Abstract
Food allergy is often understood as an IgE-mediated hypersensitivity, characterized by allergic symptoms which occur “immediately” after the ingestion of a relevant food allergen. Increasingly, however, other food-related immune-mediated disorders are recognized in which symptoms can have a delayed onset and IgE does not play a central role. One of the described examples of the latter is eosinophilic esophagitis (EoE) – a disease defined pathologically by local eosinophilic inflammation in the esophagus in the setting of symptoms of esophageal dysfunction. The evidence that EoE is a food-mediated allergic disease includes i) almost all patients respond to an elemental diet and many respond to a diet in which dairy, wheat, eggs and/or soy are eliminated, ii) the presence of food-specific IgE and Th2 cells are consistent with a loss of tolerance to trigger foods and iii) many EoE patients have concomitant IgE-mediated food allergy and other allergic co-morbidities. This narrative review focuses on the hypothesis that EoE is a form of chronic food allergy. The goal is to describe similarities and differences in EoE and IgE-mediated food allergy, and to consider ways that these two increasingly common forms of food allergy are related to each other.
Keywords: food allergy, eosinophilic esophagitis, IgE, IgG4, Th2, Treg, barrier hypothesis
Introduction
Over the last three decades food allergy has emerged as a significant health burden, particularly in children.1,2 Food allergy is often understood as an IgE-mediated hypersensitivity that is characterized by a constellation of symptoms, such as hives, itch, swelling, wheeze and/or anaphylaxis, which occur rapidly after the ingestion of a relevant food allergen. However, other forms of food-related immune-mediated disorders in which symptoms do not occur “immediately” after food ingestion and IgE does not play a central role are increasingly recognized.3,4 One of the best described examples is eosinophilic esophagitis (EoE) – a disease defined by local eosinophilic inflammation in the esophagus in the setting of symptoms of esophageal dysfunction.5,6 Dysphagia and food impaction are classic symptoms associated with EoE, but symptoms can vary by age and sex.7 Nonetheless, the symptoms are not perceived by patients or providers as being “allergic” in nature. A variety of foods have been causally linked with EoE but cow’s milk and wheat are most consistently identified as the major food triggers.8–10 The goal of this narrative review is to consider the evidence that EoE represents a form of chronic food allergy and to describe parallels and distinctions between EoE and IgE-mediated food allergy. For details regarding diagnosis and management of EoE and in-depth discussion geared toward IgE-mediated food allergy (herein, referred to as FA) the reader is directed to a number of recent reviews.1,6,11–16
Overview of EoE
The first report of EoE can be dated to 1977, but it was not recognized as a distinct entity until the 1990s.17–19 Since that time, the reported prevalence of EoE has increased markedly.20 In North America, Europe and Australia, the prevalence is now estimated at ~50 per 100,000, though there can be significant geographic variability.20–22 EoE has consistently been identified more frequently in males than females, with most studies indicating a male to female ratio ~2:1.21–23 The basis for this sex difference is not completely understood, but similar observations have been made in children with other allergic diseases.24 Heritability studies suggest a role for genetics in EoE, but on the other hand the rapid rise in prevalence indicates environmental effects must also be important.20,25 The data in this area are limited but C-section delivery, early life use of antibiotics and acid-suppressive medications have been consistently associated with pediatric EoE.26–29
EoE classically presents with dysphagia or food impactions in older children and adults, though other manifestations can occur. Feeding dysfunction, failure to thrive, abdominal pain, nausea, and vomiting are manifestations that are particularly pronounced in young children.30 In addition to symptoms of esophageal dysfunction, the diagnosis of EoE requires pathologic identification of ≥15 eosinophils per high power field on esophageal biopsy obtained via upper endoscopy.5 EoE is generally considered to be a chronic disease and, if left untreated, can result in esophageal remodeling.20 Sequelae of this remodeling can include diminished esophageal distensibility and the development of strictures. The mainstays of treatment are proton pump inhibitors (PPIs), preparations of swallowed steroids, and/or avoidance of select trigger foods. The majority of patients will respond to one or all of these approaches.6
While the pathophysiology of EoE is not completely understood, Th2-related cytokines (eg, IL-5 and IL-13), antibodies (ie, IgE and IgG4) and cells (eg, mast cells, Th2 cells, eosinophils) are consistently identified in the inflammatory milieu of the esophageal mucosa and submucosa in patients with EoE.31 Thus, it is not surprising that EoE is highly associated with atopic diseases, including asthma, allergic rhinitis, IgE-mediated food allergy and atopic dermatitis.9 Because of this link, it has recently been proposed that EoE is a late manifestation of the “allergic march”.32 A number of studies have evaluated whether conventional allergy tests, such as skin prick testing, patch testing or serum food-specific IgE, can be used to predict trigger foods.33 Unfortunately, these studies have had mixed results, and recent guidelines from the AGA and joint task force give only a conditional recommendation for allergy testing for EoE.6 The development of diagnostic tests that can identify trigger foods is thus a critical knowledge gap in the field and an active area of study.
The Evidence for EoE as a Form of Chronic Food Allergy
According to an International Consensus statement published in 2012, food allergy is defined as “an adverse health effect arising from a specific immune response that occurs reproducibly on exposure to a given food”.34 On this basis, there is strong rationale to consider EoE as a form of food allergy (see Table 1). First, >90% of patients respond to an elemental amino acid-based diet and a large majority will respond to avoidance of one or more foods, most commonly dairy, wheat, egg or soy.35–39 Importantly, re-introduction of identified trigger foods leads to disease recurrence. The fact that a targeted avoidance diet is sufficient to induce remission, and that the process is reversible, is persuasive that food antigens are an important driver of EoE in many patients. Moreover, there is ample evidence of an exaggerated food-specific adaptive immune response in EoE, including both antibodies and T cells. Food-specific IgE, which is a sine qua non in FA, is commonly detected in EoE.40 Despite the fact that the levels of these IgE antibodies are often low, that IgE has not been mechanistically linked with EoE pathogenesis, and that food-specific IgE testing has a limited ability to identify food triggers in EoE, the presence of IgE indicates the loss of food-specific immune tolerance.8,40–43 The loss of tolerance is also supported by increased levels of food-specific IgG4 antibodies and increased food-specific Th2 cells.41,44
Table 1.
Evidence That EoE is a Form of Chronic Food Allergy
| Form of Evidence | Finding |
|---|---|
| Epidemiologic | EoE is highly associated with allergic diseases, including IgE-mediated food allergies |
| EoE is reported in ~3% of individuals with a history of FA undergoing oral immunotherapy; Symptoms and esophageal inflammation normalize upon discontinuing immunotherapy | |
| EoE has been reported in subjects with a history of FA who achieve natural “tolerance” and consistently introduce the culprit food into their diet; Removal of the offending food results in remission of EoE | |
| Immune specificity | Inflammatory milieu in esophagus is enriched for cytokines, antibodies and cells associated with allergic (ie – Th2-related) immunity |
| Presence of antibodies (IgE and IgG4) and T cells which recognize food allergens that are causally-related with EoE | |
| Diet specificity | Avoidance of specific foods can lead to disease remission |
| Reversibility | Re-introduction of specific foods leads to recurrence of disease |
The fact that both FA and EoE have increased in parallel as major health problems in industrialized countries, and are associated with each other, also suggest shared underpinnings.1,20 Hill et al reported that in a cohort of children with FA, 4.7% had EoE, which is ~100-fold higher than population-based estimates.45 Conversely, 68% of children with EoE had self or parental reported FA. In a similar vein, an investigation that relied on physician diagnosis of FA found that 29% of children with EoE had a history of FA.46 Barbosa et al conducted endoscopies on subjects with a history of anaphylaxis to cow’s milk. Strikingly, 34% of the patients, many of whom did not report chronic GI or esophageal symptoms, had evidence of esophageal eosinophilia (>15 Eos/hpf).47 Among adults with peanut allergy, a recent report identified 14% with subclinical esophageal eosinophilia.48
Connections between FA and EoE can also be seen when examining the natural history and treatment of FA. Maggadottir et al described 17 pediatric EoE cases in which an established food trigger for EoE was the same food that had historically caused immediate FA but had been “successfully” introduced into the diet.49 EoE also appears to be over-represented among FA patients undergoing oral or sublingual immunotherapy.50 A systematic review indicated that ~3% of subjects treated with oral immunotherapy developed EoE, though some estimates have been higher.50,51 It is also possible that rates of sub-clinical esophageal eosinophilia would be higher, which is supported by a recent report from Wright et al that looked at adults receiving oral immunotherapy to peanut.52 Among 7 adults treated with oral immunotherapy, two had esophageal eosinophilia (>15 Eos/hpf) at baseline (29%) which increased to four at 52 weeks (57%). Interestingly, in two of these cases, the eosinophil count normalized at 104 weeks despite continuing with treatment.
Similarities and Differences Between EoE and IgE-Mediated Food Allergy
EoE and FA have a number of shared pathophysiologic features, but also important differences. Here we consider several of these features (see Table 2).
Table 2.
Summary of Characteristics in EoE and IgE-Mediated Food Allergy
| Characteristics | IgE-Mediated Food Allergy | Eosinophilic Esophagitis |
|---|---|---|
| Sensitization site | Good evidence for Skin; airway or gut may contribute |
Uncertain |
| Effector site | Gut, skin, cardiovascular and respiratory systems | Esophagus |
| Major allergens in adult patients |
Peanut = tree nuts > shellfish >> cow’s milk | Cow’s milk > wheat = egg > soy |
| Relevant genes | Filaggrin, HLA, IL-13, TSLP, STAT6 | TSLP, Calpain 14, LRRC32, EMSY, STAT6 |
| Environmental Factors | Early acid suppression medications and delayed exposure to allergenic foods | C-section, early antibiotics and acid suppression medications |
| Specific Antibodies | IgE high, IgG4 low | IgE low, IgG4 high |
| T cells | Th2 and Treg | Th2 and Treg |
| Effector Cells | Mast cells, basophils? | Eosinophils, mast cells |
Tissue-Specific vs Systemic
FA results from activation of mast cells, a cell population present in many tissues and organs throughout the body. As a consequence, allergic reactions related to FA often involve multiple organ systems. By contrast, EoE is best understood as a localized allergic disease. Although this framework has caveats, it is at least useful for thinking about where the respective disease processes are initiated and where the dominant pathogenic effects occur. Recent evidence points to the skin as a major site of IgE induction in FA, though the gastrointestinal tract may also be important.15,53 IgE, which is produced predominantly by plasma cells or plasmablasts, transits via the blood to become bound to mast cells (via FcεR1 engagement) in a variety of mucosal and peri-vascular tissues.54–56 Subsequent allergen exposure can lead to systemic manifestations via IgE-dependent degranulation of these mast cells in the skin, gut, airway and/or cardiovascular system. The details of how and where sensitization occurs in EoE is very much an open question. Mouse studies have suggested that sensitization can occur via the skin, airway or gut.57 The question of whether the esophagus itself could play an important role is not clear; however, the dilated intercellular spaces that are consistently observed in the esophageal epithelium of EoE patients suggests an impaired barrier that could be permissive for sensitization.52,58 While some EoE-related markers, such as food-specific antibodies and T cells, can be identified in peripheral blood, these are likely produced primarily or exclusively in the esophagus.59–61 Thus, allergic inflammation in EoE is likely a consequence of immune cells localized to the esophagus being consistently exposed to relevant food antigens. Left untreated (eg, with swallowed steroids), the inflammation can culminate in fibrosis and remodeling. In contrast to EoE, investigation of chronic sequelae of FA has received little attention. Most investigations into FA focus on acute outcomes, but there are reasons to consider that food-specific IgE could impact chronic inflammatory diseases.56 For example, we have reported on an association between the presence of IgE to α-Gal and atherosclerosis.62
Allergens
Relevant allergens vary by region and also age, but in the USA, Europe and Australia major food allergens in FA include peanut, tree nuts, hen’s egg, cow’s milk, fish, and shellfish.1 Of these, clinical sensitivity to hen’s egg and cow’s milk is much less in adults than children. The top four allergens in most studies of EoE have been cow’s milk, wheat, egg and soy. As such, these are foods that are included in the so-called “four-food” elimination diet.11 Some patients benefit from additional avoidance of nuts (peanuts and tree nuts) and fish (shellfish and fin fish), which collectively constitute the “six-food” elimination diet.63 Interestingly, nuts and fish were included in the original six-food elimination largely because of their connection with IgE-mediated food allergy rather than an a priori strong association with EoE.64,65 One of the reasons that foods such as shellfish, fin fish and tree nuts may be less relevant to EoE is that these are usually consumed less frequently and/or in smaller quantities than the top four foods. Supporting this idea, foods such as chicken and beef, which are major foods in the Western diet, appear to be more relevant to EoE than FA (with the exception of areas where tick-acquired “red meat” allergy, ie, α-Gal syndrome, is common).66,67
Genes
A recent systematic review described genetic determinants of pediatric FA.68 The strongest evidence of an association was for genes that encode filaggrin, HLA and IL-13, however SPINK5, SERPINB, STAT6 and LRRC32 also had some evidence for a linkage. Notably, many of these genes have also emerged as risk factors for atopic dermatitis (AD), which is consistent with the mechanistic and epidemiologic association between AD and FA.69 In EoE, associations with the genes encoding for calpain 14 and thymic stromal lymphopoietin (TSLP) have emerged, but also STAT6, LRRC32.31 One study investigated specific genes in FA that had previously been associated with EoE. They showed that TSLP and STAT6, but not CAPN14 (which encodes for calpain 14), were associated with FA.70 This finding makes sense in that calpain 14 is an esophageal-specific proteolytic enzyme, whereas TSLP and STAT6 play a role in promoting induction of Th2 cells.31 Although filaggrin and IL-13 mutations have not been strongly linked with EoE in candidate-gene or genome-wide association studies, it is notable that filaggrin is transcriptionally down-regulated and IL-13-responsive genes are transcriptionally upregulated in EoE.31 Collectively, genetic and transcriptome studies suggest a number of conserved elements that are shared between AD, FA and EoE.
Environmental Factors
Consistent with all allergic diseases, the rapid rise in both FA and EoE suggests that environmental factors must be playing an important role in disease pathogenesis.71 Studies over the past decade have begun to identify risk factors for EoE and FA. Early life use of antibiotics and acid-suppressive medications (ie, H2 histamine receptor blockers and proton-pump inhibitors), and also C-section delivery, have been reproducibly associated with pediatric EoE.27–29 Risk factors for FA include early life use of acid-suppressive medications and delayed introduction of foods.72,73 Other dietary aspects, including recent changes in farming practices and food processing, have only begun to be explored. A small study of patients with EoE demonstrated that diet had an impact on esophageal inflammation and permeability.74 Omega-6 fatty acids, total fat and phosphorus had an unfavorable effect, whereas dietary fiber, iron, dairy, pasta/rice and soy were favorable. A recent investigation of Old Order Mennonites in Upstate New York who practiced single-family farming and lived a traditional lifestyle found that FA rates were lower in this study population than age-similar subjects in the National Health and Nutrition Examination Survey (NHANES).75 This “farm effect” is consistent with studies evaluating risk factors for childhood asthma, but has yet to be investigated in EoE.76 On a related note, some studies have shown that EoE is more common in rural areas, though others have found the opposing result.21,29
Antibodies
IgE antibodies which are specific for food allergens are fundamental to FA pathophysiology.1,15 Often, the relevant IgE which is responsible for allergic reactions can be detected at high or very high levels.77 IgE to food antigens which are disease-relevant are also commonly detected in EoE. However, in contrast to the IgE levels observed in FA, the levels in EoE are usually quite low, often class 2 or less (ie, <3.5 IU/mL). It is perhaps little surprise then that IgE is not central to EoE pathogenesis and measurement of food-specific IgE does not have strong performance characteristics for identifying relevant food triggers.8,41
Another antibody isotype which is often linked with Th2-related immunity is IgG4. Commonly associated with tolerance to allergens (including food allergens), IgG4 has a number of distinct features. IgG4 does not fix complement, has low affinity for activating FcγR receptors, and can be functionally monovalent because of Fab arm-exchange.78,79 Several reports have convincingly shown that IgG4 is abundant in EoE tissue and also that IgG4 specific for causally relevant foods is elevated in the serum and tissue of patients with EoE.41,42,59,61,80,81 Whether food-specific IgG4 is important in the pathogenesis of EoE is unclear.79 Although contrary to its understood role in FA, the high levels of allergen food-specific IgG4 which accumulate locally could nonetheless contribute to the inflammatory lesion in EoE.82 The relative IgE to IgG4 ratio could also explain why some patients with food-specific IgE mount a rapid allergic reaction to that food while others do not.83
T Cells
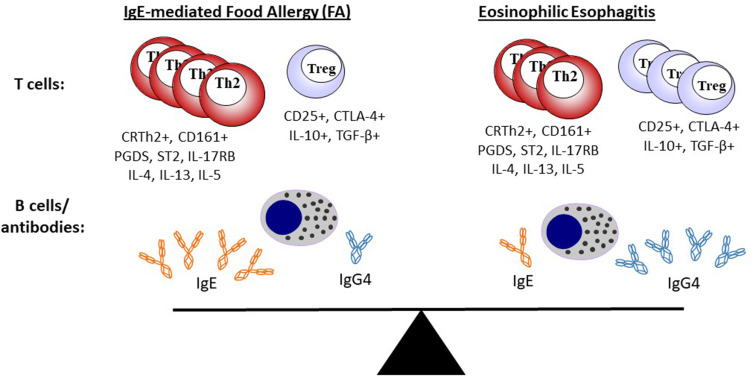
The role of T cells is almost certainly less well understood, albeit no less important, than the role of antibodies and B cells in the development of allergic disease. Historically, the study of T cells has been challenging, but a number of technical advances including tetramer technology, polychromatic flow cytometry and single-cell RNA sequencing have begun to shed important insights into T cells in both EoE and FA. Th2 cells, as articulated in early ideas about the Th1/Th2 paradigm, are still understood to be important players in allergic disease, including food allergy, but our understanding of T cells is now much more nuanced.84 Recent studies make clear that there are specific subsets of Th2 which confer pathogenic activity, but also that T follicular helper cells (Tfh),85,86 regulatory T cells (Tregs)87 and other T cell populations88,89 may be important in allergic disease. Wambre et al recently described features of a circulating allergen-specific Th2 cell subset, coined as Th2A (“Th2 allergic”), that was highly associated with peanut allergy and other IgE-mediated allergic conditions.90 Features of the Th2A cell included high levels of CRTh2 (prostaglandin D2 receptor) and CD161 expression, but low or undetectable levels of CD27 and CD45RB. This population expressed canonical-Th2-related cytokines (ie, IL-4, IL-5 and IL-13) and was markedly reduced in peanut-allergic patients who underwent oral immunotherapy. In an investigation of EoE, Cianferoni et al identified a population of T cells in EoE patients that elaborated type-2 cytokines upon stimulation with cow’s milk. However, detailed characterization of the T cell phenotype was not carried out.44 Prussin et al described a population of food-specific “pathogenic effector Th2” (peTh2) cells in patients with eosinophilic gastrointestinal disease.91 Interestingly, these IL-5 secreting peTh2 cells shared many features with the Th2A cells described by Wambre and colleagues. Wen et al recently used FACS and RNA-seq to characterize lymphocytes present in esophageal tissue of patients with EoE. They found a highly enriched population of Th2 cells (compared to relevant controls) that also had many shared features with peTh2 and Th2A.92 Genes encoding CRTh2, CD161 and IL-5 were all upregulated. Collectively these studies suggest that a highly activated population of allergen-specific IL-5-secreting Th2 cells may be an important feature that underlies many allergic diseases, including both EoE and FA (Figure 1).93
Figure 1.
EoE is a local inflammatory disease of the esophagus and FA is a systemic allergic disorder, but both represent a continuum of food-related allergic disease. Th2 cells and Tregs are present in different relative amounts, but share similar surface markers and cytokine-expression profiles in both EoE and FA. Food-specific IgE and IgG4 are common in both EoE and FA, but the relative ratio of IgE is higher in FA. Changes that occur with diet or treatment (eg, oral immunotherapy) will influence the adaptive immune response and may change the dynamic equilibrium between EoE and FA.
Notes: Adapted from Annals of Allergy, Asthma & Immunology, Vol 22/edition 6, McGowan EC, Platts-Mills TAE, Wilson JM. Food allergy, eosinophilic esophagitis, and the enigma of IgG4, pages 563–564, Copright 2019, with permission from Elsevier.82
Tregs are a population of CD4+ T cells that express anti-inflammatory mediators such as IL-10 and TGF-β and which are important for maintaining tolerance to exogenous and endogenous antigens. A protective role for CD4+CD25+ Tregs in food allergy is exemplified by studies of milk allergic children in which those who developed natural tolerance were found to have increased frequency of this cell type.87 Interestingly, multiple groups have shown that Tregs are abundant in the esophageal tissue of EoE patients.92,94,95 On its face, the increased number of Tregs in the inflammatory milieu of the esophagus in EoE is counter-intuitive.82 The Tregs could reflect an inadequate regulatory response, but it is also possible that these Tregs could acquire “Th2-like” functions, such as IL-4 and/or IL-13 production, which contribute to EoE pathogenesis. The latter idea has been reported in studies of FA in humans and mice and also in a cohort of EoE patients with Loey-Dietz syndrome.87,92,96,97 In their recent study, Wen et al also found that Foxp3+ Treg-like cells that were present in esophageal tissue of EoE patients co-expressed GATA-3.92 These dual-expressing cells were not found in healthy controls, leading the authors to suggest that these Treg-like cells could have acquired Th2 effector activity.
The Rise of EoE and FA in the Modern Era: Relevance of the Exposome, Microbiome and Barrier Dysfunction
While the history and origins of food allergy may be fodder for debate, there is good evidence that the incidence of immune-mediated reactions to food, including EoE and FA, have risen over recent decades.2,20,98,99 The explanation(s) for this rise represents an important question in the field and is likely to inform future efforts at management and prevention. Several hypotheses have been proposed which provide a framework for considering the general emergence of allergic disease, ideas which are also important for thinking about EoE and FA. These include the “hygiene hypothesis” and more recently the “old friends hypothesis”, “biodiversity hypothesis” and “dysregulated barrier hypothesis”.100,101 There are important distinctions between these proposals, but they share the idea that industrialization and technologic advances have led to myriad changes in lifestyle and the environment, now often referred to as the “exposome”, that have impacted human health.102–104 Because the skin and mucosal epithelium represent the major interface between the host and environment, it is logical to think that a dysregulated epithelial barrier could play a role in promoting allergic immunity. The hypotheses also largely share the idea that modernization has led to profound shifts in our relationship to the microbial world. The hygiene hypothesis emphasizes a role for decreased early childhood viral and bacterial infections, whereas the old friends and biodiversity hypotheses describe a role for perturbations in our commensal microbiome. The role of the microbiome in the esophagus is not well understood, but bacteria have been shown to exert regulatory effects on the intestine.101,105 Thus, it is possible that shifts in the microbiome could contribute to dysregulation of the esophageal epithelium. As previously mentioned, and in support of this hypothesis, early antibiotic use and also C-section delivery represent risk factors for EoE.
When thinking about epithelial barrier dysfunction, it is also important to consider a host of other exposures which have been relatively newly introduced as part of the Western lifestyle, eg, detergents, chemicals, pesticides, diesel particulates and other forms of air pollution.106 In many cases, the food we eat has been markedly transformed from the parent food which was consumed by our ancestors over preceding millennia. This can include extensive processing of the food itself (eg, homogenization and ultra-heat pasteurization of cow’s milk) but also changes in breeding and cultivation practices. One or a combination of these factors, especially in an individual with genetic susceptibility, could be sufficient to lead to EoE and/or FA. Whether EoE or FA emerges as the dominant allergic manifestation may relate to which epithelial barrier is dysregulated (ie, skin, airway, gut or esophagus) or the specific genetic susceptibility of the host (eg, Calpain 14 versus filaggrin).
Conclusion
There is considerable evidence that EoE, at least in most cases, is a form of chronic food allergy. Unlike FA, which is mediated by food-specific IgE bound to mast cells, IgE does not play a major role in EoE pathogenesis. Nonetheless, cells and antibodies which are part of Th2-related immunity are present in both EoE and FA. Both diseases share a number of genetic and environmental linkages, with EoE being favored in situations where impairment of the epithelial barrier is localized to the esophagus. EoE and FA represent two forms of food allergy that can be in dynamic equilibrium with each other, presenting challenges but also opportunities in management (Figure 1). An interesting possibility is that novel therapies which target mediators of Th2-related immunity may be able to simultaneously address both EoE and FA.
Disclosure
Dr Jeffrey M Wilson reports personal fees, non-financial support from Thermo Fisher/Phadia, outside the submitted work. Dr Emily C McGowan reports consulting fees from Shire, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Sicherer SH, Sampson HA. Food allergy: a review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immunol. 2018;141(1):41–58. doi: 10.1016/j.jaci.2017.11.003 [DOI] [PubMed] [Google Scholar]
- 2.Sampson HA. Food allergy: past, present and future. Allergol Int. 2016;65(4):363–369. doi: 10.1016/j.alit.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 3.Cianferoni A. Non-IgE mediated food allergy. Curr Pediatr Rev. 2019. [DOI] [PubMed] [Google Scholar]
- 4.Nowak-Wegrzyn A, Katz Y, Mehr SS, Koletzko S. Non-IgE-mediated gastrointestinal food allergy. J Allergy Clin Immunol. 2015;135(5):1114–1124. doi: 10.1016/j.jaci.2015.03.025 [DOI] [PubMed] [Google Scholar]
- 5.Furuta GT, Katzka DA, Ingelfinger JR. Eosinophilic esophagitis. N Engl J Med. 2015;373(17):1640–1648. doi: 10.1056/NEJMra1502863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirano I, Chan ES, Rank MA, et al. AGA institute and the joint task force on allergy-immunology practice parameters clinical guidelines for the management of eosinophilic esophagitis. Ann Allergy Asthma Immunol. 2020;124(5):416–423. doi: 10.1016/j.anai.2020.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miehlke S. Clinical features of Eosinophilic esophagitis in children and adults. Best Pract Res Clin Gastroenterol. 2015;29(5):739–748. doi: 10.1016/j.bpg.2015.09.005 [DOI] [PubMed] [Google Scholar]
- 8.Spergel J, Aceves SS. Allergic components of eosinophilic esophagitis. J Allergy Clin Immunol. 2018;142(1):1–8. doi: 10.1016/j.jaci.2018.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spergel JM, Brown-Whitehorn TF, Cianferoni A, et al. Identification of causative foods in children with eosinophilic esophagitis treated with an elimination diet. J Allergy Clin Immunol. 2012;130(2):461–467e465. doi: 10.1016/j.jaci.2012.05.021 [DOI] [PubMed] [Google Scholar]
- 10.Kagalwalla AF, Shah A, Li BU, et al. Identification of specific foods responsible for inflammation in children with eosinophilic esophagitis successfully treated with empiric elimination diet. J Pediatr Gastroenterol Nutr. 2011;53(2):145–149. doi: 10.1097/MPG.0b013e31821cf503 [DOI] [PubMed] [Google Scholar]
- 11.Wilson JM, McGowan EC. Diagnosis and management of eosinophilic esophagitis. Immunol Allergy Clin North Am. 2018;38(1):125–139. doi: 10.1016/j.iac.2017.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirano I. How i approach the management of eosinophilic esophagitis in adults. Am J Gastroenterol. 2017;112(2):197–199. doi: 10.1038/ajg.2016.515 [DOI] [PubMed] [Google Scholar]
- 13.Lucendo AJ, Molina-Infante J, Arias A, et al. Guidelines on eosinophilic esophagitis: evidence-based statements and recommendations for diagnosis and management in children and adults. United Eur Gastroenterol J. 2017;5(3):335–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Renz H, Allen KJ, Sicherer SH, et al. Food allergy. Nat Rev Dis Primers. 2018;4:17098. [DOI] [PubMed] [Google Scholar]
- 15.Tordesillas L, Berin MC, Sampson HA. Immunology of food allergy. Immunity. 2017;47(1):32–50. doi: 10.1016/j.immuni.2017.07.004 [DOI] [PubMed] [Google Scholar]
- 16.Eiwegger T, Hung L, San Diego KE, O’Mahony L, Upton J. Recent developments and highlights in food allergy. Allergy. 2019;74(12):2355–2367. doi: 10.1111/all.14082 [DOI] [PubMed] [Google Scholar]
- 17.Dobbins JW, Sheahan DG, Behar J. Eosinophilic gastroenteritis with esophageal involvement. Gastroenterology. 1977;72(6):1312–1316. doi: 10.1016/S0016-5085(77)80034-6 [DOI] [PubMed] [Google Scholar]
- 18.Attwood SE, Smyrk TC, Demeester TR, Jones JB. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci. 1993;38(1):109–116. [DOI] [PubMed] [Google Scholar]
- 19.Straumann A, Spichtin HP, Bernoulli R, Loosli J, Vogtlin J. Idiopathic eosinophilic esophagitis: a frequently overlooked disease with typical clinical aspects and discrete endoscopic findings. Schweiz Med Wochenschr. 1994;124(33):1419–1429. [PubMed] [Google Scholar]
- 20.Dellon ES, Hirano I. Epidemiology and natural history of eosinophilic esophagitis. Gastroenterology. 2018;154(2):319–332e313. doi: 10.1053/j.gastro.2017.06.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGowan EC, Keller JP, Dellon ES, Peng R, Keet CA. Prevalence and geographic distribution of pediatric eosinophilic esophagitis in the 2012 US medicaid population. J Allergy Clin Immunol Pract. 2020;8(8):2796–2798.e4. doi: 10.1016/j.jaip.2020.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robson J, O’Gorman M, McClain A, et al. Incidence and prevalence of pediatric eosinophilic esophagitis in utah based on a 5-year population-based study. Clin Gastroenterol Hepatol. 2019;17(1):107–114e101. doi: 10.1016/j.cgh.2018.06.028 [DOI] [PubMed] [Google Scholar]
- 23.Mansoor E, Cooper GS. The 2010–2015 prevalence of eosinophilic esophagitis in the USA: a population-based study. Dig Dis Sci. 2016;61(10):2928–2934. doi: 10.1007/s10620-016-4204-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nowak-Wegrzyn A, Ellis A, Castells M. Sex and allergic diseases. Ann Allergy Asthma Immunol. 2019;122(2):134–135. doi: 10.1016/j.anai.2018.12.010 [DOI] [PubMed] [Google Scholar]
- 25.Kottyan LC, Rothenberg ME. Genetics of eosinophilic esophagitis. Mucosal Immunol. 2017;10(3):580–588. doi: 10.1038/mi.2017.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radano MC, Yuan Q, Katz A, et al. Cesarean section and antibiotic use found to be associated with eosinophilic esophagitis. J Allergy Clin Immunol Pract. 2014;2(4):475–477e471. doi: 10.1016/j.jaip.2014.02.018 [DOI] [PubMed] [Google Scholar]
- 27.Jensen ET, Kappelman MD, Kim HP, Ringel-Kulka T, Dellon ES. Early life exposures as risk factors for pediatric eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2013;57(1):67–71. doi: 10.1097/MPG.0b013e318290d15a [DOI] [PubMed] [Google Scholar]
- 28.Witmer CP, Susi A, Min SB, Nylund CM. Early infant risk factors for pediatric eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2018;67(5):610–615. doi: 10.1097/MPG.0000000000002123 [DOI] [PubMed] [Google Scholar]
- 29.Jensen ET, Dellon ES. Environmental factors and eosinophilic esophagitis. J Allergy Clin Immunol. 2018;142(1):32–40. doi: 10.1016/j.jaci.2018.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sorser SA, Barawi M, Hagglund K, Almojaned M, Lyons H. Eosinophilic esophagitis in children and adolescents: epidemiology, clinical presentation and seasonal variation. J Gastroenterol. 2013;48(1):81–85. doi: 10.1007/s00535-012-0608-x [DOI] [PubMed] [Google Scholar]
- 31.O’Shea KM, Aceves SS, Dellon ES, et al. Pathophysiology of eosinophilic esophagitis. Gastroenterology. 2018;154(2):333–345. doi: 10.1053/j.gastro.2017.06.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill DA, Grundmeier RW, Ramos M, Spergel JM. Eosinophilic esophagitis is a late manifestation of the allergic march. J Allergy Clin Immunol Pract. 2018;6(5):1528–1533. doi: 10.1016/j.jaip.2018.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anyane-Yeboa A, Wang W, Kavitt RT. The role of allergy testing in eosinophilic esophagitis. Gastroenterol Hepatol (N Y). 2018;14(8):463–469. [PMC free article] [PubMed] [Google Scholar]
- 34.Burks AW, Tang M, Sicherer S, et al. ICON: food allergy. J Allergy Clin Immunol. 2012;129(4):906–920. doi: 10.1016/j.jaci.2012.02.001 [DOI] [PubMed] [Google Scholar]
- 35.Kelly KJ, Lazenby AJ, Rowe PC, Yardley JH, Perman JA, Sampson HA. Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino acid-based formula. Gastroenterology. 1995;109(5):1503–1512. doi: 10.1016/0016-5085(95)90637-1 [DOI] [PubMed] [Google Scholar]
- 36.Peterson KA, Byrne KR, Vinson LA, et al. Elemental diet induces histologic response in adult eosinophilic esophagitis. Am J Gastroenterol. 2013;108(5):759–766. doi: 10.1038/ajg.2012.468 [DOI] [PubMed] [Google Scholar]
- 37.Arias A, Gonzalez-Cervera J, Tenias JM, Lucendo AJ. Efficacy of dietary interventions for inducing histologic remission in patients with eosinophilic esophagitis: a systematic review and meta-analysis. Gastroenterology. 2014;146(7):1639–1648. doi: 10.1053/j.gastro.2014.02.006 [DOI] [PubMed] [Google Scholar]
- 38.Molina-Infante J, Gonzalez-Cordero PL, Arias A, Lucendo AJ. Update on dietary therapy for eosinophilic esophagitis in children and adults. Expert Rev Gastroenterol Hepatol. 2017;11(2):115–123. doi: 10.1080/17474124.2017.1271324 [DOI] [PubMed] [Google Scholar]
- 39.Warners MJ, Vlieg-Boerstra BJ, Verheij J, et al. Elemental diet decreases inflammation and improves symptoms in adult eosinophilic oesophagitis patients. Aliment Pharmacol Ther. 2017;45(6):777–787. doi: 10.1111/apt.13953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Erwin EA, Tripathi A, Ogbogu PU, et al. IgE antibody detection and component analysis in patients with eosinophilic esophagitis. J Allergy Clin Immunol Pract. 2015;3(6):896–904e893. doi: 10.1016/j.jaip.2015.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clayton F, Fang JC, Gleich GJ, et al. Eosinophilic esophagitis in adults is associated with IgG4 and not mediated by IgE. Gastroenterology. 2014;147(3):602–609. doi: 10.1053/j.gastro.2014.05.036 [DOI] [PubMed] [Google Scholar]
- 42.Schuyler AJ, Wilson JM, Tripathi A, et al. Specific IgG4 antibodies to cow’s milk proteins in pediatric patients with eosinophilic esophagitis. J Allergy Clin Immunol. 2018;142(1):139–148e112. doi: 10.1016/j.jaci.2018.02.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chehade M, Aceves SS. Food allergy and eosinophilic esophagitis. Curr Opin Allergy Clin Immunol. 2010;10(3):231–237. doi: 10.1097/ACI.0b013e328338cbab [DOI] [PubMed] [Google Scholar]
- 44.Cianferoni A, Ruffner MA, Guzek R, et al. Elevated expression of activated TH2 cells and milk-specific TH2 cells in milk-induced eosinophilic esophagitis. Ann Allergy Asthma Immunol. 2018;120(2):177–183e172. doi: 10.1016/j.anai.2017.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hill DA, Dudley JW, Spergel JM. The prevalence of eosinophilic esophagitis in pediatric patients with IgE-mediated food allergy. J Allergy Clin Immunol Pract. 2017;5(2):369–375. doi: 10.1016/j.jaip.2016.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pelz BJ, Wechsler JB, Amsden K, et al. IgE-associated food allergy alters the presentation of paediatric eosinophilic esophagitis. Clin Exp Allergy. 2016;46(11):1431–1440. doi: 10.1111/cea.12776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barbosa AC, Castro FM, Meireles PR, et al. Eosinophilic esophagitis: latent disease in patients with anaphylactic reaction to cow’s milk. J Allergy Clin Immunol Pract. 2017. [DOI] [PubMed] [Google Scholar]
- 48.Wright BL, Fernandez-Becker NQ, Kambham N, et al. Baseline gastrointestinal eosinophilia is common in oral immunotherapy subjects with IgE-mediated peanut allergy. Front Immunol. 2018;9:2624. doi: 10.3389/fimmu.2018.02624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maggadottir SM, Hill DA, Ruymann K, et al. Resolution of acute IgE-mediated allergy with development of eosinophilic esophagitis triggered by the same food. J Allergy Clin Immunol. 2014;133(5):1487–1489, 1489 e1481. doi: 10.1016/j.jaci.2014.02.004 [DOI] [PubMed] [Google Scholar]
- 50.Cafone J, Capucilli P, Hill DA, Spergel JM. Eosinophilic esophagitis during sublingual and oral allergen immunotherapy. Curr Opin Allergy Clin Immunol. 2019;19(4):350–357. doi: 10.1097/ACI.0000000000000537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lucendo AJ, Arias A, Tenias JM. Relation between eosinophilic esophagitis and oral immunotherapy for food allergy: a systematic review with meta-analysis. Ann Allergy Asthma Immunol. 2014;113(6):624–629. doi: 10.1016/j.anai.2014.08.004 [DOI] [PubMed] [Google Scholar]
- 52.Wright BL, Fernandez-Becker NQ, Kambham N, et al. Gastrointestinal eosinophil responses in a longitudinal, randomized trial of peanut oral immunotherapy. Clin Gastroenterol Hepatol. 2020. doi: 10.1016/j.cgh.2020.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoh RA, Joshi SA, Lee JY, et al. Origins and clonal convergence of gastrointestinal IgE+B cells in human peanut allergy. Sci Immunol. 2020;5(45):45. doi: 10.1126/sciimmunol.aay4209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tordesillas L, Goswami R, Benede S, et al. Skin exposure promotes a Th2-dependent sensitization to peanut allergens. J Clin Invest. 2014;124(11):4965–4975. doi: 10.1172/JCI75660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walker MT, Green JE, Ferrie RP, Queener AM, Kaplan MH, Cook-Mills JM. Mechanism for initiation of food allergy: dependence on skin barrier mutations and environmental allergen costimulation. J Allergy Clin Immunol. 2018;141(5):1711–1725e1719. doi: 10.1016/j.jaci.2018.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Galli SJ, Gaudenzio N, Tsai M. Mast cells in inflammation and disease: recent progress and ongoing concerns. Annu Rev Immunol. 2020;38(1):49–77. doi: 10.1146/annurev-immunol-071719-094903 [DOI] [PubMed] [Google Scholar]
- 57.Wechsler JB, Bryce PJ. Allergic mechanisms in eosinophilic esophagitis. Gastroenterol Clin North Am. 2014;43(2):281–296. doi: 10.1016/j.gtc.2014.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ravelli A, Villanacci V, Cadei M, Fuoti M, Gennati G, Salemme M. Dilated intercellular spaces in eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2014;59(5):589–593. doi: 10.1097/MPG.0000000000000491 [DOI] [PubMed] [Google Scholar]
- 59.Wright BL, Kulis M, Guo R, et al. Food-specific IgG4 is associated with eosinophilic esophagitis. J Allergy Clin Immunol. 2016;138(4):1190–1192e1193. doi: 10.1016/j.jaci.2016.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vicario M, Blanchard C, Stringer KF, et al. Local B cells and IgE production in the oesophageal mucosa in eosinophilic oesophagitis. Gut. 2010;59(1):12–20. doi: 10.1136/gut.2009.178020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rosenberg CE, Mingler MK, Caldwell JM, et al. Esophageal IgG4 levels correlate with histopathologic and transcriptomic features in eosinophilic esophagitis. Allergy. 2018;73(9):1892–1901. doi: 10.1111/all.13486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilson JM, McNamara CA, Platts-Mills TAE. IgE, alpha-Gal and atherosclerosis. Aging (Albany NY). 2019;11(7):1900–1902. doi: 10.18632/aging.101894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Molina-Infante J, Arias A, Alcedo J, et al. Step-up empiric elimination diet for pediatric and adult eosinophilic esophagitis: the 2-4-6 study. J Allergy Clin Immunol. 2018;141(4):1365–1372. doi: 10.1016/j.jaci.2017.08.038 [DOI] [PubMed] [Google Scholar]
- 64.Spergel JM, Beausoleil JL, Mascarenhas M, Liacouras CA. The use of skin prick tests and patch tests to identify causative foods in eosinophilic esophagitis. J Allergy Clin Immunol. 2002;109(2):363–368. doi: 10.1067/mai.2002.121458 [DOI] [PubMed] [Google Scholar]
- 65.Kagalwalla AF, Sentongo TA, Ritz S, et al. Effect of six-food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2006;4(9):1097–1102. doi: 10.1016/j.cgh.2006.05.026 [DOI] [PubMed] [Google Scholar]
- 66.Zhan T, Ali A, Choi JG, et al. Model to determine the optimal dietary elimination strategy for treatment of eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2018;16(11):1730–1737e1732. doi: 10.1016/j.cgh.2018.04.013 [DOI] [PubMed] [Google Scholar]
- 67.Platts-Mills TAE, Commins SP, Biedermann T, et al. On the cause and consequences of IgE to galactose-alpha-1,3-galactose: a report from the national institute of allergy and infectious diseases workshop on understanding IgE-mediated mammalian meat allergy. J Allergy Clin Immunol. 2020;145(4):1061–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suaini NHA, Wang Y, Soriano VX, et al. Genetic determinants of paediatric food allergy: a systematic review. Allergy. 2019;74(9):1631–1648. doi: 10.1111/all.13767 [DOI] [PubMed] [Google Scholar]
- 69.Tham EH, Leung DY. Mechanisms by which atopic dermatitis predisposes to food allergy and the atopic march. Allergy Asthma Immunol Res. 2019;11(1):4–15. doi: 10.4168/aair.2019.11.1.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hirota T, Nakayama T, Sato S, et al. Association study of childhood food allergy with genome-wide association studies-discovered loci of atopic dermatitis and eosinophilic esophagitis. J Allergy Clin Immunol. 2017;140(6):1713–1716. doi: 10.1016/j.jaci.2017.05.034 [DOI] [PubMed] [Google Scholar]
- 71.Platts-Mills TA. The allergy epidemics: 1870–2010. J Allergy Clin Immunol. 2015;136(1):3–13. doi: 10.1016/j.jaci.2015.03.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mitre E, Susi A, Kropp LE, Schwartz DJ, Gorman GH, Nylund CM. Association between use of acid-suppressive medications and antibiotics during infancy and allergic diseases in early childhood. JAMA Pediatr. 2018;172(6):e180315. doi: 10.1001/jamapediatrics.2018.0315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Du Toit G, Sayre PH, Roberts G, et al. Effect of avoidance on peanut allergy after early peanut consumption. N Engl J Med. 2016;374(15):1435–1443. doi: 10.1056/NEJMoa1514209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.de Kroon MLA, Warners MJ, van Ampting MTJ, et al. The relationship of habitual diet with esophageal inflammation and integrity in eosinophilic esophagitis. Allergy. 2019;74(5):1005–1009. doi: 10.1111/all.13695 [DOI] [PubMed] [Google Scholar]
- 75.Phillips JT, Stahlhut RW, Looney RJ, Jarvinen KM. Food allergy, breastfeeding, and introduction of complementary foods in the New York old order mennonite community. Ann Allergy Asthma Immunol. 2020;124(3):292–294e292. doi: 10.1016/j.anai.2019.12.019 [DOI] [PubMed] [Google Scholar]
- 76.von Mutius E, Vercelli D. Farm living: effects on childhood asthma and allergy. Nat Rev Immunol. 2010;10(12):861–868. doi: 10.1038/nri2871 [DOI] [PubMed] [Google Scholar]
- 77.Platts-Mills TA, Schuyler AJ, Erwin EA, Commins SP, Woodfolk JA. IgE in the diagnosis and treatment of allergic disease. J Allergy Clin Immunol. 2016;137(6):1662–1670. doi: 10.1016/j.jaci.2016.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aalberse RC, Stapel SO, Schuurman J, Rispens T. Immunoglobulin G4: an odd antibody. Clin Exp Allergy. 2009;39(4):469–477. doi: 10.1111/j.1365-2222.2009.03207.x [DOI] [PubMed] [Google Scholar]
- 79.Aalberse RC, Platts-Mills TA, Rispens T. The developmental history of IgE and IgG4 antibodies in relation to atopy, eosinophilic esophagitis, and the modified TH2 response. Curr Allergy Asthma Rep. 2016;16(6):45. doi: 10.1007/s11882-016-0621-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pope AE, Stanzione N, Naini BV, et al. Esophageal IgG4: clinical, endoscopic, and histologic correlations in eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2019;68(5):689–694. doi: 10.1097/MPG.0000000000002227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weidlich S, Nennstiel S, Jesinghaus M, et al. IgG4 is elevated in eosinophilic esophagitis but not in gastroesophageal reflux disease patients. J Clin Gastroenterol. 2020;54(1):43–49. doi: 10.1097/MCG.0000000000001154 [DOI] [PubMed] [Google Scholar]
- 82.McGowan EC, Platts-Mills TAE, Wilson JM. Food allergy, eosinophilic esophagitis, and the enigma of IgG4. Ann Allergy Asthma Immunol. 2019;122(6):563–564. doi: 10.1016/j.anai.2019.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wilson JM, Platts-Mills TAE. Alpha-Gal and other recent findings that have informed our understanding of anaphylaxis. Ann Allergy Asthma Immunol. 2020;124(2):135–142. doi: 10.1016/j.anai.2019.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Romagnani S. Immunologic influences on allergy and the TH1/TH2 balance. J Allergy Clin Immunol. 2004;113(3):395–400. doi: 10.1016/j.jaci.2003.11.025 [DOI] [PubMed] [Google Scholar]
- 85.Varricchi G, Harker J, Borriello F, Marone G, Durham SR, Shamji MH. T follicular helper (Tfh) cells in normal immune responses and in allergic disorders. Allergy. 2016;71(8):1086–1094. doi: 10.1111/all.12878 [DOI] [PubMed] [Google Scholar]
- 86.Gowthaman U, Chen JS, Eisenbarth SC. Regulation of IgE by T follicular helper cells. J Leukoc Biol. 2020;107(3):409–418. doi: 10.1002/JLB.3RI1219-425R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Noval Rivas M, Chatila TA. Regulatory T cells in allergic diseases. J Allergy Clin Immunol. 2016;138(3):639–652. doi: 10.1016/j.jaci.2016.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Del Moral MG, Martinez-Naves E. The role of lipids in development of allergic responses. Immune Netw. 2017;17(3):133–143. doi: 10.4110/in.2017.17.3.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Saidova A, Hershkop AM, Ponce M, Eiwegger T. Allergen-specific T cells in IgE-mediated food allergy. Arch Immunol Ther Exp (Warsz). 2018;66(3):161–170. doi: 10.1007/s00005-017-0501-7 [DOI] [PubMed] [Google Scholar]
- 90.Wambre E, Bajzik V, DeLong JH, et al. A phenotypically and functionally distinct human TH2 cell subpopulation is associated with allergic disorders. Sci Transl Med. 2017;9(401):401. doi: 10.1126/scitranslmed.aam9171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mitson-Salazar A, Yin Y, Wansley DL, et al. Hematopoietic prostaglandin D synthase defines a proeosinophilic pathogenic effector human T(H)2 cell subpopulation with enhanced function. J Allergy Clin Immunol. 2016;137(3):907–918e909. doi: 10.1016/j.jaci.2015.08.007 [DOI] [PubMed] [Google Scholar]
- 92.Wen T, Aronow BJ, Rochman Y, et al. Single-cell RNA sequencing identifies inflammatory tissue T cells in eosinophilic esophagitis. J Clin Invest. 2019;129(5):2014–2028. doi: 10.1172/JCI125917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mitson-Salazar A, Prussin C. Pathogenic effector Th2 cells in allergic eosinophilic inflammatory disease. Front Med (Lausanne). 2017;4:165. doi: 10.3389/fmed.2017.00165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tantibhaedhyangkul U, Tatevian N, Gilger MA, Major AM, Davis CM. Increased esophageal regulatory T cells and eosinophil characteristics in children with eosinophilic esophagitis and gastroesophageal reflux disease. Ann Clin Lab Sci. 2009;39(2):99–107. [PubMed] [Google Scholar]
- 95.Fuentebella J, Patel A, Nguyen T, et al. Increased number of regulatory T cells in children with eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2010;51(3):283–289. [DOI] [PubMed] [Google Scholar]
- 96.Noval Rivas M, Burton OT, Wise P, et al. Regulatory T cell reprogramming toward a Th2-cell-like lineage impairs oral tolerance and promotes food allergy. Immunity. 2015;42(3):512–523. doi: 10.1016/j.immuni.2015.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Frischmeyer-Guerrerio PA, Guerrerio AL, Oswald G, et al. TGFbeta receptor mutations impose a strong predisposition for human allergic disease. Sci Transl Med. 2013;5(195):195ra194. doi: 10.1126/scitranslmed.3006448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cohen SG. Food allergens: landmarks along a historic trail. J Allergy Clin Immunol. 2008;121(6):1521–1524, 1524 e1521. doi: 10.1016/j.jaci.2008.04.027 [DOI] [PubMed] [Google Scholar]
- 99.Wuthrich B. History of food allergy. Chem Immunol Allergy. 2014;100:109–119. [DOI] [PubMed] [Google Scholar]
- 100.Lambrecht BN, Hammad H. The immunology of the allergy epidemic and the hygiene hypothesis. Nat Immunol. 2017;18(10):1076–1083. doi: 10.1038/ni.3829 [DOI] [PubMed] [Google Scholar]
- 101.Iweala OI, Nagler CR. The microbiome and food allergy. Annu Rev Immunol. 2019;37(1):377–403. doi: 10.1146/annurev-immunol-042718-041621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wild CP. Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1847–1850. doi: 10.1158/1055-9965.EPI-05-0456 [DOI] [PubMed] [Google Scholar]
- 103.Renz H, Holt PG, Inouye M, Logan AC, Prescott SL, Sly PD. An exposome perspective: early-life events and immune development in a changing world. J Allergy Clin Immunol. 2017;140(1):24–40. doi: 10.1016/j.jaci.2017.05.015 [DOI] [PubMed] [Google Scholar]
- 104.Trumble BC, Finch CE. The exposome in human evolution: from dust to diesel. Q Rev Biol. 2019;94(4):333–394. doi: 10.1086/706768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.May M, Abrams JA. Emerging insights into the esophageal microbiome. Curr Treat Options Gastroenterol. 2018;16(1):72–85. doi: 10.1007/s11938-018-0171-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Celebi Sozener Z, Cevhertas L, Nadeau K, Akdis M, Akdis CA. Environmental factors in epithelial barrier dysfunction. J Allergy Clin Immunol. 2020;145(6):1517–1528. doi: 10.1016/j.jaci.2020.04.024 [DOI] [PubMed] [Google Scholar]