Abstract
Lay Abstract
One of the earliest “red flags” for autism spectrum disorder (ASD) is the child not turning towards social sounds (e.g., caregiver calling their name). A key component of turning attention to sounds is auditory spatial attention, or the ability to pay attention to sounds at a specific location in space while ignoring nearby sounds. We examined auditory spatial attention in children with ASD and neurotypical children by playing sounds from an arrangement of speakers placed in front of the child and spanning to the side. Children were asked to attend to a particular location and press a button whenever they heard a target sound coming from that location, while ignoring all other sounds. Additionally, to explore whether the acoustic complexity of speech sounds (e.g., variability in pitch and timing of sound waves) makes it particularly difficult for children with ASD to locate and pay attention to speech, we tested simple tones, speech sounds (vowels), and complex non-speech sounds. Children with ASD had less finely-tuned (i.e., more diffuse) auditory spatial attention than neurotypical children when attending to sounds in front of them. In other words, they were more likely to also pay attention to distracting sounds near the target. Their spatial attention was more diffuse for speech and for non-speech sounds. Children with ASD with more diffuse spatial attention also had more severe ASD symptoms. This general difficulty with auditory spatial attention may help explain why children with ASD have difficulty directing attention to important sounds, particularly in noisy environments.
Scientific Abstract
One of the earliest observable impairments in autism spectrum disorder (ASD) is a failure to orient to speech and other social stimuli. Auditory spatial attention, a key component of orienting to sounds in the environment, has been shown to be impaired in adults with ASD. Additionally, specific deficits in orienting to social sounds could be related to increased acoustic complexity of speech. We aimed to characterize auditory spatial attention in children with ASD and neurotypical controls, and to determine the effect of auditory stimulus complexity on spatial attention. In a spatial attention task, target and distractor sounds were played randomly in rapid succession from speakers in a free-field array. Participants attended to a central or peripheral location, and were instructed to respond to target sounds at the attended location while ignoring nearby sounds. Stimulus-specific blocks evaluated spatial attention for simple non-speech tones, speech sounds (vowels), and complex non-speech sounds matched to vowels on key acoustic properties. Children with ASD had significantly more diffuse auditory spatial attention than neurotypical children when attending front, indicated by increased responding to sounds at adjacent non-target locations. No significant differences in spatial attention emerged based on stimulus complexity. Additionally, in the ASD group, more diffuse spatial attention was associated with more severe ASD symptoms but not with general inattention symptoms. Spatial attention deficits have important implications for understanding social orienting deficits and atypical attentional processes that contribute to core deficits of ASD.
Keywords: autism spectrum disorder, children, auditory spatial attention, acoustic complexity, speech
Introduction
One of the earliest observable deficits in children with autism spectrum disorder (ASD) is the failure to reliably orient spontaneously to social stimuli in their environment (e.g., human speech, facial expressions, and gestures), called the social orienting deficit (Baranek, 1999; Dawson, Meltzoff, Osterling, Rinaldi, & Brown, 1998; Maestro et al., 2002; Zwaigenbaum et al., 2005). Early social attention deficits may deprive children with ASD of learning opportunities in social interactions, with deleterious effects on subsequent social learning and development (Mundy & Neal, 2001), and have been linked to later impairments in joint attention and language (Dawson et al., 2004). Understanding mechanisms underlying this social orienting deficit will allow for earlier identification and aid in developing targeted interventions, and may also provide insight into the neural underpinnings of ASD.
Auditory Spatial Attention
A key component of the ability to successfully orient to social information is auditory spatial attention, the ability to focus auditory perception on the specific location of a sound source in the environment. There has been little work on children’s auditory spatial attention; however, there has been more work characterizing children’s ability to localize sounds. Studies of auditory localization frequently examine the minimum audible angle (MAA), the minimum distance between two sounds that is necessary for them to be perceived as coming from different locations (Mills, 1958). In typically developing (TD) children, the ability to localize sounds presented directly ahead improves significantly between infancy and age five; the MAA for sounds presented centrally is about 19° in 26- to 30-weeks-old infants (Ashmead, Clifton, & Perris, 1987), 5.7° in 18-month-old toddlers, and 1.5° in 5-year-old children, when it becomes comparable to adult MAA of approximately 1° (Litovsky, 1997). This rapid development of auditory localization abilities coincides with critical periods for other key developmental processes, including acquisition of language and social communication skills. Additionally, there is evidence that localization abilities diminish significantly for sounds that are further from center; when 12–15 year-olds were asked to localize sounds presented 45° horizontally from center, their MAA was about 4–5° (Ashmead et al., 1998). For adults, for sounds heard between 75° and 90° from center, the MAA was estimated to be 8° or more (Grantham, 1995).
Orienting is impacted not only by sound localization abilities, but also by the distribution of auditory attention in space. Evidence from free-field studies with adults shows that auditory spatial attention is distributed in a gradient, where processing efficiency declines with distance from the attended location (Mondor & Zatorre, 1995). Teder-Sälejärvi, Hillyard, Röder, and Neville (1999) examined how diffusion of spatial attention varied based on where participants directed their attention, and found that spatial attention was more finely-tuned when participants were attending to target sounds directly in front compared to the periphery.
Teder-Sälejärvi and colleagues also investigated auditory spatial attention in adults with ASD (Teder-Sälejärvi, Pierce, Courchesne, & Hillyard, 2005). Both adults with ASD and controls showed more finely-tuned spatial attention gradients when attending to sounds at the front than at the periphery. However, adults with ASD had more diffuse auditory spatial attention gradients than controls, and were slower and less accurate in identifying the target sound both at the front and the periphery. This ability to focus on auditory information at a single location in space (e.g., someone calling your name) while ignoring nearby sounds (e.g., others talking nearby) is critical in most environments encountered in daily life, and particularly in social situations. Impairments in auditory spatial attention in ASD may have a significant impact on social functioning across the lifespan.
Stimulus Complexity
Characteristics of the auditory stimulus may also impact an individual’s ability to orient to that sound. There is evidence that children with ASD are more impaired in orienting to speech than to nonsocial stimuli. For example, Dawson and colleagues found that children with ASD have impairments in orienting both to social and nonsocial auditory stimuli compared to both control groups, but this impairment was more pronounced for social compared to nonsocial stimuli (Dawson et al., 1998; Dawson et al., 2004). Research examining processing of auditory stimulus properties has shown that while children with ASD are able to discriminate changes in pitch in both speech and non-speech sounds as well or better than TD controls (Čeponienė et al., 2003), they have more difficulty than controls discriminating changes in the duration of sounds (Lepistö et al., 2005; Lepistö et al., 2006) and changes in consonant-vowel combinations (Kuhl, Coffey-Corina, Padden, & Dawson, 2005). These temporal acoustic cues are key components of speech processing (Shannon, Zeng, Kamath, Wygonski, & Ekelid, 1995). It has been hypothesized that difficulty processing more complex acoustic stimuli may help explain observed speech processing deficits in ASD (Mottron, Dawson, Soulières, Hubert, & Burack, 2006; Samson, Mottron, Jemel, Belin, & Ciocca, 2006).
Children with ASD have also been shown to have impaired pre-attentive involuntary orienting to speech sounds, but not to non-speech sounds (Lepistö et al., 2005; Lepistö et al., 2006). Čeponienė et al. (2003) used event related potentials to examine basic sensory processing and involuntary attentional orienting by comparing neural responses to vowel sounds, acoustically-matched complex sounds, and simple tones. Compared to TD children, children with ASD showed similar neural responses at sensory processing stages, while the ASD group showed a diminished neural response to vowel sounds but not simple tones at the involuntary attentional orienting stage, with no significant group difference in involuntary attentional orienting to the complex non-speech sound. Their results suggested that children with ASD have impairments in pre-attentive processing of complex speech stimuli; however, the impact of the acoustic complexity of speech at later stages of attentional processing is still unknown. A critical area for further study identified in the field is to investigate the role of acoustic complexity in observed processing differences for social stimuli in ASD (O’Connor, 2012).
The present study
We aimed to characterize auditory spatial attention in children with ASD and TD controls, and to determine the effect of auditory stimulus complexity on spatial attention using speech sounds, simple non-speech sounds, and complex non-speech sounds matched to the speech sounds on key acoustic properties. We hypothesized that children with ASD would show more diffuse spatial attention compared to TD controls, and that auditory spatial attention for both groups would be more finely-tuned when attending front than when attending to the side. Based on previous studies of speech and non-speech orienting, we predicted that children with ASD would show differentially more diffuse spatial attention than controls in the vowel condition compared to the simple tone. Additionally, by examining spatial attention to complex non-speech sounds, we could also explore whether any differences in spatial attention to speech were related to acoustic complexity.
Method
Participants
Thirty-nine children and adolescents ages 10 to 17 were recruited for this study, including 21 participants with high functioning ASD and 18 TD controls. Three participants had significant difficulty understanding verbal directions throughout the study, and therefore did not complete study tasks. Data from one participant was unusable due to equipment failure. Additionally, data from one participant who completed the task strongly indicated that he was not performing the task (i.e., pressing the response button continuously throughout the tasks). Specifically, inspection of his data indicated that his total number of button presses was approximately 4.5 SDs above the mean rate across task conditions. Therefore, the final sample included 16 participants with ASD and 18 TD controls.
All participants were male in order to control for potential gender differences in brain lateralization of auditory processing, particularly for complex and speech sounds (Obleser, Eulitz, Lahiri, & Elbert, 2001). Participants were matched on age (see Table 1 for participant characteristics). Written consent was obtained from parents, and assent was obtained from participants. This study was approved by the Institutional Review Board.
Table 1.
Characterization of participants with autism spectrum disorder (ASD) and typically developing controls (TD).
| Measure | ASD (n = 16) | TD (n = 18) | F | p | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | |||
| Age (years) | 13.78 | 1.93 | 10.6–17.5 | 13.94 | 1.85 | 11.1–16.8 | 0.06 | 0.81 |
| Full Scale IQ | 111.00 | 12.30 | 93–133 | 110.89 | 16.40 | 84–148 | 0.00 | 0.98 |
| PPVT-4 | 113.63 | 16.84 | 76–133 | 115.65 | 14.66 | 87–133 | 0.14 | 0.72 |
| SRS-2 T-score | 75.38 | 8.60 | 61–87 | 44.39 | 5.37 | 37–56 | 159.22 | <0.001 |
| SNAP-IV Inattention | 1.44 | 0.81 | 0–2.44 | 0.40 | 0.40 | 0–1.33 | 22.40 | <0.001 |
| SNAP-IV Hyperactivity | 0.94 | 0.58 | 0–1.89 | 0.14 | 0.18 | 0–.56 | 29.38 | <0.001 |
Note: Missing data from one TD participant for the PPVT-4 and the SNAP-IV.
Exclusion criteria for both groups included any known auditory impairment, genetic disorder, neurological or structural brain abnormality or injury as reported by parents, or lab-measured audiometric thresholds in either ear of ≥ 20 dB HL for 250, 500, 1000, 2000, and 4000 Hz tones, and ≥ 25 dB HL for 8000 Hz tones (measured via audiometry). Additional exclusion criteria for the TD group included a diagnosis of a psychiatric, learning, or behavioral disorder, based on parent-reported history and scores on the Child Behavior Checklist (Achenbach & Rescorla, 2001), or a first- or second-degree relative with ASD. All participants were native English speakers and were right-handed.
Presence or absence of ASD was confirmed using a combination of the Autism Diagnostic Observation Schedule (ADOS; Lord, Rutter, DiLavore, & Risi, 1999), Autism Diagnostic Interview-Revised (Lord, Rutter, & Le Couteur, 1994), and clinician judgment. Intellectual ability was assessed using abbreviated versions of the Wechsler scales (WISC-IV; Wechsler, 2003; WAIS-IV; Wechsler, 2008). Receptive vocabulary skills were assessed with the Peabody Picture Vocabulary Test, 4th edition (Dunn & Dunn, 2007). The groups did not differ on Full Scale IQ or receptive vocabulary (see Table 1).
Symptom Measures
Participants’ parents completed two standardized measures regarding their children’s behavioral symptoms related to ASD and inattention/hyperactivity (see Table 1).
Social Responsiveness Scale-2 (SRS-2, Constantino & Gruber, 2012)
The SRS-2 is a widely used parent-report measure of ASD symptoms. The SRS-2 is comprised of 65 items scored on a 4-point scale. It yields a total T-score that provides a measure of overall ASD symptom severity, with higher scores indicating more severe ASD symptoms. Total T-scores of 60 and above indicate symptoms consistent with ASD, while scores of 59 and below are considered to be in the typical range.
Swanson, Nolan, and Pelham Questionnaire, revised (SNAP-IV, Bussing et al., 2008)
Parents also completed the SNAP-IV Inattention and Hyperactivity subscales measuring symptoms of ADHD based on the DSM-IV (American Psychiatric Association, 2000). Each subscale has 9 items rated on a 4-point scale. Average scores are computed for each subscale, with higher scores indicating more severe symptoms. Scores > 1.2 on either subscale indicate potential clinical concern, while an Inattention score > 1.8 and a Hyperactivity score > 2.4 indicate a higher likelihood of ADHD diagnosis (Bussing et al., 2008).
Stimuli
Auditory stimuli consisted of three classes of sounds: natural spoken vowel, synthetic complex sound, and pure tone. Each of these sounds classes had a “standard” version (85% of trials) and a higher-pitched “oddball” version (15% of trials). All sounds were of equal duration (250 ms) and intensity (60 dBA).
Stimuli were recorded or produced and edited using Praat speech editing software (Boersma & Weenink, 2013). The standard vowel stimulus /a/was recorded from a female speaker in a quiet room at a sampling frequency of 44100 Hz. The oddball vowel stimulus was created by raising the fundamental frequency of the standard vowel stimulus by 20% (using Praat’s PSOLA algorithm), so that it would have a noticeably higher pitch.
The standard complex sound was produced by first creating a broadband harmonic tone complex with a fundamental frequency equal to that of the standard spoken vowel stimulus (F0 = 223 Hz). The sound complex was then filtered to have a single formant matching the standard vowel’s first formant (F1 = 870 Hz) and a bandwidth setting of 100 Hz (Boersma & Weenink, 2013). The oddball complex sound was created by increasing the fundamental frequency by 20%.
The frequency of the standard pure tone was matched to the 4th harmonic of the standard complex sound, and the frequency of the oddball pure tone was matched to the 4th harmonic of the oddball complex sound, causing the standard and oddball pure tones to have the same pitch difference as the complex and vowel sounds. The 4th harmonic was selected because the frequency of this component fell near the first formant of the vowel. The pure tone stimuli had 10 ms cosine-squared onset and offset ramps.
Apparatus
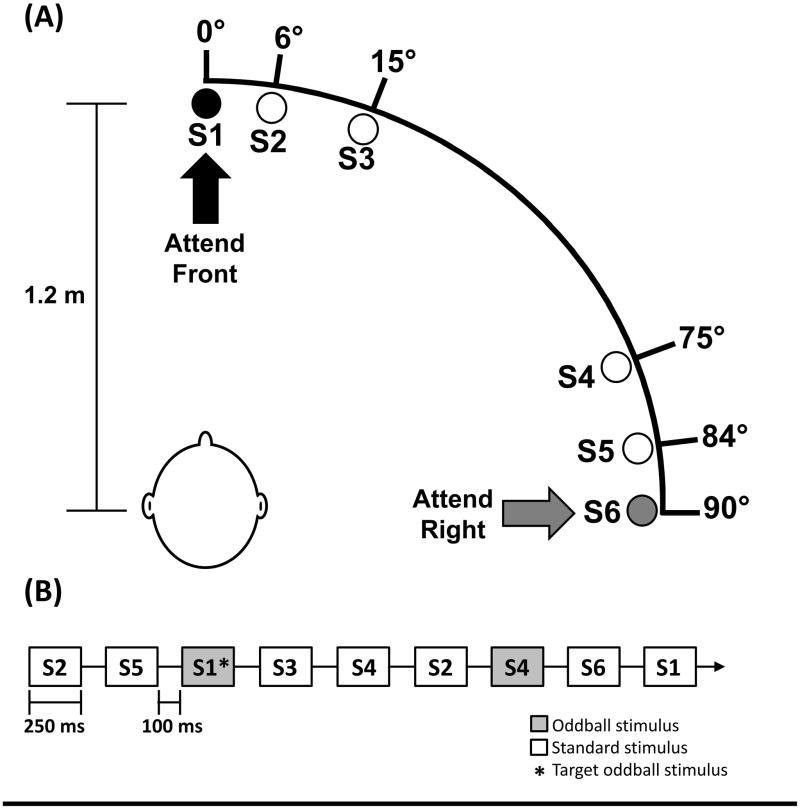
Participants were seated in a sound-attenuated booth. Stimuli were presented through two arrays composed of three speakers each (see Figure 1a). Speakers were placed along a horizontal arc spanning from directly in front (S1) to directly to the right of the participant’s head (S6), at a distance of 1.2 meters. The speaker positions on the arc in relation to the central speaker (S1, 0°) were: S2 at 6°, S3 at 15°, S4 at 75°, S5 at 84°, and S6 at 90°. These speaker locations were chosen based on speaker locations used by Teder-Sälejärvi and colleagues (1999, 2005); however, as they found no differences in response rates between speakers at 12° and 15° (Attend Front) and 78° and 72° (Attend Right) we selected the midpoint locations for the current study. Speaker locations only spanned the right hemi-field of auditory space, similar to the apparatus used by Teder-Sälejärvi and colleagues (1999, 2005), due to space constraints of the sound-attenuated booth. The height of the arrays was adjusted to participant’s height while seated such that speakers were at ear level.
Figure 1.
(A) Experimental apparatus depicting speakers arranged along a horizontal arc spanning from directly in front of participant (0°) to directly to the right of the participant (90°). Speakers are labeled S1–S6 based on position along the arc. Participant was instructed to face forward towards S1 for all tasks. (B) Schematic depicting timing of auditory stimuli in spatial attention task in Attend Front condition. Oddball and standard stimuli are labeled by speaker (S1–S6). Target oddball stimulus is indicated by an asterisk (*).
Procedure
Pitch and Location Discrimination Testing
Prior to the main experimental task, a brief pitch discrimination task was used to confirm participants’ ability to discriminate between our standard and oddball stimuli in the absence of high attentional and spatial discrimination demands. Pairs of stimuli were presented from either the front (S1) or the right speaker (S6), in two separate blocks for each of the three stimulus classes. The sounds in the pair were either the same pitch (both standard stimuli or both oddball) or different pitches (one standard and one oddball). The SOA between the two sounds was 1 second, and the time between the start of the second sound in the pair and the start of the first sound in the next pair was 3 seconds. In this go/no-go task, participants were instructed to press a button only when the sounds in the pair were of different pitch. Signal detection theory (Green & Swets, 1966) was used to determine participants’ ability to differentiate between pitches, where d′ was calculated using hit rate (proportion responses to stimulus pairs of different pitch) and false alarm rate (proportion responses to stimulus pairs of same pitch). A d′ ≥ 1 is typically considered adequate sensitivity. All participants in both groups had d′ scores ≥ 1 for all stimulus types (simple, complex, and vowel) at both attended locations (front and right), meaning they were adequately able to discriminate between the pitches of the standard and oddball sounds for each stimulus type.
A similar location discrimination task assessed participants’ ability to differentiate between speakers in close proximity without pitch discrimination demands and with significantly reduced attentional demands. Pairs of stimuli were presented from S1 and S2 in the Attend Front condition, and S5 and S6 in the Attend Right condition, in two separate blocks for each of the three stimulus classes. In each pair, both stimuli were either played consecutively from the same speaker, or consecutively from different speakers, with the same timing between sounds as in the pitch discrimination task. Participants were instructed to press the button when the two sounds were played from different speakers. Again, d′ was calculated to determine whether participants were able to discriminate between adjacent speakers. Averaged across tone type, in the Attend Front condition, all participants (100%) had an average d′ > 1. Additionally, there was no group difference in the ability to differentiate between S1 and S2 at the front, t(32)=.27, p=.79 [mean d′(SD): ASD=3.53(1.00); TD=3.60(.51)]. In the Attend Right condition, 35.3% of participants had an average d′ < 1, indicating that participants in both groups had significant difficulty discriminating between these two peripheral speakers. Again, there was no group difference in discrimination ability at the right, t(32)=.26, p=.80 [mean d′(SD): ASD=1.49(1.21); TD=1.59(1.02)]. We present auditory spatial attention findings from the Attend Right condition of the main experimental task; however, caution should be used in interpreting findings in this condition because some participants may not have sufficient auditory spatial acuity to distinguish these locations.
Spatial Attention Tasks
Prior to the main experimental tasks, brief blocks of practice trials similar to the experimental task were administered to provide participants an opportunity to experience the task demands and verify that they understood the instructions. Before moving on to the experimental tasks, participants’ responses to the practice trials were examined, and feedback was provided if participants did not press the button to the majority of target oddball stimuli or if participants pressed frequently to non-target stimuli. Participants were then given another opportunity to complete the same practice block before moving on to the experimental trials.
For the main experimental blocks (Figure 1b), stimuli were randomized in single-sound-class blocks of 700 stimuli each. In each block, 200 stimuli were played from the target speaker (S1 in the Attend Front condition, S6 in the Attend Right condition), and 100 stimuli were played from each non-target speaker. Fifteen percent of stimuli at each speaker were oddball stimuli, meaning that in each block, 28.6% of all oddball stimuli were played from the target speaker, and the remaining 71.4% were equally distributed across the remaining five non-target speakers. Thus, in each block, oddball stimuli played from the target speaker constituted 4.3% of total stimuli. The stimulus onset asynchrony (SOA) was 350 ms, and each block lasted 4.08 minutes with a brief pause halfway through. Stimuli were presented and participant responses were collected using Presentation software (Version 17.0, www.neurobs.com). The task consisted of two Attend conditions, where participants were instructed to attend to either S1 (Attend Front) or S6 (Attend Right). For both conditions, participants were instructed to remain still and look directly forward at S1; head position was monitored by the experimenter throughout the tasks.
Participants were instructed to press a button as quickly as possible only when they heard the oddball stimulus from the attended (target) speaker, and to ignore all other stimuli from all other speakers. Within each Attend condition (Front, Right), there was a separate block for each of the three stimulus classes (pure tone, complex sound, vowel). These six experimental blocks were presented in a semi-randomized, counterbalanced order across participants (three of the same Attend condition blocks were never presented consecutively in order to minimize response bias at either attended location). Participants’ performance on the main experimental tasks were measured by the proportion of correct responses to oddball stimuli at the attended location (hits) and the proportion of responses to oddball stimuli at each of the non-target speakers (false alarms). Because approximately three stimuli were presented per second, it was not possible to unambiguously assign a response to a single stimulus. Therefore, each button press was related to any stimuli that fell 200–1000 ms before the button press. Within each block, randomization patterns were checked to ensure that no two oddball stimuli were presented within the same 1000 ms time window such that a button press could not be associated with more than one oddball stimulus. Examination of overall button presses showed that 90.1% of TD participants’ responses and 85.6% of ASD participants’ responses were to oddball stimuli (both target and non-target), which did not differ significantly, t(32)=1.33, p=.19. Similar percentages of button presses to oddball stimuli were observed in the Attend Right condition (TD: 90.5%, ASD: 86.6%, t(32)=1.47, p=.15). These data demonstrate that, as expected, participants in both groups were primarily responding to oddball stimuli.
Data Analysis
Spatial Attention–Task Performance
Performance on the spatial attention task was quantified using d′, where hits were correct button presses to oddball stimuli at the target speaker (S1 in the Attend Front condition, S6 in the Attend Right condition) and false alarms were button presses to oddball stimuli at non-target speakers (S2–S6 in Attend Front, S1–S5 in Attend Right). Higher values of d′ represent better task performance, or better ability to distinguish the signal (oddball stimuli at the target location) from the noise (oddball stimuli at non-target locations). This d′ metric was calculated for each subject in each of the six experimental conditions. Group differences in task performance (d′) were examined using mixed-model analysis of variance (ANOVA). Follow-up univariate analyses were conducted for significant main effects and interactions.
Spatial Attention–Response Analysis
In order to better understand the pattern of responses across speakers, the response rate—the proportion of button presses to oddball stimuli out of the total number of oddball stimuli—was calculated for each subject at each of the six speakers for each experimental block, which were used in calculations of group means (see Table 3). At the target speaker (i.e., S1 for Attend Front, S6 for Attend Right), the response rate represents hit rate (i.e., correct responding), while the response rate at each non-target speaker represents a false alarm rate (i.e., incorrect responding). Proportion data were transformed using the arcsine square root procedure due to non-normality. Analyses were conducted on transformed variables, but untransformed proportion means are presented in figures for ease of interpretation.
Table 3.
Mean response rate data, averaged across Sound Type by Attend condition and separately by Sound Type, for participants with autism spectrum disorder (ASD) and typically developing controls (TD). Response rates reflect the proportion of responses to oddball stimuli at each speaker (S1–S6) out of the total number of oddball stimuli presented at that speaker.
| Condition | ASD (n = 16) | TD (n = 18) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | S5 | S6 | S1 | S2 | S3 | S4 | S5 | S6 | |
| Attend Front* | 0.68 | 0.65 | 0.32 | 0.04 | 0.04 | 0.05 | 0.71 | 0.43 | 0.11 | 0.02 | 0.01 | 0.01 |
| Simple | 0.78 | 0.73 | 0.49 | 0.05 | 0.07 | 0.07 | 0.76 | 0.46 | 0.19 | 0.03 | 0.03 | 0.02 |
| Complex | 0.64 | 0.67 | 0.23 | 0.03 | 0.04 | 0.04 | 0.72 | 0.42 | 0.05 | 0.01 | 0.01 | 0.01 |
| Vowel | 0.62 | 0.57 | 0.24 | 0.05 | 0.03 | 0.05 | 0.64 | 0.4 | 0.09 | 0.02 | 0.00 | 0.01 |
|
| ||||||||||||
| Attend Right* | 0.08 | 0.10 | 0.07 | 0.58 | 0.62 | 0.68 | 0.03 | 0.03 | 0.05 | 0.54 | 0.57 | 0.62 |
| Simple | 0.13 | 0.11 | 0.09 | 0.62 | 0.64 | 0.74 | 0.06 | 0.04 | 0.04 | 0.61 | 0.64 | 0.69 |
| Complex | 0.06 | 0.90 | 0.07 | 0.57 | 0.65 | 0.65 | 0.02 | 0.02 | 0.06 | 0.51 | 0.49 | 0.57 |
| Vowel | 0.05 | 0.11 | 0.04 | 0.57 | 0.58 | 0.65 | 0.03 | 0.04 | 0.04 | 0.50 | 0.58 | 0.58 |
mean response rate across all Sound Types
Group differences in spatial attention were compared using mixed-model analysis of variance (ANOVA) examining response rates at each of the six speakers. Due to participants’ significant localization difficulties on the location discrimination task at the Right, and poor overall spatial attention task performance (as indexed by d′) in the Attend Right condition in both groups and across stimulus types, analyses of group differences in mean response rates were conducted separately by Attended Location. Planned follow-up univariate analyses were conducted at each speaker. Paired samples t-tests examined within-group effects between speakers of interest (S1–S3). The Bonferroni correction was used to correct for multiple comparisons where appropriate. Effect sizes are reported as partial eta squared ( ).
Relationship to Symptom Severity in ASD Group
Exploratory analyses also examined the relationship between spatial attention and severity of ASD symptoms, as well as general inattention and hyperactivity/impulsivity symptoms, which are highly comorbid in children with ASD (Simonoff et al., 2008). To do so, a summary value of performance on the spatial attention task (d′) in the Attend Front condition was calculated for each participant by averaging d′ values across stimulus type. Bivariate correlations were used to examine the relationship between spatial attention task performance and ASD symptoms (SRS-2 Total T-score), inattention symptom severity (SNAP-IV Inattention scale), and hyperactivity/impulsivity symptom severity (SNAP-IV Hyperactivity scale).
Results
Spatial Attention–Task Performance
Task performance (d′) on the spatial attention task was examined via a Group × Attended Location × Stimulus Type ANOVA, which yielded a significant effect of Attended Location, F(1,32)=61.24, p<.001, , such that participants across both groups performed significantly worse in the Attend Right condition compared to the Attend Front condition. The Attended Location × Group interaction was also significant, F(1,32)=16.45, p<.001, , due to a significant group difference in task performance in the Attend Front condition (ASD participants had significantly poorer performance than TD participants, F(1,32)=8.94, p=.005, ), with no group differences in task performance in the Attend Right condition, F(1,32)=.76, p=.39, (Figure 2).
Figure 2.
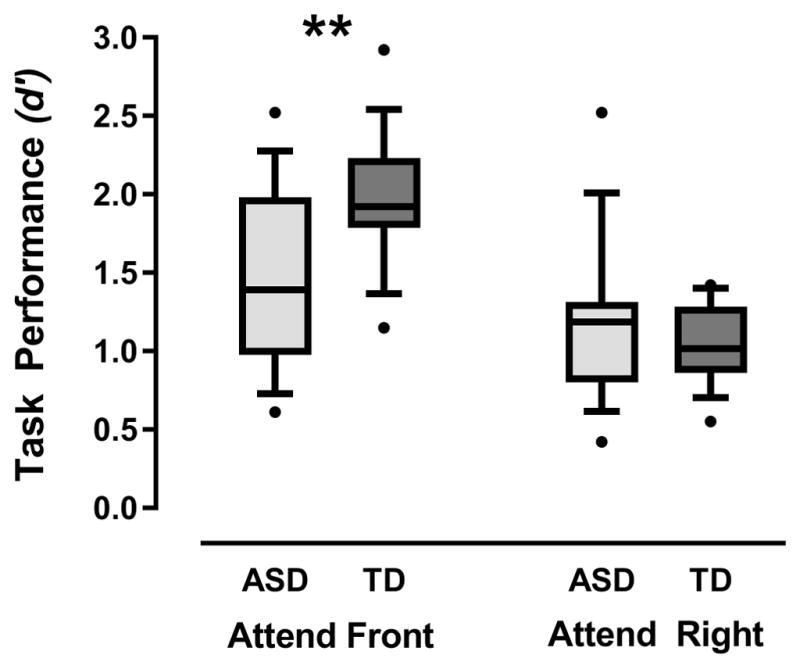
Spatial attention task performance (as measured by d′) for both groups in the Attend Front and Attend Right conditions, where d′ values calculated based on hits to oddball sounds at the target speaker and false alarms to oddball sounds at non-target speakers. Box plots depict the full range of d′ scores. Significant group differences in task performance are indicated, **p<.01.
There was also a significant main effect of Stimulus Type, F(2,64)=4.90, p=.01, , with task performance across both experimental groups being significantly better for the simple sound as compared to both the complex non-speech and vowel sounds, which yielded similar task performance. However, contrary to our original hypothesis, there was no significant Stimulus Type × Group interaction, F(2,64)=1.35, p=.26, , suggesting that differences in overall task performance based on stimulus type did not differ significantly between groups.
The effect size for this interaction was small; however, because of our a priori hypothesis regarding an ASD-specific difficulty for speech compared to non-speech sounds, we performed a post hoc Bayes factor analysis (Dienes, 2014) to determine whether this null finding could be considered definitive based on our data. A Bayes factor value < .33 is considered definitive in favor of the null hypothesis, a value > 3.0 is considered definitive in favor of the alternative hypothesis, and values in between .33 and 3.0 indicate that the data may not be sensitive enough to detect an effect (Jeffreys, 1961). We calculated the Bayes factor comparing the mean difference in task performance between the simple sound and the vowel sound in the Attend Front condition for each group, the primary comparison of interest. This analysis yielded a Bayes factor value of 0.38, suggesting that our data may not be sensitive enough to definitively conclude that stimulus type has no effect on spatial attention in ASD.
Finally, neither of the other interactions was significant: Attended Location x Stimulus Type, F(2,64)=1.88, p=.16, Equation ; Attended Location × Stimulus Type × Group, F(2,64)=0.48, p=.62, . To better understand the nature of group differences in task performance and characterize the spatial attention gradient for both TD and ASD groups, response rates across locations were further analyzed.
Spatial Attention–Response Analysis
Attend Front
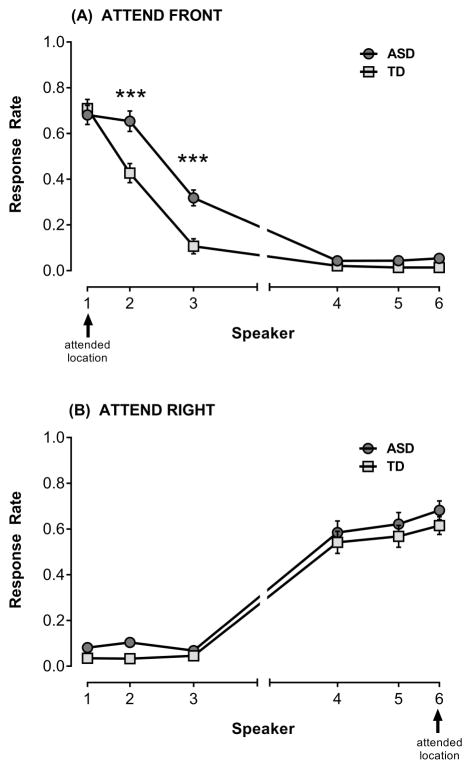
The Group × Stimulus Type × Speaker ANOVA of mean response rates in the Attend Front condition yielded an expected effect of Speaker, F(5,160)=342.72, p<.001, , with the target speaker having the highest response rate, and the speakers on the right (S4–S6) having very low response rates. There was a significant main effect of Group, F(1,32)=10.69, p=.003, , where the ASD group had a higher average response rate than the TD group.
Importantly, the Group × Speaker interaction was significant, F(5,160)=10.02, p<.001, (see Figure 3a), indicating patterns of spatial attention (averaged across stimulus type) differed between groups. Follow-up univariate analyses at each speaker, corrected for multiple comparisons (significant p<.0083), revealed comparable performance for the groups at the target speaker (S1), F (1,32)=.17, p=.69, , but showed that the ASD group pressed significantly more than the TD group to oddball stimuli at S2, F(1,32)=13.68, p<.001, , and S3, F(1,32)=19.95, p<.001, . Responses to speakers S4–S6 did not reach significance after correction for multiple comparisons, S4: F(1,32)=2.70, p=.11, , S5: F(1,32)=7.85, p=.009, , S6: F(1,32)=5.35, p=.03, . Thus, the Group × Speaker interaction effect reflects an increased number of false alarms to oddball sounds at nearby non-target speakers in the ASD group compared to the TD children.
Figure 3.
Mean proportion responses (i.e., response rate) ± SEM to oddball stimuli at each speaker collapsed across stimulus type, where response rate at target represents hits and response rate at peripheral speakers are false alarms. The drop-off in responding from target to distant speakers represents the spatial attention gradient. (A) Attend Front condition (target=S1), (B) Attend Right condition (target=S6). Only group differences in mean response rates at speakers that were significant after correcting for multiple comparisons are indicated, ***p<.001.
To understand the pattern of spatial attention within groups, paired t-tests examined differences in response rate at S1–S3. In the TD group, response rates dropped off as expected from S1 to S2 (p<.001), and from S2 to S3 (p<.001), indicating a finely-tuned spatial attention gradient. However, in the ASD group, there was no difference in proportion responding between S1 and S2 (p=.16), providing further evidence of more diffuse spatial attention. Response rate at S3 was significantly lower than S2 (p<.001).
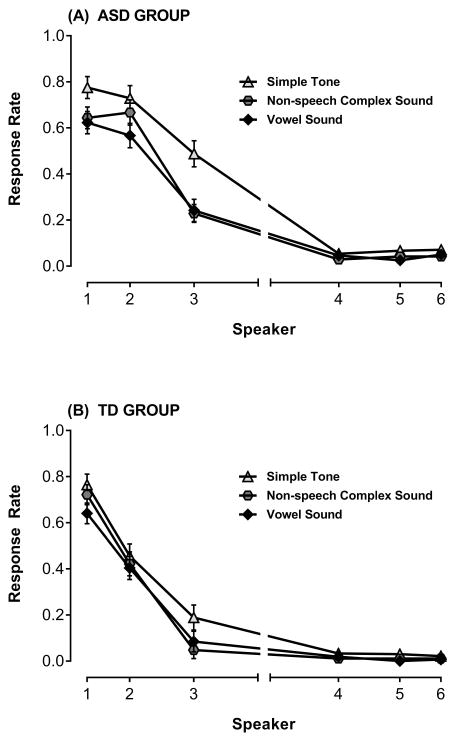
Stimulus Type
We also examined the impact of stimulus type on auditory spatial attention. There was a significant main effect of stimulus type F(2,64)=17.88, p<.001, , with the simple tone having a higher overall response rate compared to the complex non-speech sound and the vowel. Additionally, the Stimulus Type × Speaker interaction was significant, F(10,320)=5.91, p<.001, , primarily due to higher response rates to the simple sound at S1, S2, S3, and S5. However, the interactions for Group × Stimulus Type, F(2,64)=1.38, p=.26, , and Group × Stimulus Type × Speaker, F(10,320)=.92, p=.51, , were not significant, indicating that overall response and spatial attention gradients for varying sound types were not significantly different across groups (see Figure 4).
Figure 4.
Mean proportion responses (i.e., response rate) ± SEM to oddball stimuli at each speaker in the Attend Front condition for each of the three stimulus types (simple tone, non-speech complex sound, vowel sound), separately for the (A) ASD group and (B) TD group. Response rate at target speaker represents hits and response rate at peripheral speakers are false alarms. Though there is a main effect of stimulus type overall (higher response rates to the simple tone), the key finding is that the pattern of the spatial attention gradient was similar across stimulus types, and across groups.
Attend Right
A similar Group × Stimulus Type × Speaker mixed-model ANOVA examined response rates when participants were attending to the right (S6). There was a significant effect of Speaker, F(5,160)=275.04, p<.001, , where the target speaker (S6) and two adjacent speakers (S5 and S4) had similarly high response rates, while the speakers more distant from the target (S3–S1) were significantly lower. However, the main effect of Group, F(1,32)=2.19, p=.15 , and the key Group × Speaker interaction, F(5,160)=.39, p=.85, , were not significant (see Figure 3b).
As in the Attend Front condition, there was a significant main effect of stimulus type, F(2,64)=8.83, p<.001, , where participants pressed significantly more for the simple tone. None of the other interactions was significant: Stimulus Type × Group, F(2,64)=.94, p=.40, . Stimulus Type × Speaker, F(10,320)=1.48, p=.15, , or Stimulus Type × Speaker × Group, F(10,320)=1.70, p=.08, .
Spatial Attention and Symptom Severity in ASD group
Next we examined how ASD symptoms and inattention symptoms were related to spatial attention abilities in the ASD group when attending front. Spatial attention (represented by d′) was significantly related to ASD symptom severity, r(14)=-.59, p=.016, correcting for multiple comparisons (significant p<.0167), such that children with more severe ASD symptoms had significantly more diffuse spatial attention. There was no relationship between spatial attention and more general inattention symptoms (as measured by the SNAP-IV Inattention scale), r(14)=-.23, p=.40. Additionally, there was no relationship between performance on spatial attention tasks and hyperactivity/impulsivity symptoms (as measured by the SNAP-IV Hyperactivity scale), r(14)=.06, p=.82.
Discussion
The present study examined auditory spatial attention in children with ASD compared to TD controls. Overall, our findings indicated that children with ASD showed general impairments in auditory spatial attention to centrally located sounds for both speech and non-speech stimuli. To our knowledge, this is the first study to characterize the auditory spatial attention gradient in children, as well as the first to do so in a population of children with ASD.
Specifically, when attending to sounds directly in front of them, children with ASD showed significantly less finely-tuned auditory spatial attention than TD children. Both groups had comparable accuracy in detecting the oddball sound from the target location, but children with ASD pressed significantly more to nearby non-target oddball sounds, with approximately equal rates of responding to the target and nearest adjacent (6°) non-target locations. In other words, this deficit in spatial attention in children with ASD is primarily due to an inability to ignore nearby competing stimuli, rather than a deficit in detecting the target sound source. Results from our study extend work by Teder-Sälejärvi et al. (2005) on auditory spatial attention in adults with ASD to a younger population. While our results are mostly consistent with their findings of more diffuse auditory spatial attention gradients in adults with ASD, that study also found that adults with ASD pressed significantly less than neurotypical adults at the target location. It is possible that the longer auditory stimuli used in the present study (250 ms, compared to 82 ms in Teder-Sälejärvi et al.) may have influenced accuracy rates in detecting the target, or that group differences in the ability to accurately detect the target sound emerge with development, such that neurotypical adults see more gains in this ability than adults with ASD.
We also found that in the children with ASD, more diffuse auditory attention when attending centrally was related to the severity of ASD symptoms. In contrast, we did not find a significant relationship between auditory spatial attention and parent-reported inattention symptoms or hyperactivity/impulsivity symptoms. While these symptoms are often comorbid in children with ASD (Simonoff et al., 2008), and are more prevalent in the ASD group than the TD group in the present study, our results suggested that it is unlikely that general inattention or impulsivity symptoms can fully explain our group differences in auditory spatial attention abilities.
While findings in the present study provide evidence for significant differences in the spatial distribution of auditory attention to centrally presented sounds in children with ASD, further work is necessary to explore possible alternative explanations for this pattern of results based on other atypical attentional processes in ASD. One possibility is that increased responding to nearby non-target oddball sounds by children with ASD in the present study could be related to reduced response inhibition. Indeed, previous work has suggested that adolescents with ASD may exhibit higher false alarm rates in go/no-go tasks due to impairments in inhibitory control (Vara et al., 2014). In our study, while children with ASD did have higher rates of false alarms than TD children, these false alarms were distributed primarily over nearby speakers 2 and 3, rather than evenly across all non-target speakers. These results suggest that the observed results are likely not due solely to a general impairment in inhibitory control. However, future studies could manipulate auditory stimulus features (e.g., increase or decrease intensity) of target vs. non-target stimuli in order to further explore the role of inhibitory control preventing exogenous shifts of attention across space in children with ASD.
Another possibility is that diffuse spatial attention observed in the ASD group may be a result of an impairment in rapid endogenous attention shifting. Courchesne et al. (1994) showed that adolescents with ASD exhibit difficulty with rapid (< 2.5 seconds) shifts in endogenous attention, with poorer target detection and slower reaction times. In our study, this phenomenon could be captured by examining whether children with ASD were more likely to miss the target oddball sound at the central speaker (S1) after hearing a non-target oddball sound from a peripheral speaker (S4–S6) within the previous 2.5 seconds. A post hoc analysis of the data in the current study suggested that groups did not differ in the proportion of target stimuli missed after hearing an oddball stimulus in the periphery within 2.5 seconds prior (ASD: Mean=.41, SD=.09; TD: Mean=.37, SD=.08; Mann-Whitney U Test, p=.18). However, it will be critical for future studies of auditory spatial attention of ASD to directly test the role of impaired rapid endogenous attention shifting in auditory spatial attention abilities in children with ASD.
Finally, it is possible that basic auditory spatial discrimination abilities in children with ASD are more impaired in the presence of noise or distractors. Evidence suggests that children with ASD demonstrate impaired processing of basic auditory information in noisy or distracting environments (Alcántara, Weisblatt, Moore, & Bolton, 2004). While performance on the location discrimination task showed that groups did not differ in their ability to discriminate between the closest speakers, this task did not require suppression of distracting auditory information and therefore does not allow us to test this hypothesis directly within the present study. Future studies should examine location discrimination abilities of children with ASD in the presence of distractor sounds that do not include a spatial component in order to further investigate this alternative explanation.
Our results also showed that children in both groups had significantly more diffuse spatial attention gradients when attending to the side compared to the front. Teder-Sälejärvi et al. (2005) also found that spatial attention was more diffuse when attending to the side; however, they reported that adults with ASD had differentially more diffuse spatial attention at the periphery than neurotypical adults. The lack of group differences in the present study may be because TD children also showed markedly diffuse spatial attention at the periphery. In our preliminary location discrimination task, a number of children in both groups showed poor differentiation between the two most peripheral speakers (separated by 6°). Thus, the speaker locations chosen for the present study limited our ability to capture the full spatial attention gradient at the side. Without knowing whether diffuse peripheral spatial attention was primarily due to difficulties in sound localization, it is imprudent to make assumptions about the lack of group differences.
Future studies of auditory spatial attention would also benefit from testing a wider range of spatial locations (e.g., equally spaced speakers spanning the full range from a central location to 90° to the side) to be able to capture the spatial attention gradient at the periphery. Additionally, the experimental apparatus used in the present study only allowed for examination of spatial attention of the right hemisphere, due to limitations of physical space. However, there is evidence that the lateralization of cortical functions may differ in individuals with ASD, particularly for language processing and language development (Lindell & Hudry, 2013). It will be important for future work to examine auditory spatial attention bilaterally across a broader range of space to fully capture differences in individuals with ASD.
A secondary goal of this study was to examine whether spatial attention in ASD differed between speech and non-speech sounds, and if so, whether acoustic complexity helped to explain that difference. Contrary to our hypothesis, we found no differences within either group in spatial attention between speech vs. non-speech sounds (both simple and complex); specifically, within children with ASD, spatial attention was similarly diffuse across all stimulus types. While this study is an initial step towards understanding the role of complexity in auditory spatial attention, results of the Bayes factor follow-up analysis indicated that the data may not have been sensitive enough to definitively conclude that acoustic complexity has no effect on spatial attention in ASD, warranting further exploration of this question. The current study was limited by the use of an isolated vowel sound, which does not capture the full range of acoustic complexity of human speech (Fant, 1971). Testing auditory spatial attention to more complex and ecologically valid types of speech sounds (e.g., consonant-vowel combinations, words) may lead to a more comprehensive understanding of whether acoustic complexity impacts auditory spatial attention in ASD. Additionally, our final sample was relatively small. Although we had sufficient power to detect differences in the pattern of spatial attention, larger samples may be needed to identify more subtle stimulus effects.
Overall, our findings suggest that while children with ASD may be able to detect important sources of auditory information in their environment, other nearby sounds may be particularly intrusive. Even when facing and attending to an auditory source (e.g., parent, teacher, peer), children with ASD may also be processing extraneous noises, with deleterious effects on learning and social communication. It should be noted that our participants were older children and adolescents, all males, and had average to above average IQ, so we do not know if findings generalize beyond this particular subgroup. Nonetheless, auditory spatial attention differences likely have a significant impact on daily functioning in this group of individuals and across the lifespan. Our results also necessitate examination of auditory spatial attention in male and female children across a wider range of intellectual abilities, as well as examination of the developmental impact of auditory spatial attention differences in infancy and early childhood. Young children’s ability to localize sounds improves significantly during early developmental periods critical for language learning (Litovsky, 1997), and the ability to orient attention based on exogenous cues continues to develop until at least age 10 (for a review, see Keehn, Müller, & Townsend, 2013). Further research in a younger sample will be essential to understanding the relationship between sensory processing of complex stimuli, auditory spatial attention differences, and atypical development of social attention and complex social communication in ASD.
Although we did not find significant differences in spatial attention to speech vs. non-speech stimuli, general auditory spatial attention deficits may have a particularly detrimental effect in social contexts, especially when combined with other ASD characteristics. For example, spatial attention difficulties may be further exacerbated by deficits in eye contact in ASD. Previous work with neurotypical populations has shown that auditory spatial fields shift depending on eye gaze location (Razavi, O’Neill, & Paige, 2007). Our results showed that children’s spatial attention is more finely-tuned when facing the sound source, so deficits in eye contact may put children with ASD at further disadvantage for auditory attention and processing, particularly in social contexts. Understanding the role of auditory spatial attention deficits in the development of ASD will help to elucidate neurocognitive mechanisms of ASD, and is likely to have important implications for both early identification and early intervention.
Table 2.
Mean spatial attention task performance (d′) averaged across Sound Type by Attend condition and separately by Sound Type, for participants with autism spectrum disorder (ASD) and typically developing controls (TD). d′ values calculated based on hits to oddball sounds at the target speaker and false alarms to oddball sounds at non-target speakers.
| Condition | ASD (n = 16) | TD (n = 18) | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Attend Front* | 1.46 | 0.58 | 1.96 | 0.40 |
| Simple | 1.73 | 1.02 | 2.12 | 0.79 |
| Complex | 1.33 | 0.60 | 2.02 | 0.53 |
| Vowel | 1.32 | 0.56 | 1.76 | 0.34 |
|
| ||||
| Attend Right* | 1.17 | 0.50 | 1.06 | 0.25 |
| Simple | 1.23 | 0.40 | 1.17 | 0.31 |
| Complex | 1.04 | 0.54 | 1.04 | 0.40 |
| Vowel | 1.24 | 0.79 | 0.96 | 0.29 |
mean d′ across all Sound Types
Acknowledgments
Grant sponsor: National Institute on Deafness and Other Communication Disorders; Grant numbers T32 DC009974, R01 DC009439 (PI: Bennetto), R21 DC011094 (PI: Bennetto).
Data collection for this manuscript was supported by the following grants from the National Institute on Deafness and Other Communication Disorders: T32 DC009974, R01 DC009439 (PI: Bennetto), R21 DC011094 (PI: Bennetto). The authors have no conflicts of interest to declare. The authors thank Kenneth Henry, Ph.D. for his contribution to the development of the auditory stimuli, and John Wilson and Rebecca Goldberg for their assistance in testing participants. We also sincerely thank the children and families who participated in this research.
References
- Achenbach T, Rescorla L. Manual for the ASEBA School-Age Forms & Profiles. Burlington, VT: Achenbach System of Empirically Based Assessment; 2001. [Google Scholar]
- Alcántara JI, Weisblatt EJ, Moore BC, Bolton PF. Speech-in-noise perception in high-functioning individuals with autism or Asperger’s syndrome. Journal of Child Psychology and Psychiatry. 2004;45(6):1107–1114. doi: 10.1111/j.1469-7610.2004.t01-1-00303.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders IV-TR. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Ashmead DH, Clifton RK, Perris EE. Precision of auditory localization in human infants. Developmental Psychology. 1987;23(5):641–647. [Google Scholar]
- Ashmead DH, Wall RS, Ebinger KA, Eaton SB, Snook-Hill MM, Yang X. Spatial hearing in children with visual disabilities. Perception. 1998;27:105–122. doi: 10.1068/p270105. [DOI] [PubMed] [Google Scholar]
- Baranek GT. Autism during infancy: A retrospective video analysis of sensory-motor and social behaviors at 9–12 months of age. Journal of Autism and Developmental Disorders. 1999;29(3):213–224. doi: 10.1023/a:1023080005650. [DOI] [PubMed] [Google Scholar]
- Bussing R, Fernandez M, Harwood M, Hou W, Garvan CW, Eyberg SM, Swanson JM. Parent and teacher SNAP-IV ratings of attention deficit hyperactivity disorder symptoms psychometric properties and normative ratings from a school district sample. Assessment. 2008;15(3):317–328. doi: 10.1177/1073191107313888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Čeponienė R, Lepistö T, Shestakova A, Vanhala R, Alku P, Näätänen R, Yaguchi K. Speech–sound-selective auditory impairment in children with autism: They can perceive but do not attend. Proceedings of the National Academy of Sciences. 2003;100(9):5567–5572. doi: 10.1073/pnas.0835631100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP. Social Responsiveness Scale, Second Edition (SRS-2) Los Angeles, CA: Western Psychological Services; 2012. [Google Scholar]
- Courchesne E, Townsend J, Akshoomoff NA, Saitoh O, Yeung-Courchesne R, Lincoln AJ, … Lau L. Impairment in shifting attention in autistic and cerebellar patients. Behavioral Neuroscience. 1994;108(5):848. doi: 10.1037//0735-7044.108.5.848. [DOI] [PubMed] [Google Scholar]
- Dawson G, Meltzoff A, Osterling J, Rinaldi J, Brown E. Children with autism fail to orient to naturally occurring social stimuli. Journal of Autism and Developmental Disorders. 1998;28(6):479–485. doi: 10.1023/a:1026043926488. [DOI] [PubMed] [Google Scholar]
- Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, Liaw J. Early social attention impairments in autism: Social orienting, joint attention, and attention to distress. Developmental Psychology. 2004;40(2):271. doi: 10.1037/0012-1649.40.2.271. [DOI] [PubMed] [Google Scholar]
- Dienes Z. Using Bayes to get the most out of non-significant results. Frontiers in Psychology. 2014;5:781. doi: 10.3389/fpsyg.2014.00781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn LM, Dunn DM. Peabody picture vocabulary test, (PPVT-4) Minneapolis, MN: Pearson Assessments; 2007. [Google Scholar]
- Fant G. Acoustic theory of speech production: with calculations based on X-ray studies of Russian articulations. Vol. 2. The Hague: de Gruyter; 1971. [Google Scholar]
- Grantham D. Spatial hearing and related phenomena. In: Moore B, editor. Hearing. 2. New York, NY: Academic Press; 1995. pp. 297–345. [Google Scholar]
- Jeffreys H. The Theory of Probability. Oxford, UK: Oxford University Press; 1961. [Google Scholar]
- Keehn B, Müller RA, Townsend J. Atypical attentional networks and the emergence of autism. Neuroscience and Biobehavioral Reviews. 2013;37(2):164–183. doi: 10.1016/j.neubiorev.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl PK, Coffey-Corina S, Padden D, Dawson G. Links between social and linguistic processing of speech in preschool children with autism: Behavioral and electrophysiological measures. Developmental science. 2005;8(1):F1–F12. doi: 10.1111/j.1467-7687.2004.00384.x. [DOI] [PubMed] [Google Scholar]
- Lepistö T, Kujala T, Vanhala R, Alku P, Huotilainen M, Näätänen R. The discrimination of and orienting to speech and non-speech sounds in children with autism. Brain Research. 2005;1066(1):147–157. doi: 10.1016/j.brainres.2005.10.052. [DOI] [PubMed] [Google Scholar]
- Lepistö T, Silokallio S, Nieminen-von Wendt T, Alku P, Näätänen R, Kujala T. Auditory perception and attention as reflected by the brain event-related potentials in children with Asperger syndrome. Clinical Neurophysiology. 2006;117(10):2161–2171. doi: 10.1016/j.clinph.2006.06.709. [DOI] [PubMed] [Google Scholar]
- Lindell AK, Hudry K. Atypicalities in cortical structure, handedness, and functional lateralization for language in autism spectrum disorders. Neuropsychology Review. 2013;23(3):257–270. doi: 10.1007/s11065-013-9234-5. [DOI] [PubMed] [Google Scholar]
- Litovsky RY. Developmental changes in the precedence effect: Estimates of minimum audible angle. Journal of the Acoustical Society of America. 1997;102(3):1739–1745. doi: 10.1121/1.420106. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism diagnostic observation schedule: Manual. Los Angeles, CA: Western Psychological Services; 1999. [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Maestro S, Muratori F, Cavallaro MC, Pei F, Stern D, Golse B, Palacio-Espasa F. Attentional skills during the first 6 months of age in autism spectrum disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(10):1239–1245. doi: 10.1097/00004583-200210000-00014. [DOI] [PubMed] [Google Scholar]
- Mills AW. On the minimum audible angle. The Journal of the Acoustical Society of America. 1958;30(4):237–246. [Google Scholar]
- Mondor TA, Zatorre RJ. Shifting and focusing auditory spatial attention. Journal of Experimental Psychology: Human Perception and Performance. 1995;21(2):387–409. doi: 10.1037//0096-1523.21.2.387. [DOI] [PubMed] [Google Scholar]
- Mottron L, Dawson M, Soulières I, Hubert B, Burack J. Enhanced perceptual functioning in autism: An update, and eight principles of autistic perception. Journal of Autism and Developmental Disorders. 2006;36(1):27–43. doi: 10.1007/s10803-005-0040-7. [DOI] [PubMed] [Google Scholar]
- Mundy P, Neal R. Neural plasticity, joint attention, and a transactional social-orienting model of autism. International Review of Research in Mental Retardation. 2001;23:139–168. [Google Scholar]
- O’Connor K. Auditory processing in autism spectrum disorder: a review. Neuroscience and Biobehavioral Reviews. 2012;36(2):836–854. doi: 10.1016/j.neubiorev.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Obleser J, Eulitz C, Lahiri A, Elbert T. Gender differences in functional hemispheric asymmetry during processing of vowels as reflected by the human brain magnetic response. Neuroscience Letters. 2001;314(3):131–134. doi: 10.1016/s0304-3940(01)02298-4. [DOI] [PubMed] [Google Scholar]
- Razavi B, O’Neill WE, Paige GD. Auditory spatial perception dynamically realigns with changing eye position. The Journal of Neuroscience. 2007;27(38):10249–10258. doi: 10.1523/JNEUROSCI.0938-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson F, Mottron L, Jemel B, Belin P, Ciocca V. Can spectro-temporal complexity explain the autistic pattern of performance on auditory tasks? Journal of Autism and Developmental Disorders. 2006;36(1):65–76. doi: 10.1007/s10803-005-0043-4. [DOI] [PubMed] [Google Scholar]
- Shannon RV, Zeng FG, Kamath V, Wygonski J, Ekelid M. Speech recognition with primarily temporal cues. Science. 1995;270(5234):303–304. doi: 10.1126/science.270.5234.303. [DOI] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47(8):921–929. doi: 10.1097/CHI.0b013e318179964f. [DOI] [PubMed] [Google Scholar]
- Teder-Sälejärvi WA, Hillyard SA, Röder B, Neville HJ. Spatial attention to central and peripheral auditory stimuli as indexed by event-related potentials. Cognitive Brain Research. 1999;8(3):213–227. doi: 10.1016/s0926-6410(99)00023-3. [DOI] [PubMed] [Google Scholar]
- Teder-Sälejärvi WA, Pierce KL, Courchesne E, Hillyard SA. Auditory spatial localization and attention deficits in autistic adults. Cognitive Brain Research. 2005;23(2):221–234. doi: 10.1016/j.cogbrainres.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Vara AS, Pang EW, Doyle-Thomas KA, Vidal J, Taylor MJ, Anagnostou E. Is inhibitory control a ‘no-go’in adolescents with autism spectrum disorder? Molecular autism. 2014;5(1):1. doi: 10.1186/2040-2392-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience. 2005;23(2–3):143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]