Abstract
The objective of this study was to investigate the effects of dietary soluble fiber (SF) or insoluble fiber (ISF) intake in late gestation on litter performance, milk composition, immune function, and redox status of sows around parturition. A total of 60 Yorkshire sows were randomly assigned into three dietary treatments: normal level of dietary fiber (CON, 16.16% dietary fiber with 1.78% soluble fiber and 14.38% insoluble fiber), high insoluble fiber (ISF, 30.12% dietary fiber with 2.97% soluble fiber and 27.15% insoluble fiber), and high soluble fiber (SF, 30.15% dietary fiber with 4.57% soluble fiber and 25.58% insoluble fiber). Digestible energy and crude protein intake were comparable among treatments via adjusting feed intake from day 90 of gestation to parturition. After parturition, all sows were fed the same lactation diet. Results showed that litter performance of sows was not markedly affected by maternal fiber intake. However, sows fed ISF or SF diet had increased concentration of plasma mmunoglobulin G at day 107 (P < 0.05) and parturition (P < 0.01), and the SF diet had a tendency to increase fat content in both colostrum and milk relative to the CON diet. Furthermore, sows fed ISF diet had increased glutathione peroxidase activity (P < 0.05) at day 107, but decreased the plasma level of malondialdehyde at parturition (P < 0.05). High maternal SF intake tended to decrease the number of weaned piglets due to the increased preweaning mortality, as compared with sows fed the ISF diet. In conclusion, high fiber intake in late gestation may improve immune function and redox status, but differentially influenced the milk composition and preweaning mortality.
Keywords: antioxidant, colostrum, dietary fiber, immunology, lactation, swine
Introduction
It has been demonstrated that fiber addition in the gestation diet may increase litter size through one or several reproductive cycles (Veum et al., 2009; Che et al., 2011). In addition, fiber-rich diet during gestation improved feed intake of sows and piglets growth rate during lactation due to a carryover effect on feed intake capacity (Guillemet et al., 2007; Li et al., 2019). Other positive effects of dietary fiber on embryo survival rate, piglet colostrum intake (CI), stillbirth rate, and intestinal microbiota composition of sows have also been reported (Theil et al., 2014; Feyera et al., 2017; Pan et al., 2017).
During pregnancy, fetal development is largely influenced by the uterine environment and umbilical supply of nutrients (Vallet et al., 2014), which determines the birth weight and litter uniformity (Wang et al., 2017). In addition, the inflammatory response and oxidative stress occurred during the perinatal period (Berchieri-Ronchi et al., 2011; Newbern and Freemark, 2011), which may impair fetal development by decreasing fetal protein deposition and skeletal formation (Prater et al., 2008). In contrast, dietary fiber has been shown to improve immune response and redox status during the perinatal period (Wang et al., 2016).
To our knowledge, however, it is rarely reported about the effects of dietary fiber sources on reproductive performance and sow physiology. In this study, therefore, we aimed to investigate the effects of high soluble or insoluble fiber intake in late gestation on litter performance, milk composition, immune function, and redox status of sows around parturition. High ISF inclusion in diet was obtained by including soybean hulls and wheat bran as main fiber ingredients with ISF/SF ratio of 9.14, which is a typical value of gestating diet in China (Sun et al., 2014; Tan et al., 2018). The high SF diet was obtained by replacing part of these ingredients with sugar beet pulp with ISF/SF ratio of 5.60, which closes to the typical value of gestating diet in European countries (Guillemet et al., 2007; Quesnel et al., 2009). To a great extent, the beneficial function of fiber depends on its fermentation and water-holding capabilities. It is widely believed that soluble fibers are fermented faster, producing higher amounts of short-chain fatty acids (SCFA) than insoluble fibers and favoring proliferation of beneficial microbiota (Jha and Berrocoso, 2015). Based on this, we hypothesized that high SF or high ISF intake in late gestation may differentially affect litter performance, milk composition, immune function, and redox status of sows.
Materials and Methods
The study was conducted at Tianfu Sow Farm, Giastar Group, Chengdu, China. The experiment followed the actual law of animal protection and was approved by the Animal Care and Use Committee of the Sichuan Agricultural University (DKY-B20121602) and was performed in accordance with the National Research Council’s Guideline for the Care and Use of Laboratory Animals.
Animals and diets
A total of 60 Yorkshire sows (20 per treatment) were included and randomly allocated into three dietary treatments, based on parity (4~6) and body weight (267.8 ± 22.6 kg, means ± SD). Three diets were formulated based on corn–soybean meal to meet or exceed the recommendation of NRC (2012) as presented in Table 1: normal dietary fiber diet (CON, 16.2 % total dietary fiber [TDF]), high insoluble fiber diet (ISF, 30.1% total dietary fiber), and high soluble fiber diet (SF, 30.1% total dietary fiber). The average parity was 4.74, 4.75, and 4.89 for CON, ISF, and SF groups, respectively. In this study, all sows were used for recording reproductive performance, but only sows in parities 4 and 5 were used for milk and blood sampling. The ISF and SF diets had similar TDF, but the SF diet had a higher amount of soluble fiber than the ISF diet (4.57% in SF vs. 2.97% in ISF, respectively) from sugar beet pulp. In addition to dietary fiber, other nutrients such as digestible energy and crude protein intake were comparable via adjusting feed intake (3.0 kg/d in CON and 3.2 kg/d in either ISF or SF group) from day 90 of gestation to parturition. The daily dietary fiber intake of ISF and SF groups was about twice as much as the CON group (485g/d in CON and 964g/d in either ISF or SF group). During day 90 to 110 of gestation, the diet was supplied once a day (0800 hours). On day 111 of gestation, sows were moved to the farrowing room and feeding frequency turned to twice a day (0800 and 1500 hours) until parturition, but the total feeding supply did not change. Within 48 h after parturition, cross-fostering was carried out within dietary treatment to standardize the litter size to 11 to 12 piglets. On the day of parturition, no feed was supplied to sows. After parturition, all sows were fed a commercial lactation diet until day 21 of lactation and feed intake was increased by 0.5 kg/d from day 2 to 7 of lactation. From day 8 of lactation, ad libitum feeding was applied. The healthcare and immunization procedures of sows and piglets followed the regulation of Tianfu Sow Farm. During the entire experimental period, sows and piglets were given free access to drinking water.
Table 1.
Ingredients and composition of gestation diets and lactation diet1
| Gestation diets | ||||
|---|---|---|---|---|
| Ingredients, % | CON | ISF | SF | Lactation diet |
| Corn (CP 8.2%) | 73.50 | 53.30 | 49.83 | 59.86 |
| Soybean meal (CP 46%) | 16.80 | 10.40 | 11.30 | 17.68 |
| Wheat bran | 2.90 | 16.40 | 12.00 | — |
| Soybean hull | 2.90 | 16.40 | 12.00 | 5.00 |
| Sugar beet pulp | 0.00 | 0.00 | 11.40 | — |
| Fish meal | — | — | — | 2.00 |
| Extruded soybean | — | — | — | 6.00 |
| Wheat | — | — | — | 3.59 |
| Soybean oil | — | — | — | 2.00 |
| Lysine-HCl (70%) | 0.02 | 0.01 | 0.00 | 0.25 |
| DL-Methionine (98.5%) | 0.01 | 0.04 | 0.04 | 0.05 |
| L-Threonine (98.5%) | 0.00 | 0.03 | 0.04 | 0.09 |
| CaCO3 | 1.08 | 0.86 | 0.67 | 0.87 |
| CaHPO4 | 1.70 | 1.56 | 1.63 | 0.93 |
| Sodium chloride | 0.40 | 0.40 | 0.40 | 0.40 |
| Potassium chloride | — | — | — | 0.50 |
| Choline chloride (50%) | 0.14 | 0.14 | 0.14 | 0.15 |
| Vitamin and mineral premix1 | 0.55 | 0.55 | 0.55 | 0.63 |
| Calculated nutrient levels | ||||
| Digestible energy, MJ/kg | 13.31 | 12.39 | 12.47 | — |
| Crude protein, % | 14.23 | 13.29 | 13.29 | — |
| NDF, % | 10.11 | 21.15 | 21.93 | — |
| SF, % | 1.78 | 2.97 | 4.57 | — |
| ISF, % | 14.38 | 27.15 | 25.58 | — |
| ISF/SF | 8.08 | 9.14 | 5.60 | — |
| Dietary fiber, % | 16.15 | 30.11 | 30.14 | — |
| Analyzed nutrient levels | ||||
| Digestible energy, MJ/kg | 13.80 | 13.22 | 13.16 | 14.27 |
| Crude protein, % | 13.59 | 12.57 | 12.48 | 17.20 |
| NDF, % | 13.25 | 26.84 | 26.47 | 11.00 |
| SF, % | 1.92 | 3.22 | 5.06 | 3.80 |
| ISF, % | 15.55 | 29.68 | 28.44 | 12.15 |
| ISF/SF | 8.10 | 9.22 | 5.62 | 3.20 |
| Dietary fiber, % | 17.47 | 32.90 | 33.50 | 15.95 |
1CON, control fiber; CP, crude protein; ISF, high insoluble fiber; NDF, neutral detergent fiber; SF, high soluble fiber.
2Provided per kg of diet: Zn 100 mg, Cu 6 mg, Fe 100 mg; Mn 10 mg, I 0.14 mg, Se 0.25 mg, VA 14 mg, VB6 14 mg, VE 30 mg, VC 100 mg, biotin 0.1 mg, folic acid 2.5 mg, carnitine 46 mg, and organic chromium 0.3 mg.
Data collection and sampling
The number of total born, born alive, low birth weight (LBW) piglets (birth weight < 0.8 kg), mummies, and stillborn were recorded at parturition. Expelled placentas were collected and weighed. The placental efficiency was calculated as the ratio of the total litter weight to the placental weight. Piglets were weighed at birth and 48 h after birth, before cross-fostering, then days 7, 14, and 21 after cross-fostering to determine average daily gain (ADG). The average daily feed intake (ADFI) of sows after cross-fostering was recorded by weighing daily feed refusals.
Blood samples (10 mL, n = 10) from ear vein of sows were collected into sodium heparinized tubes 4 h after providing the morning meal on day 107 of gestation and on 1 h after the first piglet born. Plasma was obtained by centrifuging at 3,000 × g for 15 min and stored immediately at −20 °C for later analysis.
Colostrum samples (20 mL, n = 10) were collected on 1 h after the first piglet born. Milk samples (20 mL, n = 10) were collected on day 7 of lactation after an intravenous injection of 20 IU of oxytocin (Qilu Animal Health Products Co., Ltd, Shandong, China). Both colostrum and milk samples were manually collected from all functional teats, then stored at −20 °C for later analysis.
CI and milk composition
CI and colostrum yield
A total of 24 sows were included for estimating CI, including 258 piglets. Ten sows (five sows in parity 4, two sows in parity 5, and three sows in parity 6), five sows (three sows in parity 4, one sow in parity 5, and one sow in parity 6), and nine sows (three sows in parity 4, five sows in parity 5, and one sow in parity 6) were used for detecting CI for CON, ISF, and SF groups, respectively. CI by individual piglet during the 24-h period after the first piglet born was estimated using the following equation developed by Theil et al. (2014):
where WG is piglet weight gain between birth and 24 h after the first piglet born (g), BWB is BW at birth (kg), and D is the duration of colostrum suckling, that is the time difference (min) from birth to 24 h after the birth of the first piglet. Piglets from these 24 sows were weighed not only at birth but also 24 h after the first piglet born for the calculation of WG. Colostrum yield was the sum of CI for all piglets in the same litter.
Composition of colostrum and milk
Frozen colostrum and milk samples were thawed at 4 °C, and then 10 mL of each sample was used for composition analysis. The fat, protein, lactose (LAC), dry matter, and urea nitrogen content of colostrum and milk were measured using a milk composition analyzer (Milkoscan FT2, Denmark).
Plasma metabolites and redox status
The plasma concentrations of glucose (GLU), LAC, triglyceride, urea, total protein, albumin (ALB), nonesterified fatty acid, total bile acids, immunoglobulin G (IgG), immunoglobulin M, complement 4, and C-reactive protein (C-RP) were determined using automatic biochemical analyzer (Model 7020, Hitachi, Tokyo, Japan). There was less than 5% variation of intra-assay and inter-assay coefficients for each assay.
Glutathione peroxidase (GSH-Px), malondialdehyde (MDA), total superoxide dismutase (T-SOD), and catalase (CAT) enzyme activities were determined as described by Wang et al. (2016), who have validated the kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) used for determination. There was less than 5% variation of intra-assay and inter-assay coefficients for each assay.
Statistical analysis
Sows were regarded as the experimental unit and piglet data were reported as a mean for the litter. The data were considered as outlier when the student residue was greater than 3. The GLIMMIX and univariate procedures of SAS (SAS Institute Inc., Cary, NC, USA) were used to analyze the variance homogeneity and normality, respectively. Reproductive performance, milk composition, and blood biochemistry were analyzed using the MIXED procedure of SAS with the diet as a fixed factor and parity as a random factor, using the following statistical model:
where Y is the parameter to be tested, α is the mean, Si is the effect of the diet (i = 1, 2, 3), Bj is the random effect of the parity (j = 1, 2), and ε ij is the error term. Means were separated using the Tukey method with a significance level of 0.05, and a tendency was considered when probability values were less than 0.10. The results were expressed as the mean and SEM.
Results
Litter performance
The results pertaining to the litter performance of sows are presented in Table 2. No significant effects of dietary fiber for number of total born, born alive, LBW piglets, mummy and stillborn, litter birth weight, born alive litter weight, placental efficiency, and CV (coefficient of variation within-litter birth weight) (P > 0.05).
Table 2.
Effect of high ISF or SF intake in late gestation on litter performance of sows1,2
| CON | ISF | SF | P-value | |
|---|---|---|---|---|
| Piglets per litter, no. | ||||
| Total born | 15.31 ± 0.61 | 16.13 ± 0.56 | 15.39 ± 0.63 | 0.59 |
| Born alive | 13.75 ± 0.70 | 14.25 ± 0.55 | 13.22 ± 0.65 | 0.52 |
| Stillborn | 1.25 ± 0.43 | 1.31 ± 0.43 | 1.72 ± 0.36 | 0.66 |
| Mummies | 0.31 ± 0.15 | 0.56 ± 0.20 | 0.44 ± 0.27 | 0.73 |
| LBW piglets3 | 0.94 ± 0.22 | 0.69 ± 0.30 | 1.11 ± 0.27 | 0.52 |
| Placental weight | 3.51 ± 0.35 | 3.55 ± 0.35 | 3.34 ± 0.28 | 0.89 |
| Placental efficiency4 | 5.36 ± 0.76 | 5.84 ± 0.95 | 5.92 ± 0.43 | 0.62 |
| CV5, % | 21.50 ± 1.60 | 20.43 ± 1.56 | 21.46 ± 1.06 | 0.83 |
| Total born, kg | ||||
| Litter birth weight | 19.28 ± 0.75 | 20.36 ± 0.72 | 19.47 ± 1.09 | 0.67 |
| Piglet birth weight | 1.27 ± 0.05 | 1.27 ± 0.03 | 1.27 ± 0.06 | 0.99 |
| Born alive, kg | ||||
| Litter birth weight | 17.67 ± 0.84 | 18.59 ± 0.85 | 17.31 ± 1.25 | 0.66 |
| Piglet birth weight | 1.31 ± 0.05 | 1.30 ± 0.03 | 1.30 ± 0.05 | 0.99 |
1 n = 20.
2CON, control fiber; ISF, high insoluble fiber; SF, high soluble fiber.
3LBW piglets refer to piglets with birth weight less than 0.8 kg.
4Placental efficiency, the ratio of total litter weight to placental weight.
5CV, coefficient of variation within-litter birth weight.
CI and milk composition
The results of CI of piglets and milk composition of sows are presented in Table 3. Feeding ISF or SF diet did not markedly affect CI of piglets and colostrum yield (P > 0.05). Sows fed the ISF diet tended to increase the concentration of urea nitrogen in colostrum when compared with sows fed the CON diet (P = 0.08). Also, sows fed ISF and SF diets tended to increase fat content in colostrum when compared with sows fed CON diet (P = 0.07). At day 7 of lactation, sows fed SF diet tended to have a higher milk fat content as compared with sows fed CON and ISF diets (P = 0.08).
Table 3.
Effects of high SF or ISF intake in late gestation on milk composition of sows and CI of piglets1
| CON | ISF | SF | P-value | |
|---|---|---|---|---|
| CI, g | 327.90 ± 20.62 | 311.92 ± 11.77 | 290.20 ± 18.47 | 0.35 |
| Colostrum yield, kg | 3.42 ± 0.31 | 4.12 ± 0.59 | 3.54 ± 0.43 | 0.54 |
| Colostrum | ||||
| Fat, % | 3.74 ± 0.25 | 4.58 ± 0.39 | 4.68 ± 0.15 | 0.07 |
| Protein, % | 15.64 ± 0.62 | 16.70 ± 0.82 | 15.34 ± 0.90 | 0.45 |
| LAC, % | 2.96 ± 0.16 | 3.09 ± 0.12 | 3.36 ± 0.12 | 0.11 |
| Dry matter, % | 25.36 ± 0.71 | 26.35 ± 0.94 | 25.47 ± 0.91 | 0.67 |
| Urea nitrogen, mg/dL | 61.20 ± 2.45 | 68.22 ± 2.33 | 63.90 ± 2.18 | 0.09 |
| Milk, 7 d | ||||
| Fat, % | 8.02 ± 0.34 | 8.32 ± 0.31 | 9.11 ± 0.36 | 0.08 |
| Protein, % | 5.86 ± 0.08 | 6.21 ± 0.25 | 6.16 ± 0.16 | 0.37 |
| LAC, % | 5.88 ± 0.09 | 5.86 ± 0.09 | 5.66 ± 0.14 | 0.33 |
| Dry matter, % | 21.22 ± 0.34 | 21.77 ± 0.49 | 22.37 ± 0.51 | 0.23 |
| Urea nitrogen, mg/dL | 66.20 ± 2.67 | 68.22 ± 1.03 | 65.10 ± 1.82 | 0.56 |
1CON, control fiber; ISF, high insoluble fiber; SF, high soluble fiber.
Lactation performance
As presented in Table 4, ADG of piglets and ADFI of sows in lactation were not different across the three dietary treatments (P > 0.05). The litter size (P = 0.06) and litter weight (P = 0.05) at weaning tended to be lower, while preweaning mortality (P = 0.06) tended to be greater in sows fed SF diet.
Table 4.
Effects of high SF or ISF intake in late gestation on lactation performance of sows1
| CON | ISF | SF | P-value | |
|---|---|---|---|---|
| Litter size, no. | ||||
| Cross-foster | 11.42 ± 0.15 | 11.64 ± 0.15 | 11.42 ± 0.15 | 0.96 |
| Weaning | 10.72 ± 0.23 | 11.38 ± 0.35 | 10.05 ± 0.26 | 0.06 |
| Piglet weight, kg | ||||
| Cross-foster | 1.80 ± 0.14 | 1.79 ± 0.10 | 1.84 ± 0.14 | 0.83 |
| Weaning | 6.85 ± 0.35 | 6.76 ± 0.25 | 6.35 ± 0.32 | 0.34 |
| ADG of piglet, g/d | ||||
| Day 1 to 7 | 165.19 ± 13.93 | 171.90 ± 8.74 | 149.03 ± 11.96 | 0.53 |
| Day 8 to 14 | 271.46 ± 11.97 | 266.54 ± 12.99 | 241.02 ± 11.70 | 0.30 |
| Day 15 to 21 | 281.33 ± 10.48 | 272.22 ± 12.50 | 255.37 ± 10.00 | 0.26 |
| Day 1 to 21 | 240.07 ± 12.06 | 236.65 ± 10.39 | 215.15 ± 10.79 | 0.33 |
| ADFI of sows, kg/d | ||||
| Day 1 to 7 | 1.50 ± 0.01 | 1.46 ± 0.03 | 1.50 ± 0.01 | 0.95 |
| Day 8 to 14 | 6.02 ± 0.16 | 5.85 ± 0.19 | 5.73 ± 0.22 | 0.76 |
| Day 15 to 21 | 7.04 ± 0.11 | 6.68 ± 0.19 | 6.88 ± 0.17 | 0.78 |
| Day 1 to 21 | 4.85 ± 0.08 | 4.66 ± 0.10 | 4.70 ± 0.11 | 0.69 |
| Litter weight at weaning, kg | 73.10 ± 5.12 | 76.96 ± 2.66 | 64.75 ± 3.32 | 0.05 |
| Preweaning mortality, % | 6.13 | 2.23 | 12.00 | 0.06 |
1CON, control fiber; ISF, high insoluble fiber; SF, high soluble fiber.
Metabolic and immunological parameters
The results pertaining to the metabolic and immunological parameters of sows are presented in Table 5. Sows fed SF had markedly increased ALB concentration in the plasma on day 107 as compared with sows fed ISF and CON diets (P < 0.05). Moreover, sows fed either ISF or SF diet had higher IgG concentration in the plasma than sows fed CON diet on both day 107 (P < 0.05) and parturition (P < 0.01). Compared with sows fed the CON diet, sows fed SF tended to have a lower C-RP concentration in plasma on day 107 of gestation (P = 0.08).
Table 5.
Effect of high ISF or SF intake in late gestation on metabolic and immunological parameters of sows1
| CON | ISF | SF | P-value | |
|---|---|---|---|---|
| GLU, mmol/L | ||||
| Day 107 | 3.72 ± 0.18 | 3.76 ± 0.34 | 3.87 ± 0.20 | 0.91 |
| Parturition | 3.88 ± 0.19 | 3.90 ± 0.22 | 3.73 ± 0.30 | 0.89 |
| Lactic acid, mmol/L | ||||
| Day 107 | 2.23 ± 0.12 | 2.20 ± 0.21 | 2.29 ± 0.12 | 0.93 |
| Parturition | 2.77 ± 0.17 | 2.40 ± 0.15 | 2.52 ± 0.08 | 0.19 |
| Triglyceride, mmol/L | ||||
| Day 107 | 0.46 ± 0.04 | 0.50 ± 0.08 | 0.54 ± 0.02 | 0.52 |
| Parturition | 0.19 ± 0.03 | 0.19 ± 0.01 | 0.17 ± 0.02 | 0.75 |
| Urea, mmol/L | ||||
| Day 107 | 4.20 ± 0.18 | 4.51 ± 0.27 | 4.32 ± 0.15 | 0.57 |
| Parturition | 3.55 ± 0.27 | 3.83 ± 0.22 | 3.55 ± 0.22 | 0.93 |
| Total protein, g/L | ||||
| Day 107 | 77.81 ± 1.79 | 76.29 ± 1.39 | 79.99 ± 1.15 | 0.20 |
| Parturition | 69.29 ± 1.82 | 65.88 ± 1.11 | 67.53 ± 1.32 | 0.29 |
| ALB, g/L | ||||
| Day 107 | 34.67 ± 0.44b | 35.64 ± 0.55b | 38.46 ± 0.54a | <0.01 |
| Parturition | 34.33 ± 0.70 | 34.19 ± 0.51 | 34.46 ± 0.43 | 0.95 |
| Nonesterified fatty acid, μmol/L | ||||
| Day 107 | 94.90 ± 6.15 | 91.22 ± 6.45 | 99.67 ± 7.74 | 0.69 |
| Parturition | 633.70 ± 132.14 | 605.50 ± 78.07 | 560.10 ± 93.38 | 0.88 |
| Total bile acid, μmol/L | ||||
| Day 107 | 101.56 ± 12.11 | 104.35 ± 4.27 | 110.18 ± 11.35 | 0.84 |
| Parturition | 85.10 ± 13.53 | 98.53 ± 10.38 | 86.04 ± 4.12 | 0.98 |
| Immunoglobulin G, g/L | ||||
| Day 107 | 2.39 ± 0.27b | 3.29 ± 0.18a | 3.14 ± 0.15a | 0.01 |
| Parturition | 2.79 ± 0.18b | 3.70 ± 0.12a | 3.39 ± 0.12a | <0.01 |
| Immunoglobulin M, g/L | ||||
| Day 107 | 0.29 ± 0.05 | 0.21 ± 0.03 | 0.30 ± 0.05 | 0.23 |
| Parturition | 0.39 ± 0.02 | 0.36 ± 0.02 | 0.37 ± 0.04 | 0.80 |
| Complement C4, mg/L | ||||
| Day 107 | 14.70 ± 1.22 | 13.60 ± 0.83 | 15.56 ± 1.61 | 0.55 |
| Parturition | 6.80 ± 1.04 | 5.90 ± 0.94 | 8.22 ± 1.14 | 0.31 |
| C-RP, mg/L | ||||
| Day 107 | 3.17 ± 0.42 | 2.36 ± 0.21 | 2.23 ± 0.23 | 0.08 |
| Parturition | 3.58 ± 0.86 | 2.83 ± 0.55 | 2.53 ± 0.53 | 0.52 |
1CON, control fiber; ISF, high insoluble fiber; SF, high soluble fiber.
a,bMean values within a row with unlike superscript letters were significantly different (P < 0.05). n = 10.
Redox status
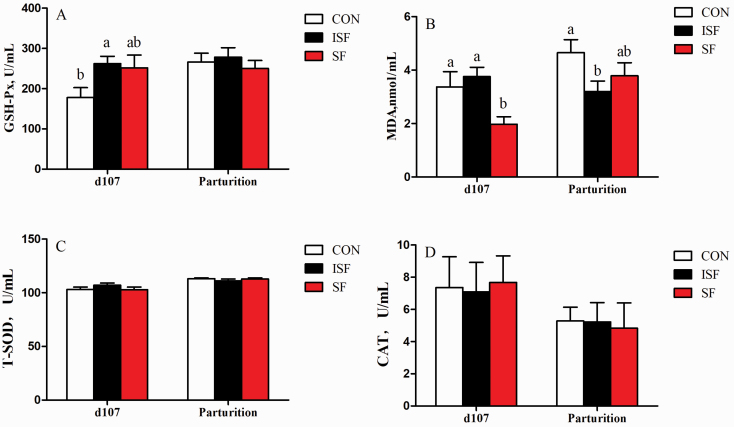
Compared with sows fed the CON diet, sow fed the ISF diet had higher activity of GSH-Px (P < 0.05) on day 107 (Figure 1A). Moreover, plasma MDA level in sows fed SF diet was lower than for sows fed ISF or CON diets on day 107 of gestation (P < 0.05). In addition, sows fed ISF diet had decreased plasma level of MDA at parturition (P < 0.05) when compared with sows fed the CON diet (Figure 1B). The T-SOD and CAT activities in the plasma were not affected by dietary treatment (Figure 1C–D; P > 0.05).
Figure 1.
Effects of high SF or ISF intake in late gestation on redox status of sows around parturition. Plasma GSH-Px activity (A), MDA concentration (B), T-SOD activity (C), and CAT activity (D) were determined on day 107 and at parturition. Values are means, with their standard errors represented by vertical bars. a,bMean values with unlike letters were significantly different (P < 0.05). n = 10.
Discussion
Litter performance
Several studies reported that high fiber intake during gestation improved the litter size of sows (Veum et al., 2009; Che et al., 2011). In this study, however, we did not observe increased litter size or increased litter weights by feeding high fiber diet, which may be related to the intervention period, as sows in the present study were provided dietary treatments only in late gestation. It is a noneffective period to change litter size in late gestation (Krogh et al., 2015; Feyera et al., 2017) as the litter size is already determined in early gestation partly (Durrant et al., 1980). In this study, moreover, dietary fiber may not be able to provide sufficient energy to influence litter weights, though dietary fiber could be fermented to produce SCFAs (Michael, 2015).
Performance during the colostrum period and lactation
In this study, maternal fiber intake at 964 g/d with ISF/SF ratio either at 9.14 or 5.60 tended to increase the fat concentration of colostrum. High fiber intake may increase the retention of fat in the mammary glands prior to parturition, which then is secreted into early colostrum (Krogh et al., 2017; Feyera et al., 2018). No evidence of increased colostrum production due to fiber inclusion in this study, which is in contrast to Theil et al. (2014), but in line with Krogh et al. (2015).
Although SCFAs, produced by high fiber intake, could be used as substrate for the synthesis of milk fat (Loisel et al., 2013), the reasons for the increased milk fat observed at day 7 of lactation for sows fed SF are unknown. Surprisingly, higher SF intake in late gestation tended to decrease the number of weaning piglets and litter weight at weaning, because the preweaning mortality was 12% in SF while it was as low as 2% in the ISF group. It is noteworthy that the amount of CI is far more important than the composition for the growth of piglet during lactation (Quesnel et al., 2012). Previous studies showed that piglet body weight gain increased concomitantly with CI during the first 24 h after birth (Devillers et al., 2011). Besides, Dyck and Swierstra (1987) reported that the preweaning mortality has been negatively associated with CI. In this study, we did find that the CI in sows fed the SF diet was numerically lower (11.6% decrease, 290 vs. 328 g on average), when compared with sows fed the CON diet. The lower amount of CI by the SF group may be partly responsible for the decreased number of weaning piglets. Supportively, Theil et al. (2014) found that sows fed pectin residue containing high insoluble fiber had markedly higher yield (approximately 1.56 kg increase) of colostrum than sows fed potato pulp containing high soluble fiber.
It is not known which specific mechanism may account for the negative effects of maternal SF intake on the survival rate of preweaning piglets. Previous studies have shown that fiber intake at 13% to 23% (16% on average) improved the piglets growth rate and intestinal function associated with the gut microbiome of neonates (Cheng et al., 2018; Li et al., 2019). In this study, however, the excessive intake of soluble fiber may not be positive on the intestinal function of the offspring. Vestergaard and Danielsen (1998) reported that both mean piglet weight and total mean litter weight at birth markedly decreased by receiving 500 g sugar beet pulp per kilogram diet. Likewise, long-term consumption of fermentable fiber leads to dysbiosis and hepatocellular carcinoma in a mice model (Singh et al., 2018).
Immunology
The markedly changed physiology occurs around parturition, such as inflammatory response, changes of endocrine and metabolism (Quesnel et al., 2009), and transferring of maternal IgG to sow colostrum (Feyera et al., 2018). High fiber intake has been shown to influence immunology by changing intestinal microbiota or fermentation metabolites (Maslowski et al., 2009). In this study, maternal high ISF or SF intake increased IgG, but decreased C-RP concentration of plasma around parturition, indicating that farrowing stress may be alleviated by high fiber intake. As a biomarker for inflammation, the lower systematic level of C-RP in sows with high fiber intake suggests that inflammatory response was alleviated, which has been shown to influence colostrum composition (Aring et al., 1999). Consistently, systematic levels of IgG were higher when sows had higher fiber intake (Kim et al., 2016), and the immune-regulatory effect of dietary fiber has been mainly ascribed to its fermentation metabolites-SCFA, which would induce maturation of B cells, through upregulating genes for promoting the differentiation of naive B cells into B cells (Kim et al., 2014; Kim and Chang, 2017). Moreover, SCFA has been shown to enhance antibody production by activating cell-surface receptors (GPR41, GPR43, and GPR109A), histone deacetylase inhibition, and metabolic integration (Licciardi et al., 2011; Ganapathy et al., 2013).
Redox status
The increased demands of energy and oxygen for the placenta of sows in late gestation lead to excessive oxidative stress, which impairs the litter performance of sows (Mueller et al., 2005). As a marker for oxidative stress, MDA is resulting from lipid peroxidation of polyunsaturated fatty acids (Che et al., 2019), and the lower MDA concentration may indicate relatively less energy supply from fatty acids, but more energy from GLU was available during farrowing. In line with Wang et al. (2016), in this study, maternal high fiber intake decreased the MDA level of plasma on day 107 of gestation or parturition and increased GSH-Px activity on day 107 of gestation, supporting the antioxidant effect of high fiber intake in sows around parturition.
Conclusions
In this study, high ISF or high SF intake in late gestation did not markedly influence litter performance, but increased fat concentration of colostrum and milk and improved immune function and redox status around parturition. Unexpectedly, however, piglets from sows fed high SF diet had higher preweaning mortality in lactation and the underlying mechanism needs further research.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (2018YDF05001002), Overseas Expertise Introduction Project for Discipline Innovation (111 Project), and the National Natural Science Foundation of China (31872372).
Glossary
Abbreviations
- ADFI
average daily feed intake
- ADG
average daily gain
- ALB
albumin
- CAT
catalase
- CI
colostrum intake
- C-RP
C-reactive protein
- GLU
glucose
- GSH-Px
glutathione peroxidase
- LAC
lactose
- LBW
low birth weight
- MDA
malondialdehyde
- SCFA
short-chain fatty acid
- TDF
total dietary fiber
- T-SOD
total superoxide dismutase
Conflict of interest statement
There is no conflict of interest for any of the authors of this manuscript.
Literature Cited
- Aring F, Ouml S B, Ttcher M, Jenmalm M C, Garofalo R P, Ouml B, Eacute R, and Bengt N. . 1999. Cytokines in breast milk from allergic and nonallergic mothers. Int. Arch. Allergy Imm. 118:319–320. doi: 10.1159/000024117 [DOI] [PubMed] [Google Scholar]
- Berchieri-Ronchi C B, Kim S W, Zhao Y, Correa C R, Yeum K J, and Ferreira A L. . 2011. Oxidative stress status of highly prolific sows during gestation and lactation. Animal 5:1774–1779. doi: 10.1017/S1751731111000772 [DOI] [PubMed] [Google Scholar]
- Che L, Feng D, Wu D, Fang Z, Lin Y, and Yan T. . 2011. Effect of dietary fibre on reproductive performance of sows during the first two parities. Reprod. Domest. Anim. 46:1061–1066. doi: 10.1111/j.1439-0531.2011.01787.x [DOI] [PubMed] [Google Scholar]
- Che L, Hu L, Wu C, Xu Q, Zhou Q, Peng X, Fang Z, Lin Y, Xu S, Feng B, . et al. 2019. Effects of increased energy and amino acid intake in late gestation on reproductive performance, milk composition, metabolic, and redox status of sows1. J. Anim. Sci. 97:2914–2926. doi: 10.1093/jas/skz149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Hongkui W, Chuanhui X, Xiaowei X, Siwen J, and Jian P. . 2018. Maternal soluble fiber diet during pregnancy changes the intestinal microbiota, improves growth performance, and reduces intestinal permeability in piglets. Appl. Environ. Microb. 84:1018–1047. doi: 10.1128/AEM.01047-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devillers N, Le Dividich J, and Prunier A. . 2011. Influence of colostrum intake on piglet survival and immunity. Animal 5:1605–1612. doi: 10.1017/S175173111100067X [DOI] [PubMed] [Google Scholar]
- Durrant B S, Eisen E J, and Ulberg L C. . 1980. Ovulation rate, embryo survival and ovarian sensitivity to gonadotrophins in mice selected for litter size and body weight. J. Reprod. Fertil. 59:329–339. doi: 10.1530/jrf.0.0590329 [DOI] [PubMed] [Google Scholar]
- Dyck G W, and Swierstra E E. . 1987. Causes of piglet death from birth to weaning. Can. J. Anim. Sci. 67:543–547. doi: 10.4141/cjas87-053 [DOI] [Google Scholar]
- Feyera T, Højgaard C K, Vinther J, Bruun T S, and Theil P K. . 2017. Dietary supplement rich in fiber fed to late gestating sows during transition reduces rate of stillborn piglets. J. Anim. Sci. 95:5430–5438. doi: 10.2527/jas2017.2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyera T, Pedersen T F, Krogh U, Foldager L, and Theil P K. . 2018. Impact of sow energy status during farrowing on farrowing kinetics, frequency of stillborn piglets, and farrowing assistance. J. Anim. Sci. 96:2320–2331. doi: 10.1093/jas/sky141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganapathy V, Thangaraju M, Prasad P D, Martin P M, and Singh N. . 2013. Transporters and receptors for short-chain fatty acids as the molecular link between colonic bacteria and the host. Curr. Opin. Pharmacol. 13:869–874. doi: 10.1016/j.coph.2013.08.006 [DOI] [PubMed] [Google Scholar]
- Guillemet R, Hamard A, Quesnel H, Père M C, Etienne M, Dourmad J Y, and Meunier-Salaün M C. . 2007. Dietary fibre for gestating sows: effects on parturition progress, behaviour, litter and sow performance. Animal 1:872–880. doi: 10.1017/S1751731107000110 [DOI] [PubMed] [Google Scholar]
- Jha R, and Berrocoso J D. . 2015. Review: Dietary fiber utilization and its effects on physiological functions and gut health of swine. Animal 9:1441–1452. doi: 10.1017/S1751731115000919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, and Chang H K. . 2017. Regulation of humoral immunity by gut microbial products. Gut Microbes. 8:1–8. doi: 10.1080/19490976.2017.1299311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C H, Park J, and Kim M. . 2014. Gut microbiota-derived short-chain fatty acids, T cells, and inflammation. Immune Netw. 14:277–288. doi: 10.4110/in.2014.14.6.277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Qie Y, Park J, and Kim C H. . 2016. Gut microbial metabolites fuel host antibody responses. Cell Host Microbe. 20:202–214. doi: 10.1016/j.chom.2016.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh U, Bruun T S, Amdi C, Flummer C, Poulsen J, and Theil P K. . 2015. Colostrum production in sows fed different sources of fiber and fat during late gestation. Can. J. Anim. Sci. 95:211–223. doi: 10.4141/CJAS-2014-060 [DOI] [Google Scholar]
- Krogh U, Bruun T S, Poulsen J, and Theil P K. . 2017. Impact of fat source and dietary fibers on feed intake, plasma metabolites, litter gain and the yield and composition of milk in sows. Animal 11:975–983. doi: 10.1017/S1751731116002585 [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang L, Liu, Y. Yang, J. He, M. Cao, M. Yang, W. Zhong, Y. Lin, Y. Zhuo H, . et al. 2019. Effects of the ratio of insoluble fiber to soluble fiber in gestation diets on sow performance and offspring intestinal development. Animals. 9:422. doi: 10.3390/ani9070422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licciardi P V, Ververis K, and Karagiannis T C. . 2011. Histone deacetylase inhibition and dietary short-chain fatty acids. ISRN Allergy 2011:869647. doi: 10.5402/2011/869647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loisel F, Farmer C, Ramaekers P, and Quesnel H. . 2013. Effects of high fiber intake during late pregnancy on sow physiology, colostrum production, and piglet performance. J. Anim. Sci. 91:5269–5279. doi: 10.2527/jas.2013-6526 [DOI] [PubMed] [Google Scholar]
- Maslowski K M, Vieira A T, Ng A, Kranich J, Sierro F, Yu D, Schilter H C, Rolph M S, Mackay F, Artis D, . et al. 2009. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461:1282–1286. doi: 10.1038/nature08530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael B. 2015. Gut microbiota and energy balance: role in obesity. Proc. Nutr. Soc. 74:227–234. doi: 10.1017/S0029665114001700 [DOI] [PubMed] [Google Scholar]
- Mueller A, Koebnick C, Binder H, Hoffmann I, Schild R L, Beckmann M W, and Dittrich R. . 2005. Placental defence is considered sufficient to control lipid peroxidation in pregnancy. Med. Hypotheses 64:553–557. doi: 10.1016/j.mehy.2004.08.008 [DOI] [PubMed] [Google Scholar]
- Newbern D, and Freemark M. . 2011. Placental hormones and the control of maternal metabolism and fetal growth. Curr. Opin. Endocrinol. Diabetes Obes. 18:409–416. doi: 10.1097/MED.0b013e32834c800d [DOI] [PubMed] [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th ed Washington (DC): National Academy Press. [Google Scholar]
- Pan Z, Yang Z, Pan Z, Yan L, Taotao G, Jun W, Chao J, Lianqiang C, Jian L, and Yan L. . 2017. Microbial mechanistic insight into the role of inulin in improving maternal health in a pregnant sow model. Front. Microbiol. 8:2242. doi: 10.3389/fmicb.2017.02242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prater M R, Laudermilch C L, Liang C, and Holladay S D. . 2008. Placental oxidative stress alters expression of murine osteogenic genes and impairs fetal skeletal formation. Placenta 29:802–808. doi: 10.1016/j.placenta.2008.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesnel H, Farmer C, and Devillers N. . 2012. Colostrum intake: influence on piglet performance and factors of variation. Livest. Sci. 146:105–114. doi: 10.1016/j.livsci.2012.03.010 [DOI] [Google Scholar]
- Quesnel H, Meunier-Salaün M C, Hamard A, Guillemet R, Etienne M, Farmer C, Dourmad J Y, and Père M C. . 2009. Dietary fiber for pregnant sows: influence on sow physiology and performance during lactation. J. Anim. Sci. 87:532–543. doi: 10.2527/jas.2008-1231 [DOI] [PubMed] [Google Scholar]
- Singh V, Yeoh B S, Chassaing B, Xiao X, Saha P, Aguilera Olvera R, Lapek J D Jr, Zhang L, Wang W B, Hao S, . et al. 2018. Dysregulated microbial fermentation of soluble fiber induces cholestatic liver cancer. Cell 175:679–694.e22. doi: 10.1016/j.cell.2018.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H Q, Zhou Y F, Tan C Q, Zheng L F, Peng J, and Jiang S W. . 2014. Effects of konjac flour inclusion in gestation diets on the nutrient digestibility, lactation feed intake and reproductive performance of sows. Animal 8:1089–1094. doi: 10.1017/S175173111400113X [DOI] [PubMed] [Google Scholar]
- Tan C Q, Sun H Q, Wei H K, Tan J J, Long G, Jiang S W, and Peng J. . 2018. Effects of soluble fiber inclusion in gestation diets with varying fermentation characteristics on lactational feed intake of sows over two successive parities. Animal 12:1388–1395. doi: 10.1017/S1751731117003019 [DOI] [PubMed] [Google Scholar]
- Theil P K, Flummer C, Hurley W L, Kristensen N B, Labouriau R L, and Sørensen M T. . 2014. Mechanistic model to predict colostrum intake based on deuterium oxide dilution technique data and impact of gestation and prefarrowing diets on piglet intake and sow yield of colostrum. J. Anim. Sci. 92:5507–5519. doi: 10.2527/jas.2014-7841 [DOI] [PubMed] [Google Scholar]
- Vallet J L, McNeel A K, Miles J R, and Freking B A. . 2014. Placental accommodations for transport and metabolism during intra-uterine crowding in pigs. J. Anim. Sci. Biotechnol. 6:163–176. doi: 10.1186/2049-1891-5-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard E M, and Danielsen V. . 1998. Dietary fibre for sows: effects of large amounts of soluble and insoluble fibres in the pregnancy period on the performance of sows during three reproductive cycles. Anim. Sci. 67:355–362. doi: 10.1017/S1357729800010134 [DOI] [Google Scholar]
- Veum T L, Crenshaw J D, Crenshaw T D, Cromwell G L, and Ellersieck M R. . 2009. The addition of ground wheat straw as a fiber source in the gestation diet of sows and the effect on sow and litter performance for three successive parities. J. Anim. Sci. 87:1003–1012. doi: 10.2527/jas.2008-1119 [DOI] [PubMed] [Google Scholar]
- Wang J, Feng C, Liu T, Shi M, Wu G, and Bazer F W. . 2017. Physiological alterations associated with intrauterine growth restriction in fetal pigs: causes and insights for nutritional optimization. Mol. Reprod. Dev. 84:897–904. doi: 10.1002/mrd.22842 [DOI] [PubMed] [Google Scholar]
- Wang Y S, Zhou P, Liu H, Li S, Zhao Y, Deng K, Cao D D, Che L Q, Fang Z F, Xu S Y, . et al. 2016. Effects of inulin supplementation in low- or high-fat diets on reproductive performance of sows and antioxidant defence capacity in sows and offspring. Reprod. Domest. Anim. 51:492–500. doi: 10.1111/rda.12707 [DOI] [PubMed] [Google Scholar]