Abstract
The objective of this study was to determine the minimum requirement (MR) for methionine (Met), when cyst(e)ine (Cys) is provided in excess, in adult dogs of three different breed sizes using the indicator amino acid (AA) oxidation (IAAO) technique. In total, 12 adult dogs were used: 1 neutered and 3 spayed Miniature Dachshunds (4.8 ± 0.4 kg body weight [BW], mean ± SD), 4 spayed Beagles (9.5 ± 0.7 kg BW, mean ± SD), and 4 neutered Labrador Retrievers (31.8 ± 1.7 kg BW, mean ± SD). A deficient Met basal diet with excess Cys was formulated. Dogs were fed the basal diet randomly supplemented with different Met-Alanine (Ala) solutions to achieve final Met concentrations in experimental diets of 0.21%, 0.26%, 0.31%, 0.36%, 0.41%, 0.46%, and 0.66% (as-fed basis). After 2 d of adaptation to the experimental diets, dogs underwent individual IAAO studies. During the IAAO study day, the total feed was divided into 13 equal meals; at the sixth meal, dogs were fed a bolus of l-[1-13C]-phenylalanine (Phe), and thereafter, l-[1-13C]-Phe was supplied with every meal. The total production of 13CO2 during isotopic steady state was determined by the enrichment of 13CO2 in breath samples, and the total production of CO2 measured using indirect calorimetry. The mean MR for Met and the upper 95% confidence limit (CL) were determined using a two-phase linear mixed-effects regression model. For Miniature Dachshunds, the MR for Met was between the first two dietary Met concentrations and is, therefore, between 35.7 and 44.1 mg.kg BW−1·d−1 (0.21% to 0.26%, as-fed basis; no requirement could be determined on a metabolic BW basis). For Beagles and Labrador Retrievers, the MR for Met was 57.5 and 50.4 mg.kg BW−1·d−1, 107.7 and 121.8 mg/kg BW^0.75, or 0.338 and 0.360%, respectively (as-fed basis). The upper 95% CL of Met requirements was 77.9 and 72.4 mg.kg BW−1·d−1, 147.8 and 159.6 mg/kg BW^0.75,or 0.458 and 0.517% for Beagles, and Labradors, respectively (as-fed basis). When pooling data from Beagles and Labrador Retrievers, the MR and upper 95% CL were 56.0 and 75.8 mg.kg BW−1·d−1 or 118.4 and 150.5 mg/kg BW^0.75 or 0.360% and 0.482% (as-fed basis). In conclusion, the MR and the upper 95% CL for Met are different for Dachshunds when compared with Beagles and Labrador Retrievers. Using this low-protein diet, the estimated upper 95% CL Met requirement for Beagles and Labrador is higher than those recommended in the National Research Council (NRC), but NRC is similar to the estimated upper 95% CL for Dachshunds.
Keywords: adult dogs, indirect amino acid oxidation, methionine requirement
Introduction
Methionine (Met) is an indispensable amino acid (AA) for domestic canines (Canis lupus familiaris; Milner, 1979) and is often the first- or second-limiting AA for dogs fed diets formulated with soybean or rendered meat meals (National Research Council; NRC, 2006). However, current recommendations by the NRC (2006) are calculated only from studies done in immature (growing) dogs (Burns and Milner, 1981; Hirawaka and Baker, 1985) or from other long-term studies where dogs were fed low-protein diets and displayed no clinical signs of deficiency (Ward, 1976; Sanderson et al., 2001; NRC, 2006).
To estimate requirements in adult dogs, the indicator AA oxidation (IAAO) is more sensitive and accurate than traditional techniques, such as nitrogen balance and growth performance (Pencharz and Ball, 2003; Levesque et al., 2010). Those techniques are directly related to increments in whole-body protein mass and, therefore, are not suitable for adult dogs at maintenance. We have previously estimated phenylalanine (Phe), tryptophan (Trp), threonine (Thr), and lysine (Lys) requirements in adult dogs of different breeds using the IAAO technique and found that Phe requirements are lower (Mansilla et al., 2018) and Trp, Thr, and Lys requirements are higher (Templeman et al., 2019; Mansilla et al., 2020; Sutherland et al., 2020) than those recommended by NRC (2006).
The objective of the present study is to estimate dietary minimum Met requirements, where cyst(e)ine (Cys) is provided in excess, in three different breeds of adult dogs using the IAAO technique. We hypothesized that the mean Met requirement for adult dogs will be different among different breeds. Second, when breeds are pooled to provide an “all breed” recommendation, this estimate will be greater than the current NRC (2006) recommendation for adult dogs at maintenance.
Materials and Methods
Animals and housing
The present experiment was approved by the Procter and Gamble Pet Care’s Institutional Animal Care and Use Committee. A total of 12 adult dogs were used, 1 neutered and 3 spayed Miniature Dachshunds (initial body weight [BW] 4.77 ± 0.44 kg; 0.93 ± 0.03 yr old, mean ± SD), 4 spayed Beagles (initial BW 9.46 ± 0.76 kg; 5.73 ± 0.25 yr old, mean ± SD), and 4 neutered Labrador Retrievers (initial BW 31.8 ± 1.66 kg; 3.98 ± 1.5 yr old, mean ± SD), and all dogs were at a body condition score of 3 on a 5-point scale. A power of greater than 0.8 was found for the two-phase linear regression model used for each breed size using a data step of SAS (SAS Institute Inc., v. 9.4, Cary, NC) and data from Templeman et al. (2019). All dogs resided at Procter and Gamble Pet Care (Lewisburg, OH) and were considered healthy based on a general health evaluation by a licensed veterinarian prior to the study. During the study, dogs were pair-housed in kennels (2.4 × 2.4 m) in a temperature-controlled building (22 °C) and with a lighting schedule of 12:2 (L: D) h. Dogs received daily socialization, exercise, and regular veterinary care as previously reported (Shoveller et al., 2017). Throughout the entire study, dogs had access to fresh water via an automatic watering system.
Study design and diets
A basal diet was formulated to meet or exceed requirements for all indispensable AA according to NRC (2006). The inclusion level of crude protein (CP) was below the Association of American Feed Control Officials (AAFCO) (2014) recommendations; however, those regulations are predicated upon meeting AA requirements with intact protein-based ingredients rather than with crystalline AA supplementation. The ingredient formula and nutrient content of this diet are detailed in Table 1. The extruded kibble basal diet was fed twice daily (0700 and 1300 hours) for 14 d prior to the beginning of the experiment (adaptation period) in amounts known to maintain dog individual BW. After the 14-d adaptation period to the basal diet, a test diet (similar to basal diet but without added crystalline Met; final Met = 0.21% as fed) was fed to the dogs at 17 g/kg of BW for Miniature Dachshunds and Beagles and at 14 g/kg of BW for Labradors for 2 d prior to each IAAO study (Moehn et al., 2004), which allowed all the dogs to adapt to the level of protein intake (Thorpe et al., 1999). Furthermore, the amount of food provided equated to about a 5% restriction of the normal intake known to support BW but was also 1.5 to 1.8 times resting energy expenditure (REE). The test diet was supplemented at feeding with one of seven Met (Skidmore Sales & distributing, West Chester Township, OH) solutions (0, 1.67, 3.33, 5.00, 6.67, 8.33, and 15.00 g/L) at 5.1 mL/kg BW for Miniature Dachshunds and Beagles and at 4.2 mL/kg BW for Labradors. To maintain similar nitrogen content among all supplemental solutions, Ala was added (8.96, 4.98, 3.98, 2.99, 1.99, 1.0, and 0 g/L for solution one to seven, respectively). The final Met contents in the test diet plus the supplemental solution were 0.21, 0.26, 0.31, 0.36, 0.41, 0.46, and 0.66% on an as-fed basis (experimental diets). After the 2-d adaptation period to the test diet (Moehn et al., 2004), the IAAO study combined with indirect calorimetry was conducted. After each IAAO study, dogs returned to the basal diet in amounts to maintain dog individual BW for 4 d before feeding the test diet supplemented with a different solution and conducting the next IAAO study. This 7-d feeding regime was repeated seven times with treatments assigned to dogs in a random order (Microsoft Excel 2010: Random function). After completion of the study, all dogs had received each of the seven supplemental Met solutions in the test diet and no dog received the same order of diets. Blood samples (3 mL) were collected from the jugular vein in serum vacutainers (Becton & Dickinson, Franklin Lakes, NJ) immediately after each IAAO study to represent fed state serum AA concentrations as samples were collected within 30 min after the dogs’ final meal.
Table 1.
Ingredient composition and analyzed nutrient contents of the test diet on an as-is basis
| Ingredient | g/kg |
|---|---|
| Corn starch | 480.6 |
| Chicken fat | 130.6 |
| Chicken meal | 63.9 |
| Yellow corn | 50.6 |
| Brewer’s rice | 50.6 |
| AA premix1 | 75.9 |
| Beet pulp | 30.4 |
| Dicalcium phosphate | 29.0 |
| Chicken flavor | 20.2 |
| Potassium chloride | 13.3 |
| Sodium bicarbonate | 10.1 |
| Chicken liver flavor | 5.06 |
| Brewer’s yeast | 5.06 |
| Ground flax | 5.06 |
| Choline chloride | 4.47 |
| Vitamin premix2 | 4.25 |
| Sodium hexametaphosphate | 4.05 |
| Calcium carbonate | 5.80 |
| Mineral premix D3 | 3.44 |
| Fish oil | 2.91 |
| Sodium chloride | 1.82 |
| Monosodium phosphate | 2.33 |
| Ethoxyquin | 0.51 |
| Nutrient content | Analyzed content |
| ME, kcal/kg (calculated)4 | 3,700 |
| DM, % | 92.22 |
| CP, % | 11.05 |
| Predicted crude fiber, % | 1.20 |
| Predicted crude fat, % (min) | 15.5 |
| Arg, % | 1.162 |
| Pro, % | 0.553 |
| Cys, % | 1.124 |
| His, % | 0.567 |
| Ile, % | 0.749 |
| Leu, % | 1.087 |
| Lys, % | 0.870 |
| Met, % | 0.210 (0.487) 5 |
| Phe, % | 0.800 |
| Tau, % | 0.040 |
| Thr, % | 0.866 |
| Trp, % | 0.444 |
| Tyr, % | 0.600 |
| Val, % | 0.755 |
| Ser, % | 0.685 |
| Gly, % | 0.598 |
| Ala, % | 0.534 |
1Provides per kg of final diet: 4.03 g of Arg, Cystine, His, Ile, Leu, Lys, Met, Phe, Thr, Trp, Tyr, and Val each. Threonine was removed for the test diets.
2Vitamin premix contained per kg: 6,650 K IU vitamin A, 365,000 IU vitamin D3, 100,400 IU vitamin E, 4,100 mg thiamine, 2,500 mg niacin, 2,000 mg pyridoxine, 7,750 mg d-pantothenic acid, 115 mg folic acid, 45 mg vitamin B12, 2,500 mg inositol, 13,750 mg vitamin C, and 1,200 mg β-carotene.
3Mineral premix contained per kg: 150 mg cobalt carbonate, 4,500 mg copper sulphate, 900 mg potassium iodine, 72,000 mg iron sulfate, 8,000 mg manganese oxide, 5,800 mg manganese sulfate, and 60,000 mg sodium selenite.
4Calculated ME based on modified Atwater values.
5Value for Met presented in brackets represents the basal test diet only.
Indicator amino acid oxidation studies
The conduct of the IAAO study has been previously described in detail (Templeman et al., 2019), and no deviations other than dietary treatment (Met rather than Trp) and food provision occurred. The total amount of diet fed during the IAAO study was based on BW measured that same day, in the morning after 18 h of fasting (17 g/kg of BW for Miniature Dachshunds and Beagles and 14 g/kg BW for Labradors). This level of feeding was on average 95% of the historical feeding allowance and ensured that dogs consumed all of the diet and equivalent isotope delivery among dogs of the same breed and among all dietary treatments. An important design principle to employing oxidation methods is that all animals are fed equivalent food with the same caloric density among all the dietary treatments as this will change the overall gas exchange and macronutrient partitioning, potentially contributing to the dilution of the tracer and increasing variability (Hoerr et al., 1989). Furthermore, equivalent single diet delivery among dietary treatments within the breed is necessary to maintain the equivalent delivery of dietary Phe and Tyr among dietary treatments since Phe oxidation is sensitive to dietary supply (Mansilla et al., 2018). Details regarding the timeline for each IAAO study have been reported in detail in Mansilla et al. (2018).
Sample collection, analysis, and calculations
Analysis of nitrogen content in the basal diet, AA content in the test diet, and calorimetry data collection have been detailed previously (Templeman et al., 2019) and no deviations occurred. The fraction of 13CO2 released per kg of BW per h (F13CO2, mmol.kg−1·h−1) was calculated using the following equation:
in which FCO2 is the average production of CO2 during the isotope steady-state phase (mL/min); ECO2 is the average 13CO2 enrichment in expired breath at isotopic steady state (atom percent excess, %); and BW is the weight of the dog (kg). The constants 44.6 (mmol/mL) and 60 (min/h) convert the FCO2 to micromoles per hour; the factor 100 changes enrichment to a fraction; and the 1.0 is the retention factor of CO2 in the body due to bicarbonate fixation as reported previously (Shoveller et al., 2017). REE and fed energy expenditure (FEE) were calculated based on the volume of O2 and CO2 produced using the modified Weir equation (Weir, 1949). Energy expenditure (EE; kcal/d) was expressed in relation to metabolic BW (BW0.75) for all dog breed sizes.
Body composition determination
Lean body mass (LBM) was determined using X-Ray Bone Densitometer (Model Delphi A, Hologic Inc., Marlborough, MA) during the 7-wk study as described in detail previously (Templeman et al., 2019). Body mass composition (i.e., mineral, fat, and lean contents) was determined in the left and right front and hind legs, trunk, and head (data not shown). Whole-body composition was determined by the sum of all regions measured on individual dogs. Following the scan, atipamezole (0.2 mg/kg; Antisedan, Pfizer, New York, NY) was administered i.m. Dogs were placed in a heated cage until fully recovered and monitored for 1 wk for complications. For additional details regarding body composition determination, refer to Templeman et al. (2019).
Serum amino acid concentrations
Collected blood samples were centrifuged (2,500 × g, 10 min, 4 °C), and serum was separated and stored for later analysis at −80 °C. On the day of analysis, serum samples were protein-precipitated using 20% sulfosalicylic acid, filtered, and analyzed by pre-column 9-fluorenylmethyloxycarbonyl and ortho-phthaldialdehyde derivatization. AAs were measured using high-performance liquid chromatography as described in Agilent publication 5980-1193E.
Statistical analysis
The effect of Met content in the test diet on F13CO2 was analyzed by fitting a mixed-effects model using the MIXED procedure of SAS with diet as a fixed effect and dog as a random effect for each individual breed. The estimate of the mean Met requirement and the upper 95% confidence limit (CL) for individual dog breeds and pooled data from Beagles and Labradors were derived by breakpoint analysis of the F13CO2/kg BW using a two-phase linear regression model using the MIXED procedure of SAS (v. 9.4; SAS Institute Inc., Cary, NC) as previously reported (Shoveller et al., 2017).
Within different breed sizes, BW, calorimetry data, and plasma AA were analyzed using a mixed-effect model with the MIXED procedure of SAS (v. 9.4; SAS Institute Inc., Cary, NC) with diet as a fixed effect and dog as a random effect. Differences in AA concentration in blood were determined comparing each dietary Met content against the lowest Met diet (0.21% as fed basis) using the Dunnett’s test. The same data were also pooled across diets and compared using a linear mixed-effects model with breed as a fixed effect and dog as a random variable. When ANOVA was P < 0.05, differences between breed AA concentrations were determined with the Tukey test. No adjustments were made for multiple comparisons. Results were considered statistically significant at P ≤ 0.05 and a trend when P ≤ 0.10.
Results
All animals remained healthy and maintained their body condition and BW during the execution of the experiment. Throughout every IAAO study, all dogs consumed their entire daily diet offerings. Comparing among experimental diets and within each breed, BW, REE, FEE, and fast and fed respiratory quotient (RQ) were not significantly different (P > 0.05; Supplemental Table S1) and demonstrate the test diets only affected the oxidation of the indicator AA. During the IAAO studies, all dogs reached isotopic steady state at every intake of Met (data not shown).
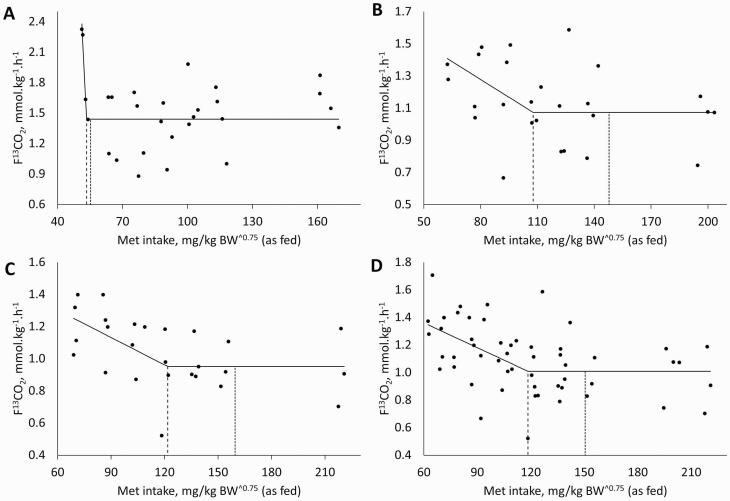
To account for differences in feed intake among breeds (17, 17, and 14 g/kg BW for Miniature Dachshunds, Beagles, and Labradors Retrievers, respectively), the two-phase linear regression was done with Met intake (mg/kg BW and mg/kg BW^0.75) as an independent variable rather than Met content (%). Based on F13CO2, the mean Met requirement for Miniature Dachshunds was at 44.2 mg/kg BW (0.260% Met as-fed basis) with an upper 95% CL of 51.6 mg/kg BW (0.304% as-fed basis; Figure 1A). The model calculated based on metabolic BW (BW^0.75) was not significant (P > 0.10; Figure 2A). For Beagles and Labradors Retrievers, the mean Met requirement was estimated at 57.5 and 50.4 mg/kg BW (107.7 and 121.8 mg/kg BW^0.75 as-fed basis) with an upper 95% CL of 77.9 and 72.4 mg/Kg BW (147.8 and 159.6 mg/kg BW^0.75 as fed basis), respectively (Table 2; Figures 1B and C and 2B and C). As the minimum requirement (MR) for Met was not significantly different for Beagles and Labrador Retrievers, data were pooled for these breeds. The mean MR for Met was estimated at 56.0 mg/kg BW with an upper 95% CL of 75.8 mg/kg BW of Met for Beagles and Labrador Retrievers together (118.4 and 150.5 mg/kg BW^0.75 as-fed basis for MR and upper 95% CL, respectively; Table 2; Figures 1D and 2D).
Figure 1.
Production of 13CO2 from the oxidation of orally administered l-[1-13C]-Phe in adult dogs of different breeds fed diets with increasing concentrations of Met with excess Cys. Miniature Dachshunds (A), Beagles (B), Labrador Retrievers (C), and Beagles and Labrador Retrievers (D). Dashed lines represent estimated mean Met requirement (44.2 mg/kg BW for A, 57.5 mg/kg BW for B, 50.4 mg/kg BW for C, and 56.0 mg/kg BW for D); dotted lines represent the upper 95% CL for Met requirement (51.6 mg/kg BW for A, 77.9 mg/kg BW for B, 72.4 mg/kg BW for C, and 75.8 mg/kg BW for D). Data points represent mean + SE of samples (n = 4).
Figure 2.
Production of 13CO2 from the oxidation of orally administered l-[1-13C]-Phe in adult dogs of different breeds fed diets with increasing concentrations of Met with excess Cys. Miniature Dachshunds (A), Beagles (B), Labrador Retrievers (C), and Beagles and Labrador Retrievers (D). Dashed lines represent estimated mean Met requirement (model was P > 0.10 for A, 107.7 mg/kg BW^0.75 for B, 121.8 mg/kg BW^0.75 for C, and 118.4 mg/kg BW^0.75 for D); dotted lines represent the upper 95% CL for Met requirement (147.8 mg/kg BW^0.75 for B, 159.6 mg/kg BW^0.75 for C, and 150.5 mg/kg BW^0.75 for D). Individual data points are represented in the graphs.
Table 2.
Recommended dietary Met inclusions for adult dogs at maintenance by AAFCO, European Pet Food Industry Federation (FEDIAF), NRC, and the present study
| NRC3 | Miniature Dachshunds | Beagles | Labrador Retrievers | Beagles and Labradors (pooled data) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AAFCO1 | FEDIAF2 | MR4 | RA | MR | CL | MR | CL | MR | CL | MR | CL | |
| Grams/100 g DM5 | 0.33 | 0.46 | 0.26 | 0.33 | [0.21 to 0.26] | 0.304 | 0.338 | 0.458 | 0.360 | 0.517 | 0.360 | 0.482 |
| Grams/Mcal ME | 0.83 | 1.16 | 0.65 | 0.83 | [0.57 to 0.70] | 0.822 | 0.914 | 1.238 | 0.973 | 1.397 | 0.973 | 1.303 |
| Milligrams/kg BW | [35.7 to 45.0] | 51.6 | 57.5 | 77.9 | 50.4 | 72.4 | 56.0 | 75.8 | ||||
| Milligrams/kg BW^0.75 | 85 | 110 | — | — | 107.7 | 147.8 | 121.8 | 159.6 | 118.4 | 150.5 | ||
1 AAFCO (2014) manual.
2 European Pet Food Industry Federation (2013) nutritional guidelines for complete and complementary pet food for cats and dogs.
3Nutrient requirements of dogs and cats (NRC, 2006).
4Upper 95% CL; MR, minimal requirement, RA, recommended allowance.
5Values for g/100 g DM are determined assuming a dietary energy density of 4,000 kcal ME/kg.
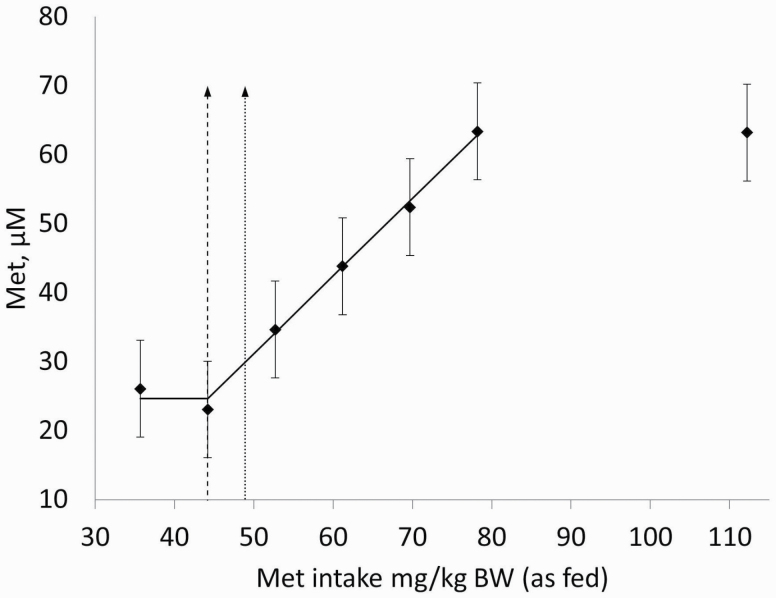
In Miniature Dachshunds, serum Met concentration was significantly greater at the three highest dietary concentrations of Met (0.41%, 0.46%, and 0.66%) when compared with 0.21% Met (Table 3). In Beagles, serum Met concentration was significantly greater at the highest level (0.66%) of Met supplementation when compared with 0.21% Met (Table 3). Serum Met concentrations were not significantly different among dietary Met intakes in Labrador Retrievers (Table 3). Cystine and taurine (Tau) were not modified at any level of Met supplementation in any of the breeds used. As there were no interactions between dietary Met content and breeds (Table 3), the overall AA concentration was compared across breeds. Cys concentration across the three breeds was not significantly different (61.4, 41.1, and 39.1 ± 10.6 µM; mean ± SEM for Miniature Dachshunds, Beagles, and Labradors, respectively; P > 0.10), while Met concentration was significantly lower for miniature Dachshunds (43.8, 238.8, 247.4 ± 14.8 µM; mean ± SEM for Miniature Dachshunds, Beagles, and Labradors, respectively; P ≤ 0.05) despite being fed more Met per kg BW than both Beagles and Labradors. Taurine was also significantly different across breeds, with significantly greater concentration for Miniature Dachshunds compared with Beagles and Labradors (225.6, 172.8, and 123.6 ± 11.8 µM; mean ± SEM for Miniature Dachshunds, Beagles, and Labradors, respectively; P ≤ 0.05) and could be a function of the total sulfur AA (SAA) intake. When the Met concentration data were analyzed using the two-phase linear regression model, data from the highest Met level were not included during the data analysis for Miniature Dachshunds as it did not follow the linear increase seen in previous levels (Figure 3). Using Met concentration in serum, the MR for Met in Miniature Dachshunds was estimated at 44.2 mg/kg BW (0.260%) with an upper 95% CL of 48.9 mg/kg (0.288%; Figure 3). Estimation of the breakpoint was not significant for Beagles or Labrador Retrievers using serum Met concentrations (P > 0.10).
Table 3.
Least square means of fed state serum Met, Cys, and Tau concentrations in adult Miniature Dachshunds, Beagles, and Labrador Retrievers fed diets containing increasing concentrations of Met with excess Cys
| Dietary Met, % (n = 4) | P-value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA, μM | Breed | 0.21 | 0.26 | 0.31 | 0.36 | 0.41 | 0.46 | 0.66 | SEM1 | Breed | Met | Interaction |
| Cys | Dachshunds | 84.6 | 67.6 | 93.1 | 49.0 | 66.2 | 23.3 | 45.7 | 34.0 | 0.276 | 0.511 | 0.794 |
| Beagles | 46.1 | 43.2 | 41.7 | 34.5 | 42.4 | 44.2 | 36.2 | 6.9 | ||||
| Labradors | 45.4 | 52.4 | 33.5 | 39.5 | 29.8 | 33.0 | 40.2 | 7.1 | ||||
| Met | Dachshunds | 26.1 | 23.1 | 34.7 | 43.9 | 52.4* | 63.4* | 63.2* | 7.0 | <0.001 | 0.016 | 0.125 |
| Beagles | 141.0 | 220.6 | 199.3 | 238.5 | 304.3 | 222.8 | 344.9* | 51.0 | ||||
| Labradors | 280.6 | 285.0 | 147.6 | 224.8 | 194.1 | 252.2 | 347.6 | 41.0 | ||||
| Tau | Dachshunds | 266.1 | 227.0 | 195.6 | 238.5 | 226.0 | 217.6 | 208.7 | 25.5 | <0.001 | 0.243 | 0.882 |
| Beagles | 175.9 | 159.4 | 141.4 | 176.6 | 184.5 | 192.2 | 177.1 | 24.0 | ||||
| Labradors | 133.8 | 131.3 | 110.1 | 126.9 | 119.3 | 116.2 | 127.4 | 12.8 | ||||
1Standard error of the mean, n = 4 at each level of dietary Met for Miniature Dachshunds, Beagles, and Labrador Retrievers.
*Significantly different (P ≤ 0.05) when compared with the lowest level of dietary Met (Met = 0.21%) using the Dunnett’s test within each breed.
Figure 3.
Methionine concentration in serum collected from adult Miniature Dachshunds fed diets supplemented with increasing levels of Met and excess Cys during the fed state. Dashed lines represent the estimated mean Met requirement (44.2 mg/kg BW); dotted lines represent the upper 95% CL (48.9 mg/kg BW). Data points represent mean + SE of samples (n = 4).
Among breeds, BW and LBM were significantly different (P ≤ 0.05; Table 4). When expressed as a proportion of BW, LBM was the greatest for Miniature Dachshunds (P ≤ 0.05), but no significant differences were found between Beagles and Labradors (P > 0.10, Table 4). Resting EE per unit of BW0.75 was the greatest for Labrador Retrievers (P ≤ 0.05), and no significant differences were found for Miniature Dachshunds and Beagles (P > 0.10, Table 4). FEE per BW0.75 increased compared with REE for all breeds (P ≤ 0.05) and was significantly greater for Miniature Dachshunds and Labrador Retrievers as compared with Beagles (P ≤ 0.05). Fasting and fed RQ was significantly higher for Beagles (P ≤ 0.05) compared with Miniature Dachshund and Labradors (Table 4).
Table 4.
Mean BW, LBM, and indirect calorimetry data of dogs used
| Miniature Dachshunds (n = 4) | Beagles (n = 4) | Labrador Retrievers (n = 4) | Pooled ANOVA | ||
|---|---|---|---|---|---|
| SEM1 | P-value | ||||
| BW2, kg | 4.66c | 9.91b | 32.6a | 0.23 | <0.001 |
| LBM, kg | 3.74c | 7.61b | 25.1a | 0.33 | <0.001 |
| LBM, % BW | 80.6a | 76.7b | 76.9b | 1.20 | 0.001 |
| REE, Kcal/BW0.75 | 63.1b | 62.1b | 70.4a | 1.87 | 0.004 |
| FEE, Kcal/BW0.75 | 91.3a | 81.5b | 89.2a | 2.69 | 0.029 |
| Fasting RQ | 0.774b | 0.795a | 0.771b | 0.007 | 0.026 |
| Fed RQ | 0.845b | 0.868a | 0.848b | 0.003 | <0.001 |
1Standard error of the mean, n = 4 for Miniature Dachshunds, Beagles, and Labrador Retrievers.
2LSmean of BW, REE, FEE, fasting RQ, and fed RQ measured on each day of each IAAO study.
a–cValues in a row with different superscript are different (P ≤ 0.05; Tukey test).
Discussion
To our knowledge, this is the first dose–response study evaluating Met requirements among different breeds of adult dogs at weight maintenance. The current study suggests that the minimum recommended allowance of the Met requirement with Cys provided in excess (0.930% as fed) is 51.6 mg/kg BW (0.304 g/100 g dry matter [DM]) for Miniature Dachshunds, 77.9 mg/kg BW (147.8 mg/kg BW^0.75 as-fed basis and 0.458 g/100 g DM) for Beagles, and 172.4 mg/kg BW (159.6 mg/kg BW^0.75 as-fed basis and 0.517 g/100 g DM) for Labrador Retrievers. NRC (2006) recommends that Met and Met + Cys should be provided at 0.26 and 0.52 g/100 g DM, respectively, for adult dogs at maintenance. The current NRC recommendations are based on the lowest Met concentrations reported in two long-term studies where mature Beagles were fed low-protein diets for extended periods of time and showed no observable signs of deficiency (Ward, 1976; Sanderson et al., 2001). In the study by Sanderson et al. (2001), feeding 0.48% of total SAA resulted in one case of dilated cardiomyopathy from Tau deficiency and suggests that SAAs were deficient, but may also be due to other factors, such as the relatively high amount of dietary fat provided. Compared with those results, the estimated Met requirement in the present experiment is much higher for Beagles and Labrador Retrievers but is similar for Miniature Dachshunds when reported in mg/kg and in percent (%) of diet on a DM basis.
Recently, Harrison et al. (2020) applied IAAO methods to estimate the dietary requirements of Met in adult Labrador Retrievers and determined an upper 95% CL of 0.71 g/Mcal, which is about 50% of the present recommendation (1.303 g/Mcal metabolizable energy [ME] for the pooled requirement of Beagles and Labrador Retrievers). One difference when comparing these estimates and presenting them as a function of dietary energy is that in North America, the ME content of the diet is calculated using factors of 3.5 kcal/g for protein and nitrogen-free extract and 8.5 kcal/g for fat (modified Atwater factors). In contrast, in the United Kingdom and Europe, factors of 4 kcal/g for protein and nitrogen-free extract and 9 kcal/g for fat are used (Atwater factors). As such, if you used the Atwater factors, rather than modified Atwater factors, the estimate of our requirement on a caloric basis would be 1.176 g/Mcal ME for the pooled requirement of Beagles and Labrador Retrievers and still greater than the estimate from Harrison et al. (2020). Further differences between these estimates may be due to the differences in digestibility (Harrison et al., 2020 = 96% total tract apparent CP digestibility), whereas the digestibility of the diets used in the present study was likely lower due to the inclusion of more intact ingredients in contrast to the diets used by Harrison et al. (2020). More specifically, the diets used in the current study included beet pulp, a moderately fermentable fiber. Fermentable carbohydrates reduce apparent ileal digestibilities, nitrogen retention, and average daily gain in pigs (Myrie et al., 2008), and the addition of dietary pectin resulted in reduced total tract CP digestibility in dogs (Silvio et al., 2000). In addition to reducing the ileal digestibility of the AA, fermentable fibers such as oligosaccharides result in greater colonic weight of rats (Campbell et al., 1997), and consequently higher Met utilization by the gut tissue. The latter is supported by the 30% lower Met requirement of piglets fed parenterally in contrast to enterally (Shoveller et al., 2003) and that the gut utilizes 20% of dietary Met in healthy pigs (Riedijk et al., 2007). It should also be noted that the diets used by Harrison et al. (2020) had a much higher protein content and a lower ratio of indispensable AA to total nitrogen content. We do not know whether greater dispensable AA may have contributed to a lower estimate of the requirement, but this is an industry-relevant question to investigate.
Related to the study designs, there are also fundamental differences between these two studies. Harrison et al. (2020) did not use the prime to constant ratio, which is based on the Phe kinetics that we conducted in dogs (Gooding et al., 2013) and also explains the significantly lower F13CO2 presented by Harrison et al. (2020). Furthermore, given that Gooding et al. (2013) demonstrated that the gastric emptying rate is approximately 25 min, the once hourly feeding in Harrison et al. (2020) may not have allowed for the achievement of a similar isotopic steady state. Finally, we validated that metabolic chambers resulted in complete CO2 collection and did not result in any fractionation of the breath sample (Shoveller et al., 2017), but it was unclear how Harrison et al. (2020) had done the same using respiratory masks. Together, these differences in the experimental diets and in the study design may explain the greater requirement determined in the present study in contrast to Harrison et al. (2020). Indeed, a limitation of the current study is that basal and test diets were low-protein, AA-supplemented diets, and as such, the total protein supply may affect the prediction of the requirement. Further research examining the effects of dietary fat and protein on single indispensable AA requirements and how the gut function is related to the estimate of AA requirements is warranted.
The Met requirements estimated herein may increase depending on other factors, such as Cys supplementation, the nature of the dietary ingredients, dietary protein and fat provision, and/or provision of transmethylation products in the diets. Methionine acts as a precursor for Cys synthesis, and supplementation of Cys can spare up to ~70% of the Met needed in rats (Finkelstein et al., 1988), whereas in pigs, Cys spares the Met requirement by 40% on a weight basis, equating to Cys comprising 50% of the total SAA (Shoveller et al., 2003). We included an excess of Cys in the diet to minimize the Met utilization for Cys synthesis; thus, the present values should be interpreted as the minimum dietary Met requirements or the lowest amount of Met needed in the diet. As well, the estimated MR for Met has been determined here using a semi-purified diet, with more than 50% of the estimated requirement coming from a crystalline AA source that is considered to be 100% bioavailable (NRC, 2006). Total Met content in the diet should be modified depending on AA digestibility and bioavailability in the ingredients used to meet the animal needs. Finally, Met is also involved in methylation reactions, and as such, direct supplementation of the final products of methylation (i.e., folate, betaine, and choline) may decrease the need for Met in the diet (Shoveller et al., 2005; Robinson et al., 2016).
The difference in estimated Met requirement between Miniature Dachshunds, Beagles, and Labrador Retrievers is not well understood. In Miniature Dachshunds, the MR (the break point in the two-phase regression model) was found at the second level of Met supplementation. This indicates that the true break point could be between 0.21% and 0.26%, values that are similar to estimates in growing dogs (NRC, 2006). Moreover, when determining Met requirements based on BW^0.75, the model was not significant. Thus, data from Miniature Dachshunds should be interpreted carefully. However, the analysis of serum Met concentration in adult Miniature Dachshunds also suggests lower Met requirements, in agreement with IAAO data. Based on the overall concentration of AA across the multiple levels of Met intake, there are consistent differences in SAA metabolism. Across all dietary Met level, Dachshunds Met concentration in plasma was approximately 20% compared with that of Beagles and Labrador, while for Tau, Dachshunds concentration was 50% higher. In the present study, given that Tau is mostly influenced by dietary supply, plasma Tau was likely greater due to greater food intake. The mechanism behind these differences in SAA metabolism supports the notion of different indispensable AA requirements among dog breeds and warrants a breed-specific investigation of the control of transmethylation, remethylation, and transsulfuration.
Similar to other studies presented by our lab (Mansilla et al., 2018, 2020; Templeman et al., 2019; Sutherland et al., 2020), calorimetry data remained similar at all levels of Met supplementation, indicating no differences in AA or nutrient metabolism with increasing levels of Met supplementation. The animals used for the present experiment have been used for similar studies (Mansilla et al., 2018, 2020; Templeman et al., 2019; Sutherland et al., 2020); therefore, the calorimetry data are comparable to data published elsewhere. The higher REE (kcal/BW0.75) in Labrador Retrievers compared with Beagles and Miniature Dachshunds is, however, not consistent with our previous results. This could be related to changes in BW and LBM. As expected, and as seen in the previous reports in this series, the fasted RQ values are indicative of fat metabolism for energy production, and increased RQ in the fed state suggests a preferential shift to dietary protein and carbohydrate metabolism in the postprandial phase.
This study is part of a series of experiments where the requirements of Phe, Trp, Thr, and Lys for adult dogs of different breeds have been previously estimated using the IAAO technique (Mansilla et al., 2018, 2020; Templeman et al., 2019; Sutherland et al., 2020). It should be noted, though, that in all these reports (including the present study), only spayed and neutered dogs were used. As such, the authors acknowledge that no conclusions can be drawn regarding how these indispensable AA requirements may differ based on hormonal status.
In conclusion, this is the second dose–response study determining Met requirements in adult dogs at maintenance and the first to use multiple breeds. The estimates presented are higher than current recommendations by the NRC (2006) for Beagles and Labrador Retrievers, but similar to Miniature Dachshunds. Values presented herein are based on semi-purified diets supplemented with excess Cys; actual dietary supply of Met (bioavailability) may vary depending on the ingredients used in the diet. The present study suggests that SAA metabolism may differ between breeds and may be greater when lower digestibility diets with greater dietary fiber are utilized, or when diets with an increased dietary fat-to-protein ratio are provided. Further research is warranted to establish Met requirements with varying inclusion levels of dietary Cys, differing splits of specific macronutrients (i.e., fat to protein), and with direct supplementation of final products of methylation. Additionally, a more thorough investigation into the metabolic differences that may underpin breed differences in Met requirements, such as breed-specific control of transmethylation and remethylation, and transsulfuration is necessary. Finally, the values presented herein were determined in a short-term study, and validation in a long-term study requires further attention.
Supplementary Data
Supplementary data are available at Journal of Animal Science online.
Supplemental Table S1. Least square means of BW, fasting and fed EE, and RQ for Miniature Dachshunds, Beagles, and Labrador Retrievers fed diets containing graded levels of Met with excess Cys
Supplementary Material
Acknowledgment
The funding for this work was provided by Procter & Gamble Co., Mason, Ohio, USA, 45040.
Glossary
Abbreviations
- AA
amino acid
- AAFCO
Association of American Feed Control Officials
- BW
body weight
- BW0.75
metabolic body weight
- CL
confidence limit
- CO2
carbon dioxide
- DM
dry matter
- ECO2
13CO2 enrichment
- EE
energy expenditure
- F13CO2
Breath 13CO2 production
- FCO2
breath CO2 production
- FEDIAF
European Pet Food Industry Federation
- FEE
fed energy expenditure
- IAAO
indicator amino acid oxidation
- LBM
lean body mass
- ME
metabolizable energy
- MR
minimum requirement
- NRC
National Research Council
- O2
oxygen
- RA
recommended allowance
- REE
resting energy expenditure
- RQ
respiratory quotient
- SAA
sulfur amino acid
Authors’ Contributions
A.K.S. designed the study; A.K.S. and L.F. conducted the study; and all authors analyzed the data and wrote the manuscript. A.K.S. had primary responsibility for the final content. All authors read and approved the final manuscript.
Conflict of interest statement
A.K.S. and L.F. were employees of the Procter & Gamble Co.; L.F. is now employed by Mars Petcare; A.K.S was employed by Mars Petcare (2014 to 2015) and is now faculty at the University of Guelph. W.D.M. and J.R.T. have no conflicts of interest.
Literature Cited
- Association of American Feed Control Officials (AAFCO) 2014. AAFCO manual. West Lafayette (IN): AAFCO Inc. [Google Scholar]
- Burns R A, and Milner J A. . 1981. Sulfur amino acid requirements of immature Beagle dogs. J. Nutr. 111:2117–2124. doi: 10.1093/jn/111.12.2117 [DOI] [PubMed] [Google Scholar]
- Campbell J M, Fahey G C, and Wolf B W. . 1997. Selected indigestible oligosaccharides affect large bowel mass, cecal and fecal short-chain fatty acids, pH, and microflora in rats. J. Nutr. 127:130–136. doi: 10.1093/jn/127.1.130 [DOI] [PubMed] [Google Scholar]
- European Pet Food Industry Federation 2013. Nutritional guidelines for complete and complementary pet food for cats and dogs. Belgium: FEDIAF. [Google Scholar]
- Finkelstein J D, Martin J J, and Harris B J. . 1988. Methionine metabolism in mammals. The methionine-sparing effect of cystine. J. Biol. Chem. 263:11750–11754. [PubMed] [Google Scholar]
- Gooding M A, Cant J P, Pencharz P B, Davenport G M, Atkinson J L, and Shoveller A K. . 2013. Oral and intravenous l-[1-13C]phenylalanine delivery measure similar rates of elimination when gastric emptying and splanchnic extraction are accounted for in adult mixed hounds. J. Anim. Physiol. Anim. Nutr. 97:181–189. doi: 10.1111/j.1439-0396.2011.01256.x [DOI] [PubMed] [Google Scholar]
- Harrison M, Thomas G, Gilham M, Gray K, Colyer A, and Allaway D. . 2020. Short-term determination and long-term evaluation of the dietary methionine requirement in adult dogs. Br. J. Nutr. 26:1–12. doi: 10.1017/S0007114520000690 [DOI] [PubMed] [Google Scholar]
- Hirawaka D A, and Baker D H. . 1985. Sulfur amino acid nutrition of the growing puppy: determination of dietary requirements for methionine and cystine. Nutr. Res. 5:631–642. doi: 10.1016/S0271-5317(85)80244-X [DOI] [Google Scholar]
- Hoerr R A, Yu Y M, Wagner D A, Burke J F, and Young V R. . 1989. Recovery of 13C in breath from NaH13CO3 infused by gut and vein: effect of feeding. Am. J. Physiol. 257(3 Pt 1):E426–E438. doi: 10.1152/ajpendo.1989.257.3.E426 [DOI] [PubMed] [Google Scholar]
- Levesque C L, Moehn S, Pencharz P B, and Ball R O. . 2010. Review of advances in metabolic bioavailability of amino acids. Livest. Sci. 133:4–9. doi: 10.1016/j.livsci.2010.06.013 [DOI] [Google Scholar]
- Mansilla W D, Gorman A, Fortener L, and Shoveller A K. . 2018. Dietary phenylalanine requirements are similar in small, medium, and large breed adult dogs using the direct amino acid oxidation technique. J. Anim. Sci. 96:3112–3120. doi: 10.1093/jas/sky208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansilla W D, Templeman J R, Fortener L, and Shoveller A K. . 2020. Adult dogs of different breed sizes have similar threonine requirements as determined by the indicator amino acid oxidation technique. J. Anim. Sci. 98:skaa066. doi: 10.1093/jas/skaa066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner J A. 1979. Assessment of the essentiality of methionine, threonine, tryptophan, histidine and isoleucine in immature dogs. J. Nutr. 109:1351–1357. doi: 10.1093/jn/109.8.1351 [DOI] [PubMed] [Google Scholar]
- Moehn S, Bertolo R F, Pencharz P B, and Ball R O. . 2004. Indicator amino acid oxidation responds rapidly to changes in lysine or protein intake in growing and adult pigs. J. Nutr. 134:836–841. doi: 10.1093/jn/134.4.836 [DOI] [PubMed] [Google Scholar]
- Myrie S B, Bertolo R F, Sauer W C, and Ball R O. . 2008. Effect of common antinutritive factors and fibrous feedstuffs in pig diets on amino acid digestibilities with special emphasis on threonine. J. Anim. Sci. 86:609–619. doi: 10.2527/jas.2006-793 [DOI] [PubMed] [Google Scholar]
- National Research Council (NRC). 2006. Nutrient requirements of dogs and cats. 2nd rev. ed. Washington (DC): National Academies Press. [Google Scholar]
- Pencharz P B, and Ball R O. . 2003. Different approaches to define individual amino acid requirements. Annu. Rev. Nutr. 23:101–116. doi: 10.1146/annurev.nutr.23.011702.073247 [DOI] [PubMed] [Google Scholar]
- Riedijk M A, Stoll B, Chacko S, Schierbeek H, Sunehag A L, van Goudoever J B, and Burrin D G. . 2007. Methionine transmethylation and transsulfuration in the piglet gastrointestinal tract. Proc. Natl. Acad. Sci. U. S. A. 104:3408–3413. doi: 10.1073/pnas.0607965104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J L, Bartlett R K, Harding S V, Randell E W, Brunton J A, and Bertolo R F. . 2016. Dietary methyl donors affect in vivo methionine partitioning between transmethylation and protein synthesis in the neonatal piglet. Amino Acids 48:2821–2830. doi: 10.1007/s00726-016-2317-x [DOI] [PubMed] [Google Scholar]
- Sanderson S L, Gross K L, Ogburn P N, Calvert C, Jacobs G, Lowry S R, Bird K A, Koehler L A, and Swanson L L. . 2001. Effects of dietary fat and l-carnitine on plasma and whole blood taurine concentrations and cardiac function in healthy dogs fed protein-restricted diets. Am. J. Vet. Res. 62:1616–1623. doi: 10.2460/ajvr.2001.62.1616 [DOI] [PubMed] [Google Scholar]
- Shoveller A K, Brunton J A, Pencharz P B, and Ball R O. . 2003. The methionine requirement is lower in neonatal piglets fed parenterally than in those fed enterally. J. Nutr. 133:1390–1397. doi: 10.1093/jn/133.5.1390 [DOI] [PubMed] [Google Scholar]
- Shoveller A K, Danelon J J, Atkinson J L, Davenport G M, Ball R O, and Pencharz P B. . 2017. Calibration and validation of a carbon oxidation system and determination of the bicarbonate retention factor and the dietary phenylalanine requirement, in the presence of excess tyrosine, of adult, female, mixed-breed dogs. J. Anim. Sci. 95:2917–2927. doi: 10.2527/jas.2017.1535 [DOI] [PubMed] [Google Scholar]
- Shoveller A K, Stoll B, Ball R O, and Burrin D G. . 2005. Nutritional and functional importance of intestinal sulfur amino acid metabolism. J. Nutr. 135:1609–1612. doi: 10.1093/jn/135.7.1609 [DOI] [PubMed] [Google Scholar]
- Silvio J, Harmon D L, Gross K L, and McLeod K R. . 2000. Influence of fiber fermentability on nutrient digestion in the dog. Nutrition 16:289–295. doi: 10.1016/s0899-9007(99)00298-1 [DOI] [PubMed] [Google Scholar]
- Sutherland K A K, Mansilla W D, Fortener L, and Shoveller A K. . 2020. Lysine requirements in small, medium, and large breed adult dogs using the indicator amino acid oxidation technique. Transl. Anim. Sci. 4:txaa082. doi: 10.1093/tas/txaa082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeman J R, Mansilla W D, Fortener L, and Shoveller A K. . 2019. Tryptophan requirements in small, medium, and large breed adult dogs using the indicator amino acid oxidation technique1. J. Anim. Sci. 97:3274–3285. doi: 10.1093/jas/skz142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe J M, Roberts S A, Ball R O, and Pencharz P B. . 1999. Prior protein intake may affect phenylalanine kinetics measured in healthy adult volunteers consuming 1 g protein. kg-1. d-1. J. Nutr. 129:343–348. doi: 10.1093/jn/129.2.343 [DOI] [PubMed] [Google Scholar]
- Ward J. 1976. The amino acid requirements of the adult dog [Ph.D. dissertation]. Oxford (UK): Wolfson College, University of Cambridge. [Google Scholar]
- Weir J B. 1949. New methods for calculating metabolic rate with special reference to protein metabolism. J. Physiol. 109:1–9. doi: 10.1113/jphysiol.1949.sp004363 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.