Abstract
Cancer is one of the most serious diseases that are harmful to human health. Systemic chemotherapy is an optimal therapeutic strategy for the treatment of cancer, but great difficulty has been encountered in its administration in the form of multidrug resistance (MDR). As an enzyme on the outer cell surface, CD13 is documented to be involved in the MDR development of tumor cells. In this review, we will focus on the role of CD13 in MDR generation based on the current evidence.
Key words: CD13, Multidrug resistance (MDR), Drug efflux, Chemotherapy, Reactive oxygen species, Immune suppression
INTRODUCTION
Chemotherapy is widely used to treat cancer, often with effective results1. Chemotherapeutic drugs can be divided into the following kinds according to their antitumor mechanisms. (1) Alkylating agents, represented by cyclophosphamide and cisplatin, prevent tumor regeneration by targeting DNA synthesis with cross-linking DNA. Hodgkin’s lymphoma and breast and ovarian cancer are sensitive to treatment with alkylating agents2. (2) Antimetabolites used in the treatment of chronic leukemia, gastric cancer (GC), and colorectal cancer are represented by 5-fluorouracil, methotrexate, and cytarabine. It has been proven that antimetabolites can interfere with the synthesis of DNA and RNA by reducing the intake of purine nucleotides3. (3) Antitumor antibiotics, including bleomycin and adriamycin, have been widely used in cancer chemotherapy and function by intercalating into DNA base pairs to inhibit the replication of DNA4. Beyond these, anticancer drugs obtained from plants, such as vincristine and paclitaxel, can restrain the mitosis that realizes cell regeneration5.
Unfortunately, the application of chemotherapeutic drugs is hindered by multidrug resistance (MDR), in which tumor cells show resistance to not only a specific drug but also other drugs with different structures and mechanisms6. Emerging evidence has demonstrated that MDR development represents an important contributor to the unfavorable prognosis of GC patients and the majority of GC deaths7. GC patients with MDR often exhibit a median survival period that ranges from 3 to 6 months8. A clinical trial was performed in which hepatocellular carcinoma (HCC) patients were randomly divided into two groups, including 112 patients who did not receive chemotherapy and 120 patients treated with cisplatin (100 mg) treatment in advance, followed by the administration of mitomycin, cisplatin, and adriamycin for more than 4 weeks. The results showed that the rate of response in HCC patients treated without chemotherapy and in those treated with cisplatin treatment was 26.7% and 11.7%, respectively, suggesting that HCC cells are also less sensitive to chemotherapeutic drugs, probably due to MDR development9. Thus, MDR development has become an urgent problem to be solved in cancer chemotherapy.
CD13, also known as aminopeptidase N (APN), is an extracellular peptide belonging to the type II zinc-dependent metalloproteinase family, the members of which are widely expressed and have catalytic activity10. CD13 promotes tumor angiogenesis, invasion, and metastasis in breast, ovarian, and prostate cancer cells by participating in enzymatic cleavage of the polypeptide chain11. More strikingly, there is a positive association between CD13 expression and MDR development in tumor cells12–14. Herein we provide comprehensive insights into the role of CD13 in promoting MDR development by mediating the increase in drug efflux, ROS inhibition, and immune suppression.
DRUG EFFLUX: THE NOTORIOUS REASON FOR MDR DEVELOPMENT
Drug efflux, facilitated by membrane transport proteins, is associated with the development of MDR in tumor cells15. Membrane transport proteins represented by the ATP-binding cassette (ABC) transport superfamily, including P-glycoprotein (P-gp), multidrug resistance-associated proteins (MRPs), lung resistance protein (LRP), and breast cancer resistance protein (BCRP/ABCG), pump out a variety of chemotherapeutic drugs to increase drug efflux and reduce intracellular concentrations, thereby inducing drug resistance in tumor cells16. P-gp has 12 transmembrane domains and two ATP binding sites that can be inserted into the cell membrane17. The transmembrane region as a membrane channel is conducive to material transport, while the ATP binding site is responsible for the energy supply18. Expression of the MDR1 gene encoding P-gp is upregulated in the chemoresistant GC cell line SCG-7901/ADR compared to that in parental SCG-7901 cells19. Additionally, P-gp is an ABC transport protein that is known to contribute to the incidence of MDR in HCC20. However, new evidence indicates that the MDR1 gene and P-gp are not detected in some tumors with MDR, but MRPs, represented by MRP1-9, were found on the cell membrane of these non-P-gp-expressing MDR cells21. MRP1 expression is used to guide the selection of chemotherapeutic regimens, consistent with the positive association between MRP1 expression and drug susceptibility22. MRP5 is involved in the development of intrinsic resistance, while MRP2 and MRP3 are associated with acquired resistance of HCC cells23. In addition, increased invasive depth and numbers of metastatic lymph nodes in GC specimens are tied to an elevated expression of ABCG224. Furthermore, protein levels of LRP are higher in GCs with high differentiation than in mucinous carcinomas with poorly differentiated cells, suggesting that LRP is necessary for the development of MDR in GC cells25. Recent evidence suggests that CD13 induces MDR in tumor cells by triggering increased drug efflux12. In particular, CD13 may activate the expression of ABC family proteins in both cancer stem cells (CSCs) and tumor cells to accelerate MDR development13,26.
CD13: THE GENESIS OF DRUG EFFLUX MEDIATED BY CANCER STEM CELLS
CSCs, which include a small number of tumor cells with self-renewal and tumor-initiating ability, slow growth, and low differentiation status, play a decisive role in the initiation of tumor formation and growth27. CSCs can induce drug resistance, recurrence, and metastasis of malignant tumors28. Nevertheless, the current research on CSCs is still in the early stages. More exciting advances in sorting and identifying cancer stem cells have been reported. CSCs can be identified by magnetic-activated cell sorting (MACS) and fluorescence-activated cell sorting (FACS) using specific surface markers29. In addition, CSCs are also characterized as side population (SP) cells that can efflux Hoechst 33342, showing low fluorescence or no fluorescence, and thus can be separated by FACS30. At present, SP cells have been successfully isolated from HCC, pancreatic cancer, lung cancer, and GC cells31–34. Cell markers, such as CD24, CD105, CD44, CD90, CD133, EpCAM, CD13, and OV6, have been identified on these SP cells35.
CD13 plays a vital role in the self-renewal capacity of liver cancer stem cells (LCSCs), which are derived from the differentiation of hepatic stem cells and oval cells36. CD13+ LCSCs maintain a semidormant state at the G0/G1 transition in the hypoxic environment of the liver fibrous capsule, and the growth of new tumors is demonstrated after these cells are transplanted subcutaneously into immune-deficient mice, but no new tumors grow after anti-CD13 antibody or the CD13 inhibitor bestatin is administered37,38. CD13+ SP cells were also found to be resistant to irinotecan, and these SP cells highly express ABCG239. In vivo results demonstrate that CD13+ cells have stronger lung metastasis ability as well as greater resistance to doxorubicin and vincristine than CD13− MHCC-97L cells due to the high expression of ABCG240. More intriguingly, Li-7, a unique CD13+ HCC line that was developed by cancer stem cell differentiation in culture, has been shown to be resistant to sorafenib due to the high expression of P-gp and MRP241.
Emerging evidence has demonstrated that the induction of ABC transporter expression mediated by CD13 in CSCs is caused by abnormal activation of the Hedgehog (Hh) signaling pathway42. The Hh pathway plays an important role in maintaining the function of CSCs but is mostly inactivated in normal cells. The Hh signal is delivered by two receptors, also known as Patched (Ptc) and Smoothened (Smo), on the cell membrane. Hedgehog signal transduction begins from Patched, is transmitted by a G protein-coupled receptor, and finally initiates the activation of the Gli family to promote gene transcription43. An epoch-making study indicates that green fluorescent protein (GFP)-labeled CD13 can be captured by CD90+ A549 cells, and this GFP–CD13 fusion protein localized to Patched44. A further study showed that human glioblastoma stem cells display significant resistance to etoposide and 5-fluorouracil with aberrant expression of CD13 and CD13345, and transcriptional activity of Gli-1 in HCC is reduced after a blocking antibody targeting CD13 is administered, accompanied by a decrease in ABCG2 expression46. Moreover, CD13 can act as a pseudoligand of Patched to sensitize the Hedgehog signaling pathway, leading to the upregulation of ABCG2, P-gp, MRP2, and MRP3, which are direct targets of Gli1 in the induction of drug resistance47.
Signal transduction initiated by Notch, including Notch-1, Notch-2, Notch-3, and Notch-4, is another pathway for maintaining normal growth and differentiation of CSCs. Notch-1 is the most commonly studied receptor, and its role in MDR formation caused by CD13 expression is a focus of much research. Notch-1 expression is upregulated in colon cancer cell lines, whereas inhibition of Notch-1 can increase the sensitivity of tumor cells to regorafenib48. A further study was carried out in which CD44+CD13+ SP cells were sorted from gastric cancer stem cells. These SP cells show sustained loss of Notch-1 after stimulation by small interfering RNA targeting CD13, but MW167, an inhibitor of Notch-1, had no effect on the expression of CD1349. In addition, constitutive expression of Notch-1 was positively related to ABCG2 protein levels in glioma stem cells50. More importantly, miR-34 has also been shown to be involved in regulating the expression of CD13, and miR-34 overexpression restrained CD13 expression to decrease Notch-1 levels, thus reversing the resistance of pancreatic cancer stem cells to gemcitabine51.
CD13: THE MODERATOR OF DRUG EFFLUX MEDIATED BY TUMOR CELLS
It is currently believed that CD13 can be used as a hub for promoting the expression of ABC transporters. Moreover, CD13 can initiate this regulation by upregulating the expression of p53 and CDX2 in HCC cells52. The activation of P-gp requires self-phosphorylation. Protein kinase C (PKC) plays important roles in several signal transduction cascades by phosphorylating the serine and threonine amino acid residues on signaling proteins53. Additionally, the MDR cell lines obtained by drug selection are often accompanied by increased PKC activity, indicating a positive correlation between PKC expression and MDR. Furthermore, the expression of CD13 in the K562 leukemia cell line can induce the upregulation of PKC-γ, which may be related to the high phosphorylation of P-gp54.
Through the comparison of the expression of MRP2, MRP3, and MRP5 in the normal liver cell line L-02, the hepatoma cell line BEL and the HCC drug-resistant cell line BEL/ADM, one study clarified that MRP2 expression in EL/ADM cells is higher than that in L-02 and BEL cells, but the expression of MRP3 and MRP5 was distinctly different in L-02, BEL, and BEL/ADM cells55. This suggests that MRP2 may be involved in the development of intrinsic MDR in HCC, and MRP5 and MRP3 expression may be related to acquired MDR in HCC. CD13 expression was positively correlated with the expression of MRP2 and MRP3 in HCC cells and LCSCs, indicating that CD13 mediates intrinsic and extrinsic MDR in HCC cells by increasing drug efflux56. MRPs belong to the ABC superfamily along with P-gp, and MRPs can pump drugs with the help of ATP-dependent glutathione-binding S carrier (GS-X pump), for which the activity of glutathione-S-transferases (GSTs), including GST-π, GST-α, and GST-μ, was essential57. The constructive findings show that alkylating agent and platinum drugs can be excreted out of the cells through this so-called “GSH–drug coupling” mechanism. GST-π is currently considered to be the most closely associated with MDR formation58. In the colorectal cancer cell line SW480, GST-π expression can be obviously induced after CD13 is overexpressed, followed by increased expression of MRP259.
Unlike P-gp and MRPs, LRP is a kind of nonglycoprotein, the gene of which is located in the short arm of the sixth chromosome, which is very close to the gene encoding MRP60. It has been proven that two mechanisms are involved in MDR mediated by LRP. First, LRP prevents drugs from passing through nuclear pores into the nucleus. Additionally, LRP can deliver chemotherapeutic drugs into transport vesicles in the cytoplasm and present atrioventricular distribution, thus expelling them into the extracellular space through exocytosis61,62. CD13 silencing can inhibit LRP expression in lung cancer, ovarian cancer, rectal cancer, and leukemia cells63. Further results indicate that the nuclear pore complex (NPC), which can mediate the nuclear transport of proteins and the nuclear translocation of mRNA, has limited assembly in lung cancer cells, which have a remarkable expression of CD1364.
In conclusion, CD13 plays a vital role in promoting drug efflux mediated by ABC transporters, in which diverse mechanisms are involved.
OXIDATIVE STRESS OBSTRUCTION BY CD13: THE IMPORTANT FACTOR OF MDR GROWTH
Reactive oxygen species (ROS) are formed as a natural byproduct of the normal metabolism of oxygen and are crucial in the maintenance of normal intracellular homeostasis65. However, ROS levels can increase dramatically under environmental stress (e.g., UV light or tumor initiation), which is characterized by oxidative stress66. Chemotherapeutic drugs mostly rely on the induction of tumor cell apoptosis to exert a therapeutic effect, and apoptosis mediated by mitochondrial pathways is largely dependent on ROS. ROS can promote the influx of Ca2+ to promote the release of CytC, which can be combined with apoptotic protease activating factor-1 (Apaf-1) to engender the formation of apoptotic bodies67. These apoptotic bodies can recruit caspase molecules, leading to the activation of caspase 3/9, which is the executor of cell apoptosis68. Unfortunately, when MDR occurs, oxidative stress is blocked. Studies have shown that there is a negative relation between CD13 expression and the level of ROS69. In addition, the expression of Apaf-1 in patients with long-term administration of chemotherapeutic drugs is significantly lower than that in patients treated with initial chemotherapy and is negatively correlated with the expression of CD1370. We have also found that the expression of BCL-XL and BCL-2 in HCC and normal tissues is not the same, and there is also a positive correlation between the BCL-XL and BCL-2 expression and the expression of CD1371.
The mitogen-activated protein kinase (MAPK) pathway is crucial in the eukaryotic signal transmission network. Signal transduction mediated by MAPK and the downstream cascade molecules p38 MAPK, JNK, and ERK1/2 can be propagated by MAP kinase, MAPK kinase, and MEK kinase72. The ERK1/2 signaling pathway regulates cell growth and differentiation, and the signaling pathways of p38 and MAPK JNK play an important role in inflammation initiation and cell apoptosis73. Interestingly, mounting evidence supports a physiological role for ROS as a second messenger responsible for regulating drug resistance together with MAPK74. The release of ROS can inhibit GST activity and reduce the synthesis of glutathione and downregulate P-gp expression, accompanied by inactivation of ERK1/2 and JNK75. Combined treatment with molybdate, 5-fluorouracil, and mitomycin C activated JNK and P38, and elevated levels of intracellular ROS and decreased expression of CD13 were demonstrated76. As such, we believe that high CD13 expression enables inhibition of ROS-induced stress, leading to the inhibition of cell apoptosis, thus generating MDR.
IMMUNE SUPPRESSION BY CD13: AN ACCELERATOR OF MDR DEVELOPMENT
As shown previously, the cancer patients with MDR to chemotherapeutic drugs often exhibited immune tolerance77. It has been reported that CD13 is expressed not only on the surface of tumor cells but also in a large number of other cells and lymphocytes, which participate in the inflammatory response of T lymphocytes78. Critically, when a tumor develops, CD13 can be expressed by antigen-presenting cells such as macrophages and monocytes to inhibit their antigen-presenting ability and the cytotoxic potential of natural killer cells against tumors79. Chemokines (e.g., fMLP), antigen-presenting molecules (e.g., CD86), and a variety of immune-activating substances (e.g., IL-8) display obvious degradation80. CD13 can also reduce the binding of major histocompatibility complex class II (MHC II), thereby reducing the ability of T cells to recognize tumor antigens. Moreover, CD13+CD4+CD25hi regulatory T cells exhibit higher suppressive function than CD4+CD25hi regulatory T cells that do not express CD13 and increase with tumor stage in non-small cell lung cancer patients81. CD13hi neutrophil-like myeloid-derived suppressor cells also exert immune suppression through arginase 1 expression in pancreatic ductal adenocarcinoma82. However, evidence to support that immune escape mediated by CD13 plays a key role in MDR is still lacking.
CONCLUSIONS
MDR of tumor cells to multiple chemotherapeutic agents is still the main reason for chemotherapy failure and is difficult to handle in the treatment of cancer. To seek effective countermeasures to reverse MDR and enhance the effectiveness of chemotherapy for malignant tumors, the mechanism involved in MDR formation should be thoroughly researched.
As an exopeptidase, CD13 works ubiquitously in different peptide metabolism pathways. Interestingly, CD13 is overexpressed in many tumor cells and plays a key role in tumor angiogenesis, invasion, and metastasis, and also has been identified as a factor encouraging MDR development.
The abnormal expression of multidrug resistance-associated proteins is a culprit of increased drug efflux. In this review, we consider that CD13 can regulate the accumulation of ABC transporters, including P-gp, MRPs, LRP, and BCRP/ABCG, from gene transcription and protein expression in tumor cells. Although LRP is a new protein related to MDR, CD13 has been revealed to have a regulatory relationship with it. Therefore, it is obvious that the tumor-associated antigen CD13 can induce the occurrence of MDR by directly targeting ABC transporters.
CSCs are considered the most challenging enemy of tumor treatment because most of them are in a resting stage in the cell cycle. It is noteworthy that CD13 may increase the expression of ABC transporters by activating the Hedgehog and Notch-1 signaling pathways in CSCs to induce MDR development, creating a challenge for treatment. Moreover, p53 and CDX2 can promote the transcriptional activity of genes encoding ABC transporters, while PKC and GSTs provide a way for ABC transporters to pump chemotherapeutic drugs out of tumor cells.
ROS are promoters of cell apoptosis, but tumor cells lag in response to apoptosis, especially when they are resistant to chemotherapeutic drugs. In addition to mediating the efflux of drugs, CD13 can also inhibit the oxidative stress reaction of tumor cells. In particular, CD13 can downregulate ROS expression, resulting in inactivation of the MAPK signaling pathway with increased expression of ABC transporters.
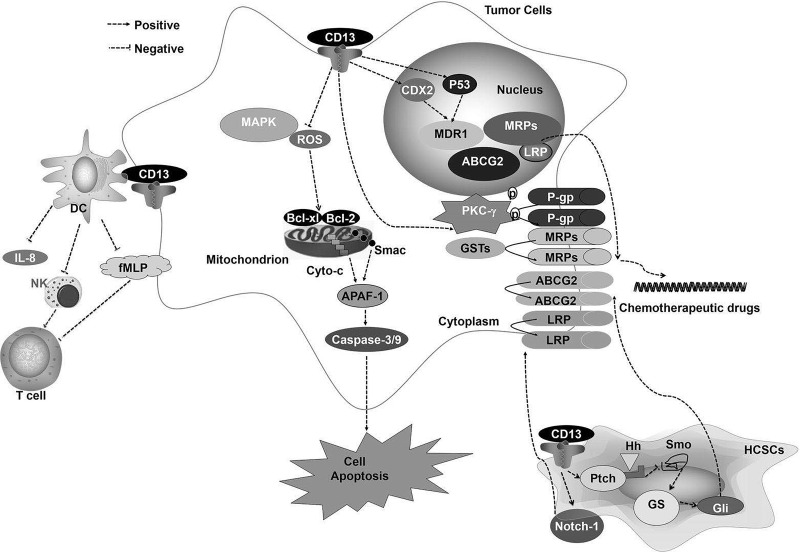
Immune suppression has been suggested as a mediator of tumor occurrence, and immune incompetence is also a cause of drug resistance. Studies have found that the expression of CD13 in lymphocytes can inhibit specific and nonspecific immunity by suppressing the secretion of inflammatory factors and preventing antigen presentation. As shown in Figure 1, CD13 mediates the production of MDR by triggering multiple mechanisms. Our discussion will provide a theoretical basis for further study of tumor MDR. Simultaneously, our review is useful in finding ways to reverse the drug resistance of tumors, and CD13 can be not only a direct target for cancer therapy but also a key point to reverse multidrug resistance. MDR might be resolved by CD13 inhibition induced by bestatin. The combination of bestatin and chemotherapeutic drugs, such as cisplatin, 5-fluorouracil, and the FOLFOX regimen (comprised of 5-fluorouracil, oxaliplatin, and leucovorin), may represent a novel therapeutic approach for GC and HCC patients12–14. However, thus far, bestatin has not received FDA approval or has been tested in clinical trials, and there are no reliable methods to distinguish CD13 expression by normal cells, drug-resistant cells, and drug-sensitive cells. Thus, more research is needed.
Figure 1.
CD13 mediates multidrug resistance (MDR) development by triggering multiple mechanisms.
ACKNOWLEDGMENT
The authors would like to thank AJE (American Journal expert) for editing our manuscript.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL, Siegel RL. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363–85. [DOI] [PubMed] [Google Scholar]
- 2. Kantner T, Watts AG. Characterization of reactions between water-soluble trialkylphosphines and thiol alkylating reagents: Implications for protein-conjugation reactions. Bioconjug Chem. 2016;27(10):2400–6. [DOI] [PubMed] [Google Scholar]
- 3. Składanowski AC. The role of soluble 5′-nucleotidases in the conversion of nucleotide analogs: Metabolic and therapeutic aspects. Curr Med Chem. 2013;20(34):4249–59. [DOI] [PubMed] [Google Scholar]
- 4. Fais S. A nonmainstream approach against cancer. J Enzyme Inhib Med Chem. 2016;31(6):882–9. [DOI] [PubMed] [Google Scholar]
- 5. Tilaoui M, Mouse HA, Jaafari A, Zyad A. Differential effect of artemisinin against cancer cell lines. Nat Prod Bioprospect. 2014;4(3):189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dong X, RJ Mumper. Nanomedicinal strategies to treat multidrug-resistant tumors: Current progress. Nanomedicine (Lond) 2010;5(4):597–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet 2016;388(10060):2654–64. [DOI] [PubMed] [Google Scholar]
- 8. Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang XS, Bannon F, Ahn JV, Johnson CJ, Bonaventure A, Marcos-Gragera R. Global surveillance of cancer survival 1995–2009: Analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries. Lancet 2015;385(9972):977–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 10. Wickström M, Larsson R, Nygren P, Gullbo J. Aminopeptidase N (CD13) as a target for cancer chemotherapy. Cancer Sci. 2011;102(3):501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang X, Fang H, Zhang J, Yuan Y, Xu W. Recent advance in aminopeptidase N (APN/CD13) inhibitor research. Curr Med Chem. 2011;18(32):5011–21. [DOI] [PubMed] [Google Scholar]
- 12. Guo Q, Sui ZG, Xu W, Quan XH, Sun JL, Li X, Ji HY, Jing FB. Ubenimex suppresses Pim-3 kinase expression by targeting CD13 to reverse MDR in HCC cells. Oncotarget 2017;8(42):72652–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guo Q, Jing FJ, Qu HJ, Xu W, Han B, Xing XM, Ji HY, Jing FB. Ubenimex Reverses MDR in gastric cancer cells by activating caspase-3-mediated apoptosis and suppressing the expression of membrane transport proteins. Biomed Res Int. 2019;2019:4390839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guo Q, Jing FJ, Xu W, Li X, Li X, Sun JL, Xing XM, Zhou CK, Jing FB. Ubenimex induces autophagy inhibition and EMT suppression to overcome cisplatin resistance in GC cells by perturbing the CD13/EMP3/PI3K/AKT/NF-κB axis. Aging (Albany NY) 2019;12(1):80–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kachalaki S, Ebrahimi M, Mohamed Khosroshahi L, Mohammadinejad S, Baradaran B. Cancer chemoresistance; biochemical and molecular aspects: A brief overview. Eur J Pharm Sci. 2016;8:20–30. [DOI] [PubMed] [Google Scholar]
- 16. Chen Z, Shi T, Zhang L, Zhu P, Deng M, Huang C, Hu T, Jiang L, Li J. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016;370(1):153–64. [DOI] [PubMed] [Google Scholar]
- 17. Li XQ, Wang L, Lei Y, Hu T, Zhang FL, Cho CH, To KK. Reversal of P-gp and BCRP-mediated MDR by tariquidar derivatives. Eur J Med Chem. 2015;101:560–72. [DOI] [PubMed] [Google Scholar]
- 18. Alfarouk KO, Stock CM, Taylor S, Walsh M, Muddathir AK, Verduzco D, Bashir AH, Mohammed OY, Elhassan GO, Harguindey S. Resistance to cancer chemotherapy: Failure in drug response from ADME to P-gp. Cancer Cell Int. 2015;15:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cui J, Yin Y, Ma Q, Wang G, Olman V, Zhang Y, Chou WC, Hong CS, Zhang C, Cao S, Mao X, Li Y, Qin S, Zhao S, Jiang J, Hastings P, Li F, Xu Y. Comprehensive characterization of the genomic alterations in human gastric cancer. Int J Cancer 2015;137(1):86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gao B, Yang FM, Yu ZT, Li R, Xie F, Chen J, Luo HJ, Zhang JC. Relationship between the expression of MDR1 in hepatocellular cancer and its biological behaviors. Int J Clin Exp Pathol. 2015;8:6995–7001. [PMC free article] [PubMed] [Google Scholar]
- 21. Keppler D. Multidrug resistance proteins (MRPs, ABCCs): Importance for pathophysiology and drug therapy. Handb Exp Pharmacol. 2011;201:299–323. [DOI] [PubMed] [Google Scholar]
- 22. Rocha Gda G, Oliveira RR, Kaplan MA, Gattass CR. 3β-Acetyl tormentic acid reverts MRP1/ABCC1 mediated cancer resistance through modulation of intracellular levels of GSH and inhibition of GST activity. Eur J Pharmacol. 2014;741:140–9. [DOI] [PubMed] [Google Scholar]
- 23. Qian JQ, Sun P, Pan ZY, Fang ZZ. Annonaceous acetogenins reverses drug resistance of human hepatocellular carcinoma BEL-7402/5-FU and HepG2/ADM cell lines. Int J Clin Exp Pathol. 2015;8(9):11934–44. [PMC free article] [PubMed] [Google Scholar]
- 24. Westover D, Li F. New trends for overcoming ABCG2/BCRP-mediated resistance to cancer therapies. J Exp Clin Cancer Res. 2015;34:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hu WQ, Peng CW, Li Y. The expression and significance of P-glycoprotein, lung resistance protein and multidrug resistance-associated protein in gastric cancer. J Exp Clin Cancer Res. 2009;28:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang Q, Chen N, Yu C, Wei Z, Li Z, Jin Z. Aberrant expression of CD13 and stem cell markers in CD133-induced liver cancer in mice. J BUON. 2019;24(4):1408–13. [PubMed] [Google Scholar]
- 27. Hoek KS, Goding CR. Cancer stem cells versus phenotype-switching in melanoma. Pigment Cell Melanoma Res. 2010;23(6):746–59. [DOI] [PubMed] [Google Scholar]
- 28. Guo Z, Li LQ, Jiang JH, Ou C, Zeng LX, Xiang BD. Cancer stem cell markers correlate with early recurrence and survival in hepatocellular carcinoma. World J Gastroenterol. 2014;20(8):2098–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nakayama M, Ogasawara S, Akiba J, Ueda K, Koura K, Todoroki K, Kinoshita H, Yano H. Side population cell fractions from hepatocellular carcinoma cell lines increased with tumor dedifferentiation, but lack characteristic features of cancer stem cells. J Gastroenterol Hepatol. 2014;29(5):1092–101. [DOI] [PubMed] [Google Scholar]
- 30. Zhang H, Xi H, Cai A, Xia Q, Wang XX, Lu C, Zhang Y, Song Z, Wang H, Li Q, Chen L, Guo Z. Not all side population cells contain cancer stem-like cells in human gastric cancer cell lines. Dig Dis Sci. 2013;58(1):132–9. [DOI] [PubMed] [Google Scholar]
- 31. Zhang N, Li R, Tao KS, Cao DY, Ti ZY, Ding R, Cai L, Zhang FQ, Dou KF. Characterization of a stem-like population in hepatocellular carcinoma MHCC97 cells. Oncol Rep. 2010;23(3):827–31. [PubMed] [Google Scholar]
- 32. Niess H, Camaj P, Renner A, Ischenko I, Zhao Y, Krebs S, Mysliwietz J, Jäckel C, Nelson PJ, Blum H, Jauch KW, Ellwart JW, Bruns CJ. Side population cells of pancreatic cancer show characteristics of cancer stem cells responsible for resistance and metastasis. Target Oncol. 2015;10(2):215–27. [DOI] [PubMed] [Google Scholar]
- 33. Ho MM, Ng AV, Lam S, Hung JY. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 2007;67(10):4827–33. [DOI] [PubMed] [Google Scholar]
- 34. Haraguchi N, Utsunomiya T, Inoue H, Tanaka F, Mimori K, Barnard GF, Mori M. Characterization of a side population of cancer cells from human gastrointestinal system. Stem Cells 2006;24(3):506–13. [DOI] [PubMed] [Google Scholar]
- 35. Shimoda M, Ota M, Okada Y. Isolation of cancer stem cells by side population method. Methods Mol Biol. 2018;1692:49–59. [DOI] [PubMed] [Google Scholar]
- 36. Tanimizu N, Mitaka T. Re-evaluation of liver stem/progenitor cells. Organogenesis 2014;10(2):208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Guzman Rojas, Rangel R, Salameh A, Edwards JK, Dondossola E, Kim YG, Saghatelian A, Giordano RJ, Kolonin MG, Staquicini FI, Koivunen E, Sidman RL, Arap W, Pasqualini R. Cooperative effects of aminopeptidase N (CD13) expressed by nonmalignant and cancer cells within the tumor microenvironment. Proc Natl Acad Sci USA 2012;109(5):1637–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Haraguchi N, Ishii H, Mimori K, Tanaka F, Ohkuma M, Ho Min K, Hirofumi A, Daisuke T, HisanoriH, Hiroaki N, Graham F. Barnard, Yuichiro, Masaki M. CD13 is a therapeutic target in human liver cancer stem cells. J Clin Invest. 2010;120(9):3326–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shishido Y, Ueno S, Yamazaki R, Nagaoka M, Matsuzaki T. ABCG2 inhibitor YHO-13351 sensitizes cancer stern/initiating-like side population cells to irinotecan. Anticancer Res. 2013;33(4):1379–86. [PubMed] [Google Scholar]
- 40. Hu C, Li H, Li J, Zhu Z, Yin S, Hao X, Yao M, Zheng S, Gu J. Analysis of ABCG2 expression and side population identifies intrinsic drug efflux in the HCC cell line MHCC-97L and its modulation by Akt signaling. Carcinogenesis 2008;29(12):2289–97. [DOI] [PubMed] [Google Scholar]
- 41. Yamada T, Abei M, Danjoh I, Shirota R, Yamashita T, Hyodo I, Nakamura Y. Identification of a unique hepatocellular carcinoma line, Li-7, with CD13(+) cancer stem cells hierarchy and population change upon its differentiation during culture and effects of sorafenib. BMC Cancer 2015;15:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Carballo GB, Honorato JR, de Lopes GPF, Spohr TCLSE. A highlight on Sonic hedgehog pathway. Cell Commun Signal. 2018;16(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kasper M, Regl G, Frischauf AM, Aberger F. GLI transcription factors: Mediators of oncogenic Hedgehog signalling. Eur J Cancer 2006;42(4):437–45. [DOI] [PubMed] [Google Scholar]
- 44. Giroux-Leprieur E, Costantini A, Ding VW, He B. Hedgehog signaling in lung cancer: From oncogenesis to cancer treatment resistance. Int J Mol Sci. 2018;19(9):2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Girardi D, Barrichello A, Fernandes G, Pereira A. Targeting the hedgehog pathway in cancer: Current evidence and future perspectives. Cells 2019;8(2):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang G, Wang Z, Luo W, Jiao H, Wu J, Jiang C. Expression of potential cancer stem cell marker ABCG2 is associated with malignant behaviors of hepatocellular carcinoma. Gastroenterol Res Pract. 2013;2013:782581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Laukkanen MO, Castellone MD. Hijacking the hedgehog pathway in cancer therapy. Anticancer Agents Med Chem. 2016;16(3):309–17. [DOI] [PubMed] [Google Scholar]
- 48. Mirone G, Perna S, Shukla A, Marfe G. Involvement of Notch-1 in resistance to regorafenib in colon cancer cells. J Cell Physiol. 2016;231(5):1097–105. [DOI] [PubMed] [Google Scholar]
- 49. Zhao Y, Chen X, Ma L, Zuo Z, Zhu Z, Zhu X, Wang Q, He E, Xiong L, Pei J, Xu L, Hou L, Chen S. Electro acupuncture pretreatment induces tolerance against focal cerebral ischemia through activation of canonical Notch pathway. BMC Neurosci. 2012;13:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pohlmann E, Murphy S. Abstract 3862: Isolation and nanoscale visualization of glioblastoma stem-like cells utilizing the Notch1 receptor. Cancer Res. 2014;74:3862. [Google Scholar]
- 51. Yabuuchi S, Pai SG, Campbell NR, de Wilde RF, De Oliveira E, Korangath P, Streppel MM, Rasheed ZA, Hidalgo M. Notch signaling pathway targeted therapy suppresses tumor progression and metastatic spread in pancreatic cancer. Cancer Lett. 2013;335(1):41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Li Y, Bi H, Zhong G, Huang L, Li G, Xia Y, Chen X, Huang M. Effect of phorbol 12-myristate 13-acetate on function and gene expression of P-glycoprotein in adriamycin-resistant K562/ADM cells. Pharmacology 2013;92(3–4):121–30. [DOI] [PubMed] [Google Scholar]
- 53. Sun Q, Li Y. The inhibitory effect of pseudolaric acid B on gastric cancer and multidrug resistance via Cox-2/PKC-α/P-gp pathway. PLoS One 2014;9(9):e107830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yang X, Li X, Duan Z, Wang X. An update on circumventing multidrug resistance in cancer by targeting P-glycoprotein. Curr Cancer Drug Targets 2018;18(7):677–96. [DOI] [PubMed] [Google Scholar]
- 55. Qian JQ, Sun P, Pan ZY, Fang ZZ. Annonaceous acetogenins reverses drug resistance of human hepatocellular carcinoma BEL-7402/5-FU and HepG2/ADM cell lines. Int J Clin Exp Pathol. 2015;8(9):11934–44. [PMC free article] [PubMed] [Google Scholar]
- 56. Liu AQ, Ge LY, Lu XL, Luo XL, Cai YL, Ye XQ, Geng FF. Silencing of the hTERT gene by shRNA inhibits HCC growth in vitro and in vivo. PLoS One 2014;9(9):e107019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lin Y, Huang Y, Zheng W, Wu K, Luo Q, Zhao Y, Xiong S, Wang F. Quantification of bindings of organometallic ruthenium complexes to GSTπ by mass spectrometry. J Inorg Biochem. 2015;146:44–51. [DOI] [PubMed] [Google Scholar]
- 58. Cree IA, Charlton P. Molecular chess? Hallmarks of anti-cancer drug resistance. BMC Cancer 2017;17(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Engl J Med. 2005;352(5):476–87. [DOI] [PubMed] [Google Scholar]
- 60. Fletcher JI, Williams RT, Henderson MJ, Norris MD, Haber M. ABC transporters as mediators of drug resistance and contributors to cancer cell biology. Drug Resist Updat. 2016;26:1–9. [DOI] [PubMed] [Google Scholar]
- 61. Kartal-Yandim M, Adan-Gokbulut A, Baran Y. Molecular mechanisms of drug resistance and its reversal in cancer. Crit Rev Biotechnol. 2016;36(4):716–26. [DOI] [PubMed] [Google Scholar]
- 62. Hagmann W, Jesnowski R, Faissner R, Guo C, Löhr JM. ATP-binding cassette C transporters in human pancreatic carcinoma cell lines. Pancreatology 2009;9(1–2):136–44. [DOI] [PubMed] [Google Scholar]
- 63. Murakami S. Molecular mechanism of multi-drug resistance. Nihon Rinsho 2008;66(1):193–203. [PubMed] [Google Scholar]
- 64. Chen YL, Yang TY, Wu CL, Chen KC, Hsu SL, Hsueh CM. Mechanisms underlying lung resistance-related protein (LRP)-mediated doxorubicin resistance of non-small cell lung cancer cells. Chin J Physiol. 2016;59(6):331–47. [DOI] [PubMed] [Google Scholar]
- 65. Bose J, Rodrigo-Moreno A, Shabala S. ROS homeostasis in halophytes in the context of salinity stress tolerance. J Exp Bot. 2014;65(5):1241–57. [DOI] [PubMed] [Google Scholar]
- 66. Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24(10):R453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jia G, Wang Q, Wang R, Deng D, Xue L, Shao N, Zhang Y, Xia X, Zhi F, Yang Y. Tubeimoside-1 induces glioma apoptosis through regulation of Bax/Bcl-2 and the ROS/cytochrome C/caspase-3 pathway. Onco Targets Ther. 2015;8:303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kaleem S, Siddiqui S, Siddiqui HH, Badruddeen, Hussain A, Arshad M, Akhtar J, Rizvi A. Eupalitin induces apoptosis in prostate carcinoma cells through ROS generation and increase of caspase-3 activity. Cell Biol Int. 2016;40(2):196–203. [DOI] [PubMed] [Google Scholar]
- 69. Cui Q, Wang JQ, Assaraf YG, Ren L, Gupta P, Wei L, Ashby CR Jr, Yang DH, Chen ZS. Modulating ROS to overcome multidrug resistance in cancer. Drug Resist Updat. 2018;41:1–2. [DOI] [PubMed] [Google Scholar]
- 70. Dharmaraja AT. Role of reactive oxygen species (ROS) in therapeutics and drug resistance in cancer and bacteria. J Med Chem. 2017;6(8):3221–40. [DOI] [PubMed] [Google Scholar]
- 71. Kesavardhana S, Kanneganti TD. Stressed-out ROS take a silent death route. Nat Immunol. 2018;19(2):103–05. [DOI] [PubMed] [Google Scholar]
- 72. Tikkanen R, Nikolic-Paterson DJ. Mitogen-activated protein kinases: Functions in signal transduction and human diseases. Int J Mol Sci. 2019;20(19):4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chen NN, Wei F, Wang L, Cui S, Wan Y, Liu S. Tumor necrosis factor alpha induces neural stem cell apoptosis through activating p38 MAPK pathway. Neurochem Res. 2016;41(11):3052–62. [DOI] [PubMed] [Google Scholar]
- 74. Jalmi SK, Sinha AK. ROS mediated MAPK signaling in abiotic and biotic stress-striking similarities and differences. Front Plant Sci. 2015;6:769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lee S, Rauch J, Kolch W. Targeting MAPK signaling in cancer: Mechanisms of drug resistance and sensitivity. Int J Mol Sci. 2020;21(3):1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yamashita M, Wada H, Eguchi H, Ogawa H, Yamada D, Noda T, Asaoka T, Kawamoto K, Gotoh K, Umeshita K, Doki Y, Mori M. A CD13 inhibitor, ubenimex, synergistically enhances the effects of anticancer drugs in hepatocellular carcinoma. Int J Oncol. 2016;49(1):89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhang Y, Xu J, Zhang N, Chen M, Wang H, Zhu D. Targeting the tumour immune microenvironment for cancer therapy in human gastrointestinal malignancies. Cancer Lett. 2019;458:123–35. [DOI] [PubMed] [Google Scholar]
- 78. Domagala-Kulawik J, Osinska I, Hoser G. Mechanisms of immune response regulation in lung cancer. Transl Lung Cancer Res. 2014;3(1):15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ghosh M, Gerber C, Rahman MM, Vernier KM, Pereira FE, Subramani J, Caromile LA, Shapiro LH. Molecular mechanisms regulating CD13-mediated adhesion. Immunology 2014;142(4):636–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ghosh M, Subramani J, Rahman MM, Shapiro LH. CD13 restricts TLR4 endocytic signal transduction in inflammation. J Immunol. 2015;19(9):4466–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ju S, Qiu H, Zhou X, Zhu B, Lv X, Huang X, Li J, Zhang Y, Liu L, Ge Y, Johnson DE, Ju S, Shu Y. CD13+CD4+CD25hi regulatory T cells exhibit higher suppressive function and increase with tumor stage in non-small cell lung cancer patients. Cell Cycle 2009;8(16):2578–85. [DOI] [PubMed] [Google Scholar]
- 82. Zhang J, Xu X, Shi M, Chen Y, Yu D, Zhao C, Gu Y, Yang B, Guo S, Ding G, Jin G, Wu CL, Zhu M. CD13hi Neutrophil-like myeloid-derived suppressor cells exert immune suppression through Arginase 1 expression in pancreatic ductal adenocarcinoma. Oncoimmunology 2017;6(2):e1258504. [DOI] [PMC free article] [PubMed] [Google Scholar]