Abstract
Osteoarthritis (OA) is a common age-related joint disorder, for which no effective disease-modifying drugs are currently available. Long non-coding RNAs (lncRNAs) are involved in the occurrence of OA. lncRNA small nucleolar RNA host gene 16 (SNHG16) has been reported to regulate inflammation; however, the exact biological function of SNHG16 in OA and its underlying mechanism of action remain unclear. In this study, gene and protein expression levels were detected using reverse transcription-quantitative PCR and western blotting, respectively. Cell apoptosis was analyzed using flow cytometry and ELISA was performed to detect TNF-α levels. The interactions between lncRNA SNHG16 and microRNA (miR)-373-3p were examined using the dual-luciferase reporter assay. lncRNA SNHG16 was upregulated in OA tissue compared with normal joint tissue. The expression levels of collagen II were significantly reduced in OA tissue compared with normal tissue. Similarly, aggrecan expression levels were significantly reduced in IL-1β-treated CHON-001 cells compared with the controls. In addition, the protein expression levels of MMP13 were significantly increased in OA tissues and IL-1β-treated CHON-001 cells compared with the controls. SNHG16 knockdown significantly increased the expression levels of aggrecan, and decreased the expression levels of MMP13, cleaved caspase-3 and p21 in IL-1β-treated CHON-001 cells. In addition, IL-1β induced CHON-001 cell apoptosis, while SNHG16 knockdown decreased IL-1β-induced apoptosis. Furthermore, the luciferase activity assay suggested that SNHG16 negatively regulated miR-373-3p in OA. Finally, the results suggested that the proinflammatory effect of IL-1β on CHON-001 cells was significantly reduced by SNHG16 knockdown. In conclusion, lncRNA SNHG16 knockdown significantly limited the progression of OA by sponging miR-373-3p in vitro, which suggested that SNHG16 may serve as a potential therapeutic target for OA.
Keywords: osteoarthritis, long non-coding RNA, small nucleolar RNA host gene 16, microRNA-373-3p, p21
Introduction
Osteoarthritis (OA) is the most common form of arthropathy in elderly individuals worldwide, and is characterized by local inflammation, articular cartilage damage and degradation of the cartilage (1). The number of patients with OA is expected to increase as the population ages, increasing the psychological and socio-economic burden of patients (2). Current therapeutic strategies for OA are aimed at symptomatic control, rather than disease modification (3); therefore, extending the existing knowledge of the pathology of OA is required for the development of effective therapeutic strategies.
OA displays typical heritability, which is why gene therapy for OA has gained much attention. Clinically, in vivo gene expression is difficult to control, and the selection and safety of vectors require confirmation; therefore, OA gene therapy is currently at the in vitro and in vivo stages of research (4). Meanwhile, a growing number of studies have indicated that non-coding RNAs (ncRNAs) are possible regulators of cell apoptosis (5,6). As a ncRNA subclass, long ncRNAs (lncRNAs) are >200 nucleotides in length, and display improved tissue and cell specificity compared with coding RNAs. lncRNAs are differentially expressed in different cells, tissues and developmental stages, and are associated with the occurrence and development of various diseases, including OA and malignancies (7–10). Moreover, it has been confirmed that enriched lncRNAs in the cytoplasm typically participate in post-transcriptional modification by binding to microRNAs (miRNAs/miRs) or mRNAs (8). For example, Li et al (11) reported that Pvt1 oncogene (PVT1) regulated chondrocyte apoptosis in OA by sponging miR-488-3p. Xiao et al (12) indicated that lncRNA miR4435-2HG, which regulated chondrocyte cell proliferation and apoptosis, may be downregulated in OA. Furthermore, it has been reported that lncRNA small nucleolar RNA host gene 16 (SNHG16) reversed the effects of miR-15a/16 on the lipopolysaccharide-induced inflammatory pathway (13). A recent study reported that SNHG16 may be associated with the occurrence of OA (14). However, the biological role of SNHG16 during the development of OA requires further investigation.
Therefore, the present study aimed to explore the roles of SNHG16 during the development of OA and the potential underlying mechanisms. The present study provided a theoretical basis for OA treatment and a novel perspective for clinical therapy.
Materials and methods
Cell culture
Previous studies have indicated that CHON-001 cells treated with interleukin (IL)-1β serve as an in vitro model of OA; therefore, this model was selected for the present study (15,16). The human chondrocyte cell line CHON-001 (American Type Culture Collection) was cultured in RPMI-1640 medium (Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Thermo Fisher Scientific, Inc.) and 2 mM glutamine (Sigma-Aldrich; Merck KGaA) at 37°C at 5% CO2. To induce the in vitro model of OA, CHON-001 cells were treated with IL-1β (10 ng/ml) (Sigma-Aldrich; Merck KGaA) for 24 h at room temperature.
Tissue collection
OA tissues (n=20) and normal joint tissues (n=20) were obtained from the Second Affiliated Hospital of Inner Mongolia Medical University (Hohhot, China) between August 2017 and April 2019. OA tissues were obtained from patients with OA. Normal joint tissues were obtained from patients who had been in a car accident. Clinical and pathological data of the patients were collected. Patients were diagnosed with OA according to the American College of Rheumatology classification criteria for the diagnosis of OA (17). The present study was approved by the Institutional Ethical Committee of the Second Affiliated Hospital of Inner Mongolia Medical University. Written informed consent was obtained from all participants. Clinical characteristics of the patients and healthy controls are presented in Table I.
Table I.
Clinical characteristics of the patients and healthy controls.
| Clinical characteristic | Patients (n=20) | Healthy controls (n=20) | P-value |
|---|---|---|---|
| Age (years) | 57.63±7.22 | 57.28±6.55 | 0.8733 |
| Sex (male/female) | 11/9 | 10/10 | 0.7515 |
| Body mass index (kg/m2) | 23.86±2.05 | 24.01±1.89 | 0.8112 |
| Family history of OA (yes/no) | 5/15 | 2/18 | 0.2119 |
OA, osteoarthritis.
Cell transfection
siRNAs targeted against SNHG16 (SNHG16 siRNA1 and SNHG16 siRNA2; 10 nM) and a negative control siRNA (siRNA-NC) were purchased from Guangzhou RiboBio Co., Ltd. and transfected into CHON-001 cells (5×103) using Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. Cells were incubated at 37°C for 6 h before subsequent experiments were performed. Transfection efficiency was determined using reverse transcription-quantitative PCR (RT-qPCR). The sequences of the siRNAs were as follows: Negative control siRNA, 5′-UUCUCCGAACGUGUCACGUTT-3′; SNHG16 siRNA1, 5′-GGAAUGAAGCAACUGAGAUUU−3′; and SNHG16 siRNA2, 5′-GGGTTACGATTGCCCAGAT-3′.
RT-qPCR
Total RNA was extracted from tissues or cells using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA (5–10 µg) was reverse transcribed into cDNA using the PrimeScript RT reagent kit (Takara Bio, Inc.), according to the manufacturer's instructions. Subsequently, qPCR was performed using the SYBR premix Ex Taq II kit (Takara Bio, Inc.). The following primer pairs were used for qPCR: SNHG16, forward, 5′-CTTCCGCCATGATTGTGAGG-3′, reverse, 5′-AACAGTCCCTCCTTGGTCTC-3′; β-actin, forward, 5′-AGCGAGCATCCCCCAAAGTT-3′, reverse, 5′-GGGCACGAAGGCTCATCATT-3′; collagen II, forward, 5′-ATGCCACACTCAAGTCCCTCA-3′, reverse, 5′-GTCTCGCCAGTCTCCATGTTG-3′; MMP13, forward, 5′-CAGAACTTCCCAACCGTATTGAT-3′, reverse, 5′-TGTATTCAAACTGTATGGGTCCG-3′; miR-373-3p, forward, 5′-GGCGGAAGTGCTTCGATTTT-3′, reverse, 5′-GTGCAGGGTCCGAGGTATTC-3′; and U6, forward, 5′-GCTTCGGCAGCACATATACT-3′ and reverse, 5′-GTGCAGGGTCCGAGGTATTC-3′. The following thermocycling conditions were used for qPCR: Initial denaturation for 10 min at 95°C; 40 cycles of 95°C for 15 sec and 60°C for 30 sec; and final extension for 1 min at 60°C. miRNA and mRNA levels were quantified using the 2−ΔΔCq method and normalized to the internal reference genes β-actin and U6, respectively (18).
Cell Counting Kit-8 (CCK-8) assay
The CCK-8 assay (Beyotime Institute of Biotechnology) was performed to investigate cell proliferation. CHON-001 cells (5×103 cells/well) were plated into 96-well plates and treated for 0, 24, 48 or 72 h at room temperature as follows: 10 ng/ml IL-1β (IL-1β + NC siRNA); 10 ng/ml IL-1β and subsequently transfected with SNHG16 siRNA1 (IL-1β + SNHG16 siRNA1). Control cells were left untreated. CCK-8 reagent (10 µl) was added to each well and incubated for 2 h at 37°C. The absorbance of each well was measured at a wavelength of 450 nm using a microplate reader.
Western blotting
Total protein was extracted from OA tissues and CHON-001 cells using RIPA lysis buffer (Beyotime Institute of Biotechnology) and quantified using a bicinchoninic acid assay kit (Thermo Fisher Scientific, Inc.). Proteins (40 µg/lane) were separated by SDS-PAGE on 10% gels and were transferred onto PVDF membranes (Thermo Fisher Scientific, Inc.). Subsequently, the membranes were blocked with 5% skim milk in TBS-Tween (10%) for 1 h at room temperature. The membranes were then incubated overnight at 4°C with primary antibodies targeted against: Collagen II (1:1,000; cat. no. ab325034; Abcam), aggrecan (1:1,000; cat. no. ab1816028; Abcam), MMP13 (1:1,000; cat. no. ab25367; Abcam), p21 (1:1,000; cat. no. ab14323; Abcam), cleaved caspase-3 (1:1,000; cat. no. ab43583; Abcam) and β-actin (1:1,000; cat. no. ab06789; Abcam). Following primary antibody incubation, the membranes were incubated with a HRP-conjugated secondary antibody (1:5,000; cat. no. ab20876; Abcam) for 1 h at room temperature. Protein bands were detected using an ECL kit (Thermo Fisher Scientific, Inc.). Protein expression was semi-quantified using Image-Pro Plus software (version 6.0; Media Cybernetics, Inc.) with β-actin as the internal control.
Target prediction
TargetScan (www.targetscan.org/vert_71/) and microRNA Target Prediction Database (miRDB) databases (www.mirdb.org) were used to identify the downstream target genes of SNHG16.
ELISA
The levels of TNF-α in the media of CHON-001 cells were detected using the TNF-α ELISA kit [cat. no. ab47956, Hangzhou Multi Sciences (Lianke) Biotech Co., Ltd.], according to the manufacturer's protocol.
Immunofluorescence
CHON-001 cells were seeded (5×104 cells/well) into 24-well plates and incubated at 37°C for 24 h. Cells were pre-fixed with 4% paraformaldehyde for 10 min at room temperature, and fixed in pre-cold methanol at 4°C for another 10 min. Following blocking with 10% BSA (Beyotime Institute of Biotechnology) at room temperature for 1 h, cells were incubated overnight at 4°C with anti-Ki67 primary antibody (1:1,000; cat. no. ab35267; Abcam) and DAPI. Following primary antibody incubation, cells were incubated with the goat anti-rabbit IgG secondary antibody (1:5,000; cat. no. ab65734; Abcam) at room temperature for 1 h. Immunofluorescence staining was observed using a CX23 fluorescence microscope (Olympus Corporation) and quantified using Image-Pro Plus software (version 6.0; Media Cybernetics, Inc.).
Cell apoptosis analysis
CHON-001 cells were seeded (5×104 cells/well) in 6-well plates. Cells were centrifuged at 15 × g for 5 min at 4°C and resuspended in 100 µl binding buffer (BD Biosciences). Subsequently, 5 µl Annexin V-FITC (BD Biosciences) and 5 µl propidium (BD Biosciences) iodide were added to the cell solution for 15 min at 0°C. Early and late apoptotic cells were analyzed using a FACS flow cytometer (BD Biosciences) and WinMDI software (version 2.9; Invitrogen; Thermo Fisher Scientific, Inc.).
Dual-luciferase reporter assay
The partial sequence of the 3′-untranslated region (UTR) SNHG16 and the 3′-UTR of p21 containing the putative binding sites of miR-373-3p were synthetized and obtained from Sangon Biotech Co., Ltd. Subsequently, the sequences were cloned into the pmirGLO Dual-Luciferase miRNA Target Expression Vector (Promega Corporation) to construct wild-type (WT) reporter vectors for SNHG16 and p21. The mutant (MUT) SNHG16 and p21-3′-UTR sequences containing the putative binding sites of miR-373-3p were created using the Q5 Site-Directed Mutagenesis kit (New England Biolabs, Inc.), according to the manufacturer's protocol, and cloned into pmirGLO vectors to construct MUT reporter vectors for SNHG16 and p21. The WT and MUT SNHG16 vectors were transfected into CHON-001 cells (5×103 cells/well) with blank (untransfected cells), 10 nM vector-control (pcDNA-3.1 vector; cat. no. ab06707; Beyotime Institute of Biotechnology) or 10 nM miR-373-3p mimics (cat. no. ab35643; Beyotime Institute of Biotechnology) using Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. Similarly, the WT and MUT p21 vectors were transfected into 293T cells with blank, vector-control or miR-373-3p mimics. At 48 h post-transfection, cells were collected and the relative luciferase activity was detected using the Dual-Glo Luciferase assay system (Promega Corporation), according to the manufacturer's protocol. Firefly luciferase activity was normalized to Renilla luciferase activity.
Statistical analysis
Experiments were performed in at least triplicate. Data are presented as the mean ± SD. Comparisons between two groups were analyzed using an unpaired Student's t-test. Comparisons among multiple groups were analyzed using one-way ANOVA followed by Tukey's post hoc test. Statistical analyses were performed using GraphPad Prism software (version 7; GraphPad Software, Inc.). P<0.05 was considered to indicate a statistically significant difference.
Results
Expression levels of SNHG16 are upregulated in OA tissues
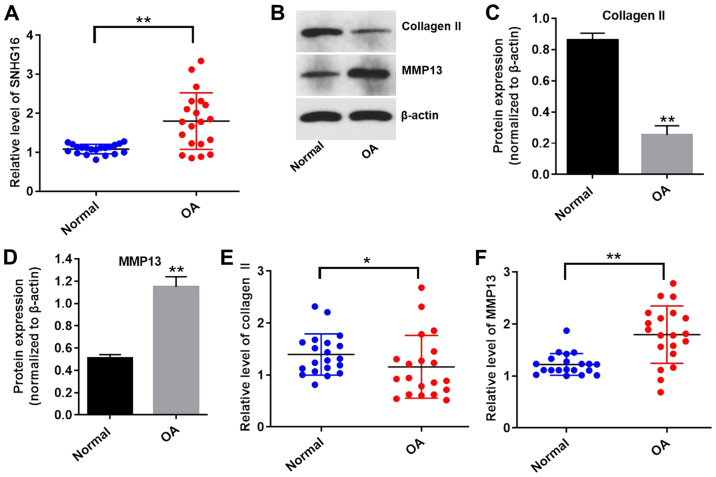
To explore whether the expression levels of lncRNA SNHG16 were altered in OA, the expression of SNHG16 in OA and normal tissues was measured using RT-qPCR. The expression levels of SNHG16 were significantly upregulated in OA tissues compared with normal tissues (Fig. 1A). The expression levels of collagen II were significantly reduced, while the expression levels of MMP13 were significantly upregulated in OA tissues compared with normal tissues (Fig. 1B-F). These results indicated that lncRNA SNHG16 was significantly upregulated in OA tissues compared with normal tissues.
Figure 1.
SNHG16 is upregulated in OA tissues. (A) Expression levels of SNHG16 in human OA tissues or normal joint tissues were detected using RT-qPCR. Protein expression levels were (B) determined by western blotting, and (C) collagen II and (D) MMP13 expression was semi-quantified. β-actin was used as an internal control. The expression levels of (E) collagen II and (F) MMP13 in OA and normal tissues were detected using RT-qPCR. *P<0.05 and **P<0.01 vs. normal group. OA, osteoarthritis; RT-qPCR, reverse transcription-quantitative PCR; SNHG16, small nucleolar RNA host gene 16.
In vitro model of OA was successfully established
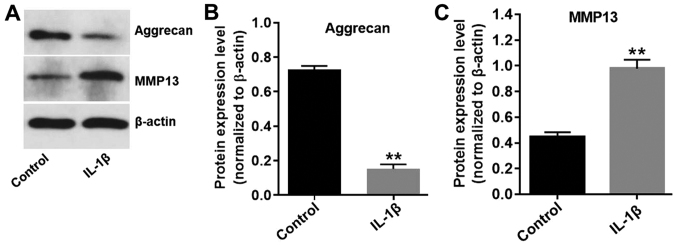
It has previously been demonstrated that IL-1β contributes to the destruction of articular cartilage (19); therefore, CHON-001 cells were treated with 10 ng/ml IL-1β for 24 h to develop an in vitro model of OA. The expression levels of aggrecan in CHON-001 cells were significantly decreased in the presence of IL-1β (Fig. 2A and B), while IL-1β significantly increased the expression levels of MMP13 in CHON-001 cells, compared with the control group (Fig. 2A and C). The results indicated that the in vitro model of OA was successfully established.
Figure 2.
Successful establishment of the in vitro model of OA. CHON-001 cells were treated with IL-1β (10 ng/ml) for 24 h to establish an in vitro model of OA. Protein expression levels were (A) determined by western blotting, and (B) aggrecan and (C) MMP13 were semi-quantified. β-actin was used as an internal control. **P<0.01 vs. control group. IL-1β, interleukin-1β; OA, osteoarthritis.
SNHG16 knockdown significantly inhibits apoptosis and enhances the proliferation of CHON-001 cells
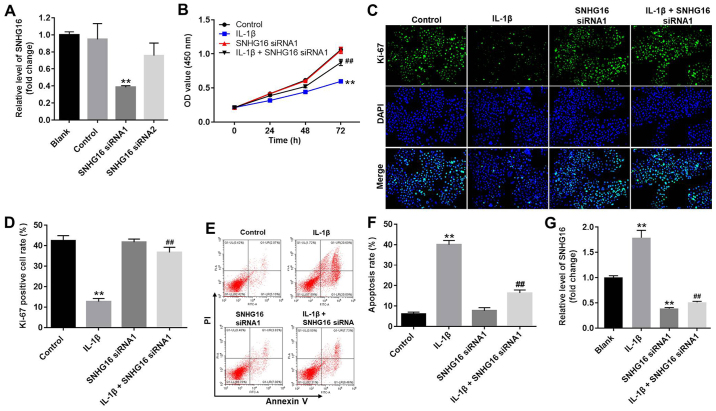
To investigate transfection efficiency, the expression levels of SNHG16 in CHON-001 cells were measured using RT-qPCR. SNHG16 siRNA1 significantly decreased the expression levels of SNHG16 in CHON-001 cells compared with the control group (Fig. 3A). However, SNHG16 siRNA2 displayed a limited effect on the expression of SNHG16 compared with the control group (Fig. 3A); therefore, SNHG16 siRNA1 was used for subsequent experiments. The CCK-8 assay and Ki-67 staining were performed to assess CHON-001 cell proliferation. CHON-001 cell proliferation was significantly decreased in the presence of IL-1β compared with the control group, but co-treatment with SNHG16 siRNA1 partially reversed the effects of IL-1β on CHON-001 cell proliferation. The SNHG16 siRNA group had a limited effect on CHON-001 cell proliferation compared with the control group (Fig. 3B). Similarly, the proportion of Ki-67-positive CHON-001 cells was significantly decreased by treatment with IL-1β compared with the control group. However, SNHG16 knockdown reversed the inhibitory effect of IL-1β on CHON-001 cell proliferation, as indicated by a significant increase in the proportion of Ki-67-positive cells (Fig. 3C and D). Flow cytometry was then performed to investigate CHON-001 cell apoptosis. The results suggested that IL-1β induced cell apoptosis and co-treatment with SNHG16 knockdown inhibited the proapoptotic effect of IL-1β on CHON-001 cells (Fig. 3E and F). Moreover, IL-1β treatment significantly increased the expression levels of SNHG16 in CHON-001 cells compared with the control group. However, the effect of IL-1β on SNHG16 expression was significantly reversed by SNHG16 knockdown in CHON-001 cells (Fig. 3G). These results suggested that SNHG16 knockdown inhibited apoptosis and enhanced the proliferation of CHON-001 cells.
Figure 3.
SNHG16 knockdown inhibits apoptosis and promotes the proliferation of CHON-001 cells. (A) CHON-001 cells were transfected with SNHG16 siRNA1 or SNHG16 siRNA2. Transfection efficiency was assessed by RT-qPCR. (B) CHON-001 cells were treated with IL-1β (10 ng/ml) and/or SNHG16 siRNA1 for 0, 24, 48 or 72 h. The effect of SNHG16 on CHON-001 cell proliferation was assessed using the Cell Counting Kit-8 assay. CHON-001 cell proliferation was (C) detected by Ki-67 staining (magnification, ×200) and (D) semi-quantified. CHON-001 cell apoptosis was detected by Annexin V/PI staining, (E) assessed by flow cytometry and (F) quantified. (G) Expression levels of SNHG16 in CHON-001 cells were detected by RT-qPCR. **P<0.01 vs. control group; ##P<0.01 vs. IL-1β group. IL-1β, interleukin-1β; OD, optical density; PI, propidium iodide; RT-qPCR, reverse transcription-quantitative PCR; siRNA, small interfering RNA; SNHG16, small nucleolar RNA host gene 16.
SNHG16 knockdown ameliorates IL-1 β -induced OA in vitro
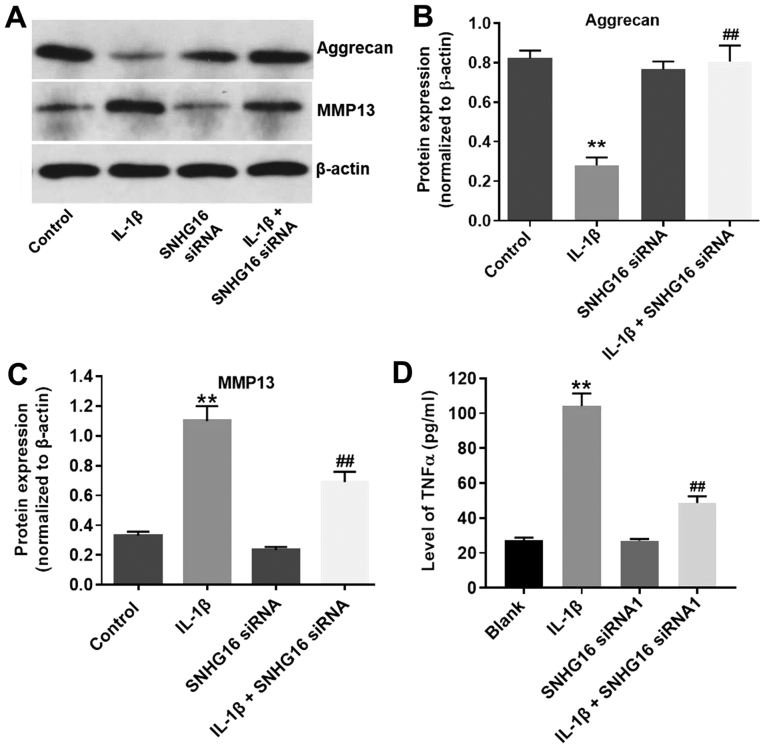
To further assess the function of lncRNA SNHG16 in IL-1β-treated CHON-001 cells, western blotting was performed. IL-1β significantly decreased the expression of aggrecan in CHON-001 cells compared with the control group, and SNHG16 knockdown decreased the inhibitory effect of IL-1β on aggrecan expression (Fig. 4A and B). Furthermore, the protein expression levels of MMP13 were significantly increased in the IL-1β group compared with the control group, and SBHG16 knockdown partially reduced the IL-1β-induced effects (Fig. 4A and C). The SNHG16 siRNA1 group displayed limited effects on the expression levels of aggrecan and MMP13 compared with the control group (Fig. 4A-C). Additionally, the levels of TNF-α in the media of CHON-001 cells were significantly increased by IL-1β treatment compared with the control group, and SNHG16 knockdown partially inhibited IL-1β-mediated effects on TNF-α (Fig. 4D). The results suggested that SNHG16 knockdown may ameliorate IL-1β-induced OA in vitro.
Figure 4.
SNHG16 knockdown ameliorates IL-1β-induced OA in vitro. CHON-001 cells were treated with IL-1β (10 ng/ml) and/or SNHG16 siRNA1 for 48 h. Protein expression levels were (A) determined by western blotting, and (B) aggrecan and (C) MMP13 were semi-quantified. β-actin was used as an internal control. (D) Levels of TNF-α in the media of CHON-001 cells were detected by ELISA. **P<0.01 vs. control group; ##P<0.01 vs. the IL-1β group. IL-1β, interleukin-1β; siRNA, small interfering RNA; SNHG16, small nucleolar RNA host gene 16.
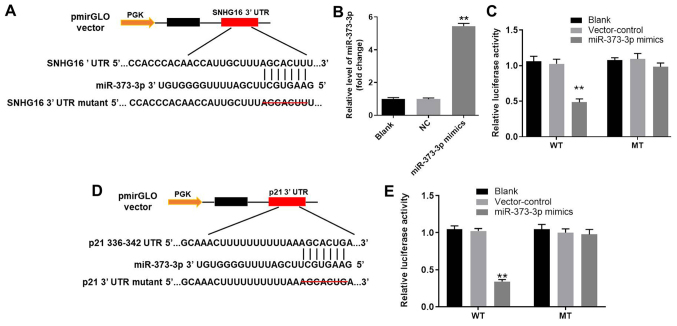
miR-373-3p is the downstream target gene of SNHG16
To investigate the potential mechanism underlying the action by which lncRNA SNHG16 ameliorated OA in vitro, TargetScan and miRDB databases were used to identify the downstream target genes of SNHG16. SNHG16 displayed a putative miR-373-3p targeting site (Fig. 5A). Furthermore, the RT-qPCR results indicated that miR-373-3p mimic was stably transfected into CHON-001 cells (Fig. 5B). In addition, the luciferase reporter assay was performed to determine whether miR-373-3p directly interacted with SNHG16 in CHON-001 cells. The results indicated that co-transfection of the WT SNHG16 vector with miR-373-3p mimic significantly decreased luciferase activities compared with the control group (Fig. 5C). Therefore, the results suggested that SNHG16 bound to miR-373-3p.
Figure 5.
miR-373-3p is the downstream target gene of SNHG16 and p21 is the direct target gene of miR-373-3p. (A) 3′-UTR gene structure of SNHG16 at the position of 1,467-1,473 contained the predicted target site of miR-373-3p, with a sequence of AUCCGCU. (B) miR-373-3p expression in CHON-001 cells was detected by RT-qPCR. (C) Luciferase activity was measured in CHON-001 cells following co-transfection with WT/MUT SNHG16 3′-UTR plasmid and miR-373-3p mimic using a dual luciferase reporter assay. (D) 3′-UTR gene structure of p21 at the position of 750–756 contained the predicted target site of miR-373-3p, with a sequence of AACCCUUG. (E) Luciferase activity was measured in CHON-001 cells following co-transfection with WT/MUT p21 3′-UTR plasmid and miR-373-3p mimic using a dual luciferase reporter assay. **P<0.01 vs. the control or blank group. 3′-UTR, 3′-untranslated region; miR, microRNA; MUT, mutant; NC, negative control; RT-qPCR, reverse transcription-quantitative PCR; SNHG16, small nucleolar RNA host gene 16; WT, wild-type.
p21 is the direct target gene of miR-373-3p
Subsequently, the TargetScan and miRDB databases were used to investigate the direct targets of miR-373-3p. The results suggested that p21 was a potential target of miR-373-3p (Fig. 5D). In addition, the luciferase assay demonstrated significantly reduced luciferase activity in CHON-001 cells post-transfection with WT p21 and miR-373-3p mimic compared with the control group (Fig. 5E). The results indicated that p21 was the direct target of miR-373-3p.
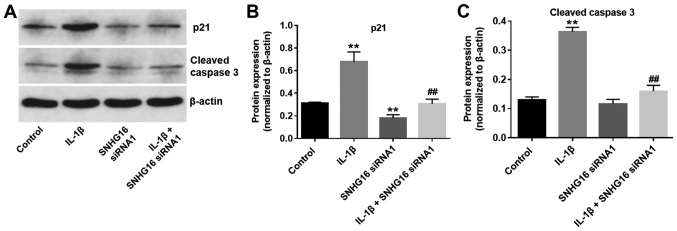
SNHG16 knockdown significantly decreases the IL-1 β -mediated activation of p21 and cleaved caspase-3
To further investigate the biological role of SNHG16 during the progression of OA, the expression levels of the cell cycle regulator, p21, and proapoptotic protein, cleaved caspase-3, were detected using western blot analysis. The expression levels of p21 in CHON-001 cells were significantly increased in the IL-1β group compared with the control group. IL-1β-mediated effects on p21 expression were significantly rescued by SNHG16 knockdown in CHON-001 cells (Fig. 6A and B). Additionally, SNHG16 knockdown significantly reduced p21 expression levels compared with the control group (Fig. 6A and B). Similarly, IL-1β significantly increased the expression levels of cleaved caspase-3 compared with the control group. However, SNHG16 knockdown significantly inhibited the proapoptotic effect of IL-1β on the expression of cleaved caspase-3 in CHON-001 cells (Fig. 6A and C). The results indicated that SNHG16 knockdown may decrease the IL-1β-mediated effects on p21 and cleaved caspase-3 expression.
Figure 6.
SNHG16 knockdown inhibits IL-1β-mediated activation of p21 and cleaved caspase-3. Protein expression levels were (A) determined by western blotting, and (B) p21 and (C) cleaved caspase-3 were semi-quantified. **P<0.01 vs. control group; ##P<0.01 vs. IL-1β group. IL-1β, interleukin 1β; siRNA, small interfering RNA; SNHG16, small nucleolar RNA host gene 16.
Discussion
Previous studies have indicated that lncRNAs serve a critical role during the progression of OA (11,20). In the present study, it was further suggested that the expression of lncRNA SNHG16 was significantly upregulated in OA tissues compared with normal joint tissues. Zhou et al (21) reported that the expression of lncRNA HOX transcript antisense RNA was significantly upregulated in OA tissue, which was similar to the results obtained in the present study. In addition, a recent study reported that the inflammatory response may have an important role during the progression of OA (22). Tan et al (23) indicated that the expression levels of IL-1β were increased in OA tissues. Furthermore, IL-1β promoted damage in arthritic cartilage and enhanced the expression levels of matrix-degradation proteins (24). The aforementioned results indicated that high expression levels of lncRNA SNHG16 were closely associated with the progression of OA.
To further explore the biological role of lncRNA SNHG16 during the development of OA, in vitro experiments were performed. Collagen II and MMP13 are matrix components of the cartilage (25,26). Loss of collagen II and upregulation of MMP13 expression can lead to the development of OA (27–29). In the present study, CHON-001 cell apoptosis was induced by IL-1β, whereas SNHG16 knockdown significantly decreased the expression of cleaved caspase-3, a proapoptotic protein, in IL-1β-induced CHON-001 cells (30). Lin et al (31) reported that luteoloside inhibited IL-1β-induced apoptosis in vitro by inactivating cleaved caspase-3 (31). The aforementioned results are consistent with the results of the present study, indicating that SNHG16 knockdown inhibited CHON-001 cell apoptosis by downregulating the expression levels of cleaved caspase-3. Similarly, the expression of collagen II was significantly decreased and the expression of MMP13 was significantly increased in OA tissues compared with normal joint tissues, which was consistent with previous studies (32,33). Based on the results of the present study, it was suggested that the in vitro model of OA was successfully established.
Further experiments suggested that SNHG16 knockdown significantly decreased apoptosis and increased the proliferation of CHON-001 cells treated with IL-1β. Furthermore, SNHG16 knockdown significantly decreased the expression levels of aggrecan and upregulated the expression levels of MMP13 in CHON-001 cells treated with IL-1β. Similarly, Tu et al (34) suppressed IL-1β-induced cartilage degradation by decreasing the expression levels of collagen II. A previous study revealed that the Bushenhuoxue formula attenuated cartilage degeneration in a mouse model of OA via the transforming growth factor-β/MMP13 signaling pathway (35). Additionally, the expression levels of PVT1 were increased in IL-1β-stimulated OA chondrocytes; therefore, PVT1 knockdown may inhibit the development of OA by altering inflammatory responses (36). The aforementioned results were consistent with the results of the present study, indicating that SNHG16 knockdown may suppress the development of OA in vitro.
The mechanism underlying the action by which SNHG16 knockdown inhibited the progression of OA in vitro was investigated. The dual-luciferase reporter assay indicated that miR-373-3p was the downstream target gene of SNHG16. miRNAs are highly conserved ncRNAs that display versatile biological functions (37,38). Lei et al (39) reported that lncRNA SNHG1 inhibited IL-1β-induced OA by suppressing the miR-16-5p-mediated p38 MAPK and NF-κB signaling pathway. In addition, lncRNA metastasis associated lung adenocarcinoma transcript 1 promoted the progression of OA by regulating the miR-150-5p/AKT3 signaling pathway (40). Meanwhile, miR-373-3p has been reported to be involved in multiple diseases (41,42). The present study also investigated the biological effect of miR-373-3p on IL-1β-treated CHON-001 cell proliferation, suggesting that it may act as a suppressor during the progression of OA. The results of the present study further suggested that SNHG16 may promote the development of OA by sponging miR-373-3p.
It has been reported that miRNAs primarily exert their function by binding to target genes (43,44). To explore the potential mechanism underlying the action of miR-373-3p during the progression of OA, TargetScan and miRDB databases were used to identify target genes of miR-373-3p. p21, a cell cycle regulator, was identified as a direct target of miR-373-3p (45). It has been reported that p21 may be partially involved in G2 arrest following cell damage (46). In addition, p21 deficiencies resulted in patient susceptibility to OA via STAT3 phosphorylation (47). In the present study, the luciferase reporter assay further indicated that p21 was a direct target gene of miR-373-3p. Furthermore, the results suggested that IL-1β significantly increased the expression of p21 in CHON-001 cells. Tang et al (48) reported that p21 promoted chondrocyte apoptosis in OA by sponging miR-451. The results obtained in the present study suggested that downregulation of p21 may contribute to the alleviation of IL-1β-induced CHON-001 cell growth inhibition. Since STAT3 has been reported to be involved in the progression of OA (49), further investigation into the effects of SNHG16 on STAT3 signaling should be conducted. The present study did not include in vivo experiments due to lack of funds; therefore, future studies should employ in vivo models to investigate therapeutic targets for OA.
In conclusion, the present study investigated the roles of SNHG16 during the development of OA and the underlying mechanisms. The results indicated that SNHG16 expression was significantly upregulated in OA tissues compared with normal tissues, and that knockdown of SNHG16 expression increased the proliferation and inhibited apoptosis of CHON-001 cells. miR-373-3p was identified as a downstream target of SNHG16, and p21 was identified as a target gene of miR-373-3p. The present study suggested that SNHG16 knockdown inhibited the progression of OA in vitro, which indicated that SNHG16 may serve as a novel therapeutic target for OA.
Acknowledgements
The authors would like to thank Dr Zhang (Department of Surgery, Medical School, Stanford University) for proofreading the manuscript.
Funding
The present study was supported by Inner Mongolia Natural Science Foundation [grant no. (2017LH)0309].
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
HF and YY conceived and supervised the study. HF, LD and YY designed the study. HF and LD performed the experiments and analyzed the data. All authors reviewed the results and approved the final version of the manuscript.
Ethics approval and consent to participate
The present study was approved by the Institutional Ethical Committee of the Second Affiliated Hospital of Inner Mongolia Medical University. Written informed consent was obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Allen KD, Choong PF, Davis AM, Dowsey MM, Dziedzic KS, Emery C, Hunter DJ, Losina E, Page AE, Roos EM, et al. Osteoarthritis: Models for appropriate care across the disease continuum. Best Pract Res Clin Rheumatol. 2016;30:503–535. doi: 10.1016/j.berh.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Cavalli E, Levinson C, Hertl M, Broguiere N, Brück O, Mustjoki S, Gerstenberg A, Weber D, Salzmann G, Steinwachs M, et al. Characterization of polydactyly chondrocytes and their use in cartilage engineering. Sci Rep. 2019;9:4275. doi: 10.1038/s41598-019-40575-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor N. Nonsurgical management of osteoarthritis knee pain in the older adult. Clin Geriatr Med. 2017;33:41–51. doi: 10.1016/j.cger.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Qiong J, Xia Z, Jing L, Haibin W. Synovial mesenchymal stem cells effectively alleviate osteoarthritis through promoting the proliferation and differentiation of meniscus chondrocytes. Eur Rev Med Pharmacol. 2020;24:1645–1655. doi: 10.26355/eurrev_202002_20338. [DOI] [PubMed] [Google Scholar]
- 5.Su Y, Wu H, Pavlosky A, Zou LL, Deng X, Zhang ZX, Jevnikar AM. Regulatory non-coding RNA: New instruments in the orchestration of cell death. Cell Death Dis. 2016;7:e2333. doi: 10.1038/cddis.2016.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Ma L, Wang C, Wang L, Guo Y, Wang G. Long noncoding RNA LINC00461 induced osteoarthritis progression by inhibiting miR-30a-5p. Aging (Albany NY) 2020;12:4111–4123. doi: 10.18632/aging.102839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ai D, Yu F. LncRNA DNM3OS promotes proliferation and inhibits apoptosis through modulating IGF1 expression by sponging MiR-126 in CHON-001 cells. Diagn Pathol. 2019;14:106. doi: 10.1186/s13000-019-0877-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen H, Wang Y, Shi W, Sun G, Hong L, Zhang Y. LncRNA SNHG5/miR-26a/SOX2 signal axis enhances proliferation of chondrocyte in osteoarthritis. Acta Biochim Biophys Sin (Shanghai) 2018;50:191–198. doi: 10.1093/abbs/gmx141. [DOI] [PubMed] [Google Scholar]
- 9.Qi K, Lin R, Xue C, Liu T, Wang Y, Zhang Y, Li J. Long non-coding RNA (LncRNA) CAIF is downregulated in osteoarthritis and inhibits LPS-Induced interleukin 6 (IL-6) upregulation by downregulation of MiR-1246. Med Sci Monit. 2019;25:8019–8024. doi: 10.12659/MSM.917135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song J, Ahn C, Chun CH, Jin EJ. A long non-coding RNA, GAS5, plays a critical role in the regulation of miR-21 during osteoarthritis. J Orthop Res. 2014;32:1628–1635. doi: 10.1002/jor.22718. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Li S, Luo Y, Liu Y, Yu N. LncRNA PVT1 regulates chondrocyte apoptosis in osteoarthritis by acting as a sponge for miR-488-3p. DNA Cell Biol. 2017;36:571–580. doi: 10.1089/dna.2017.3678. [DOI] [PubMed] [Google Scholar]
- 12.Xiao Y, Bao Y, Tang L, Wang L. LncRNA MIR4435-2HG is downregulated in osteoarthritis and regulates chondrocyte cell proliferation and apoptosis. J Orthop Surg Res. 2019;14:247. doi: 10.1186/s13018-019-1278-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang W, Lou C, Gao J, Zhang X, Du Y. LncRNA SNHG16 reverses the effects of miR-15a/16 on LPS-induced inflammatory pathway. Biomed Pharmacother. 2018;106:1661–1667. doi: 10.1016/j.biopha.2018.07.105. [DOI] [PubMed] [Google Scholar]
- 14.Cheng W, Hao CY, Zhao S, Zhang LL, Liu D. SNHG16 promotes the progression of osteoarthritis through activating microRNA-93-5p/CCND1 axis. Eur Rev Med Pharmacol Sci. 2019;23:9222–9229. doi: 10.26355/eurrev_201911_19414. [DOI] [PubMed] [Google Scholar]
- 15.Cheng F, Hu H, Sun K, Yan F, Geng Y. miR-455-3p enhances chondrocytes apoptosis and inflammation by targeting COL2A1 in the in vitro osteoarthritis model. Biosci Biotechnol Biochem. 2020;84:695–702. doi: 10.1080/09168451.2019.1690974. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Fan J, Ding X, Sun Y, Cui Z, Liu W. Tanshinone I inhibits IL-1β-induced apoptosis, inflammation and extracellular matrix degradation in chondrocytes CHON-001 cells and attenuates murine osteoarthritis. Drug Des Devel Ther. 2019;13:3559–3568. doi: 10.2147/DDDT.S216596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palmieri B, Lodi D, Capone S. Osteoarthritis and degenerative joint disease: Local treatment options update. Acta Biomed. 2010;81:94–100. [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Li M, Zhao J, Jia L. USP14-mediated IkBα degradation exacerbates NF-kB activation and IL-1β-stimulated chondrocyte dedifferentiation. Life Sci. 2019;218:147–152. doi: 10.1016/j.lfs.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 20.Xu J, Xu Y. The lncRNA MEG3 downregulation leads to osteoarthritis progression via miR-16/SMAD7 axis. Cell Biosci. 2017;7:69. doi: 10.1186/s13578-017-0195-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou W, He X, Chen Z, Fan D, Wang Y, Feng H, Zhang G, Lu A, Xiao L. LncRNA HOTAIR-mediated Wnt/β-catenin network modeling to predict and validate therapeutic targets for cartilage damage. BMC Bioinformatics. 2019;20:412. doi: 10.1186/s12859-019-2981-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paish HL, Baldock TE, Gillespie CS, Del Carpio Pons A, Mann DA, Deehan DJ, Borthwick LA, Kalson NS. Chronic, active inflammation in patients with failed total knee replacements undergoing revision surgery. J Orthop Res. 2019;37:2316–2324. doi: 10.1002/jor.24398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan C, Zhang J, Chen W, Feng F, Yu C, Lu X, Lin R, Li Z, Huang Y, Zheng L, et al. Inflammatory cytokines via up-regulation of aquaporins deteriorated the pathogenesis of early osteoarthritis. PLoS One. 2019;14:e0220846. doi: 10.1371/journal.pone.0220846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Liu S, Guo W, Hao C, Wang M, Li X, Zhang X, Chen M, Wang Z, Sui X, et al. Coculture of hWJMSCs and pACs in oriented scaffold enhances hyaline cartilage regeneration in vitro. Stem Cells Int. 2019;2019:5130152. doi: 10.1155/2019/5130152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang M, Sampson ER, Jin H, Li J, Ke QH, Im HJ, Chen D. MMP13 is a critical target gene during the progression of osteoarthritis. Arthritis Res Ther. 2013;15:R5. doi: 10.1186/ar4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ji B, Ma Y, Wang H, Fang X, Shi P. Activation of the P38/CREB/MMP13 axis is associated with osteoarthritis. Drug Des Devel Ther. 2019;13:2195–2204. doi: 10.2147/DDDT.S209626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng W, Feng Z, Lou Y, Chen C, Zhang C, Tao Z, Li H, Cheng L, Ying X. Silibinin protects against osteoarthritis through inhibiting the inflammatory response and cartilage matrix degradation in vitro and in vivo. Oncotarget. 2017;8:99649–99665. doi: 10.18632/oncotarget.20587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lorenz J, Seebach E, Hackmayer G, Greth C, Bauer RJ, Kleinschmidt K, Bettenworth D, Böhm M, Grifka J, Grässel S. Melanocortin 1 receptor-signaling deficiency results in an articular cartilage phenotype and accelerates pathogenesis of surgically induced murine osteoarthritis. PLoS One. 2014;9:e105858. doi: 10.1371/journal.pone.0105858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crowley LC, Waterhouse NJ. Detecting cleaved caspase-3 in apoptotic cells by flow cytometry. Cold Spring Harb Protoc. 2016;2016 doi: 10.1101/pdb.prot087312. [DOI] [PubMed] [Google Scholar]
- 31.Lin J, Chen J, Zhang Z, Xu T, Shao Z, Wang X, Ding Y, Tian N, Jin H, Sheng S, et al. Luteoloside inhibits il-1β-induced apoptosis and catabolism in nucleus pulposus cells and ameliorates intervertebral disk degeneration. Front Pharmacol. 2019;10:868. doi: 10.3389/fphar.2019.00868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chakkalakal SA, Heilig J, Baumann U, Paulsson M, Zaucke F. Impact of arginine to cysteine mutations in collagen II on protein secretion and cell survival. Int J Mol Sci. 2018;19:E541. doi: 10.3390/ijms19020541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niebler S, Schubert T, Hunziker EB, Bosserhoff AK. Activating enhancer binding protein 2 epsilon (AP-2ε)-deficient mice exhibit increased matrix metalloproteinase 13 expression and progressive osteoarthritis development. Arthritis Res Ther. 2015;17:119. doi: 10.1186/s13075-015-0648-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tu C, Huang X, Xiao Y, Song M, Ma Y, Yan J, You H, Wu H. Schisandrin A inhibits the IL-1β-induced inflammation and cartilage degradation via suppression of MAPK and NF-kB signal pathways in rat chondrocytes. Front Pharmacol. 2019;10:41. doi: 10.3389/fphar.2019.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang PE, Zhang L, Ying J, Jin X, Luo C, Xu S, Dong R, Xiao L, Tong P, Jin H. Bushenhuoxue formula attenuates cartilage degeneration in an osteoarthritic mouse model through TGF-β/MMP13 signaling. J Transl Med. 2018;16:72. doi: 10.1186/s12967-018-1437-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao Y, Zhao J, Guo X, She J, Liu Y. Long non-coding RNA PVT1, a molecular sponge for miR-149, contributes aberrant metabolic dysfunction and inflammation in IL-1β-simulated osteoarthritic chondrocytes. Biosci Rep. 2018;38:BSR20180576. doi: 10.1042/BSR20180576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eguchi T, Kuboki T. Cellular reprogramming using defined factors and MicroRNAs. Stem Cells Int. 2016;2016:7530942. doi: 10.1155/2016/7530942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tam C, Wong JH, Tsui SK, Zuo T, Chan TF, Ng TB. LncRNAs with miRNAs in regulation of gastric, liver, and colorectal cancers: Updates in recent years. Appl Microbiol Biotechnol. 2019;103:4649–4677. doi: 10.1007/s00253-019-09837-5. [DOI] [PubMed] [Google Scholar]
- 39.Lei J, Fu Y, Zhuang Y, Zhang K, Lu D. LncRNA SNHG1 alleviates IL-1β-induced osteoarthritis by inhibiting miR-16-5p-mediated p38 MAPK and NF-kB signaling pathways. Biosci Rep. 2019;39:BSR20191523. doi: 10.1042/BSR20191523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Wang F, Chen G, He R, Yang L. LncRNA MALAT1 promotes osteoarthritis by modulating miR-150-5p/AKT3 axis. Cell Biosci. 2019;9:54. doi: 10.1186/s13578-019-0302-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeon M, Byun J, Kim H, Kim M, Jung HS, Jeon D, Kim Y, Jeoung D. CAGE binds to beclin1, regulates autophagic flux and CAGE-derived peptide confers sensitivity to anti-cancer drugs in non-small cell lung cancer cells. Front Oncol. 2018;8:599. doi: 10.3389/fonc.2018.00599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith-Vikos T, Liu Z, Parsons C, Gorospe M, Ferrucci L, Gill TM, Slack FJ. A serum miRNA profile of human longevity: Findings from the baltimore longitudinal study of aging (BLSA) Aging (Albany NY) 2016;8:2971–2987. doi: 10.18632/aging.101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adlakha YK, Saini N. Brain microRNAs and insights into biological functions and therapeutic potential of brain enriched miRNA-128. Mol Cancer. 2014;13:33. doi: 10.1186/1476-4598-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ple H, Landry P, Benham A, Coarfa C, Gunaratne PH, Provost P. The repertoire and features of human platelet microRNAs. PLoS One. 2012;7:e50746. doi: 10.1371/journal.pone.0050746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pozzoli G, Petrucci G, Navarra P, Marei HE, Cenciarelli C. Aspirin inhibits proliferation and promotes differentiation of neuroblastoma cells via p21(Waf1) protein up-regulation and Rb1 pathway modulation. J Cell Mol Med. 2019;23:7078–7087. doi: 10.1111/jcmm.14610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koyano T, Namba M, Kobayashi T, Nakakuni K, Nakano D, Fukushima M, Nishiyama A, Matsuyama M. The p21 dependent G2 arrest of the cell cycle in epithelial tubular cells links to the early stage of renal fibrosis. Sci Rep. 2019;9:12059. doi: 10.1038/s41598-019-48557-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayashi S, Fujishiro T, Hashimoto S, Kanzaki N, Chinzei N, Kihara S, Takayama K, Matsumoto T, Nishida K, Kurosaka M, Kuroda R. p21 deficiency is susceptible to osteoarthritis through STAT3 phosphorylation. Arthritis Res Ther. 2015;17:314. doi: 10.1186/s13075-015-0828-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang L, Ding J, Zhou G, Liu Z. LncRNAp21 promotes chondrocyte apoptosis in osteoarthritis by acting as a sponge for miR451. Mol Med Rep. 2018;18:5295–5301. doi: 10.3892/mmr.2018.9506. [DOI] [PubMed] [Google Scholar]
- 49.Xu X, Lv H, Li X, Su H, Zhang X, Yang J. Danshen attenuates cartilage injuries in osteoarthritis in vivo and in vitro by activating JAK2/STAT3 and AKT pathways. Exp Anim. 2018;67:127–137. doi: 10.1538/expanim.17-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.