Abstract
The present study aimed to investigate the effect of miR-186 on proliferation, migration, invasion, and epithelial–mesenchymal transition (EMT) of hepatocellular carcinoma (HCC). In this work, miR-186 was downregulated in HCC tissues and cells, and low miR-186 level helped predict the occurrence of vascular invasion and poor prognosis in patients with HCC. miR-186 overexpression inhibited cell proliferation and tumor growth in nude mice, repressed migration and invasion abilities, and enhanced apoptosis in HCC cells. miR-186 also retarded progression of EMT. miR-186 directly bound to the 3′-untranslated regions of cyclin-dependent kinase 6 (CDK6) to inhibit its expression. Overexpression of CDK6 markedly reversed inhibitory effects of miR-186 on proliferation, apoptosis, migration, and invasion of HCC cells. Conversely, inhibition of CDK6 exerted synergic effect on the biological functions of miR-186. In conclusion, miR-186 represses proliferation, migration, invasion, and EMT, and induces apoptosis through targeting CDK6 in HCC, which may provide a new therapeutic target for HCC.
Key words: miR-186, Cyclin-dependent kinase 6 (CDK6), Epithelial–mesenchymal transition (EMT), Hepatocellular carcinoma (HCC)
INTRODUCTION
Hepatocellular carcinoma (HCC) is the most common malignancy in the world with increasing incidence in East Asia1,2. Although some therapeutic strategies have improved the prognosis in recent decades, including surgical resection, radiofrequency, and chemotherapy, the 5-year survival rate is still low due to vascular invasion, metastasis, drug resistance, and recurrence3,4. Therefore, it is crucial to understand the molecular mechanisms underlying the progression of HCC to identify novel potential molecular targets for the treatment of HCC5,6.
miRNAs are endogenous small noncoding RNAs consisting of 19–23 nucleotides that regulate gene expression via its interaction with the 3′-untranslated region (3′-UTR) of mRNA targets, causing translation suppression or mRNA degradation7,8. miRNAs could act as tumor suppressor genes or oncogenes and have been implicated in human carcinogenesis9,10. Previous reports demonstrated deregulated miRNAs were involved in angiogenesis, tumor cell proliferation, tumor suppressor, epithelial–mesenchymal transition (EMT), and metastasis in some cancers, such as bladder cancer and breast cancer. Of these miRNAs, miR-186 has been extensively investigated in various tumors, and its expression in cancer tissues varies depending on the cancer type and might act as a biomarker for the diagnosis and prognosis of cancers11. Various biological processes in human cancers are affected by miR-18612. However, more detailed mechanisms underlying miR-186-mediated HCC remain to be elucidated.
In this work, we focused on the clinical and biological significance of miR-186 in HCC. The proliferation, apoptosis, migration, and invasion of HCC cells were determined by in vitro assays. The interaction between miR-186 and cyclin-dependent kinase 6 (CDK6) was confirmed with the dual-luciferase reporter system. A mouse model experiment was conducted to determine the effect of miR-186 on tumor growth. The present study will verify the regulatory mechanism of the miR-186/CDK6 axis in the progression of HCC.
MATERIALS AND METHODS
Human HCC Tissue Collection
HCC tissues and adjacent normal liver tissues were obtained from 50 cases of patients who had received surgical resection at Shandong Provincial Third Hospital. All the patients signed informed consent. The examination and informed consents were approved by the Ethics Committee of Shandong Provincial Third Hospital. All fresh tissues were histologically verified by two pathologists. The surgically removed tissue samples were rapidly frozen in liquid nitrogen and kept at −80°C until use.
Cell Culture
HCC cell lines (HepG2, Hep3B, HUH7, MHCC-97, and SMMC-7721) were purchased from the American Type Culture Collection (ATCC) (Manassas, VA, USA) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/ml of penicillin, and 0.1 mg/ml of streptomycin. Human LO2 cells were purchased from Bogoo Biotechnology (Shanghai, China) and cultured in RPMI-1640 medium containing 10% FBS. All cells were incubated at 37°C in a humidified atmosphere containing 5% CO2.
Cell Transfection
miR-186 mimics, negative control (NC) mimics, small interfering RNA (siRNA) of CDK6 (si-CDK6), as well as pcDNA3.1-CDK6 were synthesized and bought from GeneChem (Shanghai, China). The pmirGLO luciferase reporter vectors (Promega, Madison, WI, USA) were used to construct dual-luciferase reporter plasmid vectors containing CDK6 3′-UTR sequences or the corresponding mutation sequences. Cell transfection was carried out using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) and Western blotting were used to verify the efficiency of transfection 48 h after transfection.
qRT-PCR Assay
Total RNAs were isolated using TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA). The RNAs were reversely transcribed to complementary DNAs (cDNAs) through the reverse transcriptase kit (Takara, Otsu, Japan) or the TaqMan® miRNA reverse transcription kit (Thermo Fisher Scientific). The qRT-PCR was performed on the ABI 7900 Detection System (Applied Biosystems, Foster City, CA, USA) using the SYBR Green PCR Master Mix Kit (Takara, Dalian, China). The relative expression levels were calculated using the 2−ΔCt method. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and U6 acted as internal controls. For RT-PCR, PCR products were evaluated for band abundance and size by agarose gel electrophoresis. The samples were run on a 2% (w/v) agarose gel in a 1× TBE buffer system and stained with ethidium bromide, and a size marker was included for molecular weight determination.
Western Blot Assay
Whole-cell protein extracts were prepared using the M-Per Mammalian Protein Extraction Reagent (Pierce Biotechnology, Woburn, MA, USA) according to the manufacturer’s instructions. Subsequently, 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed. The proteins were then transferred onto polyvinylidene difluoride membranes and mounted using 5% nonfat dried milk. The blots were incubated with primary antibodies at 4°C overnight and then subjected to the second antibody for another 2 h. Finally, membranes were developed in enhanced chemiluminescence reagent (Pierce, Rockford, IL, USA). GAPDH was used as a loading control.
Cell Counting Kit-8 (CCK-8) Assay
Briefly, cells (1 × 103 cells/well) were seeded on a 96-well plate. After the cells were incubated for 0, 12, 24, and 48 h, 10 μl of CCK-8 solution (Dojindo, Tokyo, Japan) was added into each well. The absorbance at 450 nm was determined through a microplate reader (Bio-Tek, Winooski, VT).
Apoptotic Analysis
Cells were digested and collected, and then stained by 2 μl annexin V-fluorescein isothiocyanate and 2 μl propidium iodide (BD Pharmingen, San Diego, CA, USA) for 100 μl of cell suspension. After incubation at room temperature in the dark for 15 min, the stained cells were detected by BD LSRFortessa to analyze the cell apoptotic distribution.
Transwell Assay
The chambers were coated with Matrigel for invasive assay. No Matrigel was added for migration. The lower chamber was filled with 600 μl complete medium containing 10% FBS. The cells in the 200 μl serum-free medium were loaded into the upper Transwell chamber (BD Biosciences, Franklin Lakes, NJ, USA). Cells that migrated and invaded into the lower side of the inserts were fixed and stained with 0.1% crystal violet. The cells were photographed and counted in 10 random visual fields.
Tumor Xenograft Experiments
The animal experiments were approved by the Animal Care and Use Committee of Shandong Provincial Third Hospital. Four-week-old male BALB/c nude mice were subcutaneously injected with 2 × 105 cells with agomir-186 or NC (RiboBio, Guangzhou, China). All mice were kept under specific pathogen-free conditions. The width and length of tumor xenografts were measured using vernier calipers every 7 days. Tumor volumes were calculated using the standard formula: V = 1/2 × length × width2. On the fifth week, the mice were euthanized, and tumor xenografts were excised and weighed.
Luciferase Reporter Assay
The pmirGLO-CDK6-wild type (WT) and pmirGLO-CDK6-mutant (MUT) reporters were designed and purchased from GenePharma (Shanghai, China). miR-186 or NC mimics were cotransfected with the above reporters into 293T cells using Lipofectamine™ 2000 reagent (Invitrogen). The transfected cells were incubated for 48 h, and then the luminescence signals were measured using the Dual-Luciferase Reporter Assay System Kit (Promega). The firefly luciferase activity was normalized to the Renilla luciferase activity.
Statistical Analysis
Data were presented as mean ± standard deviation (SD) or standard error of the mean (SEM) for continuous variables. Statistical analysis was performed using independent Student’s t-test or one-way analysis of variance via GraphPad Prism 6.0 (GraphPad Software Inc., La Jolla, CA, USA). Overall survival (OS) curve was constructed using the Kaplan–Meier methods and compared using a log-rank test. A value of p < 0.05 was considered as statistically significant.
RESULTS
miR-186 Is Downregulated in HCC Tissues and Cells and Correlates With Poor Prognosis
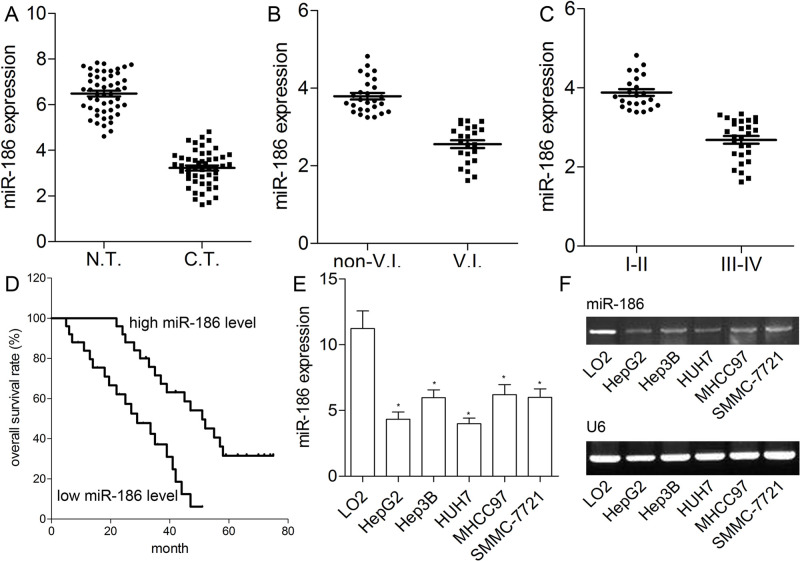
Here we detected the expression level of miR-186 in 50 HCC tissues and matched adjacent liver tissues. The qRT-PCR analysis showed that miR-186 was obviously reduced in HCC tissues in contrast with matched normal liver tissues (p < 0.01) (Fig. 1A). As illustrated in Figure 1B and C, compared with HCC tissues with I–II stage or nonvascular invasion, miR-186 expression was obviously lower in HCC tissues with III–IV stage or vascular invasion (p < 0.01). All patients were then divided into the high miR-186 expression group (n = 25) and the low miR-186 expression group (n = 25) according to the median miR-186 expression value (Fig. 1D). In contrast with HCC patients (median survival time: 51 months) who had higher expression level of miR-186, the median survival time was decreased to 29 months in HCC patients with lower miR-186 expression (p < 0.01). In addition, we also measured the miR-186 expression level in five HCC cell lines (HepG2, Hep3B, HUH7, MHCC-97, and SMMC-7721). The results showed that, compared with LO2, the expression level of miR-186 was indeed reduced in all HCC cell lines (p < 0.01) (Fig. 1E and F). HepG2 and HUH7 cells exhibited the lowest level of miR-186 (p < 0.05), which was subsequently used for in vitro assays. These findings suggested that miR-186 may be involved in the progression of HCC.
Figure 1.
Expression of miR-186 in hepatocellular carcinoma (HCC) tissues and cell lines. (A) Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) analysis for the relative expression of miR-186 in 50 cases of HCC tissues and adjacent normal tissues. (B) The expression of miR-186 in HCC with (n = 23) or without vascular invasion (V.I.) (n = 27). (C) The expression of miR-186 in HCC with low (I–II) (n = 23) and high TNM stage (III–IV) (n = 27). Data represent means ± standard deviation (SD) of three independent experiments. (D) Kaplan–Meier survival curves for patients with high and low miR-186 expression are shown. (E) The qRT-PCR was used to quantitatively detect miR-186 expression in HCC cells. (F) RT-PCR bands for miR-186 and U6 in HCC cell lines are shown. Data represent means ± standard error of the mean (SEM) of three independent experiments. *p < 0.01.
miR-186 Decreases Cell Proliferation and Promotes Apoptosis
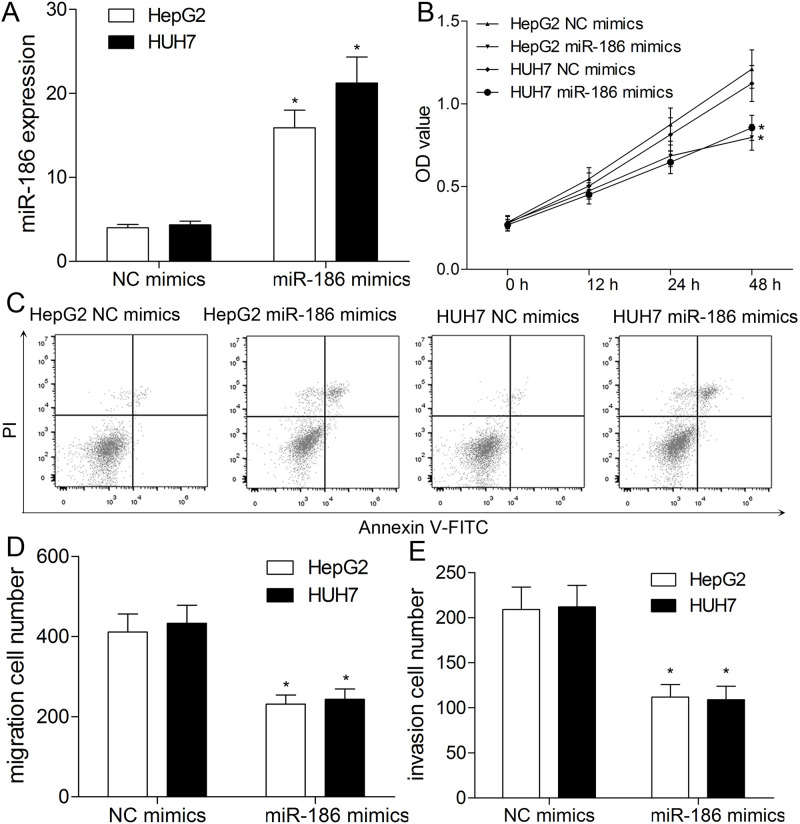
Because of downregulation of miR-186 in HepG2 and HUH7 cells, a series of gain-of-function assays were designed to elucidate the role of miR-186. As exhibited in Figure 2A, miR-186 was specifically upregulated in HepG2 and HUH7 cells transfected with miR-186 mimics compared with the NC mimics group (p < 0.01). The CCK-8 assay showed that in comparison with the NC mimics group, the proliferation ability of miR-186-upregulated HepG2 and HUH7 cells was markedly decreased (p < 0.01) (Fig. 2B). Flow cytometry assay was performed to determine the cell apoptosis. The flow cytometry analysis indicated that the rate of apoptosis in miR-186 mimics-transfected HepG2 and HUH7 cells was significantly increased than that in the NC mimics group (p < 0.01) (Fig. 2C).
Figure 2.
miR-186 inhibits proliferation, apoptosis, migration, and invasion of HCC cells. HepG2 and HUH7 cells were transfected with miR-186 or negative control (NC) mimics. (A) miR-186 expression level in HepG2 and HUH7 cells was detected using qRT-PCR. (B) Cell proliferation was determined by cell counting kit-8 (CCK-8) assay. (C) The apoptosis of HCC cells was validated using flow cytometry. Transwell assay was used to analyze migration (D) and invasion (E) capacities of HCC cells. All results are expressed as the mean ± SEM from independent experiments (n = 3). *p < 0.01.
miR-186 Inhibits Migration and Invasion in HepG2 and HUH7 Cells
Subsequently, the migration and invasion capacities of HepG2 and HUH7 cells were detected following the transfection of miR-186/NC mimics. The Transwell assay revealed that the migratory numbers of HepG2 and HUH7 cells were significantly decreased by the miR-186 mimics (p < 0.01) (Fig. 2D). In addition, the invasive numbers of HepG2 and HUH7 cells were also markedly reduced in the miR-186 mimics-treated group in contrast with both in the NC mimics group (p < 0.01) (Fig. 2E). These results suggested the potential suppressive effect of miR-186 on the migration and invasion in HCC cells in vitro.
miR-186 Inhibits EMT in HepG2 and HUH7 Cells
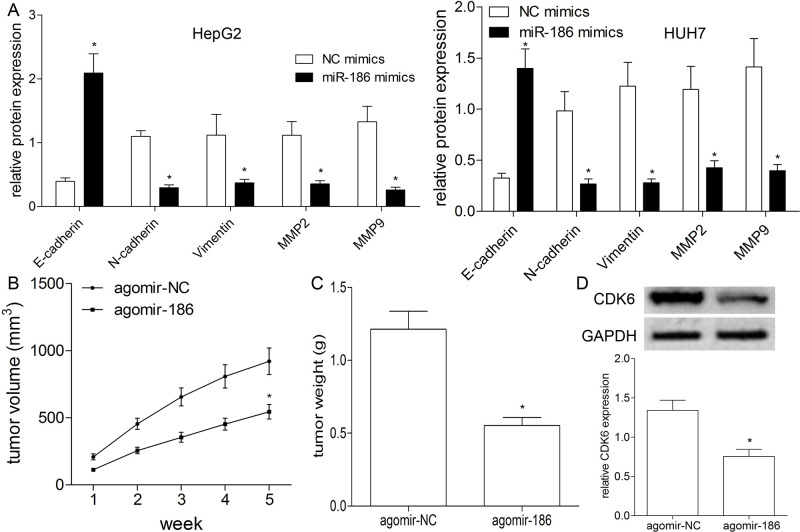
To elucidate the effect of miR-186 on EMT in HepG2 and HUH7 cells, we investigated the expression levels of E-cadherin, N-cadherin, vimentin, and MMP2/9 after transfection of miR-186/NC mimics. Western blot results indicated that the expression of E-cadherin was elevated, and expressions of N-cadherin, vimentin, and MMP2/9 were decreased by miR-186 mimics compared with those in the NC mimics group (p < 0.01) (Fig. 3A). These results showed the suppressive effect of miR-186 on the progression of EMT in HCC cells.
Figure 3.
miR-186 has an inhibitory effect on epithelial–mesenchymal transition (EMT) and tumor growth. (A) Western blotting was used to detect the expression levels of related proteins of N-cadherin, E-cadherin, vimentin, and MMP2/9 in HepG2 and HUH7 cells transfected with miR-186 or NC mimics. (B) The tumor volume was measured weekly after transfection with agomir-186 or agomir-NC. (C) Tumor-bearing mice were killed after 5 weeks’ cultivation, and tumors were stripped and weighed. (D) Western blotting was used to detect the expression of CDK6 protein in tumor tissues of nude mice. All results are expressed as the mean ± SEM from independent experiments (n = 3). *p < 0.01.
miR-186 Suppresses Tumor Growth In Vivo
To investigate the tumorigenic effects of miR-186 on the BALB/C nude mice, we conducted a xenograft mouse model using transfection with agomir-186 or agomir-NC by tail vein injection. Tumor volumes were then measured weekly, and tumor weight was detected in week 5. The results showed that the mice with agomir-186 exhibited significantly inhibited tumor volume (p < 0.01) (Fig. 3B) and tumor weight (p < 0.01) (Fig. 3C). Moreover, we measured the expression level of CDK6 in the tumor tissues using Western blotting, and the results indicated CDK6 expression was apparently decreased in the tumor tissue of nude mice with agomir-186 compared with that with agomir-NC (p < 0.01) (Fig. 3D), thus suggesting miR-186 suppresses tumor growth in vivo.
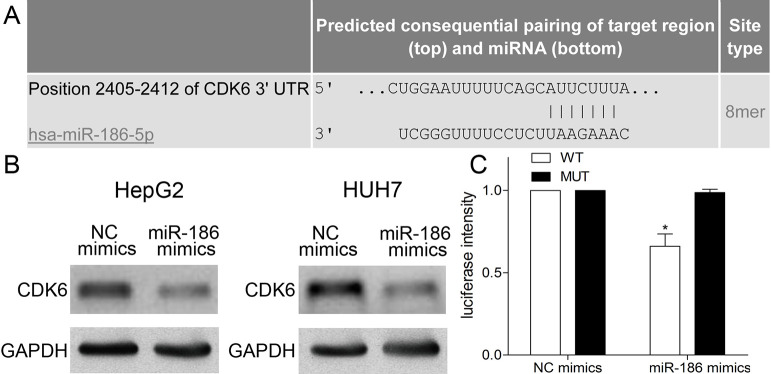
miR-186 Directly Targeted CDK6
TargetScan and functional analyses were used to predict the target gene of miR-186. CDK6 was chosen as the object for further study owing to its potential target relationship (Fig. 4A). We found that CDK6 was obviously downregulated in miR-186-transfected HepG2 and HUH7 cells compared with NC mimics using Western blotting (p < 0.01) (Fig. 4B). To verify this prediction, luciferase reporter assay was conducted to observe the luciferase activity of 293T cells cotransfected with miR-186 mimics and CDK6 WT or MUT vector. In the present work, the luciferase activity of 293T cells cotransfected with miR-186 mimics and CDK6 WT was obviously decreased in contrast with that in NC mimics and CDK6 WT group (p < 0.01) (Fig. 4C), whereas miR-186 mimics and the CDK6 MUT group exhibited no difference in the luciferase activity of 293T cells from NC mimics and CDK6 MUT group (p > 0.05) (Fig. 4C). These results suggested that miR-186 directly targeted CDK6 in HCC cells.
Figure 4.
miR-186 directly targets CDK6. (A) The putative conserved miR-186-binding site in the CDK6 3′-untranslated region (3′-UTR). (B) Western blotting was used to analyze the relative expression of CDK6 in HepG2 and HUH7 cells transfected with miR-186 or NC mimics, respectively. (C) The relative luciferase activity of 293T cells cotransfected with miR-186 mimics/NC and CDK6 wild type (WT)/mutant (MUT) was detected using luciferase reporter assay. Data represent means ± SEM of three independent experiments. *p < 0.01.
miR-186 Regulates Proliferation and Apoptosis by Targeting CDK6 in HepG2 and HUH7 Cells
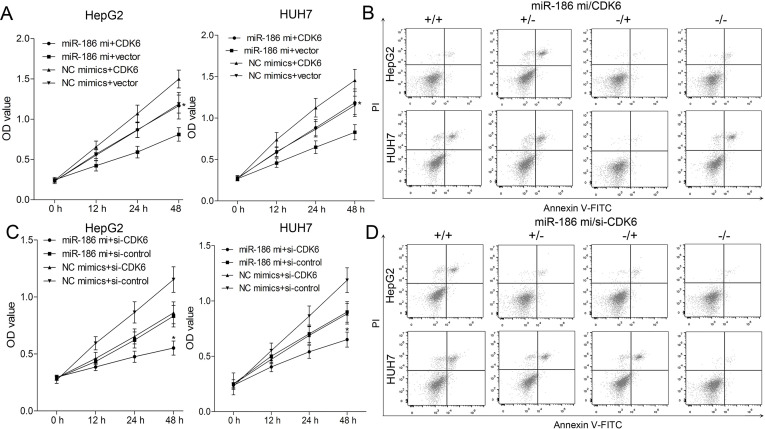
To explore whether miR-186 acts as a tumor suppressor in the proliferation of HCC via targeting CDK6, we conducted the gain- and loss-of-function analyses in HepG2 and HUH7 cells. CDK6 expression was rescued in miR-186-upregulated HepG2 and HUH7 cells in the presence of pcDNA3.1-CDK6, but further inhibited in HepG2 and HUH7 cells transfected with si-CDK6. Ectopic expression of CDK6 promoted the cell viability in miR-186-upregulated HepG2 and HUH7 cells (p < 0.01) (Fig. 5A). Moreover, the apoptosis rates of HepG2 and HUH7 cells increased by miR-186 mimics were further reduced by CDK6 overexpression (p < 0.01) (Fig. 5B). Compared with control siRNAs and NC mimics, the viability of HepG2 and HUH7 cells was markedly weaker in the si-CDK6 group, while in the miR-186 mimics + si-CDK6 group, HepG2 and HUH7 cells exhibited the weakest viability (p < 0.01) (Fig. 5C). Flow cytometry analysis indicated CDK6 knockdown further enhanced cell apoptosis induced by miR-186 in HepG2 and HUH7 cells (p < 0.01) (Fig. 5D). These results confirmed that miR-186 regulates proliferation and apoptosis by targeting CDK6 in HepG2 and HUH7 cells.
Figure 5.
Cyclin-dependent kinase 6 (CDK6) is involved in miR-186-regulated proliferation and apoptosis of HepG2 and HUH7 cells. HepG2 and HUH7 were transfected with pcDNA3.1-CDK6 (loss of WT 3′-UTR) and/or miR-186 mimics. (A) The proliferation ability of HepG2 and HUH7 cells was determined by CCK-8 assay. (B) The apoptosis rate of HepG2 and HUH7 cells was assessed using flow cytometry. HepG2 and HUH7 cells were then cotransfected with miR-186 mimics and/or si-CDK6. (C) CCK-8 assay was used to analyze cell proliferation in HepG2 and HUH7 cells. (D) The apoptosis rates of HepG2 and HUH7 cells were assessed using flow cytometry. Data represent means ± SEM of three independent experiments. *p < 0.01.
Involvement of CDK6 in miR-186-Inhibited Migration and Invasion of HepG2 and HUH7 Cells
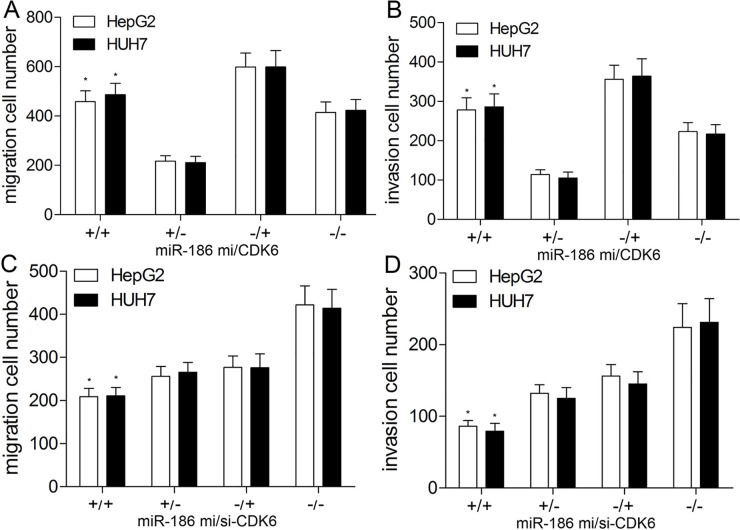
In order to further evaluate whether miR-186 inhibited the migration and invasion of HCC cells by regulating CDK6, pcDNA3.1-CDK6 or si-CDK6 was cotransfected with miR-186 mimics to explore biological changes of HCC cells. The Transwell assay revealed that the suppression of migration and invasion abilities of HepG2 and HUH7 cells by miR-186 mimics was partially abrogated by transfection of pcDNA3.1-CDK6 (p < 0.01) (Fig. 6A and B). Compared with control siRNAs and NC mimics, the migration and invasion capacities in the si-CDK6 group were reduced by approximately 45%. However, in the miR-186 mimics + si-CDK6 group, the inhibition effect of miR-186 was obviously strengthened by CDK6 silencing compared with miR-186 mimics or si-CDK6 group (Fig. 6C and D). The above findings revealed that miR-186 suppressed the migration and invasion of HCC cells via inhibiting CDK6.
Figure 6.
CDK6 is involved in miR-186-inhibited migration and invasion of HepG2 and HUH7 cells. HepG2 and HUH7 were transfected with pcDNA3.1-CDK6 (loss of WT 3′-UTR) and/or miR-186 mimics. Transwell assay was conducted to detect cell migration (A) and invasion (B) in HepG2 and HUH7 cells. HepG2 and HUH7 cells were then cotransfected with miR-186 mimics and/or si-CDK6. The migration (C) and invasion (D) abilities of HepG2 and HUH7 cells were detected by Transwell assay. Data represent means ± SEM of three independent experiments. *p < 0.01.
DISCUSSION
A large number of miRNAs have been demonstrated to play a vital role in the occurrence and progression of tumors in humans6. Therefore, more effort should be made to elucidate the expression profile and utility of miRNAs in various cancers. miR-186, a widely studied miRNA, has been proved to attend in the progression of tumors, such as renal cell carcinoma (RCC)12, lung adenocarcinoma13, and cholangiocarcinoma14. In the present study, we found that miR-186 was downregulated in HCC tissues and cell lines, and HCC patients with lower miR-186 expression have lower OS rate and shorter survival time. Therefore, we demonstrated miR-186 might be involved in the progression of HCC.
A growing body of evidence shows that miR-186 acts as a critical regulator of biological function in a variety of cancers10. For instance, overexpression of miR-186 could markedly repress proliferation, migration, and invasion and induce apoptosis in RCC cells12. miR-186 upregulation was able to repress proliferation and migration of cholangiocarcinoma cells14. Consistent with these studies, we found that overexpression of miR-186 could inhibit the growth of HCC cells and xenograft tumors and decrease migrated and invasive cell numbers. EMT is involved in the process of migration and invasion in many cancers and has been reported to be regulated directly or indirectly by miRNAs15,16. Cao et al. demonstrated that ectopic overexpression of miR-186 inhibited gastric cancer cell migration and invasion by inhibiting EMT biomarker Twist115. Gou et al. found that miR-186 mimics attenuated RUNX3-induced increase in E-cadherin and inhibited metastasis and invasion, thereby influencing EMT progression16. Consistent with the above findings, our study further identified that miR-186 inhibited EMT in HepG2 and HUH7 cells. These results inferred that miR-186 acts as a critical suppressor of biological function in HCC.
miRNAs bind with specific mRNAs to regulate the target gene expression7. Results from TargetScan showed that miR-186 has complementary bases paring with CDK6 mRNA. Generally, CDK6 is coexpressed and copurified with cyclin D1 and functions as a cell cycle initiator protein to regulate cell proliferation17,18. Additionally, overexpression of CDK6 has been demonstrated to be associated with the development and carcinogenesis of HCC from cirrhosis19. In this study, we identified that CDK6 3′-UTR was a direct target of miR-186 using dual-luciferase reporter assay. In vitro gain- and loss-of-function assays revealed that overexpression of CDK6 undermined miR-186-induced effects on proliferation, apoptosis, migration, and invasion of HCC cells. Conversely, inhibition of CDK6 exerted synergic effect on biological functions of miR-186. These findings indeed demonstrate the inhibitor effect of miR-186 on CDK6. At present, CDK6 inhibitor palbociclib has been approved for hormone receptor-positive breast cancer patients. The effect of palbociclib on HCC is under active investigation20. Thus, miR-186 may serve as a biological inhibitor of CDK6 for future treatment of HCC.
In summary, we have identified for the first time that miR-186 inhibits proliferation, migration, invasion, and EMT by exerting its negative regulation on the expression of CDK6 in HCC. Exploration of miR-186-orientated pathway networks may help us develop novel potential therapeutic options for HCC.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Quaglia A. Hepatocellular carcinoma: A review of diagnostic challenges for the pathologist. J Hepatocell Carcinoma 2018;5:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sim HW, Knox J. Hepatocellular carcinoma in the era of immunotherapy. Curr Probl Cancer 2018;42:40–8. [DOI] [PubMed] [Google Scholar]
- 3. Armengol C, Sarrias MR, Sala M. Hepatocellular carcinoma: Present and future. Med Clin. (Barc) 2018;150:390–7. [DOI] [PubMed] [Google Scholar]
- 4. Ronot M, Purcell Y, Vilgrain V. Hepatocellular carcinoma: Current imaging modalities for diagnosis and prognosis. Dig Dis Sci. 2019;64(4):934–50. [DOI] [PubMed] [Google Scholar]
- 5. Hayes CN, Chayama K. MicroRNAs as biomarkers for liver disease and hepatocellular carcinoma. Int J Mol Sci. 2016;17(3):280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rupaimoole R, Slack FJ. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16(3):203–22. [DOI] [PubMed] [Google Scholar]
- 7. Lima CR, Gomes CC, Santos MF. Role of microRNAs in endocrine cancer metastasis. Mol Cell Endocrinol. 2017;456:62–75. [DOI] [PubMed] [Google Scholar]
- 8. Tang J, Li Y, Wang J, Wen Z, Lai M, Zhang H. Molecular mechanisms of microRNAs in regulating epithelial–mesenchymal transitions in human cancers. Cancer Lett. 2016;371(2):301–13. [DOI] [PubMed] [Google Scholar]
- 9. Zaravinos A, Radojicic J, Lambrou GI, Volanis D, Delakas D, Stathopoulos EN, Spandidos DA. Expression of miRNAs involved in angiogenesis, tumor cell proliferation, tumor suppressor inhibition, epithelial–mesenchymal transition and activation of metastasis in bladder cancer. J Urol. 2012;188(2):615–23. [DOI] [PubMed] [Google Scholar]
- 10. Petrovic N, Davidovic R, Bajic V, Obradovic M, Isenovic RE. MicroRNA in breast cancer: The association with BRCA1/2. Cancer Biomark. 2017;19(2):119–28. [DOI] [PubMed] [Google Scholar]
- 11. Wang Z, Sha HH, Li HJ. Functions and mechanisms of miR-186 in human cancer. Biomed Pharmacother. 2019;119:109428. [DOI] [PubMed] [Google Scholar]
- 12. Jiao D, Wu M, Ji L, Liu F, Liu Y. MicroRNA-186 suppresses cell proliferation and metastasis through targeting sentrin-specific protease 1 in renal cell carcinoma. Oncol Res. 2018;26(2):249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cai J, Wu J, Zhang H, Fang L, Huang Y, Yang Y, Zhu X, Li R, Li M. miR-186 downregulation correlates with poor survival in lung adenocarcinoma, where it interferes with cell-cycle regulation. Cancer Res. 2013;73(2):756–66. [DOI] [PubMed] [Google Scholar]
- 14. Zhang M, Shi B, Zhang K. miR-186 suppresses the progression of cholangiocarcinoma cells through inhibition of Twist1. Oncol Res. 2019;27:1061–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cao C, Sun D, Zhang L, Song L. miR-186 affects the proliferation, invasion and migration of human gastric cancer by inhibition of Twist1. Oncotarget 2016;7:79956–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gou Y, Zhai F, Zhang L, Cui L. RUNX3 regulates hepatocellular carcinoma cell metastasis via targeting miR-186/E-cadherin/EMT pathway. Oncotarget 2017;8:61475–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang H, Nicolay BN, Chick JM, Gao X, Geng Y, Ren H, Gao H, Yang G, Williams JA, Suski JM, Keibler MA, Sicinska E, Gerdemann U, Haining WN, Roberts TM, Polyak K, Gygi SP, Dyson NJ, Sicinski P. The metabolic function of cyclin D3-CDK6 kinase in cancer cell survival. Nature 2017;546:426–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kollmann K, Heller G, Schneckenleithner C, Warsch W, Scheicher R, Ott RG, Schäfer M, Fajmann S, Schlederer M, Schiefer AI, Reichart U, Mayerhofer M, Hoeller C, Zöchbauer-Müller S, Kerjaschki D, Bock C, Kenner L, Hoefler G, Freissmuth M, Green AR, Moriggl R, Busslinger M, Malumbres M, Sexl V. A kinase-independent function of CDK6 links the cell cycle to tumor angiogenesis. Cancer Cell 2013;24:167–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Masaki T, Shiratori Y, Rengifo W, Igarashi K, Yamagata M, Kurokohchi K, Uchida N, Miyauchi Y, Yoshiji H, Watanabe S, Omata M, Kuriyama S. Cyclins and cyclin-dependent kinases: Comparative study of hepatocellular carcinoma versus cirrhosis. Hepatology 2003;37(3): 534–43. [DOI] [PubMed] [Google Scholar]
- 20. Sherr CJ, Beach D, Shapiro GI. Targeting CDK4 and CDK6: From discovery to therapy. Cancer Discov. 2016;6(4):353–67. [DOI] [PMC free article] [PubMed] [Google Scholar]