Abstract
Objective
The aim of this study was to construct the immunoscore (IS) to facilitate the prediction of postoperative survival and benefit from adjuvant chemotherapy (ACT) in esophageal squamous cell carcinoma (ESCC).
Methods
A total of 249 patients who received radical esophagectomy at Fudan University Shanghai Cancer Center were divided into training set and testing set. Eighty-nine patients with ESCC from TCGA database were enrolled into the validation set. Myeloid cells in tumor microenvironment were evaluated by immunohistochemistry or CIBERSORT, and then were included into a LASSO Cox regression model to construct the immunoscore. The predictive value of the immunoscore for prognosis after surgery or ACT was analyzed.
Results
The immunoscore was constructed by four types of myeloid cells including macrophages, neutrophils, mast cells, and dendritic cells and was demonstrated as IS=2^(0.527719*Mφ −0.2604269*MC-0.4812935*DC-0.4519706*Neu). The overall survival was significantly different between two immunotypes, which were divided according to the immunoscore, in all sets (P<0.001, P=0.005, and P=0.002, respectively). Immunotype A was identified as an independent predictor for survival benefit in all three sets (HR=2.068, P=0.005; HR=2.028, P=0.007; HR=6.474, P=0.007; respectively). In patients who received ACT, immunotype A was significantly related to longer overall survival both in the training set (P<0.001) and in the testing set (P=0.011). The nomogram based on immunotype and other clinicopathological factors showed good efficiency of predicting response to ACT. Finally, several important cytokines and pathways were highly enriched in immunoscore A subgroup.
Conclusion
The immunoscore was an effective prognostic predictor in ESCC for patients undergoing surgical resection and receiving ACT.
Keywords: adjuvant chemotherapy, prognosis, immunoscore, esophageal squamous cell carcinoma
Introduction
Esophageal cancer (EC), the seventh most common cancer, remained a life-threatening malignancy with dismal prognosis, in spite of recent advances in medical treatment.1 Esophageal squamous cell carcinoma (ESCC), known as the predominant historic type worldwide, occurred in the majority of EC patients in Asia.2
Although surgical resection was regarded as dispensable in treatment of ESCC, surgery alone failed to achieve satisfactory outcomes,3 due to the high frequency of recurrence and metastasis, which suggested the potential importance of adjuvant chemotherapy (ACT). However, the role of ACT was controversial, partly because few of traditional clinicopathological factors, such as tumor size and differentiation grade, was able to identify suitable patients who could benefit from ACT.4–6
Instead of the characteristics of cancer cells, evidence showed the important impact of immune status in tumor microenvironment on tumor progression and metastasis.7–9 A growing number of studies revealed the myeloid cells infiltrating into the tumor were significantly related with patients’ prognosis, and more interestingly, the response to ACT in several types of cancer, which might provide a novel sight to seek for sensitive predictors for ACT effect and to identify the suitable population for ACT in ESCC treatment.10–12 By far, only a few articles suggested the possible influence of tumor-associated myeloid cells on the survival of ESCC patients, including macrophages,13 neutrophils,14 mast cells,15 and dendritic cells.16 As for the comprehensive immune status featured by the interaction of theses myeloid cells in the tumor microenvironment, no study has evaluated its impact on survival or effect of ACT.
Therefore, the aim of this study was to confirm the prognostic value of myeloid cells in patients with ESCC, and construct a novel classifier based on myeloid cells using a LASSO Cox regression model17,18 in order to predict survival, and more importantly, the benefits of ACT in ESCC patients.
Methods
Study Population
Patients who received radical esophagectomy between 2001 and 2009 at Fudan University Shanghai Cancer Center (FUSCC) were enrolled in this study. The inclusion criteria were: (1) histopathological diagnosis of ESCC; (2) without distant metastases; (3) complete resection of tumors; (4) without preoperative antitumor treatment; (5) available clinical and survival data; (6) sufficient tissue samples for tissue microarray. Finally, a total of 249 eligible patients were included and assigned to the training set (124 patients) and the testing set (125 patients) by computer-generated random numbers. The regimen for the patients who received ACT in this study was based on four to six cycles of 5-fluorouracil and cisplatin/oxaliplatin. The median follow-up of all the patients was 70 months. In addition, 89 patients with ESCC from TCGA database were enrolled as validation set. The inclusion criteria were: (1) pathological stage I to stage III; (2) available clinical data and mRNA expression data; (3) available overall survival data. The clinicopathological characteristics of the patients in all three sets were shown in Table 1.
Table 1.
Clinical Characteristics of Patients in the Training, Testing, and Validation Sets
| Characteristics | Training Set | Testing Set | Validation Set |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| Age | |||
| ≤60 years | 74 (59.7) | 79 (63.2) | 54 (60.7) |
| >60 years | 50 (40.3) | 46 (36.8) | 35 (39.3) |
| Gender | |||
| Female | 23 (18.5) | 22 (17.6) | 13 (14.6) |
| Male | 101 (81.5) | 103 (82.4) | 76 (85.4) |
| Immunotype | |||
| Type A | 92 (74.2) | 92 (73.6) | 28 (31.5) |
| Type B | 32 (25.8) | 33 (26.4) | 61 (68.5) |
| Tumor size | |||
| ≤3 cm | 53 (42.7) | 41 (32.8) | – |
| >3 cm | 71 (57.3) | 84 (67.2) | – |
| pTNM stage | |||
| I | 18 (14.5) | 20 (16.0) | 7 (7.9) |
| II | 45 (36.3) | 34 (27.2) | 55 (61.8) |
| III | 61 (49.2) | 71 (56.8) | 27 (30.3) |
| Tumor grade | |||
| G1/G2 | 103 (83.1) | 92 (73.6) | 60 (67.4) |
| G3 | 21 (16.9) | 33 (26.4) | 20 (22.5) |
| Gx | 0 (0.0) | 0 (0.0) | 9 (10.1) |
| LVI | |||
| Negative | 38 (30.6) | 44 (35.2) | – |
| Positive | 86 (69.4) | 81 (64.8) | – |
Note: The data of tumor size and LVI were not available in the validation set (TCGA cohort).
Tissue Microarray and Immunohistochemistry (IHC)
Tissue microarray (TMA) was established with formalin-fixed, paraffin embedded surgical specimens derived from the enrolled patients at FUSCC. Two tissue cores were selected from two distinct areas of tumor specimens for each patient. IHC was performed on TMA using selected biomarkers including: macrophages (CD68), neutrophils (CD66b), dendritic cells (CD1a) and mast cells (tryptase). The information of antibodies and dilution was provided in Table S1. The detailed IHC protocol was described in the supplementary document.
Evaluations of Immune Cells
The IHC results were evaluated by two independent observers who were blinded to the clinical information. The nucleated stained cells infiltrating into the tumor tissue of each tissue core were counted under a high magnification field (HPF, 400×). The mean count derived from the two observers was adopted as the cell count of each case. In the validation set, the mRNA expression data was converted to the estimated number of different types of immune cells in each tumor sample using CIBERSORT method (an online tool and a computational approach for estimating proportion of immune cells in the given sample using mRNA expression data).19
Construction of Immunoscore
The establishment of immunoscore can be concisely divided into three steps. First, a cutoff value of cell counts was derived for each type of immune cell. Second, the abundance of immune cells in each patient was compared with the cutoff value in order to determine the expression status (the low expression status was equivalent to 0, and high expression status was equivalent to 1). Third, the expression status of selected immune cells in all patients was included in a LASSO Cox regression model to construct a formula (immunoscore).
Statistical Analysis
The optimal cutoff value for count of each immune cell was calculated with “survminer” package in R. The LASSO Cox regression model was generated to integrate features of prognostic related immune cells with ideal coefficient for survival prediction using training set by “glmnet” package. The OS was analyzed by Kaplan–Meier method and log rank test. Univariate and multivariate Cox regression analysis were performed to identify independent prognostic variables for survival. In the validation set, differential gene expression was analyzed using “edgeR” package, and the pathways which enriched between the two groups were calculated with gene set enrichment analysis (GSEA).20 Statistical analysis was performed with R software (version 3.5.1) and SPSS (version 19.0). Statistical significance was set at 0.05.
Results
Construction of Immunoscore and Definition of Immunotype
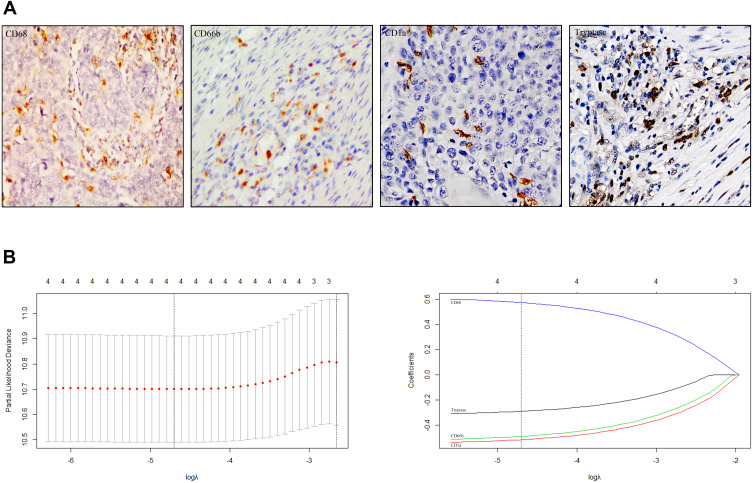
Tumor-infiltrated macrophages, neutrophils, mast cells and dendritic cells were stained using IHC as shown in Figure 1A. The prognostic value of these myeloid cells was examined in the training set using Kaplan–Meier survival analysis. The results indicated that high density of neutrophils, mast cells and dendritic cells was positively related to better survival, on the contrary, abundant macrophages represented a sign of poorer survival (Figure S1). Given the confirmed correlation between cell counts and prognosis, all the four types of myeloid cells were included in the LASSO Cox regression model (Figure 1B), then a formula to calculate the immunoscore (IS) for each patient was constructed, where IS=2 ^(0.527719*Mφ -0.2604269*MC-0.4812935*DC-0.4519706*Neu). In this formula, Mφ, Neu, MC, and DC represented the expression status of macrophages, neutrophils, mast cells and dendritic cells. In addition, the low expression status was equivalent to 0, and high expression status was equivalent to 1. According to the immunoscore, patients with IS <1 were classified into immunotype A, and those with IS ≥1 were classified into immunotype B.
Figure 1.
Construction of immunoscore using LASSO Cox regression. (A) Tumor infiltration of macrophages, neutrophils, dendritic cells and mast cells in ESCC patients with 400× magnification. (B) LASSO coefficient profiles of the four selected stromal immune features. Vertical lines were drawn at the optimal values by minimum criteria and 1-se.
Immunotype and Survival
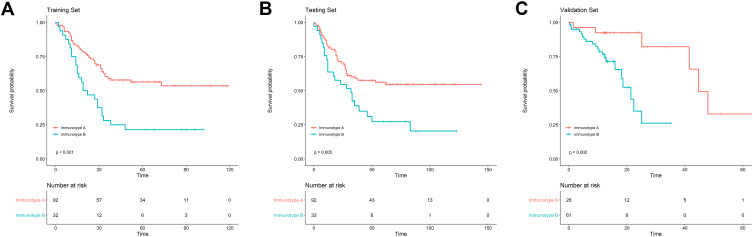
To assess the prognostic value of immunotype, Kaplan–Meier survival analysis was applied to compare OS between patients with immunotype A and immunotype B. In the training set, patients with immunotype A had significantly better OS than those with immunotype B (P<0.001), and the similar results were confirmed both in the testing set (P=0.005) and in the validation set (P=0.002; Figure 2). In univariate analysis, immunotype A had a beneficial effect on OS in all three sets (P<0.01; Table 2). All the survival related variables identified in univariate analysis were included in the multivariate Cox regression analysis. As shown in Table 3, immunotype A remained an independent predictor for survival benefit in training set (HR=2.068, 95%CI: 1.243–3.440, P=0.005), testing set (HR=2.028, 95%CI: 1.210–3.397, P=0.007), and validation set (HR=6.474, 95%CI: 1.744–24.038, P=0.007).
Figure 2.
The Kaplan–Meier survival analysis showed the difference of postoperative OS between patients with immunotype A (IS <1) and immunotype B (IS 1) in the training set (A), the testing set (B) and the validation set (C).
Table 2.
Univariate Analyses for Characteristics Related to Overall Survival
| Characteristics | Training Set | Testing Set | Validation Set | |||
|---|---|---|---|---|---|---|
| HR (95%CI) | P-value | HR (95%CI) | P-value | HR (95%CI) | P-value | |
| Age | 0.982 | 0.761 | 0.343 | |||
| ≤60 years | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| >60 years | 0.994 (0.602–1.643) | 1.083 (0.650–1.805) | 1.459 (0.668–3.188) | |||
| Gender | 0.139 | 0.567 | 0.046* | |||
| Female | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Male | 1.705 (0.841–3.456) | 0.837 (0.455–1.539) | 4.490 (1.025–19.665) | |||
| Immunotype | 0.001* | 0.009* | 0.005* | |||
| Type A | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Type B | 2.445 (1.476–4.052) | 2.020 (1.216–3.356) | 5.951 (1.717–20.632) | |||
| Tumor size | 0.289 | 0.828 | – | |||
| ≤3 cm | 1.000 (reference) | 1.000 (reference) | – | |||
| >3 cm | 1.312 (0.794–2.170) | 0.944 (0.564–1.528) | – | |||
| pTNM stage | <0.001* | 0.120 | 0.108 | |||
| I | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| II | 9.012 (1.206–67.329) | 0.843 (0.365–1.948) | 1.110 (0.245–5.028) | |||
| III | 20.057 (2.759–145.802) | 1.528 (0.742–3.146) | 2.486 (0.549–11.251) | |||
| Tumor grade | 0.237 | 0.344 | 0.595 | |||
| G1/G2 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| G3 | 1.463 (0.779–2.750) | 1.296 (0.758–2.217) | 0.597 (0.202–1.762) | |||
| Gx | – | – | 1.136 (0.378–3.419) | |||
| LVI | <0.001* | 0.018* | – | |||
| Negative | 1.000 (reference) | 1.000 (reference) | – | |||
| Positive | 4.228 (2.011–8.888) | 1.960 (1.124–3.419) | – | |||
Notes: The data of tumor size and LVI were not available in the validation set (TCGA cohort). *P<0.05.
Table 3.
Multivariate Analyses for Characteristics Related to Overall Survival
| Characteristics | Training Set | Testing Set | Validation Set | |||
|---|---|---|---|---|---|---|
| HR (95%CI) | P-value | HR (95%CI) | P-value | HR (95%CI) | P-value | |
| Immunotype | 0.005* | 0.007* | 0.005* | |||
| Type A | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Type B | 2.068 (1.243–3.440) | 2.028 (1.210–3.397) | 6.474 (1.744–24.038) | |||
| pTNM stage | 0.048* | 0.430 | 0.104 | |||
| I | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| II | 6.756 (0.851–53.638) | 0.591 (0.241–1.452) | 0.659 (0.137–3.160) | |||
| III | 11.534 (1.337–99.486) | 0.820 (0.304–2.211) | 1.657 (0.334–8.221) | |||
| LVI | 0.304 | 0.141 | – | |||
| Negative | 1.000 (reference) | 1.000 (reference) | – | |||
| Positive | 1.624 (0.645–4.089) | 1.823 (0.820–4.052) | – | |||
| Gender | – | – | 0.245 | |||
| Female | – | – | 1.000 (reference) | |||
| Male | – | – | 2.563 (0.524–12.538) | |||
Notes: The variables identified with statistical significance were included into the multivariate analysis. *P<0.05.
Immunotype and Adjuvant Chemotherapy Therapy
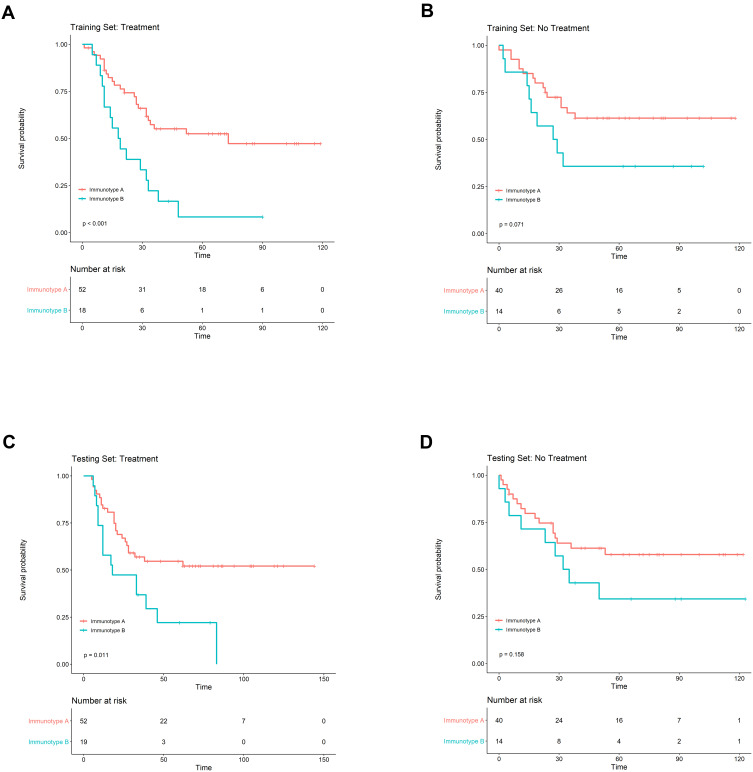
The predictive value of immunotype for ACT outcomes was evaluated by subgroup analysis in the training set and the testing set. For patients who received ACT, significant survival difference could be found between immunotype A and immunotype B subgroups both in the training set (P<0.001) and in the testing set (P=0.011; Figure 3). Cox regression analysis demonstrated that survival benefit was significant in patients with immunotype A in comparison with immunotype B group (training set: HR=2.873, 95%CI: 1.511–5.461, P=0.001; testing set: HR=2.265, 95%CI: 1.181–4.345, P=0.014). However, for patients without ACT, there was only a trend of better OS in the immunotype A subgroup, while the survival difference did not achieve statistical significance (training set: P=0.071, testing set: P=0.158; Figure 3).
Figure 3.
Subgroup analysis to assess the predictive value of immunotype for ACT. The survival curves according to immunotype A (IS <1) and immunotype B (IS ≥1) in ESCC patients with ACT (A and C) and without ACT (B and D) in the training set and the testing set.
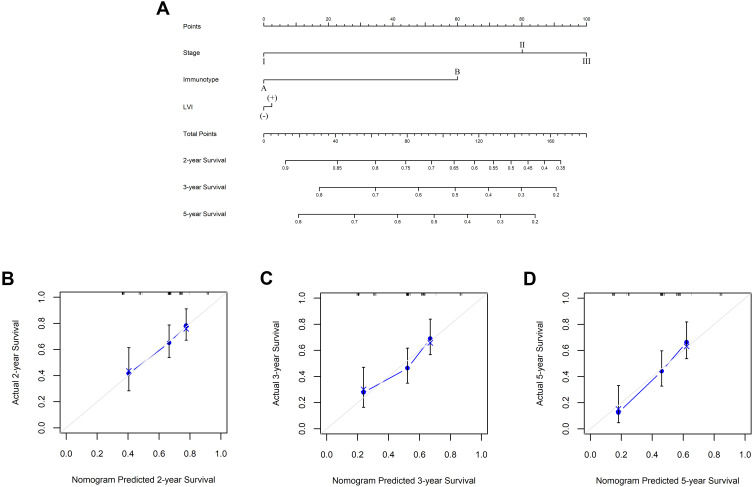
Due to the promising prognostic value of immunotype for patients who received ACT, a nomogram based on immunotype was established to calculate the probability of two, three, and five-year OS in patients receiving ACT (Figure 4A). Calibration plots demonstrated that the nomogram performed well in comparison with an ideal model (Figure 4B–4D).
Figure 4.
Nomogram to predict survival probability in ESCC patients receiving ACT. (A) Nomogram based on immunotype A (IS <1) and immunotype B (IS ≥1) and clinicopathological factors to predict two, three, and 5five-year survival in ESCC patients after ACT. Two-year (B), three-year (C) and five-year (D) nomogram evaluated by calibration curves. The dashed line represented an ideal evaluation, whereas the blue line represented the performance of the nomogram.
Immunotype Related Immune Molecules and Biological Pathways
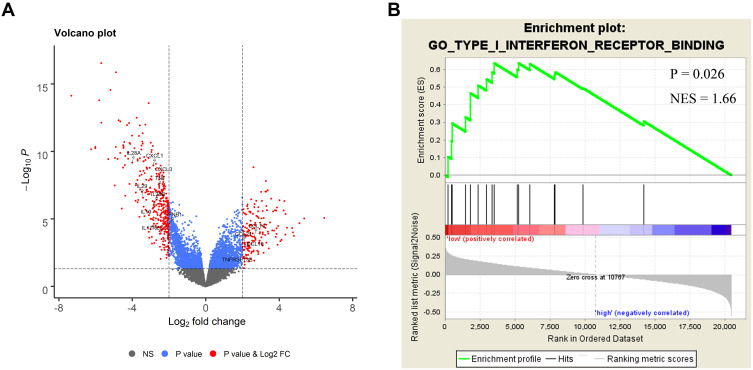
In the validation set, gene expression profile was compared between immunotype A and immunotype B using TCGA data. Interestingly, several important cytokines, such as TNF and IFN-β1 were significantly enriched in immunotype A subgroup (Figure 5A), which might partly explain the survival difference between the two groups. The GSEA showed that type I interferon receptor binding pathway was enriched in immunotype A subgroup (Figure 5B), which suggested the possibility of positive antitumor regulation in these patients.
Figure 5.
Bioinformatics analysis of the characteristics and signal pathway of immunotypes. (A) Volcano plot comparing the FDR vs fold-change for genes from immunotype A (IS <1) group relative to immunotype B (IS ≥1) group using TCGA data. (B) Enriched biology pathways related with immunotype A in the validation cohort.
Discussion
Appropriate selection of therapeutic and follow-up strategy depended on precise prognostic evaluation of cancer patients. In clinical practice, survival prediction of ESCC patients was mostly based on clinicopathological features. As for tumor-associated immune cells, which were proven to have significant impact on cancer progression and patients’ survival in several malignancies by growing evidence,10–12 the relevant literature was limited in ESCC. Our data indicated that myeloid cells infiltrating in cancer microenvironment, including macrophages, neutrophils, mast cells and dendritic cells were related with prognosis. More specifically, macrophages mostly played a protumoral role in the microenvironment of ESCC, while neutrophils, mast cells and dendritic cells tended to have an antitumoral impact on patients. To improve the predictive power, the features of the immune cells were integrated using a LASSO Cox regression model so that it could reflect the contexture of immune status in the microenvironment, reduce the deviation caused by variable function of immune cells, and partly demonstrate the comprehensive interaction of immune cells. Interestingly, as shown in the results, two different types of immune status represented distinctively two different characteristics of survival status of patients. Patients with immunotype A, characterized as low density of macrophages and high density of neutrophils, mast cells, and dendritic cells, were identified to be an independent prognostic predictor from the clinicopathological factors that mainly focused on the features of tumor itself. The immunotype, however, described the malignant disease from another perspective, known as tumor microenvironment, which was of equal importance to tumoral characteristics in cancer progression.21,22
Substantial controversy existed regarding the effectiveness of adjuvant chemotherapy in patients with ESCC postoperatively, to a certain extent, owing to the lack of precise prognostic predictors. Recent studies demonstrated that ACT might prolong the DFS in patients with positive lymph nodes, which became the main indication for ACT. However, this clinicopathological feature seemed not to be sufficient to distinguish the suitable candidates for ACT postoperatively, since no advantage of overall survival was observed in prior studies.23,24 With the deepening of understanding of the immune mechanism, it provided a new perspective to reinterpret the mechanism of chemotherapy. Signatures consisting of appropriate immune markers showed a good predictive value of ACT response in several other types of cancer.7,8 In the present study, the patients with a low immunoscore had better response to ACT than those with a high immunoscore, while the survival difference did not achieve statistical significance in patients without ACT. The different role of immunoscore according to whether ACT was performed or not might partly be explained by the enhanced anticancer immunity, for instance, dendritic cells were attracted into the tumor bed, which was triggered by chemotherapy.25,26 For immunotype A, characterized as a high density of anticancer immune cells infiltrating into the tumor microenvironment, it was plausible that the stronger anticancer immune responses occurred after chemotherapy. In contrast, chemotherapy agents, including cisplatin, selectively inhibited regulatory and suppressor cells at low doses based on the previous study.27 The results of our bioinformatics analysis showed that several antitumor chemokines28,29 were identified to be enriched in the immunotype A group by bioinformatics analysis, while their role might be veiled by the coexistence of some protumor molecules.30,31 With the performance of ACT, the protumor feature might be inhibited by chemotherapy agents so that the antitumor function had the advantage. To strengthen the prognostic value of immunotype, we combined it with tumoral characteristics including pathologic stage and LVI, and generated a nomogram to predict ACT outcomes, since both tumoral features and microenvironment status were critical to anticancer treatment, and the calibration plots demonstrated its efficiency.
There were some limitations of our study. First, it was a retrospective study with all specimens from only one center, although the results were verified using public database, the efficiency should be further confirmed by multicenter data. Second, our model was constructed by myeloid cells, however, some important immune molecules were not included.
In conclusion, the immunoscore was an effective prognostic predictor in ESCC for patients undergoing surgical resection and receiving ACT. Thus it could be used as a predictive tool facilitating follow-up strategy and a classifier to select suitable patients who might benefit from ACT.
Funding Statement
This work was supported by Shanghai Science and Technology Commission Foundation key project (14JC1401400), Grant from Science and Technology Commission of Shanghai Municipality (No. 15411951602; No. 16401970704), and Shanghai Sailing Program (19YF1408800).
Data Sharing Statement
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institution (Fudan University Shanghai Cancer Center Institutional Review Board no. 090977-1), and informed consent was obtained from the study participants prior to study commencement.
Consent for Publication
Not applicable.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Arnold M, Soerjomataram I, Ferlay J, et al. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64(3):381–387. doi: 10.1136/gutjnl-2014-308124 [DOI] [PubMed] [Google Scholar]
- 3.Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet. 2013;381(9864):400–412. doi: 10.1016/S0140-6736(12)60643-6 [DOI] [PubMed] [Google Scholar]
- 4.Chen H, Wu Z, Chen J, et al. Postoperative adjuvant therapy for resectable thoracic esophageal squamous cell carcinoma: a retrospective analysis of 426 cases. Med Oncol. 2015;32:417. doi: 10.1007/s12032-014-0417-6 [DOI] [PubMed] [Google Scholar]
- 5.Gwynne S, Wijnhoven BP, Hulshof M, et al. Role of chemoradiotherapy in oesophageal cancer: adjuvant and neoadjuvant therapy. Clin Oncol (R Coll Radiol). 2014;26(9):522–532. doi: 10.1016/j.clon.2014.05.015 [DOI] [PubMed] [Google Scholar]
- 6.Pasquer A, Gronnier C, Renaud F, et al. Impact of adjuvant chemotherapy on patients with lymph node-positive esophageal cancer who are primarily treated with surgery. Ann Surg Oncol. 2015;22(Suppl S3):S1340–9. doi: 10.1245/s10434-015-4658-1 [DOI] [PubMed] [Google Scholar]
- 7.Jiang Y, Zhang Q, Hu Y, et al. ImmunoScore signature: a prognostic and predictive tool in gastric cancer. Ann Surg. 2018;267(3):504–513. doi: 10.1097/SLA.0000000000002116 [DOI] [PubMed] [Google Scholar]
- 8.Fu H, Zhu Y, Wang Y, et al. Identification and validation of stromal immunotype predict survival and benefit from adjuvant chemotherapy in patients with muscle-invasive bladder cancer. Clin Cancer Res. 2018;24(13):3069–3078. doi: 10.1158/1078-0432.CCR-17-2687 [DOI] [PubMed] [Google Scholar]
- 9.Turley SJ, Cremasco V, Astarita JL, et al. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat Rev Immunol. 2015;15(11):669–682. doi: 10.1038/nri3902 [DOI] [PubMed] [Google Scholar]
- 10.Liu Z, Zhu Y, Xu L, et al. Tumor stroma-infiltrating mast cells predict prognosis and adjuvant chemotherapeutic benefits in patients with muscle invasive bladder cancer. Oncoimmunology. 2018;7(9):e1474317. doi: 10.1080/2162402X.2018.1474317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H, Liu H, Shen Z, et al. Tumor-infiltrating neutrophils is prognostic and predictive for postoperative adjuvant chemotherapy benefit in patients with gastric cancer. Ann Surg. 2018;267(2):311–318. doi: 10.1097/SLA.0000000000002058 [DOI] [PubMed] [Google Scholar]
- 12.Fu Q, Xu L, Wang Y, et al. Tumor-associated macrophage-derived interleukin-23 interlinks kidney cancer glutamine addiction with immune evasion. Eur Urol. 2019;75(5):752–763. doi: 10.1016/j.eururo.2018.09.030 [DOI] [PubMed] [Google Scholar]
- 13.Sugimura K, Miyata H, Tanaka K, et al. High infiltration of tumor-associated macrophages is associated with a poor response to chemotherapy and poor prognosis of patients undergoing neoadjuvant chemotherapy for esophageal cancer. J Surg Oncol. 2015;111:752–759. [DOI] [PubMed] [Google Scholar]
- 14.Chen CL, Wang Y, Huang CY. IL-17 induces antitumor immunity by promoting beneficial neutrophil recruitment and activation in esophageal squamous cell carcinoma. Oncoimmunology. 2018;7(1):e1373234. doi: 10.1080/2162402X.2017.1373234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fakhrjou A, Niroumand-Oscoei SM, Somi MH, et al. Prognostic value of tumor-infiltrating mast cells in outcome of patients with esophagus squamous cell carcinoma. J Gastrointest Cancer. 2014;45(1):48–53. doi: 10.1007/s12029-013-9550-2 [DOI] [PubMed] [Google Scholar]
- 16.Ishigami S, Natsugoe S, Matsumoto M, et al. Clinical implications of intratumoral dendritic cell infiltration in esophageal squamous cell carcinoma. Oncol Rep. 2003;10:1237–1240. [PubMed] [Google Scholar]
- 17.Tibshirani R. The lasso method for variable selection in the Cox model. Stat Med. 1997;16(4):385–395. doi: [DOI] [PubMed] [Google Scholar]
- 18.Tibshirani R. Regression shrinkage and selection via the lasso: a retrospective. J R Stat Soc Series B Stat Methodol. 2011;73(3):273–282. doi: 10.1111/j.1467-9868.2011.00771.x [DOI] [Google Scholar]
- 19.Newman AM, Liu CL, Green MR, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12(5):453–457. doi: 10.1038/nmeth.3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102(43):15545–15550. doi: 10.1073/pnas.0506580102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ceelen W, Ramsay RG, Narasimhan V, et al. Targeting the tumor microenvironment in colorectal peritoneal metastases. Trends Cancer. 2020;6(3):236–246. doi: 10.1016/j.trecan.2019.12.008 [DOI] [PubMed] [Google Scholar]
- 22.Wu T, Dai Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017;387:61–68. [DOI] [PubMed] [Google Scholar]
- 23.Ando N, Iizuka T, Kakegawa T, et al. A randomized trial of surgery with and without chemotherapy for localized squamous carcinoma of the thoracic esophagus: the Japan Clinical Oncology Group Study. J Thorac Cardiovasc Surg. 1997;114(2):205–209. doi: 10.1016/S0022-5223(97)70146-6 [DOI] [PubMed] [Google Scholar]
- 24.Pouliquen X, Levard H, Hay JM, et al. 5-Fluorouracil and cisplatin therapy after palliative surgical resection of squamous cell carcinoma of the esophagus. A multicenter randomized trial. French Associations for Surgical Research. Ann Surg. 1996;223(2):127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shinto E, Hase K, Hashiguchi Y, et al. CD8+ and FOXP3+ tumor-infiltrating T cells before and after chemoradiotherapy for rectal cancer. Ann Surg Oncol. 2014;21(Suppl S3):S414–S421. doi: 10.1245/s10434-014-3584-y [DOI] [PubMed] [Google Scholar]
- 26.Mickaël M, Isabelle M, AbdulQader S, et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334(6062):1573–1577. [DOI] [PubMed] [Google Scholar]
- 27.Bindea G, Mlecnik B, Tosolini M, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39(4):782–795. doi: 10.1016/j.immuni.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 28.Provance OK, Lewis-Wambi J. Deciphering the role of interferon alpha signaling and microenvironment crosstalk in inflammatory breast cancer. Breast Cancer Res. 2019;21(1):59. doi: 10.1186/s13058-019-1140-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindner DJ, Kalvakolanu DV, Borden EC, et al. Increasing effectiveness of interferon-alpha for malignancies. Semin Oncol. 1997;24:S9-99–S9–104. [PubMed] [Google Scholar]
- 30.Zhang Z, Chen Y, Jiang Y, et al. Prognostic and clinicopathological significance of CXCL1 in cancers: a systematic review and meta-analysis. Cancer Biol Ther. 2019;20(11):1380–1388. doi: 10.1080/15384047.2019.1647056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qi YL, Li Y, Man XX, et al. CXCL3 overexpression promotes the tumorigenic potential of uterine cervical cancer cells via the MAPK/ERK pathway. J Cell Physiol. 2020;235(5):4756–4765. doi: 10.1002/jcp.29353 [DOI] [PubMed] [Google Scholar]