Abstract
Background
Although investigations on different pharmacological activities of the experimental plant, Ficus sur have been conducted, its folklore use for diuresis has not yet been validated. The current study, therefore, focused on the diuretic activity of aqueous and 80% methanol extracts of F. sur Forssk. leaves in rats.
Methods
Rats were randomly assigned into eight groups each consisting of six rats. Test groups received either 100 mg/kg, 200 mg/kg, or 400 mg/kg of aqueous or 80% methanol leaves extract. The negative control group and positive control were treated with 2 mL/100 g of distilled water and furosemide (10 mg/kg), respectively. Thereafter urine volume was recorded every hour until the end of the fifth hour, and cumulative urine volume of each rat was measured. Then, diuretic activity, diuretic index, saliuretic index, natriuretic index and carbonic anhydrase inhibition index in each group were calculated, and results were compared among the groups.
Results
The middle (200 mg/kg) and the highest (400 mg/kg) doses of both extracts significantly increased diuresis at the fifth hour (p<0.001) compared to the negative control, although the diuretic activity was less than that of the positive control. Regarding electrolyte excretion, all dose levels of both extracts showed significant natriuresis (p<0.001) and chloriuresis (p<0.01) compared to the negative control. Aqueous extract displayed more significant diuretic effect than 80% methanol extract. The aqueous and 80% methanol extracts produced alkaline urine.
Conclusion
The crude leaves extracts of F. sur increased urinary excretion and concentration of urinary electrolytes in a dose-dependent manner. These findings are in agreement with the traditional claim for use of F. sur as diuretic agent.
Keywords: Ficus sur, diuretic activities, urinary electrolyte, furosemide, Na+/K+ ratio
Background
Medicinal plants have been widely used as a source for the treatment of human disorders since the ancient times to this date.1 Between 70 and 95% of people in developing nations rely on herbal medicines for managing different disease ailments and their health benefits are growing rapidly in recent time.2 One of the application areas of botanicals is their diuretic effect. Therefore, herbal medicines are employed to tackle edematous condition such as such as cardiac failure, cirrhosis, and nephritic syndrome that lead to fluid overload in the body.3
With currently available conventional drugs, several adverse effects are associated. For example loop and thiazide diuretics cause electrolyte abnormalities (hypokalemia, hyperuricemia and hyponatremia), acid base balance, metabolic abnormalities and acute hypovolemia.4 Therefore, it is imperative to look a source for a diuretic with relatively free of such unfavorable and unwanted side effects.
Ficus sur Forssk is also known by other names as Ficus capensis Thunb, Ficus mallotocarpa Warb, and Ficus riparia (Miq. Fi.s sur L.).5,6 It belongs to the family of Moraceae and genus of ficus. The genus ficus consists of over 800 species.7 In Ethiopia, this plant is known by different vernacular names as Shola in Amharic, Harbuu in Afan Oromo and Odakko in Sidamenya.8
F. sur is used for treatment of different diseases in different countries. In Sudan and Nigeria, its leaves and roots are used for treatment of leukoderma, leprosy, wounds, edema, respiratory disorders, diarrhea, sexually transmitted diseases, tuberculosis, anemia, epilepsy, rickets, dysentery, male infertility and gonorrhea.9,10 Ethnobotanical studies also showed F. sur is used to treat swellings and edema,11 In South Africa and other countries it is traditionally used in kidney problems and as adiuretic.5,12
In Ethiopia, the pounded fresh leaves of F. sur, mixed with water are given orally as a traditional medication for urine retention thereby to alleviate the problem via increasing urine output. There is also a traditional claim for the root part of this plant to be used for urinary problems.8,13 In addition, experimentally evaluated plants of the same genus for their diuretic effect showed promise, which includes among others Ficus glumosa,14 Ficus exasperate and Ficus carica.15
Albeit ample ethnobotanical evidence for the use of F. sur as a diuretic its claim is not yet validated experimentally. The aim of the present study was, therefore, to evaluate the diuretic activities of aqueous and 80% methanol leaves extracts of F. sur.
Materials and Methods
Chemicals and Drugs
Chemicals and solvents used in this study include absolute methanol (Lova Chemie, India), Distilled water (Social Pharmacy and Pharmaceutics Laboratory, Addis Ababa University), Normal saline (Addis Pharmaceutical Factory, Ethiopia) and the standard drug furosemide (Epharm, Ethiopia).
Collection of Plant Material
The fresh leaves of F. sur were collected from Dugda district, Oromia Region, situated 134 km from Addis Ababa, Ethiopia on April 27, 2019. Ato Melaku Wondafrash (MSc) did taxonomic identification and a voucher specimen MA/001 was given and deposited at the National Herbarium, College of Natural and Computational Sciences, Addis Ababa University for future reference.
Experimental Animals
Healthy Wistar albino rats (200–245 g) of either sex reared in the animal house of the School of Pharmacy, Addis Ababa University were used for the experiment. The animals were housed in plastic cages (six to eight rats per cage). The animals had free access to rodent pellets and water ad libitum. Before initiation of the experiment, the animals were acclimatized to laboratory conditions for a period of five days. The care and handling of animals were in accordance with internationally accepted guidelines for use of the experimental animals,16,17 and the study was approved by the Ethics Committee of the School of Pharmacy, Addis Ababa University.
Extraction of the Plant
The leaves were cleaned from the dust and debris and washed gently with water. To decrease the size, they were cut into smaller pieces using scissors. The cut pieces of the leaves were then pounded using mortar and pestle and extracted.
Aqueous Extraction
Five hundred grams of the pounded fresh leaves of F. sur were cold macerated in 1000 mL of distilled water and allowed to stand at room temperature for 72 h using shaker. The macerate was first filtered using cotton gauze and later through Whatman filter paper No. 1. Subsequently, the filtrate was then freeze-dried with a lyophilizer (OPR-FDU-5012, Korea) and finally 72.5 g of semi-solid pasty mass of dark brown color with a yield of 14.5 (w/w) was obtained.
Methanol Extraction
Five hundred grams of the pounded fresh leaves of F. sur were macerated with 1000 mL of 80% methanol and allowed to stand at room temperature for 72 h using shaker. Seventy-two hours later, the macerate was filtered using Whatman filter paper No. 1. The methanol was then evaporated from the extract under reduced pressure using Rota vapour (BUCHI Rota vapour R-200, Switzerland) at 40°C. The extract obtained was filtered and frozen at −20°C and lyophilized until dried. The yield of the dry extract was found to be 10.5% (w/w).
Acute Toxicity Study
For acute toxicity test, healthy nonpregnant Wistar albino rats (age of 8–12 weeks) weighing 200–245 g was used. The test was done according to the OECD 425 Guideline with a limit dose of 2000 mg/kg. Ten rats, five for each extract were used. On day one, a rat (from each group) was given an oral dose of 2000 mg/kg. Then rats were closely observed for behavioral or physical changes during the first 30 min (with special attention during the first four hours), thereafter for 24 h. After 24 h, another four rats (from each group) were given the same dose and observed for general sign of toxicity.
Grouping and Dosing of Animals
Rats were randomly assigned into eight groups each consisting of six rats for diuretic test. Group I was negative control treated with 2 mL/100 g of distilled water used for reconstitutions. Group II was positive control treated with 10 mg/kg furosemide (FURO10). Groups III–V were treated with doses of 100 mg/kg, 200 mg/kg and 400 mg/kg of F. sur aqueous extract, respectively. Whereas, Groups VI–VII were treated with doses of 100 mg/kg, 200 mg/kg, and 400 mg/kg of F. sur 80% methanol extract, respectively. Dose selections was made based on the acute oral toxicity test performed, taking one tenth of the limit dose as the middle dose. Route of administration used for all groups was oral using gavage.
Determination of Diuretic Activity
Diuretic activity was determined following the methods used by the previous study, with slight modification.18
Each rat was placed in an individual metabolic cage (metabolic cage for rats, UGO BASILE, Italy) 24 h prior to initiation of the experiment for acclimatization and then fasted for 18 h with free access to water ad libitum.
Before treatment, all animals were given oral saline with a volume of 15 mL/kg body weight.19 Each group then received standard drug, water and various doses of extract. Immediately after dosing, the rats were placed individually in a metabolic cage. The urine was then collected, measured and the pH determined at one, two, three, four, and five hours. Finally, the urine was stored at −20°C for electrolyte analysis.
The following parameters were calculated in order to compare the effects of extracts with those of the vehicle and standard. The urinary excretion, independent of the animal weight, was computed as total urinary output divided by total liquid administered (formula −1). The ratio of urinary excretion in test group to urinary excretion in the control one was used as a measure of diuretic action of a given dose of an agent (formula −2). A parameter known as diuretic activity was also calculated as the ratio of diuretic action of the extracts in the test group to that of the standard drug (formula – 3)20 as given below:
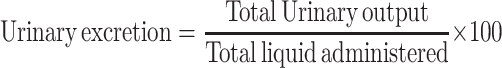 |
Formula 1
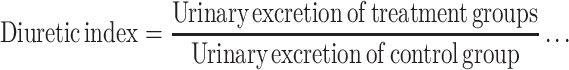 |
Formula 2
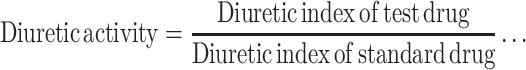 |
Formula 3
Analytical Procedure
Urinary Na+, K+, and Cl− concentrations of the plant extracts, control, and standard groups were analyzed using Ion Selective Electrode (ISE) analyzer (AVL 9181 Electrolyte Analyzer, Roche, Germany). The instrument was automatically calibrated prior to analysis with different standard solutions. The ratios of electrolytes, Na+/K+ and Cl−/K++Na+, were calculated to evaluate the saluretic activity of the different extracts. In addition, urine pH was directly measured on fresh urine using a pH meter. Furthermore, the salt content of the extract was determined to rule out its contribution on urinary electrolyte concentration.
Statistical Analysis
Analyses were performed using international business machine of statistical package for the social Sciences, version 25 for windows (IBM Corporation, Armonk, NY, USA). Experimental results were expressed as mean ± SEM (standard error of the mean) and statistical significance test was carried out by one-way ANOVA followed by the Tukey's post hoc test to compare results among groups. Values of p<0.05 were considered statistically significant.
Results
Acute Toxicity Test
From the acute toxicity test, no visible sign of toxicity was observed indicating that the median lethal oral dose of F. sur in rats is greater than 2000 mg/kg body weight.
Diuretic Activity
Effects of the Extracts at Each Time Point
The aqueous extract produced significant diuresis with FSAE400 (p<0.05) at the third hour. Standard drug (FURO10) also produced apparent urine output at the same time point (Table 1).
Table 1.
Effect of Aqueous and 80% Methanol Extracts of the Leaves of F. sur at Each Time Point of Five-hour Urine Volume in Rats
| Group | Volume of Urine (mL/100 g) | Diuretic Action | Diuretic Activity | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 h | 2 h | 3 h | 4 h | 5 h | |||||
| DW/NC | 1.83±0.32 | 2.53±0.42 | 0.43±0.21 | 0.62±0.31 | 0.52±0.23 | 1.00 | |||
| FURO10 | 2.75±0.36 | 1.87±0.40 | 3.15±0.74a** | 1.67±0.44 | 0.67±0.33 | 1.52 | 1.00 | ||
| FSAE100 | 1.0±0.51 | 1.85±0.38 | 2.33±0.24 | 1.88±0.38 | 1.27±0.32 | 1.26 | 0.83 | ||
| FSAE200 | 1.63±0.39 | 2.0±0.25 | 2.33±0.34 | 1.92±0.15 | 1.38±0.17 | 1.39 | 0.91 | ||
| FSAE400 | 1.77±0.18 | 2.38±0.14 | 2.43±0.24a* | 2.07±0.23 | 1.57±0.35 | 1.47 | 0.97 | ||
| FSME100 | 2.4±0.77 | 2.27±0.59 | 1.97±0.67 | 0.92±0.41 | 0.47±0.21 | 1.25 | 0.82 | ||
| FSME200 | 2.55±0.31 | 2.38±0.31 | 1.93±0.29 | 0.92±0.43 | 0.42±0.15 | 1.27 | 0.83 | ||
| FSME400 | 2.47±0.19 | 2.42±0.47 | 1.95±0.35 | 1±0.34 | 0.77±0.32 | 1.41 | 0.92 | ||
Notes: Each value represents mean ± S.E.M; n=6; Analysis was performed by one way ANOVA; acompared to negative control; *p<0.05; **p<0.01; number followed by FURO, FSAE and FSME indicates dose/kg.
Abbreviations: DW, distilled water; NC, negative control; FURO, furosemide; FSAE, F. sur aqueous extract; FSME, F. sur methanol extract.
Effect of the Extracts on Cumulative Urine Volume
The aqueous extract of F. sur produced dose-dependent diuresis, albeit insignificant across the first three time points (Table 2). It showed significant urine output starting from the fourth hour with the middle dose (45.3%, p<0.05) and higher dose (59.6%, p<0.01). Besides, all three doses continued to be significant until the fifth hour, FSAE200 (56.3% p<0.001) and FSAE400 (72.3%, p<0.001). Unlike the middle and higher doses of extract, FASE100 produced apparent urine output at the last time point only (40.4%, p<0.01). Inter and intragroup analysis also revealed that, FSAE400 showed a significant difference in urine output at the fifth hour compared to FSAE100 (22.6%, p<0.05), FSME100 (27.4%, p<0.01) and FSME200 (24.6%, p<0.05). Likewise, FURO10 treated rats exhibited a significant increment in urine volume setting in the third hour (61.8%, p<0.01) and onward. In addition, it displayed a significantly greater effect against FSAE100, FSME100 and FSME200 (p<0.05) at the fifth hour.
Table 2.
Effect of Aqueous and 80% Methanol Extracts of the Leaves of F. sur on Five-hour Cumulative Urine Volume in Rats
| Group | Volume of Urine (mL/100gm) | Diuretic Index | Diuretic Activity | ||||
|---|---|---|---|---|---|---|---|
| 1 h | 2 h | 3 h | 4 h | 5 h | |||
| DW/NC | 1.83±0.32 | 4.37±0.22 | 4.80±0.30 | 5.42±0.35 | 5.93±0.30 | 1 | 0.65 |
| FURO10 | 2.75±0.36 | 4.62±0.54 | 7.77±0.97a**,b* | 9.43±0.80a***,b* | 10.10±0.60a***,b*,c*,d* | 1.52 | 1 |
| FSAE100 | 1.00±0.52 | 2.85±0.59 | 5.18±0.50 | 7.07±0.68 | 8.33±0.50a** | 1.26 | 0.83 |
| FSAE200 | 1.63±0.40 | 3.63±0.53 | 5.97±0.36 | 7.88±0.30a* | 9.27±0.37a*** | 1.39 | 0.91 |
| FSAE400 | 1.77±0.18 | 4.15±0.24 | 6.58±0.24 | 8.65±0.37a** | 10.22±0.17a***,b*,c**,d* | 1.47 | 0.97 |
| FSME100 | 2.40±0.77 | 4.67±0.37 | 6.63±0.41 | 7.55±0.23 | 8.02±0.15a* | 1.25 | 0.82 |
| FSME200 | 2.55±0.32 | 4.93±0.51B* | 6.87±0.46 | 7.78±0.33a* | 8.20±0.38a** | 1.27 | 0.83 |
| FSME400 | 2.47±0.19 | 4.88±0.53 | 6.83±0.47 | 7.83±0.57a* | 8.60±0.42a*** | 1.41 | 0.92 |
Notes: Each value represents mean ±SEM (n=6). Analysis was performed by one-way ANOVA followed by Tukey's post hoc multiple comparison test; aagainst control, bagainst FSAE100, cagainst FSME100, dagainst FSME200; *p<0.05; **p<0.01; ***p<0.001; number followed by FURO, FSAE and FSME indicates dose/kg.
Abbreviations: DW, distilled water; NC, negative control; FURO, furosemide; FSAE, F. sur aqueous extract; FSME, F. sur methanol extract.
Regarding the methanolic extract, significant difference in urine output was noted with FSME200 (43.5%) and FSME400 (44.5%) at the fourth hour and (p<0.05) at the fifth hour 38.2% and 45% (p<0.01 and p<0.001), respectively. On the contrary, FSME100 produced insignificant urine output in all but at the fifth hour 35.24% (p<0.05). Intergroup analysis revealed that FSME200 (72.9%, p<0.05) produced significant difference compared to FSAE100.
Saluretic Activity
The cumulative urine samples collected over five hours were analyzed for the electrolytes content (Na+, K+, and Cl–) (Table 3). All the three doses of aqueous extract tended to increase Na+ excretion by at least 201% (p<0.001) compared to NC group. Likewise, the methanolic extract produced significant Na+ excretion with all doses by 159.8%, (p<0.01), 294.6% and 339.2% (p<0.001) from low to high dose, respectively. The standard drug, however, increased Na+ level significantly (542.5%, p<0.001) compared to NC.
Table 3.
Effect of Aqueous and 80% Methanol Extracts of the Leaves of F. sur on Five-hour Urinary Electrolyte Excretion in Rats
| Group | Urinary Electrolyte Concentration (mmol/L) | Saluretic Index | Na+/K+ | Cl–/ Na++K+ |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Na+ | K+ | Cl– | Na+ | K+ | Cl– | ||||
| DW/NC | 15.33±2.38 | 27.68±5.80 | 20.73±3.43 | 0.55 | 0.48 | ||||
| FURO10 | 98.5±2.03a***,b***,e*** | 80.27±10.51a**,d*,e* | 97.92±3.27a***,b***,e*** | 6.42 | 2.90 | 4.72 | 1.22 | 0.54 | |
| FSAE100 | 46.17±5.12a*** | 39.33±8.79 | 52.63±7.83a** | 3.01 | 1.42 | 2.54 | 1.17 | 0.61 | |
| FSAE200 | 55.67±4.63a*** | 58.89±4.48 | 46.77±3.91a* | 3.63 | 2.13 | 2.26 | 0.95 | 0.41 | |
| FSAE400 | 82.33±5.61a***,b***,e*** | 44.32±4.96 | 74.93±3.88a***,c*,e*** | 5.37 | 1.60 | 3.61 | 1.86 | 0.59 | |
| FSME100 | 39.83±1.74a** | 39.14±2.97 | 33.52±2.71 | 2.60 | 1.41 | 1.62 | 1.02 | 0.42 | |
| FSME200 | 60.5±2.74a***,e** | 51.59±6.70 | 51.90±6.36a** | 3.95 | 1.86 | 2.50 | 1.17 | 0.46 | |
| FSME400 | 67.33±3.56a***,b**,e*** | 62.58±13.33 | 55.38±9.36a** | 4.39 | 2.26 | 2.67 | 1.08 | 0.43 | |
Notes: Each value represents mean ±SEM (n=6). Analysis was performed by one way ANOVA followed by Tukey's post hoc multiple comparison test; aagainst control, bagainst FSAE100, cagainst FSAE200, dagainst FSAE400, eagainst FSME100; *p<0.05; **p<0.01; ***p<0.001; number followed by FURO, FSAE and FSME indicates dose/kg.
Abbreviations: DW, distilled water; NC, negative control; FURO, furosemide; FSAE, F. sur aqueous extract; FSME, F. sur methanol extract.
Inter- and intragroup analysis showed that, compared to FSAE100 and FSME100, FSAE400 significantly increased Na+ excretion by 78.30% and 106.7%, while FURO10 increased Na+ loss by 113.3% and 147.3% (p<0.001) compared to FSAE100 and FSME100, respectively.
FURO10 significantly increased K+ loss by 189.9% (p<0.01), 81.1% and 105 (p<0.05) compared with the NC, FSAE400 and FSME100, respectively. In the case of Cl− excretion, all doses of extract except FSME100 produced significant loss compared to NC. The Cl− loss produced by FURO10 was significantly higher compared to NC and the lowest doses of both extracts.
Electrolyte Content of the Extract
Water soluble salts that could present in the extract may interfere with the urinary excretion of electrolytes. The result revealed that there were very low amounts, ie, 7.69, 8.3, and 10.4 mmol/L of Na+, K+ and Cl− respectively, with the higher (400 mg/kg) dose of aqueous extract whereas the concentrations of these electrolytes in 200 mg/kg and 100 mg/kg were 4.7, 6.9, and 5.4mmol/L and 3.4, 6, and 7 mmol/L, respectively. The quantitative determination of Na+, K+, Cl− in these same doses of 80% methanol extract showed nondetectable levels.
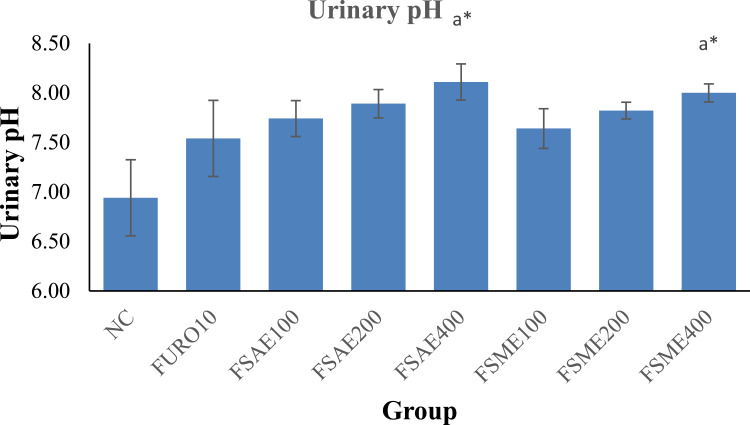
Urinary pH
Urinary pH measurement revealed that the different treatment groups of both extracts produced relatively alkaline urine. The NC group produced the lowest pH and the standard group an intermediate pH (7.50) between vehicle and extract-treated groups.
As for the trend, the urine of rats treated with aqueous extract showed an increase in pH from 7.70 (FSAE100) to 8.10 (FSAE400). In contrast, treatment with 80% methanol extract increased pH from 7.60 (FSME100) to 8.00 (FSME400). Group comparison revealed that the pH of urine from rats treated with the higher doses of both extracts (FSAE400 and FSME400) displayed a significant (p<0.05) increase compared to negative control (Figure 1).
Figure 1.
Urinary pH of rats treated with aqueous and 80% methanol extracts of the leaves of F. sur.
Notes: Each value represents mean ±SEM (n=6). Error bars indicate SEM. Analysis was performed by one-way ANOVA followed by Tukey's post hoc multiple comparison test; aagainst control, *p<0.05.
Abbreviations: DW, distilled water; NC, negative control; FURO, furosemide; FSAE, F. sur aqueous extract; FSME, F. sur methanol extract.
Discussion
Diuresis could be beneficial for treatment of a number of disease conditions such as congestive heart failure, nephritic syndrome, hypertension, liver cirrhosis, poisoning, and certain kidney diseases. It is also important in kidney stone treatment. An increased fluid volume flowing through the kidney could dissolve the stones, and flush out the deposits.21 In addition to conventional drugs, numerous herbal preparations are used as diuretics. There are also scientific studies that have been carried out to support the diuretic effects of many traditional medicinal herbal products.22
The leaves of F. sur has a range of promising medicinal claims among which its diuretic potential is yet to be supported by scientific evidence.10 Therefore, the aim of this study was to evaluate the diuretic activity of both aqueous and 80% methanol extract in saline-loaded rats.
The present study revealed that F. sur has notable diuretic effects in the given animal model. As diuretics are employed clinically in treatment of edema, it would be most important to demonstrate effectiveness in the presence of electrolyte and water.23 Accordingly, saline was given to impose water and salt load or simulate edema.
Regarding acute toxicity, leaf extract of F. sur does not produce a toxic response in rats at a dose of 2000 mg/kg in both extracts.
F. sur was studied for presence of a variety of secondary metabolites. In a previous study, the quantitative phytochemical analysis carried out on aqueous leaf extract of F. sur showed presence of flavonoids (1367.42 mg/100 g), terpenoids (1280.39 mg/100g), tannins (687.64 mg/100g) and alkaloids (422.12 mg/100g) in larger amounts; whilst saponins, steroids, and glycosides are in trace amounts.24 However, methanol extract of this plant contains tannins, alkaloids, flavonoids, phenols, cardenolides, steroids, and anthraquinones.25,26 Quantification of secondary metabolites showed methanol extracts of F. sur contains phenol (462.65 mg gallic acid equivalent/g extract) and flavonoids (46.0 mg quercetin equivalent/g extract).27
Several studies have explained that flavonoids, saponins, terpenoids, tannins, alkaloids, and organic acids were responsible for the diuretic activity of a plant extract.21,28 For example, flavonoids were known to be A1R antagonists.29 Thus, the diuretic effect of this plant extract may be due to stimulation of regional blood flow, or by inhibiting tubular reabsorption of water and anions. Moreover a number of compounds including a flavone, 4,5,7-trihydroxyflavone-3-ol, were identified from leaf extract of this plant and, flavones are known by their effect of diuresis.30,31 Although, it is almost impossible to pin down to the particular phytoconstituents that cause the plant’s observed diuretic activity with this study, it can be suggested that compounds can act individually or synergistically.
Loop diuretics like furosemide increase urinary flow rate and urinary excretion of sodium, potassium and chloride by inhibiting Na+–K+–2Cl− symporter and carbonic anhydrase enzyme.32 The result obtained from this study showed a marked excretory effect both on water and electrolytes, typical of saluretic diuretic types.33 The larger doses of both extracts of F. sur produced similar diuresis and saluretic (Na+ and Cl–) excretion profile to that of furosemide. The diuretic activity is considered to be good if the diuretic index is greater than 1.50, moderate if the values is between 1.00 and 1.50, mild if the values lies between 0.72 and 1.00 and nil if the value is <0.72.34 Both extracts accordingly, at all doses, have shown a moderate diuretic activity as evidenced by the diuretic index values. The aqueous and 80% methanol extracts at 400 mg/kg displayed a diuretic activity of 97% and 92%, respectively, compared to the reference drug, furosemide (10 mg/kg).
Both extracts of F. sur revealed an increase in urine volume that appeared to vary with dose and nature of the extract. The aqueous extract produced a better diuretic effect compared to the 80% methanol extract, particularly with increasing dose, FSAE400 vs FSME100 (27.4%, p<0.01) and FSME200 (24.6%, p<0.05). Therefore, it is plausible to suggest that the secondary metabolites responsible for increasing urine output could be more polar. In addition to the cumulative diuretic effect of the plant, the highest dose of aqueous extract produced significant diuresis at the third hour, showing the dose-dependent effect of the plant. This may also suggest that the maximal effect of the aqueous extract may reach at this time point.
Quantitative determinations of potassium ion in both extracts of F. sur showed very low concentration suggesting, the diuretic effect of the plant extract was not due to its content of potassium salt, rather due to intrinsic ability of the plant. In addition, potassium, the effect of extracts on sodium was evaluated. Sodium retention and increased amount of water are key players in hypertension pathogenesis, as well as in edematous conditions such as heart failure and cirrhosis.35 The effect of the extracts on urinary electrolyte excretion was clearly observed by the water and sodium excretion which reinforces the idea that the diuretic effect of F. sur was of the saluretic type.36 In addition, this saluretic diuretics activity of F. sur was similar to the result observed on Ficus glumosa.14 Based on the finding, the diuretic action of the plant might be through inhibition Na+–K+–2Cl– cotransporter 2. Nevertheless, future studies should explore the mechanism of action at molecular level. It was noted that both extracts exhibited a weak excretion of potassium less than the standard suggesting that it has potassium-sparing diuretic activity as well.
The Na+/K+ ratio is a biomarker of mineralocorticoid receptor antagonism.37 The greater Na+/K+ indicates as the substance has greater proportion of Na+ excretion capacity than K+ and signaled good diuretic activities.38 Therefore, comparatively FSAE400 has an interesting natriuretic activity (ie 1.86), preventing hypokalemia, a common problem associated with most diuretic agents.
The Cl−/(Na++K+), however, indicates carbonic anhydrase inhibition activity, and substances having a value between 0.8 and 1.0 could be excluded.20 The present study, therefore, indicates that the extracts might work through independent of carbonyl anhydrase inhibition.
The extracts exhibited a relative increase in urine pH, suggesting that carbonic anhydrase inhibition to be one of the possible mechanisms of action. This alkalinization of urine also gives a clue that the plant has potassium-sparing diuretic activity.14
Conclusion
The present study revealed both aqueous and methanol extracts of F. sur possessed diuretic and natriuretic activities. The present finding, therefore, supports the traditional claim of the plant as a diuretic agent. The urinary PH and electrolytes analysis results hint that the extracts to have multiple modes of action. The safety profile of the extract was an added advantage that calls for conducting further research to ascertain the findings reported in this study.
Acknowledgments
The authors would like to thank Addis Ababa University for the financial support of this study.
Funding Statement
Addis Ababa University funded this study as part of funding an MSc work.
Data Sharing Statement
The datasets used for the current study are available from the corresponding author on reasonable request .
Ethics Approval
The protocol was approved by Institutional Review Board of the School of Pharmacy, College of Health Sciences, Addis Ababa University with reference no. ERB/SOP/213/03/2019.
Consent for Publication
Not applicable.
Author Contributions
All authors made significant contributions to data conception and design, data collection or analysis and interpretation; took part in the drafting or critical review of the article for significant intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be responsible for all aspects of the work.
Disclosure
The authors report no financial or nonfinancial conflicts of interest in this work.
References
- 1.Yadav RH. Medicinal plants in folk medicine system of Ethiopia. J Poisonous Med Plant Res. 2013;1(1):7–11. [Google Scholar]
- 2.Organization WH. WHO Traditional Medicine Strategy 2002–2005. Geneva: World Health Organization; 2002:2015. [Google Scholar]
- 3.Vazir A, Cowie MR. The use of diuretics in acute heart failure: evidence based therapy? World J Cardiovascular Diseases. 2013;03:25–34. doi: 10.4236/wjcd.2013.32A004 [DOI] [Google Scholar]
- 4.Dhondup T, Qian Q. Acid-base and electrolyte disorders in patients with and without chronic kidney disease: an update. Kidney Diseases. 2017;3(4):136–148. doi: 10.1159/000479968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lansky EP, Paavilainen HM. Figs: The Genus Ficus. CRC Press; 2010. [Google Scholar]
- 6.Ojukwu U. Phytochemical and antimicrobial screening and nutritional qualities of ficus sur (forssk). Int J Engineering Technologies Management Res. 2018;5(10):35–44. doi: 10.29121/ijetmr.v5.i10.2018.300 [DOI] [Google Scholar]
- 7.Sirisha N, Sreenivasulu M, Sangeeta K, Chetty CM. Antioxidant properties of Ficus species-a review. Int j Pharmtech Res. 2010;2(4):2174–2182. [Google Scholar]
- 8.Beyi M. Ethnobotanical investigation of traditional medicinal plants in dugda district, Oromia Regio. SM J Med Plant Stud. 2018;2(1):1007. [Google Scholar]
- 9.Clark AM. Natural products as a resource for new drugs. Pharm Res. 1996;13(8):1133–1141. doi: 10.1023/A:1016091631721 [DOI] [PubMed] [Google Scholar]
- 10.Muhammad M. A pharmacognostical efficacy of five plants used traditionally for the treatment of cancer in Northern Nigeria. A Master Thesis in the Department of Pharmacognosy, Institute of Health Science. Bayero University, Kano: 2017. [Google Scholar]
- 11.Esievo KB, Anthony SO, Fatokun OT, Kunle OF. Ficus capensis Thumb.(Moraceae): review of its ethnomedicinal uses, pharmacological activities and phytochemical constituents. Archives Current Res Int. 2018;1–7. [Google Scholar]
- 12.Sabiu S, O’Neill FH, Ashafa AOT. The purview of phytotherapy in the management of kidney disorders: a systematic review on Nigeria and South Africa. African Journal of Traditional, Complementary and Alternative Medicines. 2016;13(5):38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Regassa R. Diversity and conservation status of some economically valued indigenous medicinal plants in hawassa college of teacher education campus, Southern Ethiopia. Int J Adv Res. 2013;1(3):308–328. [Google Scholar]
- 14.Ntchapda F, Abakar D, Kom B, et al. Diuretic activity of the aqueous extract leaves of ficus glumosa Del. (Moraceae) in Rats. Scientific World J. 2014;2014:1–10. doi: 10.1155/2014/693803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sruthi B, Sunny G, Hajra N, Sakthivel S. Diuretic activity of ethanolic extracts of Ficus carica L. fruits. Int J Res Pharmacol Pharmacother. 2012;1:25–28. [Google Scholar]
- 16.Herling AW, Maas J, Seeger K. Guidelines for the care and use of laboratory animals. Drug Discovery Evaluation. 1997;p. 726–40. [Google Scholar]
- 17.Vogel HG, Vogel WH. Drug Discovery and Evaluation: Pharmacological Assays. Springer Science & Business Media; 2013. [Google Scholar]
- 18.Lahlou S, Tahraoui A, Israili Z, Lyoussi B. Diuretic activity of the aqueous extracts of Carum carvi and Tanacetum vulgare in normal rats. J Ethnopharmacol. 2007;110(3):458–463. doi: 10.1016/j.jep.2006.10.005 [DOI] [PubMed] [Google Scholar]
- 19.Sayana SB, Christina T, Patil P. Study of diuretic activity of ethonolic extract of leaves of Cissampelos pareira in rats. Asian J Pharmaceutical Clinical Res. 2014;7:157–159. [Google Scholar]
- 20.Kebamo S, Makonnen E, Debella A, Geleta B. Evaluation of diuretic activity of different solvent fractions of methanol extract of carissa edulis root bark in rats. J Med Chem. 2015;5:472–478. [Google Scholar]
- 21.Yuliana ND, Khatib A, Link-Struensee AMR, et al. Adenosine A1 receptor binding activity of methoxy flavonoids from Orthosiphon stamineus. J Med Plant Natural Product Res. 2009;75(02):132–136. [DOI] [PubMed] [Google Scholar]
- 22.Dutta KN, Chetia P, Lahkar S, Das S. Herbal plants used as diuretics: a comprehensive review. J Pharm Chem Biol Sci. 2014;2(1):27–32. [Google Scholar]
- 23.Nedi T, Mekonnen N, Urga K. Diuretic effect of the crude extracts of Carissa edulis in rats. J Ethnopharmacol. 2004;95(1):57–61. doi: 10.1016/j.jep.2004.06.017 [DOI] [PubMed] [Google Scholar]
- 24.Achi NK, Onyeabo C, Ekeleme-Egedigwe CA, Onyeanula JC. Phytochemical, proximate analysis, vitamin and mineral composition of aqueous extract of Ficus capensis leaves in South Eastern Nigeria. J Appl Pharmaceutical Sci. 2017;7(3):117–122. [Google Scholar]
- 25.Odusanmi J. Phytoconstituents, proximate and mineral investigations of the ethanol extracts of the bark and leaves of ficus sur forssk. J Scientific Res Development. 2017;17(1):9–14. [Google Scholar]
- 26.Solomon-Wisdom G, Shittu G, Agboola Y. Antimicrobial and phytochemical screening activities of Ficus sur (Forssk). New York Science J. 2011;4(1):15ñ8. [Google Scholar]
- 27.Omoregie ES, Okugbo OT. In vitro antioxidant activity and phytochemical screening of methanol extracts of Ficus capensis and Dacryodes edulis leaves. J Pharmacy Bioresources. 2014;11(2):66–75. doi: 10.4314/jpb.v11i2.6 [DOI] [Google Scholar]
- 28.Saxena M, Saxena J, Nema R, Singh D, Gupta A. Phytochemistry of medicinal plants. J Pharmacognosy Phytochemistry. 2013;1:6. [Google Scholar]
- 29.Moro S, van Rhee AM, Sanders LH, Jacobson KA. Flavonoid derivatives as adenosine receptor antagonists: a comparison of the hypothetical receptor binding site based on a comparative molecular field analysis model. J Med Chem. 1998;41(1):46–52. doi: 10.1021/jm970446z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mena P, Ludwig IA, Tomatis VB, Acharjee A, Calani L, Rosi A, et al. Inter-individual variability in the production of flavan-3-ol colonic metabolites. European Journal of neutrition 2019;58:1529–1543 [DOI] [PubMed] [Google Scholar]
- 31.Saloufou KI, Boyode P, Simalou O, et al. Chemical composition and antioxidant activities of different parts of Ficus sur. J Herbmed Pharmacology. 2018;7(3):185–192. doi: 10.15171/jhp.2018.30 [DOI] [Google Scholar]
- 32.Sarafidis PA, Georgianos PI, Lasaridis AN. Diuretics in clinical practice. Part I: mechanisms of action, pharmacological effects and clinical indications of diuretic compounds. Expert Opin Drug Saf. 2010;9(2):243–257. doi: 10.1517/14740330903499240 [DOI] [PubMed] [Google Scholar]
- 33.Al-Saikhan FI, Ansari MN. Evaluation of the diuretic and urinary electrolyte effects of methanolic extract of Peganum harmala L. in Wistar albino rats. Saudi j Biol Sci. 2016;23(6):749–753. doi: 10.1016/j.sjbs.2016.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asif M, Jabeen Q, Atif M, Majid AMSA, Qamar-Uz-Zaman M. Diuretic activity of achyranthes aspera linn crude aqueous extract in albino rats. Tropical J Pharmaceutical Res. 2014;13(12):2039–2045. doi: 10.4314/tjpr.v13i12.14 [DOI] [Google Scholar]
- 35.Schrier RW. Water and sodium retention in edematous disorders: role of vasopressin and aldosterone. Am J Med. 2006;119(7):S47–S53. doi: 10.1016/j.amjmed.2006.05.007 [DOI] [PubMed] [Google Scholar]
- 36.Martín-Herrera D, Abdala S, Benjumea D, Pérez-Paz P. Diuretic activity of withania aristata: an endemic canary island species. J Ethnopharmacol. 2007;113(3):487–491. doi: 10.1016/j.jep.2007.07.005 [DOI] [PubMed] [Google Scholar]
- 37.Eudy RJ, Sahasrabudhe V, Sweeney K, et al. The use of plasma aldosterone and urinary sodium to potassium ratio as translatable quantitative biomarkers of mineralocorticoid receptor antagonism. J Transl Med. 2011;9(1):180. doi: 10.1186/1479-5876-9-180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar B, Swamy B, Behara GM, Baidya M, Bilal S, Swamy A. Diuretic activity of the root of Homonoia retusa (GRAH. EX WT.) MUELL. Pharmacologyonline. 2010;3:276–284. [Google Scholar]