Abstract
Oral cancer is one of the most common malignancies in the world. The present study aimed to investigate the effects of dexmedetomidine on immune response in patients undergoing radical and reconstructive surgery for oral cancer. Patients were randomly divided into the dexmedetomidine and control groups. Within 15 min before anesthesia induction, dexmedetomidine was infused with a 0.5 µg·kg−1 loading dose followed by a maintenance dose of 0.4 µg·kg−1·h−1 to the end of operation in the dexmedetomidine group, whereas the same volume of saline was administered in the control group. Blood samples were obtained at five time-points: 30 min Before induction (T0), 1 h after induction (T1), end of the operation (T2) and 24 (T3) and 48 h (T4) after the operation. The T lymphocyte subsets (including CD3+, CD4+ and CD8+ cells) and CD4+/CD8+ ratio, B lymphocytes, dendritic cells and myeloid-derived suppressor cells (MDSCs) were analyzed by flow cytometry. All immunological indicators, except CD8+ cells, significantly decreased between the two groups at T1–3 compared with T0 (P<0.05). The percentages of CD3+, CD4+, dendritic cells and the CD4+/CD8+ ratios were significantly higher at T2–4 and the percentages of MDSCs were significantly lower at T2–4 in the dexmedetomidine group compared with the control group (all P<0.05). These findings suggested that dexmedetomidine can attenuate immunosuppression in patients undergoing radical and reconstructive surgery for oral cancer.
Keywords: dexmedetomidine, immunity, oral neoplasms
Introduction
Oral cancer is one of the most common tumors with the 5-year survival rate that has remained at 50% in the last few decades (1). Oral cancer is primarily caused by habits of betel quid chewing, smoking and alcohol consumption in Southeast Asia (1). Though it is typically regarded as a disease of the elderly, it has been reported that the numbers of young patients have been increasing worldwide in recent years (2). Radical resection and immediate reconstruction are the mainstays of treatment for oral cancer. However, the surgical stress response is considered to directly induce immunosuppression by activating the hypothalamus-pituitary-adrenal axis and sympathetic nervous system (3), and residual tumor cells after surgery are likely to metastasize due to decreased immunity (4). Meanwhile, some anesthetics have direct suppressive impacts on innate and adaptive immunity (5–8). Therefore, selection of suitable anesthetics is important during the perioperative period. Dexmedetomidine is an α2-adrenergic receptor agonist with analgesic, sedative, anxiolytic and anti-sympatholytic properties (9,10). Several studies have indicated that dexmedetomidine can regulate perioperative immune response in radical surgeries of breast, colon and gastric cancer (11–13). Nevertheless, the influence of dexmedetomidine on immune response in patients with oral malignant tumors during operation remains unclear.
The present study aimed to observe the level of immune cells through flow cytometry and to evaluate the effects of dexmedetomidine on immune response in patients undergoing radical operation and immediate reconstruction with forearm flap for oral cancer.
Materials and methods
Patients and grouping
The study was carried out at the School and Hospital of Stomatology of Wuhan University and conducted accordance with the Declaration of Helsinki between September 2018 and January 2019. It was approved by The Ethics Committee of School and Hospital of Stomatology, Wuhan University (Wuhan, China; approval no. IRB-2018B23) and registered in the Chinese Clinical Trial Registry (ChiCTR-1800018367). Written informed consent was provided by all participants before the trial. Patients classified as American Society of Anesthesiologist (ASA) physical status I to II (14) and aged 30–70 years were enrolled. All patients were scheduled for radical operation and immediate reconstruction with forearm flap under general anesthesia. None had a history of endocrine, immune or circulatory system diseases. Other exclusion criteria included recent or concurrent chemotherapy and a requirement for perioperative blood transfusion or perioperative treatment with immunomodulatory agents. Patients who developed severe surgical complications including infection or secondary surgery, were also excluded from study. Patients were randomly allocated to two groups using a computer-generated randomization list, the procedure of details was following: Before the patients were allocated into group, random numbers were generated by computer. Patients who were enrolled in this study were allocated in the order of random numbers, the even numbers were divided into control group and the odd numbers were divided into experimental group (15).
Methods of anesthesia
All patients were premedicated with anintramuscular injection of 0.01 mg·kg−1 atropine at 30 min before anesthesia. In the operating room, the right subclavian vein was cannulated for central venous pressure monitoring and the radial artery was cannulated for real-time blood pressure monitoring. The electrocardiography, blood oxygen saturation (SaO2), end-tidal carbon dioxide (PetCO2), and cerebral state index were continuously monitored during the operation. Within 15 min before anesthesia induction, dexmedetomidine (lot no. 180604BP; Jiangsu Hengrui Medicine Co. Ltd.) was infused with a 0.5 µg·kg−1 loading dose followed by a maintenance dose of 0.4 µg·kg−1·h−1 to the end of operation in the dexmedetomidine group. According to its clinical pharmacokinetics and pharmacodynamics (10), dexmedetomidine was actually discontinued 30 min before the surgery. But in the present study research, it was difficult to determine 30 min before the operation is completed. Therefore, in order to reduce deviation, the dexmedetomidine infusion was stopped immediately after the surgery with the opinion of making the baseline of every patient the same. The same volume of normal saline was administered to the patients in the control group. Anesthesia induction was performed by intravenous injection of propofol 1.5–2.0 mg·kg−1, sufentanil 0.4 µg·kg−1 and cisatracurium 0.2 mg·kg−1 to facilitate nasal tracheal intubation. Anesthesia maintenance included sevoflurane 2–3%, remifentanil 0.2–0.3 µg·kg−1·min−1 and intermittent injection of cisatracurium 0.1 mg·kg−1. Mechanical ventilation was performed to maintain the PetCO2 at 35–40 mmHg and SaO2>98%. Blood pressure and heart rate were fluctuated within ±120% of baseline values. The depth of anesthesia was monitored to maintain the cerebral state index between 40 and 60. Sufentanil 0.1 µg·kg−1 was administered as a loading dose for postoperative analgesia in each patient 30 min before the end of surgery, and patients received the same intravenous analgesia for postoperative pain therapy.
Peripheral blood mononuclear cell separation
Saphenous vein blood (2 ml) was obtained at five time-points: 30 min before induction (T0), 1h after induction (T1), end of the operation (T2) and 24 (T3) and 48 h (T4) after the operation. Peripheral blood mononuclear cells (PBMC) were isolated by centrifugation at 800 × g for 20 min at room temperature using Lymphoprep™ (Stemcell Technologies, Inc.).
Flow cytometry
The PBMCs were stained by the following antibodies at 4°C for 30 min: APC-eFluor 780-conjugated anti-CD45 (clone: HI30; 1:100), Alexa Fluor 700-conjugated anti-CD3 (clone: UCHT1; 1:50), FITC-conjugated anti-CD4 (clone: RPA-T4; 1:200), PE-Cy7-conjugated anti-CD8 (clone: SK1; 1:200), PC5.5-conjugated anti-CD19 (clone: SJ25C1; 1:50), APC-conjugated anti-HLA-DR (clone: LN3; 1:50), PC5.5-conjugated anti-CD14 (clone: 61D3; 1:50), PE-conjugated anti-CD15 (clone: HI98; 1:50) and BV421-conjugated anti-CD11C (clone: 3.9; 1:50). PE-conjugated anti-CD15 were obtained from Becton, Dickinson and Company; BV421-conjugated anti-CD11C were obtained from BioLegend, Inc; APC-eFluor 780-conjugated anti-CD45, Alexa Fluor 700-conjugated anti-CD3, FITC-conjugated anti-CD4, PE-Cy7-conjugated anti-CD8, PC5.5-conjugated anti-CD19, PC5.5-conjugated anti-CD14 and APC-conjugated anti-HLA-DR were obtained from eBioscience; Thermo Fisher Scientific, Inc. Isotype-matched IgG controls (cat. no. 56-0038-41) were purchased from eBioscience; Thermo Fisher Scientific, Inc., and were incubated at 4°C for 30 min. Dead cells were excluded using staining with Fixable Viability Dye eFluor™ 506 at 4°C (eBioscience; Thermo Fisher Scientific, Inc.). Flow cytometry detection were performed on CytoFLEX flow cytometer (Beckman Coulter). The data were analyzed using FlowJo version 10 (Tree Star, Inc.) and gated by the side scatter and forward scatter filters.
Observational index
The levels of T lymphocyte subsets, B lymphocytes, dendritic cells and myeloid-derived suppressor cells (MDSCs) of the patients in the two groups were observed by flow cytometry. The level of agitation during emergence was assessed using the Richmond Agitation-Sedation Scale (RASS), which is defined as RASS score ≥+2 (16). The incidence of emergence agitation, hypotension and bradycardia during surgery were also recorded.
Statistical analysis
According to the percentages of CD3+ cells at T4 between two groups from our preliminary experiment, using Chinese High Intellectualized Statistical Software, with a type-I error of 5% and a power of 80%, 29 patients were needed in each group. Anticipating a 10% dropout rate, a total of 70 patients were recruited. All statistical analyses were performed using GraphPad Prism 7 (GraphPad Software, Inc.). Data are expressed as mean ± standard deviation or value. Differences in sex, ASA status and stage of tumor were compared using χ2 tests and Fisher's exact test as appropriate. Student's unpaired t-tests were performed to compare age, body mass index (BMI), duration of surgery, blood loss and liquid infusion volume. The differences in percentages of immune cells between time-points and the two groups are compared using two-way ANOVA and Bonferroni's correction. P<0.05 was considered to indicate a statistically significant difference.
Results
Comparison of general data between two groups
A total of 70 patients were recruited, two refused to consent and two patients from each group were excluded due to blood transfusions. Therefore, 32 patients were left in each group (Fig. 1). The demographics and surgical profiles of the patients were similar in the two groups. The patients in the two groups showed no significant differences in age, sex, BMI, ASA, stage of tumor, duration of surgery, blood loss or liquid infusion volume (all P>0.05; Table I).
Figure 1.
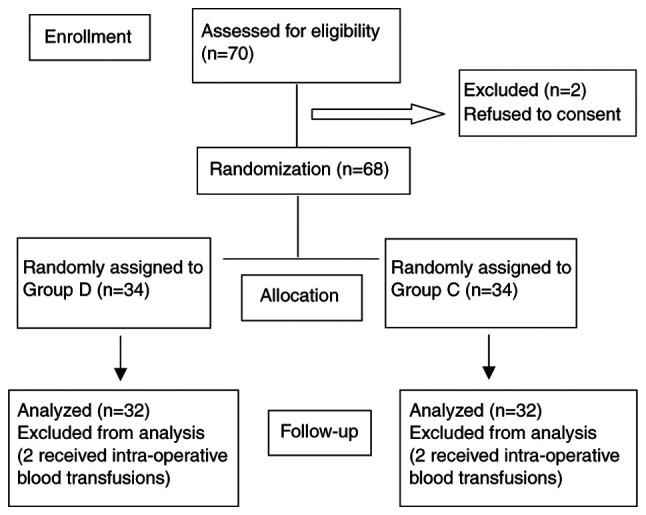
Consolidated Standards of Reporting Trials flow diagram of the patients included in the study. D, dexmedetomidine; C, control.
Table I.
Demographics and surgical profiles of the patients.
| Variables | Group D | Group C | P-value |
|---|---|---|---|
| Age, years | 49±10 | 51±11 | 0.454 |
| Sex, male/female | 25/7 | 24/8 | 0.768 |
| BMI, kg/m2 | 22.9±3.5 | 21.81±2.7 | 0.159 |
| ASA status, I/II | 22/10 | 23/9 | 0.784 |
| Stage of tumor | 0.963 | ||
| T1N0M0 | 12 | 11 | |
| T2N0M0 | 15 | 16 | |
| T3N0M0 | 5 | 5 | |
| Duration of surgery, h | 6.6±0.5 | 6.4±0.6 | 0.108 |
| Blood loss, ml | 302±53 | 280±54 | 0.080 |
| Liquid infusion volume, ml | 3006±320 | 3072±295 | 0.336 |
BMI, Body Mass Index; ASA, American Society of Anesthesiologists; D, dexmedetomidine, C, control.
Comparison of T lymphocyte subsets between the two groups
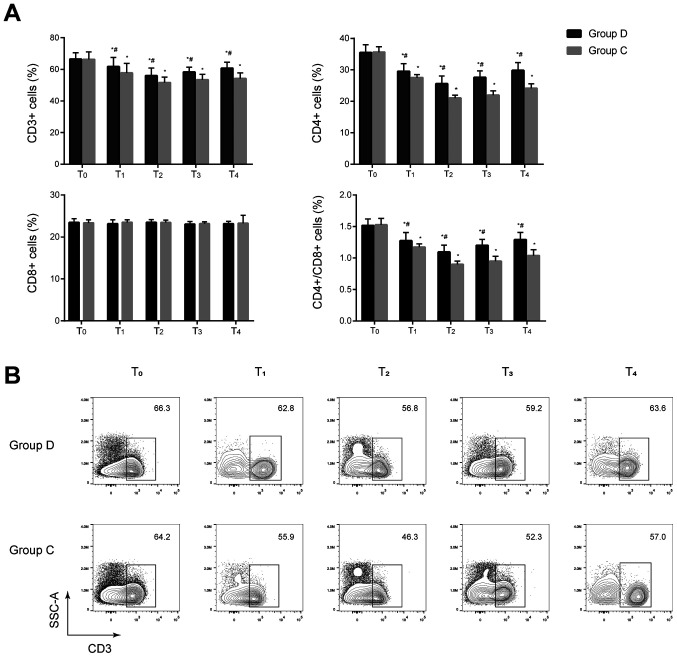
No significant differences in the percentages of CD3+ and CD4+ cells and the CD4+/CD8+ ratios were observed between the two groups before anesthesia induction (P>0.05). The percentages of CD3+ and CD4+ cells, and the CD4+/CD8+ ratios significantly decreased at T1–4 in the two groups compared with the baseline value at T0 and significantly increased at T1–4 in the dexmedetomidine group compared with the control group (all P<0.05). No significant difference in the percentage of CD8+ cells was found between the two groups at T0–4 (P>0.05) (Fig. 2 and Fig. S1). These results indicated that cellular immunity was suppressed in the two groups after anesthesia and dexmedetomidine may be associated with less impairment of cellular immunity in these patients.
Figure 2.
Comparison of T lymphocyte subsets at different time-points between the two groups. (A) T lymphocyte subsets including CD3+, CD4+ and CD8+ cells were analyzed by flow cytometry. Percentages of CD3+ and CD4+ cells, and the CD4+/CD8+ ratios significantly decreased at T1–4 in the two groups compared with the baseline value at T0 and significantly increased at T1–4 in the dexmedetomidine group compared with the control group. *P<0.05 vs. T0, #P<0.05 vs. group C. (B) Representative flow cytometry contour plots of CD3+ T cells in CD45+ cells of group C and group D at T0–4. D, dexmedetomidine; C, control; T0, 30 min before induction; T1, 1 h after induction; T2, end of the operation; T3, 24 h after operation; T4, 48 h after operation.
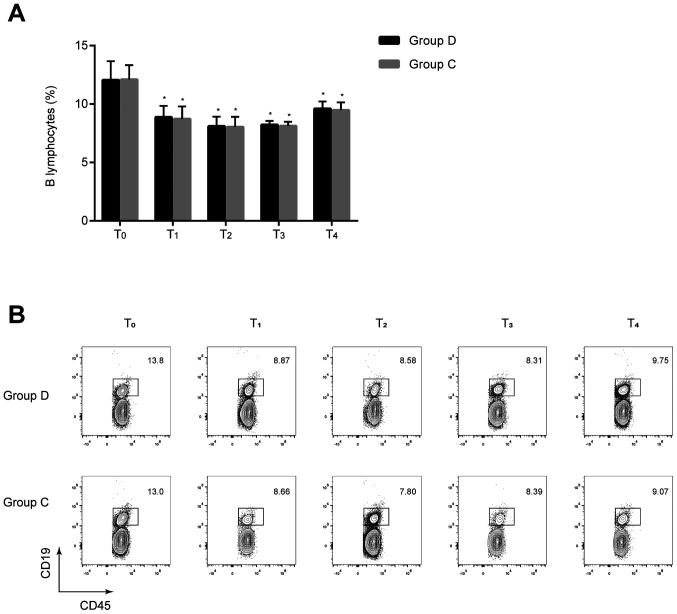
Comparison of B lymphocytes between the two groups
The percentages of B lymphocytes at T1–4 were significantly lower compared with those at T0 in the two groups (all P<0.05), but no statistically significant differences were found between the two groups at the same time-points (P>0.05) (Fig. 3). These results implied that dexmedetomidine exerts minimal effect on the humoral immune response of patients undergoing radical and reconstructive surgery for oral cancer.
Figure 3.
Comparison of B lymphocytes at different time-points between the two groups. (A) B lymphocytes were analyzed using flow cytometry. No significant differences were found between the two groups at the same time-points. *P<0.05 vs. T0. (B) Representative flow cytometry contour plots of B lymphocytes in CD45+ cells of group C and group D. D, dexmedetomidine; C, control; T0, 30 min before induction; T1, 1 h after induction; T2, end of the operation; T3, 24 h after operation; T4, 48 h after operation.
Comparison of dendritic cells between the two groups
The percentages of dendritic cells were significantly decreased at T1–4 in the two groups compared with the baseline value at T0, and they were significantly higher at T2–4 in the dexmedetomidine group compared with the respective control group (0.5±0.2% vs. 0.3±0.2% at T2, 0.5±0.1% vs. 0.4±0.2% at T3, 0.6±0.2% vs. 0.5±0.3% at T4; all P<0.05; Fig. 4). These results suggested that dexmedetomidine may attenuate the inhibition of immune response and may be beneficial to antitumor therapy.
Figure 4.
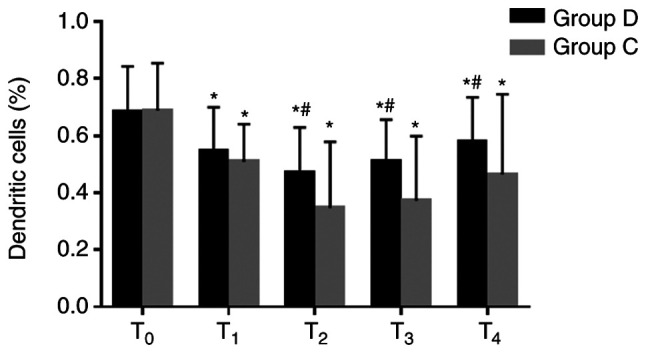
Comparison of dendritic cells at different time-points between the two groups. Percentages of dendritic cells were significantly higher at T2–4 in the group D compared with the group C. *P<0.05 vs. T0, #P<0.05 vs. group C. D, dexmedetomidine; C, control; T0, 30 min before induction; T1, 1 h after induction; T2, end of the operation; T3, 24 h after operation; T4, 48 h after operation.
Comparison of MDSCs between the two groups
The percentages of MDSCs significantly decreased at T1–3 in the two groups compared with the respective baseline value at T0, and they were significantly lower at T2–4 in the dexmedetomidine group compared with the respective control group (2.6±1.4% vs. 3.4±1.4% at T2, 3.2±1.1% vs. 4.0±1.1% at T3 and 4.2±1.1% vs. 4.7±1.% at T4; all P<0.05; Fig. 5). These results suggested that dexmedetomidine may decrease the percentages of MDSCs and improve the immunosuppressive state of patients.
Figure 5.
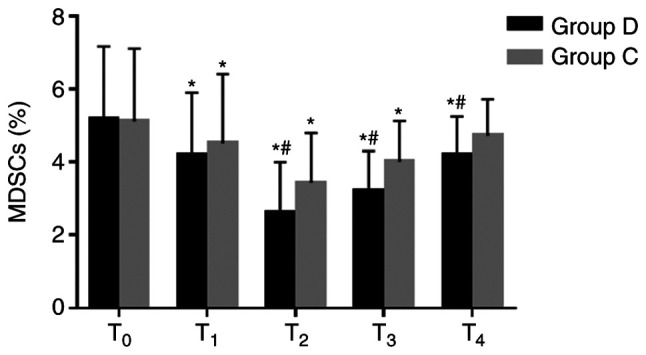
Comparison of MDSCs at different time-points between the two groups. MDSCs were analyzed using flow cytometry. Percentages of MDSCs significantly decreased at T1-3 in the two groups compared with the baseline value at T0, and they were significantly lower at T2-4 in the dexmedetomidine group compared with the control group. *P<0.05 vs. T0, #P<0.05 vs. group C. MDSCs, myeloid-derived suppressor cells; D, dexmedetomidine; C, control; T0, 30 min before induction; T1, 1 h after induction; T2, end of the operation; T3, 24 h after operation; T4, 48 h after operation.
Comparison of the incidence of emergence agitation, hypotension and bradycardia between the two groups
The incidence of emergence agitation was lower in the dexmedetomidine group compared with the control group (31.2 vs. 59.4%; P=0.044). However, the incidence of hypotension and bradycardia during surgery were significantly higher in the dexmedetomidine group compared with in the control group (P<0.05) (Table II).
Table II.
Incidence of emergence agitation, hypotension and bradycardia between the D (n=32) and C (n=32) groups.
| Variable | Group D, n (%) | Group C, n (%) | P-value |
|---|---|---|---|
| Emergence agitation | 10 (31.2) | 19 (59.4) | 0.044 |
| Hypotension in OR | 14 (43.8) | 5 (15.6) | 0.027 |
| Bradycardia in OR | 12 (37.5) | 3 (9.4) | 0.016 |
D, dexmedetomidine, C, control; OR, operating room.
Discussion
The present results showed that dexmedetomidine alleviated the decrease in the percentages of CD3+, CD4+ and dendritic cells as well as the CD4+/CD8+ ratios and reduced the percentages of MDSCs, indicating that dexmedetomidine can attenuate immunosuppression in patients undergoing radical and reconstructive surgery for oral cancer.
Oral cancer is a major life-threatening disease with a high incidence in Southeast Asian countries (1), and radical operation and immediate reconstruction are the common treatment for patients. The surgical stress response is considered to directly induce immunosuppression by activating the hypothalamus-pituitary-adrenal axis and sympathetic nervous system, which results in the increased production of glucocorticoids and catecholamines (3). Glucocorticoids are known to decrease the number and activity of natural killer cells and reduce T cell proliferation in a dose-dependent manner (17). Catecholamines can also inhibit T cell proliferation by decreasing IL-2 expression and secretion and reduce natural killer cell activity (3). Meanwhile, it is reported that some anesthetics have direct suppressive impacts on innate and adaptive immunity (5–8). Sevoflurane is a popular inhaled anesthetic that can inhibit the activity of natural killer cells and induce the apoptosis of T and B lymphocytes (8). Opioids are commonly used analgesic agents in surgery and have immunosuppressive effects by suppressing the activity of natural killer cells, neutrophils and macrophages and the proliferation of T lymphocytes (7). Surgery and anesthesia-induced immunosuppression have been implicated in the development of post-operative septic complications and tumor metastasis (3,4).
Considering that surgery is necessary in cancer treatment, extensive research has been conducted to determine the effect of anesthetics on immune cell population (5–8). Dexmedetomidine is a highly selectiveα2-adrenoceptor agonist with sedative, anxiolytic, analgesic and anti-sympatholytic properties (9,10). Ebert et al (18) reported that dexmedetomidine reduces the concentration of circulating plasma catecholamines by 60–80% in a dose-dependent manner, and this reduction is consistent with long-lasting anti-sympathetic effect. Other studies have indicated that dexmedetomidine exerts anti-inflammatory effects and organ-protective effects against ischemic and hypoxic injury, which can also regulate immune response (10,13,19).
Cellular immunity is closely associated with antitumor effects and cancer metastasis surveillance (6,20). Improving perioperative cellular immune status contributes to attenuation of immunosuppression and resistance to metastasis. T lymphocytes are important in cellular immunity (20). All mature peripheral T lymphocytes, labeled by CD3+, comprise of CD4+ and CD8+. The former represents cellular immune function, whereas the latter recognizes and kills tumor cells. The reduction of CD4+/CD8+ ratio implies that the cellular immune function is downregulated (6). The present study showed that the percentages of CD3+ and CD4+ cells and the CD4+/ CD8+ ratios significantly decreased from T1 to T4 in the two groups and significantly decreased in the control group. This result implied that cellular immunity was suppressed in the two groups after anesthesia and dexmedetomidine may be associated with less impairment of cellular immunity in these patients. These results are aligned with previous reports. For example, Yang et al (11) found a significant difference in lymphocyte count after anesthesia between the dexmedetomidine and control groups in radical mastectomy. A recent clinical trial on 141 patients receiving radical operation of colon carcinoma has revealed that dexmedetomidine can decrease the inhibition of T lymphocyte subsets and reduce the secretion of inflammatory factors (12). Another study by Wang et al (13) indicated that dexmedetomidine can preserve the balance of T helper (h)1/Th2 ratio 24 h after surgery and attributed this result to the increased response of Th1 in patients undergoing radical gastrectomy. The decrease in Th1/Th2 ratio after surgery suggests a suppressed cell-mediated immunity (13).
B lymphocytes producing antibodies mediate humoral immunity (6). Surgery-induced immunosuppression is mainly caused by the effect on the cellular immune system, and T lymphocytes are most affected with B lymphocytes numbers changing little (3). In the present study, the percentage of B lymphocytes was slightly decreased in the two groups after anesthesia, and there was no significant difference between the two groups at T1–4. The results suggested that dexmedetomidine exerts minimal effect on the humoral immune response of patients undergoing radical and reconstructive surgery for oral cancer. Further studies are needed to find the influence of dexmedetomidine on the humoral immunity of patients with malignant tumors during operation.
Dendritic cells, first identified by Steinman in 1973, are antigen-presenting cells that are considered a critical factor in anti-tumor immunity (21). As an immune surveillance cell, dendritic cells can efficiently cluster and activate T cells inhibiting the occurrence and development of tumors (22). Clinical studies on dendritic cells have focused on therapeutic vaccination against cancer, immunotherapy applying ex vivo-generated and tumor antigen-loaded dendritic cells has been successfully introduced in clinical vaccination protocols and has proven to be feasible and effective (23). The present study demonstrated that the percentages of dendritic cells were significantly higher in the dexmedetomidine group compared within the control group from T2to T4. This result suggested that dexmedetomidine may attenuate the inhibition of immune response and may be beneficial to antitumor therapy.
MDSCs are a subset of immune cells that have a myeloid origin with immunosuppressive abilities (24). Studies have reported that MDSCs suppress CD8+ T cells and IFN-γ and IL-2 production by T cells (24,25). In addition, MDSCs inhibit B cell proliferation and antibody production (25) and the expansion of MDSCs is associated with tumor progression (26). The present study showed that the percentages of MDSCs significantly decreased from T1 to T3 in the two groups, indicating that surgical resection of the tumor has an anti-tumor effect. Meanwhile, the percentages of MDSCs in the dexmedetomidine group significantly decreased at T2 to T4, suggesting that dexmedetomidine may decrease the percentages of MDSCs and improve the immunosuppressive state of patients.
The level of agitation during emergence was assessed using RASS scores (16). The incidence of emergence agitation was lower in the dexmedetomidine group compared within the control group. This result may be associated with the sedation and analgesia effects of dexmedetomidine. Thus, patients are likely to tolerate tracheal catheter and other discomfort during the recovery period. However, the incidence of hypotension and bradycardia was higher in the dexmedetomidine group compared within the control. This result is in accordance with previous research and the reason is related to the inhibitory effect of dexmedetomidine on the sympathetic system (27,28).
The present study has some limitations. The clinical outcomes and cancer-free survival time were not measured. In addition, the specific mechanisms of immunosuppressive effects of dexmedetomidine were not explored. Therefore, cellular and molecular level studies are also needed to resolve these underlying mechanisms. Immune cells could be analyzed to detect mRNA expression levels of transcription factors and chemokines.
In conclusion, dexmedetomidine alleviated the decrease in the percentages of CD3+, CD4+ and dendritic cells as well as the CD4+/CD8+ ratios and reduced the percentages of MDSCs. Thus, dexmedetomidine can attenuate immunosuppression in patients undergoing radical and reconstructive surgery for oral cancer.
Supplementary Material
Acknowledgements
The authors would like to thank their colleague Dr Liang Mao (School and Hospital of Stomatology, Wuhan University, Wuhan, Hubei, China) for his excellent technical assistance.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
LH designed the study, performed the experiments and wrote the manuscript. CQ performed the experiments and revised the manuscript. LW designed the study and revised the manuscript. TZ collected and analyzed the data. JL designed the study and revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by The Ethics Committee of School and Hospital of Stomatology, Wuhan University (Wuhan, China; approval noIRB-2018B23) and registered in the Chinese Clinical Trial Registry (ChiCTR-1800018367). Written informed consent was provided by all participants before the trial.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Kumar M, Nanavati R, Modi TG, Dobariya C. Oral cancer: Etiology and risk factors: A review. J Cancer Res Ther. 2016;12:458–63. doi: 10.4103/0973-1482.186696. [DOI] [PubMed] [Google Scholar]
- 2.Hussein AA, Helder MN, de Visscher JG, Leemans CR, Braakhuis BJ, de Vet HCW, Forouzanfar T. Global incidence of oral and oropharynx cancer in patients younger than 45 years versus older patients: A systematic review. Eur J Cancer. 2017;82:115–127. doi: 10.1016/j.ejca.2017.05.026. [DOI] [PubMed] [Google Scholar]
- 3.Hogan BV, Peter MB, Shenoy HG, Horgan K, Hughes TA. Surgery induced immunosuppression. Surgeon. 2011;9:38–43. doi: 10.1016/j.surge.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Kim R. Anesthetic technique and cancer recurrence in oncologic surgery: Unraveling the puzzle. Cancer Metastasis Rev. 2017;36:159–177. doi: 10.1007/s10555-016-9647-8. [DOI] [PubMed] [Google Scholar]
- 5.Kurosawa S, Kato M. Anesthetics, immune cells, and immune responses. J Anesth. 2008;22:263–277. doi: 10.1007/s00540-008-0626-2. [DOI] [PubMed] [Google Scholar]
- 6.Zhang T, Fan Y, Liu K, Wang Y. Effects of different general anaesthetic techniques on immune responses in patients undergoing surgery for tongue cancer. Anaesth Intensive Care. 2014;42:220–227. doi: 10.1177/0310057X1404200209. [DOI] [PubMed] [Google Scholar]
- 7.Plein LM, Rittner HL. Opioids and immune system-friend or foe. Br J Pharmacol. 2018;175:2717–2725. doi: 10.1111/bph.13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stollings LM, Jia LJ, Tang P, Dou H, Lu B, Xu Y. Immune modulation by volatile anesthetics. Anesthesiology. 2016;125:399–411. doi: 10.1097/ALN.0000000000001195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahmoud M, Mason KP. Dexmedetomidine: Review, update, and future considerations of pediatric perioperative and periprocedural applications and limitations. Br J Anaesth. 2015;115:171–182. doi: 10.1093/bja/aev226. [DOI] [PubMed] [Google Scholar]
- 10.Maud AS, Michel MR, Laura NH, Clements RM, Anthony RA, Pieter C. Clinical pharmacokinetics and pharmacodynamics of dexmedetomine. Clin Pharmacokinet. 2017;56:893–913. doi: 10.1007/s40262-017-0507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang XH, Bai Q, Lv MM, Fu HG, Dong TL, Zhou Z. Effect of dexmedetomidine on immune function of patients undergoing radical mastectomy. Eur Rev Med Pharmacol Sci. 2017;21:1112–1116. [PubMed] [Google Scholar]
- 12.Wang K, Li C. Effects of dexmedetomidine on inflammatory factors, T lymphocyte subsets and expression of NF-κB in peripheral blood mononuclear cells in patients receiving radical surgery of colon carcinoma. Oncol Lett. 2018;15:7153–7157. doi: 10.3892/ol.2018.8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Xu X, Liu H, Ji F. Effects of dexmedetomine on patients undergoing radical gastrectomy. J Surg Res. 2015;194:147–153. doi: 10.1016/j.jss.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Mayhew D, Mendonca V, Murthy BVS. A review of ASA physical status-historical perspectives and modern developments. Anesthesia. 2019;74:373–379. doi: 10.1111/anae.14569. [DOI] [PubMed] [Google Scholar]
- 15.Scott NW, McPherson GC, Ramsay CR, Campbell MK. The method of minimization for allocation to clinical trials: A review. Control Clin Trials. 2002;23:662–674. doi: 10.1016/S0197-2456(02)00242-8. [DOI] [PubMed] [Google Scholar]
- 16.Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, Davidson JE, Devlin JW, Kress JP, Joffe AM, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the Intensive Care Unit. Crit Care Med. 2013;41:263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 17.Chen L, Jondal M, Yakimchuk K. Regulatory effects of dexamethasone on NK and T cell immunity. Inflammopharmacology. 2018;26:1331–1338. doi: 10.1007/s10787-017-0418-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ebert TJ, Hall JE, Barnev JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93:382–394. doi: 10.1097/00000542-200008000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Xiang H, Hu B, Li Z, Li J. Dexmedetomine controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Inflammation. 2014;37:1763–1770. doi: 10.1007/s10753-014-9906-1. [DOI] [PubMed] [Google Scholar]
- 20.Goldfarb Y, Sorski L, Benish M, Levi B, Melamed R, Ben-Eliyahu S. Improving postoperative immune status and resistance to cancer metastasis: A combined perioperative approach of immunostimulation and prevention of excessive surgical stress responses. Ann Surg. 2011;253:798–810. doi: 10.1097/SLA.0b013e318211d7b5. [DOI] [PubMed] [Google Scholar]
- 21.Steinman RM. Decisions about dendritic cells: Past, present, and future. Annu Rev Immunol. 2012;30:1–22. doi: 10.1146/annurev-immunol-100311-102839. [DOI] [PubMed] [Google Scholar]
- 22.Mildner A, Jung S. Development and function of dendritic cell subsets. Immunity. 2014;40:642–656. doi: 10.1016/j.immuni.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 23.Den Brok MH, Nierkens S, Figdor CG, Ruers TJ, Adema GJ. Dendritic cells: Tools and targets for antitumor vaccination. Expert Rev Vaccines. 2005;4:699–710. doi: 10.1586/14760584.4.5.699. [DOI] [PubMed] [Google Scholar]
- 24.Dai J, El Gazzar M, Li GY, Moorman JP, Yao ZQ. Myeloid-derived suppressor cells: Paradoxical roles in infection and immunity. J Innate Immun. 2015;7:116–126. doi: 10.1159/000368233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamadaho RSE, Hoerauf A, Layland LE. Immunomodulatory effects of myeloid-derived suppressor cells in diseases: Role in cancer and infections. Immunobiology. 2018;223:432–442. doi: 10.1016/j.imbio.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: Expect the unexpected. J Clin Invest. 2015;125:3356–3364. doi: 10.1172/JCI80005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang X, Li Z, Gao C, Liu R. Effect of dexmedetomidine on preventing agitation and delirium after microvascular free flap surgery: A randomized, double-blind, control study. J Oral Maxillofac Surg. 2015;73:1065–1072. doi: 10.1016/j.joms.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 28.Riker RR, Shehabi Y, Bokesch PM, Ceraso D, Wisemandle W, Koura F, Whitten P, Margolis BD, Byrne DW, Ely EW, et al. Dexmedetomidine vs. midazolam for sedation of critically ill patients: A randomized trial. JAMA. 2009;301:489–499. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.