Abstract
Objectives:
Non-alcoholic fatty liver disease (NAFLD) is closely associated with diseases, such as obesity, diabetes mellitus, metabolic syndrome, which are characterized by insulin resistance. NAFLD is thought to be a manifestation of metabolic syndrome in the liver. Liver fibrosis has a high prognostic significance in non-alcoholic steatohepatitis (NASH). In this study, the relationship between insulin resistance and the histopathological changes in the liver was investigated in biopsy-proven NAFLD patients.
Methods:
In this study, 85 biopsy-proven NAFLD patients (64 NASH, 21 non-NASH) and 40 healthy control subjects were enrolled. Insulin resistance was calculated using the “homeostasis model assessment of insulin resistance” (HOMA-IR).
Results:
C reactive protein, total cholesterol, low-density lipoprotein, triglyceride, body mass index (BMI), HOMA-IR levels were significantly higher in the NAFLD group compared to the control group. In the NASH group, the HOMA-IR level was significantly higher than the non-NASH group (p=0.026). When NAFLD patients with advanced fibrosis (stage 3-4, n=27) and without fibrosis (stage 0-2, n=58) are compared, in advanced fibrosis group BMI (35.2±4.6 kg/m2 and 32.7±4.1 kg/m2, respectively; p=0.031) and HOMA-IR (6.3 [5.8-6.8] and 3.4 [2.6-4.8], respectively, p=0.001) levels were higher significantly. In the covariance analysis, when confounding factors, such as BMI, age and gender, were corrected, it was observed that the elevation of HOMA-IR level in the advanced fibrosis group continued statistically significantly.
Conclusion:
HOMA-IR levels were high in NAFLD patients with advanced fibrosis. HOMA-IR, which can be easily measured in daily practice, is an independent predictor for fibrosis.
Keywords: HOMA-IR, insulin resistance, Non-alcoholic fatty liver
With its increasing incidence, non-alcoholic fatty liver disease (NAFLD) is the most common liver disease in the world.[1] NAFLD has a broad spectrum from simple hepatic steatosis to non-alcoholic steatohepatitis (NASH), liver cirrhosis and hepatocellular carcinoma.
Evaluation of fibrosis in NAFLD is clinically critical because patients with advanced fibrosis are at increased risk concerning liver cirrhosis and hepatocellular carcinoma. Therefore, NAFLD cases should be followed closely with screening programs.[2] It has been shown with long-term prospective studies that general and specifically cardiovascular mortality increase in NASH patients with liver fibrosis accompanied by intense inflammation.[3, 4] Therefore, early diagnosis of patients at risk concerningcomplications is important for reducing mortality and morbidity.
As the hepatic component of metabolic syndrome, NAFLD is also closely associated with other clinic features of metabolic syndrome.[5] It is reported that NAFLD prevalence is above 75% in populations with common obesity, metabolic syndrome and type 2 diabetes mellitus (DM), which are characterized by insulin resistance.[6]
Type 2 DM and insulin resistance facilitate lipolysis in adipose tissue and lead to the release of free fatty acids and their storage in the liver, and in consequence, to the development of hepatic steatosis.[7] Type 2 DM is a significant risk factor that leads to progression in the spectrum from NASH to liver cirrhosis. Current data show that obesity and type 2 DM are also risk factors for hepatocellular carcinoma.[8] Moreover, it has been revealed that DM is an independent risk factor for hepatic mortality as well as general mortality in NAFLD.[9]
The present study aims to investigate insulin resistance in patients with NAFLD, the prevalence of which has been increasing in society, and its relationship with histopathological changes of the liver.
Methods
A group of 85 biopsy-proven NAFLD patients and a control group of 40 healthy individuals were included in this study, which was planned as an observational case-control study. The group of patients consisted of NAFLD-diagnosed individuals that applied to Şişli Hamidiye Etfal Training and Research Hospital gastroenterology clinic as outpatients between May 2016 and October 2017.
Patients diagnosed with viral/autoimmune hepatitis, Wilson’s disease, hemochromatosis, alpha-1 antitrypsin deficiency, primary sclerosing cholangitis, biliary system disorders, diagnosed diabetes, acute/chronic kidney damage, ischemic cardiac or cerebrovascular disease and malignancy were not included in the present study. In addition, Individuals using hepatotoxic drugs, herbal products, hormone replacement therapy, antidiabetic drugs and those with a history of alcohol consumption >20 g/day were also excluded from this study.
To determine insulin resistance, HOMA-IR (Homeostasis Model Assessment of Insulin Resistance) index was found with the formula “fasting glucose (mg/dL) x fasting plasma insulin level (μIU/ml)/405.”
Venous blood samples of all participants were taken after an overnight fast, simultaneously with liver biopsy, which was performed under the guidance of ultrasound using a 16-gauge Hepafix needle. Histological findings in biopsy samples were evaluated by an experienced hematopathologist blinded to clinical and laboratory data of participants. The hematopathologist evaluated NAFLD-diagnosed cases according to steatosis, ballooning degeneration, presence and degree of lobular inflammation with the scoring system “National Institute of Diabetes and Digestive and Kidney Diseases Nonalcoholic Steatohepatitis (NIDDK NASH) Clinical Research Network Scoring System”, and classified the patients into two subgroups as NASH and non-NASH.[10] In addition, fibrosis scoring was made as follows: stage 0, no fibrosis; stage 1, perisinusoidal or periportal fibrosis; stage 2, perisinusoidal and portal/periportal fibrosis; stage 3, bridging fibrosis; and stage 4, cirrhosis. Fibrosis scores between 0-2 were evaluated as mild fibrosis, and ≥3 were evaluated as advanced fibrosis.
Ethical Aspects
This study was conducted in accordance with the “Declaration of Helsinki” and approved by Şişli Hamidiye Etfal Training and Research Hospital Ethics Committee. Informed consent of all participants of this study was received orally and in written.
Statistical Analysis
All statistical analyses were conducted using Statistical Package for the Social Sciences (SPSS, version 21.0; IBM Corp, Armonk, NY, USA). Visual (histograms, probability graph) and analytical methods (Shapiro-Wilk test) were used to determine the distribution of variables. Mann-Whitney U test was used to compare non-normally distributed variables and continuous variables. Normally distributed continuous variables, such as age and BMI between the two study groups, were evaluated using Student’s t-test. The p-value calculated with Bonferroni correction and considered statistically significant in post-hoc comparisons was <0.01. Correlations between variables were analyzed with Pearson and Spearman tests.
The capacity of HOMA-IR to predict the presence of advanced fibrosis was assessed by performing ROC (receiver operating characteristics) curve analysis. The point closest to the upper left corner on the ROC curve was defined as the optimal cut-off value; sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated accordingly. In order to test the significance of HOMA-IR in showing advanced fibrosis, confounding factors, such as BMI, age and gender, were corrected, and covariance analysis was performed. A p<0.05 value was considered statistically significant.
Results
Basic clinical and biochemical features of NAFLD patients and control group are summarized in Table 1. The distribution of gender and age was similar in both groups. It was seen that alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyltransferase (GGT), C-reactive protein (CRP), total cholesterol, low-density lipoprotein (LDL), triglyceride, BMI and HOMA-IR levels were statistically significantly higher in the NAFLD group. It was also observed that the high density lipoprotein (HDL) level in the NAFLD group was significantly lower than the control group.
Table 1.
Clinical and biochemical characteristics of the NAFLD and Control Groups
| NAFLD (n=85) | Control(n=40) | p | |
|---|---|---|---|
| Age | 45.2±10.2 | 41.9±9.6 | 0.179 |
| Gender, F/M | 53/32 | 22/18 | 0.173 |
| BMI, kg/m2 | 33.5±4.1 | 22.8±1.8 | <0.001 |
| ALT, IU/L | 90.4 [48.2-98.6] | 16.2 [10.0-29.7] | <0.001 |
| AST, IU/L | 62.2 [42.1-82.5] | 18.6 [12.4-23.1] | <0.001 |
| GGT, IU/L | 64.7 [24.7-69.5] | 13.4 [7.1-19.6] | <0.001 |
| HOMA-IR | 4.3 [2.9-6.1] | 1.8 [1.2-2.8] | <0.001 |
| CRP, mg/dL | 4.3 [2.6-7.7] | 1.4 [1.0-2.1] | <0.001 |
| Total cholesterol, | 198.7 [175.5-249.2] | 153.1 [130.4-179.7] | 0.002 |
| mg/dL | |||
| LDL, mg/dL | 121.9 [93.6-166.4] | 94.3 [81.4-124.3] | 0.031 |
| HDL, mg/dL | 43.4 [33.6-51.6] | 53.2 [47.6-59.5] | 0.022 |
| Triglyceride, mg/dL | 151.3 [100.4-218.6] | 79.7 [54.6-134.5] | 0.002 |
The values were shown as average±standard deviation for variables that had normal distribution; For non-normally distributed variables, the median; first and third quartile were shown in parentheses. BMI: Body mass index; ALT: alanine aminotransferase; AST: aspartate aminotransferase; GGT: gamma-glutamyltransferase; HOMA-IR: homeostasis model assessment of insulin resistance; CRP: C-reactive protein; LDL: low-density lipoprotein; HDL: high-density lipoprotein.
When NASH (n=64) and non-NASH (n=21) patient groups were compared, it was seen that HOMA-IR levels were statistically significantly higher (p=0.026) in the NASH group. Comparison of clinical and biochemical data of NASH and non-NASH patient groups is summarized in Table 2.
Table 2.
Clinical and biochemical characteristics of NASH and non-NASH groups
| NASH(n=64) | Non-Nash(n=21) | p | |
|---|---|---|---|
| Age | 44.9±10.4 | 46.2±11.1 | 0.610 |
| Gender, F/M | 36/28 | 17/4 | 0.542 |
| BMI, kg/m2 | 34.9±4.2 | 29.4±3.5 | 0.062 |
| ALT, IU/L | 89.7 [49.5-101.3] | 91.1 [47.4-102.4] | 0.357 |
| AST, IU/L | 65.0 [44.6-85.5] | 58.4 [42.7-73.2] | 0.514 |
| GGT, IU/L | 66.0 [30.4-76.7] | 60.7 [28.5-69.1] | 0.642 |
| HOMA-IR | 4.6 [2.9-6.3] | 3.2 [2.5-4.0] | 0.026 |
| CRP, mg/dL | 4.8 [2.4-7.9] | 4.7 [2.1-5.9] | 0.567 |
| Total cholesterol, | 203.3 [161.0-246.4] | 194.7 [160.3-251.0] | 0.583 |
| mg/dL | |||
| LDL, mg/dL | 128.7 [99.3-169.8] | 117.3 [91.8-137.6] | 0.211 |
| HDL, mg/dL | 43.9 [32.8-49.0] | 47.2 [37.2-53.8] | 0.389 |
| Triglyceride, mg/dL | 156.8 [93.5-227.2] | 152.1 [98.0-188.5] | 0.625 |
The values were shown as average±standard deviation for variables that had normal distribution; For non-normally distributed variables, the median, first and third quartile were shown in parentheses. BMI: Body mass index; ALT: alanine aminotransferase; AST: aspartate aminotransferase; GGT: gamma-glutamyltransferase; HOMA-IR: homeostasis model assessment of insulin resistance, CRP: C-reactive protein; LDL: low-density lipoprotein; HDL: high-density lipoprotein.
When NAFLD patients were classified into two subgroups according to their fibrosis levels, there were 27 patients with advanced fibrosis (stage 3-4) and 58 patients with no/mild fibrosis (stage 0-2) (Table 3). In the comparison of these two subgroups, it was seen that BMI (35.2±4.6 kg/m2 and 32.7±4.1 kg/m2, respectively, p=0.031) and HOMA-IR (6.3 [5.8-6.8] and 3.4 [2.6-4.8], respectively, p=0.001) levels of the group with stage 3-4 fibrosis was statistically significantly higher than the group with stage 0-2 fibrosis; and the mean age was also higher (47.3±7.7 and 44.2±12.2, respectively, p=0.026).
Table 3.
Clinical and biochemical characteristics of NAFLD patients with no/mild fibrosis and with advanced fibrosis
| No/mild fibrosis (Stage 0-2, n=58) | Advanced Fibrosis (Stage 3-4, n=27) | p | |
|---|---|---|---|
| Age | 44.2±12.2 | 47.3±7.7 | 0.026 |
| Gender, F/M | 37/20 | 16/12 | 0.745 |
| BMI, kg/m2 | 32.7±4.1 | 35.2±4.6 | 0.031 |
| ALT, IU/L | 90.8 [53.0-116.0] | 86.0 [49.0-102.0] | 0.958 |
| AST, IU/L | 63.6 [40.0-71.0] | 59.1 [40.0-89.0] | 0.979 |
| GGT, IU/L | 64.2 [29.0-67.0] | 65.7 [26.0-67.0] | 0.579 |
| HOMA-IR | 3.4 [2.6-4.8] | 6.3 [5.8-6.8] | 0.001 |
| CRP, mg/dL | 4.5 [2.2-6.9] | 3.6 [2.1-5.9] | 0.456 |
| Total cholesterol, | 210.0 [172.0-234.0] | 189.2 [157.0-217.0] | 0.127 |
| mg/dL | |||
| LDL, mg/dL | 128.8 [98.8-150.0] | 111.8 [94.0-147.0] | 0.273 |
| HDL, mg/dL | 44.2 [36.0-53.0] | 39.8 [38.0-47.0] | 0.341 |
| Triglyceride, mg/dL | 156.1 [100.0-205.0] | 140.9 [85.0-168.0] | 0.210 |
The values were shown as average±standard deviation for variables that had normal distribution; For non-normally distributed variables, the median, first and third quartile were shown in parentheses. BMI: Body mass index; ALT: alanine aminotransferase; AST: aspartate aminotransferase; GGT: gamma-glutamyltransferase; HOMA-IR: homeostasis model assessment of insulin resistance, CRP: C-reactive protein; LDL: low-density lipoprotein; HDL: high-density lipoprotein.
In the covariance analysis, when confounding factors, such as BMI, age and gender, were corrected, the elevation of HOMA-IR level in the advanced fibrosis group continued statistically significantly.
When HOMA-IR was evaluated for the differentiation of cases with advanced fibrosis and mild fibrosis, the area under the curve (AUC) obtained by ROC analysis was 0.68. (p=0.002) (Fig. 1). The optimal cut-off value for HOMA-IR was 3.32, with a sensitivity of 69% and specificity of 64%.
Figure 1.
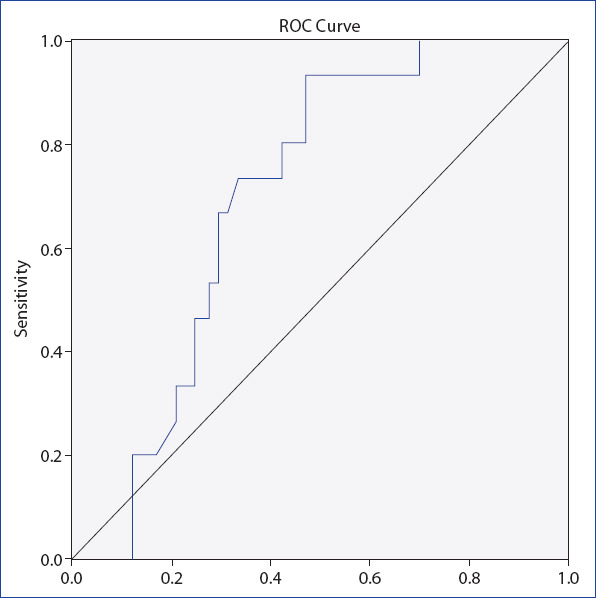
ROC analysis to distinguish NAFLD patients with advanced fibrosis from NAFLD patients with no/mild fibrosis according to HOMA-IR values (AUC=0.68, p=0.002).
Discussion
NAFLD has a broad spectrum from simple hepatic steatosis (simple fatty liver) to NASH (fat accumulation with hepatocellular damage and inflammation), liver cirrhosis and hepatocellular carcinoma.[11] While simple fatty liver is a more benign situation, the progression rate to cirrhosis is around 15-25% in NASH patients.[12] Ekstedt et al.[13] showed the fibrosis stage is the most potent data in predicting disease-specific mortality in NAFLD. Therefore, early and rapid recognition of NASH and fibrosis is important in terms of reducing mortality and morbidity.
In this study, we investigated the relationship of insulin resistance with non-alcoholic fatty liver disease and related histopathological changes. We found that insulin resistance statistically significantly increases as simple fatty liver progresses to NASH and fibrosis level increases. In addition, we showed that HOMA-IR level is an independent risk factor for fibrosis after correction of confounding factors.
There are several studies showing that metabolic disorders, such as obesity, insulin resistance, type 2 diabetes mellitus and hypertriglyceridemia, are closely associated with NAFLD; therefore, non-alcoholic fatty liver disease (NAFLD) is considered to be the manifestation of metabolic syndrome in the liver.[11-13] The insulin resistance plays an important role in NAFLD pathogenesis by enabling the storage of free fatty acids in the liver[14-16] and one of the most important factors of the increase in NAFLD prevalence is the increase in insulin resistance in developed countries.[11]
It has been reported that more than 75% of diabetic patients were diagnosed with NAFLD.[13] Paradis et al.[17] argued that it had a role in hepatic fibrosis by leading to incubation of liver stellate cells with glucose or to cause insulin overstimulate connective tissue growth factor.
The majority of studies show that insulin resistance has a predictive value for fibrosis,[18-21] while there are studies expressing opinions on the contrary.[22] Our study supports the opinion that insulin resistance is an independent risk factor in predicting liver fibrosis.
There are studies showing that the ongoing inflammation due to insulin resistance lead to higher levels of inflammatory markers in NAFLD patients.[23, 24] In our study, it was shown that CRP, one of the inflammatory markers, was significantly higher in the NAFLD group than the control group (p<0.001).
Our study has some limitations. Comprehensiveness of the results will increase if the study is conducted with a broader group of patients. Furthermore, more reliable data on fibrosis progression can be obtained with follow-up biopsies or intermittent FibroScan analyses in long-term prospective studies.
As a result, this study shows that HOMA-IR is an independent risk factor for liver fibrosis. Today, hepatic fibrosis is also accepted as an important mortality marker in NAFLD. Therefore, it is possible to state that NAFLD patients with high insulin resistance will be a priority candidate for liver biopsy compared to other cases. Inclusion of NAFLD patients with high HOMA-IR levels in closer follow-up programs will be effective in reducing mortality and morbidity rates.
Disclosures
Ethics Committee Approval: The study was approved by Şişli Hamidiye Etfal Training and Research Hospital Local Ethics Commitee.
Peer-review: Externally peer-reviewed.
Conflict of Interest: None declared.
Authorship Contributions: Concept – I.S.; Design – I.S., E.G.C., N.D.; Supervision – I.S.; Materials – E.G.C., N.D.; Data collection &/or processing – E.G.C., N.D.; Analysis and/or interpretation – E.G.C., I.S.; Literature search – N.D.; Writing – E.G.C., I.S.; Critical review – I.S.
References
- 1.Milić S, Lulić D, Štimac D. Non-alcoholic fatty liver disease and obesity:biochemical, metabolic and clinical presentations. World J Gastroenterol. 2014;20:9330–7. doi: 10.3748/wjg.v20.i28.9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. American Association for the Study of Liver Diseases;American College of Gastroenterology;American Gastroenterological Association. The diagnosis and management of non-alcoholic fatty liver disease:Practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Am J Gastroenterol. 2012;107:811–26. doi: 10.1038/ajg.2012.128. [DOI] [PubMed] [Google Scholar]
- 3.Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–73. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 4.Söderberg C, Stål P, Askling J, Glaumann H, Lindberg G, Marmur J, et al. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51:595–602. doi: 10.1002/hep.23314. [DOI] [PubMed] [Google Scholar]
- 5.Milić S, Stimac D. Nonalcoholic fatty liver disease/steatohepatitis:epidemiology pathogenesis, clinical presentation and treatment. Dig Dis. 2012;30:158–62. doi: 10.1159/000336669. [DOI] [PubMed] [Google Scholar]
- 6.Baran B, Akyüz F. Non-alcoholic fatty liver disease:what has changed in the treatment since the beginning?World J Gastroenterol. 2014. 20:14219–29. doi: 10.3748/wjg.v20.i39.14219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shields WW, Thompson KE, Grice GA, Harrison SA, Coyle WJ. The Effect of Metformin and Standard Therapy versus Standard Therapy alone in Nondiabetic Patients with Insulin Resistance and Nonalcoholic Steatohepatitis (NASH):A Pilot Trial. Therap Adv Gastroenterol. 2009;2:157–63. doi: 10.1177/1756283X09105462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Streba LA, Vere CC, Rogoveanu I, Streba CT. Nonalcoholic fatty liver disease, metabolic risk factors, and hepatocellular carcinoma:an open question. World J Gastroenterol. 2015;21:4103–10. doi: 10.3748/wjg.v21.i14.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stepanova M, Rafiq N, Younossi ZM. Components of metabolic syndrome are independent predictors of mortality in patients with chronic liver disease:a population-based study. Gut. 2010;59:1410–5. doi: 10.1136/gut.2010.213553. [DOI] [PubMed] [Google Scholar]
- 10.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 11.Brunt EM. Nonalcoholic steatohepatitis:pathologic features and differential diagnosis. Semin Diagn Pathol. 2005;22:330–8. doi: 10.1053/j.semdp.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 12.McCullough AJ. Pathophysiology of nonalcoholic steatohepatitis. J Clin Gastroenterol. 2006;40(Suppl 1):S17–29. doi: 10.1097/01.mcg.0000168645.86658.22. [DOI] [PubMed] [Google Scholar]
- 13.Ekstedt M, Hagström H, Nasr P, Fredrikson M, Stål P, Kechagias S, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547–54. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 14.Bugianesi E, McCullough AJ, Marchesini G. Insulin resistance:a metabolic pathway to chronic liver disease. Hepatology. 2005;42:987–1000. doi: 10.1002/hep.20920. [DOI] [PubMed] [Google Scholar]
- 15.Fracanzani AL, Valenti L, Bugianesi E, Andreoletti M, Colli A, Vanni E, et al. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels:a role for insulin resistance and diabetes. Hepatology. 2008;48:792–8. doi: 10.1002/hep.22429. [DOI] [PubMed] [Google Scholar]
- 16.Bugianesi E, Gastaldelli A, Vanni E, Gambino R, Cassader M, Baldi S, et al. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease:sites and mechanisms. Diabetologia. 2005;48:634–42. doi: 10.1007/s00125-005-1682-x. [DOI] [PubMed] [Google Scholar]
- 17.Paradis V, Perlemuter G, Bonvoust F, Dargere D, Parfait B, Vidaud M, et al. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression:a potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology. 2001;34:738–44. doi: 10.1053/jhep.2001.28055. [DOI] [PubMed] [Google Scholar]
- 18.Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356–62. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- 19.Rosso C, Mezzabotta L, Gaggini M, Salomone F, Gambino R, Marengo A, et al. Peripheral insulin resistance predicts liver damage in nondiabetic subjects with nonalcoholic fatty liver disease. Hepatology. 2016;63:107–16. doi: 10.1002/hep.28287. [DOI] [PubMed] [Google Scholar]
- 20.Svegliati-Baroni G, Bugianesi E, Bouserhal T, Marini F, Ridolfi F, Tarsetti F, et al. Post-load insulin resistance is an independent predictor of hepatic fibrosis in virus C chronic hepatitis and in non-alcoholic fatty liver disease. Gut. 2007;56:1296–301. doi: 10.1136/gut.2006.107946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bugianesi E, Manzini P, D'Antico S, Vanni E, Longo F, Leone N, et al. Relative contribution of iron burden, HFE mutations, and insulin resistance to fibrosis in nonalcoholic fatty liver. Hepatology. 2004;39:179–87. doi: 10.1002/hep.20023. [DOI] [PubMed] [Google Scholar]
- 22.Korkmaz H, Unler GK, Gokturk HS, Schmidt WE, Kebapcilar L. Noninvasive estimation of disease activity and liver fibrosis in nonalcoholic fatty liver disease using anthropometric and biochemical characteristics, including insulin, insulin resistance, and 13C-methionine breath test. Eur J Gastroenterol Hepatol. 2015;27:1137–43. doi: 10.1097/MEG.0000000000000407. [DOI] [PubMed] [Google Scholar]
- 23.Hanley AJ, Williams K, Festa A, Wagenknecht LE, D'Agostino RB, Jr, Haffner SM. Liver markers and development of the metabolic syndrome:the insulin resistance atherosclerosis study. Diabetes. 2005;54:3140–7. doi: 10.2337/diabetes.54.11.3140. [DOI] [PubMed] [Google Scholar]
- 24.Park SH, Kim BI, Yun JW, Kim JW, Park DI, Cho YK, et al. Insulin resistance and C-reactive protein as independent risk factors for non-alcoholic fatty liver disease in non-obese Asian men. J Gastroenterol Hepatol. 2004;19:694–8. doi: 10.1111/j.1440-1746.2004.03362.x. [DOI] [PubMed] [Google Scholar]


