Abstract
Objective
TMED2 is a member of the transmembrane emp24 domain (Tmed)/p24 protein family, which is significantly upregulated in breast cancer, ovarian cancer and other tumour tissues. The purpose of this study was to investigate the expression of TMED2 in MM cell lines and its effect on the biological behaviour of MM cell lines.
Methods
Real-time quantitative PCR (RT-qPCR) was used to detect the expression of TMED2 in MM cell lines, including MM.1S and RPMI 8226 cells, and lentivirus vector-mediated TMED2 gene silencing was used to further study the effect of the downregulation of TMED2 expression on cell viability, the cell cycle, and apoptosis.
Results
Based on the RT-qPCR results, the expression of the TMED2 mRNA was increased in the MM cell lines MM.1S and RPMI 8226 compared with endogenous control GAPDH. The expression of the TMED2 mRNA was substantially reduced after transfection of the shRNA targeting TMED2 (shTMED2) in both MM cell lines. The CCK-8 assay showed significant decreases in the viability of MM.1S and RPMI 8226 cells, suggesting that the TMED2 gene plays an important role in the proliferation of these two cell lines. The cell cycle of MM.1S and RPMI 8226 cells was substantially altered by shTMED2, as evidenced by the increased number of cells in G1 phase and decreased number of cells in S and G2/M phases. The FACS analysis revealed a significant increase in the apoptosis of MM.1S and RPMI 8226 cells due to the increased activity of Caspase 3/7, suggesting that the TMED2 gene is significantly related to the apoptosis of these two cell lines.
Conclusion
Based on these results, TMED2 may play an important role in the pathogenesis of MM. This novel study may contribute to further investigations of useful biomarkers and potential therapeutic targets in patients with MM.
Keywords: multiple myeloma, TMED2, FACS, shRNA
Introduction
Multiple myeloma (MM) is a malignant proliferative disease of bone marrow plasma cells with a highly heterogeneous pathogenesis, clinical manifestations, efficacy, and prognosis.1–3 MM accounts for approximately one-tenth of all haematological malignancies and is mainly diagnosed in older male patients.4,5 Despite the development of novel agents such as lenalidomide,6 thalidomide7 and bortezomib,8 the five-year overall survival rate is still approximately 50%.9–11 Thus, interest in the development of new and effective therapies, biomarkers and targets for the prediction and treatment of MM has increased.4,12
TMED2 is a member of the transmembrane emp24 domain (Tmed)/p24 protein family13 that is involved in embryonic and placental development. TMED2 expression is significantly upregulated in breast cancer,14 ovarian cancer15 and other tumour tissues.13 The expression of TMED2 in myeloma cells has not been reported in the literature. The purpose of this study was to detect the expression of TMED2 in MM cell lines and its effect on the biological behaviour of MM cell lines.
In this study, the expression of TMED2 in MM cell lines, including MM.1S and RPMI 8226 cells, was investigated using real-time quantitative PCR (RT-qPCR). The functions of TMED2 in cell proliferation, cell cycle, apoptosis and cell viability were further investigated using a lentivirus vector-mediated TMED2 gene silencing technique.
Through this study, we hope to determine whether TMED2 potentially represents a new diagnostic marker for the treatment of multiple myeloma, which will lay a foundation for further development of TMED2-based reagents for clinical applications in patients with multiple myeloma.
Materials and Methods
Cell Lines
The human myeloma cell lines RPMI 8226 and MM.1S were obtained from ATCC (Rockville, MD). The cells were incubated in RPMI 1640 medium supplemented with 10% (v/v) foetal bovine serum (FBS) (Ausbian), sodium pyruvate and 2.5 mg/mL glucose at 37°C in an incubator with 5% CO2.
Quantitative Real-Time PCR
MM.1S and RPMI 8226 cells grown in 6-well plates were collected and centrifuged at 2000 rpm for 5 min. The cell pellet was mixed with TRIzol reagent to obtain the total RNA according to the manufacturer’s instructions (Shanghai Pufei, Co. Ltd., Shanghai, China). The RNA was reverse transcribed to cDNAs using the M-MLV kit from Promega (Madison, WI) according to the manufacturer’s instructions. The qPCR assays were performed using the MX3000p Real-Time PCR system (Agilent, Santa Clara, CA) in a 20 μL reaction system containing 10 μL of SYBR premix ex Taq (Takara, Kusatsu, Shiga, Japan), 0.5 μL of each forward and reverse primer, 1.0 μL of the cDNA templates and 8 μL of RNase-free H2O. RT-PCR was conducted at 95°C for 30 s, followed by 45 cycles of 95°C for 5 s and 60°C for 30 s, and a final step at 95°C for 15 s, 55°C for 30 s, and 95°C for 15 s. The forward and reverse primers for GAPDH were 5ʹ-TGACTTCAACAGCGACACCCA and 5ʹ-CACCCTGTTGCTGTAGCCAAA, respectively. The forward and reverse primers for TMED2 were GGTGACGCTTGCTGAACTGC and 5ʹ-AGATGAGGCCCATCTTGGTG, respectively. The mRNA levels of specific genes were normalized to the level of the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA using the ΔCt method described in a previous study.16 The ΔCt value is the difference between the Ct values of TMED2 and GAPDH.
Construction of the Lentivirus Vector Containing the shRNA Targeting TMED2
The shRNA targeting TMED2 was designed by Shanghai Genechem Co., Ltd. (Shanghai, China) using the TMED2 gene sequence from GenBank (Access number: NM_006815) as the template. After the evaluation and determination, several 19–21 nt sequences were designed, among which, the 5ʹ-ATGGATGGAACATACAAAT-3ʹ sequence flanked with CCGG at the 5ʹ-end and TTTTTG at the 3ʹ-end as the terminal signal was selected and sent to Jierui Co., Ltd. (Shanghai, China) to synthesize double-stranded DNA. A lentivirus vector (GV115) containing a U6 promoter and a CMV-driven GFP reporter was used to express the shRNA targeting TMED2. The forward (5ʹ-CCGGTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAATTTTTG-3ʹ) and reverse (5ʹ-AATTCAAAAATTCTCCGAACGTGTCACGTTCTCTT GAAACGTGACACGTTCGGAGAA-3ʹ) primers were used to incorporate the shRNA oligomer into the vector predigested with the restriction enzymes EcoRI and AgeI. The oligomer 5ʹ-TTCTCCGAACGTGTCACGT-3ʹ was used to construct a negative control vector. The ligated vector was transformed into commercial competent TOP10 E. coli cells purchased from TIANGEN BIOTECH (Beijing) Co., Ltd., according to the manufacturer’s instructions. The shRNA sequence was amplified by PCR using a 20 μL reaction system with the following primers: F-CCTATTTCCCATGATTCCTTCAT and R-GTAATACGGTTATCCACGCG. The PCR was initiated with a step at 94°C for 3 min, followed by 22 cycles of 94°C for 3 s, 55°C for 30 s and 72°C for 30 s, and a final step of 72°C for 5 min. The PCR product was verified by 1% agarose gel electrophoresis. The vector was extracted using the EndoFree Maxi Plasmid Kit (Qiagen, Shanghai, China) according to the manufacturer’s instructions, and stored at −20°C until further analysis.
Knockdown of TMED2
MM.1S and RPMI 8226 cells (2 × 105 cell/mL) grown in RPMI 1640 medium supplemented with 10% (v/v) foetal bovine serum (FBS) (Ausbian), sodium pyruvate and 2.5 mg/mL glucose were plated in 12-well plates. The cells were transfected with lentivirus vectors expressing siRNA targeting TEMD2 and a negative control sequence. The plates were placed in an incubator with 5% CO2 at 37°C, and the transfection rate was determined by monitoring the fluorescence of GFP. Using the quantitative real-time PCR method described in the previous section, the levels of the TMED2 mRNA were normalized to the level of the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA using the 2−ΔΔCt method described in a previous study.16 The ΔCt value is the difference between the Ct values of TMED2 and GAPDH.
Western Blot Analysis
The cells (2 × 105 cell/mL) were lysed in pre-cooled protein extraction buffer (1 M Tris-HCl (pH 6.8), 2% mercaptoethanol, 20% glycerin, and 4% SDS) for 30 min, followed by centrifugation at 12,000 g for 30 min to remove the nuclei and other components. The protein concentrations were measured using the BCA Protein Assay Kit (Beyotime Biotechnology Co. Ltd., Shanghai, China) and the absorbance was measured at 562 nm. The supernatant was subjected to electrophoresis on a 12% SDS-polyacrylamide gel and then transferred to polyvinylidene difluoride (PVDF) membranes, which were blocked with 5% low-fat milk for 60 min. Primary antibodies against TMED2 (Anti-TMED2 (1:500), Abcam, Cambridge, MA) and GAPDH (Anti-GAPDH (1:2000), Santa-Cruz Biotechnology) were added to the membranes and incubated overnight at 4°C. The secondary antibodies (anti-mouse IgG (1:2000), Abcam, Cambridge, MA and anti-rabbit IgG (1:2000), Santa-Cruz Biotechnology) were added and incubated for 2 h at room temperature after the membrane was washed three times with 0.1% TBS. The bands were visualized with a Pierce ECL Western Blotting Substrate kit (Thermo Scientific, Waltham, MA) according to the manufacturer’s instructions.
Analysis of Cell Viability Using the CCK-8 Assay
MM.1S and RPMI 8226 cells in the logarithmic phase were digested with 0.25% trypsin before being resuspended in DMEM containing 10% foetal bovine serum (Ausbian). The cells were plated in 96-well plates after being adjusted to a density of 4.0 x 103 cells/mL and infected with lentivirus containing the shRNA targeting TMED2 (shTMED2). After an incubation for 9 d, the cells were treated with reagents in the Cell Counting Kit (CCK-8) (Sigma, Shanghai, China) to determine the proliferation rate. After 3 h, the absorbance at 450 nm was measured using an Infinite200 PRO microplate reader (Männedorf, Switzerland). For the control group, the lentivirus with the negative control sequence (shCtrl) was transfected into cells instead.
Analysis of the Cell Cycle Distribution Using Fluorescence-Activated Cell Sorting (FACS)
Cells in the logarithmic phase were collected and digested with 0.25% trypsin before being resuspended in pre-cooled D-Hanks balanced salt solution (pH 7.2–7.4), which were then centrifuged at 1300 rpm for 5 min and fixed with pre-cooled 70% ethanol. The cells were infected with the lentivirus containing the shRNA targeting TMED2 (shTMED2), and the control group was infected with the lentivirus containing the negative control sequence (shCtrl). The cell pellet was washed before being stained with D-Hanks solution containing 50 μg/mL PI and 100 μg/mL RNase at 37°C for 30 min. The cell cycle was analysed using a Guava easyCyte HT Flow Cytometer (LUMINEX, Shanghai, China).
Cell Apoptosis
Cell apoptosis was analysed using the eBioscience™ Annexin V Apoptosis Detection Kit (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer’s instructions. Briefly, the cells in the logarithmic phase were collected and digested with 0.25% trypsin before being resuspended in pre-cooled Binding Buffer at a density of 5x106 cells/mL. The cells were infected with the lentivirus containing the shRNA targeting TMED2 (shTMED2), and cells in the control group were infected with the lentivirus containing the negative control sequence (shCtrl). Ten microliters of fluorochrome-conjugated Annexin V was added to 200 μL of the cell suspension, which was incubated for 15 min at 25°C. The cells were resuspended in 200 μL of 1X Binding Buffer, stained with the Propidium Iodide Staining Solution (10 μL) and analysed using a Guava easyCyte HT flow cytometer (LUMINEX, Shanghai, China). Caspase 3/7 activity was measured using the Caspase-Glo 3/7 Assay kit (Promega, Madison, WI) according to the manufacturer’s instructions.
Statistical Analyses
All statistical analyses were performed using SPSS 16.0 software (Armonk, New York). Each experiment was performed in triplicate. The data are presented as the means ± standard deviations (SD). An unpaired t-test was used to compare two mean values. Significance was defined as **P<0.01 and *P<0.05.
Results
Expression of the TMED2 mRNA in MM Cell Lines
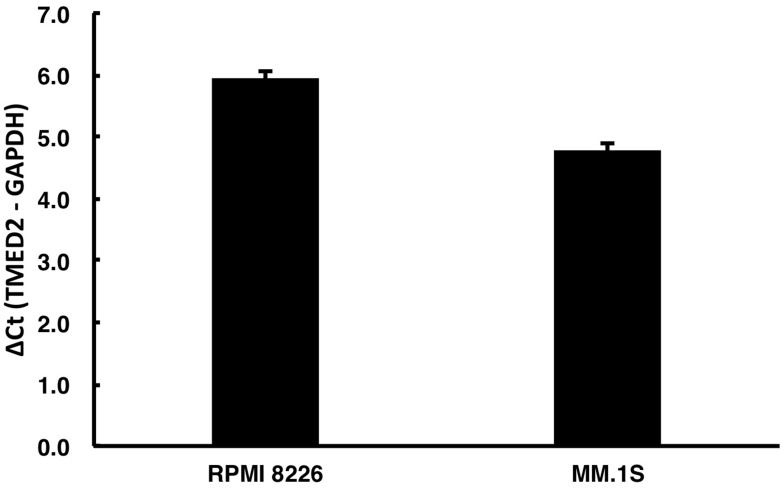
The expression of the TMED2 mRNA in MM.1S and RPMI 8226 cell lines was investigated using RT-qPCR, and the TMED2 mRNA was expressed at a relatively high level in both MM cell lines compared with the endogenous control GAPDH (Figure 1). Cells with a higher ΔCt value express a lower level of the target gene. The ΔCt value ≤12 for the TMED2 mRNA in both cell lines indicates that TMED2 was expressed at high levels.17
Figure 1.
The level of the TMED2 mRNA in RPMI 8226 and MM.1S cell lines. The expression of TMED2 mRNA was measured using RT-qPCR, with the constitutively expressed GAPDH mRNA serving as an internal control.
Effects of the shRNA on TMED2 Expression
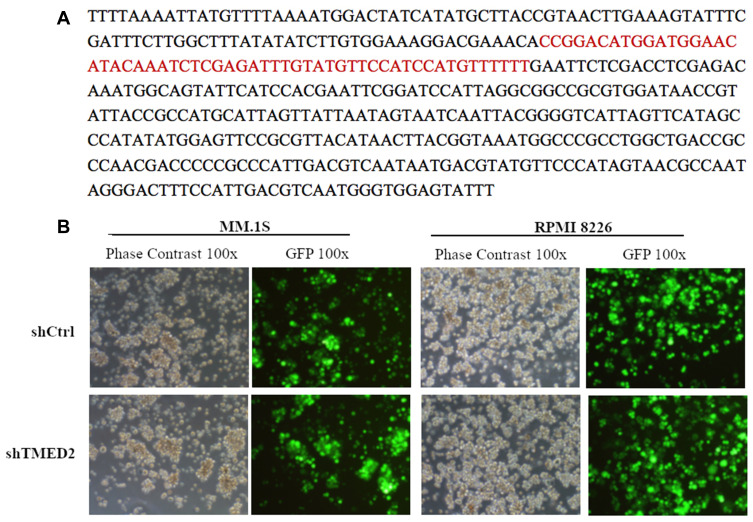
The shRNA-containing lentivirus was sequenced, and the shRNA sequence was successfully incorporated into the lentivirus (Figure 2A). The fluorescence images shown in Figure 2B revealed GFP expression in both MM.1S and RPMI 8226 cells, indicating that the cells were transfected with the lentivirus 72 h after infection with an infection efficiency of >70%.
Figure 2.
Establishment of stable TMED2-silenced MM.1S and RPMI 8226 cell lines. (A) The designed shRNA sequence was successfully incorporated into the lentivirus. (B) GFP expression in MM.1S and RPMI 8226 cells was detected using a fluorescence microscope (x100).
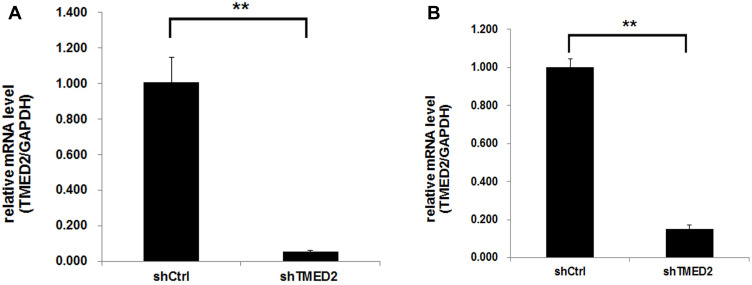
Based on the results of quantitative PCR (Figure 3), the expression of the TMED2 mRNA was significantly inhibited in cells infected with the lentivirus containing the shRNA (p<0.01), and the knockdown efficiency was more than 94.5% and 84.9% for MM.1S and RPMI 8226 cells, respectively.
Figure 3.
The knockdown efficiency of the TMED2 mRNA by the shRNA was detected using quantitative PCR. The expression of the specific shRNA significantly inhibited TMED2 expression in MM.1S (A) and RPMI 8226 cells (B). **p<0.01.
Western Blot Analysis
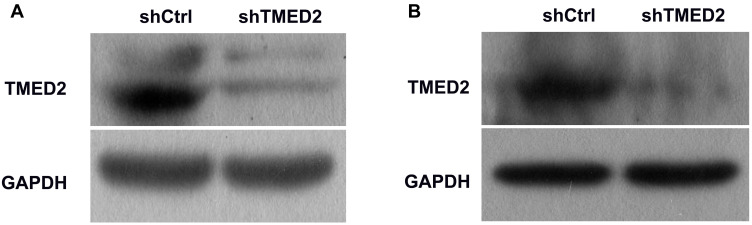
Western blot analyses showed a substantial decrease in the level of the TMED2 protein in the cells transfected with the shRNA targeting TMED2 (Figure 4), suggesting that the shRNA successfully targeted TMED2. In contrast, the lentivirus expressing the negative control sequence did not exert obvious effects on the expression of TMED2.
Figure 4.
Western blot analyses of the levels of the TMED2 protein in MM.1S (A) and RPMI 8226 (B) cells. GAPDH served as a loading control.
Analysis of Cell Viability Using the CCK-8 Assay
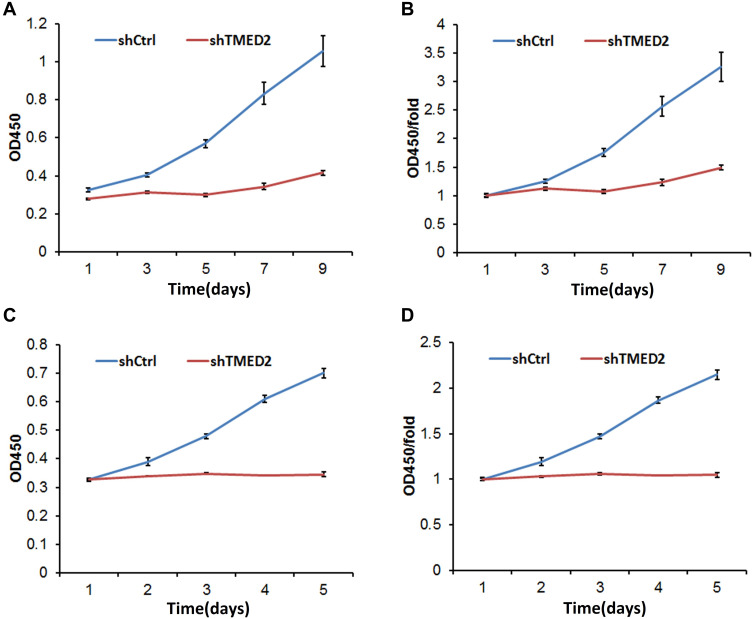
After infection with the shRNA-containing lentivirus, MM.1S and RPMI 8266 cells were cultured for 9 and 5 d, respectively, and treated with CCK-8 reagents for 3 h. The absorbance at 450 nm was substantially reduced for the cells in the shTMED2 group compared with the control group (shCtrl) in both cell lines (Figure 5), indicating that cell viability was significantly reduced due to the inhibition of TMED2 by the shRNA.
Figure 5.
Cell viability was affected by the inhibition of TMED2 in MM.1S (A, B) and RPMI 8266 cells (C, D).
Cell Cycle Distribution
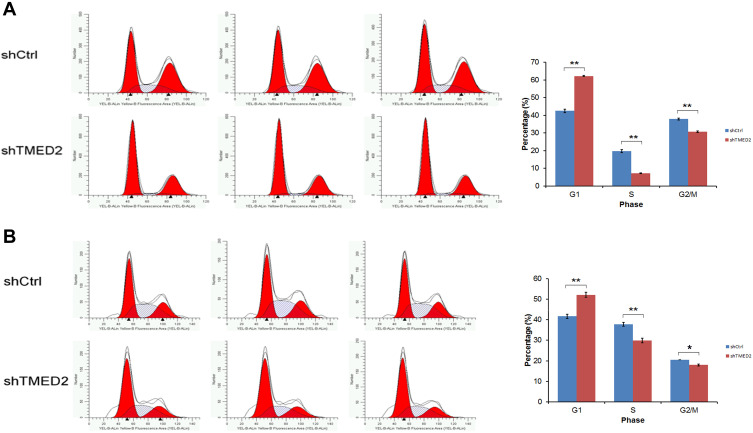
The transfection of the shRNA-containing lentivirus exerted a substantial effect on the cell cycle distribution of MM.1S and RPMI 8226 cells by increasing the percentage of MM.1S and RPMI 8226 cells in G1 phase, while significantly decreasing the percentage of cells in S and G2 phases compared with the negative control (Figure 6).
Figure 6.
Cell cycle distribution of MM.1S and RPMI 8226 cells transfected with shTMED. The transfection of the lentivirus containing the shRNA substantially increased the percentages of MM.1S (A) and RPMI 8226 (B) cells in G1 phase, while the percentages of cells in S and G2 phases were significantly decreased compared with the negative control. **p<0.01, *p<0.05.
Apoptosis of MM.1S and RPMI Cells Transfected with the shRNA Targeting TMED2
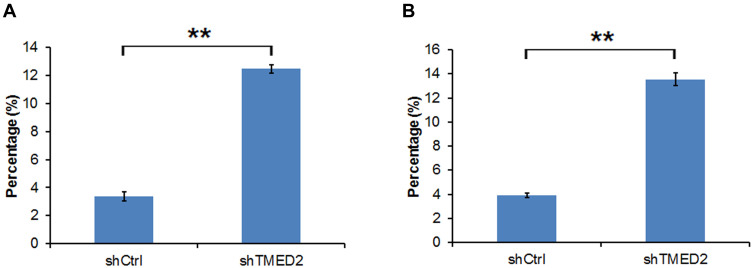
The apoptosis status of MM.1S and RPMI cells was investigated five days after infection with the lentivirus containing the shRNA. The apoptosis rate increased by more than 3-fold compared with the control group (shCtrl) (Figure 7).
Figure 7.
Apoptosis of MM.1S (A) and RPMI 8226 cells (B) transfected with the shRNA targeting TMED2. **p<0.01.
Caspase 3/7 Activity Was Increased by shTMED2
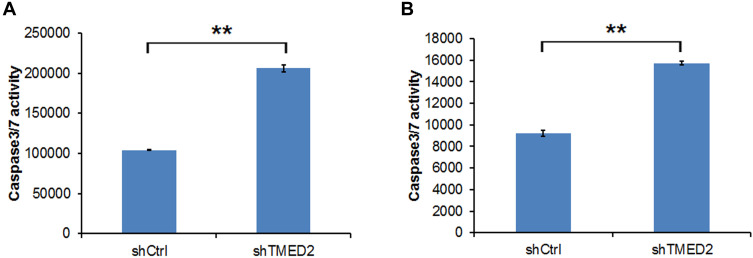
The activity of Caspases 3/7 was investigated. The activity of Caspase 3/7 increased by more than 2- and 1.7-fold in MM.1S and RPMI 8226 cells, respectively, compared with the control group (shCtrl), indicating that apoptosis was induced by the inhibition of TMED2 (Figure 8).
Figure 8.
Caspase 3/7 activity in MM.1S (A) and RPMI 8226 cells (B) transfected with the shRNA targeting TMED2. **p<0.01.
Discussion
Although multiple myeloma is still an incurable disease,18 the progression-free survival and overall survival of patients with multiple myeloma have steadily increased due to the introduction of novel medications in recent years.19,20 Novel therapies for MM have steadily been developed over the years, from early medicines, such as alkylators and corticosteroids, to the more recent drugs panobinostat, pomalidomide, and carfilzomib. However, many patients have developed resistance to some of these treatments, and thus the development of new medicines and novel targets is urgently needed and necessary. Our work presented in this study is designed to contribute to this field by investigating whether TMED2 represents a diagnostic marker and therapeutic strategy for multiple myeloma. TMED2 was expressed at high levels in both MM cell lines, and knockdown of the TMED2 gene by an shRNA inhibited cell proliferation and promoted the apoptosis of MM cell lines.
TMED2 is located in many organelles, such as the Golgi, ER, and endosomes, and plays an important role in regulating the transport of cargo proteins, such as Anterior gradient 2 (AGR2),21 glucagon receptor (GCGR),22 and Gas1p.23 Abnormal expression of TMED2 may lead to uncontrolled protein transport, leading to diseases such as cancer. Aberrant expression of TMEDs is linked to a number of diseases, such as colon cancer,24 non-alcoholic fatty liver disease,14 hepatocellular carcinoma progression,25 breast cancer,26 and prostate cancer.27 The regulatory mechanism of TMED2 in cancer remains unclear. According to one study, TMED2 promotes epithelial ovarian cancer growth by modulating the IGF2/IGF1R/PI3K/AKT pathway.14 However, the function of TMED2 in MM is unclear. TMED2 belongs to the transmembrane emp24 domain (Tmed)/p24 protein family. In addition to TMED2, other members in this family may also play a similar role, which should be investigated in further studies. For example, TMED3 has been revealed to be a tumour suppressor in prostate cancer, colon cancer, and other tumours24,27 by increasing endogenous WNT-TCF activity. Knockdown or loss of function of TMED proteins may activate the unfolded protein response during ER stress. For example, a loss of TMED promotes the splicing of the XBP1 pre-mRNA, which in turn leads to unfolded protein response.
In summary, the TMED2 mRNA is expressed at high levels in MM cell lines, and interference with the target TMED2 mRNA inhibits cell proliferation and promotes the apoptosis of MM cell lines by regulating the activity of Caspase 3/7. Based on the results of our study, TMED2 may play an important role in the pathogenesis of multiple myeloma, and thus further studies should be conducted to develop new and useful biomarkers and potential therapeutic targets for MM.
Acknowledgments
This study was funded by the National Natural Science Foundation (No. 81302044, No. 81270598, No. 81473486 and No. 81770210), the Projects of Medical and Health Technology Development Program of Shandong Province (No. 2016WS0407), the Promotive Research Fund for Excellent Young and Middle-aged Scientists of Shandong Province (No. BS2013YY009), the Key Research and Development Program of Shandong Province (No. 2018CXGC1213 and No. 2018GSF118157), the Technology Development Projects of Shandong Province (No. 2017GSF18189), the Taishan Scholars Program of Shandong Province, the Shandong Provincial Engineering Research Center of Lymphoma, and the Academic Promotion Program of Shandong First Medical University.
Disclosure
The authors have no conflicts of interest to declare.
References
- 1.Angtuaco EJC, Fassas ABT, Walker R, Sethi R, Barlogie B. Multiple myeloma: clinical review and diagnostic imaging. Radiology. 2004;231(1):11–23. doi: 10.1148/radiol.2311020452 [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21–33. doi: 10.4065/78.1.21 [DOI] [PubMed] [Google Scholar]
- 3.Kazandjian D. Multiple myeloma epidemiology and survival: a unique malignancy. Semin Oncol. 2016;43(6):676–681. doi: 10.1053/j.seminoncol.2016.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vincent Rajkumar S, Kumar S. Multiple myeloma: diagnosis and treatment. Mayo Clin Proc. 2016;91(1):101–119. doi: 10.1016/j.mayocp.2015.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanagarajah P, Cao K, Chen L, Rumbak M. Malignant multiple myeloma of the pleura. J Bronchology Interv Pulmonol. 2018;25(4):332–334. doi: 10.1097/LBR.0000000000000498 [DOI] [PubMed] [Google Scholar]
- 6.Chen C, Baldassarre F, Kanjeekal S, Herst J, Hicks L, Cheung M. Lenalidomide in multiple myeloma—a practice guideline. Curr Oncol. 2013;20(2):e136–e149. doi: 10.3747/co.20.1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singhal S, Mehta J, Desikan R, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341(21):1565–1571. doi: 10.1056/NEJM199911183412102 [DOI] [PubMed] [Google Scholar]
- 8.Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352(24):2487–2498. doi: 10.1056/NEJMoa043445 [DOI] [PubMed] [Google Scholar]
- 9.Blimark CH, Turesson I, Genell A, et al. Outcome and survival of myeloma patients diagnosed 2008–2015. real-world data on 4904 patients from the swedish myeloma registry. Haematologica. 2018;103(3):506–513. doi: 10.3324/haematol.2017.178103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pulte D, Jansen L, Castro FA, et al. Trends in survival of multiple myeloma patients in Germany and the United States in the first decade of the 21st century. Br J Haematol. 2015;171(2):189–196. doi: 10.1111/bjh.13537 [DOI] [PubMed] [Google Scholar]
- 11.Tomasson MH, Ali M, De Oliveira V, et al. Prevention is the best treatment: the case for understanding the transition from monoclonal gammopathy of undetermined significance to myeloma. Int J Mol Sci. 2018;19(11):3621. doi: 10.3390/ijms19113621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paul B, Lipe B, Ocio EM, Usmani SZ. Induction therapy for newly diagnosed multiple myeloma. Am Soc Clin Oncol Educ Book. 2019;39(39):e176–e186. doi: 10.1200/EDBK_238527 [DOI] [PubMed] [Google Scholar]
- 13.Aber R, Chan W, Mugisha S, Jerome-Majewska LA. Transmembrane Emp24 domain proteins in development and disease. Genet Res (Camb). 2019;27(101):e14. doi: 10.1017/S0016672319000090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia L, Liu J, Hu SF, Xun H. Increased expression of TMED2 is an unfavorable prognostic factor in patients with breast cancer. Cancer Manag Res. 2019;11:2203–2214. doi: 10.2147/CMAR.S192949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi-Peng G, Chun-Lin C, Huan W, et al. TMED2 promotes epithelial ovarian cancer growth. Oncotarget. 2017;8(55):94151–94165. doi: 10.18632/oncotarget.21593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 17.Kapellos TS, Recio C, Greaves DR, Iqbal AJ. Cannabinoid receptor 2 modulates neutrophil recruitment in a murine model of endotoxemia. Mediators Inflamm. 2017;2017:4315412. doi: 10.1155/2017/4315412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ravi P, Kumar SK, Cerhan JR, et al. Defining cure in multiple myeloma: a comparative study of outcomes of young individuals with myeloma and curable hematologic malignancies. Blood Cancer J. 2018;8(3):26. doi: 10.1038/s41408-018-0065-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landgren O, Iskander K. Modern multiple myeloma therapy: deep, sustained treatment response and good clinical outcomes. J Intern Med. 2017;281(4):365–382. doi: 10.1111/joim.12590 [DOI] [PubMed] [Google Scholar]
- 20.Kristinsson SY, Anderson WF, Landgren O. Improved long-term survival in multiple myeloma up to the age of 80 years. Leukemia. 2014;28(6):1346–1348. doi: 10.1038/leu.2014.23 [DOI] [PubMed] [Google Scholar]
- 21.Maurel M, Obacz J, Avril T, et al. Control of Anterior GRadient 2 (AGR2) dimerization links endoplasmic reticulum proteostasis to inflammation. EMBO Mol Med. 2019;11(6):e10120. doi: 10.15252/emmm.201810120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han J, Zhang M, Froese S, et al. The identification of novel protein-protein interactions in liver that affect glucagon receptor activity. PLoS One. 2015;10(6):e0129226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jerome-Majewska LA, Achkar T, Luo L, Lupu F, Lacy E. The trafficking protein Tmed2/P24β1 is required for morphogenesis of the mouse embryo and placenta. Dev Biol. 2010;341(1):154–166. doi: 10.1016/j.ydbio.2010.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duquet A, Melotti A, Mishra S, et al. A novel genome-wide in vivo screen for metastatic suppressors in human colon cancer identifies the positive WNT-TCF pathway modulators TMED3 and SOX12. EMBO Mol Med. 2014;6(7):882–901. doi: 10.15252/emmm.201303799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng H, Yang Y, Han J, et al. TMED3 promotes hepatocellular carcinoma progression via IL-11/STAT3 signaling. Sci Rep. 2016;6(1):37070. doi: 10.1038/srep37070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hou W, Gupta S, Beauchamp M-C, et al. Non-alcoholic fatty liver disease in mice with heterozygous mutation in TMED2. PLoS One. 2017;12(8):e0182995. doi: 10.1371/journal.pone.0182995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vainio P, Mpindi J-P, Kohonen P, et al. High-throughput transcriptomic and RNAi analysis identifies AIM1, ERGIC1, TMED3 and TPX2 as potential drug targets in prostate cancer. PLoS One. 2012;7(6):e39801. [DOI] [PMC free article] [PubMed] [Google Scholar]