Abstract
Objective
The purpose of this study is to review CDK 4/6 inhibitors used to treat metastatic breast cancer for patient safety, cost and utilization. By evaluating patient outcomes and payer influence, this study will provide critical information to aid prescribers in therapeutic decisions.
Methods
This retrospective cohort study included patients from a national specialty pharmacy with a diagnosis of breast cancer and received either palbociclib, abemaciclib, or ribociclib for treatment. Patients were stratified into four subgroups based on their total oncolytic regimen at the time of their first eligible study medication dispense. Pharmacy claims data were reviewed to determine cost and therapy adherence.
Results
The mean proportion of days covered was highest in patients on combination therapy with a hormone agent, 81.0%. While secondary insurances largely affected final patient out-of-pocket costs, final copays were significantly lower than the average wholesale price (AWP) of each CDK 4/6 inhibitor. When analyzing patient reported side effects, over 60% of the study population did not experience an adverse drug event (ADE) during the study time period. Ribociclib had the fewest number of reported side effects with abemaciclib patients reporting the most. Although reported ADE profiles were similar across all three study medications, difference in frequency should be evaluated when considering medication choice with specific comorbidities.
Conclusion
CDK 4/6 inhibitors have demonstrated safety and tolerability in HR-positive/HER2-negative breast cancer patients. Real world safety data and out-of-pocket patient costs in addition patient specific comorbidities should be considered when developing a treatment plan that includes a CDK 4/6 inhibitor selection.
Keywords: Palbociclib, abemaciclib, ribociclib, breast cancer, cancer, side effects, safety, adverse events
Background
Breast cancer is the second most commonly diagnosed cancer in women; equaling approximately 30% of all newly diagnosed cancers in women in the United States. Breast cancer is typically invasive in nature, overtaking breast tissue, but can also be noninvasive, contained to the milk ducts and lobules of the breast. It is predicted that over 276,000 new cases of invasive breast cancer and 48,000 new cases of noninvasive breast cancer will be diagnosed in women in 2020. Males have a 1 in 883 lifetime risk of breast cancer with a predictive 2600 new cases to be diagnosed in 20201. If unmanaged, breast cancer typically metastasizes to the bones, brain, and lungs leading to worsening prognosis and disease state complications. Although the number of breast cancer deaths has decreased over the past decade, breast cancer is the second highest cause of death due to cancer in the female population. Current data report the 5-year survival rate of invasive breast cancer for women is 91%. This number decreases to 86% when metastasized to the lymph nodes and 27% in metastases to distant organs1,2. Men typically have a worse prognosis and lower survival rates when compared to women. Due to lack of trials and resulting data, as well as absence of screening recommendations for male breast cancer patients, males are often diagnosed much later after cancer development. A recently published study reported 5 year survival of male patients to be 9% lower than their female counter parts as well as having a 19% increased all-cause mortality rate3.
The most common form of breast cancer is hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative which makes up 60% of all breast cancer diagnosis4. HR-positive patients are predicted to have a better response to hormonal therapy. However, the lack of HER2 proteins in this cancer type eliminates medications that are specifically designed to target these proteins as therapy options for this patient population group. The normal cell replication process is broken down into four main phases including G1, when cell growth occurs through RNA and protein synthesis, which then transitions into the S phase, allowing DNA synthesis to occur before ultimately duplicating and replicating in the G2 and mitosis phases. The retinoblastoma (Rb) protein, a tumor suppressor, tightly regulates the transcription of RNA during the G1 phase to halt further development and proliferation of malignant cells5. HR-positive breast cancer has high expression of cyclin D1 proteins due to positive estrogen receptors (ER)6. The cyclin D1 proteins binds to cyclin-dependent kinase (CDK) enzymes 4 and 6 during the transition of phases G1 to S of the cycle. This bound D1-CDK 4/6 complex then inhibits the regulatory function of Rb protein. The high frequency of cyclin D1 in HR-positive cancer leads to an abundance of D1-CDK 4/6 complexes resulting in lack of regulatory function by the Rb protein and amplified cell proliferation. A newer class of oral oncolytics, CDK 4/6 inhibitors, arrest the transition of G1 to S by preventing the cyclin D1-CDK 4/6 complex from forming, ultimately allowing Rb to properly to control proliferation5,7.
The National Comprehensive Cancer Network (NCCN) recognizes CDK 4/6 inhibitors palbociclib, abemaciclib, and ribociclib in combination with aromatase inhibitors or fulvestrant as category 1 first-line therapy regimens in the treatment of HR-positive/HER2-negative advanced or metastatic breast cancer in postmenopausal or premenopausal women receiving ovarian ablation or suppression4. CDK 4/6 inhibitors are also utilized with hormone therapy for male patients diagnosed with HR-positive breast cancer8. Together, combination therapy treats HR-positive/HER2-negative advanced or metastatic breast cancer by lowering estrogen levels to block cell growth and inhibiting cyclin-dependent kinase to interrupt malignant cell division and proliferation9.
Previous studies have determined that both abemaciclib and ribociclib have demonstrated a relative reduction of the risk of death by 25–30%10,11.The progression free survival (PFS) of CDK 4/6 inhibitors with an aromatase inhibitor vs aromatase inhibitor monotherapy has also been reviewed. When an aromatase inhibitor was combined with palbociclib the PFS increased by 10 months, as compared to hormone monotherapy12. Similarly, the addition of palbociclib to fulvestrant yielded a two fold increase in PFS compared to those taking fulvestrant alone13. Although palbociclib and ribociclib are only FDA approved for use in combination with a hormone therapy agent, there are incidences of practitioners prescribing CDK 4/6 inhibitors as monotherapy. In a recently published study, it was reported that of the 75.8% of female HR-positive patients, only 70.2% of these patients received hormone therapy as part of their oncolytic regimen. The same study reported that although 84.5% of male breast cancer patients were HR-positive, only 57.9% of positive patients were prescribed dual therapy with a hormone therapy agent, a much larger difference than female patients14.
Due to lack of head to head studies and reported patient cases comparing efficacy of CDK 4/6 inhibitors alone versus in combination therapy, there is limited evidence to support the utilization of CDK 4/6 inhibitors as monotherapy. At this time, there is also inadequate data that treatment with a subsequent CDK 4/6 containing regimen would be beneficial if disease progression were to occur during treatment with a CDK 4/6 as first line treatment4. Since diagnosis of breast cancer in males is lower than that of other cancer diagnosis, there is a small portion of studies evaluating breast cancer therapies in men. Most data used to determine therapies for the male population are based on results of female only studies, therefore limiting viable therapy options for males14.
The objective of this study was to review current targeted CDK 4/6 inhibitors palbociclib, ribociclib, and abemaciclib, in real-world patients to treat metastatic breast cancer for patient safety, cost and utilization. Increasing data on safety and tolerability of CDK 4/6 inhibitors in real world patients can aid prescribers in their selection of initial therapy for HR-positive/HER2-negative breast cancer patients. Cost and side effects are large patient barriers15. By having a better understanding of average patient copay costs as well as the side effect profile of each medication, medication choice may be influenced to meet the needs of the HR-positive/HER2-negative breast cancer patients to ultimately help reduce therapy discontinuation rates or poor adherence. Additionally, by analyzing male patients specifically, we can increase insight on a clinical gap.
Methods
Study design
A retrospective analysis was performed using data collected from a national specialty pharmacy database. Patients included in the study were over the age of 18 and receiving a CDK 4/6 inhibitor for breast cancer. Patients were excluded from the study if they did not have two consecutive fills (two separate fills within 45 days of each other) of a CDK 4/6 inhibitor between January 1, 2019 and October 31, 2019. Patients prohibited from partaking in studies by their insurance provider were excluded prior to initial data pull. Patients who were initiated on therapy with either palbociclib, abemaciclib, or ribociclib prior to 2019 were included in study provided they still had two consecutive fills within the specified 10 month study period. Patients were then stratified into four subgroups based on their total oncolytic regimen prescribed at the time of their first eligible study medication dispense. These four groups consisted of CDK 4/6 inhibitor monotherapy, CDK 4/6 inhibitor in combination with hormone therapy, CDK 4/6 inhibitor in combination with another oncolytic class, or CDK 4/6 inhibitor combined with both hormone therapy and another oncolytic class.
Data collection
Data was collected from a specialty pharmacy’s operating database utilizing pharmacy dispensing software, clinical patient management applications, and communications provided from physician offices. Data collected included patient demographic information, prescription dispense history, patient reported adverse events and concurrent medications, patient reported hospitalizations, and cost of CDK 4/6 inhibitor treatment. Clinical patient management operations are utilized by both pharmacists and care coordinators responsible for scheduling deliveries and triaging patients to pharmacists. These clinical applications prompt for documentation of patient reported current medications and recent changes, side effects since previous dispense, and missed doses prior to each medication fill. These clinical assessments are applied by pharmacists to conduct full medication counseling sessions specific to the prescribed therapy prior to medication dispensing both at initiation of therapy and subsequent refill dispenses. These applications are adaptable to gear counseling based on patient specific responses. The captured clinical data from these assessments can then be extracted for research purposes. Patients whom were prescribed palbociclib or ribociclib and reported they were unsure or not prescribed adjunct hormone therapy, were intervened on via facsimile to physician offices for clarification as to whether or not the patient would be receiving the full FDA approved combination therapy. Therapy changes, therapy discontinuations, and dose changes were also confirmed with physician offices via facsimile or outreach calls.
Data analysis
Statistical data analysis was completed utilizing SAS (SAS Institute Inc., Cary, NC) and Microsoft Excel (Microsoft Corporation, Redmond, WA) software applications. Study results were compared across specified study groups, CDK 4/6 inhibitor specific populations, and clinical trials for respective therapies. Primary study outcome was to compare tolerability and safety of real world data versus clinical trials. Additional outcomes focused on medication adherence, utilization, and patient cost. Male patients were also separately analyzed to evaluate for gender specific utilization and safety outcomes.
This study was evaluated and approved by a university’s institutional review board prior to initiation, protocol number 2019/11/10. All patients included in this study received a Notice of Privacy Practice and Health Insurance Portability and Accountability Act authorization form with their first medication dispense to inform each patient that his or her protected health information may be used for research purposes as authorized by law. No funding was received from any outside source for this study.
Results
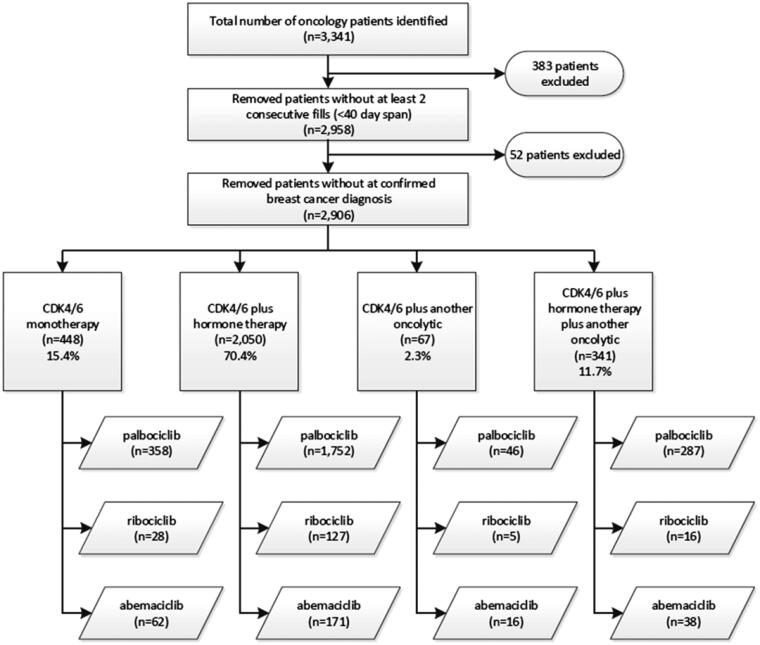
A total of 3341 adult patients received either palbociclib, abemaciclib, or ribociclib within the study period. 383 patients were excluded for not receiving two consecutive fills within 45 days, and an additional 52 patients were excluded due to off label prescribing. The remaining 2906 patients were then stratified into four categories based on their prescribed therapy regimen including CDK 4/6 inhibitor monotherapy, CDK 4/6 inhibitor with hormone therapy, CDK 4/6 inhibitor with another oncolytic agent, and CDK 4/6 inhibitor with both an oncolytic and hormone therapy. The majority of study patients were prescribed combination therapy with a CDK 4/6 and a hormonal therapy agent (Figure 1). Demographics across all patient subgroups were similar in respect to gender and patient age. The majority of study patients received albociclib (84.1%) (Table 1).
Figure 1.
Study population patient subcategory inclusion criteria.
Table 1.
Patient characteristics and CDK 4/6 inhibitor selection per therapy category.
| Therapy category | Patients, n (%) |
Female, n (%) |
Age, mean (range) | Prescribed CDK 4/6 Inhibitor | Patient Count by CDK 4/6 inhibitor |
|---|---|---|---|---|---|
| CDK4/6 monotherapy | 448 (15.4) | 439 (98.0) | 64 (29–100) | Palbociclib | 358 |
| Ribociclib | 28 | ||||
| Abemaciclib | 62 | ||||
| CDK4/6 + hormone therapy | 2050 (70.4) | 2034 (99.2) | 61 (25–99) | Palbociclib | 1752 |
| Ribociclib | 127 | ||||
| Abemaciclib | 171 | ||||
| CDK4/6 + oncolytic agent | 67 (2.3) | 64 (95.5) | 61 (38–84) | Palbociclib | 46 |
| Ribociclib | 5 | ||||
| Abemaciclib | 16 | ||||
| CDK4/6 + another agent + hormone therapy | 341 (11.7) | 337 (98.8) | 60 (29–86) | Palbociclib | 287 |
| Ribociclib | 16 | ||||
| Abemaciclib | 38 | ||||
| Total patients | 2906 (100) | 2874 (98.9) | 61 (25–100) | Palbociclib | 2443 (84.1%) |
| Ribociclib | 176 (6.1%) | ||||
| Abemaciclib | 287 (9.8%) |
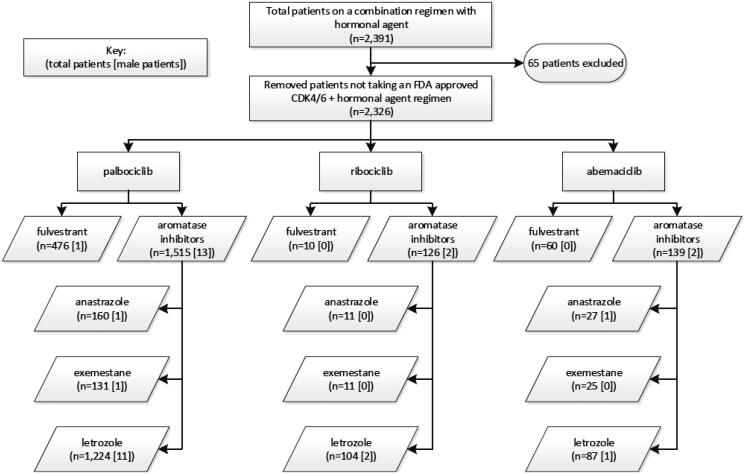
Of the 2391 patients prescribed both a CDK 4/6 inhibitor and hormone therapy with or without another oncolytic agent, 65 (2.2%) received a hormone agent not included in the FDA dosing guidelines. The majority of the remaining 2326 dual therapy patients were on an aromatase inhibitor, 1780 (76.5%) over fulvestrant, 546 (23.5%). Letrozole was the most prescribed aromatase inhibitor with all three CDK 4/6 inhibitors and was utilized in 79.5% of aromatase inhibitor receiving patients. The prescribing frequency of anastrozole and exemestane were similar, 11.1% and 9.4%, respectively (Figure 2). Of the study’s 32 male patients, 20 (62.5%) received hormone therapy; 17 (85%) of which was an aromatase inhibitor: letrozole (14), exemestane (1), and anastrozole (2).
Figure 2.
CDK 4/6 inhibitor and hormone combination therapy agent frequency.
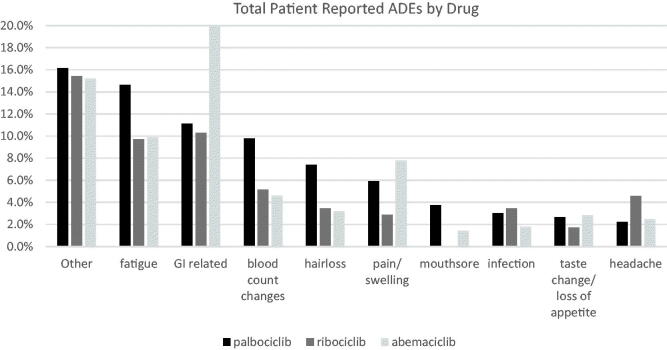
Throughout the study period, a total of 2200 adverse drug events (ADEs) were reported by 1141 patients. During the study 28 patients died and were removed from ADEs analysis leaving a final patient count of 2878 to be reviewed, 60% of which reported no ADEs. Ribociclib had the least reported side effects with just over 32% of patients reporting one or more ADEs. Most commonly reported ADE by ribociclib patients was GI related events, 18 (10.3%), closely followed by fatigue, 17 (9.7%). All other reported side effects occurred in fewer than 10 patients each, < 5% of the medication group. Patients in the palbociclib group reported higher rates of fatigue, blood count changes, and hair loss. The highest percentage of ADE reports per medication occurred in abemaciclib with over half of its patients experiencing at least one ADE. Gastrointestinal (GI) related ADEs were experienced by 37.6% of all abemaciclib study patients, affecting almost four times as many patients prescribed either palbociclib or ribociclib. Patients in the palbociclib group reported higher rates of fatigue, blood count changes, and hair loss. Headache and taste change frequency was consistent across the CDK 4/6 study medications (Figure 3).
Figure 3.
Frequency of patient reported adverse events.
Specific CDK 4/6 inhibitor therapy with either an aromatase inhibitor or fulvestrant was also analyzed. However, due to the small sample size of abemaciclib and ribociclib dual therapy patients, the analysis could not determine statistical significance of safety within these subgroups. The palbociclib patient population for analysis was not only the majority of this study’s patients, but also is much greater than the number of patients receiving palbociclib in the PALOMA-2 (1506 vs 444) and PALOMA-3 (473 vs 345) trials. Patients on combination therapy with fulvestrant had higher rates of fatigue and GI events compared those receiving an aromatase inhibitor across all three medications.
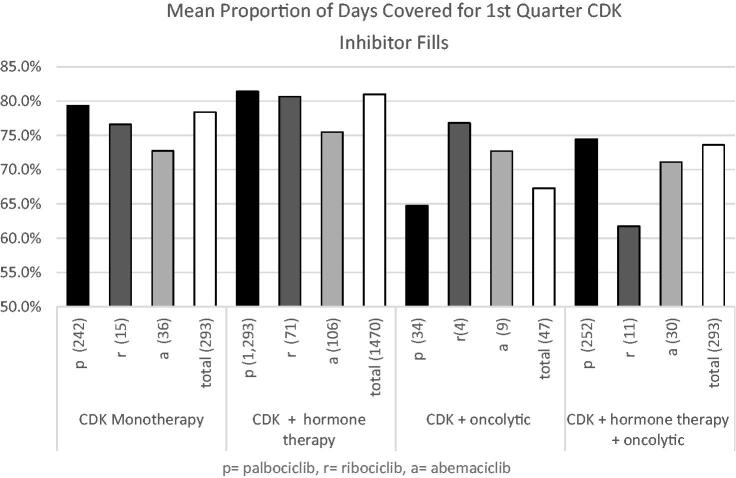
The proportion of days covered (PDC) was reported over a 6 month period and included patients that had their first fill within the first quarter of the study period. The mean PDC of patients was highest for patients on CDK 4/6 monotherapy or combination therapy with a hormone agent, 78.4% or 81.0%, respectively. The lowest PDC rate was the CDK 4/6 + another oncolytic agent category with a mean of 67.3% (Figure 4). This same trend was seen with palbociclib patients within these respective categories. The PDC for patient prescribed abemaciclib ranged from 71.1% to 75.5% across all patient categories. Throughout the 10 month period, 110 (7.5%) of all study patients discontinued CDK 4/6 therapy, a third of which switched to a new oncolytic agent. Dose changes were reported in 74 patients (5.0%) of the population and were highest in abemaciclib patients 19 (6.7%).
Figure 4.
Mean six-month proportion of days covered for CDK 4/6 inhibitors.
During the study, 52 patients switched from one CDK 4/6 study medication to another. Of those, 40% had reduced frequency or severity of ADEs. At the end of the study period, 62% of switched patients were still receiving the second prescribed inhibitor. Due to the small population size of patients switching CDK 4/6 inhibitors during the study, follow up studies need to be completed to adequately determine the safety and therapeutic benefit of continued utilization of CDK 4/6 inhibitors for a second line treatment.
Financially, when evaluating the same patients from the PDC analysis, the patient out of pocket copay was highest for ribociclib patients with commercial insurance accompanied with commercial secondary or a prescription assistance program (PAP). However, in patients lacking a secondary payer, ribociclib had the lowest final copay. Patients on abemaciclib with a secondary payer had the lowest levels of final copay (Table 2). Additionally, 9% of patients had a zero dollar final copay. When reviewing the average copay of each patient’s first CDK 4/6 inhibitor dispense, palbociclib and abemaciclib had a lower out-of-pocket cost compared to the AWP of the medications at $68 and $83. The first fill average cost of ribociclib was more than 4x abemaciclib at $376. Although these products are expensive with an average 30 day average wholesale price (AWP) of $14,089 the final patient out-of-pocket cost is much lower.
Table 2.
Average patient copays broken down by payer type.
| Primary payer types | Secondary payer types |
|||
|---|---|---|---|---|
| CDK4/6 | Primary payer | Commercial | PAP | None |
| Palbociclib | Commercial | $0 ($0–$50) | $108 ($0–$7312) | $65 ($0–$12,058) |
| Government | $21 ($0–$2345) | $73 ($0–$5259) | ||
| Ribociclib | Commercial | $298 ($0–$6474) | $245 ($0–$5975) | $32 ($0–$1859) |
| Government | $0 ($0–$0) | $0 ($0–$8) | ||
| Abemaciclib | Commercial | $0 ($0–$0) | $2 ($0–$10) | $53 ($0–$5914) |
| Government | $0 ($0–$0) | $160 ($0–$2492) | ||
Note: coupon and government secondary columns are either not present or at $0 costs. Means and ranges are based on 6-month period for first quarter 2019.
Discussion
The high prevalence of breast cancer and accompanying morbidities leads to continuing evolution of new technologies and real world studies to better understand and treat this cancer. Breast cancer guidelines are frequently updated to provide treatment recommendations based on the most recent information for this disease state4. The results of this study have provided additional insight in the management of HR-positive/HER2-negative treatment in both female and male patients.
Utilization
Each patient has unique characteristics that can impact the state of their disease. Due to patient specific comorbidities, allergies, or site of metastasizes, not all therapy regimens are the same. The use of therapy subgroups allowed for a better representation of variety of therapies used in real-world patients. Prior to separating groups, the theory was that the majority of patients would fall into the CDK 4/6 inhibitor plus hormone therapy combination subgroup due to the HR-positive component of their diagnosis. It was also hypothesized that the smallest portion of patients would fall into the CDK 4/6 inhibitor plus another oncolytic group.
Although this study focused on HR-positive/HER2-negative patients, there were 515 patients that were not documented to have been prescribed a concurrent hormone therapy agent. Unlike palbociclib and ribociclib, abemaciclib is FDA approved as a monotherapy agent in HR-positive patients16. Therefore, the study population included 437 (15.0%) patients on palbociclib or ribociclib without a recommended hormone agent. Of these patients, multiple had documentation that they were either hormone-resistant or allergic to an aromatase inhibitor and/or fulvestrant. Additionally, due to patient reported medications, improper documentation, or lack of provider response, the percentage of patients not on recommended combination therapy is potentially lower that determined in this study. Of those that did receive combination hormone therapy, the large majority, 74.4%, received endocrine therapy with an aromatase inhibitor. Patients that fail endocrine therapy or become resistant then transitioned to fulvestrant. When analyzing our HR-positive patients by gender, 2371 (82.4%) of women and 20 (62.5%) of men received a form of hormone therapy. Although our sample size was small, these percentages are higher than the previously discussed retrospective, national study which reported 70.2% and 57.9%, respectively14,17.
Adverse events
The frequency and severity of ADEs in the study population was much lower than that reported in the clinical trials for all three CDK 4/6 inhibitors. With over 60% of patient’s not reporting any adverse effect, this medication class has shown to be tolerable and safe. By evaluating reported ADEs of real-world patients, clinicians are able to provide patients with recent and up to date information to increase their knowledge on the probability of ADE occurrence. Since the fear of side effects can deter patients from initiating therapy, reporting accurate risk percentages can help reduce prescription abandonment. Additionally, this updated information can be beneficial for physicians to use prior to initiating a specific CDK 4/6 inhibitor. When choosing a CDK 4/6 inhibitor, physicians should evaluate the comorbidities and past medical history of a patient. Ribociclib had the least reported side effects and unlike the other CDK 4/6 inhibitors, there were no reports of mouth sores, which can lead to malnutrition and pain. Patients with prior history of mouth sores or stomatitis as well as concerns of side effect occurrence may experience higher tolerability with ribociclib. Additionally, patients with a history of bowel disease such as Crohn’s disease or ulcerative colitis, should be considered for treatment with palbociclib or ribociclib due to the high percentage of patients experiencing GI related ADEs while on abemaciclib.
Unmanaged or severe adverse events can also lead to termination of therapy. Therefore, by choosing an agent with a more tolerable side effect profile for a specific patient, there is a likelihood that risk of therapy discontinuation due to adverse events is reduced. Current guidelines recommend chemotherapy agents after failure of a CDK 4/6 inhibitor for HR-positive/HER2-negative patients4. Chemotherapy agents, especially infusion, are most often accompanied with a diverse side effect profile with risks of events not associated with oral therapies such as infusion reactions. Additionally, transition to infusion chemotherapies will impact each patient’s daily life due to need for additional blood work and physician appointments for chemotherapy administration.
Adherence
Adherence rates related to oncolytic agents is a challenge given the various types of cancers treated with oncolytic agents, the variable mechanisms of actions of oncolytic agents, the variations in administration and dosing, and the difficulty in measuring dose adjustments or held doses. Abemaciclib which is dosed continuously twice daily, no rest periods per cycle, had consistent adherence rates across all patient subgroups. Palbociclib and ribociclib both consist of dosing that includes three weeks of therapy followed by a week without medication. Patients on these medications experienced high adherence rates in the monotherapy and combination with hormone therapy subgroups. However, these rates decreased in the subgroups that included another oncolytic, potentially due to conflicting or varying dosing regimens being a challenge for some patients.
Adherence among patients receiving oral therapies for cancer ranges from 15% to 97%, depending on the type of therapy17. Up to 60% of patients with breast cancer have reported non adherence18. Although expected that cancer therapies would have higher adherence due to disease severity, the responsibility of taking medication at home versus in the hospital as well as having less direct care by a physician leads to increased non adherence17. The mean PDC for patients on CDK 4/6 monotherapy or in combination with a hormonal therapy during this study was 79.3% and 81.4%, higher than previous studies. Although not conclusive, the CDK 4/6 inhibitors were dispensed solely by a specialty pharmacy that completes monthly refill reminder calls and counseling at each fill which could impact patient adherence. In a study comparing oncolytic dispensing at traditional pharmacy versus specialty pharmacy, it was found that patients filling at the specialty pharmacy had a 15.2% higher adherence rate and overall lower prescription abandonment rates19.
Patient cost
An additional challenge this patient population faces is high out-of-pocket costs. Oral oncolytic medications are typically covered under a patient’s pharmacy benefit, unlike chemotherapy infused medications which are typically covered under a patient’s medical benefit. Patient out-of-pocket costs for medications covered under the pharmacy benefit are typically higher than those under the medical benefit due to either a high copay or co-insurance based on a percentage of the medication’s cost. Varying average copays were reported due to insurance plan specifics such as differing deductible amounts and percentage of medication coverage per plan. If patients are concerned about their out-of-pocket cost associated with their prescribed oral oncolytic mediation they may intentionally skip doses to make the medication last longer or extend out the off periods in their cycles delaying refills which may have a negative impact on outcomes. A previous outcome report stated that abandonment rates of new patients for both commercial and Medicare begins to steadily increase once patient out-of-pocket costs reach $5020. Fortunately, though higher than $50, the majority of patients experienced copays significantly lower than the AWP of each medication. Through the use of patient financial services, specialty pharmacies are able to assist patients with high out-of-pocket costs by coordinating benefits across payers or working with manufacturers and charitable foundation for additional financial assistance helping patients to start and stay on these therapeutic regimens.
Study limitations
Limitations of this study include possible incomplete documentation resulting in missing patient specific data. Additionally, potential risk of recall bias through utilization of patient reported information could have occurred. All included patients were filling the study drug at a single specialty pharmacy and assessed through its specific clinical applications. Therefore, since pharmacy patient population is influenced by payer contracts and managed care organizations, the data may not be representative of the entire breast cancer population. Additionally, due to the study period, PDC and copays were only analyzed for those patients filling in the first quarter of 2019 and not representative of the entire study population. Hormone therapy agents are non-specialty and typically filled at local retail stores. Due to inability to access outside databases, the copay and adherence of hormone agents could not be assessed.
Study importance
This study supplements the currently available real-world data elated to the use of CDK 4/6 inhibitors. Unlike previous studies that have focused on efficacy, this study’s emphasis on tolerability and safety allowed for another perspective when evaluating this therapy class. These results can better aid physicians in patient based medication outcomes. Post-approval safety data, patient out-of-pocket costs and clinical efficacy outcomes should all be evaluated by physicians and patients to determine the appropriate medication to prescribe. Additionally, although small in number, this study provided needed data on the use of CDK 4/6 inhibitors in the male population.
Conclusion
CDK 4/6 inhibitors have demonstrated safety and tolerability in both female and male HR-positive/HER2-negative breast cancer patients. Prescribers and patients should consider real world safety data and out-of-pocket patient costs in addition patient specific comorbidities when developing a treatment plan that includes a CDK 4/6 inhibitor selection.
Acknowledgements
The authors would like to acknowledge Francis Staskon, Ph.D. who helped with the data analysis for the article.
Transparency
Declaration of funding
No funding was received to produce this manuscript.
Declaration of financial/other relationships
The authors report no conflicts of interest. The contents of the paper and the opinions expressed within are those of the authors, and it was the decision of the authors to submit the manuscript for publication.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Data availability statement
All study data are AllianceRx Walgreens Prime proprietary data – which is available for additional internal study but is not accessible external to the organization.
References
- 1.[ACS] About Breast Cancer . How common is breast cancer? [Internet]. Atlanta (GA): American Cancer Society; 2020. Jan 8 [cited 2020 May 1]; [about 2 screens]. Available from: https://www.cancer.org/cancer/breast-cancer/about/how-common-is-breast-cancer.html
- 2.U.S. Breast Cancer Statistics [Internet] . Ardmore (PA): Breastcancer.org; 2020. Jun 25 [cited 2020 Jul 1]; [about 1 screen]. Available from: https://www.breastcancer.org/symptoms/understand_bc/statistics
- 3.Wang F, Shu X, Meszoely I, et al. . Overall mortality after diagnosis of breast cancer in men vs women. JAMA Oncol. 2019;5:1589–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NCCN Clinical Practice Guidelines in Oncology (US) . Breast Cancer. Rev. ed. Plymouth Meeting (PA): National Comprehensive Cancer Network (US); 2020. [Google Scholar]
- 5.Spring LM, Wander SA, Zangardi M, et al. . CDK 4/6 inhibitors in breast cancer: current controversies and future directions. Curr Oncol Rep. 2019;21:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marra A, Curigliano G.. Are all cyclin-dependent kinases 4/6 inhibitors created equal? NPJ Br Can. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller T.W, Balko J, Fox E, et al. . ERα-dependent E2F transcription can mediate resistance to estrogen deprivation in human breast cancer. Cancer Discov. 2011;1:338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.U.S. FDA approves IBRANCE® (palbociclib) for the treatment of men with HR+, HER2-metastatic breast cancer [Internet]. New York (NY): Pfizer; c2002–2020. 2019. Apr 4 [cited 2020 Mar 2]; [about 2 screens]. Available from: https://www.pfizer.com/news/press-release/press-releasedetail/u_s_fda_approves_ibrance_palbociclib_for_the_treatment_of_men_with_hr_her2_metastatic_breast_cancer
- 9.Hormone therapy for breast cancer [Internet]. Chicago (IL): Mayo Clinic; c1998–2020. 2019. Feb 5 [cited 2020 May 28]; [about 4 screens]. Available from: https://www.mayoclinic.org/tests-procedures/hormone-therapy-for-breast-cancer/about/pac-20384943
- 10.Im SA, Lu YS, Bardia A, et al. . Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019;381:307–316. [DOI] [PubMed] [Google Scholar]
- 11.CDK4/6 inhibitors reach new benchmarks in overall survival in HR + breast cancer nts [Internet] . Cranbury (NJ): Onc Live; 2020. Mar 6 [cited 2020 May 2]; [about 4 screens]. Available from: http://www.ama-assn.org/ama/pub/category/17469.ht
- 12.Finn RS, Martin M, Rugo HS, et al. . PALOMA-2: primary results from a phase III trial of palbociclib (P) with letrozole (L) compared with letrozole alone in postmenopausal women with ER+/HER2–advanced breast cancer (ABC). J Clin Oncol. 2016;34:507–507. [Google Scholar]
- 13.Verma S, Bartlett CH, Schnell P, et al. . Palbociclib in combination with fulvestrant in women with hormone receptor-positive/HER2-negative advanced metastatic breast cancer: detailed safety analysis from a multicenter, randomized, placebo-controlled, phase III study (PALOMA-3). Oncologist. 2016;21:1165–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landhuis E. Men with breast cancer fare worse. Sci News. 2019;196:8–9. [Google Scholar]
- 15.Paranjpe R, John G, Trivedi M, et al. . Identifying adherence barriers to oral endocrine therapy among breast cancer survivors. Breast Cancer Res Treat. 2019;174:297–305. [DOI] [PubMed] [Google Scholar]
- 16.Marra A, Curigliano G.. Are all cyclin-dependent kinases 4/6 inhibitors created equal? NPJ Breast Cancer. 2019;5:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen LA. Impact of nonadherence to cancer therapy. J Hematol Oncol Phar. [2012. Mar 26]:[6 p.]. Author’s manuscript available at http://jhoponline.com/ton-online-first/3639-ton-3639
- 18.Foulon V, Schöffski P, Wolter P.. Patient adherence to oral anticancer drugs: an emerging issue in modern oncology. Acta Clin Belg. 2011;66:85–96. [DOI] [PubMed] [Google Scholar]
- 19.Stokes M, Reyes C, Xia Y, et al. . Impact of pharmacy channel on adherence to oral oncolytics. BMC Health Serv Res. 2017;17:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.IQVIA Institute for Human Data Science . Medicine use and spending in the U.S. – a review of 2018 and outlook to 2023. Parsippany (NJ): IQVIA Institute; 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All study data are AllianceRx Walgreens Prime proprietary data – which is available for additional internal study but is not accessible external to the organization.