Abstract
5-Fluorouracil (5-FU) is a widely used chemotherapeutic agent for breast cancer. However, acquired chemoresistance leads to a loss of its efficacy; methods to reverse are urgently needed. Some studies have shown that pyrotinib, an ErbB receptor tyrosine kinase inhibitor, is effective against HER2+ breast cancer. However, whether pyrotinib sensitizes 5-FU-resistant breast cancer cells to 5-FU is unknown. We hypothesized that the combination of pyrotinib and 5-FU would show synergistic antitumor activity, and pyrotinib could reverse 5-FU resistance in HER2+ breast cancer cells in vitro and in vivo. Our data showed that pyrotinib inhibited the growth of 5-FU-resistant SKBR-3/FU and MDA-MB-453/FU cell lines and the parental cell lines. 5-FU remarkably suppressed the growth of SKBR-3 and MAD-MB-453 cells. However, SKBR-3/FU and MAD-MB-453/FU cells showed resistance to 5-FU. A combination of pyrotinib and 5-FU resulted in the synergistic inhibition of the growth of the 5-FU-resistant SKBR-3/FU and MDA-MB-453/FU cell lines and the parental cell lines. Pyrotinib decreased significantly the IC50 values of 5-FU and the thymidylate synthase (TS) mRNA expression levels in the 5-FU-resistant SKBR-3/FU and MDA-MB-453/FU cell lines and the parental cell lines and increased significantly the intracellular concentration of 5-FU in SKBR-3/FU and MDA-MB-453/FU cells. In addition, pyrotinib reduced the ABCG2 mRNA and protein expression levels in SKBR-3/FU and MDA-MB-453/FU cells and downregulated the protein expression levels of pAKT, pHER2, and pHER4 in all four cell lines. After TS or ABCG2 in 5-FU-resistant breast cancer cells was knocked down, the sensitivity of SKBR-3/FU and MDA-MB-453/FU cells to 5-FU was restored. Moreover, in vivo experiments demonstrated that pyrotinib in combination with 5-FU more effectively inhibited SKBR-3/FU tumor growth than either pyrotinib or 5-FU alone. In conclusion, our findings suggest that pyrotinib could restore sensitivity of 5-FU-resistant HER2+ breast cancer cells to 5-FU through downregulating the expression levels of TS and ABCG2.
Key words: Pyrotinib, 5-Fluorouracil (5-FU), HER2, Breast cancer, Chemoresistance
INTRODUCTION
Breast cancer is a common tumor in women1, and it is sensitive to chemotherapeutic agents. Among chemotherapeutic agents, 5-fluorouracil (5-FU) is widely used for the treatment of patients with breast cancer, and it transforms into FdUMP, which blocks thymidylate synthase (TS) and thus inhibits DNA synthesis in cells2. However, acquired chemoresistance to 5-FU frequently leads to a loss of its effectiveness against breast cancer. The mechanisms of chemoresistance to 5-FU are not fully known. Some reports have revealed that chemoresistance to 5-FU is associated with overexpression of TS and increasing efflux of 5-FU from cancer cells3,4. Thus, it is necessary to overcome chemoresistance to 5-FU and improve the outcomes of chemotherapy.
The HER2 family comprises HER1/ErbB1, HER2/ErbB2, HER3/ErbB3, and HER4/ErbB45, and 15%–30% of breast cancers overexpress HER26. HER2-targeted therapies, including trastuzumab, pertuzumab, trastuzumab-emtansine, and lapatinib, have been developed over the past two decades and have significantly improved outcomes in patients with HER2+ breast cancer6–8. Recently, a novel drug, pyrotinib, was developed by Jiangsu Hengrui Pharmaceutical (Lianyungang, People’s Republic of China); this drug is an oral irreversible inhibitor of pan-ErbB receptor tyrosine kinase and targets HER1, HER2, and HER4. Clinical trials have indicated that pyrotinib is effective in patients with HER2+ breast cancer9,10. Developing a combination treatment of targeted therapies with cytotoxic agents is a critical strategy for HER2+ breast cancer. Some studies have shown that the combination of pyrotinib with capecitabine significantly improved progression-free survival and objective response rate in patients with HER2+ metastatic breast cancer11,12. These results revealed the synergistic anticancer activities of pyrotinib plus capecitabine against HER2+ breast cancers. However, it is not known whether pyrotinib can sensitize 5-FU-resistant breast cancer cells to 5-FU. A previous report has shown that lapatinib could sensitize 5-FU-resistant breast cancer to 5-FU by downregulating TS activity13. In this study, we established HER2+ breast cancer cell lines with resistance to 5-FU and investigated the synergistic antitumor effect of pyrotinib plus 5-FU on HER2+ breast cancer with resistance to 5-FU. Our results showed that pyrotinib could sensitize 5-FU-resistant breast cancer cells to 5-FU, which provides new clues for combination treatment with pyrotinib and 5-FU in HER2+ breast cancer with resistance to 5-FU.
MATERIALS AND METHODS
Chemicals and Antibodies
Pyrotinib was obtained from Hengrui Medicine Co. (Jiangsu, China). Fetal bovine serum, RNAzol® RT Kit, Reverse Transcription System Kit, cell lysis buffer, BCA Protein Assay Kit, chemiluminescence reagents, Lipofectamine 3000, and 5-FU were purchased from Sangon Biotech (Shanghai, China). Antibodies against pAKT (Ser473), AKT, breast cancer resistance protein (BCRP), pHER2 (Tyr1221/1222), HER2, pHER4 (Tyr1284), HER4, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were purchased from Cell Signaling Technology (Beverly, MA, USA).
Cell Lines and Cell Cultures
Human HER2 breast cancer cell line MDA-MB-468 and human HER2+ breast cancer cell lines SKBR-3 and MAD-MB-453 were purchased from the American Type Culture Collection (ATCC) (Manassas, VA, USA). The cell lines were maintained in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum and 100 U/ml penicillin/streptomycin.
Establishment of 5-FU-Resistant SKBR-3 and MAD-MB-453 Sublines
SKBR-3 and MAD-MB-453 cells were cultured in Dulbecco’s modified Eagle’s medium with gradually increasing amounts of 5-FU for approximately 3 months. The final concentration of 5-FU was 10 μM. The 5-FU-resistant subclone cells were then cloned using the limiting dilution method. Two 5-FU-resistant SKBR-3 and MAD-MB-453 subclones, named SKBR-3/FU and MAD-MB-453/FU, respectively, showed the same growth ability as the parental cells.
Growth Inhibitory Activity Assay and Combination Studies
The CellTiter-Glo® nonradioactive cell proliferation kit (Promega Beijing Biotech Co., Beijing, China) was used to determine cell viability. Briefly, 3 × 104 cells were cultured in 96-well plates for 24 h. These cells were then treated with different concentrations of pyrotinib and/or 5-FU for different durations. The plates were incubated at 25°C for 30 min. We then added 100 μl of CellTiter-Glo® reagent to each well, shook the plates for 2 min, and placed them at 25°C for 10 min to stabilize the luminescence signal, which was recorded with a GloMax® luminometer (Promega). The percentage of viable cells was calculated using the formula: ratio (%) = [luminescence (treatment) – luminescence (blank)]/[luminescence (control) – luminescence (blank)] × 100%. IC50 was determined as the drug concentration that inhibited cell proliferation by 50%. The dose–effect curves were analyzed using Combidrug14.
Interactions between pyrotinib and 5-FU were evaluated using dose–effect curves and fraction affected (FA)–combination index (CI) curves. The FA was evaluated using the formula: FA = (100 – growth inhibition)/100. The CI for each cell line was computed using Chou–Talalay means, where CI <1, =1, and >1 indicated synergism, additivity, and antagonism, respectively14.
Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
Total RNA from the cells was extracted using RNAzol® RT, and the RNA was reverse transcribed using the Reverse Transcription System Kit according to the manufacturer’s instructions. The sequences of the primers used were as follows: 5′-GATCACAGTCTTCAAGGAGATC-3′ and 5′-CAGTCCCAGTACGACTGTGACA-3′ for ABCG2, 5′-CAGATTATTCAGGACAGGGAGTT-3′ and 5′-CATCAGAGGAAGATCTCTTGGATT-3′ for TS, and 5′-CTCATGACCACAGTCCATGCCATC-3′ and 5′-CTGCTTCACCACCTTCTTGATGTC-3′ for GAPDH. The thermocycling conditions for PCR amplification were as follows: 96°C for 1 min, then 40 cycles of 96°C for 10 s, and 60°C for 40 s. GAPDH was included as an internal control, and relative gene expression was determined as previously described15.
Detection of 5-FU Accumulation by High-Performance Liquid Chromatography (HPLC)
Cells (1 × 108) were cultured in 12-well plates for 24 h and were then treated with or without pyrotinib for 24 h. After washing with phosphate-buffered saline, 400 mg/L 5-FU was added, and the cells were cultured for 2 h. The cells were then washed with phosphate-buffered saline and fresh medium was added; they were then cultured for an additional 1 h. After washing with trypsin in phosphate-buffered saline, the cells were collected by centrifugation at 3,000 × g for 5 min. The collected cells were mixed with 200 μl of sterile water, sonicated for 20 s, and then centrifuged at 10,000 × g for 30 min at 4°C. The 5-FU in the supernatant was assessed by HPLC as previously described16.
Western Blotting
Cells were lysed with cell lysis buffer. Protein concentrations were measured using a BCA Protein Assay Kit according to the manufacturer’s instructions. The proteins were then separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electrophoretically transferred onto polyvinylidene fluoride membranes. The membrane was incubated with the corresponding primary antibodies for 24 h at 4°C, and then with the secondary antibodies for 2 h at 20°C. Immunoreactive bands were determined using enhanced chemiluminescence, and GAPDH was used as an internal standard.
Signal Silencing RNA (siRNA) Transfection
Signal silencing TS RNA (TS siRNA) (5′-GCCACTGGCAACATCCTTAA-3′, complementary to human TS mRNA), negative control TS siRNA (5′-ATGCGCCAACGGTTCCTAAA-3′, identical base composition in random order), ABCG2 siRNA (5′-ACAAGGUAGAAAGCCACUCUU-3′, complementary to human ABCG2 mRNA), and negative control ABCG2 siRNA (5′-AAGAGTGGCTTTCTACCTTGT-3′, identical base composition in random order) were synthesized by Sangon Biotech. These siRNAs were transfected into the SKBR-3/FU or MAD-MB-453/FU cell lines using Lipofectamine 3000. The suppression efficiencies of each siRNA were determined using RT-qPCR and Western blotting.
Animal Experiments
Female 6- to 8-week-old BALB/c null mice were purchased from Sipper-BK Experimental Animal Co. (Shanghai, China) and raised in a pathogen-free laboratory. All animal experiments were approved by the Ethics Committee of The First Affiliated Hospital of Hunan Normal University/Hunan Provincial People’s Hospital. For xenograft experiments, SKBR-3/FU cell suspensions (1 × 107 cells/100 μl) were injected into the subcutaneous tissue of the left flank of each mouse. Tumor size was measured with a pair of calipers, and the tumor volume was calculated using the formula: 1/2 (length × width2). When the tumor size reached ∼300 mm3, the mice were randomly divided into four groups (control, FU, PYR, and FU + PYR; n = 5 mice each). The mice in the control group received normal saline via tail vein injection three times a week for 2 weeks. The mice in the FU group were administered 5-FU (20 mg/kg) daily via tail vein injection three times a week for 2 weeks. The mice in the PYR group were administered a daily dose of pyrotinib (10 mg/kg) by oral gavage for 24 days and normal saline via tail vein injection three times a week for 2 weeks. The mice in the FU + PYR group were administered a daily dose of pyrotinib (10 mg/kg) by oral gavage for 24 days and 5-FU (20 mg/kg) via tail injection vein three times a week for 2 weeks. Tumors were measured at 2-day intervals. Twenty-seven days after the treatments, all mice were euthanized and weighed. The tumor samples were collected and analyzed by Western blotting.
The study was approved by the Medical Ethics Committee of The First Affiliated Hospital of Hunan Normal University/Hunan Provincial People’s Hospital. We obtained consent to publish this article from all the participants of this study.
Statistical Analysis
Data are presented as the means ± standard deviations (SDs), and statistical significance was determined using the Student’s t-tests. All statistical analyses were performed using SPSS 21.0 (SPSS, Chicago, IL, USA). A value of p < 0.05 was considered to be statistically significant.
RESULTS
Inhibitory Effect of 5-FU or Pyrotinib on the Growth of Breast Cancer Cells
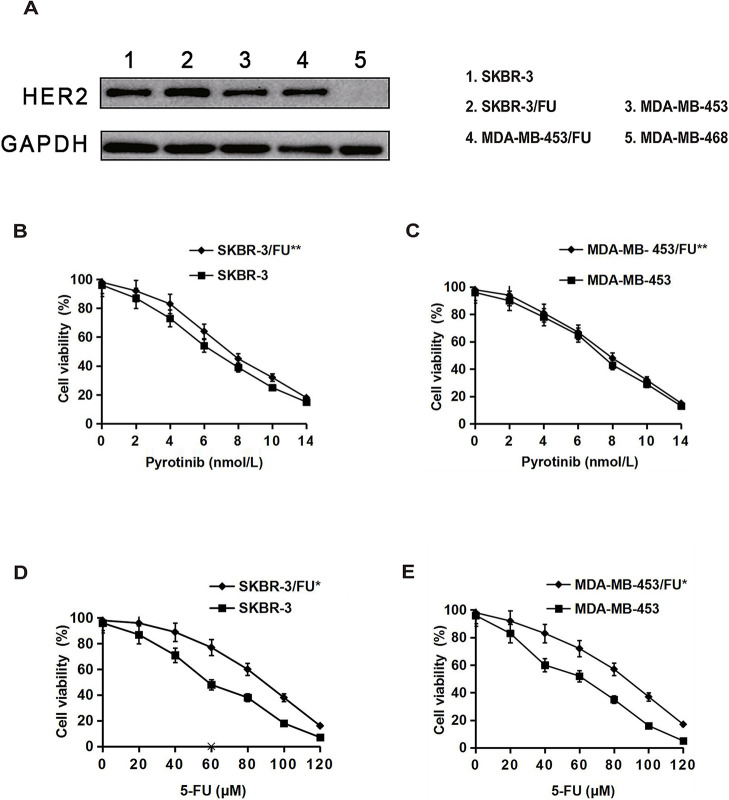
We first detected HER2 protein expression in breast cancer cell lines. The results showed high HER2 protein expression in SKBR-3, MAD-MB-453, SKBR-3/FU, and MAD-MB-453/FU, but not in MDA-MB-468 cell lines (Fig. 1A). We further examined the inhibitory effects of 5-FU and pyrotinib on the growth of the HER2+ SKBR-3, MAD-MB-453, SKBR-3/FU, and MAD-MB-453/FU cell lines. The results showed that pyrotinib inhibited the growth of SKBR-3 and SKBR-3/FU cells similarly in a dose-dependent manner, and pyrotinib showed a growth inhibitory effect on MAD-MB-453 and MAD-MB-453/FU cells in a concentration-dependent manner (Fig. 1B and C). The IC50 values of pyrotinib in the SKBR-3/FU and MAD-MB-453/FU cell lines were the same as those in the parental cell lines (p > 0.05) (Table 1). In addition, 5-FU remarkably suppressed the growth of SKBR-3 and MAD-MB-453 cells in a dose-dependent manner; however, the inhibitory effect of 5-FU on the growth of SKBR-3/FU and MAD-MB-453/FU cells was significant lower than that on the growth of the parental cell lines (p < 0.05) (Fig. 1D and E). The IC50 values of 5-FU in the SKBR-3/FU and MAD-MB-453/FU cell lines were significantly higher than those in the parental cell lines (p < 0.05) (Table 1), indicating that SKBR-3/FU and MAD-MB-453/FU cells were considerably less sensitive to 5-FU than the corresponding parental cell lines, with 11-fold and 10-fold increases in resistance, respectively.
Figure 1.
Growth inhibitory effects of 5-fluorouracil (5-FU) or pyrotinib on breast cancer cell lines. (A) HER2 protein expression in breast cancer cell lines was detected by Western blotting. (B–E) Cells were treated with various concentrations of 5-FU or pyrotinib for 72 h, and then cell viability was measured. Each point represents the mean ± standard deviation (SD) (n = 3). *p < 0.05 versus the parental cell line; **p > 0.05 versus the parental cell line.
Table 1.
IC50 Values of 5-Fluorouracil (5-FU) and Pyrotinib in Breast Cancer Cell Lines
| Cell Lines | Pyrotinib (nM) | 5-FU (μM) |
|---|---|---|
| SK-BR-3 cell | 3.93 ± 0.18 | 8.34 ± 0.32 |
| SK-BR-3 cell/FU | 3.86 ± 0.15* | 91.62 ± 1.35*** |
| MDA-MB-453 cell | 4.25 ± 0.21 | 9.83 ± 0.38 |
| MDA-MB-453 cell/FU | 4.36 ± 0.27** | 98.72 ± 1.75# |
The IC50 values (mean ± SD) were calculated based on the results of three independent experiments.
p > 0.05 versus SKBR-3 cells,
p > 0.05 versus. MDA-MB-453 cells,
p < 0.05 versus SKBR-3 cells,
p < 0.05 versus MDA-MB-453 cells.
Pyrotinib Could Sensitize 5-FU-Resistant Breast Cancer Cells to 5-FU In Vitro
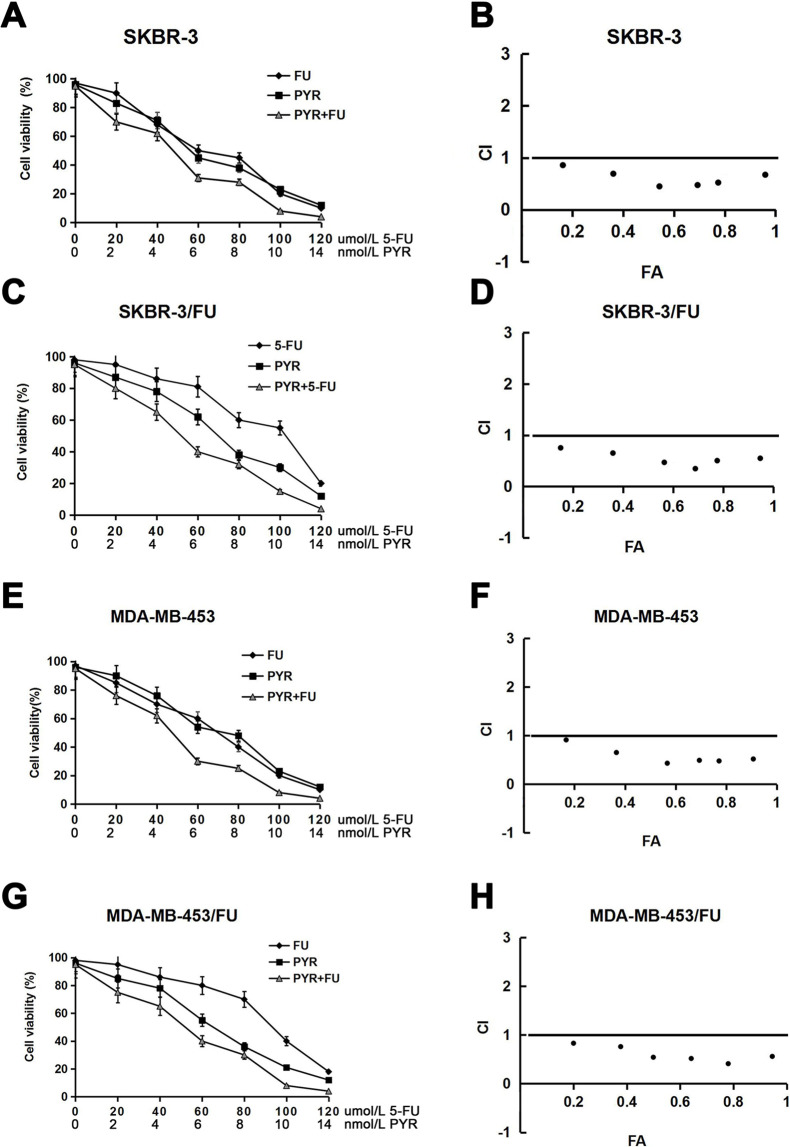
Next, to investigate whether pyrotinib in combination with 5-FU has a synergistic influence on the growth of HER2+ breast cancer cells, we measured the efficacy of different concentrations of the combination of pyrotinib and 5-FU in suppressing the proliferation of SKBR-3, SKBR-3/FU, MDA-MB-453, and MDA-MB-453/FU cells. To assess whether the antitumor activities were synergistic, we calculated the drug CI values for each cell line. The results showed that pyrotinib in combination with 5-FU resulted in the inhibition of the growth of not only the parental SKBR-3 and MDA-MB-453 cell lines but also the 5-FU-resistant SKBR-3/FU and MDA-MB-453/FU cell lines (Fig. 2A, C, E, and G). Moreover, these cotreatments indicated synergistic CI values that were all less than 1 (Fig. 2B, D, F, and H). In addition, the IC50 values of 5-FU in the parental SKBR-3 and MDA-MB-453 cell lines were significantly decreased by pyrotinib. Similar phenomena were observed in the 5-FU-resistant SKBR-3/FU and MDA-MB-453/FU cell lines (Table 2). These findings indicated that pyrotinib could sensitize 5-FU-resistant HER2+ breast cancer cells to 5-FU.
Figure 2.
Growth inhibitory effects of pyrotinib (PYR) combined with 5-FU on breast cancer cell lines. The cells were treated with various concentrations of 5-FU and/or pyrotinib for 72 h, and then cell viability was measured and the dose–effect curves were drawn (A, C, E, and G). The combined drug effect was calculated using the combination index (CI) equation, the fraction affected (FA) of the combinations was presented, and the FA–CI curves were drawn (B, D, F, and H). (B), (D), (F), and (H) corresponds to (A), (C), (E), and (G), respectively. Synergy was defined as a CI value <1.0, antagonism was defined as a CI value >1.0, and additivity was defined as a CI value of 1.0. The data points were the mean ± SD (from three independent experiments). CI, FA = (100 – growth inhibition)/100.
Table 2.
IC50 Values for 5-FU in Breast Cancer Cell Lines in the Presence of Pyrotinib
| Cell Lines | Control | Pyrotinib (6 nM) | p * | Pyrotinib (14 nM) | p * |
|---|---|---|---|---|---|
| SK-BR-3 cells | 12.61 ± 1.81 | 6.34 ± 0.42 | <0.05 | 3.23 ± 0.31 | <0.05 |
| SK-BR-3 cells/FU | 91.82 ± 1.76 | 62.15 ± 1.35 | <0.05 | 18.17 ± 0.82 | <0.05 |
| MDA-MB-453 cells | 13.63 ± 1.52 | 8.26 ± 0.52 | <0.05 | 4.62 ± 0.27 | <0.05 |
| MDA-MB-453 cells/FU | 96.82 ± 1.59 | 68.49 ± 1.87 | <0.05 | 17.51 ± 0.78 | <0.05 |
The IC50 values (mean ± SD) were calculated based on the results of three independent experiments.
Versus control.
Pyrotinib Could Increase the Intracellular Concentrations of 5-FU in 5-FU-Resistant Breast Cancer Cells
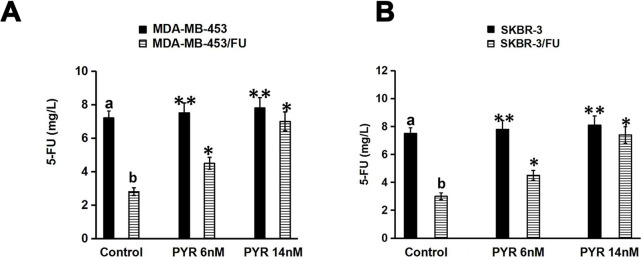
The intracellular concentrations of 5-FU were examined using HPLC. As shown in Figure 3, the intracellular concentrations of 5-FU were remarkably higher in SKBR-3 and MDA-MB-453 cells than in SKBR-3/FU and MDA-MB-453/FU cells, respectively, in the absence of pyrotinib (p < 0.01). After induction with pyrotinib, the intracellular concentration of 5-FU in SKBR-3/FU and MDA-MB-453/FU cells increased significantly (p < 0.05). However, pyrotinib had no significant influence on the intracellular concentration of 5-FU in SKBR-3 and MDA-MB-453 cells (p > 0.05) (Fig. 3A and B).
Figure 3.
Effect of PYR on the accumulation of 5-FU in breast cancer cells. After induction with PYR (6 or 14 nM; control group without induction with PYR), cells were treated with 5-FU (400 mg/L). The accumulation of 5-FU in these cells was detected by high-performance liquid chromatography (HPLC). The data shown were representative of three independent experiments. (A) Accumulation of 5-FU in SKBR-3 and SKBR-3/FU cells. (B) Accumulation of 5-FU in MDA-MB-453 and MDA-MB-453/FU. *p < 0.05 versus controlb; **p > 0.05 versus controla; and b p < 0.05 versus controla.
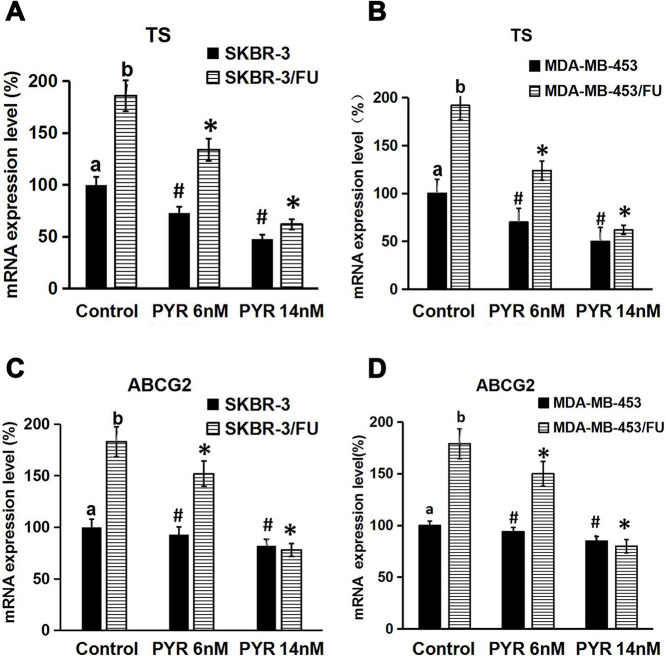
Effect of Pyrotinib on TS and ABCG2 mRNA Expression Levels
To investigate the potential mechanisms responsible for the sensitization of 5-FU-resistant HER2+ breast cancer cells to 5-FU by pyrotinib, we evaluated the effect of pyrotinib on the TS and ABCG2 mRNA expression levels using RT-qPCR. The results showed that TS and ABCG2 mRNA expression levels were higher in SKBR-3/FU and MDA-MB-453/FU cells than in SKBR-3 and MDA-MB-453 cells, respectively (p < 0.05) (Fig. 4A–D). We also observed that pyrotinib decreased the TS mRNA expression levels in SKBR-3, MDA-MB-453, SKBR-3/FU, and MDA-MB-453/FU cells (p < 0.05 vs. control group) (Fig. 4A and B). In addition, pyrotinib reduced the ABCG2 mRNA expression levels in SKBR-3/FU and MDA-MB-453/FU cells (p < 0.05 vs. control group) (Fig. 4C and D); however, it had no influence on the ABCG2 mRNA expression levels in SKBR-3 and MDA-MB-453 cells (p > 0.05 vs. control group) (Fig. 4C and D).
Figure 4.
Effect of PYR on thymidylate synthase (TS) and ABCG2 mRNA expression in breast cancer cells. Cells (4 × 105 cells/well) were seeded in 24-well plates, cultured for 24 h, and treated with PYR (6 or 14 nM; control group without treatment with PYR) for 24 h. Total RNA was extracted, mRNA expression was measured by reverse transcription quantitative polymerase chain reaction (RT-qPCR). (A, B) TS mRNA expression in breast cancer cell lines. (C, D) ABCG2 mRNA expression in breast cancer cell lines. Each bar represented the mean ± SD (n = 3). *p < 0.05 versus controlb; #p < 0.05 versus controla; b p < 0.05 versus controla.
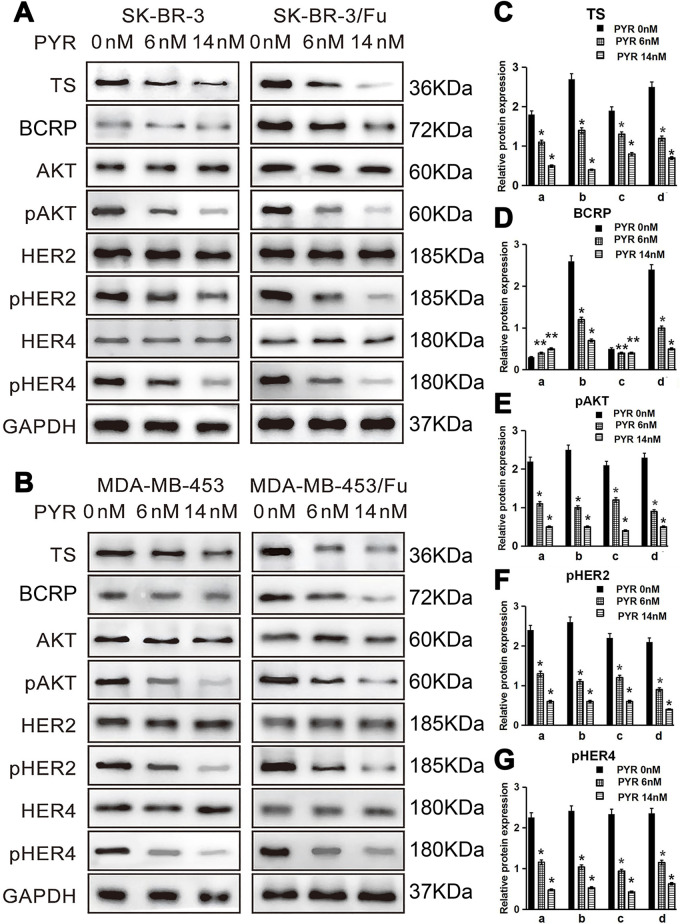
Effects of Pyrotinib on the Protein Expression of AKT, pAKT, HER2, pHER2, HER4, pHER4, TS, and BCRP
We next examined the effects of pyrotinib on the protein expression of AKT, pAKT, HER2, pHER2, HER4, pHER4, TS, and BCRP. The results showed that pAKT, pHER2, pHER4, TS, and BCRP protein expression levels were higher in SKBR-3/FU and MDA-MB-453/FU cells than in SKBR-3 and MDA-MB-453 cells, respectively (p < 0.05) (Fig. 5A–G). Pyrotinib resulted in a reduction in the protein expression levels of pAKT, pHER2, and pHER4 in all four cell lines (p < 0.05) (Fig. 5A, B, and E–G). In addition, pyrotinib downregulated the BCRP protein expression levels in SKBR-3/FU and MDA-MB-453/FU cells (p < 0.05) (Fig. 5A, B, and D); however, BCRP protein expression levels in SKBR-3 and MDA-MB-453 cells were not influenced (p > 0.05) (Fig. 5A, B, and D).
Figure 5.
Effects of PYR on the protein expression of AKT, pAKT, HER2, pHER2, HER4, pHER4, TS, and breast cancer resistance protein (BCRP). Cells (4 × 105 cells/well) were seeded in 24-well plates, cultured for 24 h, and treated with PYR (0, 6, or 14 nM) for 24 h. Total protein was extracted. (A, B) Western blot analysis of AKT, pAKT, TS, BCRP, HER2, pHER2, HER4, and pHER4. (C–G) Quantitation of protein bands [relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) protein] (n = 3).*p < 0.05 versus PYR 0 nM group; **p > 0.05 versus PYR 0 nM group. a: SKBR-3, b: SKBR-3/FU, c: MDA-MB-453, d: MDA-MB-453/FU.
TS and ABCG2 Knockdown Restored Sensitivity to 5-FU in 5-FU-Resistant Breast Cancer Cells
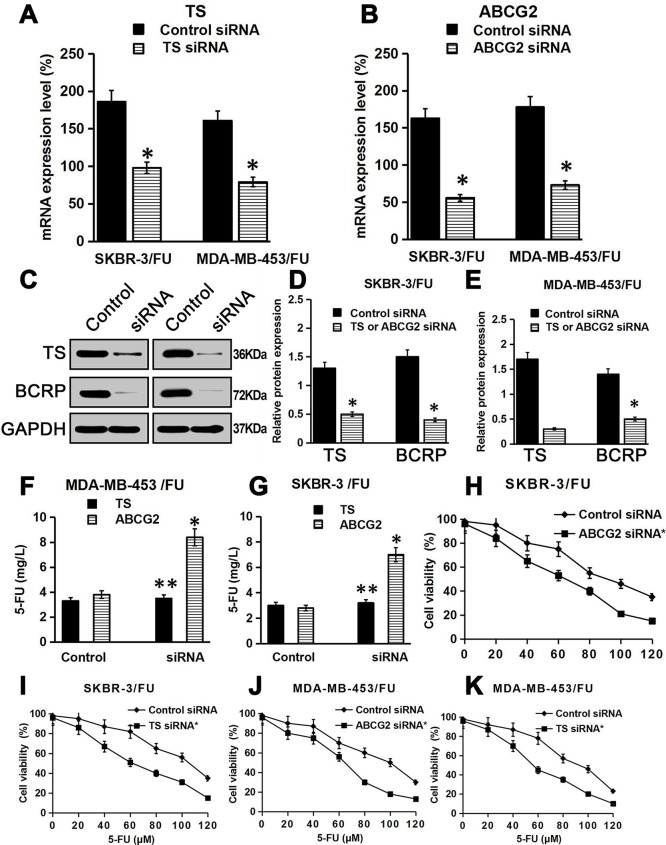
To further confirm the relationship between TS or ABCG2 and 5-FU resistance in breast cancer cells, we knocked down TS or ABCG2 in 5-FU-resistant breast cancer cells and then treated these cells with various concentrations of 5-FU. The results showed that TS or ABCG2 mRNA expression levels significantly decreased in TS or ABCG2 siRNA-transfected SKBR-3/FU cells and MDA-MB-453/FU cells, respectively, compared with that in control siRNA-transfected cells, and there was a concomitant reduction in the TS or BCRP protein expression (p < 0.05) (Fig. 6A–E), which indicated that TS siRNA or ABCG2 siRNA specifically downregulated TS or ABCG2 expression. In addition, the intracellular concentration of 5-FU was remarkably higher in ABCG2 knockdown SKBR-3/FU and MDA-MB-453/FU cells than that in the control siRNA-transfected cells (p < 0.05) (Fig. 6F and G). However, TS knockdown had no significant effect on the intracellular concentration of 5-FU (p > 0.05) (Fig. 6F and G). These findings confirmed that 5-FU is pumped out by BCRP, but not by TS. Next, we measured the changes in 5-FU sensitivity in control cells and TS/ABCG2 siRNA-transfected cells, as shown in (Fig. 6H–K) and (Table 3). TS or ABCG2 knockdown increased the sensitivity of SKBR-3/FU and MDA-MB-453/FU cells to 5-FU, compared with that of the control cells. The IC50 values of 5-FU in SKBR-3/FU and MDA-MB-453/FU cells with TS or ABCG2 knockdown were significantly lower than those in the control siRNA-transfected cells (p < 0.05). These results demonstrate that TS and ABCG2 play an important role in 5-FU resistance in breast cancer cells.
Figure 6.
Effect of TS and ABCG2 knockdown on the 5-FU sensitivity of 5-FU-resistant breast cancer cells. (A, B) TS mRNA or ABCG2 mRNA was measured by RT-qPCR. Data are presented as the mean ± SD of triplicate experiments. (C) TS or BCRP protein was measured by Western blotting. (D, E) Quantitation of protein bands (relative to GAPDH protein) (n = 3). (F, G) The accumulation of 5-FU in the cells was detected by HPLC. Shown are representative data from three independent experiments. (H–K) Cells were knocked down using siRNA, then treated with 5-FU for 72 h, and cell viability was measured. Data are presented as the mean ± SD of triplicate experiments. *p < 0.05 versus the control; **p > 0.05 versus the control.
Table 3.
IC50 Values for 5-FU in SKBR-3/FU and MDA-MB-453/FU Cells in the Knockdown Thymidylate Synthase (TS) and ABC Transporter Subfamily G member 2 (ABCG2)
| Knockdown | TS | ABCG2 |
|---|---|---|
| SKBR-3 cells/FU/control | 98.4 ± 4.9a | 91.3 ± 6.2b |
| SKBR-3 cells/FU/siRNA | 52.6 ± 3.1* | 43.1 ± 3.2** |
| MDA-MB-453 cells/FU/control | 86.5 ± 5.7c | 92.6 ± 6.2d |
| MDA-MB-453 cells/FU/siRNA | 42.8 ± 3.1*** | 39.7 ± 4.5# |
Cells were treated with various concentrations of 5-FU for 72 h. The cell viability was then measured. The IC50 values (mean ± SD) were calculated based on the results of three independent experiments. *p < 0.05 versus controla, **p < 0.05 versus controlb, ***p < 0.05 versus controlc, #p < 0.05 versus controld.
Pyrotinib Restored Sensitivity to 5-FU in 5-FU-Resistant Breast Cancer In Vivo
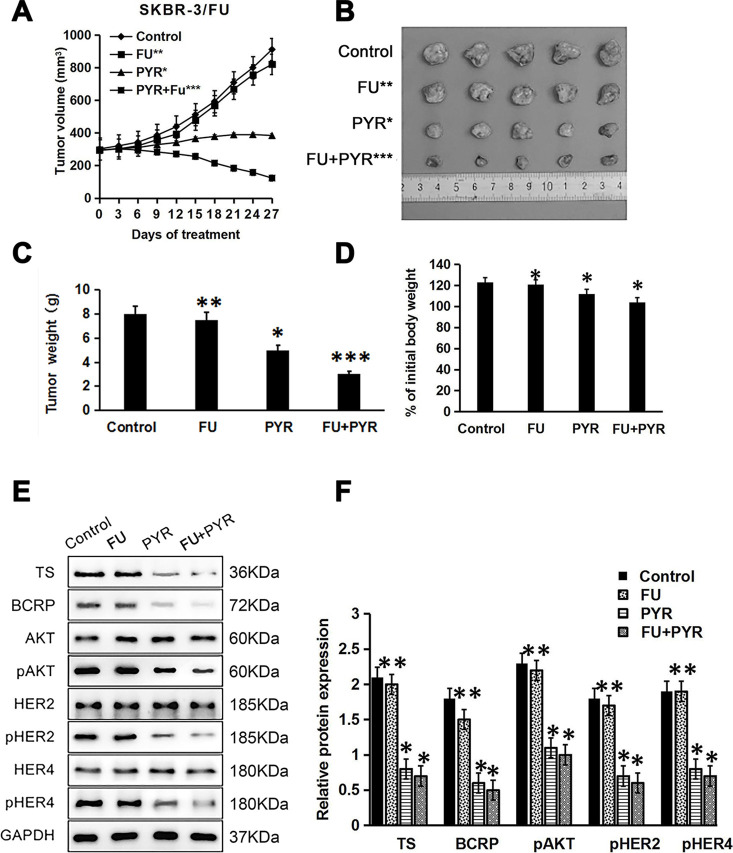
In a xenograft mouse model, we examined whether pyrotinib could sensitize HER2+ breast cancer with 5-FU resistance to 5-FU. Mice with SKBR-3/FU xenografts were randomly divided into four groups. The results showed that pyrotinib in combination with 5-FU more effectively inhibited SKBR-3/FU tumor growth than either pyrotinib or 5-FU alone (p < 0.05) (Fig. 7A–C). The mice in the combination treatment group showed body weights similar to those in the group treated with pyrotinib or 5-FU alone (p > 0.05) (Fig. 7D), indicating that pyrotinib could restore sensitivity to 5-FU in 5-FU-resistant breast cancer without increased toxicity. Western blot analysis of tumor tissues also showed that the levels of TS, BCRP, pHER2, pHER4, and pAKT decreased significantly in the groups treated with pyrotinib (p < 0.05) (Fig. 7E and F), which was consistent with the results obtained in the cell lines in vitro.
Figure 7.
Pyrotinib sensitizes 5-FU-resistant breast cancer cells to 5-FU in vivo. Nude mice bearing SKBR-3/FU tumors were treated with saline (control), FU (20 mg/kg), PYR (10 mg/kg), or FU (20 mg/kg) + PYR (10 mg/kg). (A) Tumor volume, which was assessed every 3 days after the onset of treatment. (B, C) Representative tumors and tumor weights at the end of the experiment (day 27). (D) Body weights on day 27. (E) Western blot analysis of TS, BCRP, pAKT, AKT, **pHER2, HER2, pHER4, HER4, and GAPDH in harvested tumors. (F) Quantitation of protein bands (relative to GAPDH protein) (n = 3). *p < 0.05 versus control; **p > 0.05 versus control; ***p < 0.01 versus control.
DISCUSSION
Breast cancers that overexpress HER2 are generally considered to have poor survival subtypes17. Recent advances in anti-HER2 drugs have significantly improved the outcomes of patients with HER2+ breast cancer18. However, the curative effects of these targeting drugs alone are limited. Therefore, they are typically administered in combination with chemotherapeutics to maximize their therapeutic effects. 5-FU is commonly used in combination with anti-HER2-targeting therapies for breast cancers19. However, some patients with breast cancer treated with 5-FU-based chemotherapy experience recurrence. Vulsteke et al.20 reported that 15.3% of patients with breast cancer experience disease relapse following treatment with 5-FU-based chemotherapy. This recurrence is predominantly because of the development of 5-FU resistance during treatment. Some studies have highlighted a few potential mechanisms underlying 5-FU resistance, such as the overexpression of TS, ABCG2, and dihydropyrimidine dehydrogenase3,4. However, much remains incompletely understood regarding this process.
In this study, we established two 5-FU-resistant breast cancer cell lines, SKBR-3/FU and MDA-MB-453/FU. In vitro, the inhibitory effect of 5-FU on SKBR-3/FU cells and MAD-MB-453/FU cells was minimal, and the IC50 values of 5-FU in these cell lines were significantly higher than those in the parental cell lines. In addition, in vivo xenograft experiments confirmed that 5-FU did not suppress the growth of SKBR-3/FU tumors. These results indicated that SKBR-3/FU cells and MAD-MB-453/FU cells were highly resistant to 5-FU, in comparison to the parental cells. Next, we investigated the relationship between 5-FU resistance and TS or ABCG2.
TS is an enzyme that targets 5-FU. Our results showed that the mRNA and protein levels of TS were higher in SKBR-3/FU and MDA-MB-453/FU cells than in the corresponding parental cell lines. Several reports have also shown TS overexpression in some types of 5-FU-resistant cancer cells21,22. We used TS siRNAs to knock down TS in SKBR-3/FU cells and MDA-MB-453/FU cells, and the TS mRNA and protein expression levels were found to be significantly lower in TS knockdown SKBR-3/FU and MDA-MB-453/FU cells than in the control cells. Moreover, the inhibition rate of 5-FU was also found to be remarkably higher in TS siRNA-transfected cells than in the control siRNA-transfected cells. Similarly, Kadota et al.23 also reported that a short hairpin RNA targeting TS effectively downregulated TS expression in 5-FU-resistant tumor cells, and the sensitivity to 5-FU was restored in these cells. Therefore, these findings suggest that high TS expression is a mechanism of 5-FU resistance.
Recent studies have shown that ATP-binding cassette (ABC) transport proteins can pump cytotoxic drugs out of cells and is a major mechanism underlying multidrug resistance24. BCRP, also known as ABCG2, is an ABC transport protein24. Its overexpression has been observed in some tumors4,25. In addition, it has been reported that there is a dose–effect relationship between resistance to 5-FU and BCRP expression in the cells and that 5-FU resistance could be reversed by knocking down BCRP through siRNA26. Our results showed that the intracellular concentration of 5-FU was remarkably lower and the mRNA and protein expression levels of ABCG2 were noticeably higher in SKBR-3/FU and MDA-MB-453/FU cells than in the parental cell lines. We used ABCG2 siRNAs to knock down ABCG2 in SKBR-3/FU and MDA-MB-453/FU cells, and ABCG2 mRNA and BCRP protein expression levels were found to be significantly lower in ABCG2 knockdown SKBR-3/FU and MDA-MB-453/FU cells than in the control cells. Moreover, the inhibition rate of the cell growth and intracellular concentrations of 5-FU were also found to be remarkably higher in ABCG2 siRNA-transfected cells than in the control siRNA-transfected cells. However, TS knockdown had no effect on the intracellular accumulation of 5-FU in SKBR-3/FU and MDA-MB-453/FU cells. The 5-FU resistance in breast cancer cells was reversed by ABCG2 knockdown. These findings indicate that 5-FU is a substrate of BCRP in breast cancer cells and is pumped out by BCRP.
Some studies have shown that a number of targets can restore sensitivity of 5-FU-resistant breast cancer cells to 5-FU21,22. For example, Minegaki et al.22 reported that valproic acid and suberanilohydroxamic acid, two histone deacetylase inhibitors, resensitized 5-FU-resistant breast cancer cells to 5-FU. In this study, our results revealed that pyrotinib not only increased the sensitivity to 5-FU in 5-FU-sensitive breast cancer cells but also resensitized 5-FU-resistant breast cancer cells to 5-FU in vitro and in vivo. In addition, mice that received the combination treatment did not show lower body weights than those in the groups treated with 5-FU or pyrotinib alone. Thus, our data show that pyrotinib restored sensitivity to 5-FU without an increase in toxicity.
We further investigated the mechanisms whereby pyrotinib resensitized 5-FU-resistant breast cancer cells to 5-FU. Our results showed that pyrotinib markedly decreased TS mRNA and protein expression in both the 5-FU-resistant cell lines and the corresponding parental cell lines, thus allowing 5-FU to better suppress the remaining TS activity. Apoptosis induced by 5-FU is related to the extent of enzymatic inhibition13. Consistent with our results, Chefrour et al.13 reported that lapatinib, a reversible inhibitor of HER1 and HER2, decreased TS activity and increased the cytotoxic effect of 5′-deoxy-5-fluorouridine (5′-dFUR) in HER2+ breast cancer cell lines. However, the molecular mechanism by which pyrotinib and lapatinib downregulate TS is unknown.
Moreover, pyrotinib also decreased ABCG2 mRNA and protein expression in both SKBR-3/FU and MDA-MB-453/FU cells. The intracellular concentration of 5-FU in 5-FU-resistant cells was increased in pyrotinib-treated cells almost to the level observed in the parental cells, and the sensitivity to 5-FU was restored. However, pyrotinib had no effect on ABCG2 mRNA and protein expression in SKBR-3 and MDA-MB-453 cells. This might be due to the low ABCG2 expression levels in these cells.
We further investigated the mechanisms by which ABCG2 expression was suppressed by pyrotinib. Our results showed that the levels of pAKT, pHER2, and pHER4 were higher in SKBR-3/FU and MDA-MB-453/FU cells than in the parental cell lines, and pyrotinib significantly decreased pAKT, pHER2, and pHER4 levels in SKBR-3/FU and MDA-MB-453/FU cells without affecting the total AKT, HER2, and HER4 levels in vitro. Pyrotinib-treated SKBR-3/FU xenograft tissues also had lower pAKT, pHER2, and pHER4 expression, which indicated that BCRP is suppressed by pyrotinib via the inhibition of the PI3K/Akt signaling pathway. Similarly, Chefrour et al.13 reported that lapatinib significantly downregulated the pHER2 and pAKT levels and increased the cytotoxic effect of 5′-dFUR in HER2+ breast cancer cell lines.
Previous studies have shown that HER2 overexpression activates the PI3K/Akt signal transduction pathway and leads to increased BCRP expression in breast cancer cells27,28. PI3K inhibition has also been reported to be particularly effective in sensitizing breast cancer cells to cytotoxic agents by decreasing ACCG2 expression as a consequence of inhibiting pAKT activity29,30. The present data strongly suggest that the downregulation of ABCG2 by pyrotinib, via inhibition of pAkt signaling and the pHER2/pHER4 pathway, could be a mechanism for the restoration of the sensitivity to 5-FU in 5-FU-resistant breast cancer cell lines.
In conclusion, we showed that the resistance to 5-FU in breast cancer cells is correlated with the overexpression of TS and ABCG2. This is the first study to demonstrate the role of pyrotinib in restoring sensitivity to 5-FU in 5-FU-resistant breast cancer cells. Our findings open a new pathway for the application of combination treatment with pyrotinib and 5-FU in patients with 5-FU-resistant HER2+ breast cancer.
ACKNOWLEDGMENTS
We thank HengRui for providing the pyrotinib used in this study. This work was supported by the National Natural Science Foundation (Grant No. 81602351), the Key Project of Hunan Provincial Health Commission (Grant No. A2017003), the Hunan Natural Science Foundation (Grant No. 2017JJ3171), the Hunan Province Clinical Medical Technology Innovation Guide Program (Grant No. 2018SK50720), and the Changsha Science and Technology Project (Grant No. kq1701045/Kq1701053). Author contributions: experimental design, J.N.Y., P.Z.F., and P.Y.Y.; experimental execution, J.N.Y., P.Y.Y., S.C., J.L.L., M.F., Y.D., and L.H.Z.; computational analysis, J.N.Y., P.Z.F., and P.Y.Y.; manuscript writing, review, and discussion, J.N.Y., P.Z.F., P.Y.Y., and L.H.Z.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Thomas A, Rhoads A, Pinkerton E, Schroeder MC, Conway KM, Hundley WG, McNally LR, Oleson J, Lynch CF, Romitti PA. Incidence and survival among young women with stage I–III breast cancer: SEER 2000–2015. JNCI Cancer Spectr. 2019;3(3):z40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat Rev Cancer 2003;3(5):330–8. [DOI] [PubMed] [Google Scholar]
- 3. Habara K, Ajiki T, Kamigaki T, Nakamura T, Kuroda Y. High expression of thymidylate synthase leads to resistance to 5-fluorouracil in biliary tract carcinoma in vitro. Jpn J Cancer Res. 2001;92(10):1127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yuan J, Lv H, Peng B, Wang C, Yu Y, He Z. Role of BCRP as a biomarker for predicting resistance to 5-fluorouracil in breast cancer. Cancer Chemother Pharmacol. 2009;63(6):1103–10. [DOI] [PubMed] [Google Scholar]
- 5. Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: Receptor heterodimerization in development and cancer. EMBO J. 2000;19(13):3159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iqbal N, Iqbal N. Human epidermal growth factor receptor 2 (HER2) in cancers: Overexpression and therapeutic implications. Mol Biol Int. 2014;2014:852748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pegram MD, Lipton A, Hayes DF, Weber BL, Baselga JM, Tripathy D, Baly D, Baughman SA, Twaddell T, Glaspy JA, Slamon DJ. Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2/neumonoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J Clin Oncol. 1998;16(8):2659–71. [DOI] [PubMed] [Google Scholar]
- 8. Gianni L, Llado A, Bianchi G, Cortes J, Kellokumpu-Lehtinen PL, Cameron DA, Miles D, Salvagni S, Wardley A, Goeminne JC, Hersberger V, Baselga J. Open-label, phase II, multicenter, randomized study of the efficacy and safety of two dose levels of pertuzumab, a human epidermal growth factor receptor 2 dimerization inhibitor, in patients with human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28(7):1131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li X, Yang C, Wan H, Zhang G, Feng J, Zhang L, Chen X, Zhong D, Lou L, Tao W, Zhang L. Discovery and development of pyrotinib: A novel irreversible EGFR/HER2 dual tyrosine kinase inhibitor with favorable safety profiles for the treatment of breast cancer. Eur J Pharm Sci. 2017;110:51–61. [DOI] [PubMed] [Google Scholar]
- 10. Ma F, Li Q, Chen S, Zhu W, Fan Y, Wang J, Luo Y, Xing P, Lan B, Li M, Yi Z, Cai R, Yuan P, Zhang P, Li Q, Xu B. Phase I study and biomarker analysis of pyrotinib, a novel irreversible pan-ErbB receptor tyrosine kinase inhibitor, in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2017;35(27):3105–12. [DOI] [PubMed] [Google Scholar]
- 11. Blair HA. Pyrotinib: First global approval. Drugs 2018;78(16):1751–5. [DOI] [PubMed] [Google Scholar]
- 12. Ma F, Ouyang Q, Li W, Jiang Z, Tong Z, Liu Y, Li H, Yu S, Feng J, Wang S, Hu X, Zou J, Zhu X, Xu B. Pyrotinib or lapatinib combined with capecitabine in HER2-positive metastatic breast cancer with prior taxanes, anthracyclines, and/or trastuzumab: A randomized, phase II study. J Clin Oncol. 2019;37(29):2610–9. [DOI] [PubMed] [Google Scholar]
- 13. Chefrour M, Milano G, Formento P, Giacometti S, Denden A, Renee N, Iliadis A, Fischel JL, Ciccolini J. Positive interaction between lapatinib and capecitabine in human breast cancer models: Study of molecular determinants. Fundam Clin Pharmacol. 2012;26(4):530–7. [DOI] [PubMed] [Google Scholar]
- 14. Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58(3):621–81. [DOI] [PubMed] [Google Scholar]
- 15. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 2001;25(4):402–8. [DOI] [PubMed] [Google Scholar]
- 16. Loos WJ, de Bruijn P, Verweij J, Sparreboom A. Determination of camptothecin analogs in biological matrices by high-performance liquid chromatography. Anticancer Drugs 2000;11(5):315–24. [DOI] [PubMed] [Google Scholar]
- 17. Eroglu Z, Tagawa T, Somlo G. Human epidermal growth factor receptor family-targeted therapies in the treatment of HER2-overexpressing breast cancer. Oncologist 2014;19(2):135–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brandes AA, Franceschi E, Tosoni A, Degli ER. Trastuzumab and lapatinib beyond trastuzumab progression for metastatic breast cancer: Strategies and pitfalls. Expert Rev Anticancer Ther. 2010;10(2):179–84. [DOI] [PubMed] [Google Scholar]
- 19. Cameron D, Casey M, Press M, Lindquist D, Pienkowski T, Romieu CG, Chan S, Jagiello-Gruszfeld A, Kaufman B, Crown J, Chan A, Campone M, Viens P, Davidson N, Gorbounova V, Raats JI, Skarlos D, Newstat B, Roychowdhury D, Paoletti P, Oliva C, Rubin S, Stein S, Geyer CE. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: Updated efficacy and biomarker analyses. Breast Cancer Res Treat. 2008;112(3):533–43. [DOI] [PubMed] [Google Scholar]
- 20. Vulsteke C, Pfeil AM, Schwenkglenks M, Pettengell R, Szucs TD, Lambrechts D, Peeters M, van Dam P, Dieudonne AS, Hatse S, Neven P, Paridaens R, Wildiers H. Impact of genetic variability and treatment-related factors on outcome in early breast cancer patients receiving (neo-) adjuvant chemotherapy with 5-fluorouracil, epirubicin and cyclophosphamide, and docetaxel. Breast Cancer Res Treat. 2014;147(3):557–70. [DOI] [PubMed] [Google Scholar]
- 21. Wang QP, Wang Y, Wang XD, Mo XM, Gu J, Lu ZY, Pan ZL, Zhu YX. Survivin up-regulates the expression of breast cancer resistance protein (BCRP) through attenuating the suppression of p53 on NF-kappaB expression in MCF-7/5-FU cells. Int J Biochem Cell Biol. 2013;45(9):2036–44. [DOI] [PubMed] [Google Scholar]
- 22. Minegaki T, Suzuki A, Mori M, Tsuji S, Yamamoto S, Watanabe A, Tsuzuki T, Tsunoda T, Yamamoto A, Tsujimoto M, Nishiguchi K. Histone deacetylase inhibitors sensitize 5-fluorouracil-resistant MDA-MB-468 breast cancer cells to 5-fluorouracil. Oncol Lett. 2018;16(5):6202–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kadota K, Huang CL, Liu D, Yokomise H, Haba R, Wada H. Combined therapy with a thymidylate synthase-inhibiting vector and S-1 has effective antitumor activity against 5-FU-resistant tumors. Int J Oncol. 2011;38(2):355–63. [DOI] [PubMed] [Google Scholar]
- 24. Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci USA 1998;95(26):15665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bessho Y, Oguri T, Achiwa H, Muramatsu H, Maeda H, Niimi T, Sato S, Ueda R. Role of ABCG2 as a biomarker for predicting resistance to CPT-11/SN-38 in lung cancer. Cancer Sci. 2006;97(3):192–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lv H, He Z, Liu X, Yuan J, Yu Y, Chen Z. Reversal of BCRP-mediated multidrug resistance by stable expression of small interfering RNAs. J Cell Biochem. 2007;102(1):75–81. [DOI] [PubMed] [Google Scholar]
- 27. Imai Y, Yoshimori M, Fukuda K, Yamagishi H, Ueda Y. The PI3K/Akt inhibitor LY294002 reverses BCRP-mediated drug resistance without affecting BCRP translocation. Oncol Rep. 2012;27(6):1703–9. [DOI] [PubMed] [Google Scholar]
- 28. Zhang W, Ding W, Chen Y, Feng M, Ouyang Y, Yu Y, He Z. Up-regulation of breast cancer resistance protein plays a role in HER2-mediated chemoresistance through PI3K/Akt and nuclear factor-kappa B signaling pathways in MCF7 breast cancer cells. Acta Biochim Biophys Sin. (Shanghai) 2011;43(8):647–53. [DOI] [PubMed] [Google Scholar]
- 29. Komeili-Movahhed T, Fouladdel S, Barzegar E, Atashpour S, Hossein GM, Nasser OS, Madjd Z, Azizi E. PI3K/Akt inhibition and down-regulation of BCRP re-sensitize MCF7 breast cancer cell line to mitoxantrone chemotherapy. Iran J Basic Med Sci. 2015;18(5):472–7. [PMC free article] [PubMed] [Google Scholar]
- 30. Clark AS, West K, Streicher S, Dennis PA. Constitutive and inducible Akt activity promotes resistance to chemotherapy, trastuzumab, or tamoxifen in breast cancer cells. Mol Cancer Ther. 2002;1(9):707–17. [PubMed] [Google Scholar]