Abstract
Mitochondrial anti-apoptotic Bcl2 and BclxL proteins, are overexpressed in multiple tumour types, and has been involved in the progression and survival of malignant cells. Therefore, inhibition of such proteins has become a validated and attractive target for anticancer drug discovery. In this manner, the present studies developed a series of novel isatin–indole conjugates (7a-j and 9a-e) as potential anticancer Bcl2 and BclxL inhibitors. The progression of the two examined colorectal cancer cell lines was significantly inhibited by all of the prepared compounds with IC50 ranges132–611 nM compared to IC50 = 4.6 µM for 5FU, against HT-29 and IC50 ranges 37–468 nM compared to IC50 = 1.5 µM for 5FU, against SW-620. Thereafter, compounds 7c and 7g were selected for further investigations. Interestingly, both compounds exhibited selective cytotoxicity against both cell lines with high safety to normal fibroblast (HFF-1). In addition, both compounds 7c and 7g induced apoptosis and inhibited Bcl2 and BclxL expression in a dose-dependent manner. Collectively, the high potency and selective cytotoxicity suggested that conjugates 7c and 7g could be a starting point for further optimisation to develop novel pro-apoptotic and antitumor agents towards colon cancer.
Keywords: Anti-proliferative indoles, apoptosis, isatin-indole, Bcl2/BclxL inhibitors, colorectal cancer, western blotting
1. Introduction
Cancer is a group of diseases that is characterised by uncontrolled and rapid cell proliferation and differentiation mechanisms with the potential to invade or spread to other body parts1. Since several decades, cancer has been one of the main world health problems and is still considered a serious leading cause of death worldwide. Early strategies of cancer treatment were based on the unspecific induction of cell death mainly targeting the replication machinery2–5 and/or the DNA synthesis6–9. Therefore, the traditional anticancer drugs were associated with severe adverse effects due to the unselective toxicity towards the normal cells in addition to the resistance emerged towards them9. Thus, the development of effective and safe new antitumour drugs with increased selectivity towards cancer cells is still an active search10,11. On the other hand, recent strategies of targeted therapies target specific biomarkers essential for the regulation of cancer cells proliferation and/or cell apoptosis such as deregulated, mutated, or overexpressed proteins12 and thus, selectively affect cancer cells or their supporting environment with minimum effects on normal cells13. Among these targets are the pro-apoptotic and anti-apoptotic proteins that control the cellular apoptosis14–16.
Apoptosis, a form of programmed cell death takes place in multicellular organisms, is a series of biochemical events that result in characteristic cell changes and death17. Apoptosis could be launched through one of two pathways (intrinsic pathway and extrinsic pathway). The mitochondria-dependent apoptotic pathway (intrinsic pathway), one of the main pathways of induction of the cell apoptosis18,19, is controlled by the Bcl2 proteins family. The Bcl2 family members have dual functions; some are anti-apoptotic Bcl2 proteins, such as Bcl2 and BclxL that inhibit apoptosis, while others are pro-apoptotic Bcl2 proteins such as Bax and Bak that promote apoptosis14. In this regard, it was reported that most of cancer cells are characterised by over-expression of the anti-apoptotic Bcl2 proteins which could lead to apoptosis prevention as well as drug resistance20,21. Thus, the development of anti-apoptotic Bcl2 proteins inhibitors has become an important strategy for introducing potential anti-cancer agents15,16. In this manner, several heterocyclic scaffolds including isatin22 were developed as candidates targeting Bcl2 proteins.
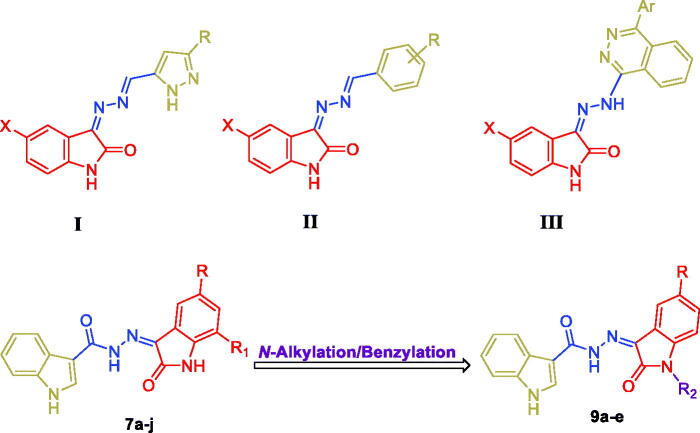
Isatin (1H-indole-2,3-dione), as a special class in drug design and discovery, represents one of the most favourable scaffolds of heterocyclic systems which possesses many interesting biological activities including anti-SARS-CoV-223, antimicrobial24, anticonvulsant25 and mainly anticancer26–28. Therefore, isatin nucleus was broadly used by our group for the development of diverse effective oxindole-based small molecules (structures I–III29–31, Figure 1) with anticancer activities that target different enzymatic and cellular targets such as inhibition of cancer-related carbonic anhydrase IX isoform32–33, inhibition of different kinases34–35, in addition to apoptosis induction in different human cancer cell lines36–37.
Figure 1.
Chemical structures for some reported isatin-based anticancer conjugates (I-III), and the target conjugates (7a-j and 9a-e).
Motivated by the aforementioned findings and in continuation to our previous work, in the present study, a novel series of isatin/indole conjugates (7a-j and 9a-e, Figure 1) were designed and synthesised. The antiproliferative effect of the new compounds against HT-29 and SW-620 colorectal cancer cell lines were examined. In addition, the levels of the mitochondria-related anti-apoptotic proteins Bcl2 and BclxL in HT-29 and SW-620 colorectal cancer cell lines after incubation with isatin derivatives 7c and 7g were determined.
2. Results and discussion
2.1. Chemistry
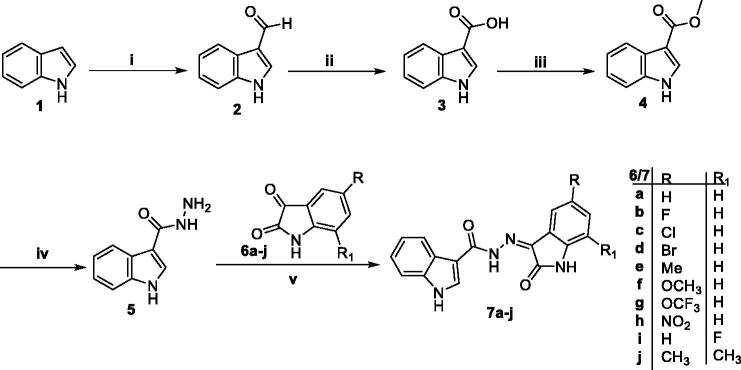
The synthetic strategies deliberate for the development of the final compounds (7a-j and 9a-e) were illustrated in Schemes 1–2. In Scheme 1, indole 1 was subjugated to formylation via Vilsmeier haack reaction to produce 1H-indole-3-carbaldehyde 2, in which the CHO functionality was oxidised by KMnO4 in acetone to furnish 1H-indole-3-carboxylic acid 3. Then, the acid analogue 3 was subjected to esterification through refluxing in dry methanol to get the carboxylate analogue 4, where the ester group reacted with hydrazine hydrate in methyl alcohol to produce the key intermediate 1H-indole-3-carbohydrazide 5. Finally, the key intermediate 5 was condensed with different isatin derivatives 6a-j in glacial acetic acid to give the final targeted compounds 7a-j.
Scheme 1.
Synthesis of target 2-oxindolin-3-ylidene-indole-3-carbohydrazide 7a-j; (i) DMF/POCl3/reflux 8 h, (ii) KMnO4/Acetone/Stirring at R.T 12 h, (iii) Dry methanol/H2SO4 (Cat.)/reflux 7 h, (iv) hydrazine hydrate/methanol/reflux 4 h, (v) Glacial acetic acid/reflux (5–7) h.
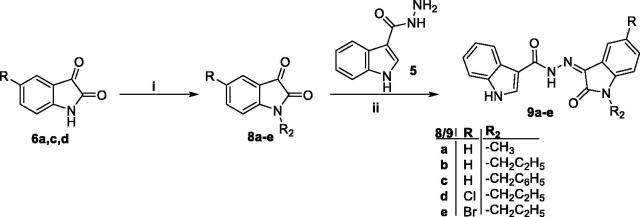
Scheme 2.
Synthesis of target N-substituted 2-oxindolin-3-ylidene-indole-3-carbohydrazide 9a-e; (i) R-Br/DMF/K2CO3/KI (Cat.)/reflux 5 h, (ii) Glacial acetic acid/reflux (5–7) h.
On the other hand, in Scheme 2, three isatins 6a, 6c and 6d were alkylated with methyl iodide, propyl bromide and benzyl bromide in DMF with the presence of K2CO3 and catalytic amount of KI to give the N-substituted isatin derivatives 8a-e, which heated under reflux with the carbohydrazide 5 in acetic acid to furnish the final compounds 9a-e, respectively. The structure of the synthesised 2-oxindolin-3-ylidene-indole-3-carbohydrazide was confirmed under the basis of spectral and elemental analyses which were in full agreement with the proposed structures.
2.2. Biological evaluation
2.2.1. Anti-proliferative activity against HT-29 and SW-620 colorectal cancer cell lines
The anti-proliferative potential of the final compounds (7a-j and 9a-e) was examined against two human colorectal cancer HT-29 and SW-620 cell lines. While, HT-29 is an adenocarcinoma cell line, SW-620 represents metastatic cancer cell line. These effects were compared with known anti-cancer drug, 5-Fluorouracil (5FU) commonly used in colorectal cancer treatment. All of the compounds were found to inhibit the cell viability of cancer cells with varied sub-micro-molar efficacy. The IC50 values ranged from 105 to 611 nM for all compounds were calculated using Graph Pad prism 8 (Table 1).
Table 1.
In vitro anti-proliferative actions of compounds 7a-j and 9a-e towards HT-29 and SW-620 colorectal cancer cell lines.
 | |||||
|---|---|---|---|---|---|
| Comp. | R | R1 | R2 |
IC50 (nM)a |
|
| HT-29 | SW-620 | ||||
| 7a | H | H | H | 408 | 145 |
| 7b | F | H | H | 611 | 175 |
| 7c | Cl | H | H | 206 | 188 |
| 7d | Br | H | H | 320 | 133 |
| 7e | CH3 | H | H | 335 | 253 |
| 7f | OCH3 | H | H | 142 | 37 |
| 7g | OCF3 | H | H | 299 | 279 |
| 7h | NO2 | H | H | 132 | 190 |
| 7i | H | F | H | 140 | 468 |
| 7j | CH3 | CH3 | H | 200 | 187 |
| 9a | H | H | CH3 | 176 | 260 |
| 9b | H | H | propyl | 525 | 105 |
| 9c | H | H | benzyl | 405 | 283 |
| 9d | Cl | H | propyl | 166 | 290 |
| 9e | Br | H | propyl | 290 | 414 |
| 5-FU | 4600 | 1500 | |||
IC50 values are the mean ± SD of three separate experiments.
Regarding the activity towards HT-29 cell line, all compounds showed superior activity to the reference drug (5FU) with IC50 ranged from 132-611 nM compared to IC50=4.6 µM for 5FU. Compound 7h was the most active against HT-29 cell line with IC50=132 nM that is approximately 35-fold more than 5FU. Also, the results revealed that the N-alkylation of isatin moiety (series 9a-e) resulted in diversified effect on the potency according to the alkyl group added and/or the substitution on indole moiety. For the unsubstituted indole (7a, IC50=408 nM), while N-alkylation with CH3- group significantly increase the potency (9a, IC50=176 nM) and N-alkylation with benzyl- group slightly increase the potency (9c, IC50=405 nM), the N-alkylation with propyl group dramatically decrease the potency (9b, IC50=525 nM). Furthermore, N-propylation of both chloro- (7c, IC50=206 nM) and bromo- (7d, IC50=320 nM) derivatives significantly increase the potency (9d, IC50=166 nM) and (9e, IC50=290 nM), respectively. Similarly, the activity towards SW-620 cell line of all compounds was more that 5FU with IC50 ranged from 37–468 nM compared to IC50=1.5 µM for 5FU. Compound 7f was the most active against SW-620 cell line with IC50=37 nM that is approximately 32 folds more than 5FU. However, N-alkylation of isatin moiety resulted in reduction of potency for all series except for compounds 9b (IC50=105 nM) compared to compound 7a (IC50=145 nM) (Table 1).
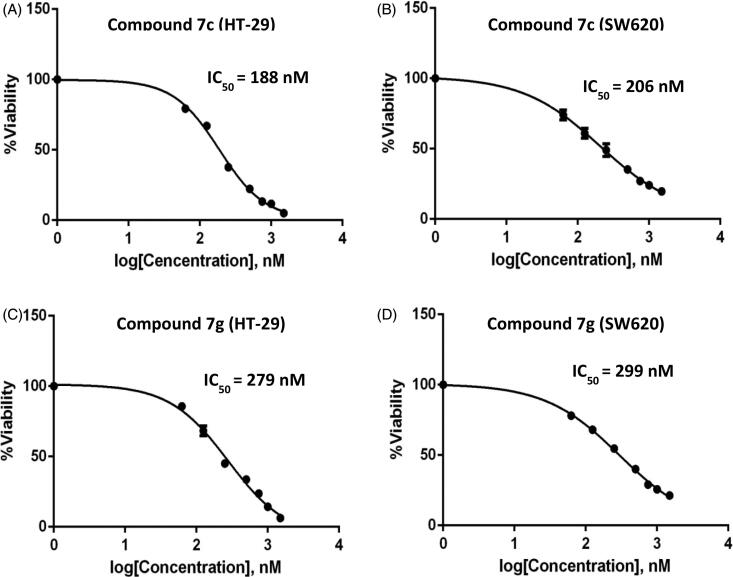
Figure 2 illustrated that treatment of HT-29 and SW-620 cell lines with different concentrations of compounds 7c and 7g resulted in a dose-dependent inhibition of cell viability. Compound 7c was found to inhibit HT-29 and SW-620 with IC50=188 nM and 206 nM, respectively (Figure 2(A,B)), whereas compound 7g was found to exhibit IC50 of 279 nM against HT-29 and 299 nM against SW-620 cell lines (Figure 2(C,D)), compared to the IC50 for 5FU that was found to be 1.5 µM against HT-29 and 4.6 µM against SW-620 cell lines.
Figure 2.
Effect of Compound 7c and 7g on the cell viability. Different concentrations of compound 7c and 7g have been used to study its effect on cell viability by MTT assay. Results of percent viability vs concentration were plotted and the IC50 was calculated for each cell line using Graph Pad prism 8. (A) HT-29 with compound 7c, (B) SW-620 with compound 7c, (C) HT-29 with compound 7g, and (D) SW-620 with compound 7g.
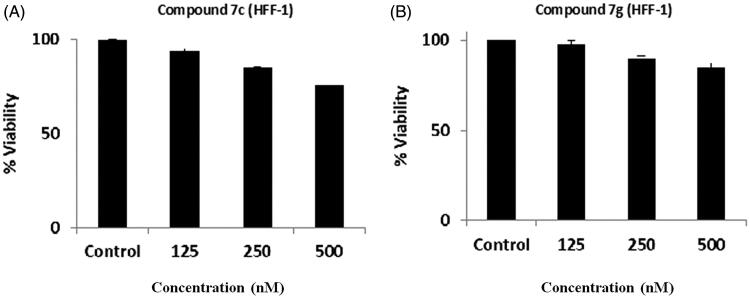
Furthermore, to investigate the selective cytotoxicity of the tested compounds, the effect of compounds 7c and 7g was studied on normal human skin fibroblast (HFF-1). Compounds 7c and 7g were found to have no/or little effect on cell viability of HFF-1 (Figure 3(A,B)). This finding indicates that compounds 7c and 7g inhibited cell viability of cancer cells without affecting normal fibroblast.
Figure 3.
Effect of Compounds 7c and 7g on HFF-1. HFF-1 fibroblast cells were treated with compound 7c and 7g for 24 h and % viability was measured using MTT assay. (A) HFF-1 with compound 7c and (B) HFF-1 with compound 7g.
2.2.2. Annexin V-FITC apoptosis assay
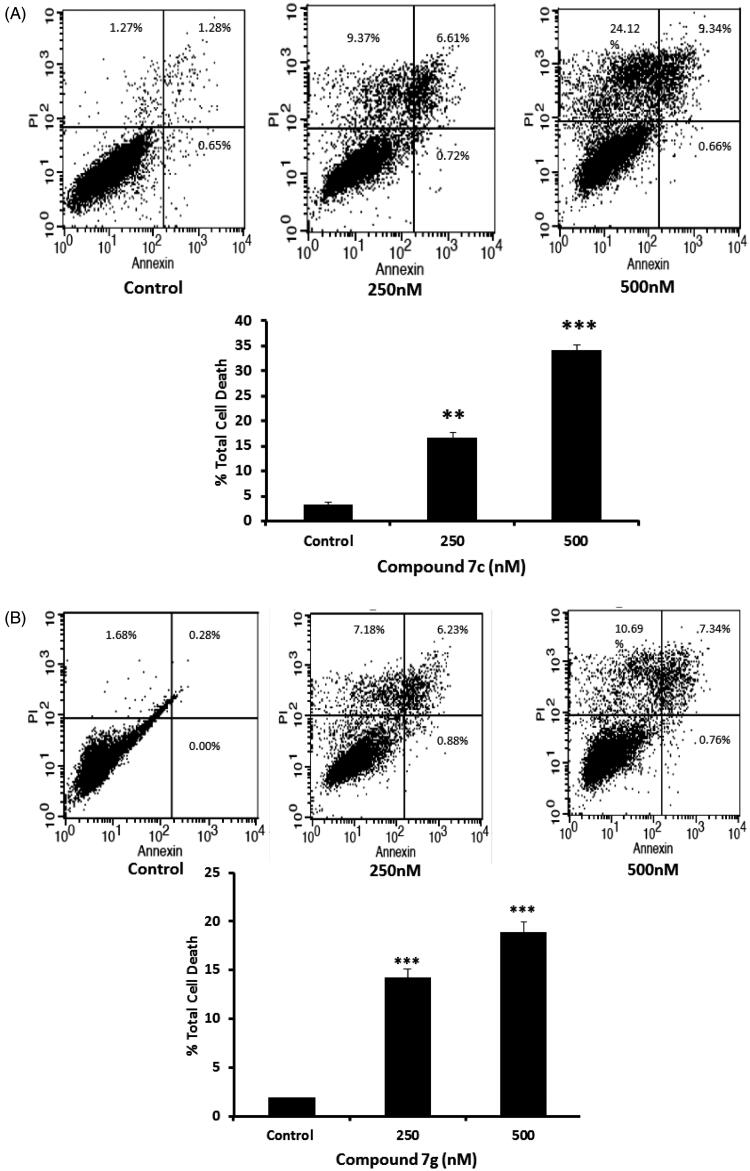
Induction of apoptosis is mainly considered as an important strategy for the development of new anti-proliferative agents38. Compounds 7c and 7g were further investigated for their potential role of apoptosis induction in the colorectal SW-620 cancer cell line (SW-620 cell line was selected because most of the new compounds showed significant potency more than HT-29 cell line). Exposure of SW-620 cells to compounds 7c and 7g resulted in the induction of apoptosis in a dose dependent manner (Figure 4). As shown in the results, compound 7c at concentrations of 250 nM and 500 nM was able to induce approximately 5.2- and 10.66-fold, respectively, total apoptosis increase compared to the control for SW-620 cell line (Figure 4(A)). Similarly, compound 7g induced approximately 7.3 and 9.6 fold total apoptosis increase compared to the control when incubated with SW-620 cell line at concentration of 250 nM and 500 nM, respectively (Figure 4(B)). These findings encouraged us to further investigate the effect of compounds 7c and 7g towards the anti-apoptotic mitochondrial markers Bcl2 and BclxL.
Figure 4.
(A) Annexin V apoptosis assay for compound 7c. SW-620 cells were treated with compound 7c (250 and 500 nM) for 24 h. Quadrant was set for the resulted dots population from the results of the negative auto-fluorescence and the statistical analysis was performed where the significance of data was assessed at p values < 0.05. **p < 0.01; ***p < 0.001 control vs treated. (B) Annexin V apoptosis assay for compound 7g. SW-620 cells were treated with compound 7g (250 and 500 nM) for 24 h. Quadrant was set for the resulted dots population from the results of the negative auto-fluorescence and the statistical analysis was performed where the significance of data was assessed at p values < 0.05. **p < 0.01; ***p < 0.001 control vs treated.
2.2.3. Impact of compounds 7c and 7g on the anti-apoptotic (Bcl2, and BclxL) markers levels
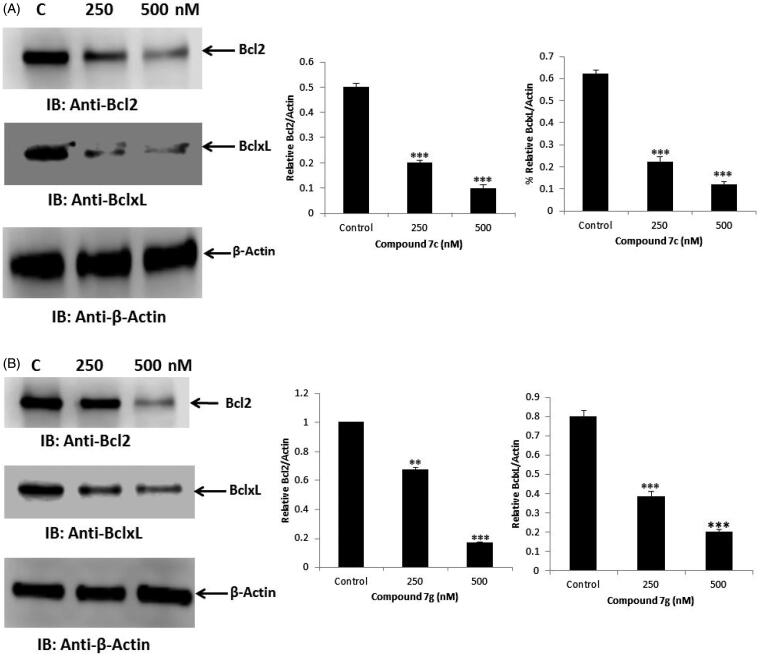
Bcl2 and BclxL, as anti-apoptotic proteins, are known to be overexpressed in diverse tumours causing cancer cell survival and drug resistance14. Inhibition of these proteins expression resulted in cancer cell death and has been exploited as a strategy for anticancer drug discovery15–16. Treatment of SW-620 with compound 7c resulted in a dose-dependent inhibition of Bcl2 and BclxL protein expression (Figure 5(A)). Compound 7g was also found to inhibit Bcl2 and BclxL expression in SW-620 cells (Figure 5(B)). These findings thus indicate that compound 7c and 7g inhibited cell viability by inhibiting Bcl2 and BclxL resulting in the apoptosis.
Figure 5.
(A) Effect of compound 7c on anti-apoptotic proteins. SW-620 cancer cells were treated with two concentrations of compound 7c for 24 h. Immunoblotting was performed with the indicated antibodies. The band densities were measured and the bar charts were created to compare the effect on proteins. The statistical analysis was performed where the significance of data was assessed at a p values < 0.05. *** p < 0.001; ** p < 0.01 control vs treated. (B) Effect of compound 7g on anti-apoptotic proteins. SW-620 cancer cells were treated with two concentrations of compound 7g for 24 h. Immunoblotting was performed with the indicated antibodies. The band densities were measured and the bar charts were created to compare the effect on proteins.
3. Conclusions
In the present work, a series of novel isatin-indole conjugates (7a-j and 9a-e) was designed and synthesised as potential antiproliferative agents towards colon cancer cells with promising inhibitory activity against the anti-apoptotic Bcl2 and BclxL proteins. The cell growth of two examined colorectal cancer (HT-29 and SW-620) cell lines was significantly inhibited by all of the prepared compounds with IC50 ranges 132–611 nM against HT-29 and IC50 ranges 37–468 nM against SW-620 as compared to IC50=4.6 and 1.5 µM for 5FU, respectively. For further mechanistic and selective cytotoxicity studies, compounds 7c and 7g were examined and proved to exhibit selective cytotoxicity against both cancer cell lines with high safety profile to normal fibroblast (HFF-1). In addition, towards SW-620 cell line, both candidates inducted apoptosis and inhibited anti-apoptotic Bcl2 and BclxL proteins in a dose dependent manner. Collectively, the high potency and selective cytotoxicity suggested that conjugates 7c and 7g could serve as lead compounds for further optimisation to develop novel antitumor agents and Bcl2/BclxL inhibitors.
4. Experimental
4.1. Chemistry
4.1.1. General
The NMR spectra have been recorded by Bruker spectrometer at 400 MHz. 13 C NMR spectra were run at 100 MHz in deuterated dimethylsulphoxide (DMSO-d6). Chemical shifts (δH) are reported relative to the solvent (DMSO-d6). Infra-red spectra were recorded on Schimadzu FT-IR 8400S spectrophotometer. Elemental analyses have been performed at the Regional Centre for Microbiology and Biotechnology, Al-Azhar University, Cairo, Egypt.
4.1.2. Synthesis of the target compounds 7a-j and 9a-e
To hot stirred solution of 1H-indole-3-carbohydrazide 5 (0.3 g, 1.7 mmole) in 15 ml of glacial acetic acid, an equivalent amount of appropriate isatin derivatives 6a-j and 8a-e was added. The reaction mixture was heated under reflux for (5–7) h, and then was cooled to room temperature. The formed precipitate was collected by filtration, washed with cold water, hexane and recrystallized from DMF-MeOH mixture to furnish the targeted novel compounds 7a-j and 9a-e, respectively. Representative NMR spectra charts were provided in the Supplementary data.
4.1.2.1. N'-(2-Oxoindolin-3-ylidene)-1H-indole-3-carbohydrazide 7a
Yellow powder; m.p. 259–261 °C; (yield 81%); 1H NMR δ ppm: 6.93 (d, 0.3H, Ar-H, J = 8.0 Hz), 6.98 (d, 0.7H, Ar-H, J = 8.0 Hz), 7.07–7.16 (m, 1H, Ar-H), 7.22–7.29 (m, 2H, Ar-H), 7.37 (t, 1H, Ar-H, J = 8.0 Hz), 7.52–7.58 (m, 1H, Ar-H), 7.65 (d, 0.7H, Ar-H, J = 8.0 Hz), 8.13 (d, 0.3H, Ar-H, J = 8.0 Hz), 8.23–8.32 (m, 1.7H, Ar-H), 8.67 (s, 0.3H, Ar-H), 10.83, 13.45 (s, 1H, NH of isatin), 11.18, 11.30 (s, 1H, hydrazide NH), 12.01, 12.05 (s, 1H, indole NH); 13 C NMR δ ppm: 107.50, 108.17, 110.96, 111.56, 112.56, 112.99, 116.14, 120.75, 120.96, 120.98, 121.73, 121.79, 121.97, 122.21, 123.03, 123.21, 126.19, 126.54, 127.75, 131.51, 132.48, 133.62, 136.22, 136.65, 136.78, 142.41, 143.91 (Aromatic carbons), 162.16, 163.37 (C=O hydrazide), 165.27, 165.65 (C=O isatin)); IR (KBr, ν cm−1) 3504, 3280, 3245 (3NH) and 1707, 1654 (2 C=O); Calcd. Anal. for C17H12N4O2: C, 67.10; H, 3.97; N, 18.41; found C, 66.83; H, 4.57; N, 18.41.
4.1.2.2. N'-(5-Fluoro-2-oxoindolin-3-ylidene)-1H-indole-3-carbohydrazide 7b
Red powder; m.p. >300 °C; (yield 78%); 1H NMR δ ppm: 6.90–9.93 (m, 0.7H, Ar-H), 6.96–7.00 (m, 0.3H, Ar-H), 7.20–7.30 (m, 3H, Ar-H), 7.49–7.58 (m, 1.3H, Ar-H), 8.15 (d, 0.7H, Ar-H, J = 8.0 Hz), 8.23 (d, 0.3H, Ar-H, J = 8.0 Hz), 8.31 (d, 1H, Ar-H, J = 8.0 Hz), 8.72 (s, 0.7H, Ar-H), 10.84, 13.41 (s, 1H, NH of isatin), 11.31, 11.35 (s, 1H, hydrazide NH), 12.03 (s, 1H, indole NH); IR (KBr, ν cm−1) 3659, 3460, 3285 (3NH) and 1712, 1669 (2C=O); Calcd. Anal. for C17H11FN4O2: C, 63.35; H, 3.44; N, 17.38; found C, 63.14; H, 4.01; N, 17.37.
4.1.2.3. N'-(5-Chloro-2-oxoindolin-3-ylidene)-1H-indole-3-carbohydrazide 7c
Yellow powder; m.p. >300 °C; (yield 75%); 1H NMR δ ppm: 6.92 (d, 0.7H, Ar-H, J = 8.4 Hz), 6.97 (d, 0.3H, Ar-H, J = 8.4 Hz), 7.20–7.29 (m, 2H, Ar-H), 7.39–7.43 (m, 1H, Ar-H), 7.51–7.58 (m, 1H, Ar-H), 7.64 (s, 0.3H, Ar-H), 8.22–8.35 (m, 2H, Ar-H), 8.71 (s, 0.7H, Ar-H), 10.95, 12.03 (s, 1H, NH of isatin), 11.43 (s, 1H, hydrazide NH), 12.03 (s, 1H, Indole NH); IR (KBr, ν cm−1) 3593, 3429, 3331 (3NH) and 1720, 1643 (2 C=O); Calcd. Anal. for C17H11ClN4O2: C, 60.28; H, 3.27; N, 16.54; found C, 59.77; H, 3.88; N, 16.37.
4.1.2.4. N'-(5-Bromo-2-oxoindolin-3-ylidene)-1H-indole-3-carbohydrazide 7d
Orange powder; m.p. 247–249 °C; (yield 83%); 1H NMR δ ppm: 6.91 (d, 1H, Ar-H, J = 8.0 Hz), 7.20–7.29 (m, 2H, Ar-H), 7.51 (d, 1H, Ar-H, J = 8.0 Hz), 7.55 (d, 1H, Ar-H, J = 8.0 Hz), 7.73 (s, 1H, Ar-H), 8.23 (d, 1H, Ar-H, J = 8.0 Hz), 8.34 (s, 1H, Ar-H), 11.34 (s, 1H, NH of isatin), 12.04 (s, 1H, hydrazide NH), 13.31 (s, 1H, Indole NH); 13 C NMR δ ppm: 107.76, 111.54, 112.97, 113.46, 114.78, 121.03, 122.02, 122.86, 123.24, 126.36, 133.58, 133.92, 136.70, 141.39, 142.39 (Aromatic carbons), 162.99 (C=O hydrazide), 163.35 (C=O isatin)); IR (KBr, ν cm−1) 3621, 3428, 3184 (3NH) and 1710, 1638 (2 C=O); Calcd. Anal. for C17H11BrN4O2: C, 53.28; H, 2.89; N, 14.62; found C, 52.79; H, 2.82; N, 14.63.
4.1.2.5. N'-(5-Methyl-2-oxoindolin-3-ylidene)-1H-indole-3-carbohydrazide 7e
Red powder; m.p. 201–203 °C; (yield 74%); 1H NMR δ ppm: 1.93, 2.33 (s, 3H, CH3), 6.85 (d, 1H, Ar-H, J = 8.0 Hz), 7.16 (d, 1H, Ar-H, J = 8.0 Hz), 7.22–7.29 (m, 2H, Ar-H), 7.45 (s, 1H, Ar-H), 7.55 (d, 1H, Ar-H, J = 8.0 Hz), 8.23–8.27 (m, 2H, Ar-H), 11.17 (s, 1H, NH of isatin), 12.04 (s, 1H, hydrazide NH), 13.44 (s, 1H, Indole NH)); IR (KBr, ν cm−1) 3451, 3322, 3252 (3NH) and 1711, 1660 (2 C=O); Calcd. Anal. for C18H14N4O2: C, 67.92; H, 4.43; N, 17.60; found C, 67.25; H, 4.98; N, 17.57.
4.1.2.6. N'-(5-Methoxy-2-oxoindolin-3-ylidene)-1H-indole-3-carbohydrazide 7f
Red powder; m.p. 197–199 °C; (yield 80%); 1H NMR δ ppm: 3.81(s, 3H, OCH3), 6.89 (d, 1H, Ar-H, J = 8.0 Hz), 6.95 (d, 1H, Ar-H, J = 8.0 Hz), 7.19 (s, 1H, Ar-H), 7.22–7.29 (m, 2H, Ar-H), 7.55 (d, 1H, Ar-H, J = 8.0 Hz), 8.22–8.26 (m, 2H, Ar-H), 11.11 (s, 1H, NH of isatin), 12.05 (s, 1H, hydrazide NH), 13.52 (s, 1H, Indole NH); 13 C NMR δ ppm: 56.09 (OCH3), 106.08, 108.20, 112.35, 112.99, 117.66, 120.94, 121.51, 121.96, 123.21, 126.12, 131.77, 136.07, 136.79, 155.87 (Aromatic carbons), 162.04 (C=O hydrazide), 163.51 (C=O isatin)); IR (KBr, ν cm−1) 3569, 3432, 3174 (3NH) and 1686, 1655 (2 C=O); Calcd. Anal. for C18H14N4O3: C, 64.67; H, 4.22; N, 16.76; found C, 64.09; H, 4.76; N, 16.56.
4.1.2.7. N'-(2-Oxo-5-(trifluoromethoxy)indolin-3-ylidene)-1H-indole-3-carbohydrazide 7g
Yellow powder; m.p. 278–280 °C; (yield 72%); 1H NMR δ ppm: 7.05 (d, 1H, Ar-H, J = 8.0 Hz), 7.23–7.29 (m, 2H, Ar-H), 7.36 (d, 1H, Ar-H, J = 8.0 Hz), 7.55–7.60 (m, 2H, Ar-H), 8.22 (d, 1H, Ar-H, J = 8.0 Hz), 8.31 (s, 1H, Ar-H), 11.46 (s, 1H, NH of isatin), 12.06 (s, 1H, hydrazide NH), 13.39 (s, 1H, Indole NH)); IR (KBr, ν cm−1) 3647, 3445, 3187 (3NH) and 1710, 1641 (2 C=O); Calcd. Anal. for C18H11F3N4O3: C, 55.68; H, 2.86; N, 14.43; found C, 55.53; H, 2.91; N, 14.39.
4.1.2.8. N'-(5-Nitro-2-oxoindolin-3-ylidene)-1H-indole-3-carbohydrazide 7h
Yellow powder; m.p. >300 °C; (yield 86%); 1H NMR δ ppm: 7.12 (d, 1H, Ar-H, J = 8.0 Hz), 7.22–7.29 (m, 2H, Ar-H), 7.54 (d, 1H, Ar-H, J = 8.0 Hz), 8.20 (d, 1H, Ar-H, J = 8.0 Hz), 8.24 (d, 1H, Ar-H, J = 8.0 Hz), 8.30–8.34 (m, 2H, Ar-H), 11.89 (s, 1H, NH of isatin), 12.11 (s, 1H, hydrazide NH), 13.19 (s, 1H, Indole NH); 13 C NMR δ ppm: 107.64, 111.74, 113.00, 115.84, 120.96, 121.46, 121.92, 122.10, 123.31, 127.21, 132.40, 133.29, 136.71, 143.24, 147.41 (Aromatic carbons), 163.65 (C=O hydrazide), 166.06 (C=O isatin)); IR (KBr, ν cm−1) 3594, 3426, 3111 (3NH) and 1704, 1671 (2 C=O); Calcd. Anal. for C17H11N5O4: C, 58.46; H, 3.17; N, 20.05; found C, 57.91; H, 3.71; N, 20.08.
4.1.2.9. N'-(7-Fluoro-2-oxoindolin-3-ylidene)-1H-indole-3-carbohydrazide 7i
Yellow powder; m.p. >300 °C; (yield 79%); 1H NMR δ ppm: 7.08–7.17 (m, 1H, Ar-H), 7.21–7.29 (m, 2H, Ar-H), 7.31 (t, 1H, Ar-H, J = 8.0 Hz), 7.50–7.58 (m, 1H, Ar-H), 8.03 (d, 1H, Ar-H, J = 8.0 Hz), 8.31 (d, 1H, Ar-H, J = 8.0 Hz), 8.68 (s, 1H, Ar-H), 11.29 (s, 1H, NH of isatin), 12.05 (s, 1H, hydrazide NH), 13.43 (s, 1H, Indole NH); 13 C NMR δ ppm: 107.34, 108.02, 112.57, 113.01, 117.04, 118.17, 118.68, 119.11, 119.28,120.96, 121.72, 122.03, 122.56, 122.97, 123.27, 123.70, 123.92, 126.20, 127.76, 129.12, 129.25, 130.80, 131.97, 133.86, 136.21, 136.78, 145.81, 146.21, 148.22, 148.63 (Aromatic carbons), 162.09, 163.17 (C=O hydrazide), 165.30, 165.44 (C=O isatin)); IR (KBr, ν cm−1) 3527, 3450, 3286 (3NH) and 1716, 1638 (2 C=O); Calcd. Anal. for C17H11FN4O2: C, 63.35; H, 3.44; N, 17.38; found C, 63.11; H, 4.08; N, 17.33.
4.1.2.10. N'-(5,7-Dimethyl-2-oxoindolin-3-ylidene)-1H-indole-3-carbohydrazide 7j
Red powder; m.p. >300 °C; (yield 74%); 1H NMR δ ppm: 2.22 (s, 3H, CH3 of position 7 for isatin), 2.30 (s, 3H, CH3 of position 5 for isatin), 7.00 (s, 1H, Ar-H), 7.15–7.28 (m, 3H, Ar-H), 7.54 (d, 1H, Ar-H, J = 8.0 Hz), 8.23–8.34 (m, 2H, Ar-H), 11.20 (s, 1H, NH of isatin), 12.03 (s, 1H, hydrazide NH), 13.45 (s, 1H, Indole NH); IR (KBr, ν cm−1) 3528, 3448, 3176 (3NH) and 1711, 1687 (2 C=O); Calcd. Anal. for C19H16N4O2: C, 68.66; H, 4.85; N, 16.86; found C, 68.14; H, 5.37; N, 16.68.
4.1.2.11. N'-(1-Methyl-2-oxoindolin-3-ylidene)-1H-indole-3-carbohydrazide 9a
Yellow powder; m.p. 273–275 °C; (yield 66%); 1H NMR δ ppm: 3.22, 3.26 (s, 3H, N-CH3), 7.10–7.18 (m, 2H, Ar-H), 7.22–7.30 (m, 2H, Ar-H), 7.43–7.49 (m, 1H, Ar-H), 7.53–7.58 (m, 1H, Ar-H), 7.66 (d, 0.3H, Ar-H, J = 8.0 Hz), 8.17 (d, 0.7H, Ar-H, J = 8.0 Hz), 8.24–8.33 (m, 1.3H, Ar-H), 8.67 (s, 0.7H, Ar-H), 11.24, 13.38 (s, 1H, hydrazide NH), 12.04, 12.07 (s, 1H, Indole NH); 13 C NMR δ ppm: 26.65 (N-CH3), 108.60, 110.78, 113.46, 120.48, 121.10, 121.34, 122.45, 123.69, 124.03, 126.55, 131.90, 132.33, 137.24, 144.14 (Aromatic carbons), 161.95 (C=O hydrazide), 162.55 (C=O isatin)); IR (KBr, ν cm−1) 3436, 3117 (2NH) and 1774, 1725 (2 C=O); Calcd. Anal. for C18H14N4O2: C, 67.92; H, 4.43; N, 17.60; found C, 67.65; H, 4.97; N, 17.54.
4.1.2.12. N'-(2-Oxo-1-propylindolin-3-ylidene)-1H-indole-3-carbohydrazide 9b
Yellow powder; m.p. 248–250 °C; (yield 69%); 1H NMR δ ppm: 0.92 (t, 3H, –CH2CH3, J = 8.0 Hz), 1.65–1.74 (m, 2H, –CH2CH3), 3.76 (t, 2H, N-CH2, J = 8.0 Hz), 7.17 (t, 1H, Ar-H, J = 8.0 Hz), 7.23–7.30 (m, 3H, Ar-H), 7.44 (t, 1H, Ar-H, J = 8.0 Hz), 7.55–7.58 (m, 1H, Ar-H), 7.68 (d, 1H, Ar-H, J = 8.0 Hz), 8.23–8.25 (m, 1H, Ar-H), 8.29 (s, 1H, Ar-H), 12.06 (s, 1H, hydrazide NH), 13.40 (s, 1H, Indole NH); 13 C NMR δ ppm: 11.67 (–CH2CH3), 20.95 (–CH2CH3), 41.24 (N-CH2), 108.10, 110.53, 113.00, 120.08, 120.78, 120.94, 122.01, 123.23, 123.45, 126.19, 131.42, 131.84, 134.42, 136.80, 143.00 (Aromatic carbons), 161.51 (C=O hydrazide), 162.10 (C=O isatin)); IR (KBr, ν cm−1) 3310, 3151 (2NH) and 1727, 1663 (2 C=O); Calcd. Anal. for C20H18N4O2: C, 69.35; H, 5.24; N, 16.17; found C, 69.20; H, 5.73; N, 16.19.
4.1.2.13. N'-(1-Benzyl-2-oxoindolin-3-ylidene)-1H-indole-3-carbohydrazide 9c
Yellow powder; m.p. 274–276 °C; (yield 72%); 1H NMR δ ppm: 5.00 (s, 2H, CH2), 7.06 (d, 1H, Ar-H, J = 8.0 Hz), 7.16 (t, 1H, Ar-H, J = 8.0 Hz), 7.24–7.31 (m, 3H, Ar-H), 7.34–7.43 (m, 5H, Ar-H), 7.57 (d, 1H, Ar-H, J = 8.0 Hz), 7.70 (d, 1H, Ar-H, J = 8.0 Hz), 8.25 (d, 1H, Ar-H, J = 8.0 Hz), 8.32 (s, 1H, Ar-H), 12.09 (s, 1H, hydrazide NH), 13.35 (s, 1H, Indole NH); 13 C NMR δ ppm: 43.03 (CH2), 108.09, 110.81, 113.03, 120.21, 120.86, 120.95, 122.06, 123.28, 123.70, 126.18, 127.83, 128.10, 129.22, 131.33, 131.89, 134.29, 136.20, 136.80, 142.62 (Aromatic carbons), 161.52 (C=O hydrazide), 162.13 (C=O isatin); IR (KBr, ν cm−1) 3450, 3210 (2NH) and 1748, 1688 (2 C=O); Calcd. Anal. for C24H18N4O2: C, 73.08; H, 4.60; N, 14.20; found C, 72.58; H, 5.14; N, 14.06.
4.1.2.14. N'-(5-Chloro-2-oxo-1-propylindolin-3-ylidene)-1H-indole-3-carbohydrazide 9d
Yellow powder; m.p. 262–263 °C; (yield 70%); IR (KBr, ν cm−1) 3450, 3221 (2NH) and 1730, 1660 (2 C=O); 1H NMR δ ppm: 0.90 (t, 3H, –CH2CH3, J = 8.0 Hz), 1.62–1.71 (m, 2H, –CH2CH3), 3.72 (t, 2H, N-CH2, J = 8.0 Hz), 7.22–7.28 (m, 3H, Ar-H), 7.45 (d, 1H, Ar-H, J = 8.0 Hz), 7.55 (d, 1H, Ar-H, J = 8.0 Hz), 7.65 (s, 1H, Ar-H), 8.22 (d, 1H, Ar-H, J = 8.0 Hz), 8.34 (s, 1H, Ar-H), 12.06 (s, 1H, hydrazide NH), 13.26 (s, 1H, Indole NH); 13 C NMR δ ppm: 11.61 (–CH2CH3), 20.89 (–CH2CH3), 41.37 (N-CH2), 107.71, 112.06, 112.55, 112.99, 120.35, 120.99, 121.81, 122.06, 123.26, 126.35, 127.70, 130.66, 133.15, 136.73, 141.63 (Aromatic carbons), 161.28 (C=O hydrazide), 162.20 (C=O isatin); IR (KBr, ν cm−1) 3450, 3210 (2NH) and 1748, 1688 (2 C=O); Calcd. Anal. for C20H17ClN4O2: C, 63.08; H, 4.50; N, 14.71; found C, 62.89; H, 4.94; N, 14.71.
4.1.2.15. N'-(5-Bromo-2-oxo-1-propylindolin-3-ylidene)-1H-indole-3-carbohydrazide 9e
Orange powder; m.p. 255–256 °C; (yield 75%); 1H NMR δ ppm: 0.95 (t, 3H, –CH2CH3, J = 8.0 Hz), 1.70–1.79 (m, 2H, –CH2CH3), 3.78 (t, 2H, N-CH2, J = 8.0 Hz), 6.94 (d, 1H, Ar-H, J = 8.0 Hz), 7.21–7.30 (m, 2H, Ar-H), 7.51 (d, 1H, Ar-H, J = 8.0 Hz), 7.55 (d, 1H, Ar-H, J = 8.0 Hz), 7.75 (s, 1H, Ar-H), 8.24 (d, 1H, Ar-H, J = 8.0 Hz), 8.35 (s, 1H, Ar-H), 12.10 (s, 1H, hydrazide NH), 13.29 (s, 1H, Indole NH); 13 C NMR δ ppm: 11.62 (–CH2CH3), 20.89 (–CH2CH3), 41.35 (N-CH2), 107.68, 112.51, 112.99, 115.33, 121.00, 122.05, 122.17, 123.04, 123.25, 126.36, 132.37, 132.98, 133.45, 136.72, 142.00 (Aromatic carbons), 161.13 (C=O hydrazide), 162.22 (C=O isatin); IR (KBr, ν cm−1) 3450, 3179 (2NH) and 1745, 1688 (2 C=O); Calcd. Anal. for C20H17BrN4O2: C, 56.48; H, 4.03; N, 13.17; found C, 56.06; H, 4.43; N, 13.16.
4.2. Biological evaluation
4.2.1. Cell culture
HT29 and SW620 colorectal cancer cell lines and HFF-1 fibroblast (ATCC, Rockville, USA) were used. HT29 cells were cultured and maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) (GIBCO, by Thermo Fischer Scientific, NY, USA) supplemented with 10% foetal bovine serum (FBS), 100 units/mL penicillin, and 100 µg streptomycin. SW620 and HFF-1 cells were cultured in Roswell Park Memorial Institute medium (RPMI-1640) (GIBCO, by Thermo scientific, NY, USA) supplemented with 10% FBS and 1% penicillin and streptomycin (Napolitano et al., 2015). All cultures were incubated at 37 °C and humidified atmosphere of 5% CO2.
4.2.2. Cell viability assay
The cytotoxicity effect of compounds on the colorectal cancer cell lines, HT29 and SW620 in addition to the normal human fibroblasts was measured by MTT (3–(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (Sigma-Aldrich, St. Louis, MO, USA) as previously described39. Briefly, cells were seeded in 96 well culture plates at 5000/well for HT29 and 10,000/well for SW620 for 24 h. Cells were then incubated with different compounds from WAG1 series (1–15) 24 h at 37 °C and humidified 5% CO2 incubator. Freshly prepared 10 µl of 3–(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT 5 mM) solution were added to the cells and further incubated for 2 h. Thereafter, 100 µl of dimethyl sulphoxide (DMSO) were added in each well and the crystals were dissolved through careful pipetting. In certain experiment, cells were treated different concentration of 5FU for 72 h. The absorbance of the product was measured at 540 nm using a Synergy™ 2 multi-mode microplate reader (Biotech, VA, USA). The experiments were performed in triplicate for each condition.
4.2.3. Measurement of apoptosis by annexin V-FITC/PI assay:
Induction of apoptosis was measured by Dead Cell Apoptosis Kit with Annexin V FITC and PI, for flow cytometry (Thermofischer scientific, OR, USA) according to the manufacturer’s instruction. As described previously (39), cells were seeded in a 6-well plate (3 × 105 cells per well) and treated with the various compound for 24 h. Both floating and adherent cells were harvested, pooled together, and incubated with Annexin V-FITC and PI for 15 min on ice in dark. The cells were analysed by BD FACSCalibur™ cell analyser (BD Biosciences, CA, USA) at an emission of 530 nm (FL1 channel) and >575 nm (FL3).
4.2.4. Western blot analysis:
All cells were seeded in a 100 mm dish (1 × 106 cells per dish) in 5% CO2 at 37 °C in the appropriate culture medium. The cells with around 50% confluency were treated with compounds for 24 h. At the experiment day, cells were washed with 1x PBS, harvested and lysed in RIPA lysis buffer, combined with protease inhibitors, (Sigma-Aldrich, St. Louis, MO, USA) as described previously39). The total protein concentration was evaluated by the colorimetric Bradford protein assay (BIO-RAD inc, CA, USA) at 595 nm absorbance. Lysates were loaded in equal concentration and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to a nitrocellulose membrane by semi-dry. Blocking of the membrane was done by 5% non-fat dried milk for one hour, incubated with the primary antibodies. The primary antibodies used were Bcl2 (cat. no. sc-7382), BclxL (cat. no. sc-8392) and β Actin (cat. no. sc-69879) from (Santa Cruz Biotechnology, Inc., Dallas, TX, USA). The secondary antibodies used were goat anti-mouse IgG-HRP (cat. no. sc-2005) and mouse anti-rabbit IgG-HRP (cat. no. sc-2357) from (Santa Cruz Biotechnology, Inc., Dallas, TX, USA). Detection was done with Luminol HRP chemiluminescence substrate (cat. no. sc-2048) from (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and then visualised by c-digit blot-scanner (LI-COR, Nebraska, USA).
4.2.5. Statistical analysis
The statistical analysis by the one-way ANOVA test was performed by GraphPad prism. Results were considered significant if the p-Values were <0.05.
Supplementary Material
Funding Statement
The authors extend their appreciation to the Deputyship for Research & Innovation, "Ministry of Education" in Saudi Arabia for funding this research work through the project number IFKSURG-1439–065.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Siegel RL, Miller KD, Jemal A.. Cancer statistics 2016. CA. Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Farber S, Diamond LK.. Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid. N Engl J Med 1948;238:787–93. [DOI] [PubMed] [Google Scholar]
- 3.Wadler S, Fuks JZ, Wiernik PH.. Phase I and II agents in cancer therapy: I. Anthracyclines and related compounds. J Clin Pharmacol 1986;26:491–509. [DOI] [PubMed] [Google Scholar]
- 4.Nitiss JL. DNA topoisomerases in cancer chemotherapy: using enzymes to generate selective DNA damage. Curr Opin Investig Drugs 2002;3:1512–6. [PubMed] [Google Scholar]
- 5.Nitiss JL. Targeting DNA topoisomerase II in cancer chemotherapy. Nat Rev Cancer 2009;9:338–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung-Ong K, Giaever G, Nislow C.. DNA-damaging agents in cancer chemotherapy: serendipity and chemical biology. Chem Biol 2013;20:648–59. [DOI] [PubMed] [Google Scholar]
- 7.Goodman LS, Wintrobe MM.. Nitrogen mustard therapy; use of methyl-bis (beta-chloroethyl) amine hydrochloride and tris (beta-chloroethyl) amine hydrochloride for Hodgkin's disease, lymphosarcoma, leukemia and certain allied and miscellaneous disorders. J Am Med Assoc 1946;132:126–32. [DOI] [PubMed] [Google Scholar]
- 8.Fischhaber PL, Gall AS, Duncan JA, Hopkins PB.. Direct demonstration in synthetic oligonucleotides that N,N'-bis(2-chloroethyl)-nitrosourea cross links N1 of deoxyguanosine to N3 of deoxycytidine on opposite strands of duplex DNA. Cancer Res 1999;59:4363–8. [PubMed] [Google Scholar]
- 9.DeVita VT, Chu E.. A history of cancer chemotherapy. Cancer Res 2008; 68:8643–53. [DOI] [PubMed] [Google Scholar]
- 10.Espinosa E, Zamora P, Feliu J, Barón MG.. Classification of anticancer drugs – a new system based on therapeutic. Cancer Treat Rev 2003;29:515–23. [DOI] [PubMed] [Google Scholar]
- 11.Mansoori B, Mohammadi A, Davudian S, et al. The different mechanisms of cancer drug resistance: a brief review. Adv Pharm Bull 2017;7:339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baudino TA. Targeted cancer therapy: the next generation of cancer treatment. Curr Drug Discov Technol 2015;12:3–20. [DOI] [PubMed] [Google Scholar]
- 13.Topcul M, Cetin I.. Endpoint of cancer treatment: targeted therapies. Asian Pac J Cancer Prev 2014; 15:4395–403. [DOI] [PubMed] [Google Scholar]
- 14.Marone M, Ferrandina G, Macchia G, et al. Bcl2, Bax, BclxL and bcl-xs expression in neoplastic and normal endometrium. Oncology 2000;58:161–8. [DOI] [PubMed] [Google Scholar]
- 15.Hata AN, Engelman JA, Faber AC.. The Bcl2 family: key mediators of the apoptotic response to targeted anticancer therapeutics. Cancer Discov 2015;5:475–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edlich F. BCL-2 proteins and apoptosis: recent insights and unknowns. Biochem Biophys Res Commun 2018;500:26–34. [DOI] [PubMed] [Google Scholar]
- 17.Green D. Means to an End: Apoptosis and other Cell Death Mechanisms. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2011. ISBN 978-0-87969-888-1. [Google Scholar]
- 18.Spierings D, Mcstay G, Saleh M, et al. Connected to death: the (unexpurgated) mitochondrial pathway of apoptosis. Science 2005;310:66–7. [DOI] [PubMed] [Google Scholar]
- 19.Wang B, Xu A.. Aryl hydrocarbon receptor pathway participates in myocardial ischemia reperfusion injury by regulating mitochondrial apoptosis. Med Hypotheses 2019;123:2–5. [DOI] [PubMed] [Google Scholar]
- 20.Placzek WJ, Wei J, Kitada S, et al. A survey of the anti-apoptotic Bcl2 subfamily expression in cancer types provides a platform to predict the efficacy of Bcl2 antagonists in cancer therapy. Cell Death Dis 2010;1:e40–e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Modugno )M, Banfi P, Gasparri F, et al. Mcl-1 antagonism is a potential therapeutic strategy in a subset of solid cancers. Exp Cell Res 2015;332:267–77. [DOI] [PubMed] [Google Scholar]
- 22. Tarfah Al-Warhi MF, Abo-Ashour H, Almahli OJ, et al. Novel [(N-alkyl-3-indolylmethylene)hydrazono]oxindoles arrest cell cycle and induce cell apoptosis by inhibiting CDK2 and Bcl2: synthesis, biological evaluation and in silico studies. J Enz Inh Med Chem 2020;35:1300–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu P, Liu H, Sun Q, et al. Potent inhibitors of SARS-CoV-2 3C-like protease derived from N-substituted isatin compounds. Eur J Med Chem 2020;206:112702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abo-Ashour MF, Eldehna WM, George RF, et al. Novel indole-thiazolidinone conjugates: Design, synthesis and whole-cell phenotypic evaluation as a novel class of antimicrobial agents. Eur J Med Chem 2018;160:49–60. [DOI] [PubMed] [Google Scholar]
- 25.Mathur G, Nain S.. Recent advancement in synthesis of isatin as anticonvulsant agents: a review. Med Chem 2014;4:417–27. [Google Scholar]
- 26.Ding Z, Zhou M, Zeng C.. Recent advances in isatin hybrids as potential anticancer agents. Arch Pharm (Weinheim) 2020;353:e1900367. [DOI] [PubMed] [Google Scholar]
- 27.Gupta AK, Tulsyan S, Bharadwaj M, Mehrotra R.. Systematic review on cytotoxic and anticancer potential of n-substituted isatins as novel class of compounds useful in multidrug-resistant cancer therapy: in silico and in vitro analysis. Top Curr Chem (Cham) 2019;377:15. [DOI] [PubMed] [Google Scholar]
- 28.Abdel-Aziz HA, Ghabbour HA, Eldehna WM, et al. Synthesis, crystal structure, and biological activity of cis/trans amide rotomers of (Z)-N'-(2-oxoindolin-3-ylidene)formohydrazide. J Chem 2014;2014:1–7. [Google Scholar]
- 29.Attia MI, Eldehna WM, Afifi SA, et al. New hydrazonoindolin-2-ones: Synthesis, exploration of the possible anti-proliferative mechanism of action and encapsulation into PLGA microspheres. PLoS One 2017;12:e0181241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eldehna WM, Al-Wabli RI, Almutairi MS, et al. Synthesis and biological evaluation of certain hydrazonoindolin-2-one derivatives as new potent anti-proliferative agents. J Enzym Inhib Med Chem 2018; 33:867–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abdel-Aziz A, Eldehna H, Keeton WM, et al. Isatin-benzoazine molecular hybrids as potential antiproliferative agents: synthesis and in vitro pharmacological profiling. Drug Des Dev Ther 2017;11:2333–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abo-Ashour MF, Eldehna WM, Nocentini A, et al. 3-Hydrazinoisatin-based benzenesulfonamides as novel carbonic anhydrase inhibitors endowed with anticancer activity: synthesis, in vitro biological evaluation and in silico insights. Eur J Med Chem 2019;184:111768. [DOI] [PubMed] [Google Scholar]
- 33.Eldehna WM, Abo-Ashour MF, Nocentini A, et al. Enhancement of the tail hydrophobic interactions within the carbonic anhydrase IX active site via structural extension: Design and synthesis of novel N-substituted isatins-SLC-0111 hybrids as carbonic anhydrase inhibitors and antitumor agents. Eur J Med Chem 2019;162:147–60. [DOI] [PubMed] [Google Scholar]
- 34.Al-Warhi T, El Kerdawy AM, Aljaeed N, et al. Synthesis, biological evaluation and in silico studies of certain oxindole–indole conjugates as anticancer CDK inhibitors. Molecules 2020;25:2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eldehna WM, El Kerdawy AM, Al-Ansary GH, et al. Type IIA - Type IIB protein tyrosine kinase inhibitors hybridization as an efficient approach for potent multikinase inhibitor development: Design, synthesis, anti-proliferative activity, multikinase inhibitory activity and molecular modeling of novel indolinone-based ureides and amides. Eur J Med Chem 2019;163:37–53. [DOI] [PubMed] [Google Scholar]
- 36.Eldehna WM, Almahli H, Al-Ansary GH, et al. Synthesis and in vitro anti-proliferative activity of some novel isatins conjugated with quinazoline/phthalazine hydrazines against triple-negative breast cancer MDA-MB-231 cells as apoptosis-inducing agents. J Enzym Inhib Med Chem 2017;32:600–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El-Naggar M, Eldehna WM, Almahli H, et al. Novel thiazolidinone/thiazolo[3,2-a]benzimidazolone-isatin conjugates as apoptotic anti-proliferative agents towards breast cancer: one-pot synthesis and in vitro biological evaluation. Molecules 2018;23:1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kasibhatla S, Tseng B.. Why target apoptosis in cancer treatment? Mol Cancer Ther 2003;2:573–80. [PubMed] [Google Scholar]
- 39.Ahmad R, Vaali-Mohammed MA, Elwatidy M, et al. Induction of ROS-mediated cell death and activation of the JNK pathway by a sulfonamide derivative. Int J Mol Med 2019;44:1552–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.