Abstract
Background
Hepatitis C virus (HCV) frequently co-occurs with symptoms of depression, which are aggravated on interferon-based regimens. However, it is unknown whether HCV treatment with direct-acting antivirals (DAAs) has effects on depressive symptoms among people who inject drugs (PWID). In this study, we examined changes in depressive symptoms during and after HCV treatment among PWID on opioid agonist therapies (OATs).
Methods
Participants were 141 PWID who achieved sustained viral response after on-site HCV treatment at 3 OAT programs.
Depressive symptoms were assessed using the Beck Depression Inventory–II (BDI-II) at baseline, every 4 weeks during treatment, and 12 and 24 weeks after treatment completion. Current diagnosis of depression or other psychiatric diagnoses were obtained through chart review. Use of illicit drugs was measured by urine toxicology screening. Alcohol use was measured using the Addiction Severity Index–Lite.
Results
Of the 141 PWID infected with HCV, 24.1% had severe, 9.9% had moderate, 15.6% had mild, and 50.4% had minimal levels of depression as per BDI-II scores at baseline. HCV treatment was significantly associated with reductions in depressive symptoms that persisted long term, regardless of symptom severity (P < .001) or presence of depression (P ≤ .01) or other psychiatric diagnoses (P ≤ .01) at baseline. Concurrent drug use (P ≤ .001) or hazardous alcohol drinking (P ≤ .001) did not interfere with reductions in depressive symptoms.
Conclusions
Depressive symptoms are highly prevalent among HCV-infected PWID. HCV treatment was associated with sustained reductions in depressive symptoms. HCV therapy with DAAs may have important implications for PWID that go beyond HCV cure.
Keywords: DAAs, depression, HCV, mental health, PWID
Hepatitis C virus treatment with direct-acting antivirals among individuals on opioid agonist therapy with a history of injecting drugs was associated with significant reductions in depressive symptoms, regardless of baseline depression severity, a history of psychiatric diagnosis, and concurrent drug or alcohol use.
Depressive disorders are highly prevalent among people infected with hepatitis C virus (HCV), ~3-fold higher than the general population (29.3% vs 5.0%–10.3%) [1]. The prevalence of depression can further rise among people with HCV when other comorbidities are present, such as substance use disorders. In particular, among HCV-infected people with a history of injection drug use, depression has been estimated to be as high as 48% [2, 3]. Concurrent depressive symptoms do not seem to have an effect on HCV treatment initiation or completion, adherence, or virologic response [4], even among PWID [2, 5, 6]. However, the presence of depressive symptoms has been known to create additional vulnerabilities among people with HCV. For example, elevated symptoms of depression have been associated with a lower likelihood of being deemed eligible for HCV treatment in individuals with or without a history of drug use [7]. HCV patients in the general population with depressive symptoms are also less likely to seek specialty HCV care compared with those without depressive symptoms [8]. Moreover, higher depression symptoms have been associated with recent injection drug use among HCV-infected PWID [2, 9].
The availability of direct-acting antivirals (DAAs) has been revolutionary in advancing HCV treatment. Besides the high rates of sustained virologic response (≥95%) associated with DAAs, DAAs also produce fewer psychiatric side effects [10]. Although there is no evidence that DAAs directly cause depressive symptoms [11], recent studies have measured depressive symptomatology during DAA treatment, either its incidence or changes related to treatment. Studies exploring incidence of depressive symptoms [12–14] showed remarkably lower rates of depression (5.6%–14.6% vs 34.8%) than reported in earlier studies with interferon-based regimens [15]. The vast majority of studies evaluating depressive symptoms during the course of DAA treatment have demonstrated that DAA therapy does not worsen depressive symptoms either during treatment or after treatment completion [11, 16–18], with some of these studies showing either reductions in depressive symptoms [19–23] or reductions in the gravity of problems related to depression [24, 25]. It is noteworthy that none of these studies included people actively using or injecting drugs, despite the fact that PWID represent the largest population infected with HCV in the United States [26]. More specifically, some of these studies included individuals with a diagnosis of a substance use disorder without information on recent or active drug use [11, 14], individuals with a history of injecting drugs but not currently injecting [18, 24, 25], and patients on methadone maintenance or other opiate substitution therapy but without information regarding current drug use [13, 21], which precludes these findings from being generalizable to individuals with recent injection practices.
Opioid agonist therapy (OAT) programs represent an ideal setting that enables providers to co-locate HCV therapy for PWID and to address other substance use disorders and/or other psychiatric comorbidities [27]. To date, 2 prospective cohort studies have assessed depressive symptoms of PWID actively using drugs attending OAT for opioid use disorder and undergoing HCV treatment, but both used interferon-based regimens [5, 28]. More specifically, Jekerman et al. showed an increase of depressive symptoms during HCV treatment compared with baseline [5], whereas Shafer et al. evidenced that depressive symptoms remained elevated within 1 year after HCV treatment completion [28]. Although neither study reported improvements in depressive symptoms during HCV treatment, this finding may not be applicable to the current state of evidence for HCV treatment using DAAs. In addition, neither study explored the effect of current drug use or psychiatric comorbidities on depressive symptoms, which further limits their findings from being generalized to a clinical population of PWID receiving DAA-based HCV treatment.
The primary aim of this study was to investigate changes in depressive symptomatology among PWID maintained on OAT (including people actively using drugs) during HCV treatment and long-term after achieving sustained virologic response (SVR). Secondary aims were to investigate whether changes in depressive symptoms were influenced by prior diagnosis of depression or any psychiatric diagnosis, the severity of depressive symptoms at baseline, and current drug and alcohol use.
METHODS
Participants
Participants were HCV-infected PWID enrolled in a randomized clinical trial (PREVAIL; NCT01857245) evaluating the effectiveness of 3 models of HCV care among PWID receiving methadone or buprenorphine maintenance treatment. Participants were recruited at 3 OAT programs in Bronx, New York. A detailed description of the overall sample of 150 PWID receiving HCV treatment in the PREVAIL study has been reported previously [29]. Participants who did not achieve sustained virologic response (n = 7) or died (n = 2) during the trial were excluded from this study. Thus, the final sample included 141 patients who successfully completed HCV treatment and achieved SVR.
All participants provided written informed consent. The study was approved by the Albert Einstein College of Medicine Institutional Review Board. The study was conducted in accordance with the Declaration of Helsinki Ethical Standards.
Patient Consent Statement
All participants underwent written informed consent. The study was approved by the Institutional Review Board (IRB) at Albert Einstein College of Medicine.
Parent Clinical Trial Design
Participants were randomized to 1 of 3 conditions: self-administered individual treatment (SIT), group treatment (GT), and directly observed therapy (DOT). Participants randomized to SIT received all their HCV medication packaged in 7-day blister packs from an Opioid Treatment Program (OTP) clinic staff member and self-administered medications at home. Participants in the GT condition, in addition to self-administering medications at home, attended an HCV support group weekly. In the DOT condition, participants attended their OAT clinic for in-person observation of medication ingestion, which was linked to OAT visits. Participants had research visits at baseline, every 4 weeks during the first 12 weeks of HCV treatment (treatment week 4, 8, and 12), at the end of treatment (if the treatment regimen was ˃12 weeks, and 4, 12, and 24 weeks after treatment completion (follow-ups 4, 12, and 24). The following regimens were provided: telaprevir, pegylated interferon, and ribavirin (TVR/IFN/RBV); sofosbuvir, pegylated interferon, and ribavirin (SOF/IFN/RBV); sofosbuvir and ribavirin (SOF/RBV); or a combination DAA regimen of sofosbuvir and simeprevir (SOF/SMV) or sofosbuvir/ledipasvir (SOF/LDV). Full study methodology has been described in detail elsewhere [30]. Since no significant differences between the 3 treatment conditions were observed in any of the baseline characteristics, or in Beck Depression Inventory (BDI) scores across research visits, data from the 3 conditions (SIT, GT, and DOT) were combined for the subsequent analyses. Given that there were no differences between the different treatment regimens in changes on BDI scores, data from all 5 regimens were also merged.
Measures
Participants Characteristics
All participants completed a survey instrument at baseline that collected data on sociodemographic characteristics including age, gender, race, ethnicity, income, education, living situation, and employment status.
Depressive Symptoms
Depressive symptoms were assessed using the Beck Depression Inventory–II (BDI-II). The BDI-II is a 21-item self-report instrument that measures severity of depressive symptomatology in the past 2 weeks. The range of possible scores is 0–63. Total scores on the BDI-II are classified as minimal depression (0–13), mild depression (14–19), moderate depression (20–28), and severe depression (29–63) [31]. Depressive symptoms, the main outcome in this study, were assessed at baseline, weeks 4, 8, and 12 of treatment, and at follow-up 12 and 24 weeks after treatment completion.
Psychiatric Diagnosis
Medical charts were systematically reviewed in order to obtain participants’ current problem list and HCV treatment evaluation notes. Data about psychiatric diagnoses were obtained through chart review at baseline. These diagnoses were made previously by other clinicians experienced in treating PWID, including primary care and addiction medicine providers, who were unrelated to the study aims and procedures. Psychiatric diagnoses included depression, anxiety, psychosis, bipolar disorder, obsessive-compulsive disorder, and post-traumatic stress disorder. Information about use of psychiatric medication at baseline was also included.
Drug and Alcohol Use
Participants provided urine specimens at each research visit to determine recent drug use. Substances tested included benzodiazepines, cocaine, methadone, opioids, and oxycodone (ABMC, Kinderhook, NY, USA). The Addiction Severity Index-Lite version (ASI-Lite) was used to collect information about self-reported hazardous drinking in the past 30 days. More specifically, participants were asked on how many days of the past 30 days they drank alcohol to intoxication, which was defined as having taken ≥5 drinks in 1 day or ≥3 in a sitting. Drug and alcohol use was assessed at all research visits.
Data Analyses
Descriptive statistics were computed to describe participants’ baseline characteristics. Categorical and continuous baseline data were compared across the 3 arms (SIT, GT, DOT) using chi-square tests and analysis of variance, respectively. Linear mixed-effect models were used to compare BDI scores over the course of the study (treatment visits and follow-ups) as a function of (1) severity of depressive symptoms (minimal, mild, moderate, severe); (2) presence of depression or any other psychiatric diagnosis at baseline (anxiety, psychosis, bipolar disorder, obsessive-compulsive disorder, and post-traumatic stress disorder); (3) use of psychiatric medication at baseline; (4) current drug use (ie, positive urine sample); and (5) reported hazardous drinking. Psychiatric medication at baseline was included as a covariate in the mixed-effects models except for those with depression or any other psychiatric diagnosis, because both were highly correlated with the psychiatric medication. Data regarding presence of depression or any other psychiatric diagnosis and use of psychiatric medication were obtained from baseline only, whereas data regarding severity of depressive symptoms and both drug use and hazardous drinking were obtained across all research visits (treatment and follow-ups). Partial eta-squared and 95% confidence intervals were used to estimate the magnitude of effects, with 0.01, 0.06, and 0.14 corresponding to small, medium, and large effect sizes, respectively. Statistical significance was set at a 2-sided P value of <.05, and all statistical analyses were conducted using the R statistical package, version 6.1.
RESULTS
Participants’ Sociodemographic Characteristics
Table 1 shows participants’ baseline measures for the overall sample and as a function of treatment condition. The sample was 64.5% male, 63.8% Hispanic, and on average 51.4 years old. Fifty-one percent reported a monthly income of ≤$900, 41.8% had less than high school education, 21.3% were homeless, and 91.5% were unemployed.
Table 1.
Participant Characteristics at Baseline Overall and as a Function of Treatment Condition
| Characteristic | Overall (N = 141) | TAU (n = 46) | Group (n = 45) | DOT (n = 50) | P |
|---|---|---|---|---|---|
| Age, y | 51.4 (10.6) | 51.2 (11.2) | 51.3 (10.8) | 51.6 (10.2) | .98 |
| Gender (male) | 91 (64.5) | 29 (63.0) | 29 (63.5) | 33 (66.0) | .96 |
| Ethnicity (Hispanic) | 90 (63.8) | 31 (67.4) | 26 (57.8) | 33 (66.0) | .58 |
| Income (≤$900) | 72 (51.1) | 23 (50.0) | 19 (42.2) | 30 (60.0) | .22 |
| Educational (<HS graduate) | 59 (41.8) | 20 (43.5) | 20 (44.4) | 19 (38.0) | .78 |
| Employment status (unemployed) | 129 (91.5) | 44 (95.7) | 43 (95.6) | 42 (84) | .07 |
| Living situation (married/living with someone) | 52 (36.9) | 13 (28.3) | 21 (46.7) | 18 (36.0) | .19 |
| Homeless | 30 (21.3) | 14 (30.4) | 8 (17.8) | 8 (16.0) | .18 |
| BDI-II scores | 17.4 (13.8) | 20.1 (45.0) | 15.2 (12.8) | 17 (13.3) | .24 |
| BDI-II severity | .41 | ||||
| Minimal | 71 (50.4) | 19 (41.3) | 24 (53.3) | 28 (56.0) | |
| Mild | 22 (15.6) | 8 (17.4) | 9 (20.0) | 5 (10.0) | |
| Moderate | 14 (9.9) | 6 (13.0) | 5 (11.1) | 3 (6.0) | |
| Severe | 34 (24.1) | 13 (28.3) | 7 (15.6) | 14 (28.0) | |
| Psychiatric condition | |||||
| Depression | 67 (47.5) | 18 (39.1) | 20 (44.4) | 29 (58.0) | .16 |
| Anxiety | 42 (29.8) | 13 (28.3) | 14 (31.1) | 15 (30.0) | .95 |
| Bipolar | 21 (14.9) | 8 (17.4) | 7 (15.6) | 6 (12.0) | .75 |
| OCD | 1 (0.7) | 0 (0) | 1 (2.2) | 0 (0) | .34 |
| Psychosis | 6 (4.3) | 2 (4.3) | 2 (4.4) | 2 (4.0) | .99 |
| PTSD | 13 (9.2) | 2 (4.3) | 6 (13.3) | 5 (10.0) | .32 |
| Any | 91 (64.5) | 29 (63.0) | 27 (60.0) | 35 (70.0) | .57 |
| Psychiatric medication | |||||
| Any | 39 (27.7) | 12 (26.1) | 11 (24.4) | 16 (32.0) | .68 |
| Alcohol use to intoxication | 35 (24.8) | 12 (26.1) | 10 (22.2) | 13 (26.0) | .88 |
| Drug use (urine screen) | |||||
| Benzodiazepines | 22 (15.6) | 9 (19.6) | 4 (8.9) | 9 (18.0) | .87 |
| Cocaine | 42 (29.8) | 15 (32.6) | 10 (22.2) | 17 (34.0) | .40 |
| Opioids (oxycodone and opiates) | 34 (24.1) | 10 (21.7) | 13 (28.9) | 11 (22.0) | .66 |
| Any | 70 (49.6) | 23 (50.0) | 21 (46.7) | 26 (52.0) | .87 |
Abbreviations: BDI, Beck Depression Inventory–II; DOT, directly observed therapy; HS, high school; OCD, obsessive compulsive disorder; PTSD, post-traumatic stress disorder; TAU, treatment as usual.
Changes in BDI Scores by Severity at Baseline
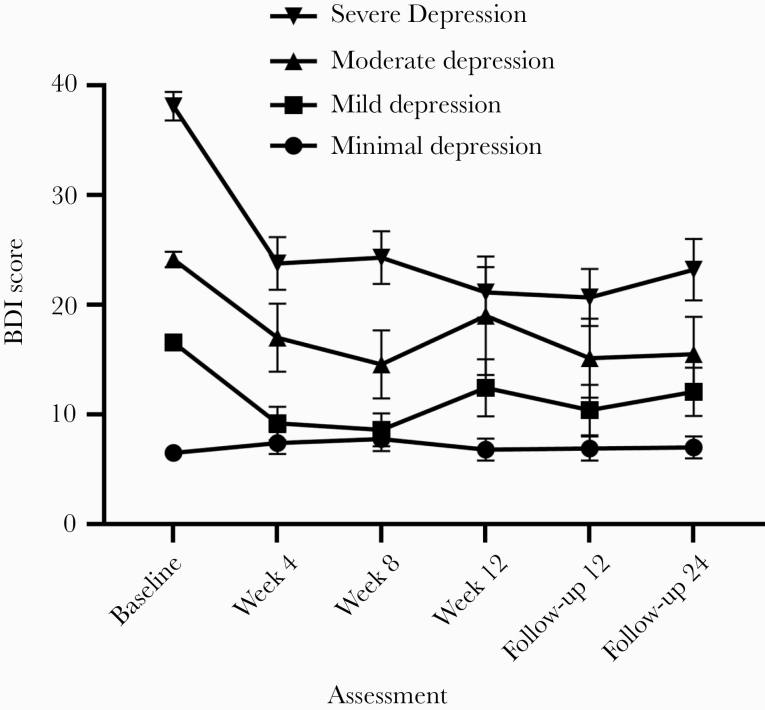
Of the 141 PWID infected with HCV, 24.1% (34/141) had severe, 9.9% (14/141) had moderate, 15.6% (22/141) had mild, and 50.4% (71/141) had minimal levels of depression as per BDI scores (Figure 1). Mixed-effects models showed there was a significant effect of time (F(5, 6105) = 13.113; P ≤ .001; = 0.045; 95% CI, 0.016–0.072) and group (F(3, 136) = 53.672; P ≤ .001; = 0.399; 95% CI, 0.347–0.444). Mixed-effects models also showed a significant group-by-time interaction with an effect size between medium and large (F(15, 605) = 7.180; P < .001; = 0.077; 95% CI, 0.028–0.097), demonstrating that BDI changes over time were different across the 4 severity levels (Figure 1). In participants with severe levels of depression at baseline (scores ≥29), BDI scores at all treatment visits and follow-ups were significantly lower than at baseline (all P < .001). Among participants with moderate levels of depression (scores 20–28), BDI scores were significantly lower at weeks 4 (P < .05) and 8 (P < .01) and at both follow-ups (P < .05) compared with baseline. In participants with mild levels of depressive symptomatology (scores 14–19), mean BDI scores at weeks 4 and 8 (all P < .001) and all follow-ups (all P < .05) were lower than at baseline. Finally, no significant changes in mean BDI scores were found among patients with minimal levels of depression (scores ≤13).
Figure 1.
Changes over time in the Beck Depression Inventory–II (BDI-II) stratified by level of severity. The figure displays the means, which are connected by lines. Error bars represent the standard error of the mean.
Changes in BDI Scores by Psychiatric Diagnosis
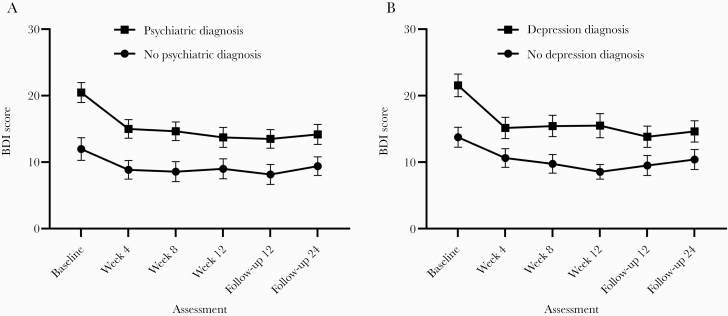
Most participants (64.5% or 91/141) had a history of a psychiatric diagnosis. Mixed-effects models showed that there was a significant effect of time (F(5, 615) = 11.471; P ≤ .001; = 0.027; 95% CI, 0.007–0.043) and small group size (F(1, 139) = 10.871; P ≤ .01; = 0.053; 95% CI, 0.30–0.081), but did not indicate a significant group-by-time interaction (F(5, 615) = 1.333; P = .25; = 0.003; 95% CI, 0.000–0.004). Among participants with a psychiatric diagnosis, BDI scores at all treatment visits and follow-ups were significantly lower than at baseline (all P < .001). Changes in BDI scores were only observed between baseline and weeks 4 and 8 (P < .05) among participants without a psychiatric diagnosis (Figure 2A).
Figure 2.
A and B, Changes over time in the Beck Depression Inventory–II (BDI-II) stratified by psychiatric status. The figure displays the means, which are connected by lines. Error bars represent the standard error of the mean.
Of the 141 patients, 67 (47.5%) had a diagnosis of depression based on the information in their medical chart. Mixed-effects models showed that there was a significant main effect of time (F(5, 615) = 11.461; P ≤ .001; = 0.027; 95% CI, 0.007–0.043) and group (depression vs no depression; F(1, 139) = 8.693; P ≤ .01; = 0.049; 95% CI, 0.027–0.076), but did not show a significant group-by-time interaction (F(5, 615) = 1.313; P = .25; = 0.003; 95% CI, 0.00–0.005). In participants with depression, BDI scores at all treatment visits (weeks 4, 8: all P < .001; week 12: P < .01) and follow-ups (follow-ups 12 and 24) were significantly lower than at baseline (all P < .001). In participants without a diagnosis of depression, BDI scores were significantly lower at weeks 4, 8 (all P < .001), and 12 (P < .01) and both follow-ups compared with baseline (P < .05) (Figure 2B).
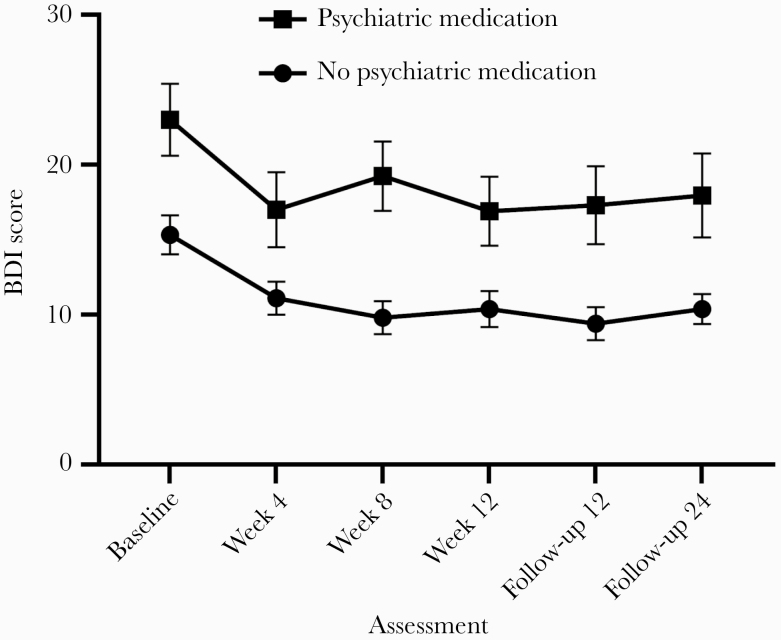
Changes in BDI Scores by Psychiatric Medication at Baseline
Overall, 27.7% of the sample (39/141) was taking psychiatric medication at baseline (Table 1). Mixed-effects models testing the effect of psychiatric medication (Figure 3) showed a significant main effect of both time (F(5, 615) = 11.403; P ≤ .001; = 0.028; 95% CI, 0.005–0.049) and group (F(1, 139) = 15.604; P ≤ .0001; = 0.073; 95% CI, 0.041–0.111), but not for group-by-time interaction (F(5, 615) = .668; P = .64; = 0.002; 95% CI, 0.000–0.003). In participants who did not report taking psychiatric medication, mean BDI scores at all treatment visits and follow-ups were significantly lower than at baseline (all P ≤ .001). In participants who reported taking medication at baseline, mean BDI scores at week 4 (P < .01) as well as both follow-ups (P < .05) were significantly lower than mean scores at baseline.
Figure 3.
Changes over time in the Beck Depression Inventory–II (BDI-II) stratified by psychiatric medication at baseline. The figure displays the means, which are connected by lines. Error bars represent the standard error of the mean.
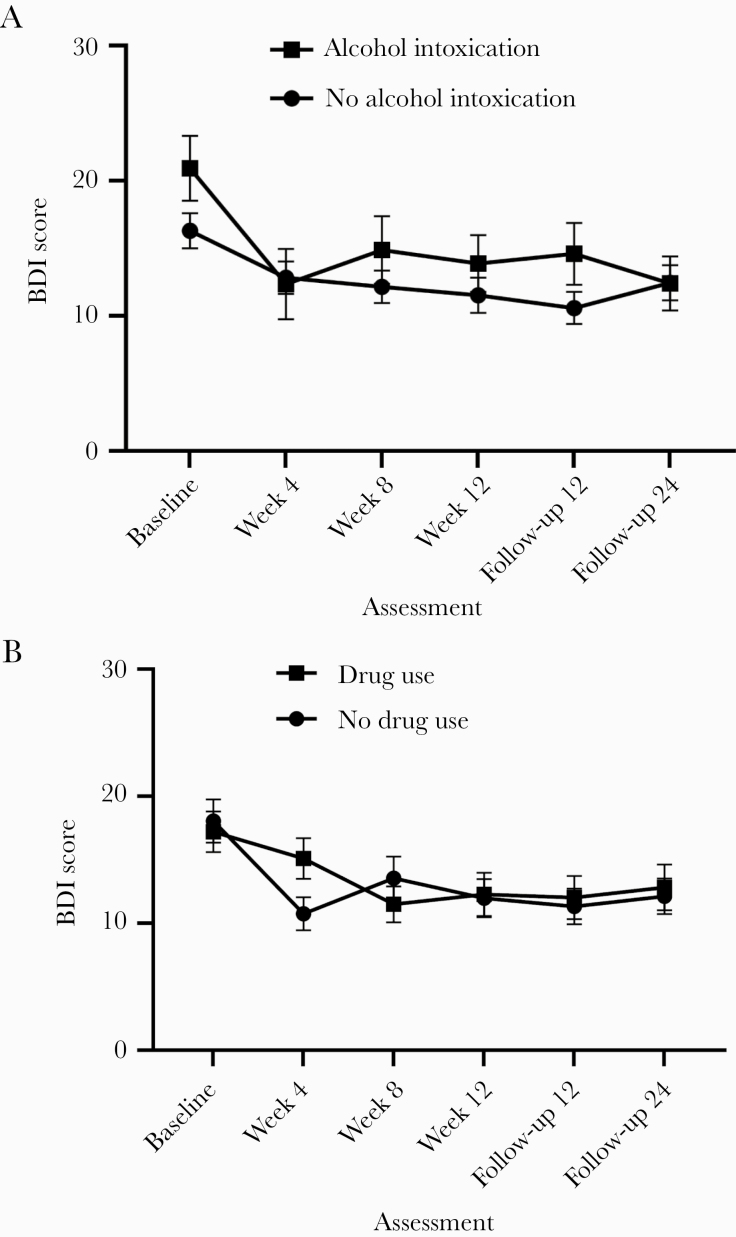
Changes in BDI Scores by Alcohol Use to Intoxication
Mixed-effects models testing the effect of alcohol use to intoxication (Figure 4A) showed a significant main effect of both time (F(5, 614) = 11.506; P ≤ .001; = 0.028; 95% CI, 0.006–0.049) and group (F(1, 614) = 4.787; P = .028; = 0.006; 95% CI, 0.000–0.022), but not for group-by-time interaction (F(5, 614) = 1.188; P = .31; = 0.006; 95% CI, 0.000–0.022). In participants who did not report alcohol use to intoxication, mean BDI scores at all treatment visits and follow-ups were significantly lower than at baseline (all P < .01). In participants who reported alcohol use to intoxication across research visits, mean BDI scores at week 4 and week 12 as well as follow-up at 24 weeks (P < .01) were significantly lower than mean scores at baseline.
Figure 4.
A and B, Changes over time in the Beck Depression Inventory–II (BDI-II) stratified by recent alcohol or drug use. The figure displays the means, which are connected by lines. Error bars represent the standard error of the mean.
Changes in BDI Scores by Drug Use
Mixed-effects models examining the effect of any drug use on depressive symptoms (Figure 4B) showed a significant effect of time (F(5, 614) = 11.502; P ≤ .001; = 0.028; 95% CI, 0.005–0.047), but not group (F(1, 614) = 2.510; P = .11; = 0.000; 95% CI, 0.000–0.008) or group-by-time interaction (F(5, 614) = 1.390; P = .22; = 0.007; 95% CI, 0.000–0.016). In participants who did not report drug use, mean BDI scores at all treatment visits and follow-ups were significantly lower than at baseline (all P < .05). In participants who reported drug use across research visits, mean BDI scores at week 8 and week 12 as well as at both follow-ups (P < .05) were significantly lower than mean scores at baseline.
CONCLUSIONS
This study is the first to examine changes in depressive symptoms among a sample of PWID (including people actively using drugs) on OAT receiving DAA treatment for HCV. Two major findings are noted: (1) HCV treatment initiation promoted significant declines in depressive symptoms that persisted long term, regardless of baseline symptom severity; (2) PWID with a diagnosis of depression or other psychiatric diagnoses, hazardous drinking, or concurrent drug use experienced improvement in depressive symptoms that was maintained over time.
Our results document that HCV treatment was associated with sustained declines in depressive symptoms among OAT-maintained PWID actively using drugs, independent of the severity of their symptoms. This result aligns with earlier studies showing that individuals with [24, 25] and without [19, 20, 22, 23] a history of injecting drugs receiving HCV care with DAA experience reductions in depressive symptoms [19, 25]. It is noteworthy that our results are the first to extend those findings to PWID with active drug use receiving DAA therapies, as earlier studies that included PWID with active drug use were performed with interferon-based therapies [5, 28]. Contrary to our findings, the 2 earlier studies conducted among PWID on OAT receiving interferon medication showed that HCV treatment was associated with an increase [5] or no change in depressive symptoms [28]. Overall, our results further support the beneficial effects of providing HCV care to PWID actively using drugs beyond SVR achievement. Various mechanisms have been proposed to explain the etiology of depression among individuals with HCV [32]. Inflammatory conditions (eg, vasculitis, inflammatory arthritis) [33] and physical symptoms (eg, pain, fatigue) associated with HCV can contribute to depression morbidity. Individuals infected with HCV frequently report feelings of stigmatization and social isolation due to their diagnoses, which can cause depression [34]. In our sample, virus clearance may have reduced HCV-induced cerebral inflammation, which could have resulted in a decrease in depressive symptoms [21]. An alternative biological pathway is that virus replication and neuro-invasion could have been reversed with successful antiretroviral treatment [33]. Finally, recurrent interactions with and social support from providers and research staff members may have facilitated a decline in depressive symptoms that remained over time [35].
Another notable finding is that individuals with a prior diagnosis of depression or any psychiatric diagnosis experienced sustained improvements in depressive symptoms. This finding matches with those observed in earlier studies that included individuals with current or lifetime psychiatric diagnoses [21, 24, 25] and, more importantly, extends the literature by analyzing changes in depressive symptoms separately for patients with and without psychiatric diagnoses. Individuals with depressive symptomatology tend to avoid pleasant situations, engage in fewer activities, and isolate themselves, which might collectively worsen depressive symptoms. It is possible that decreasing isolation and increasing activities during treatment (eg, attending research visits, treatment visits) may have helped to improve our participants’ mood [36]. The fact that these HCV patients may have received additional support from providers during treatment due to their psychiatric diagnosis could also partially explain this finding [37]. Overall, this study indicates that when PWID with a psychiatric diagnosis engage in HCV treatment, not only do they not experience a worsening in symptoms, but they also show improvements.
The finding that drug use and alcohol drinking did not interfere with reductions in depressive symptoms during DAA treatment is consistent with earlier studies that included patients with a history of drug injection and current alcohol use or a diagnosis of a substance use disorder [21, 24, 25]. A major difference is that this study included active drug users, whereas these prior studies involved individuals with history of drug use or a clinical diagnosis [21, 24, 25]. It is noteworthy that the participants in our study were exposed to clinical settings where illicit drug use and alcohol drinking were acknowledged and not stigmatized. PWID infected with HCV frequently experience stigma related to injection drug use and diagnosis [38]. Stigma is associated with mental illness, and further, there is evidence that experiencing stigma is associated with an increased risk of a diagnosis of depression and increased depressive symptomatology in people with HCV [39]. It is possible that interactions within a nonjudgmental health care setting may have decreased their perceived stigma, which may also have led to improvements in their depressive symptomatology.
This study has a number of limitations. First, participants in this study had a low socioeconomic status, so it may not be possible to generalize our findings to HCV-infected PWID who are more affluent and educated. Second, changes in depressive symptomatology were solely assessed using the BDI. Third, diagnosis of depression or other psychiatric diagnosis was based on clinician judgment, not via a structured diagnostic tool. Fourth, our study assessed depressive symptoms up to 24 weeks after the end of treatment. Future studies should examine whether reductions in depressive symptomatology are maintained over longer periods of time. Fifth, the specific type of psychiatric medication that participants were taking was not available in participants’ charts, so we were unable to include this information in the analyses. Sixth, all patients were maintained on OAT (either methadone or buprenorphine), so the results may not be generalizable to PWID not on OAT. Finally, the PREVAIL study only had 7 participants who did not achieve SVR (and were available for follow-up visits), so it is impossible to evaluate the effect of HCV cure on depressive symptoms. Future studies with larger samples of PWID including failed SVR should seek to evaluate this question, including the ongoing HERO study [40].
In summary, among PWID on OAT who had been treated for HCV, HCV treatment initiation was associated with significant reductions in depressive symptoms that persisted long term, regardless of symptom severity, history of depression or other psychiatric diagnosis, and concurrent drug use or alcohol drinking. Therefore, our data point out the need to develop and implement formal interventions to address the burden of psychiatric symptoms among PWID infected with HCV while they receive treatment.
Acknowledgments
We thank the study participants.
Financial support. This work was supported by the National Institute on Drug Abuse (R01DA034086) and Gilead Sciences (IN-337–1779).
Potential conflicts of interest. A.H.L. reports grant support from Gilead Sciences during the conduct of this study and grants and personal fees from Gilead Sciences and Merck Pharmaceuticals. The rest of the authors declare no conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. Conception and design: A.H.L., I.P.-V., M.H.; analysis and interpretation of the data: I.P.-V., J.N., M.H., A.H.L.; drafting of the article: I.P.-V., M.H., A.H.L.; critical revision for important intellectual content: M.H., A.H.L., M.J.A., B.L.N.; final approval of the article: I.P.-V., J.N., M.J.A., B.L.N., L.A., M.H., A.H.L.; provision of study materials or patients: A.H.L.; statistical expertise: M.H.; obtaining funding: M.H., A.H.L.; administrative, technical, or logistic support: L.A., A.H.L.; collection and assembly of data: I.P.-V., J.N., L.A., M.H., A.H.L.
References
- 1. Basseri B, Yamini D, Chee G, et al. Comorbidities associated with the increasing burden of hepatitis C infection. Liver Int 2010; 30:1012–8. [DOI] [PubMed] [Google Scholar]
- 2. Fortier E, Alavi M, Bruneau J, et al. ; ETHOS Study Group Depression, anxiety, and stress among people with chronic hepatitis C virus infection and a history of injecting drug use in New South Wales, Australia. J Addict Med 2017; 11:10–8. [DOI] [PubMed] [Google Scholar]
- 3. Grebely J, Dore GJ, Alami NN, et al. Safety and efficacy of glecaprevir/pibrentasvir in patients with chronic hepatitis C genotypes 1-6 receiving opioid substitution therapy. Int J Drug Policy 2019; 66:73–9. [DOI] [PubMed] [Google Scholar]
- 4. Hauser P, Morasco BJ, Linke A, et al. Antiviral completion rates and sustained viral response in hepatitis C patients with and without preexisting major depressive disorder. Psychosomatics 2009; 50:500–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jerkeman A, Norkrans G, Lidman C, et al. Treatment for chronic hepatitis C in a cohort of opiate substitution therapy recipients in three Swedish cities – completion rates and efficacy. Eur J Gastoenterol Hepatol 2014; 26:523–31. [DOI] [PubMed] [Google Scholar]
- 6. Guadagnino V, Trotta MP, Carioti J, et al. ; Nocchiero Study Group Does depression symptomatology affect medication compliance during the first weeks of anti-HCV therapy in intravenous drug users? Dig Liver Dis 2006; 38:119–24. [DOI] [PubMed] [Google Scholar]
- 7. Rogal SS, Arnold RM, Chapko M, et al. The patient-provider relationship is associated with hepatitis C treatment eligibility: a prospective mixed-methods cohort study. PLoS One 2016; 11:e0148596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Evon DM, Simpson KM, Esserman D, et al. Barriers to accessing care in patients with chronic hepatitis C: the impact of depression. Aliment Pharmacol Ther 2010; 32:1163–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alavi M, Grebely J, Matthews GV, et al. ; ATAHC Study Group Effect of pegylated interferon-α-2a treatment on mental health during recent hepatitis C virus infection. J Gastroenterol Hepatol 2012; 27:957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hajarizadeh B, Cunningham EB, Reid H, et al. Direct-acting antiviral treatment for hepatitis C among people who use or inject drugs: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2018; 3:754–67. [DOI] [PubMed] [Google Scholar]
- 11. Gallach M, Vergara M, da Costa JP, et al. Impact of treatment with direct-acting antivirals on anxiety and depression in chronic hepatitis C. PLoS One 2018; 13:e0208112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Farooq MA, Mehmood A, Haque IU, et al. Drug induced anxiety and depression in patients treated for chronic hepatitis C. J Fatima Jinnah Med Univ 2018; 12:130–2. [Google Scholar]
- 13. Miarons M, Sánchez-Ulayar A, Sempere G, et al. New direct-acting antivirals for hepatitis C treatment and neuropsychiatric symptoms in psychiatric risk groups. Eur J Hosp Pharm 2019; 26:135–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Egmond E, Mariño Z, Navines R, et al. Incidence of depression in patients with hepatitis C treated with direct-acting antivirals. Braz J Psychiatry 2020; 42:72–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Machado MO, Oriolo G, Bortolato B, et al. Biological mechanisms of depression following treatment with interferon for chronic hepatitis C: a critical systematic review. J Affect Disord 2017; 209:235–45. [DOI] [PubMed] [Google Scholar]
- 16. Evon DM, Sarkar S, Amador J, et al. Patient-reported symptoms during and after direct-acting antiviral therapies for chronic hepatitis C: the PROP UP study. J Hepatol 2019; 71:486–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tunçel ÖK, Akyol D, Pullukçu H, et al. Impact of direct acting antiviral agents on psychiatric and sexual health of patients with hepatitis C virus. Viral Hepatit Dergisis 2019; 25:25–31. [Google Scholar]
- 18. Kleefeld F, Heller S, Ingiliz P, et al. Interferon-free therapy in hepatitis C virus (HCV) monoinfected and HCV/HIV coinfected patients: effect on cognitive function, fatigue, and mental health. J Neurovirol 2018; 24:557–69. [DOI] [PubMed] [Google Scholar]
- 19. Hahn D, Stokes CS, Kaiser R, et al. Antidepressant effects of direct-acting antivirals against hepatitis C virus—results from a pilot study. Eu J Clin Invest 2018; 48:e13024. [DOI] [PubMed] [Google Scholar]
- 20. Kesen O, Kani HT, Yanartaş Ö, et al. Evaluation of depression, anxiety and quality of life in hepatitis C patients who treated with direct acting antiviral agents. Turk J Gastroenterol 2019; 30:801–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sundberg I, Lannergård A, Ramklint M, Cunningham JL. Direct-acting antiviral treatment in real world patients with hepatitis C not associated with psychiatric side effects: a prospective observational study. BMC Psychiatry 2018; 18:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takeda K, Noguchi R, Namisaki T, et al. Efficacy and tolerability of interferon-free regimen for patients with genotype-1 HCV infection. Exp Ther Med 2018; 16:2743–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tang LS, Masur J, Sims Z, et al. Safe and effective sofosbuvir-based therapy in patients with mental health disease on hepatitis C virus treatment. World J Hepatol 2016; 8:1318–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goñi Esarte S, Juanbeltz R, Martínez-Baz I, et al. Long-term changes on health-related quality of life in patients with chronic hepatitis C after viral clearance with direct-acting antiviral agents. Rev Esp Enferm Dig 2019; 111:445–52. [DOI] [PubMed] [Google Scholar]
- 25. Juanbeltz R, Martínez-Baz I, San Miguel R, et al. Impact of successful treatment with direct-acting antiviral agents on health-related quality of life in chronic hepatitis C patients. PLoS One 2018; 13:e0205277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Degenhardt L, Peacock A, Colledge S, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health 2017; 5:e1192–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Norton BL, Akiyama MJ, Zamor PJ, Litwin AH. Treatment of chronic hepatitis C in patients receiving opioid agonist therapy: a review of best practice. Infect Dis Clin North Am 2018; 32:347–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schäfer A, Wittchen H-U, Backmund M, et al. Psychopathological changes and quality of life in hepatitis C virus-infected, opioid-dependent patients during maintenance therapy. Addiction 2009; 104:630–40. [DOI] [PubMed] [Google Scholar]
- 29. Akiyama MJ, Norton BL, Arnsten JH, et al. Intensive models of hepatitis C care for people who inject drugs receiving opioid agonist therapy: a randomized controlled trial. Ann Intern Med 2019; 170:594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Akiyama MJ, Agyemang L, Arnsten JH, et al. Rationale, design, and methodology of a trial evaluating three models of care for HCV treatment among injection drug users on opioid agonist therapy. BMC Infect Dis 2018; 18:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Beck AT, Steer RA, Brown G. BDI-II, Beck Depression Inventory: Manual. San Antonio, TX: Psychological Corp; 1996. [Google Scholar]
- 32. Yeoh SW, Holmes ACN, Saling MM, et al. Depression, fatigue and neurocognitive deficits in chronic hepatitis C. Hepatol Int 2018; 12:294–304. [DOI] [PubMed] [Google Scholar]
- 33. Adinolfi LE, Nevola R, Rinaldi L, et al. Chronic hepatitis C virus infection and depression. Clin Liver Dis 2017; 21:517–34. [DOI] [PubMed] [Google Scholar]
- 34. Córdoba J, Flavià M, Jacas C, et al. Quality of life and cognitive function in hepatitis C at different stages of liver disease. J Hepatol 2003; 39:231–8. [DOI] [PubMed] [Google Scholar]
- 35. Nakimuli-Mpungu E, Wamala K, Okello J, et al. Group support psychotherapy for depression treatment in people with HIV/AIDS in Northern Uganda: a single-centre randomised controlled trial. Lancet HIV 2015; 2:e190–9. [DOI] [PubMed] [Google Scholar]
- 36. Solomonov N, Bress JN, Sirey JA, et al. Engagement in socially and interpersonally rewarding activities as a predictor of outcome in “Engage” behavioral activation therapy for late-life depression. Am J Geriatr Psychiatry 2019; 27:571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huckans M, Mitchell A, Ruimy S, et al. Antiviral therapy completion and response rates among hepatitis C patients with and without schizophrenia. Schizophr Bull 2010; 36:165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Treloar C, Rhodes T. The lived experience of hepatitis C and its treatment among injecting drug users: qualitative synthesis. Qual Health Res 2009; 19:1321–34. [DOI] [PubMed] [Google Scholar]
- 39. Golden J, Conroy RM, O’Dwyer AM, et al. Illness-related stigma, mood and adjustment to illness in persons with hepatitis C. Soc Sci Med 2006; 63:3188–98. [DOI] [PubMed] [Google Scholar]
- 40. Litwin AH, Jost J, Wagner K, et al. ; HERO Study Group Rationale and design of a randomized pragmatic trial of patient-centered models of hepatitis C treatment for people who inject drugs: The HERO study. Contemp Clin Trials 2019; 87:105859. [DOI] [PMC free article] [PubMed] [Google Scholar]