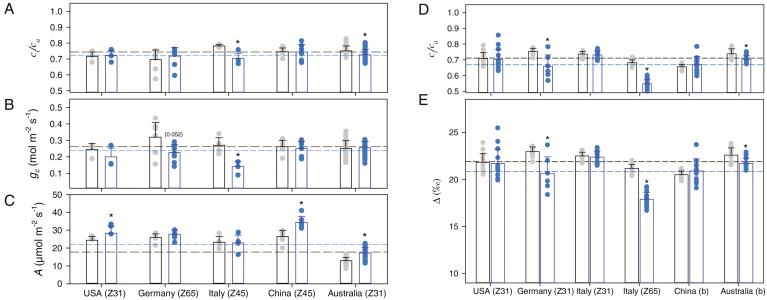
The photosynthetic activity of flag leaves was assessed using measurements of gas exchange at various times of the development cycle (shown as BBCH stage numbers), at the CO2 mole fraction (ca) used during growth (Fig. 1). Overall, there was little variation in the photosynthetic rate, which was ~22 µmol m−2 s−1 regardless of CO2, with the following properties (Fig. 1C): (i) in China, the USA, and Australia, there was a significantly higher photosynthetic rate at elevated CO2, and (ii) in Australia, plants generally had lower photosynthetic rates compared with other countries. These effects were unrelated to stomatal conductance (Fig. 1B), which was roughly constant at 0.25 mol m−2 s−1; however, CO2 had a significant depressing effect in plants grown in Italy. As a result, there was little variation in the intercellular-to-external CO2 ratio (ci/ca) (Fig. 1A), which was always ~0.75, except in Italy (ci/ca <0.7) and in Australia (ci/ca ≈0.7) at elevated CO2. The natural carbon isotope abundance (δ 13C) in raw leaf matter was measured, and ‘average’ ci/ca (i.e. across the leaf lifespan) was estimated using the simplified equation of Farquhar et al. (1982) (Fig. 1D, E). The 12C/13C fractionation (raw matter versus source CO2) was within 20–22‰, showing that average ci/ca was always ~0.7. However, the isotope fractionation was significantly lower (and therefore so was average ci/ca) in Germany, Italy, and Australia at elevated CO2, suggesting a long-term decrease in stomatal (or internal) conductance and/or an increase in photosynthetic capacity at elevated CO2. In addition, there were no significant changes either between CO2 conditions or among countries in ABA content (data not shown).
Fig. 1.
Photosynthetic parameters in wheat grown under ambient (grey) or elevated (550 µmol mol−1, blue) CO2 mole fraction. (A–C) Leaf gas exchange properties: intercellular-to-atmospheric CO2 ratio (ci/ca) (A), stomatal conductance for CO2 (B), and net photosynthesis (C). (D, E) 12C/13C carbon isotope fractionation (Δ) measured using leaf total organic matter δ 13C (E) and calculated average ci/ca values (E). Asterisks indicate significant differences (P<0.05) between ambient and elevated CO2. P-values very close to statistical significance are given in parentheses. Dashed lines show average values across all countries. The developmental stage (BBCH) for plants in each country is given in parentheses; for China and Australia, values obtained at both BBCH stages 31 and 65 were pooled together (indicated by b). ND, not determined. (This figure is available in colour at JXB online.)
Leaf metabolism
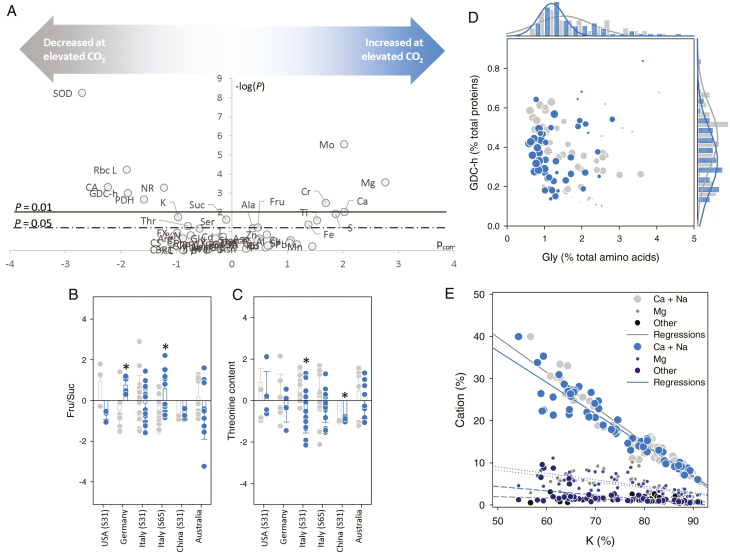
The contents of amino acids, sugars, macro- and microelements, and some target proteins were analyzed in flag leaves (contents were expressed relative to dry weight). A statistical analysis combining multivariate (OPLS) and univariate (two-way ANOVA) statistics was performed and results showing the effect of CO2 are represented as a volcano plot in Fig. 2A. In both the OPLS and ANOVA, two factors were considered: CO2 and ‘conditions’ (comprising country, species/cultivar, and growth stage). Growth at elevated CO2 led to an increased content of several elements (Mo, Mg, Ca, Cr, Ti, Fe, and S) and a decrease in K. Several enzymes [superoxide dismutase, Rubisco (large subunit), carbonic anhydrase, pyruvate dehydrogenase, and nitrate reductase] had a lower content at elevated CO2, and so had threonine. There was a tendency to have more fructose and less sucrose at elevated CO2 (significant changes in ANOVA) but OPLS loadings were rather small, showing that the change in sugar composition was not a strong marker of growth at elevated CO2. When expressed as a percentage of total amino acids and proteins, glycine and glycine decarboxylase (GDH subunit h) were lower (P<0.001) under elevated CO2 (Fig. 2B), likely reflecting lower photorespiration activity. When examined separately for each country, the fructose-to-sucrose ratio appeared to be significantly different in Germany and Italy (Fig. 2C), and threonine content appeared to be significantly different in Italy and China (Fig. 2D), showing that the effect of elevated CO2 was country-specific (i.e. it depended on environmental local conditions). This phenomenon was clearly visible in the results of the two-way ANOVA, where several features appeared to be significant for the CO2 × conditions effect (Supplementary Fig. S2). For example, under elevated CO2, there was a notably lower content of amino acids in Australia, nearly opposite effects on starch content in the USA and Australia, and a strong decrease in the content of several microelements in China. Nevertheless, the patterns of mineral elements were the same across all countries and CO2 conditions. In fact, there was as expected an antagonism between major cations, with Ca + Na or Mg being negatively correlated with K (Fig. 2E). However, there was little difference in this correlation between ambient and elevated CO2. In other words, the significant decrease in K and increase in Ca and Mg at elevated CO2 (Fig. 2A) followed the same cation balance relationship as under ambient CO2.
Fig. 2.
Metabolism of wheat leaves in plants grown under ambient (grey) or elevated (550 µmol mol−1, blue) CO2 mole fraction. (A) Volcano plot [–log(P-value) from ANOVA versus the loading pcorr from OPLS] showing the best discriminating components (metabolites, proteins, and elements) associated with the effect of CO2 enrichment. Threshold P-values (0.01 and 0.05) are shown with horizontal solid and dashed lines. (B, C) Relative fructose-to-sucrose ratio (B) and relative threonine content (C). In these panels, cultivars are pooled together for each country. Asterisks indicate significant differences (P<0.05) between the different average values. (D) Distribution of data points in the bi-plot showing the relative content of glycine decarboxylase h subunit (GDC-h) and glycine (Gly) (as a percentage of total proteins and total amino acids, respectively), with frequency plots on each axis. Countries are represented by different symbol sizes. (E) Relationship between K content and other cations under elevated and ambient CO2, with linear regressions (all significant, P<0.05). (This figure is available in colour at JXB online.)
Grain metabolism
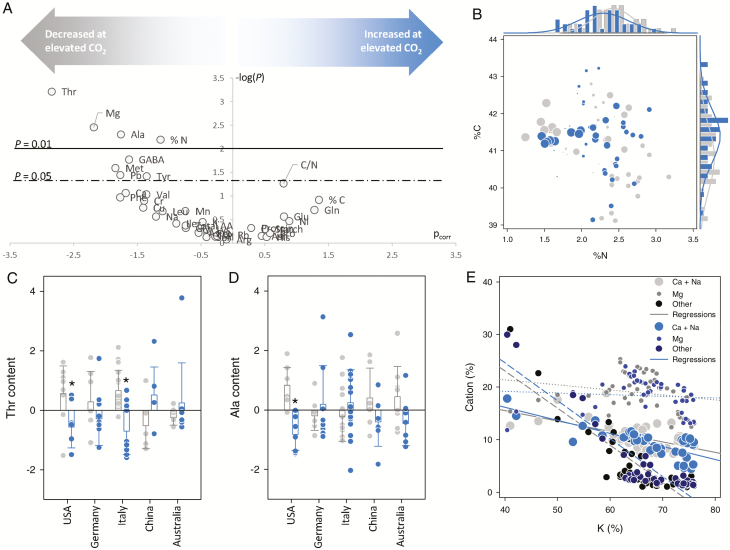
The contents of free amino acids, macro- and microelements, and total proteins and starch were quantified in mature grains (relative to dry weight). As in leaves, an analysis combining univariate (ANOVA) and multivariate (OPLS) statistics was performed and presented as a volcano plot (Fig. 3A). Interestingly, no component appeared to be significantly increased at elevated CO2. By contrast, there were significant decreases in several elements (N, Mg, and Pb), amino acids (methionine, threonine, alanine, and tyrosine), and the glutamate derivative γ-aminobutyrate (GABA). Although nitrogen content (%N) was found to be lower, there was no significant change in protein content. Such a decrease in N content could be explained by changes in the concentration of many N-containing compounds (e.g. free amino acids, polyamines, or insoluble proteins not accounted for in our assay), which in total sum up to a larger and significant change in overall %N. When plotted together, %N and carbon content (%C) showed considerable scattering across countries (Fig. 3B), with a difference greater than 1.5% in %N between samples. The effect of elevated CO2 on alanine and threonine appeared to be condition-specific, with significant differences between conditions found in the USA and Italy (Fig. 3C, D). More generally, the two-way ANOVA showed that amino acids, %N, and Pb were significant for the CO2 × conditions effect (Supplementary Fig. S3). Elevated CO2 did not lead to significant changes in major cations (K, Ca, Na, and Mg) (Fig. 3E). In fact, Ca + Na and Mg were negatively correlated with K, reflecting cation balance, and the correlation was not changed by elevated CO2; that is, the lower Mg content at elevated CO2 (Fig. 3A) was not associated with a change in the Mg/K balance in grains.
Fig. 3.
Metabolism of wheat grains in plants grown under ambient (grey) or elevated (550 µmol mol−1, blue) CO2 mole fraction. (A) Volcano plot [–log(P-value) from ANOVA versus the loading pcorr from OPLS] showing the best discriminating components (metabolites and elements) associated with the effect of CO2 enrichment. Threshold P-values (0.01 and 0.05) are shown with horizontal solid and dashed lines. (B) Distribution of data points in the bi-plot showing elemental C and N content, with frequency plots on each axis. Countries are represented by different symbol sizes. (C, D) Relative threonine (C) and alanine (D) content. In these panels, cultivars are pooled together for each country. Asterisks indicate significant differences (P<0.05) between control and elevated CO2. (E) Relationship between K content and other cations under elevated and ambient CO2, with linear regressions (all significant, P<0.05, except for Mg). (This figure is available in colour at JXB online.)
Relationships with yield
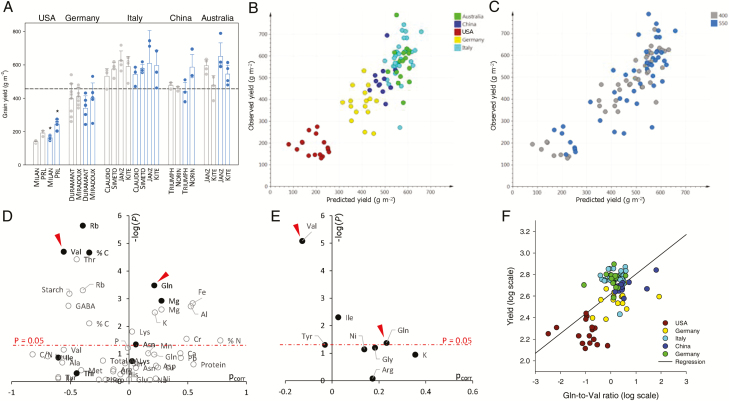
There were considerable differences in grain yield between countries, with low values in the USA (≈200 g grain m−2) and high values in Australia and Italy (≈600 g grain m−2) (Fig. 4A). The difference in yield between countries was unsurprising and resulted from seasonal differences, as well as differences in climatic conditions, fertilization (Table 1), and soil composition (Supplementary Fig. S1), or sowing density (which ranged within 120–350 m−2). To gain insight into the metabolic determinants of yield, we conducted both a multivariate OPLS analysis using yield as a predicted quantitative Y variable, and a univariate analysis by linear regression. The OPLS analysis generated a good statistical model (R2=0.75) with good robustness (Q2=0.67) and very high significance (PCV-ANOVA=10–16). When observed and predicted yield were plotted together, it was clear that the relationship was driven partly by differences between countries (Fig. 4B) and not at all by the CO2 treatment (Fig. 4C). Accordingly, there were no significant CO2 effects, except in the USA (Fig. 4A). The best drivers of yield were investigated using volcano plots. Two volcano plots are shown here: the first (Fig. 4D) is associated with an analysis that disregards ‘conditions’ (countries and species/cultivars), while the second (Fig. 4E) incorporates ‘conditions’ as a variable. In Fig. 4D, P-values are associated with a variable-by-variable linear regression or with a multiple linear regression including variable elimination (where variables that are not best correlated with yield are discarded) (Supplementary Fig. S4). Regardless of country and cultivar, the yield appeared to be significantly and positively related to micro- and macroelements (Fe, Al, K, Mg, Cr, and N) and negatively related to starch, threonine, Rb, and %C. After variable elimination upon multiple linear regression, only six features were significantly related to yield: Mg, glutamine, and asparagine (positively related), and Rb, valine, and %C (negatively related). Among these features, some appeared to be significant because of the confounding factor of country of origin (Fig. 4B, C). When this effect was removed by incorporating ‘conditions’ as a variable, the two most significant drivers were glutamine (increased) and valine (decreased) (Fig. 4E). Tyrosine and isoleucine were found to be significant (P<0.05) but their loading value was close to zero, showing that their impact (in multivariate analysis) was numerically very small. When plotted separately, there was a positive relationship between yield and the glutamine-to-valine ratio in grains (Fig. 4F).
Fig. 4.
Yield analysis. (A) Yield (in g grains m−2) of the different cultivars and countries. Asterisks indicate significant differences (P<0.05) between control (grey) and elevated (blue) CO2. (B, C) Relationship between observed yield and yield predicted using the OPLS model (comprising country+cultivar as a qualitative X variable), differentiating countries (B) or CO2 levels (C) (R2=0.75). (D) Volcano plot [–log(P-value) versus OPLS loading pcorr] showing the importance of variables for statistical analysis: P-values from separate linear regressions (each variable taken separately) (white circles), or P-values from variable elimination and linear model (black circles) against OPLS loadings (both univariate and OPLS models without country+cultivar as a qualitative X variable). (E) P-values from variable elimination and linear model against OPLS loadings (both univariate and OPLS models with country+cultivar as a qualitative X variable). In D and E, the horizontal dashed line indicates the P-value threshold of 0.05, and arrowheads indicate valine (Val) and glutamine (Gln). (F) Relationship between observed yield and the glutamine-to-valine ratio (log scales). The solid line represents the linear regression (R2=0.37). (This figure is available in colour at JXB online.)
Discussion
Here, we used five FACE sites in different countries, with different wheat cultivars/species, to investigate the metabolic effects of elevated CO2 in leaves and grains. Our results show that elevated CO2 (i) had a limited effect on photosynthesis rate and grain yield, reflecting photosynthetic acclimation; (ii) caused a decline in proteins involved in photosynthesis, photorespiration, or N assimilation in leaves; and (iii) altered grain quality, with lower contents of amino acids and mineral nutrients. In addition, a quantitative analysis of yield suggested that high yield values correlate with higher glutamine, K, and Mg contents and lower valine content.
Growth at elevated CO2 had a limited impact on crop yield
Crop yield and photosynthetic responsiveness to elevated CO2 depend considerably on surrounding (local) environmental conditions. Here, we found that with the exception of the FACE site in the USA, grain yield was not significantly affected by elevated CO2. In practice, changes in wheat yield in response to high CO2 are determined by the cultivar(s), fertilization protocols, and interactions with other environmental factors such as water availability and temperature. The absence of an effect of CO2 was probably linked to the lack of significant changes in assimilation (mechanisms are further discussed below). Accordingly, several previous studies have shown that the initial stimulation by CO2 can be compensated for by acclimation (reviewed in Ainsworth and Rogers, 2007). The meta-analyses carried out by Galmés et al. (2013) showed that under elevated CO2 conditions, Rubisco content is the primary driver in the regulation of Rubisco activity and, consequently, photosynthetic activity. Within this context, the absence of an effect of elevated CO2 on crop yield could be linked to limitations in N assimilation and altered leaf C sink/source balance (Ainsworth and Rogers, 2007). Photosynthetic performance is believed to be affected by two key factors: the CO2 concentration in the chloroplast (cc) and the carboxylation capacity, which is linked to leaf N content. Here, the absence of significant differences in stomatal conductance (accompanied by the lack of an effect on leaf ABA content) suggests that stomatal limitation of CO2 diffusion was a minor component of acclimation under our conditions. The lower content of nitrate reductase (and other proteins) suggests an inhibitory effect of elevated CO2 on N metabolism, as previously observed in wheat leaves (Jauregui et al., 2015). The inhibition of nitrate assimilation under elevated CO2 in wheat has been suggested to reflect lower electron allocation to nitrite reductase in the chloroplast and/or the inhibition of the GS/GOGAT cycle driven by photorespiration (Bloom et al., 2014). Here, the lower contents of ferredoxin, chlorophyll binding proteins, and glycine decarboxylase under elevated CO2 would be consistent with a down-regulation of N assimilation.
Besides the effect of CO2 (or lack thereof), our study shows important differences in yield between locations and cultivars. The lowest values were found in the USA, where sowing density (120 m−2), precipitation, and cumulated day·°C values were low (Table 1), and seedling mortality was pronounced in the year of the FACE experiment due to bad weather. The high yield values observed in China were probably explained by higher water availability. We note that the FACE site in the USA, where elevated CO2 had a significant effect on yield, had the highest N fertilization rate (250 kg ha−1) despite low plant sowing density and low cumulated days∙°C; this suggests that perhaps the two most important factors for CO2 responsiveness were available N and intercepted light (minimal shading) under our conditions. Differences between locations could also have originated from the contrasting behaviour of the two species of wheat studied, with bread wheat exhibiting generally higher yield values than durum wheat. This species effect comes from the fact that durum wheat is more water conservative and thus more suitable to grow in stressful environments, while bread wheat is believed to have a higher yield potential (Slafer et al., 1996; Marti and Slafer, 2014).
Photosynthetic effects of elevated CO2
Growth at elevated CO2 generally leads to a down-regulation of some photosynthetic parameters, such as carboxylation efficiency, maximal carboxylation velocity, and CO2 conductance (see Introduction). Here, photosynthesis increased by a modest but significant amount at elevated CO2 (in the USA, China, and Australia) or did not increase at all (in Italy and Germany). In Italy, the lack of a stimulating effect of CO2 mole fraction was associated with a decrease in stomatal conductance (Fig. 1B). It is also possible that internal conductance decreased under elevated CO2, and this could contribute to explaining why the 12C/13C isotope fractionation did not increase much (with apparent ci/ca staying at ~0.7 despite the increase in CO2 mole fraction) (Fig. 1E). In addition, there was probably a decrease in carboxylation capacity, as suggested by the significantly lower Rubisco content in leaves (Fig. 2), and A/ci response curves for wheat grown in Germany suggest a lower Vcmax (non-significant) and Jmax (P<0.05) (Supplementary Fig. S5). More generally, elevated CO2 led to a down-regulation of the photosynthetic and photorespiratory machinery, with less carbonic anhydrase, glycine decarboxylase, and superoxide dismutase, an enzyme of redox metabolism (as reported previously in Aranjuelo et al., 2015). As a result, the lower content of major enzymes such as Rubisco was accompanied by a decline in %N in some countries (the USA, China, and Australia) but not others. The origin of this effect of elevated CO2 could be lower biosynthesis of proteins or an increase in protein degradation, for example, earlier remobilization of proteins to facilitate export of N from leaves to developing grains (Sicher and Bunce, 1998; Zhu et al., 2009). Earlier remobilization seems unlikely, since leaves were sampled at stage Z31 (stem elongation with first node above tillering node) or Z65 (anthesis, 50% of anthers mature), that is, before the onset of remobilization. Therefore, it is probable that elevated CO2 down-regulated N metabolism, causing a general decrease in the biosynthesis of major proteins and thus potentially photosynthesis. Despite the change in Rubisco content, the net effect on photosynthesis also depends on other enzyme activities, because net assimilation is not highly sensitive to Rubisco content. For example, other enzymes, such as Rubisco activase, are crucial for carboxylation efficiency. We have previously shown using proteomics that elevated CO2 leads to a decline in Rubisco activase content (Aranjuelo et al., 2015). Here, our analysis focused on a number of specific proteins, which did not include Rubisco activase. Photosynthetic products (sugars) were affected by CO2 mole fraction, with significantly less sucrose and generally more fructose (P=0.06); as a result, the fructose-to-sucrose ratio was larger at elevated CO2 in two countries (Germany and Italy) (Fig. 2B). In other words, elevated CO2 was associated with a reconfiguration of sugar metabolism, likely including a larger allocation of carbon to starch and a futile sucrose synthesis–degradation cycle.
Effect of CO2 on grain composition
We found that elevated CO2 had a clear effect on grain composition, leading to lower contents of N, amino acids, and microelements (Fig. 3). The general effect of elevated CO2 on N content (and proteins) in grains has been documented before, and has been suggested to be linked to perturbations in both leaf-to-grain N transport (e.g. glutamine) and amino acid metabolism in grains (e.g. in the lysine degradation pathway; see the Introduction and Soba et al., 2019). Here, it is remarkable that Mg was found to be more abundant in leaves but less abundant in grains, suggesting that elevated CO2 compromised microelement remobilization (the same occurred with Ca but this was not significant in grains). Nutrient quantitation during grain filling in different cultivars of spring wheat has suggested that Mg is remobilized from the lower stem and leaves to grains, with little effect of cultivar (Tiryakioglu et al., 2014). Growth at elevated CO2 has been found to affect Mg content in wheat (Sanchez de la Puente et al., 2000; Högy et al., 2013; Aranjuelo et al., 2015; Beleggia et al., 2018). Mg remobilization involves chlorophylls and Rubisco degradation in leaves, and Mg circulation via the phloem. This raises the question of whether phloem transport was affected by elevated CO2 in the current study. On the one hand, glutamine and glutamate were found to be more abundant in grains at elevated CO2 (Fig. 3), suggesting that phloem N export from leaves was not affected. On the other hand, leaves contained significantly less K, which is the cornerstone of phloem ion movement. Therefore, our data do not provide a firm answer to this question. Nevertheless, we recognize that the higher glutamine (glutamate) content in grains could also have originated from a lower efficiency of glutamine utilization and thus a lower incorporation of N to anabolism. Grains were specifically depleted in GABA, methionine, tyrosine, alanine, and threonine. Interestingly, these amino acids belong to distinct pathways: aspartate metabolism (methionine and threonine), glutamate degradation/GABA shunt (GABA and alanine), and aromatics (tyrosine). The common branching point is pyruvate, since aspartate can come from anaplerotic phosphoenolpyruvate (PEP) carboxylase fixation (which forms oxaloacetate), alanine comes from pyruvate, and the biosynthesis of aromatics requires PEP. Therefore, it is possible that the decrease in amino acids was caused by a specific effect of elevated CO2 on (phosphoenol)pyruvate synthesis via glycolysis. For example, pyruvate Pi dikinase, which resynthesizes PEP from pyruvate, has been shown to be essential for starch accumulation at the late grain-filling stage (Prioul et al., 2012).
Importantly, these metabolic effects depended on ‘conditions’, that is, country and species/cultivar (Supplementary Figs S2, S3). It was not possible here to separate the specific contributions of country and species/cultivar, since not all plant lines were cultivated in each country. There was nevertheless a strong effect of location, with the effect of elevated CO2 on some micro- and macro-elements being country specific. For example, the effect of elevated CO2 on grain Pb content was largest in Australia, where Pb was the least abundant at the field location (Supplementary Fig. S1). Similarly, there was a very limited effect of CO2 on grain N content (with generally low values) in Australia (Supplementary Fig. S3), where N fertilization was the lowest (Table 1), while the effect on leaf N content was strong (Supplementary Fig. S2). There was also a strong effect of CO2 on grain free amino acid content in both Australia and the USA, despite the fact that the sites in the two countries used different fertilization levels and wheat species. Therefore, our results show the importance of growth conditions (i.e. location) to delineate the effects of elevated CO2; in particular, soil composition (and not only N fertilization) seems to be important. Yet, the decrease in threonine and Mg content in grains was independent of ‘conditions’, that is, it was not associated with a CO2 × conditions interaction effect.
Potential metabolic markers of yield
We used the dataset to examine possible relationships between yield and grain metabolic properties, using multivariate and univariate statistics (Fig. 4). Because the country of origin (and associated conditions such as climate, fertilization, and sowing density) and species/cultivar (i.e. ‘conditions’) represented a confounding factor, we carried out two types of analyses: (i) independent of and (ii) accounting for ‘conditions’. In doing so, we assumed that the variable ‘conditions’ can be a driver of yield regardless of other variables (e.g. contents of metabolites or micro-/macro-elements). Growth at elevated CO2 was not a confounding factor, since there was no significant effect of CO2 on yield overall (Fig. 4A, C). Only two significant and strong drivers of yield remained after the elimination of the ‘conditions’ effect: glutamine and valine (Fig. 4E, F).
This result highlights the well-known role of glutamine in grain protein synthesis: glutamate is a major amino acid in wheat stem phloem (Hayashi and Chino, 1986) used as a primary source of N for conversion to glutamine and, furthermore, glutamine is the most represented residue in accumulated proteins. It has been shown that glutamine accounts for ~40% of free amino acids not only in phloem sap from the spikelet peduncle but also in endosperm cavity sap, showing its crucial role as a N source during grain development (Fisher and Macnicol, 1986). In addition, glutamine has the highest supply rate from the vascular bundle to the developing endosperm, at ~0.5 µmol grain−1 d−1 (Ugalde and Jenner, 1990).
Valine was negatively related to yield (Fig. 4E), suggesting that increased valine degradation rather than valine synthesis is beneficial to grain production. Proteomics analyses have shown that acetolactate synthase, which catalyses the first step of valine and leucine biosynthesis, is most abundant at the beginning of grain development (pre-filling stage) and then declines up to maturity (Tasleem-Tahir et al., 2012), while methylmalonate semialdehyde dehydrogenase, which is involved in branched-chain amino acid degradation, appears at the last stage of grain filling (Vensel et al., 2005) and its content increases with ABA (Zhang et al., 2012). Accordingly, metabolic profiling has shown that branched-chain amino acids (valine, leucine and isoleucine) tend to increase during grain filling and then sharply decline (Shewry et al., 2012). Valine metabolism can have several roles. First, valine degradation generates acetyl-CoA (via methylmalonate semialdehyde), which can in turn be used for respiration or lipid synthesis. Second, valine can be converted to leucine via 2-oxoisovalerate. Labelling with 14C-valine in developing wheat spikes has shown up to 30% conversion to leucine (Kolderup, 1978), and during grain development, the valine content correlates with that of leucine (Martín Del Molino et al., 1988). Interestingly, a quantitative trait locus analysis has shown that 2-methylmaleate, an intermediate of leucine synthesis, correlates positively with the number of tillers and thermal time to heading, and negatively with yield (Hill et al., 2015).
Conclusions
Taken as a whole, our results show that growth at elevated CO2 in FACE experiments had different effects on leaf and grain metabolism, but no significant effect on yield. Elevated CO2 appeared to be detrimental to photosynthesis and leaf proteins (e.g. Rubisco large subunit) and to alter grain composition, in particular %N, amino acids (e.g. threonine and alanine), and minerals (Mg). The yield was related to metabolic features of grains but this relationship was not influenced by elevated CO2.
The lack of a positive effect of CO2 fertilization can be explained not only by the down-regulation of carbon fixation (i.e. of several photosynthetic parameters) but also by an effect on grain metabolism itself. In particular, elevated CO2 appeared to be detrimental to N metabolism, with a decrease in several amino acids and, accordingly, a positive effect of elevated CO2 on yield was observed only in the USA, where N fertilization was the highest.
Elevated CO2 also had an effect on Mg redistribution between source leaves and grains. The detrimental effect of CO2 on the content of microelements has been found elsewhere for Zn (Myers et al., 2014). A meta-analysis has recently highlighted the decrease in the content of many elements (including macroelements such as S) in crops cultivated at elevated CO2 (Loladze, 2014). Interestingly, recent experiments in which Mg availability was varied have shown that low Mg causes a significant decline in grain starch content and yield (Ceylan et al., 2016). Here, despite significantly lower Mg content in grains at elevated CO2, starch content and yield were not significantly affected, suggesting that the decrease in Mg associated with elevated CO2 was too small (<10%) to affect yield. In addition, the relationship between Mg content in grains and yield is not driven by genotype (Oury et al., 2006). We nevertheless recognize that further work is required to determine the reason for the decreased Mg content in grains found here. For example, isotopic labelling with 25Mg (or 26Mg) would be helpful to examine the role played by phloem Mg transport during remobilization from leaves at elevated CO2.
The overall effect on N, K, and Mg nutrition found here raises the question of whether specific fertilization management strategies could compensate for the lack of effect of elevated CO2 observed at most of the FACE sites. Previous studies compared ammonium-based and nitrate-based fertilizers and found that with nitrate, plants tended to show higher photosynthetic acclimation (Bowler and Press, 1996; Geiger et al., 1999; Cruz et al., 2014), which in turn limits the response of production or yield to elevated CO2. In addition, high nitrate availability tends to exaggerate photosynthetic acclimation, due to down-regulation of the expression of photosynthetic genes (Vicente et al., 2017). Therefore, in an effort to find optimal fertilization strategies adapted to local field conditions, both the quantity and the quality of N fertilizer seems to be important. The fact that nutrients other than N are also affected by elevated CO2 further indicates that the nutrient balance itself is also of importance. A specific multifactorial experiment (nutrient compositions × CO2) would be necessary to determine the best solutions to improve yield responsiveness to CO2. In addition, the selection of varieties with a high harvest index, which is associated with optimal allocation and efficient nutrient remobilization capacity, might be desirable to obtain better grain yield and quality at elevated CO2.
Finally, we found that wheat grain yield was related to glutamine and valine grain content, regardless of country and cultivar, showing the importance of amino acid metabolism for grain maturation. However, while the role of glutamine is clear (as a N source and utilization for storage proteins, which are glutamine rich), understanding the metabolic role(s) of valine requires further work, such as tracing with 13C-valine and isotope-assisted metabolomics. This will be addressed in a future study.
Supplementary data
The following supplementary data are available at JXB online.
Fig. S1. Elemental composition of soil (top layer) at the different sites used in this study.
Fig. S2. Leaf features significant for a CO2 × conditions (country, cultivar, and time) effect.
Fig. S3. Grain features significant for a CO2 × conditions (country, cultivar, and time) effect.
Fig. S4. Correlation plot showing the best variables obtained by subset sampling in linear models of yield.
Fig. S5. Photosynthetic response curve for wheat cultivated in Germany.
Acknowledgements
This work was supported by the Department of Industry, Energy and Innovation of the Government of Navarre (PI040 TRIGOCLIM). The technical support given by Inés Urretavizcaya, Petra Högy, and Jürgen Franzaring in harvesting and sample management is acknowledged. JC was supported by an Australia Awards PhD Scholarship. GT was supported by a Connect Talent Award from the Region Pays de la Loire – Angers Loire Metropole (France). Research at the Australian Grains Free Air CO2 Enrichment (AGFACE) facility was jointly run by the University of Melbourne and Agriculture Victoria with funding from the Grains Research and Development Corporation (under contract no. DAV00137) and the Australian Commonwealth Department of Agriculture and Water Resources (under contract no. FtRG 1193982-41). CAAS-FACE was supported by the National Key Research and Development Project (under contracts 2016YFD0300401 and 2019YFA0607403). The FACE experiment in Italy was supported by the AGER project ‘Durum wheat adaptation to global change: effect of elevated CO2 on yield and quality traits’ and by the collaboration CREA-CNR. Finally, the authors also acknowledge support of the publication fee by the CSIC Open Access Publication Support Initiative through its Unit of Information Resources for Research (URICI).
References
- Adam NR, Wall GW, Kimball BA, et al. 2000. Acclimation response of spring wheat in a free-air CO2 enrichment (FACE) atmosphere with variable soil nitrogen regimes. 1. Leaf position and phenology determine acclimation response. Photosynthesis Research 66, 65–77. [DOI] [PubMed] [Google Scholar]
- Ainsworth EA, Long SP. 2005. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytologist 165, 351–371. [DOI] [PubMed] [Google Scholar]
- Ainsworth EA, Rogers A. 2007. The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant, Cell & Environment 30, 258–270. [DOI] [PubMed] [Google Scholar]
- Akin DE, Kimball BA, Windham WR, Pinter PJ, Wall GW, Garcia RL, LaMorte RL, Morrison WH. 1995. Effect of free-air CO2 enrichment (FACE) on forage quality of wheat. Animal Feed Science and Technology 53, 29–43. [Google Scholar]
- Aranjuelo I, Erice G, Sanz-Sáez A, et al. 2015. Differential CO2 effect on primary carbon metabolism of flag leaves in durum wheat (Triticum durum Desf.). Plant, Cell & Environment 38, 2780–2794. [DOI] [PubMed] [Google Scholar]
- Beleggia R, Fragasso M, Miglietta F, Cattivelli L, Menga V, Nigro F, Pecchioni N, Fares C. 2018. Mineral composition of durum wheat grain and pasta under increasing atmospheric CO2 concentrations. Food Chemistry 242, 53–61. [DOI] [PubMed] [Google Scholar]
- Bhatti AU, Mulla DJ, Frazier BE. 1991. Estimation of soil properties and wheat yields on complex eroded hills using geostatistics and thematic mapper images. Remote Sensing of Environment 37, 181–191. [Google Scholar]
- Bloom AJ, Burger M, Kimball BA, Pinter Jr PJ. 2014. Nitrate assimilation is inhibited by elevated CO2 in field-grown wheat. Nature Climate Change 4, 477–480. [Google Scholar]
- Bourgault M, Dreccer MF, James AT, Chapman SC. 2013. Genotypic variability in the response to elevated CO2 of wheat lines differing in adaptive traits. Functional Plant Biology 40, 172–184. [DOI] [PubMed] [Google Scholar]
- Bowes G. 1993. Facing the inevitable: plants and increasing atmospheric CO2. Annual Review of Plant Biology 44, 309–332. [Google Scholar]
- Bowler JM, Press MC. 1996. Effects of elevated CO2, nitrogen form and concentration on growth and photosynthesis of a fast- and slow-growing grass. New Phytologist 132, 391–401. [DOI] [PubMed] [Google Scholar]
- Cabrerizo PM González EM Aparicio-Tejo PM Arrese-Igor C. 2001. Continuous CO2 enrichment leads to increased nodule biomass, carbon availability to nodules and activity of carbon-metabolising enzymes but does not enhance specific nitrogen fixation in pea. Physiologia Plantarum 113, 33–40. [Google Scholar]
- Ceylan Y, Kutman UB, Mengutay M, Cakmak I. 2016. Magnesium applications to growth medium and foliage affect the starch distribution, increase the grain size and improve the seed germination in wheat. Plant and Soil 406, 145–156. [Google Scholar]
- Cheng L, Zhu J, Chen G, Zheng X, Oh NH, Rufty TW, Richter DD, Hu S. 2010. Atmospheric CO2 enrichment facilitates cation release from soil. Ecology Letters 13, 284–291. [DOI] [PubMed] [Google Scholar]
- Cruz JL, Alves AA, LeCain DR, Ellis DD, Morgan JA. 2014. Effect of elevated CO2 concentration and nitrate: ammonium ratios on gas exchange and growth of cassava (Manihot esculenta Crantz). Plant and Soil 374, 33–43. [Google Scholar]
- Erbs M, Franzaring J, Högy P, Fangmeier A. 2009. Free-air CO2 enrichment in a wheat-weed assembly – effects on water relations. Basic and Applied Ecology 10, 358–367. [Google Scholar]
- Ethier GJ, Livingston NJ. 2004. On the need to incorporate sensitivity to CO2 transfer conductance into the Farquhar–von Caemmerer–Berry leaf photosynthesis model. Plant, Cell and Environment 27, 137–153. [Google Scholar]
- Fangmeier A, Torres-Toledo V, Franzaring J, Damsohn W. 2016. Design and performance of a new FACE (free air carbon dioxide enrichment) system for crop and short vegetation exposure. Environmental and Experimental Botany 130, 151–161. [Google Scholar]
- Fares C, Menga V, Badeck F, Rizza F, Miglietta F, Zaldei A, Codianni P, Iannucci A, Cattivelli L. 2016. Increasing atmospheric CO2 modifies durum wheat grain quality and pasta cooking quality. Journal of Cereal Science 69, 245–251. [Google Scholar]
- Farquhar GD, O’Leary MH, Berry JA. 1982. On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Functional Plant Biology 9, 121–137. [Google Scholar]
- Fernando N, Panozzo J, Tausz M, Norton RM, Fitzgerald GJ, Myers S, Nicolas ME, Seneweera S. 2014. Intra-specific variation of wheat grain quality in response to elevated [CO2] at two sowing times under rain-fed and irrigation treatments. Journal of Cereal Science 59, 137–144. [Google Scholar]
- Fisher DB, Macnicol PK. 1986. Amino acid composition along the transport pathway during grain filling in wheat. Plant Physiology 82, 1019–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galmés J, Aranjuelo I, Medrano H, Flexas J. 2013. Variation in Rubisco content and activity under variable climatic factors. Photosynthesis Research 117, 73–90. [DOI] [PubMed] [Google Scholar]
- Geiger M, Haake V, Ludewig F, Sonnewald U, Stitt M. 1999. The nitrate and ammonium nitrate supply have a major influence on the response of photosynthesis, carbon metabolism, nitrogen metabolism and growth to elevated carbon dioxide in tobacco. Plant Cell and Environment 22, 1177–1199. [Google Scholar]
- Grant RF, Kimball BA, Brooks TJ, Wall GW, Pinter Jr PJ, Hunsaker DJ, Adamsen FJ, La Morte RL, Leavitt SW, Thompson TL. 2001. CO2 effects on mass and energy exchange of wheat under different N fertilization: model theory and testing with a free air CO2 enrichment (FACE) experiment. Plant Cell and Environment 93, 630–649. [Google Scholar]
- Harley PC, Loreto F, Marco GD, Sharkey TD. 1992. Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2. Plant Physiology 98, 1429–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H, Chino M. 1986. Collection of pure phloem sap from wheat and its chemical composition. Plant and Cell Physiology 27, 1387–1393. [Google Scholar]
- He Z, Joshi AK, Zhang W. 2013. Climate vulnerabilities and wheat production. In: Pielke RA, ed. Climate vulnerability. Oxford: Academic Press, 57–67. [Google Scholar]
- Hill CB, Taylor JD, Edwards J, Mather D, Langridge P, Bacic A, Roessner U. 2015. Detection of QTL for metabolic and agronomic traits in wheat with adjustments for variation at genetic loci that affect plant phenology. Plant Science 233, 143–154. [DOI] [PubMed] [Google Scholar]
- Högy P, Brunnbauer M, Koehler P, Schwadorf K, Breuer J, Franzaring J, Zhunusbayeva D, Fangmeier A. 2013. Grain quality characteristics of spring wheat (Triticum aestivum) as affected by free-air CO2 enrichment. Environmental and Experimental Botany 88, 11–18. [Google Scholar]
- Högy P, Fangmeier A. 2008. Effects of elevated atmospheric CO2 on grain quality of wheat. Journal of Cereal Science 48, 580–591. [Google Scholar]
- Houshmandfar A, Fitzgerald GJ, Macabuhay AA, Armstrong R, Tausz-Posch S, Löw M, Tausz M. 2016. Trade-offs between water-use related traits, yield components and mineral nutrition of wheat under Free-Air CO2 Enrichment (FACE). European Journal of Agronomy 76, 66–74. [Google Scholar]
- Hunsaker J, Kimball A, Pinter J Jr, LaMorte M, Wall W. 1996. Carbon dioxide enrichment and irrigation effects on wheat evapotranspiration and water use efficiency. Transactions of the ASAE 39, 1345–1355. [Google Scholar]
- Jauregui I, Aroca R, Garnica M, Zamarreño ÁM, García-Mina JM, Serret MD, Parry M, Irigoyen JJ, Aranjuelo I. 2015. Nitrogen assimilation and transpiration: key processes conditioning responsiveness of wheat to elevated [CO2] and temperature. Physiologia Plantarum 155, 338–354. [DOI] [PubMed] [Google Scholar]
- Kimball BA. 2010. Lessons from FACE: CO2 effects and interactions with water, nitrogen and temperature. In: Hillel D, Rosenzweig C, eds. Handbook of climate change and agroecosystems: impacts, adaptation, and mitigation. London: Imperial College Press, 87–107. [Google Scholar]
- Kimball BA. 2016. Crop responses to elevated CO2 and interactions with H2O, N, and temperature. Current Opinion in Plant Biology 31, 36–43. [DOI] [PubMed] [Google Scholar]
- Kimball BA, Morris CF, Pinter PJ Jr, et al. 2001a. Elevated CO2, drought and soil nitrogen effects on wheat grain quality. New Phytologist 150, 295–303. [Google Scholar]
- Kimball BA, Morris CF, Pinter PJ Jr, Wall GW, Hunsaker DJ, Adamsen FJ, Lamorte RL, Leavitt SW, Thompson TL, Matthias AD. 2001b. Wheat grain quality as affected by elevated CO2, drought, and soil nitrogen. Plant Pathology 150, 295–303. [Google Scholar]
- Kimball BA, Pinter PJ Jr, Garcia RL, LaMorte RL, Wall GW, Hunsaker DJ, Wechsung G, Wechsung F, Kartschall T. 1995. Productivity and water use of wheat under free-air CO2 enrichment. Global Change Biology 1, 429–442. [Google Scholar]
- Kolderup F. 1978. Interconversions of amino acids in maturing wheat grains. In: Seed protein improvement in cereals and grain legumes, Vol. 1 Vienna: International Atomic Energy Agency, 187–204. [Google Scholar]
- Li A-G, Hou Y-S, Wall GW, Trent A, Kimball BA, Pinter PJ. 2000. Free-air CO2 enrichment and drought stress effects on grain filling rate and duration in spring wheat. Crop Science 40, 1263–1270. [Google Scholar]
- Loladze I. 2014. Hidden shift of the ionome of plants exposed to elevated CO2 depletes minerals at the base of human nutrition. eLife 3, e02245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Ferrer D, Martínez-Bartolomé S, Villar M, Campillos M, Martín-Maroto F, Vázquez J. 2004. Statistical model for large-scale peptide identification in databases from tandem mass spectra using SEQUEST. Analytical Chemistry 76, 6853–6860. [DOI] [PubMed] [Google Scholar]
- MacLean B Tomazela DM Shulman N Chambers M Finney GL Frewen B Kern R Tabb DL Liebler DC MacCoss MJ. 2010. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 26, 966–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manderscheid R, Dier M, Erbs M, Sickora J, Weigel H-J. 2018. Nitrogen supply – a determinant in water use efficiency of winter wheat grown under free air CO2 enrichment. Agricultural Water Management 210, 70–77. [Google Scholar]
- Marti J, Slafer GA. 2014. Bread and durum wheat yields under a wide range of environmental conditions. Field Crops Research 156, 258–271. [Google Scholar]
- Martín Del Molino IM, Rojo B, Martinez-Carrasco R, Pérez P. 1988. Amino acid composition of wheat grain. 1: Changes during development. Journal of the Science of Food and Agriculture 42, 29–37. [Google Scholar]
- Meehl G, Stocker T, Collins W, et al. 2007. Global climate projections. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt K, Tignor M, Miller H, eds. Climate change 2007: the physical science basis. Contribution of working group I to the fourth assessment report of the Intergovernmental Panel on Climate Change (IPCC). New York: Cambridge University Press, 747–845. [Google Scholar]
- Miglietta F, Giuntoli A, Bindi M. 1996. The effect of free air carbon dioxide enrichment (FACE) and soil nitrogen availability on the photosynthetic capacity of wheat. Photosynthesis Research 47, 281–290. [DOI] [PubMed] [Google Scholar]
- Miller MP, Singer MJ, Nielsen DR. 1988. Spatial variability of wheat yield and soil properties on complex hills. Soil Science Society of America Journal 52, 1133–1141. [Google Scholar]
- Mollah M, Norton R, Huzzey J. 2009. Australian grains free-air carbon dioxide enrichment (AGFACE) facility: design and performance. Crop and Pasture Science 60, 697–707. [Google Scholar]
- Moulin AP, Anderson DW, Mellinger M. 1994. Spatial variability of wheat yield, soil properties and erosion in hummocky terrain. Canadian Journal of Soil Science 74, 219–228. [Google Scholar]
- Myers SS, Zanobetti A, Kloog I, et al. 2014. Increasing CO2 threatens human nutrition. Nature 510, 139–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie G, Hendrix DL, Webber AN, Kimball BA, Long SP. 1995. Increased accumulation of carbohydrates and decreased photosynthetic gene transcript levels in wheat grown at an elevated CO2 concentration in the field. Plant Physiology 108, 975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary GJ, Christy B, Nuttall J, et al. 2015. Response of wheat growth, grain yield and water use to elevated CO2 under a Free-Air CO2 Enrichment (FACE) experiment and modelling in a semi-arid environment. Global Change Biology 21, 2670–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira HR, Campana MG, Jones H, Hunt HV, Leigh F, Redhouse DI, Lister DL, Jones MK. 2012. Tetraploid wheat landraces in the Mediterranean basin: taxonomy, evolution and genetic diversity. PLoS One 7, e37063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oury F-X, Leenhardt F, Remesy C, Chanliaud E, Duperrier B, Balfourier F, Charmet G. 2006. Genetic variability and stability of grain magnesium, zinc and iron concentrations in bread wheat. European Journal of Agronomy 25, 177–185. [Google Scholar]
- Pacholski A, Manderscheid R, Weigel H-J. 2015. Effects of free air CO2 enrichment on root growth of barley, sugar beet and wheat grown in a rotation under different nitrogen supply. European Journal of Agronomy 63, 36–46. [Google Scholar]
- Pandey V, Sharma M, Deeba F, Maurya VK, Gupta SK, Singh SP, Mishra A, Nautiyal CS. 2017. Impact of elevated CO2 on wheat growth and yield under free air CO2 enrichment. American Journal of Climate Change 6, 573–596. [Google Scholar]
- Pendall E, Leavitt SW, Brooks T, et al. 2001. Elevated CO2 stimulates soil respiration in a FACE wheat field. Basic and Applied Ecology 2, 193–201. [Google Scholar]
- Peñuelas J, Estiarte M, Kimball BA. 2000. Flavonoid responses in wheat grown at elevated CO2: green versus senescent leaves. Photosynthetica 37, 615–619. [Google Scholar]
- Prioul J, Manicacci D, Damerval C, Méchin V. 2012. Proteomics in identifying new regulatory mechanisms involved in seed development and ultimately seed quality. In: Agrawal G, Rakwal R eds. Seed development: OMICS technologies toward improvement of seed quality and crop yield. Dordrecht: Springer, 247–264. [Google Scholar]
- Ray DK, Ramankutty N, Mueller ND, West PC, Foley JA. 2012. Recent patterns of crop yield growth and stagnation. Nature Communications 3, 1293. [DOI] [PubMed] [Google Scholar]
- Reynolds M, Foulkes J, Furbank R, Griffiths S, King J, Murchie E, Parry M, Slafer G. 2012. Achieving yield gains in wheat. Plant, Cell & Environment 35, 1799–1823. [DOI] [PubMed] [Google Scholar]
- Rogers A, Humphries SW. 2000. A mechanistic evaluation of photosynthetic acclimation at elevated CO2. Global Change Biology 6, 1005–1011. [Google Scholar]
- Sanchez de la Puente L, Pérez P, Martinez-Carrasco R, Morcuende R, Martín Del Molino IM. 2000. Action of elevated CO2 and high temperatures on the mineral composition of two varieties of wheat. Agrochimica 44, 221–230. [Google Scholar]
- Shewry PR, Mitchell RAC, Tosi P, Wan Y, Underwood C, Lovegrove A, Freeman J, Toole GA, Mills ENC, Ward JL. 2012. An integrated study of grain development of wheat (cv. Hereward). Journal of Cereal Science 56, 21–30. [Google Scholar]
- Sicher RC, Bunce JA. 1998. Evidence that premature senescence affects photosynthetic decline of wheat flag leaves during growth in elevated carbon dioxide. International Journal of Plant Sciences 159, 798–804. [Google Scholar]
- Slafer G, Calderini D, Miralles D. 1996. Yield components and compensation in wheat: opportunities for further increasing yield potential. In: Reynolds MP, Rajaram S, McNab A, eds. Increasing yield potential in wheat: breaking the barriers. Mexico DF: CIMMYT, 101–133. [Google Scholar]
- Soba D, Ben Mariem S, Fuertes-Mendizábal T, Méndez-Espinoza AM, Gilard F, González-Murua C, Irigoyen JJ, Tcherkez G, Aranjuelo I. 2019. Metabolic effects of elevated CO2 on wheat grain development and composition. Journal of Agricultural and Food Chemistry 67, 8441–8451. [DOI] [PubMed] [Google Scholar]
- Tasleem-Tahir A, Nadaud I, Chambon C, Branlard G. 2012. Expression profiling of starchy endosperm metabolic proteins at 21 stages of wheat grain development. Journal of Proteome Research 11, 2754–2773. [DOI] [PubMed] [Google Scholar]
- Tausz-Posch S, Borowiak K, Dempsey RW, Norton RM, Seneweera S, Fitzgerald GJ, Tausz M. 2013a. The effect of elevated CO2 on photochemistry and antioxidative defence capacity in wheat depends on environmental growing conditions – a FACE study. Environmental and Experimental Botany 88, 81–92. [Google Scholar]
- Tausz-Posch S, Norton RM, Seneweera S, Fitzgerald GJ, Tausz M. 2013b. Will intra-specific differences in transpiration efficiency in wheat be maintained in a high CO2 world? A FACE study. Physiologia Plantarum 148, 232–245. [DOI] [PubMed] [Google Scholar]
- Thilakarathne CL, Tausz-Posch S, Cane K, Norton RM, Fitzgerald GJ, Tausz M, Seneweera S. 2015. Intraspecific variation in leaf growth of wheat (Triticum aestivum) under Australian Grain Free Air CO2 Enrichment (AGFACE): is it regulated through carbon and/or nitrogen supply? Functional Plant Biology 42, 299–308. [DOI] [PubMed] [Google Scholar]
- Thilakarathne CL, Tausz-Posch S, Cane K, Norton RM, Tausz M, Seneweera S. 2013. Intraspecific variation in growth and yield response to elevated CO2 in wheat depends on the differences of leaf mass per unit area. Functional Plant Biology 40, 185–194. [DOI] [PubMed] [Google Scholar]
- Tiryakioglu M, Yildirim M, Karanlik S. 2014. Macronutrient concentration and remobilization in spring wheat organs during grain filling. Turkish Journal of Agriculture and Forestry 38, 488–494. [Google Scholar]
- Torres N, Goicoechea N, Zamarreño AM, Antolín MC. 2018. Mycorrhizal symbiosis afects ABA metabolism during berry ripening in Vitis vinifera L. cv. Tempranillo grown under climate change scenarios. Plant Science 274, 383–393. [DOI] [PubMed] [Google Scholar]
- Tubiello FN, Rosenzweig C, Kimball BA, Pinter PJ Jr, Wall GW, Hunsaker DJ, LaMorte RL, Garcia RL. 1999. Testing CERES–Wheat with Free-Air Carbon Dioxide Enrichment (FACE) experiment data: CO2 and water interactions. Agronomy Journal 91, 247–255. [Google Scholar]
- Ugalde T, Jenner C. 1990. Substrate gradients and regional patterns of dry matter deposition within developing wheat endosperm. II. Amino acids and protein. Functional Plant Biology 17, 395–406. [Google Scholar]
- Uprety DC, Dwivedi N, Raj A, Jaiswal S, Paswan G, Jain V, Maini HK. 2009. Study on the response of diploid, tetraploid and hexaploid species of wheat to the elevated CO2. Physiology and Molecular Biology of Plants 15, 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vensel WH, Tanaka CK, Cai N, Wong JH, Buchanan BB, Hurkman WJ. 2005. Developmental changes in the metabolic protein profiles of wheat endosperm. Proteomics 5, 1594–1611. [DOI] [PubMed] [Google Scholar]
- Veres S, Malik AI, Rengel Z. 2017. Differential nitrogen supply causes large variability in photosynthetic traits in wheat germplasm. Crop and Pasture Science 68, 703–712. [Google Scholar]
- Verrillo F, Badeck F-W, Terzi V, et al. 2017. Elevated field atmospheric CO2 concentrations affect the characteristics of winter wheat (cv. Bologna) grains. Crop and Pasture Science 68, 713–725. [Google Scholar]
- Vicente R, Pérez P, Martínez-Carrasco R, Morcuende R. 2017. Improved responses to elevated CO2 in durum wheat at a low nitrate supply associated with the upregulation of photosynthetic genes and the activation of nitrate assimilation. Plant Science 260, 119–128. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Farquhar GD. 1981. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153, 376–387. [DOI] [PubMed] [Google Scholar]
- Walker C, Armstrong R, Panozzo J, Partington D, Fitzgerald G. 2017. Can nitrogen fertiliser maintain wheat (Triticum aestivum) grain protein concentration in an elevated CO2 environment? Soil Research 55, 518–523. [Google Scholar]
- Wechsung F, Garcia RL, Wall GW, et al. 2000. Photosynthesis and conductance of spring wheat ears: field response to free-air CO2 enrichment and limitations in water and nitrogen supply. Plant Cell and Environment 23, 917–929. [Google Scholar]
- Wechsung G, Wechsung F, Wall GW, Adamsen FJ, Kimball BA, Garcia RL, Pinter PJ, Kartschall T. 1995. Biomass and growth rate of a spring wheat root system grown in free-air CO2 enrichment (FACE) and ample soil moisture. Journal of Biogeography 22, 623–634. [Google Scholar]
- Wieser H, Manderscheid R, Erbs M, Weigel HJ. 2008. Effects of elevated atmospheric CO2 concentrations on the quantitative protein composition of wheat grain. Journal of Agricultural and Food Chemistry 56, 6531–6535. [DOI] [PubMed] [Google Scholar]
- Wolf J, van Oijen M, Kempenaar C. 2002. Analysis of the experimental variability in wheat responses to elevated CO2 and temperature. Agriculture, Ecosystems and Environment 93, 227–247. [Google Scholar]
- Wroblewitz S, Hüther L, Manderscheid R, Weigel HJ, Wätzig H, Dänicke S. 2013. The effect of free air carbon dioxide enrichment and nitrogen fertilisation on the chemical composition and nutritional value of wheat and barley grain. Archives of Animal Nutrition 67, 263–278. [DOI] [PubMed] [Google Scholar]
- Yang L-X, Li S-F, Wang Y-L, Huang J-Y, Yang H-J, Dong G-C, Zhu J-G, Liu G. 2007a. Effects of free-air CO2 enrichment (FACE) on yield formation of wheat. Journal of Applied Ecology 18, 75–80. [PubMed] [Google Scholar]
- Yang L-X, Wang Y-L, Li S-F, Huang J-Y, Dong G-C, Zhu J-G, Liu G, Han Y. 2007b. Effects of free-air CO2 enrichment (FACE) on dry matter production and allocation in wheat. Journal of Applied Ecology 18, 339–346. [PubMed] [Google Scholar]
- Zhang D-Y, Chen G-Y, Chen J, Yong Z-H, Zhu J-G, Xu D-Q. 2009. Photosynthetic acclimation to CO2 enrichment related to ribulose-1,5-bisphosphate carboxylation limitation in wheat. Photosynthetica 47, 152–154. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Chen J, Lin S, Li Z, Cheng R, Fang C, Chen H, Lin W. 2012. Proteomic and phosphoproteomic determination of ABA’s effects on grain-filling of Oryza sativa L. inferior spikelets. Plant Science 185–186, 259–273. [DOI] [PubMed] [Google Scholar]
- Zhu C, Zhu J, Zeng Q, Liu G, Xie Z, Tang H, Cao J, Zhao X. 2009. Elevated CO2 accelerates flag leaf senescence in wheat due to ear photosynthesis which causes greater ear nitrogen sink capacity and ear carbon sink limitation. Functional Plant Biology 36, 291–299. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.