Abstract
Rationale:
Chemokine-controlled arterial leukocyte recruitment is a crucial process in atherosclerosis. Formyl peptide receptor 2 (FPR2) is a chemoattractant receptor that recognizes proinflammatory and proresolving ligands. The contribution of FPR2 and its proresolving ligand annexin A1 to atherosclerotic lesion formation is largely undefined.
Objective:
Because of the ambivalence of FPR2 ligands, we here investigate the role of FPR2 and its resolving ligand annexin A1 in atherogenesis.
Methods and Results:
Deletion of FPR2 or its ligand annexin A1 enhances atherosclerotic lesion formation, arterial myeloid cell adhesion, and recruitment. Mechanistically, we identify annexin A1 as an endogenous inhibitor of integrin activation evoked by the chemokines CCL5, CCL2, and CXCL1. Specifically, the annexin A1 fragment Ac2-26 counteracts conformational activation and clustering of integrins on myeloid cells evoked by CCL5, CCL2, and CXCL1 through inhibiting activation of the small GTPase Rap1. In vivo administration of Ac2-26 largely diminishes arterial recruitment of myeloid cells in a FPR2-dependent fashion. This effect is also observed in the presence of selective antagonists to CCR5, CCR2, or CXCR2, whereas Ac2-26 was without effect when all 3 chemokine receptors were antagonized simultaneously. Finally, repeated treatment with Ac2-26 reduces atherosclerotic lesion sizes and lesional macrophage accumulation.
Conclusions:
Instructing the annexin A1-FPR2 axis harbors a novel approach to target arterial leukocyte recruitment. With the ability of Ac2-26 to counteract integrin activation exerted by various chemokines, delivery of Ac2-26 may be superior in inhibition of arterial leukocyte recruitment when compared with blocking individual chemokine receptors.
Keywords: annexin A1, atherosclerosis, chemokine, leukocytes
Atherosclerosis is a chronic inflammation of the arterial vessel wall characterized by continuous leukocyte recruitment. Arterial leukocyte accumulation is controlled by various mechanisms, including apoptosis, egress, proliferation, and recruitment.1 The latter process is thought to be a dominant mechanism occurring at all stages of atherosclerosis.2 Indeed, inhibition of arterial myeloid cell recruitment was shown to reduce atherogenesis, atheroprogression, and plaque destabilization in mouse models of atherosclerosis.3,4 Recruitment of arterial leukocytes, predominantly myeloid cells, is regulated by interaction of leukocytic and endothelial cell adhesion molecules. The valency of leukocyte adhesion molecules, namely integrins, is essentially controlled by chemotactic molecules, which bind to G-protein–coupled chemokine receptors unleashing an intracellular signaling cascade ultimately fostering integrin activation. Although ligation of most chemokine receptors follows this activation pattern, other chemoattractant receptors bind ligands with opposing functions.
Formyl peptide receptor 2 (FPR2) is a G-protein–coupled receptor predominantly expressed by myeloid cells and to a lower degree by endothelial cells.5 In contrast to classical chemokine receptors, FPR2 is promiscuous and recognizes ligands with proinflammatory (eg, cathelicidin, serum amyloid A) or anti-inflammatory, proresolving (eg, annexin A1 [AnxA1]) properties6 and may hence exert ambivalent effects during leukocyte recruitment in atherosclerosis. Previous work has underscored the importance of the inflammatory FPR2 ligands cathelicidin and serum amyloid A in accelerating macrophage accumulation during atherogenesis.7,8 In contrast, clinical data point toward a protective role of AnxA1.9,10 Thus, we here investigate the importance of FPR2 in atherogenesis and identify a prominent protective effect of its resolving ligand AnxA1.
Methods
Mice
Fpr2−/−11 and Anxa1−/−12 were intercrossed with Apoe−/− mice to generate double-mutant Apoe−/−Fpr2−/− and Apoe−/−Anxa1−/− mice. Apoe−/−, Apoe−/−Fpr2−/−, and Apoe−/−Anxa1−/− mice were fed a high-fat diet (HFD) containing 21% fat (ssniff) for 4 weeks to induce early atherosclerotic lesions. In a separate set of experiments, Apoe−/− mice were treated with Ac2-26 (3× per week; 50 μg IP per injection) or vehicle control during 4 weeks of HFD feeding. All genetically modified animals were backcrossed to C57Bl/6 background for ≥10 generations. All animal experiments were approved by the local ethical committee for animal experimentation. A detailed Methods section is available in the Online Data Supplement.
Results
Lack of FPR2 or its Ligand Annexin A1 Accelerates Atherogenesis
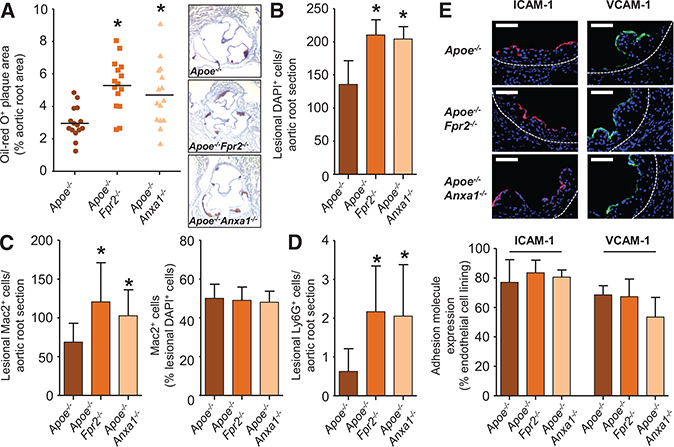
To investigate the role of FPR2 in early atherosclerosis, we fed Apoe−/− and Apoe−/−Fpr2−/− mice a HFD for 4 weeks and assessed atherosclerotic lesion sizes in aortic roots. Lack of FPR2 significantly increased lesion sizes (Figure 1A) characterized by increased cellularity and a matching increase in macrophage content (Figure 1B and 1C). Although infrequent in atherosclerotic lesions, neutrophils were also found in larger numbers in plaques of Apoe−/−Fpr2−/− mice (Figure 1D). In addition, no changes in the number of intercellular adhesion molecule-1 (ICAM-1) or vascular cell adhesion molecule-1 (VCAM-1)–expressing endothelial cells were found (Figure 1E), and the number of apoptotic cells as assessed by TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) staining was not different between the strains (Online Figure IA). Finally, no differences were detected for counts of leukocyte subsets, plasma cholesterol, and triglyceride levels in Apoe−/− and Apoe−/−Fpr2−/− mice (Online Table I).
Figure 1. Formyl peptide receptor 2 and its ligand annexin A1 protect from atherogenesis.
Apoe−/−, Apoe−/−Fpr2−/−, and Apoe−/−Anxa1−/− mice were fed a high-fat diet for 4 weeks. A, Quantification of atherosclerotic lesion sizes in Oil-Red-O–stained aortic root sections. Representative images are shown aside. B, Enumeration of lesional DAPI (4’,6-diamidino-2-phenylindole)-positive cells. C, Quantification of Mac2+ cells indicating macrophage accumulation in absolute numbers (left) and relative to lesional cell count (right). D, Quantification of lesional Ly6G+ neutrophils. E, Assessment of luminal expression of endothelial adhesion molecules intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1). Displayed are representative images (top) and quantification of luminal coverage (bottom). Scale bar, 100 μm. *P<0.05 compared to Apoe−/− mice. All data are presented as mean±SD. Experiments were performed 3× independently with a total of 15 mice. Data were analyzed with 1-way ANOVA with Dunnett post test.
These data are in striking contrast to the previously reported lesion phenotype of mice lacking the neutrophil-borne FPR2 ligand CRAMP (Cathelicidin-related Antimicrobial Peptide).7 Thus, we hypothesized that resolution-inducing FPR2 ligands may hold an important role during early atherosclerosis and consequently studied the development of atherosclerosis in mice lacking the proresolving FPR2 ligand AnxA1. Apoe−/−A nxa1−/− mice were fed a HFD for 4 weeks, and atherosclerosis development was compared with Apoe−/− mice. In parallel to what we observed in Apoe−/−Fpr2−/− mice, aortic root lesions of Apoe−/−Anxa1−/− mice were larger in size and contained more myeloid cells when compared with Apoe−/− mice (Figure 1).
Annexin A1-FPR2 Axis Prevents Arterial Myeloid Cell Recruitment
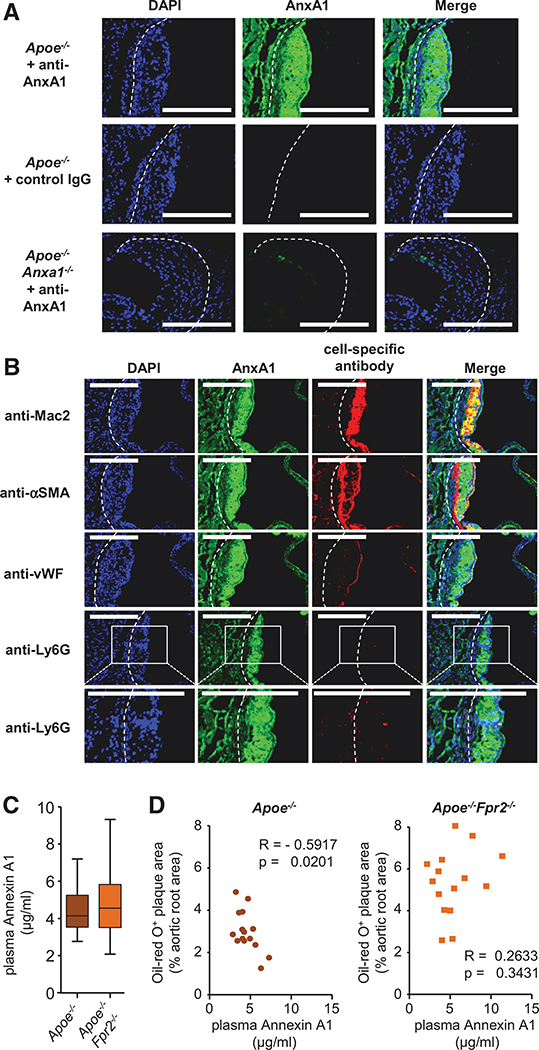
The similarity of atherosclerotic lesion characteristics in Apoe−/−Fpr2−/− and Apoe−/−Anxa1−/− mice points toward the importance of an endogenous AnxA1-FPR2 axis preventing atherogenesis. To study the presence of AnxA1 in atherosclerotic lesions, we stained murine plaques with an antibody to AnxA1. We could evidence abundant, specific staining of AnxA1 in murine lesions of Apoe−/− mice, whereas no staining was observed in lesions Apoe−/−Anxa1−/− mice (Figure 2A). To study the possible origin of AnxA1, we costained murine atherosclerotic lesions with an antibody recognizing AnxA1 and with antibodies toward macrophages (Mac2), neutrophils (Ly6G), smooth muscle cells (α-smooth muscle actin), or endothelial cells (von Willebrand Factor). Specific immunofluorescence was prominent in myeloid cells and in the endothelium, whereas scarce staining was found in smooth muscle cells (Figure 2B). Importantly, a similar distribution of AnxA1 staining was evidenced in human endarterectomy specimens (Online Figure IIA and IIB). In addition, AnxA1 plasma levels showed no differences between Apoe−/− and Apoe−/−Fpr2−/− mice (Figure 2C), and plasma AnxA1 levels were negatively correlated with lesion sizes in the aortic root of Apoe−/− mice, a relationship absent in Apoe−/−Fpr2−/− mice (Figure 2D).
Figure 2. Plasma annexin A1 (AnxA1) negatively correlates with atherosclerotic lesion sizes.
A, Apoe−/− and Apoe−/−Anxa1−/− mice were fed a high-fat diet for 4 weeks, and aortic root sections were stained with an antibody to AnxA1 or an isotype control antibody. B, Apoe−/− mice were fed a high-fat diet (HFD) for 4 weeks and AnxA1 was costained with markers for macrophages (anti-Mac2), smooth muscle cells (anti-αSMA), endothelial cells (anti-von Willebrand Factor [vWF]), or neutrophils (anti-Ly6G) in aortic root sections. Scale bar, 100 μm. C and D, Apoe−/− and Apoe−/−Fpr2−/− mice were fed a HFD for 4 weeks and AnxA1 was quantified in the plasma (B). Correlation between aortic root lesion sizes and plasma AnxA1 levels in Apoe−/− and Apoe−/−Fpr2−/− mice (Pearson correlation; D). DAPI indicates 4’,6-diamidino-2-phenylindole.
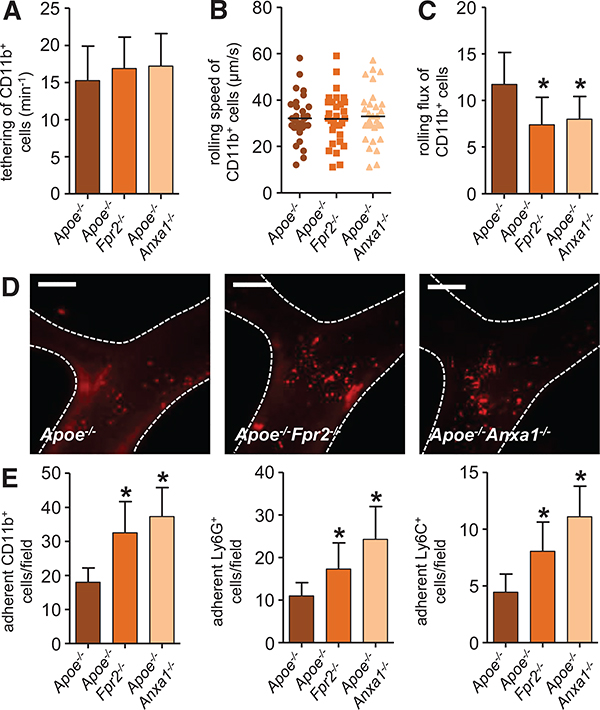
To assess the role of the AnxA1-FPR2 axis on arterial leukocyte recruitment, we performed intravital microscopy of the carotid artery. Herein, myeloid cells were visualized by administration of antibodies to Ly6G (neutrophils), Ly6C (classical monocytes), and CD11b (myeloid cells). Although tethering and rolling speed of myeloid cell subsets were not affected in mice lacking FPR2 or AnxA1 (Figure 3A and 3B; Online Figure IIIA and IIIB), rolling flux was reduced in both strains when compared with Apoe−/− mice (Figure 3C; Online Figure IIIC). Concomitantly, adhesion of myeloid cell subsets was significantly increased in both Apoe−/−Fpr2−/− and Apoe−/−Anxa1−/− mice when compared with that in Apoe−/− mice (Figure 3D and 3E). Because endothelial adhesion molecule expression did not differ between the mouse strains and with the largely myeloid cell–restricted expression of FPR2, we suspected the adhesion defect to be leukocyte intrinsic. In fact, the pattern of alterations in leukocyte adhesion with enhanced adhesion and reduced rolling flux pointed toward a modification of chemokine-mediated leukocyte integrin activation and subsequent firm adhesion.
Figure 3. Annexin A1-formyl peptide receptor 2 axis prevents arterial myeloid cell recruitment.
Apoe−/−, Apoe−/−Fpr2−/−, and Apoe−/−Anxa1−/− mice were fed a high-fat diet for 4 weeks, and intravital microscopy of the carotid artery was performed to assess luminal leukocyte endothelial interactions. Myeloid cell subsets were identified by intravenous injection of antibodies to Ly6G, Ly6C, and CD11b 10 minutes before recording. Displayed are quantification of tethering (A), rolling speed (B), and rolling flux (C) of CD11b+ cells. Number of adherent cells (E) is displayed for CD11b+ cells (left), Ly6G+ cells (middle), and Ly6C+ cells (right). Adhesion of CD11b+ myeloid cells to carotid arteries of Apoe−/−, Apoe−/−Fpr2−/−, and Apoe−/−Anxa1−/− mice is depicted in D. Scale bar, 100 μm. *P<0.05 compared with Apoe−/− mice. Experiments were performed 3× independently with a total of ≥15 mice. Data were analyzed using 1-way ANOVA with Dunnett post test.
Annexin A1 Abolishes Chemokine-Mediated Integrin Activation
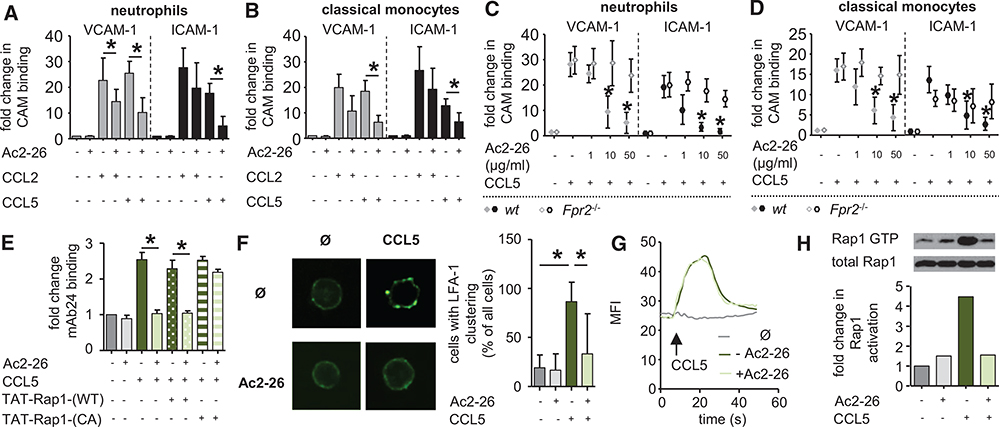
Integrin activation was further studied in murine myeloid cells after stimulation with CCL2, CCL5, CXCL1, and leukotriene B4 (LTB4), all of which are chemotactic molecules with reported importance in arterial myeloid cell recruitment.2 Activation of β1 and β2 integrins was assayed by coincubation with their natural ligands VCAM-1 and ICAM-1, which were fused with a human Fc fragment allowing for detection of binding by flow cytometry. In this setting, CCL2 and CCL5 induced a robust activation of β1 and β2 integrins (Figure 4A and 4B), whereas CXCL1 and LTB4 exerted only moderate effects (Online Figure IVA and IVB). To assess the effect of AnxA1 on chemokine-evoked integrin activation, we made the use of the AnxA1 fragment Ac2-26, which was reported to act via FPR2. In homoligand competition binding experiments, we could demonstrate that Ac2-26 competed with [125I-Tyr]-Ac2-26 at mouse or human FPR2 overexpressed in HEK293 cells (Online Figure IVC and IVD). Furthermore, we used a biochemical approach to confirm the interaction of Ac2-26 and FPR2. Herein, we performed surface plasmon resonance experiments using FPR2 expressed in proteoliposomes, thus maintaining the 3-dimensional chemokine receptor structure. Ac2-26 was immobilized on a CM4 chip and superfused with receptor-containing proteoliposomes (Online Figure IVE). With this approach, we were able to detect a clear binding of Ac2-26 to FPR2. Interestingly, pretreatment of myeloid cells with the FPR2-binding AnxA1 fragment Ac2-26 vastly reduced CCL5-inflicted activation of β1 and β2 integrins on neutrophils and classical monocytes (Figure 4A and 4B). In addition, the presence of Ac2-26 significantly lowered CCL2-induced binding of VCAM-1 on neutrophils, whereas there was only a trend toward reduced binding of ICAM-1 on neutrophils (P=0.3) and VCAM-1 (P=0.07) or ICAM-1 on classical monocytes (P=0.2). Finally, CXCL1-mediated binding of ICAM-1 was significantly lowered by Ac2-26 (Online Figure IVA and IVB), whereas there was only a trend toward reduction for LTB4-stimulated binding of ICAM-1 (P=0.12). To study the effect of Ac2-26 on adhesion of human myeloid cells, isolated human monocytes and neutrophils were perfused over tumor necrosis factor–activated human umbilical vein endothelial cells in the absence or in the presence of increasing amounts of Ac2-26 (Online Figure IVF and IVG). In these experiments, the presence of Ac2-26 caused a significant, dose-dependent decrease in myeloid cell adhesion with significant reductions at doses of 10 and 50 μg/mL. With the robust effect of Ac2-26 on CCL5-evoked integrin activation (Figure 4A and 4B) and the reported importance of CCL5 in arterial recruitment of both neutrophils and monocytes,13,14 we limited further mechanistic studies to studying the effect of Ac2-26 on CCL5-evoked integrin activation. Here, we find that Ac2-26 dose dependently reduces the affinity for ICAM-1 and VCAM-1 when neutrophils and classical monocytes are activated with CCL5 (Figure 4C and 4D). This response was annulled in myeloid cells harvested from Fpr2−/− mice (Figure 4C and 4D). Full integrin activation is accompanied by a shape change to the fully extended conformation that can be monitored in human cells by the use of antibodies recognizing the activation epitope. Here, Ac2-26 inhibited the CCL5-induced switch of β2 integrin conformation into its activated state in human neutrophils and monocytes (Figure 4E; Online Figure IVH). In addition, cell surface expression of β1 and β2 integrins was neither increased by CCL5 treatment (not shown) nor decreased by Ac2-26 treatment (Online Figure IVI). Likewise, Ac2-26 treatment did not affect the expression of the CCL5 receptors, CCR1, CCR3, or CCR5 (Online Figure IVK). Another well-described aspect of integrin activation is their clustering on the cell surface. Using confocal microscopy, we found that the relatively dispersed distribution of lymphocyte function-associated antigen 1 (LFA1), on mouse neutrophils, as well as on human neutrophils, and monocytes became much more clustered on treatment with CCL5 and that this clustering was strongly reduced by Ac2-26 (Figure 4F; Online Figure IVL and IVM). Thus, Ac2-26 inhibits the adhesiveness of myeloid cell β1 and β2 integrins by downmodulating their affinity and valency. Chemokine-triggered integrin activation is mediated by an early increase in cytosolic Ca2+ followed by a later activation of the small GTPase Rap1.15 To clarify how Ac2-26 interferes with chemokine-driven integrin activation, we analyzed whether either of these 2 steps is affected. Although the treatment of neutrophils with Ac2-26 did not affect the CCL5-stimulated increase in intracellular free Ca2+ (Figure 4G), Ac2-26 treatment reduced CCL5-triggered Rap1 activation in murine neutrophils (Figure 4H; Online Figure IVN) and in human neutrophils and monocytes (Online Figure IVO and IVP), as assessed by measuring the level of GTP-bound Rap1. Thus, Ac2-26 inhibits integrin activation by interfering with the chemokine-driven activation of Rap1, an essential step in integrin activation. To test the involvement of Rap1 in Ac2-26–triggered chemokine-evoked integrin deactivation further, we transferred a wild-type or a constitutively active Rap1 fusion protein into human neutrophils or monocytes before the treatment with Ac2-26 and CCL5. Although the transfer of the fusion proteins did not affect LFA1 activation in response to CCL5 alone, the constitutively active Rap1 fusion protein fully abolished the inhibitory effect of Ac2-26 (Figure 4E; Online Figure IVH), thus confirming that Ac2-26 acts through antagonizing Rap1 activation.
Figure 4. Annexin A1 counteracts chemokine-induced integrin activation.
A and B, Annexin A1 inhibits chemokine-evoked integrin activation on myeloid cells. Neutrophils (A) and classical monocytes (B) from C57Bl/6 mice were treated with CCL2 or CCL5, and binding of soluble vascular cell adhesion molecule-1 (VCAM-1)-Fc or ICAM-1-Fc was assessed by flow cytometry. Effect of Ac2-26 was determined by pretreatment for 30 minutes before chemokine stimulation. C and D, Dose-dependent response of Ac2-26 on CCL5-evoked binding of VCAM-1-Fc or intercellular adhesion molecule-1 (ICAM-1)-Fc on neutrophils (C) and monocytes (D) harvested from C57Bl/6 (wild-type [WT]) or Fpr2−/− mice. E, β2 integrin activation assessed by mAb24 binding and flow cytometry in human neutrophils incubated with CCL5 and preincubated with Ac2-26. Where indicated, neutrophils were pretreated with WT or constitutively active (CA) Rap1-Tat peptides. F, Redistribution of surface LFA1 on mouse neutrophils stimulated for 90 s with CCL5 and pretreated for 30 minutes with Ac2-26. G, Mean fluorescence intensity (MFI) reflecting intracellular calcium in mouse neutrophils before and after addition of CCL5 with or without Ac2-26, which was added 30 minutes before CCL5 stimulation. Data are representative recordings from 5 independent runs. H, GTP-bound Rap1 protein in mouse neutrophils 30 s after stimulation with CCL5 and prestimulation for 30 minutes with Ac2-26. Representative blots from 3 experiments are shown. All data are presented as mean±SD. Experiments were performed ≥6× independently for A–E. *P<0.05 in C and D *Differences compared with CCL5 stimulation. Data were analyzed using Kruskal–Wallis test with Dunn post test. CAM indicates cell adhesion molecule.
Delivery of the Annexin A1 Fragment Ac2-26 Reduces Atherogenesis
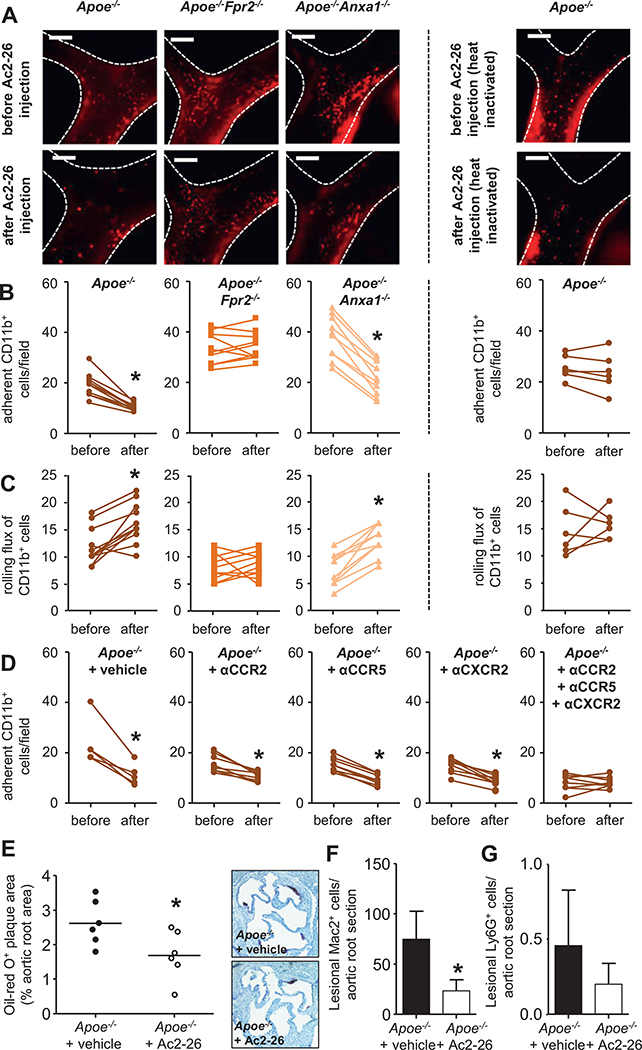
With the powerful abilities of AnxA1 to abrogate integrin activation induced by chemokines, we further aimed at using this knowledge in a translational approach. Hence, we hypothesized that in vivo delivery of Ac2-26 might reduce arterial myeloid cell recruitment. Intravital microscopy in Apoe−/−, Apoe−/−Fpr2−/−, and Apoe−/−Anxa1−/− mice was used to study leukocyte dynamics before and after intravenous Ac2-26 injection. To define the optimal time and dose for such experiments, we performed dose–response and time–course analyses using the arterial adhesion of CD11b+ myeloid cells to the carotid artery of Apoe−/− mice fed a HFD for 4 weeks as read-out. In these experiments, Ac2-26 at 0.1 and 1 μg was without effect throughout the observation period of ≤30 minutes after Ac2-26 administration. In contrast, Ac2-26 at 10 and 50 μg significantly reduced the number of adherent cells at 10 and 30 minutes after injection (Online Figure VA). On the basis of this, we decided to study arterial leukocyte adhesion before and 30 minutes after a single dose of Ac2-26 (50 μg). Although Ac2-26 shifted the adherent CD11b+ cell fraction in Apoe−/− mice toward the rolling cell fraction, an effect observed even more pronounced in Apoe−/−Anxa1−/− mice, this effect was abolished in Apoe−/−Fpr2−/− mice (Figure 5A–5C). Similar effects were also observed for Ly6G+ and Ly6C+ cells (Online Figure VB–VE). In addition, boiled Ac2-26 failed to affect adhesion of myeloid cells (Figure 5A–5C; Online Figure VB–VE).
Figure 5. In vivo delivery of the annexin A1 fragment Ac2-26 reduces atherosclerosis.
A–C, Apoe−/−, Apoe−/−Fpr2−/−, and Apoe−/−Anxa1−/− mice were fed a high-fat diet (HFD) for 4 weeks. Intravital microscopy of the carotid artery was used for assessment of luminal leukocyte endothelial interactions. Myeloid cells were identified by intravenous injection of an antibody to CD11b 10 minutes before recording. Myeloid cell adhesion (B) and rolling flux (C) in Apoe−/− (left), Apoe−/−Fpr2−/− (middle), and Apoe−/−Anxa1−/− (right) mice were assessed before and 30 minutes after injection of native (panels to the left) or boiled Ac2-26 (far right panel; 50 μg, IV). Representative images are shown in A. Scale bar, 100 μm. Each dot represents 1 mouse. *P<0.05 compared with read-out before Ac2-26 administration. Data were analyzed with paired t test. D, Apoe−/− mice were fed a HFD for 4 weeks and mice received a single dose of antagonist to CCR2 (RS504393, 5 mg/kg), CCR5 (DAPTA, 1 mg/kg), or CXCR2 (SB225002, 5 mg/kg), or a combination of all, or vehicle control. Thirty minutes later arterial adhesion of CD11b+ cells was studied (before). Immediately after recording, mice received Ac2-26 (50 μg IV) and adhesion was studied 30 minutes later (after). Each dot represents 1 mouse. *P<0.05 compared with read-out before Ac2-26 administration. Data were analyzed with Wilcoxon matched-pairs signed rank test. E–G, Ac2-26 reduces atherogenesis. Apoe−/− mice (n=6 per group) were repeatedly injected with Ac2-26 (3× per week; 50 μg IP per injection) or vehicle control during 4 weeks of HFD. E, Quantification of atherosclerotic lesion sizes in Oil-Red-O–stained aortic root sections. Representative images are shown aside. F, Quantification of Mac2+ macrophages. G, Quantification of lesional Ly6G+ neutrophils. *P<0.05 compared with vehicle treatment. Data in F and G are presented as mean±SD. Data in E–G were analyzed with Mann-Whitney test.
With the potent effect of Ac2-26 to reduce arterial myeloid cell adhesion and to diminish integrin activation evoked by chemokines, we aimed at comparing the effect of Ac2-26 and chemokine receptor antagonists. Because Ac2-26 largely diminished CCL5-evoked integrin activation triggered by CCL2 and CXCL1 to a lower degree, we used specific antagonists to CCR5, CCR2, and CXCR2 to study the effect of Ac2-26 on arterial myeloid cell adhesion. Apoe−/− mice receiving a HFD for 4 weeks were treated with an antagonist to CCR5, CCR2, or CXCR2, a combination of all 3, or vehicle only 30 minutes before intravital microscopy. Adhesion of myeloid cell subsets along the carotid artery was studied before and 30 minutes after intravenous injection of Ac2-26. Although Ac2-26 treatment reduced adhesion of myeloid cell subsets when mice were treated with just 1 chemokine receptor antagonist, this was abrogated in the presence of the combination of antagonists to CCR2, CCR5, and CXCR2 (Figure 5D; Online Figure VF and VG).
These data indicate that Ac2-26 is able to counteract myeloid cell adhesion evoked by various chemokines, and hence we suspected that Ac2-26 should be a powerful tool in reducing early atherosclerotic lesion burden. Thus, we performed a therapeutic experiment with repeated injections of Ac2-26 (3× per week; 50 μg IP per injection) or vehicle control during 4 weeks of HFD. Ac2-26 did not have any obvious side effects and did not alter circulating leukocyte counts or plasma lipid levels (Online Table II). Histomorphometry of atherosclerotic lesion sizes in aortic root sections, however, revealed a clear-cut reduction in mice receiving Ac2-26 (Figure 5E). In line, Ac2-26 administration lowered lesional macrophage counts, whereas neutrophil numbers were low in both groups and Ac2-26 treatment only exerted a trend toward reduction (Figure 5F and 5G).
Discussion
AnxA1 is a molecule that belongs to a large and structurally heterogeneous family of proresolving molecules. Other family members include resolving lipid mediators, proresolving cytokines, and proresolving hormones. Although these molecules are structurally different they share functional similarities. They terminate inflammatory leukocyte accumulation by reducing leukocyte recruitment and enhancing egress; they stimulate clearance of aged and apoptotic cells and actively promote tissue repair.16 Here, we show that AnxA1 is an endogenous inhibitor of arterial leukocyte recruitment. Because this process is a mechanism essential to atherogenesis, it is not surprising that mice lacking AnxA1 or its receptor FPR2 have accelerated atherosclerosis. Mechanistically, we show that the AnxA1 fragment Ac2-26 counteracts chemokine-mediated integrin activation in neutrophils and monocytes and could hence be a potential tool for the treatment of atherosclerosis. Direct interference with integrins, cell adhesion molecules, or chemokines to prevent arterial leukocyte recruitment has only achieved limited success in translational studies.17,18 Reasons for such limitations include the striking redundancy of cell adhesion molecules and chemokines during atherogenic recruitment, rendering interference with just 1 molecule insufficient. Indeed, it has been shown that combined inhibition of chemokine signaling has an additive effect in the treatment of atherosclerosis in mice.19 By counteracting arterial myeloid cell adhesion instructed by a variety of chemokines, AnxA1-based therapeutic approaches may be superior to the use of individual chemokine receptor antagonists. However, systemic delivery of Ac2-26 may also affect myeloid cell recruitment in acute inflammation and thus impair host defense. Thus, delivery strategies with arterial tropism may be favorable for future translational approaches.20,21
Beyond its ability to reduce myeloid cell adhesion, AnxA1 exerts several proresolving effects that may be beneficial during atheroprogression and plaque destabilization. In this context, AnxA1 was identified as a bridging molecule opsonizing apoptotic cells to mediate efferocytosis.22 In addition, AnxA1 induces a favorable macrophage M2a phenotype,23 which is characterized by the release of the cytokines interleukin-10 and TGFβ. Thus, AnxA1 and its fragments counteract inflammatory processes of central importance during advanced stages of atherosclerosis,4 and delivery of AnxA1 fragments may promote plaque stability. Of note, analyses of human atherosclerotic plaques revealed a correlation between lesional AnxA1 and signs of plaque stability.9 Interestingly, treatment with the annexin family member annexin A5 has been shown to reduce inflammation during advanced stages of atherosclerosis24 and in models of arterial injury.25,26 These indications point toward the possible importance of AnxA1 in plaque stabilization. However, a recent study indicates that mice lacking FPR2 and FPR3 are partially protected from atherosclerosis at a later stage.27 Thus, subsequent studies are required to define the role of AnxA1 and its therapeutic potential during atheroprogression and plaque destabilization clearly.
An additional point of interest is the origin of AnxA1 in the context of hypercholesterolemia-induced atherosclerosis. Although neutrophils release AnxA1 after establishing interactions with the endothelium,28 it appears unlikely that neutrophils are a major source during atherogenesis because they have been shown to promote early lesion formation.13 Instead, we here show that the arterial endothelium expresses large amounts of AnxA1. Given that the interaction between AnxA1 and myeloid cells is likely to occur in the blood or at the interface between blood and the atherosclerotic lesion, the endothelium could be an important source of AnxA1 in atherosclerosis. In fact, it has been shown that endothelial expression of AnxA1 decreases during endothelial dysfunction and vascular inflammation.29,30 Interestingly, AnxA1 is primarily a cytosolic protein lacking signal sequences that could direct it into the classical secretory pathway. Nevertheless, AnxA1 can be detected in the plasma under conditions of inflammation or in the supernatant of activated endothelial cells29,31 and hence alternative pathways for the secretion of AnxA1 have been proposed. One of these pathways includes the externalization via ABCA132 and endothelial expression of this transporter is protective in a mouse model of atherosclerosis.33 However, additional studies are needed to understand the endogenous source of AnxA1 during hypercholesterolemia.
A striking observation of the here presented study is the differential effect of FPR2 ligands. Although previous work highlighted the proatherogenic role of FPR2 ligands, such as cathelicidin and serum amyloid A,7,8 we show that AnxA1exerts antiatherogenic effects. Such opposing effects are not just shown in disease models but have also been reported on a cellular level. For example, acute-phase protein serum amyloid A and cathelicidin mediate FPR2-dependent proinflammatory leukocyte activation, including leukocyte trafficking, cytokine secretion, and inhibition of neutrophil apoptosis.34–36 In contrast, AnxA1, Ac2-26, and lipoxin A4 also signal through FPR2 to inhibit leukocyte recruitment, enhance neutrophil apoptosis, and macrophage efferocytosis,35,37 key events in inflammatory resolution. Lipid and peptide ligands act with different affinities and bind to distinct pockets on the receptor, thus making a direct competition unlikely.38 One mechanistic explanation for divergent effects of FPR2 ligands may reside in the ability of FPR2 to form homo- and heterodimers.39 Although dimerization of G-protein–coupled receptors may not generally be required for ligand recognition, emerging evidence indicates that dimers of G-protein–coupled receptors may affect signaling functions and that ligand selectivity may be directly related to different receptor conformation.40 FPR2 homodimers and FPR2/FPR1 heterodimers constitutively occur in human leukocytes.39 In addition, dimer formation and change in receptor conformation can be induced in a ligand-specific fashion, and this does not seem to be simply a consequence of ligand–receptor interaction. As an example, Ac2-26 was found to activate the JNK/caspase-3 pathway, leading to apoptosis in neutrophils. Importantly, this pathway over-rode the apoptosis suppressing action of serum amyloid A and cathelicidin.39
Recent reports have suggested the tight control of leukocyte recruitment by endogenous inhibitors. In this context, leukocyte-derived pentraxin-3 binds to endothelial P-selectin thereby abrogating leukocyte rolling.41 Del-1 directly inhibits the interaction of leukocytic LFA1 and ICAM-1.42 Finally, GDF-15 (growth differentiation factor 15) counteracts chemokine-induced activation of β2 integrins by interfering with the activity of Rap1-GTPase.43 Hence, in the context of leukocyte recruitment, the latter molecule seems to share much of its functionality with AnxA1. However, the role of these molecules in atherosclerosis has not been investigated yet. Thus, the presented study is the first report on the role of an endogenous inhibitor of leukocyte recruitment in atherosclerosis. Local delivery of AnxA1, its Ac2-26 fragment, or other endogenous inhibitors of leukocyte recruitment, may harbor valuable opportunities for the prevention and treatment of atherosclerosis.
Supplementary Material
Novelty and Significance.
What Is Known?
Neutrophils and monocytes enter atherosclerotic lesions in a process involving chemokine-mediated integrin activation.
Annexin A1 is an endogenous inhibitor of leukocyte recruitment preventing firm adhesion of neutrophils in the microcirculation.
What New Information Does This Article Contribute?
Annexin A1 and its receptor formyl peptide receptor 2 are protective during early stages of atherosclerosis in Apoe null mice fed a high-fat diet.
The annexin A1 fragment Ac2-26 inhibits chemokine-evoked integrin activation and with this arterial adhesion of neutrophils and monocytes.
Repeated treatment with Ac2-26 reduces early atherosclerosis.
Endogenous inhibitors of leukocyte recruitment have been identified as a set of breaks to fine-tune inflammatory leukocyte trafficking. However, their role in atherosclerosis is largely unknown. Here, we show that annexin A1, a protein known to inhibit leukocyte adhesion in microvascular inflammation, and its receptor formyl peptide receptor 2, are protective during early stages of atherosclerosis in Apoe null mice. Mechanistically, the annexin A1 fragment Ac2-26 counteracts chemokine-induced integrin activation, a process important in arterial leukocyte recruitment. Notably, Ac2-26 overrides the chemokine signaling exerted by several chemokines. Hence, Ac2-26 fragment of annexin1 might be of clinical importance in settings where redundant chemokine signaling guides leukocytes to sites of inflammation.
Acknowledgments
We thank Yvonne Jansen, Patricia Lemnitzer, and Gitte Zimmer for excellent technical assistance.
Sources of Funding
The study was supported by the DFG (Deutsche Forschungsgemeinschaft; SO876/3-1, SO876/6-1, FOR809, SFB914 TP B08, SFB1123 TP A06, ZA428/6-1, SFB1009 TP A05), the Else Kröner Fresenius Stiftung, the NWO (Nederlandse Organisatie voor Wetenschappelijk Onderzoek; VIDI project 91712303), the LMUexcellence and the FöFoLe program of the Ludwig-Maximilians-Universität München.
Nonstandard Abbreviations and Acronyms
- AnxA1
annexin A1
- FPR2
formyl peptide receptor 2
- HFD
high-fat diet
- ICAM-1
intercellular adhesion molecule-1
- VCAM-1
vascular cell adhesion molecule-1
Footnotes
Disclosures
None.
The online-only Data Supplement is available with this article at http://circres.ahajournals.org/lookup/suppl/doi:10.1161/CIRCRESAHA.116.305825/-/DC1.
Contributor Information
Maik Drechsler, Institute for Cardiovascular Prevention (IPEK), LMU Munich, Munich, Germany; Department of Pathology, Academic Medical Center (AMC), Amsterdam University, Amsterdam, The Netherlands.
Renske de Jong, Institute for Cardiovascular Prevention (IPEK), LMU Munich, Munich, Germany.
Jan Rossaint, Department of Anaesthesiology, University Münster, Münster, Germany; Max Planck Institute, Münster, Germany.
Joana R. Viola, Institute for Cardiovascular Prevention (IPEK), LMU Munich, Munich, Germany
Giovanna Leoni, Institute for Cardiovascular Prevention (IPEK), LMU Munich, Munich, Germany.
Ji Ming Wang, Laboratory of Molecular Immunoregulation, NCI, Frederick, MD.
Jochen Grommes, Institute for Cardiovascular Prevention (IPEK), LMU Munich, Munich, Germany; European Vascular Center Aachen-Maastricht, University Hospital RWTH Aachen, Aachen, Germany.
Rabea Hinkel, Medizinische Klinik und Poliklinik I, Klinikum Großhadern, LMU Munich, Munich, Germany; DZHK, Partner Site Munich Heart Alliance, Munich, Germany.
Christian Kupatt, Medizinische Klinik und Poliklinik I, Klinikum Großhadern, LMU Munich, Munich, Germany; DZHK, Partner Site Munich Heart Alliance, Munich, Germany.
Christian Weber, Institute for Cardiovascular Prevention (IPEK), LMU Munich, Munich, Germany; DZHK, Partner Site Munich Heart Alliance, Munich, Germany.
Yvonne Döring, Institute for Cardiovascular Prevention (IPEK), LMU Munich, Munich, Germany.
Alexander Zarbock, Department of Anaesthesiology, University Münster, Münster, Germany; Max Planck Institute, Münster, Germany.
Oliver Soehnlein, Institute for Cardiovascular Prevention (IPEK), LMU Munich, Munich, Germany; Department of Pathology, Academic Medical Center (AMC), Amsterdam University, Amsterdam, The Netherlands; DZHK, Partner Site Munich Heart Alliance, Munich, Germany.
References
- 1.Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339:161–166. doi: 10.1126/science.1230719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17:1410–1422. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- 3.Potteaux S, Gautier EL, Hutchison SB, van Rooijen N, Rader DJ, Thomas MJ, Sorci-Thomas MG, Randolph GJ. Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of Apoe−/− mice during disease regression. J Clin Invest. 2011;121:2025–2036. doi: 10.1172/JCI43802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silvestre-Roig C, de Winther MP, Weber C, Daemen MJ, Lutgens E, Soehnlein O. Atherosclerotic plaque destabilization: mechanisms, models, and therapeutic strategies. Circ Res. 2014;114:214–226. doi: 10.1161/CIRCRESAHA.114.302355. [DOI] [PubMed] [Google Scholar]
- 5.Migeotte I, Communi D, Parmentier M. Formyl peptide receptors: a promiscuous subfamily of G protein-coupled receptors controlling immune responses. Cytokine Growth Factor Rev. 2006;17:501–519. doi: 10.1016/j.cytogfr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Norling LV, Perretti M. Control of myeloid cell trafficking in resolution. J Innate Immun. 2013;5:367–376. doi: 10.1159/000350612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Döring Y, Drechsler M, Wantha S, Kemmerich K, Lievens D, Vijayan S, Gallo RL, Weber C, Soehnlein O. Lack of neutrophil-derived CRAMP reduces atherosclerosis in mice. Circ Res. 2012;110:1052–1056. doi: 10.1161/CIRCRESAHA.112.265868. [DOI] [PubMed] [Google Scholar]
- 8.Dong Z, Wu T, Qin W, An C, Wang Z, Zhang M, Zhang Y, Zhang C, An F. Serum amyloid A directly accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. Mol Med. 2011;17:1357–1364. doi: 10.2119/molmed.2011.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheuk BL, Cheng SW. Annexin A1 expression in atherosclerotic carotid plaques and its relationship with plaque characteristics. Eur J Vasc Endovasc Surg. 2011;41:364–371. doi: 10.1016/j.ejvs.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 10.Viiri LE, Full LE, Navin TJ, Begum S, Didangelos A, Astola N, Berge RK, Seppälä I, Shalhoub J, Franklin IJ, Perretti M, Lehtimäki T, Davies AH, Wait R, Monaco C. Smooth muscle cells in human atherosclerosis: proteomic profiling reveals differences in expression of Annexin A1 and mitochondrial proteins in carotid disease. J Mol Cell Cardiol. 2013;54:65–72. doi: 10.1016/j.yjmcc.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Chen K, Le Y, Liu Y, Gong W, Ying G, Huang J, Yoshimura T, Tessarollo L, Wang JM. A critical role for the g protein-coupled receptor mFPR2 in airway inflammation and immune responses. J Immunol. 2010;184:3331–3335. doi: 10.4049/jimmunol.0903022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hannon R, Croxtall JD, Getting SJ, Roviezzo F, Yona S, Paul-Clark MJ, Gavins FN, Perretti M, Morris JF, Buckingham JC, Flower RJ. Aberrant inflammation and resistance to glucocorticoids in annexin 1−/− mouse. FASEB J. 2003;17:253–255. doi: 10.1096/fj.02-0239fje. [DOI] [PubMed] [Google Scholar]
- 13.Drechsler M, Megens RT, van Zandvoort M, Weber C, Soehnlein O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation. 2010;122:1837–1845. doi: 10.1161/CIRCULATIONAHA.110.961714. [DOI] [PubMed] [Google Scholar]
- 14.Soehnlein O, Drechsler M, Döring Y, et al. Distinct functions of chemokine receptor axes in the atherogenic mobilization and recruitment of classical monocytes. EMBO Mol Med. 2013;5:471–481. doi: 10.1002/emmm.201201717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergmeier W, Goerge T, Wang HW, Crittenden JR, Baldwin AC, Cifuni SM, Housman DE, Graybiel AM, Wagner DD. Mice lacking the signaling molecule CalDAG-GEFI represent a model for leukocyte adhesion deficiency type III. J Clin Invest. 2007;117:1699–1707. doi: 10.1172/JCI30575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ortega-Gómez A, Perretti M, Soehnlein O. Resolution of inflammation: an integrated view. EMBO Mol Med. 2013;5:661–674. doi: 10.1002/emmm.201202382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horuk R Chemokine receptor antagonists: overcoming developmental hurdles. Nat Rev Drug Discov. 2009;8:23–33. doi: 10.1038/nrd2734. [DOI] [PubMed] [Google Scholar]
- 18.Ling S, Nheu L, Komesaroff PA. Cell adhesion molecules as pharmaceutical target in atherosclerosis. Mini Rev Med Chem. 2012;12:175–183. [DOI] [PubMed] [Google Scholar]
- 19.Combadière C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A, Mallat Z. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–1657. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 20.Schiener M, Hossann M, Viola JR, Ortega-Gomez A, Weber C, Lauber K, Lindner LH, Soehnlein O. Nanomedicine-based strategies for treatment of atherosclerosis. Trends Mol Med. 2014;20:271–281. doi: 10.1016/j.molmed.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Kamaly N, Fredman G, Subramanian M, Gadde S, Pesic A, Cheung L, Fayad ZA, Langer R, Tabas I, Farokhzad OC. Development and in vivo efficacy of targeted polymeric inflammation-resolving nanoparticles. Proc Natl Acad Sci U S A. 2013;110:6506–6511. doi: 10.1073/pnas.1303377110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arur S, Uche UE, Rezaul K, Fong M, Scranton V, Cowan AE, Mohler W, Han DK. Annexin I is an endogenous ligand that mediates apoptotic cell engulfment. Dev Cell. 2003;4:587–598. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Cai L, Wang H, Wu P, Gu W, Chen Y, Hao H, Tang K, Yi P, Liu M, Miao S, Ye D. Pleiotropic regulation of macrophage polarization and tumorigenesis by formyl peptide receptor-2. Oncogene. 2011;30:3887–3899. doi: 10.1038/onc.2011.112. [DOI] [PubMed] [Google Scholar]
- 24.Burgmaier M, Schutters K, Willems B, van der Vorst EP, Kusters D, Chatrou M, Norling L, Biessen EA, Cleutjens J, Perretti M, Schurgers LJ, Reutelingsperger CP. AnxA5 reduces plaque inflammation of advanced atherosclerotic lesions in apoE(−/−) mice. J Cell Mol Med. 2014;18:2117–2124. doi: 10.1111/jcmm.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ewing MM, de Vries MR, Nordzell M, Pettersson K, de Boer HC, van Zonneveld AJ, Frostegård J, Jukema JW, Quax PH. Annexin A5 therapy attenuates vascular inflammation and remodeling and improves endothelial function in mice. Arterioscler Thromb Vasc Biol. 2011;31:95–101. doi: 10.1161/ATVBAHA.110.216747. [DOI] [PubMed] [Google Scholar]
- 26.Ewing MM, Karper JC, Sampietro ML, de Vries MR, Pettersson K, Jukema JW, Quax PH. Annexin A5 prevents post-interventional accelerated atherosclerosis development in a dose-dependent fashion in mice. Atherosclerosis. 2012;221:333–340. doi: 10.1016/j.atherosclerosis.2012.01.037. [DOI] [PubMed] [Google Scholar]
- 27.Petri MH, Laguna-Fernandez A, Tseng CN, Hedin U, Perretti M, Bäck M. Aspirin-triggered 15-epi-lipoxin A4 signals through FPR2/ALX in vascular smooth muscle cells and protects against intimal hyperplasia after carotid ligation. Int J Cardiol. 2015;179:370–372. doi: 10.1016/j.ijcard.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perretti M, Croxtall JD, Wheller SK, Goulding NJ, Hannon R, Flower RJ. Mobilizing lipocortin 1 in adherent human leukocytes downregulates their transmigration. Nat Med. 1996;2:1259–1262. [DOI] [PubMed] [Google Scholar]
- 29.Cristante E, McArthur S, Mauro C, Maggioli E, Romero IA, Wylezinska-Arridge M, Couraud PO, Lopez-Tremoleda J, Christian HC, Weksler BB, Malaspina A, Solito E. Identification of an essential endogenous regulator of blood-brain barrier integrity, and its pathological and therapeutic implications. Proc Natl Acad Sci U S A. 2013;110:832–841. doi: 10.1073/pnas.1209362110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paravicini TM, Yogi A, Mazur A, Touyz RM. Dysregulation of vascular TRPM7 and annexin-1 is associated with endothelial dysfunction in inherited hypomagnesemia. Hypertension. 2009;53:423–429. doi: 10.1161/HYPERTENSIONAHA.108.124651. [DOI] [PubMed] [Google Scholar]
- 31.Römisch J, Schüler E, Bastian B, Bürger T, Dunkel FG, Schwinn A, Hartmann AA, Pâques EP. Annexins I to VI: quantitative determination in different human cell types and in plasma after myocardial infarction. Blood Coagul Fibrinolysis. 1992;3:11–17. [PubMed] [Google Scholar]
- 32.Omer S, Meredith D, Morris JF, Christian HC. Evidence for the role of adenosine 5’-triphosphate-binding cassette (ABC)-A1 in the externalization of annexin 1 from pituitary folliculostellate cells and ABCA1-transfected cell models. Endocrinology. 2006;147:3219–3227. doi: 10.1210/en.2006-0099. [DOI] [PubMed] [Google Scholar]
- 33.Vaisman BL, Demosky SJ, Stonik JA, Ghias M, Knapper CL, Sampson ML, Dai C, Levine SJ, Remaley AT. Endothelial expression of human ABCA1 in mice increases plasma HDL cholesterol and reduces dietinduced atherosclerosis. J Lipid Res. 2012;53:158–167. doi: 10.1194/jlr.M018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wantha S, Alard JE, Megens RT, van der Does AM, Döring Y, Drechsler M, Pham CT, Wang MW, Wang JM, Gallo RL, von Hundelshausen P, Lindbom L, Hackeng T, Weber C, Soehnlein O. Neutrophil-derived cathelicidin promotes adhesion of classical monocytes. Circ Res. 2013;112:792–801. doi: 10.1161/CIRCRESAHA.112.300666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El Kebir D, József L, Khreiss T, Pan W, Petasis NA, Serhan CN, Filep JG. Aspirin-triggered lipoxins override the apoptosis-delaying action of serum amyloid A in human neutrophils: a novel mechanism for resolution of inflammation. J Immunol. 2007;179:616–622. [DOI] [PubMed] [Google Scholar]
- 36.Wan M, Godson C, Guiry PJ, Agerberth B, Haeggström JZ. Leukotriene B4/antimicrobial peptide LL-37 proinflammatory circuits are mediated by BLT1 and FPR2/ALX and are counterregulated by lipoxin A4 and resolvin E1. FASEB J. 2011;25:1697–1705. doi: 10.1096/fj.10-175687. [DOI] [PubMed] [Google Scholar]
- 37.Dalli J, Consalvo AP, Ray V, Di Filippo C, D’Amico M, Mehta N, Perretti M. Proresolving and tissue-protective actions of annexin A1-based cleavage-resistant peptides are mediated by formyl peptide receptor 2/lipoxin A4 receptor. J Immunol. 2013;190:6478–6487. doi: 10.4049/jimmunol.1203000. [DOI] [PubMed] [Google Scholar]
- 38.Chiang N, Serhan CN, Dahlén SE, Drazen JM, Hay DW, Rovati GE, Shimizu T, Yokomizo T, Brink C. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev. 2006;58:463–487. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- 39.Cooray SN, Gobbetti T, Montero-Melendez T, McArthur S, Thompson D, Clark AJ, Flower RJ, Perretti M. Ligand-specific conformational change of the G-protein-coupled receptor ALX/FPR2 determines proresolving functional responses. Proc Natl Acad Sci U S A. 2013;110:18232–18237. doi: 10.1073/pnas.1308253110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mary S, Damian M, Louet M, Floquet N, Fehrentz JA, Marie J, Martinez J, Banères JL. Ligands and signaling proteins govern the conformational landscape explored by a G protein-coupled receptor. Proc Natl Acad Sci U S A. 2012;109:8304–8309. doi: 10.1073/pnas.1119881109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deban L, Russo RC, Sironi M, et al. Regulation of leukocyte recruitment by the long pentraxin PTX3. Nat Immunol. 2010;11:328–334. doi: 10.1038/ni.1854. [DOI] [PubMed] [Google Scholar]
- 42.Choi EY, Chavakis E, Czabanka MA, et al. Del-1, an endogenous leukocyte-endothelial adhesion inhibitor, limits inflammatory cell recruitment. Science. 2008;322:1101–1104. doi: 10.1126/science.1165218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kempf T, Zarbock A, Widera C, et al. GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat Med. 2011;17:581–588. doi: 10.1038/nm.2354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.