Abstract
Lupus nephritis (LN) is a frequent and severe manifestation of SLE. Along the decades, the epidemiology of LN and its clinical presentation have been changing. However, even though retrospective cohort studies report a decreased mortality rate and an improvement in the disease prognosis, the percentage of patients progressing into end stage renal disease (ESRD) keeps steady despite the improvements in therapeutic strategies. Current in-use medications have been available for decades now, yet over the years, regimens for optimizing their efficacy and minimizing toxicity have been developed. Therapeutic research is now moving towards the direction of precision medicine and several new drugs, targeting selectively different pathogenetic pathways, are currently under evaluation with promising results. In this review, we address the main changes and persistent unmet needs in LN management throughout the past decades, with a focus on prognosis and upcoming treatments.
Keywords: lupus nephritis, renal biopsy, classification, risk factors, prognosis, B cells, calcineurin inhibitors
Rheumatology key messages
LN is still a risk factor for chronic kidney injury and end-stage renal disease in SLE.
Kidney biopsy is fundamental to characterize the renal involvement and define the patient prognosis.
New target therapies are under evaluation and may provide effective alternatives to currently available treatments.
Introduction
The kidney is often affected in SLE and the impairment of renal function results from glomerular, tubule-interstitial and vascular lesions [1]. LN occurs in about 40% of SLE patients [2], mostly within 5 years from the diagnosis, and still presents a rate of progression to end stage renal disease (ESRD) of 4.3–10.1% [3]. Renal failure, along with infections, cancer and cardiovascular events, is one of the most common causes of death in SLE patients [4]. The incidence of LN varies with ethnicity [5] and the spectrum of clinical presentation ranges from silent urinary abnormalities to highly symptomatic cases of nephritic syndrome or rapidly progressive renal insufficiency [6].
Overview on clinical manifestations
Clinical presentation may be silent with urinalysis, renal function and 24 h-proteinuria within the normal range [7]. Otherwise, LN can be characterized by urinary abnormalities, e.g. haematuria, leukocyturia, cellular casts and mild proteinuria or more overt presentations including nephrotic syndrome or acute nephritic syndrome or rapidly progressive renal failure [8].
An Italian study evaluating a cohort of 499 LN patients from 1970 to 2016 recently reported significant changes in demographic distribution, clinical presentation and laboratory abnormalities in LN during the past 45 years [9]. The number of male patients and the age at LN diagnosis progressively increased along the decades with concomitant evidence of later onset of LN during the course of SLE. Over the years, clinical presentation became milder with a significant decrease in nephritic syndrome and rapidly progressive forms, a decrease in serum creatinine values and an increase in isolated urinary abnormalities, likely due to an earlier SLE diagnosis and to the identification of LN as soon as it appears during the follow-up [9], thereby highlighting the importance of a regular assessment of renal function, 24 h-proteinuria and urinary sediment in all SLE patients.
The role of kidney biopsy
The definition of renal involvement in SLE gained particular importance in the most recent sets of SLE classification criteria, where histology, together with a consistent SLE serology, is sufficient for disease classification [10, 11]. Nevertheless, the role of kidney biopsy has been questioned as most forms of LN can adequately be treated with glucocorticoids (GC) plus mycophenolate (MMF) [12]. However, because of the lack of univocal correlation between clinical presentation and histological abnormalities, renal biopsy remains fundamental in the evaluation and management of LN [13, 14]. It allows differentiation into pathological classes, the definition of the severity of renal involvement in terms of active and chronic lesions [15–17] and the identification of other rare non-LN conditions such as anti-phospholipid antibody-associated nephropathy, IgA nephropathy, thrombotic microangiopathies, drug-induced tubulo-interstitial nephritis, diabetes nephropathy or hypertensive nephroangiosclerosis [18]. A comprehensive assessment of renal biopsy may therefore guide clinicians in the choice of the more appropriate therapeutic strategy [13, 19, 20]. A threshold of proteinuria to perform a renal biopsy has not yet been defined, but when it stably reaches or overcomes the level of 500 mg/24 h or spot urine protein to creatinine ratio (UPCR) >500 mg/g (50 mg/mmol), especially with impaired renal function or active urinary sediment, it is reasonable to perform invasive investigations [17, 21]. Biopsy can also be considered in the presence of persistent haematuria or pyuria after exclusion of other potential causes or in case of unexplained renal insufficiency with normal urinalysis [15, 22].
An adequate biopsy sample should include at least 10 glomeruli and the analysis be performed by light microscopy (LM), immunofluorescence (IF) and electron microscopy (EM) when available [23].
With the exception of cases with severe activity and chronicity indexes [9], the prognostic value of the basal kidney biopsy on long-term outcome is quite modest, while a second biopsy could be more informative. Nevertheless, no standardized protocols have been defined so far [24] and re-biopsy is not part of routine care. According to international recommendations [17], a second biopsy can be considered in the presence of renal flare, unresponsiveness to treatment, worsening of renal function, persistent proteinuria or haematuria or to exclude an alternative diagnosis [25]. Recent small LN studies suggest it may be highly informative also in patients in complete clinical renal response to stratify the risk of relapse and to guide immunosuppression withdrawal [26]. Despite a clinical response to treatment, some patients still maintain mild histological signs of activity on repeated kidney biopsy [26–28] which can lead to irreversible nephron loss [29].
International Society of Nephrology/Renal Pathology Society (ISN/RPS) 2003 classification of lupus nephritis and 2018 revision
The last published classification of LN (Table 1) aimed at standardizing definitions and reducing interobserver variability. Six histological classes are defined by specific microscopic lesions and distribution of immune complexes (IC) [30]. Class I LN (Minimal mesangial) has a prevalence of 1% in adults [32] and 2.3% in children [33] and represents <20% of all cases of nephrotic syndrome that undergo renal biopsy [34]. Class II LN (Mesangial proliferative) accounts for 7–22% of all cases [35–37] and it generally presents with isolated haematuria, low-grade proteinuria and normal renal function. It is considered mild but it is associated with the risk of further progression to focal or diffuse LN [38, 39], with a cumulative incidence rate between 14.8% and 47.4% [40]. Class III and IV LN (Focal and Diffuse LN) bear the most severe prognosis and require prompt immunosuppressive treatment. A metanalysis evaluating the prevalence of biopsy-proven LN [37] identified class IV as the most prevalent and the one associated with the highest risk of progression to ESRD. A total of 15–30% of patents with class IV do not reach remission and 15–30% of those reaching remission will develop a relapse. Class V (Membranous) is characterized by IC deposits, occurring mostly in the subepithelium, and frequent podocyte loss. Pure Class V clinically presents with nephrotic or non-nephrotic proteinuria and normal or only slightly elevated serum creatinine [41] and can be often found in association with proliferative forms. It carries a relatively low rate of progression to ESRD but it is accompanied by a high rate of complications secondary to the nephrotic syndrome such as hypoalbuminemia, thrombophilia, hyperlipidemia and infections [42]. Class VI (Advanced sclerosing) is defined by 90% of sclerotic glomeruli, often resulting in impaired renal function and variable amount of proteinuria.
Table 1.
| Class | Nomenclature | Lesions description according to 2003 ISN/RPS | 2018 Revision |
|---|---|---|---|
| Class I | Minimal mesangial LN | Normal appearance of the glomeruli on LM, mesangial IC deposits on IF and fusion or effacement of podocyte on EM. |
|
| Class II | Mesangial proliferative LN | Pure mesangial hypercellularity or mesangial matrix expansion with mesangial IC deposits. Absence of subepithelial or subendothelial IC deposits visible on LM. |
Mesangial hypercellularity: ≥4 nuclei fully surrounded by matrix in the mesangial area not including the hilar region. |
| Class III | Focal LN [50% of the glomeruli involved] | Segmental or global extracapillary or endocapillary proliferative lesions [hypercellularity]b or inactive glomerular scars with focal subendothelial IC deposits with or without mesangial alterations. Such lesions involve <50% of the glomeruli. |
|
| Class IV | Diffuse LN [50% of the glomeruli involved] Diffuse segmental LNc Diffuse global LN |
|
|
| Class V | Membranous LN | Continuous or granular subepithelial IC deposits with or without mesangial alterations and IC deposits |
|
| Class VI | Advanced sclerosing LN [90% glomeruli involved] | Globally sclerosed glomeruli without residual activity |
|
| Tubulointerstitial lesions: indicate whether interstitial inflammation occurs in presence or absence of interstitial fibrosis. | |||
| Podocytopathy: glomerular changes consistent with minimal change disease, mesangial proliferation or focal segmental glomerulosclerosis on LM and podocyte effacement on EM, with or without mesangial IC deposits. No evidence of IC deposition in peripheral glomerular capillaries or endocapillary proliferation. |
see Table 2. bThe term ‘proliferative’ has been substituted by hypercellularity in the 2018 revision. cDifferentiation among diffuse segmental and diffuse global has been eliminated in the 2018 revision. EM: electron microscopy; IC: immune complex; IF: immune fluorescence; LN: lupus nephritis; LM: light microscopy.
Over the last 45 years, the prevalence of the histological classes has remained stable with class IV accounting for 50%, class III for 25% and class V for 20%. Nevertheless, an increase of mixed forms (III+IV and IV+V) has been reported along the years, probably reflecting the classification updating process [9]. Unchanged activity index over time was consistent with histological classes stability, while a progressive reduction of chronicity was demonstrated [9].
The last revision of the current classification, published in 2018 but not endorsed yet [31], proposes a series of adjustments based on evidence and consensus opinions. It submits the introduction of a semiquantitative activity and chronicity score for class III e IV (Table 2), cancels the distinction between predominantly segmental and predominantly global lesions in class IV as no relevant outcome difference was reported [43], highlights the importance of necrotizing lesions and provides more precise definitions of histologic findings (mesangial hypercellularity, cellular, fibrocellular and fibrous crescents) [44]. Another key point is the proposal to introduce a sub-classification of different vascular and tubule-interstitial abnormalities on the basis of their pathogenetic mechanism.
Table 2.
Proposed modified National Institute of Health (NIH) lupus nephritis activity and chronicity scoring system [31]
| Index type | Definition | Score |
|---|---|---|
| Modified NHI activity index | ||
| Endocapillary hypercellularity | Endocapillary hypercellularity in <25% (1+), 25%–50% (2+), or >50% (3+) of glomeruli | 0–3 |
| Neutrophils/karyorrhexis | Neutrophils and/or karyorrhexis in <25% (1+), 25%–50% (2+), or >50% (3+) of glomeruli | 0–3 |
| Fibrinoid necrosis | Fibrinoid necrosis in <25% (1+), 25%–50% (2+), or >50% (3+) of glomeruli | (0–3) × 2 |
| Hyaline deposits | Wire loop lesions and/or hyaline thrombi in <25% (1+), 25%–50% (2+), or >50% (3+) of glomeruli | 0–3 |
| Cellular/fibrocellular crescents | Cellular and/or fibrocellular crescents in <25% (1+), 25%–50% (2+), or >50% (3+) of glomeruli | (0–3) × 2 |
| Interstitial inflammation | Interstitial leukocytes in <25% (1+), 25%–50% (2+), or >50% (3+) in the cortex | 0–3 |
|
Tot |
0–24 | |
| Modified NHI chronicity index | ||
| Total glomerulosclerosis score | Global and/or segmental sclerosis in <25% (1+), 25%–50% (2+), or >50% (3+) of glomeruli | 0–3 |
| Fibrous crescents | Fibrous crescents in <25% (1+), 25%–50% (2+), or >50% (3+) of glomeruli | 0–3 |
| Tubular atrophy | Tubular atrophy in <25% (1+), 25%–50% (2+), or >50% (3+) of the cortical tubules | 0–3 |
| Interstitial fibrosis | Interstitial fibrosis in <25% (1+), 25%–50% (2+), or >50% (3+) in the cortex | 0–3 |
|
Tot |
0–12 | |
Growing interest also focuses on podocytopathy, defined on EM by extensive effacement of podocyte foot processes in association with specific histological features at LM (Table 1), so that it has been proposed as a distinct subtype of LN [45–47]. It clinically presents with nephrotic syndrome and may associate to a higher risk of developing chronic kidney disease (CKD) and severe tubulointerstitial injury when coupled with focal segmental glomerulosclerosis at LM [47]. The degree and type (functional or structural) of podocyte damage could correlate with the severity of proteinuria [48] and podocytopathy may represent an extreme form of podocyte alteration [49].
Standard of care (SoC)
The improvement in survival over the past decades among LN patients was mainly due to the introduction of effective and less toxic drugs and more tolerated regimens. GC monotherapy, especially in the form of intravenous (i.v.) pulses, was implemented after 1980 with the association of immunosuppressants such as azathioprine (AZA) or CYC [9, 50, 51]. The concept of maintenance therapy has established beside induction, becoming part of the clinical practice. In the past decade, the use of AZA for induction and maintenance has decreased in favour of MMF [9], which has become a mainstay in the treatment as it was shown to be as efficacious but less toxic than CYC in the induction phase [52, 53] and more effective than AZA during maintenance for flare prevention [9, 54]. At the turn of the new millennium, in view of optimizing efficacy and reducing side effects, the low-dose i.v. CYC induction protocol was approved for the treatment of LN as it was demonstrated to be safer and equally effective compared with the high-dose regimen hitherto in use [55].
According to current recommendations, the therapeutic strategy for LN aims to achieve rapid remission or at least partial response within 6–12 months (discussed below), to prevent flares and preserve renal function, reduce morbidity and mortality and preserve fertility [17, 56]. The choice of the treatment mainly depends on the histological class, on activity and chronicity indexes and includes immunosuppressants, adjuvants and symptomatic drugs.
For Class II, current recommendations do not suggest immunosuppression [15, 17], while previous recommendations indicated low-to-moderate dose of GC alone or in combination with AZA if proteinuria >1 g/24 h, especially in the presence of glomerular haematuria [22].
For class III and IV, there is unanimous agreement on an induction treatment based on high dose pulses of methyl-prednisolone followed by oral GC and MMF or i.v. CYC [17].
During the past 10–15 years, the efficacy and safety of Tacrolimus (Tac) + GC and Tac + MMF + GC vs CYC + GC and MMF + GC was evaluated, mostly in Chinese patients, as induction and maintenance therapy, demonstrating a good response in terms of remission and normalization of serological abnormalities [57–60]. Calcineurin inhibitors (CNI), already mentioned in the 2012 EULAR and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for LN [22], have a more prominent spot in the 2019 update as they demonstrated a favourable efficacy/toxicity profile especially in patients with nephrotic range proteinuria [61] and could be a valid option in combination with MMF for induction as multitarget therapy [17]. In non-responding or refractory patients, it is advised to switch to another first-line treatment including MMF, CYC or CNI as monotherapy or multitarget therapy. An alternative option for refractory disease [62, 63], even though not approved for LN due to its failure in randomized control trials (RCTs) [64, 65] is rituximab (RTX), a chimeric anti-CD20 monoclonal antibody. Its use is suggested by EULAR and ACR recommendations [15, 17] and it is prescribed off-label in clinical practice as a rescue treatment in a remarkable proportion of SLE and LN patients [65–69]. A recent meta-analysis assessing the clinical efficacy and safety of RTX in the treatment of LN [62] reported a significant decrease in renal disease activity and proteinuria as well as efficacy in preventing organ damage. Moreover, RTX may help GC tapering [70], potentially reducing treatment complications.
Maintenance treatment is based on oral GC plus MMF or AZA for at least 3–5 years after complete remission. Notably, the 2019 updated LN recommendations state that, after this timespan, a gradual tapering of GC and then of immunosuppressants should be attempted, keeping a tight follow-up during de-escalation [17, 71, 72].
For Class V LN, immunosuppressive treatment is indicated in patients with nephrotic syndrome and in those with proteinuria >1 g/day despite renin-angiotensin-aldosterone system therapy in order to reduce proteinuria and avoid nephron loss, with a particular endorsement for CNI which stabilize podocyte structure and function [73, 74]; MMF or i.v. CYC could be equally valid alternatives [17]. Additionally, symptomatic therapy with inhibitors of renin-angiotensin-aldosterone system is also recommended [17, 75]. Importantly, when Class V is combined with class III or IV it should be treated as class III and IV.
Histologic class VI usually requires preparation for renal replacement therapy [15, 17, 22].
In LN, HCQ in addition to the conventional immunosuppressants was shown to retard damage accrual, increase the probability of renal remission [76] and prevent renal flares in particular at a target plasma level of 0.6 mg/l [77]. If given prior to LN development, HCQ is associated with a lower risk of renal failure and death [78]. The use of HCQ is emphasized in 2012 ACR guidelines for LN and 2019 update of the EULAR recommendations for the management of SLE and LN [17, 56] where at a dose ≤5 mg/kg, adjusted in patients with glomerular filtration rate (GFR) <30 ml/min, it is strongly suggested as a background therapy in all patients in absence of contraindications, performing a regular ophthalmologic assessment. HCQ is also allowed and recommended during pregnancy as it reduces flares, thrombosis and the risk of congenital heart block in anti-Ro positive mothers [79]. Pregnancy is a condition that should be properly planned, especially in patients with active LN [17, 22] as renal disease activity at the time of conception is associated with a poor mother and foetus outcome [80]. Gestational planning aims at scheduled withdrawal of those immunosuppressants incompatible with pregnancy and at safe programming of conception during LN remission (GFR and UPCR in the preceding 6 months should be <50 ml/min and <500 mg/g (<50 mg/mmol), respectively). Immunosuppressants allowed during pregnancy and lactation are prednisone, AZA and CNI; acetylsalicylic acid is recommended in active LN to reduce the risk of pre-eclampsia in absence of contraindications [17, 22].
Renal flares, partial remission and complete remission
A complete renal response is defined, according to the 2019 update of EULAR/ERA-EDTA recommendations for LN, by a UPCR <500–700 mg/g (<50–70 mg/mmol) by 12 months with normal or near-normal GFR, while partial renal response is defined by a reduction in proteinuria of at least 25% by 3 months or 50% by 6 months; notably, the reported timeframes should be extended to 6 and 12 moths, respectively, for patients who present with nephrotic-range proteinuria [17]. Although current induction therapy is overall effective for the achievement of a complete or partial remission after 6–12 months, renal flares are quite common with a cumulative reported rate ranging from 27% to 66% [81] and are associated with impaired renal survival [82]. Renal flares are categorized, according to a European consensus statement, into proteinuric and nephritic, the latter in turn subdivided into non-severe and severe [83].
The main factors associated with the risk of renal relapse are young age at onset (<30 years), male sex, African-American ethnicity, delayed treatment initiation, long time to remission, low C4, partial response, high SLE disease activity score, rise in anti-dsDNA titer, arterial hypertension, severe extrarenal SLE (central nervous system involvement) and low-dose immunosuppression [82].
Some studies evaluated the difference of prognosis between patients who underwent partial remission and those who did not achieve any remission status, finding that partial renal remission at 24 months after biopsy, according to the modified Aspreva Lupus Management Study (mALMS) and Belimumab Lupus Nephritis Study (BLISS-LN), is significantly less likely to result in ESRD or mortality in comparison to absence of remission [84]. In a small study involving 86 patients with diffuse LN, the renal survival at 10 years was 94% in patients with complete remission, 43% in case of partial remission and 19% in those with no remission [85]. Similarly, CKD-free survival in a large multicentric cohort did not differ significantly between partial or complete responders while being significantly improved vs non-responders, thereby confirming that any degree of renal response is always advisable [86].
Importantly, time to response is likely to influence renal prognosis. Although some studies reported that proteinuria of 0.7–0.8g/day at 12 months was the individual best predictor of long-term renal outcome in LN [87, 88], it was demonstrated that the optimal cut-off varied based on baseline proteinuria values [89]. In the Hopkins Lupus Cohort, two composite remission scores including serum creatinine and proteinuria performed better in predicting renal survival than proteinuria alone [84]. Similarly, in 550 LN patients, 12-month proteinuria and 12-month serum creatinine were able to predict the risk of developing CKD at three years [90].
Prognosis, survival and outcome trends in patients with LN: past and present
LN plays a major role in defining prognosis and survival of SLE patients. Data form the last 50 years highlight a substantial decrease in mortality rate with a concomitant increase in CKD- and ESRD-free survival at 10 and 20 years. Accordingly, the number of patients achieving a complete renal remission increased from 48.5% in 1970s to 58.5% in the mid-2010s [9]. Prior to the introduction of GC, the 5-year survival rate of patients with LN was 44% but after their routine use in combination with immunosuppressants, it improved to 80% in the 1980s and to >90% now [9, 91, 92]. These results are mainly attributable to the concept of early diagnosis and the use of more effective and early treatments along with the increased knowledge in the management of complications (i.e. infections) and comorbidities [9, 93].
Nevertheless, in the last 10 years trends stabilized both in terms of ESRD and survival rates [91, 94, 95]. In a retrospective study of a cohort of 325 SLE patients followed in Oslo from 1999 to 2008 [96], the incidence rate of ESRD was 2.3 per 1000 patient-years. The cohort presented an overall standardized mortality ratio (SMR) of 2.1 but the SMR significantly differed between patients who did and did not develop LN (SMR 3.8 and 1.7, respectively). The highest estimates were noted in patients aged between 16 and 39 years (SMR 13.4) and with ESRD (SMR 5.4). These data confirm that LN is a major determinant in SLE prognosis and that once ESRD has established, the disease markedly worsens.
Risk factors for progressive renal disease
Demographic risk factors
Renal outcome of patients with LN varies among different ethnic groups, with the best prognosis for Caucasians and the worst for Africans, whereas Asians have an intermediate prognosis [5, 97–99]. Black patients present, along with Hispanic patients, worse outcomes with increased rates of ESRD and mortality [100]. This is probably the result of higher incidence of proliferative diffuse LN, burdened by nephritic syndrome with severe hypertension mediated by a genetic predisposition [101], as well as to limited access to adequate care and lower adherence to treatment [93, 97, 102]. Male gender is another established demographic risk factor for worse renal outcome [103, 104].
Clinical risk factors
To date, the main clinical risk factors for the development of CKD are baseline hypertension and poor control of cardiovascular risk factors during the follow-up; nephrotic range proteinuria, young age, anaemia and elevated serum creatinine at the time of biopsy [9, 99, 105]; an inadequate immunosuppressive treatment at diagnosis that could lead to a lack of a complete renal remission and to repeated nephritic flares [9, 82, 106].
A multicentric study on 381 LN patients has recently shown that lack of EULAR/ERA-EDTA complete or partial renal response at 12 months [22] and uncontrolled hypertension independently predicted CKD development [86].
The aforementioned risk factors, along with the histological class, are also the main contributors to the development of ESRD [9, 35].
Histopathological risk factors
A well-recognized association links histopathological findings on kidney biopsy to the clinical course of LN, with mesangial nephritis (class II) carrying the best renal prognosis while proliferative nephritis (class III and IV) presenting with a more aggressive course and deterioration of renal function [9]. Membranous (class V) nephritis has long been considered mild; however, it may lead to severe protein loss, nephrotic syndrome, prolonged hospitalization and eventually chronic renal damage [107].
High activity and chronicity indexes are independent predictors of CKD and ESRD [9]. Considering histopathological changes individually, cellular crescents (active injury) and interstitial fibrosis (chronic damage) in the initial renal biopsy bear the highest predictive value [108, 109]. Extracapillary proliferation at repeated renal biopsy is an even stronger predictor of renal function deterioration, therefore requiring an aggressive treatment [24]. Alterations of the tubulointerstitium including inflammatory infiltrate and tubular atrophy appear to also be predictors of poor prognosis, independent on class [110, 111].
The histological analysis of kidney biopsy of LN patients over the last five decades showed significant decrease in chronicity indexes, probably due to a tighter surveillance of renal function in SLE patients, likely contributing to the improvement of overall renal survival [9].
It should be mentioned, however, that some limitations in the definition of the risk factors still remain, in particular owing to the intra- and inter-observer variability in the evaluation of the biopsy specimens, the absence of a validated cut-off point for active and chronic lesions to predict renal failure or mortality and the fluctuating course of renal involvement with a possible reversibility of some histological features [112].
What’s in the pipeline?
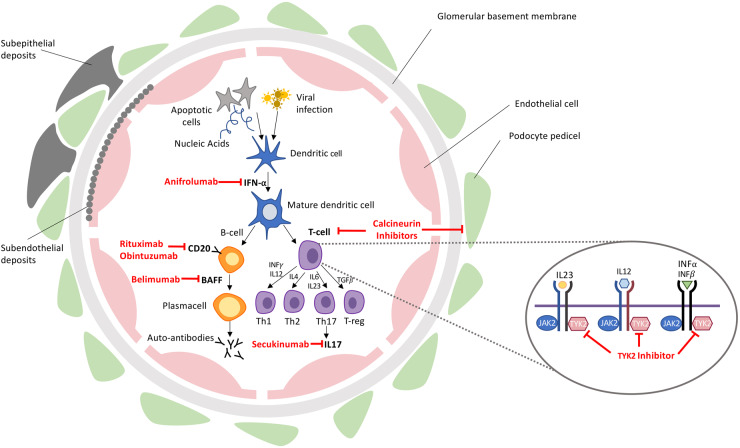
To better stratify patients and overcome the limits of current therapies, the research is now focusing on the comprehension of pathogenetic mechanisms underlying LN. Therefore, single-cell analysis, biomarker research, characterization of the role and function of autoantibodies and genetic and epigenetic profiling are providing essential information [1, 113–115]. Thanks to these achievements, further advances, increasingly oriented towards precision medicine, are going to enrich the available therapeutic armamentarium [116, 117] (Table 3) (Fig. 1).
Table 3.
Ongoing and recently completed clinical trials of new therapies for LN
| Name of the trial | Drug tested | Therapeutic target | Phase of the trial | Number of enrolled patients | Primary outcome | Ref |
|---|---|---|---|---|---|---|
| AURA-LV (completed) | Voclosporin | T-cell | II | 265 | Number of subjects achieving complete remission at 24 weeks | [118] |
| AURORA (completed) | III | 358 | Number of subjects achieving renal response within a time frame of 52 weeks | [119] | ||
| AURORA 2 extension study (completed) | III | 227 | Adverse event profile and routine biochemical and haematological assessment within a time frame of 36 months | [120] | ||
| BLISS-LN | Belimumab | BAFF | III | 448 | Number of participants with primary efficacy renal response at week 104 | [121] |
| CALIBRATE (completed) | Rituximab + Belimumab | CD20, BAFF | II | 43 | Proportion of patients with a SLEDAI-2K score of <2 without the use of additional immunosuppression | [122] |
| NCT04221477 | Obinutuzumab | CD20 | III | 250 | Percentage of patients with complete renal response at week 76 | [123] |
| NCT02550652 | II | 126 | Percentage of patients with complete renal response at week 52 | [124] | ||
| TULIP-LN1 | Anifrolumab | IFN-I receptor | II | 146 | Relative change from baseline in 24-hour urine protein to creatinine within a time frame of 1–52 weeks | [125] |
| NCT03943147 | TYK2-inhibitor | TYK2 | II | 78 | Incidence of adverse events, incidence of laboratory abnormalities, partial renal response | [126] |
| SELUNE | Secukinumab | IL-17 | III | 460 | Proportion of subjects achieving complete renal response | [127] |
BAFF: B-cell activating factor; CD: cluster of differentiation; IL: interleukin; Ref, reference; TYK2: tyrosine kinase 2.
Fig. 1.
Pathogenetic targets of new therapeutic strategies
BAFF, B-cell activating factor; IL, interleukine; TYK2, tyrosine kinase 2; JAK 2, Janus kinase 2; Th, T-helper.
The AURA-LV study [118, 128] evaluated the efficacy of high-dose and low-dose voclosporin + MMF vs MMF alone in inducing complete remission at 24 weeks, reaching its primary end point and showing a relevant steroid sparing effect. Importantly, steroid tapering in the AURA trial was dramatically fast, reaching 2.5 mg/day at 16 weeks. This not only did not affect the renal outcome of participants, but rendered apparent the incremental benefit of voclosporin [72]. Two other phase III trials are further evaluating effectiveness and long-term safety and tolerability of voclosporin [119, 120].
Among monoclonal antibodies, belimumab, a fully human IgG1-lambda antibody against B-lymphocyte stimulator (BLyS), is the only biologic drug so far approved for SLE patients with active disease despite SoC. The positive results of a phase III RCT in LN involving 448 patients [121] have recently been announced. Comparing patients receiving belimumab plus SoC to those taking placebo plus SoC, a significantly higher proportion of patients in the belimumab arm achieved the primary end point, i.e. renal response at 104 weeks, and some relevant secondary end points. These positive results were expected on the basis of the outcomes of real-life observational studies where belimumab was effective in the decrease of proteinuria levels and number of renal flares [129, 130].
Another potential option under evaluation is the sequential treatment with RTX followed by belimumab [122, 131]. It is based on the assumption that BLyS levels increase following RTX administration as a feedback mechanism; hence, a sequential therapy should drive to a depletion of CD20 expressing B cell and then avoid the rebounded B cell enhanced repopulation [132, 133]. The co-administration of the two drugs may also have a synergic effect [134].
Among biologic drugs tested for LN, obinutuzumab [123, 124] is a type II anti-CD20 monoclonal antibody that differs from RTX because it enhances CD20-expressing B-cell depletion via antibody-dependent cell-mediated cytotoxicity and programmed cell death, instead of complement-mediated cytotoxicity. Positive results of a phase II trial for the treatment of proliferative LN aiming at complete or partial renal response at week 52 have been presented at the 2019 ACR meeting in Atlanta [135,136].
Given the role of type I IFN in the pathogenesis of SLE, anifrolumab, a fully human monoclonal antibody that blocks type I IFN response through binding to type I IFN receptor, is currently under evaluation. Phase III RCT TULIP-1 was proven effective in decreasing disease activity in non-renal SLE, especially in patients with an enhanced IFN gene signature [136], and a phase II RCT is ongoing in LN [125].
Additionally, JAK inhibition is underway in SLE, culminating in down-regulation of inflammatory cytokines-driven pathways and IFN-dependent genes. Despite an overall good safety profile, JAK inhibitors have been burdened by signals of reactivation of opportunistic infections, probably due to their broad inhibitory potential [137]. In this regard, a study started in July 2019 [126] is testing safety and effectiveness of a selective Tyk-2 inhibitor in LN. Notably, the molecule stabilizes a regulatory pseudokinase domain of Tyk-2 without affecting its catalytic activity and thereby more selectively blocking the IL-12/23 and type I IFN pathways, likely raising fewer safety issues.
Concerning the selective inhibitors of interleukins, recent studies began to shed light on the role of IL-17 in the pathogenesis of SLE and LN [138–140] and a phase III RCT has just started to evaluate efficacy and safety of subcutaneous 300 mg secukinumab compared with placebo in combination of SoC therapy in active proliferative LN [127].
Conclusions
Despite important advances, LN is still a serious risk factor for the development of ESRD and for early mortality and disability in SLE. A proper management of LN by an expert dedicated team should lead to a preserved renal function in the long term, but requires an early recognition and evaluation through renal biopsy, followed by the optimized use of available treatments. Minimization/withdrawal of GC treatment is endorsed by the updated recommendations and should be attempted after an adequate time spent in renal remission. Besides traditional immunosuppression, biological drugs targeting selected pathways as well as multitargeted therapies are under evaluation and some already provided evidence of efficacy in RCTs and clinical practice, submitting a likely widespread use in the near future. This should be coupled with a personalized approach, taking into account global patients as well as renal features, in order to overcome the current limits to a truly improved prognosis.
Funding: No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript. This paper was published as part of a supplement supported by an educational grant from GSK.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. Davidson A. What is damaging the kidney in lupus nephritis? Nat Rev Rheumatol 2016;12:143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hoover PJ, Costenbader KH. Insight into the epidemiology and management of lupus nephritis from the US rheumatologist’s perspective. Kideny Int 2016;90:487–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hanly JG, O’Keeffe AG, Su L et al. The frequency and outcome of lupus nephritis: results from an international inception cohort study. Rheumatology 2016;55:252–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Doria A, Iaccarino L, Ghirardello A et al. Long-term prognosis and causes of death in systemic lupus erythematosus. Am J Med 2006;119:700–6. [DOI] [PubMed] [Google Scholar]
- 5. Bastian HM, Roseman JM, McGwin GJ et al. Systemic lupus erythematosus in three ethnic groups. XII. Risk factors for lupus nephritis after diagnosis. Lupus 2002;11:152–60. [DOI] [PubMed] [Google Scholar]
- 6. Moroni G, Depetri F, Ponticelli C. Lupus nephritis: when and how often to biopsy and what does it mean? J Autoimmun 2016;74:27–40. [DOI] [PubMed] [Google Scholar]
- 7. Wada Y, Ito S, Ueno M et al. Renal outcome and predictors of clinical renal involvement in patients with silent lupus nephritis. Nephron Clin Pract 2004;98:c105–11. [DOI] [PubMed] [Google Scholar]
- 8. Balow JE. Clinical presentation and monitoring of lupus nephritis. Lupus 2005;14:25–30. [DOI] [PubMed] [Google Scholar]
- 9. Moroni G, Vercelloni PG, Quaglini S et al. Changing patterns in clinical–histological presentation and renal outcome over the last five decades in a cohort of 499 patients with lupus nephritis. Ann Rheum Dis 2018;77:1318–25. [DOI] [PubMed] [Google Scholar]
- 10. Petri M, Orbai A-M, Alarcón GS et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aringer M, Costenbader K, Daikh D et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis 2019;78:1151–9. [DOI] [PubMed] [Google Scholar]
- 12. Rovin BH. Glomerular disease: lupus nephritis treatment: are we beyond cyclophosphamide? Nat Rev Nephrol 2009;5:492–4. [DOI] [PubMed] [Google Scholar]
- 13. Mittal B, Rennke H, Singh AK. The role of kidney biopsy in the management of lupus nephritis. Curr Opin Nephrol Hypertens 2005;14:1–8. [DOI] [PubMed] [Google Scholar]
- 14. Zabaleta-Lanz M, Vargas-Arenas RE, Tápanes F et al. Silent nephritis in systemic lupus erythematosus. Lupus 2003;12:26–30. [DOI] [PubMed] [Google Scholar]
- 15. Hahn BH, McMahon MA, Wilkinson A et al. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res 2012;64:797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haładyj E, Cervera R. Do we still need renal biopsy in lupus nephritis? Reumatologia 2016;2:61–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fanouriakis A, Kostopoulou M, Cheema K. 2019 update of the Joint European League Against Rheumatism and European Renal Association–European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis 2020;79:713–23. [DOI] [PubMed] [Google Scholar]
- 18. Hebert LA, Parikh S, Prosek J et al. Differential diagnosis of glomerular disease: a systematic and inclusive approach. Am J Nephrol 2013;38:253–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bihl GR, Petri M, Fine DM. Kidney biopsy in lupus nephritis: look before you leap. Nephrol Dial Transplant 2006;21:1749–52. [DOI] [PubMed] [Google Scholar]
- 20. Giannico G, Fogo AB. Lupus nephritis: is the kidney biopsy currently necessary in the management of lupus nephritis? Clin J Am Soc Nephrol 2013;8:138–45. [DOI] [PubMed] [Google Scholar]
- 21. Christopher-Stine L, Siedner M, Lin J et al. Renal biopsy in lupus patients with low levels of proteinuria. J Rheumatol 2007;34:332–5. [PubMed] [Google Scholar]
- 22. Bertsias GK, Tektonidou M, Amoura Z et al. Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of adult and paediatric lupus nephritis. Ann Rheum Dis 2012;71:1771–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fogo AB. Approach to renal biopsy. Am J Kidney Dis 2003;42:826–36. [PubMed] [Google Scholar]
- 24. Moroni G, Pasquali S, Quaglini S et al. Clinical and prognostic value of serial renal biopsies in lupus nephritis. Am J Kidney Dis 1999;34:530–9. [DOI] [PubMed] [Google Scholar]
- 25. Anders H-J. Re-biopsy in lupus nephritis. Ann Transl Med 2018;6:S41–S41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. De Rosa M, Azzato F, Toblli JE et al. A prospective observational cohort study highlights kidney biopsy findings of lupus nephritis patients in remission who flare following withdrawal of maintenance therapy. Kidney Int 2018;94:788–94. [DOI] [PubMed] [Google Scholar]
- 27. Zickert A, Sundelin B, Svenungsson E, Gunnarsson I. Role of early repeated renal biopsies in lupus nephritis. Lupus Sci Med 2014;1:e000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Malvar A, Pirruccio P, Alberton V et al. Histologic versus clinical remission in proliferative lupus nephritis. Nephrol Dial Transplant 2017;32:1338–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Anders H-J, Rovin B. A pathophysiology-based approach to the diagnosis and treatment of lupus nephritis. Kidney Int 2016;90:493–501. [DOI] [PubMed] [Google Scholar]
- 30. Weening JJ, D'agati VD, Schwartz MM et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int 2004;65:521–30. [DOI] [PubMed] [Google Scholar]
- 31. Bajema IM, Wilhelmus S, Alpers CE et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions and modified National Institutes of Health activity and chronicity indices. Kidney Int 2018;93:789–96. [DOI] [PubMed] [Google Scholar]
- 32. Al Arfaj AS, Khalil N, Al Saleh S. Lupus nephritis among 624 cases of systemic lupus erythematosus in Riyadh, Saudi Arabia. Rheumatol Int 2009;29:1057–67. [DOI] [PubMed] [Google Scholar]
- 33. Mok C, Cheung T, Lo W. Minimal mesangial lupus nephritis: a systematic review. Scand J Rheumatol 2010;39:181–9. [DOI] [PubMed] [Google Scholar]
- 34. Chang JH, Kim DK, Kim HW et al. Changing prevalence of glomerular diseases in Korean adults: a review of 20 years of experience. Nephrol Dial Transplant 2009;24:2406–10. [DOI] [PubMed] [Google Scholar]
- 35. Yang J, Liang D, Zhang H et al. Long-term renal outcomes in a cohort of 1814 Chinese patients with biopsy-proven lupus nephritis. Lupus 2015;24:1468–78. [DOI] [PubMed] [Google Scholar]
- 36. Huong DL, Papo T, Beaufils H et al. Renal involvement in systemic lupus erythematosus. A study of 180 patients from a single center. Medicine 1999;78:148–66. [DOI] [PubMed] [Google Scholar]
- 37. Wang H, Ren Y, Chang J et al. A systematic review and meta-analysis of prevalence of biopsy-proven lupus nephritis. Arch Rheumatol 2018;33:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tam LS, Li EK, Lai FM et al. Mesangial lupus nephritis in Chinese is associated with a high rate of transformation to higher grade nephritis. Lupus 2003;12:665–71. [DOI] [PubMed] [Google Scholar]
- 39. Lee SG, Cho YM, So MW et al. ISN/RPS 2003 class II mesangial proliferative lupus nephritis: a comparison between cases that progressed to class III or IV and cases that did not. Rheumatol Int 2012;32:2459–64. [DOI] [PubMed] [Google Scholar]
- 40. Collado MV, Dorado E, Rausch S et al. Long-term Outcome of Lupus Nephritis Class II in argentine patients: an open retrospective analysis. J Clin Rheumatol Pract Rep Rheum Musculoskelet Dis 2016;22:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moroni G, Quaglini S, Gravellone L et al. Membranous nephropathy in systemic lupus erythematosus: long-term outcome and prognostic factors of 103 patients. Semin Arthritis Rheum 2012;41:642–51. [DOI] [PubMed] [Google Scholar]
- 42. Austin HA, Illei GG. Membranous lupus nephritis. Lupus 2005;14:65–71. [DOI] [PubMed] [Google Scholar]
- 43. Haring CM, Rietveld A, Van den Brand J, Berden J. Segmental and global subclasses of class IV lupus nephritis have similar renal outcomes. J Am Soc Nephrol 2012;23:149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stokes MB, D'Agati VD. Classification of Lupus Nephritis; Time for a Change? Adv Chronic Kidney Dis 2019;26:323–9. [DOI] [PubMed] [Google Scholar]
- 45. Chen D, Hu W. Lupus podocytopathy: a distinct entity of lupus nephritis. J Nephrol 2018;31:629–34. [DOI] [PubMed] [Google Scholar]
- 46. Bomback AS, Markowitz GS. Lupus podocytopathy: a distinct entity. Clin J Am Soc Nephrol 2016;11:547–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hu W, Chen Y, Wang S et al. Clinical–morphological features and outcomes of lupus podocytopathy. Clin J Am Soc Nephrol 2016;11:585–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rezende G, Viana V, Malheiros D et al. Podocyte injury in pure membranous and proliferative lupus nephritis: distinct underlying mechanisms of proteinuria? Lupus 2014;23:255–62. [DOI] [PubMed] [Google Scholar]
- 49. Yu F, Haas M, Glassock R, Zhao M-H. Redefining lupus nephritis: clinical implications of pathophysiologic subtypes. Nat Rev Nephrol 2017;13:483–95. [DOI] [PubMed] [Google Scholar]
- 50. Felson DT, Anderson J. Evidence for the superiority of immunosuppressive drugs and prednisone over prednisone alone in lupus nephritis. N Engl J Med 1984;311:1528–33. [DOI] [PubMed] [Google Scholar]
- 51. Austin HA, Klippel JH, Balow JE et al. Therapy of lupus nephritis. N Engl J Med 1986;314:614–9. [DOI] [PubMed] [Google Scholar]
- 52. Ginzler EM, Dooley MA, Aranow C et al. Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N Engl J Med 2005;353:2219–28. [DOI] [PubMed] [Google Scholar]
- 53. Appel GB, Contreras G, Dooley MA et al. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol 2009;20:1103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lenz O, Waheed AA, Baig A et al. Lupus nephritis: maintenance therapy for lupus nephritis—do we now have a plan? Clin J Am Soc Nephrol 2013;8:162–71. [DOI] [PubMed] [Google Scholar]
- 55. Houssiau FA, Vasconcelos C, D’Cruz D et al. The 10-year follow-up data of the Euro Lupus Nephritis Trial comparing low-dose and high-dose intravenous cyclophosphamide. Ann Rheum Dis 2010;69:61–4. [DOI] [PubMed] [Google Scholar]
- 56. Fanouriakis A, Kostopoulou M, Alunno A et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis 2019;78:736–45. [DOI] [PubMed] [Google Scholar]
- 57. Zhou T, Lin S, Yang S, Lin W. Efficacy and safety of tacrolimus in induction therapy of patients with lupus nephritis. Drug Des Devel Ther 2019;13:857–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liu Z, Zhang H, Liu Z et al. Multitarget therapy for induction treatment of lupus nephritis: a randomized trial. Ann Intern Med 2015;162:18–26. [DOI] [PubMed] [Google Scholar]
- 59. Zhang H, Liu Z, Zhou M et al. Multitarget therapy for maintenance treatment of lupus nephritis. J Am Soc Nephrol 2017;28:3671–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chen W, Liu Q, Chen W et al. Outcomes of maintenance therapy with tacrolimus versus azathioprine for active lupus nephritis: a multicenter randomized clinical trial. Lupus 2012;21:944–52. [DOI] [PubMed] [Google Scholar]
- 61. Moroni G, Doria A, Ponticelli C. Cyclosporine (CsA) in lupus nephritis: assessing the evidence. Nephrol Dial Transplant 2008;24:15–20. [DOI] [PubMed] [Google Scholar]
- 62. Zhong Z, Li H, Zhong H, Zhou T. Clinical efficacy and safety of rituximab in lupus nephritis. Drug Des Devel Ther 2019;13:845–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Alshaiki F, Obaid E, Almuallim A et al. Outcomes of rituximab therapy in refractory lupus: a meta-analysis. Eur J Rheumatol 2018;5:118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Merrill JT, Neuwelt CM, Wallace DJ et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum 2010;62:222–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rovin BH, Furie R, Latinis K et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum 2012;64:1215–26. [DOI] [PubMed] [Google Scholar]
- 66. Condon MB, Ashby D, Pepper RJ et al. Prospective observational single-centre cohort study to evaluate the effectiveness of treating lupus nephritis with rituximab and mycophenolate mofetil but no oral steroids. Ann Rheum Dis 2013;72:1280–6. [DOI] [PubMed] [Google Scholar]
- 67. Moroni G, Raffiotta F, Trezzi B et al. Rituximab vs mycophenolate and vs cyclophosphamide pulses for induction therapy of active lupus nephritis: a clinical observational study. Rheumatology 2014;53:1570–7. [DOI] [PubMed] [Google Scholar]
- 68. Sanz I. Extent and patterns of off-label use of rituximab for SLE. Nat Rev Rheumatol 2016;12:700–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Iaccarino L, Bartoloni E, Carli L et al. Efficacy and safety of off-label use of rituximab in refractory lupus: data from the Italian Multicentre Registry. Clin Exp Rheumatol 2015;33:499–56. [PubMed] [Google Scholar]
- 70. Ezeonyeji A, Isenberg D. Early treatment with rituximab in newly diagnosed systemic lupus erythematosus patients: a steroid-sparing regimen. Rheumatology 2012;51:476–81. [DOI] [PubMed] [Google Scholar]
- 71. Moroni G, Gatto M, Raffiotta F et al. Can we withdraw immunosuppressants in patients with lupus nephritis in remission? An expert debate. Autoimmun Rev 2018;17:11–8. [DOI] [PubMed] [Google Scholar]
- 72. Lightstone L, Doria A, Wilson H et al. Can we manage lupus nephritis without chronic corticosteroids administration? Autoimmun Rev 2018;17:4–10. [DOI] [PubMed] [Google Scholar]
- 73. Shen X, Jiang H, Ying M et al. Calcineurin inhibitors cyclosporin A and tacrolimus protect against podocyte injury induced by puromycin aminonucleoside in rodent models. Sci Rep 2016;6:32087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Faul C, Donnelly M, Merscher-Gomez S et al. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med 2008;14:931–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wilhelmus S, Bajema IM, Bertsias GK et al. Lupus nephritis management guidelines compared. Nephrol Dial Transplant 2016;31:904–13. [DOI] [PubMed] [Google Scholar]
- 76. Pons-Estel GJ, Alarcón GS, McGwin G Jr et al. Protective effect of hydroxychloroquine on renal damage in patients with lupus nephritis: LXV, data from a multiethnic US cohort. Arthritis Rheum 2009;61:830–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cunha C, Alexander S, Ashby D et al. Hydroxycloroquine blood concentration in lupus nephritis: a determinant of disease outcome? Nephrol Dial Transplant 2018;33:1604–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Siso A, Ramos-Casals M, Bove A et al. Previous antimalarial therapy in patients diagnosed with lupus nephritis: influence on outcomes and survival. Lupus 2008;17:281–8. [DOI] [PubMed] [Google Scholar]
- 79. Clowse ME, Magder L, Witter F, Petri M. Hydroxychloroquine in lupus pregnancy. Arthritis Rheum 2006;54:3640–7. [DOI] [PubMed] [Google Scholar]
- 80. Lightstone L, Hladunewich M. Lupus nephritis and pregnancy: concerns and management. Semin Nephrol 2017. Jul;37:347–53. [DOI] [PubMed] [Google Scholar]
- 81. Sprangers B, Monahan M, Appel GB. Diagnosis and treatment of lupus nephritis flares—an update. Nat Rev Nephrol 2012;8:709–17. [DOI] [PubMed] [Google Scholar]
- 82. Moroni G, Quaglini S, Maccario M et al. “Nephritic flares” are predictors of bad long-term renal outcome in lupus nephritis. Kidney Int 1996;50:2047–53. [DOI] [PubMed] [Google Scholar]
- 83. Gordon C, Jayne D, Pusey C et al. European consensus statement on the terminology used in the management of lupus glomerulonephritis. Lupus 2009;18:257–63. [DOI] [PubMed] [Google Scholar]
- 84. Davidson JE, Fu Q, Ji B et al. Renal remission status and longterm renal survival in patients with lupus nephritis: a retrospective cohort analysis. J Rheumatol 2018;45:671–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Chen Y, Korbet S, Katz R et al. Value of a complete or partial remission in severe lupus nephritis. Clin J Am Soc Nephrol 2008;3:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Moroni G. Gatto M. Tamborini F et al. Lack of EULAR/ERA-EDTA response at 1 year predicts poor long-term renal outcome in patients with lupus nephritis [published online ahead of print, 2020 Jun 5]. Ann Rheum Dis. 2020;annrheumdis-2020-216965. 10.1136/annrheumdis. [DOI] [PubMed] [Google Scholar]
- 87. Houssiau FA, Vasconcelos C, D'Cruz D et al. Early response to immunosuppressive therapy predicts good renal outcome in lupus nephritis: lessons from long-term follow-up of patients in the Euro-Lupus Nephritis Trial. Arthritis Rheum 2004;50:3934–40. [DOI] [PubMed] [Google Scholar]
- 88. Tamirou F, Lauwerys BR, Dall'Era M et al. A proteinuria cut-off level of 0.7 g/day after 12 months of treatment best predicts long-term renal outcome in lupus nephritis: data from the MAINTAIN Nephritis Trial. Lupus Sci Med 2015;2:e000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Fung WA, Su J, Touma Z. Predictors of good long-term renal outcomes in lupus nephritis: results from a single lupus cohort. Biomed Res Int 2017;2017:5312960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Mackay M, Dall'Era M, Fishbein J et al. Establishing surrogate kidney end points for lupus nephritis clinical trials: development and validation of a novel approach to predict future kidney outcomes. Arthritis Rheumatol 2019;71:411–9. [DOI] [PubMed] [Google Scholar]
- 91. Mak A, Cheung M-L, Chiew HJ et al. Global trend of survival and damage of systemic lupus erythematosus: meta-analysis and meta-regression of observational studies from the 1950s to 2000s. Semin Arthritis Rheum 2012;41:830–9. [DOI] [PubMed] [Google Scholar]
- 92. Cameron JS. Lupus nephritis. J Am Soc Nephrol 1999;10:413–24. [DOI] [PubMed] [Google Scholar]
- 93. Croca SC, Rodrigues T, Isenberg DA. Assessment of a lupus nephritis cohort over a 30-year period. Rheumatology 2011;50:1424–30. [DOI] [PubMed] [Google Scholar]
- 94. Ward MM. Changes in the incidence of endstage renal disease due to lupus nephritis in the United States, 1996-2004. J Rheumatol 2009;36:63–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Tektonidou MG, Dasgupta A, Ward MM. Risk of end-stage renal disease in patients with lupus nephritis, 1971–2015: a systematic review and Bayesian meta-analysis. Arthritis Rheumatol 2016;68:1432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Reppe Moe SE, Molberg Ø, Strøm EH, Lerang K. Assessing the relative impact of lupus nephritis on mortality in a population-based systemic lupus erythematosus cohort. Lupus 2019;28:818–25. [DOI] [PubMed] [Google Scholar]
- 97. Jakes RW, Bae S-C, Louthrenoo W et al. Systematic review of the epidemiology of systemic lupus erythematosus in the Asia-Pacific region: prevalence, incidence, clinical features, and mortality. Arthritis Care Res 2012;64:159–68. [DOI] [PubMed] [Google Scholar]
- 98. Korbet SM, Schwartz MM, Evans J, Lewis EJ. Severe lupus nephritis: racial differences in presentation and outcome. J Am Soc Nephrol 2007;18:244–54. [DOI] [PubMed] [Google Scholar]
- 99. Contreras G, Pardo V, Cely C et al. Factors associated with poor outcomes in patients with lupus nephritis. Lupus 2005;14:890–5. [DOI] [PubMed] [Google Scholar]
- 100. Contreras G, Lenz O, Pardo V et al. Outcomes in African Americans and Hispanics with lupus nephritis. Kidney Int 2006;69:1846–51. [DOI] [PubMed] [Google Scholar]
- 101. Almaani S, Meara A, Rovin BH. Update on lupus nephritis. Clin J Am Soc Nephrol 2017;12:825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Barr RG, Seliger S, Appel GB et al. Prognosis in proliferative lupus nephritis: the role of socio-economic status and race/ethnicity. Nephrol Dial Transplant 2003;18:2039–46. [DOI] [PubMed] [Google Scholar]
- 103. Hsu C-Y, Chiu W-C, Yang T-S et al. Age- and gender-related long-term renal outcome in patients with lupus nephritis. Lupus 2011;20:1135–41. [DOI] [PubMed] [Google Scholar]
- 104. De Carvalho JF, Do Nascimento AP, Testagrossa LA et al. Male gender results in more severe lupus nephritis. Rheumatol Int 2010;30:1311–5. [DOI] [PubMed] [Google Scholar]
- 105. Donadio JVJ, Hart GM, Bergstralh EJ, Holley KE. Prognostic determinants in lupus nephritis: a long-term clinicopathologic study. Lupus 1995;4:109–15. [DOI] [PubMed] [Google Scholar]
- 106. Moroni G, Quaglini S, Gallelli B et al. The long-term outcome of 93 patients with proliferative lupus nephritis. Nephrol Dial Transplant 2007;22:2531–9. [DOI] [PubMed] [Google Scholar]
- 107. Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol 2006;17:2974–84. [DOI] [PubMed] [Google Scholar]
- 108. Austin HA 3rd, Boumpas DT, Vaughan EM, Balow JE. Predicting renal outcomes in severe lupus nephritis: contributions of clinical and histologic data. Kidney Int 1994;45:544–50. [DOI] [PubMed] [Google Scholar]
- 109. HA A 3rd, Boumpas DT, Vaughan EM, Balow JE. High-risk features of lupus nephritis: importance of race and clinical and histological factors in 166 patients. Nephrol Dial Transplant 1995;10:1620–8. [PubMed] [Google Scholar]
- 110. Leatherwood C, Speyer CB, Feldman CH et al. Clinical characteristics and renal prognosis associated with interstitial fibrosis and tubular atrophy (IFTA) and vascular injury in lupus nephritis biopsies. Semin Arthritis Rheum 2019;49:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hsieh C, Chang A, Brandt D et al. Predicting outcomes of lupus nephritis with tubulointerstitial inflammation and scarring. Arthritis Care Res 2011;63:865–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Mok CC. Prognostic factors in lupus nephritis. Lupus 2005;14:39–44. [DOI] [PubMed] [Google Scholar]
- 113. Maria NI, Davidson A. Protecting the kidney in systemic lupus erythematosus: from diagnosis to therapy. Nat Rev Rheumatol 2020;16:255–67. [DOI] [PubMed] [Google Scholar]
- 114. Gatto M, Iaccarino L, Ghirardello A, Punzi L, Doria A. Clinical and pathologic considerations of the qualitative and quantitative aspects of lupus nephritogenic autoantibodies: a comprehensive review. J Autoimmun 2016;69:1–11. [DOI] [PubMed] [Google Scholar]
- 115. Gatto M, Ghirardello A, Luisetto R et al. Immunization with pentraxin 3 (PTX3) leads to anti-PTX3 antibody production and delayed lupus-like nephritis in NZB/NZW F1 mice. Compr Perspect Syst Lupus Erythematosus 2016;74:208–16. [DOI] [PubMed] [Google Scholar]
- 116. Gatto M, Zen M, Iaccarino L, Doria A. New therapeutic strategies in systemic lupus erythematosus management. Nat Rev Rheumatol 2019;15:30–48. [DOI] [PubMed] [Google Scholar]
- 117. Gatto M, Saccon F, Zen M et al. Success and failure of biological treatment in systemic lupus erythematosus: a critical analysis. Compr Perspect Syst Lupus Erythematosus 2016;74:94–105. [DOI] [PubMed] [Google Scholar]
- 118.US National Library of Medicine. Aurinia Urinary Protein Reduction Active - Lupus With Voclosporin (AURA-LV). https://clinicaltrials.gov/ct2/show/NCT02141672.
- 119.US National Library of Medicine. Aurinia Renal Response in Active Lupus with Voclosporin (AURORA). https://www.clinicaltrials.gov/ct2/show/NCT03021499.
- 120.US National Library of Medicine. Aurinia Renal Assessments 2: Aurinia Renal Response in Lupus With Voclosporin (AURORA2). https://www.clinicaltrials.gov/ct2/show/NCT03597464.
- 121.US National Library of Medicine. Efficacy and safety of Belimumab in patients with active Lupus Nephritis (BLISS_LN). https://clinicaltrials.gov/ct2/show/NCT01639339? term=NCT01639339&rank=1.
- 122.US National Library of Medicine. Rituximab and Belimumab for Lupus Nephritis (CALIBRATE). http://clinicaltrials.gov/ct2/show/NCT02260934.
- 123.US National Library of Medicine. A study to evaluate the efficacy and safety of obinutuzumab in patients with ISN/RPS 2003 Class III or IV lupus nephritis. https://www.clinicaltrials.gov/ct2/show/NCT04221477.
- 124.US National Library of Medicine. A study to evaluate the safety and efficacy of obinutuzumab compared with placebo in participants with Lupus Nephritis (LN). https://clinicaltrials.gov/ct2/show/NCT02550652.
- 125.US National Library of Medicine. Safety and Efficacy of Two Doses of Anifrolumab Compared to Placebo in Adult Subjects With Active Proliferative Lupus Nephritis (TULIP-LN1). https://www.clinicaltrials.gov/ct2/show/NCT02547922 [Google Scholar]
- 126.US National Library of Medicine. An investigational study to evaluate the safety and effectiveness of BMS-986165 with background treatment in participants with lupus nephritis. https://www.clinicaltrials.gov/ct2/show/NCT03943147.
- 127.US National Library of Medicine. Study of safety, efficacy and tolerability of secukinumab versus placebo, in combination with SoC Therapy, in Patients With Active Lupus Nephritis (SELUNE). https://www.clinicaltrials.gov/ct2/show/NCT04181762.
- 128. Dooley MA, Pendergraft W III, Ginzler EM et al. Speed of remission with the use of voclosporin, MMF and low dose steroids: results of a global lupus nephritis study [abstract]. Arthritis Rheumatol 2016;68 (suppl 10): Abstract number: 5L. [Google Scholar]
- 129. Iaccarino L, Bettio S, Reggia R et al. Effects of belimumab on flare rate and expected damage progression in patients with active systemic lupus erythematosus. Arthritis Care Res 2017;69:115–23. [DOI] [PubMed] [Google Scholar]
- 130. Sciascia S, Radin M, Yazdany J et al. Efficacy of belimumab on renal outcomes in patients with systemic lupus erythematosus: a systematic review. Autoimmun Rev 2017;16:287–93. [DOI] [PubMed] [Google Scholar]
- 131. Dall’Era M, Aranow C, Byron M et al. Phase 2 trial of induction therapy with anti-CD20 (rituximab) followed by maintenance therapy with anti-BAFF (Belimumab) in patients with active lupus nephritis [abstract]. Arthritis Rheumatol 2018;70:(suppl 10): Abstract number: 1870. [Google Scholar]
- 132. Gualtierotti R, Borghi MO, Gerosa M et al. Successful sequential therapy with rituximab and belimumab in patients with active systemic lupus erythematosus: a case series. Clin Exp Rheumatol 2018;36:643–7. [PubMed] [Google Scholar]
- 133. Murphy G, Isenberg DA. New therapies for systemic lupus erythematosus—past imperfect, future tense. Nat Rev Rheumatol 2019;15:403–12. [DOI] [PubMed] [Google Scholar]
- 134.US National Library of Medicine. A study to evaluate the efficacy and safety of belimumab administered in combination with rituximab to adult subjects with systemic lupus erythematosus (SLE) - BLISS-BELIEVE. https://clinicaltrials.gov/ct2/show/NCT03312907.
- 135. Schindler T, Rovin B, Furie R et al. AB0423 Nobility, A Phase 2 Trial To Assess The Safety and Efficacy of Obinutuzumab, A Novel Type 2 Anti-CD20 Monoclonal Antibody (MAB), in Patients (PTS) with ISN/RPS Class III or IV Lupus Nephritis (LN). Ann Rheum Dis 2016;75:1051.2.26823530 [Google Scholar]
- 136. Furie RA Morand EF Bruce IN. et al. Type I interferon inhibitor anifrolumab in active systemic lupus erythematosus (TULIP-1): a randomised, controlled, phase 3 trial. Lancet Rheumatol 2019;1:e208–19. [DOI] [PubMed] [Google Scholar]
- 137. Winthrop KL. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat Rev Rheumatol 2017;13:234–43. [DOI] [PubMed] [Google Scholar]
- 138. Apostolidis SA, Crispín JC, Tsokos GC. IL-17-producing T cells in lupus nephritis. Lupus 2011;20:120–4. [DOI] [PubMed] [Google Scholar]
- 139. Crispín JC, Oukka M, Bayliss G et al. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol 2008;181:8761–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Pisitkun P, Ha H-L, Wang H et al. Interleukin-17 cytokines are critical in development of fatal lupus glomerulonephritis. Immunity 2012;37:1104–15. [DOI] [PMC free article] [PubMed] [Google Scholar]