Abstract
Poly(ADP-ribose) polymerase-1 (PARP-1) plays a critical role in inflammatory pathways. The PARP-1 inhibitor, 5-aminoisoquinolinone (5-AIQ), has been demonstrated to exert significant pharmacological effects. The present study aimed to further examine the potential mechanisms of 5-AIQ in a mouse model of dextran sodium sulfate (DSS)-induced colitis. Colitis conditions were assessed by changes in weight, disease activity index, colon length, histopathology and pro-inflammatory mediators. The colonic expression of PARP/NF-κB and STAT3 pathway components was measured by western blot analysis. Flow cytometry was used to analyze the proportion of T helper 17 cells (Th17) and regulatory T cells (Tregs) in the spleen. Western blot analysis and reverse transcription-quantitative PCR were employed to determine the expression of the transcription factors retinoic acid-related orphan receptor and forkhead box protein P3. The results demonstrated that 5-AIQ reduced tissue damage and the inflammatory response in mice with experimental colitis. Moreover, 5-AIQ increased the proportion of Treg cells and decreased the percentage of Th17 cells in the spleen. Furthermore, following 5-AIQ treatment, the main components of the PARP/NF-κB and STAT3 pathways were downregulated. Collectively, these results demonstrate that the PARP-1 inhibitor, 5-AIQ, may suppress intestinal inflammation and protect the colonic mucosa by modulating Treg/Th17 immune balance and inhibiting PARP-1/NF-κB and STAT3 signaling pathways in mice with experimental colitis.
Keywords: ulcerative colitis, poly(ADP-ribose) polymerase-1 inhibitor, 5-aminoisoquinolinone, regulatory T cell, T helper 17 cell
Introduction
Ulcerative colitis (UC) is a non-specific, chronic, relapsing inflammatory disorder of the colonic mucosa, which affects the rectum and colon, and its incidence is rising worldwide (1,2). The underlying causes of UC are complex and have not been fully elucidated. The most accepted view is that UC is a complex disease resulting from interactions between genes, the gut flora, host immune system and environmental factors (3,4). Currently, inappropriate activation of T cells has been deemed as a crucial factor that contributes to the pathogenesis of UC (5). The imbalance of the immune axis formed by T helper 17 (Th17) cells that contribute to the immune response and regulatory T cells (Tregs) that mediate immune tolerance may play a main role in the pathogenesis of UC (6).
Th17 cells exert a pro-inflammatory effect on the inflammatory reaction by secreting pro-inflammatory cytokines, such as IL-17; their excessive activation causes intestinal inflammation and damages the intestinal mucosa (7). Tregs have immunosuppressive functions in autoimmune diseases, regulate self-tolerance and limit excessive immune reaction (8). An increasing number of studies have indicated that the balance of Th17 cell and Treg function is essential for host immunity and immune tolerance (9,10). During the transformation of initial T cells into Th17 cells and Tregs, the JAK/STAT pathway, particularly STAT3, plays an important role in promoting this transformation (11). Moreover, some cytokines and transcription factors are essential; IL-6 signaling and TGF-β1 act synergistically to program Th17-related genes through STAT3, thereby inducing Th17 cell development (12). The vital transcription factor mediating Th17 cell differentiation is retinoic acid-related orphan receptor (RORγt). The biological function of Tregs is controlled by the expression of the transcription factor forkhead box protein P3 (FOXP3) (5,13). An increase in the number of Th17 cells in UC has been reported to lead to an increase in serum IL-17 levels, and the reduction of Tregs leads to weakness of anti-inflammatory function (14). Therefore, promoting Tregs and suppressing Th17 cells to regulate Th17/Treg cell balance may be an efficient strategy for the treatment of UC.
PolyADP-ribose polymerase-1 (PARP)-1 is a ribozyme with significant biological activity in eukaryotic cells (15). It can catalyze the polyADP ribosylation of DNA-binding proteins involved in surveillance and genomic integrity maintenance (16). A number of studies have demonstrated that PARP-1 can regulate the inflammatory response (17,18). In particular, PARP-1 can regulate and enhance NF-κB transcriptional activity (18). Therefore, the inhibition of PARPs has been extensively studied; in several acute models of kidney injury and organ transplantation, PARP-1 knockout animals or pharmacological inhibitors of PARP-1 have been shown to lead to reduced inflammatory response (19,20). Although PARP-1 does not participate in the differentiation of natural T cells into Th17 cells, it does affect the development of Tregs (21). It has been demonstrated that PARP-1 negatively regulates Treg function through FOXP3 poly(ADP-ribosyl) (22). 5-aminoisoquinolinone (5-AIQ), a water-soluble PARP-1 inhibitor, has been demonstrated to provide important protection against multiple forms of tissue injury induced by reperfusion injury, the inflammatory response and neurotoxicity (23). In addition, the pharmacological inhibition of PARP-1 by 5-AIQ has been shown to inhibit NF-κB activity with the subsequent downregulation of the expression of several gene products (24). Therefore, the present study aimed to examine the regulatory effects of 5-AIQ on Th17/Tregs in experimental colitis and to elucidate the potential mechanisms involved.
Materials and methods
Pharmacological compounds and reagents
The water-soluble compound 5-AIQ, was obtained from Matrix Science, Inc. Dextran sulfate sodium (DSS) was purchased from MP Biomedicals, LLC (molecular weight, 36-50 kDa). The following antibodies were purchased from BD Biosciences: Anti-CD3 FITC (1:50; cat. no. 561827), anti-CD4 (1:50; cat. no. 566408) and anti-CD25 (1:50; cat. no. 561065) phycoerythrin, anti-Foxp3 (1:50; cat. no. 560402) and anti-IL-17A (1:50; cat. no. 560224) allophycocyanin.
Animals
A total of 30 C57BL/6 mice (males; 6-8 weeks old; weighing 20-25 g) were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd.. The mice were housed under constant environmental conditions (12-h light/dark cycle; 21±2˚C) and were provided with standard laboratory food and water ad libitum. All experimental procedures were approved by the Ethics Committee at the Renmin Hospital of Wuhan University.
Experimental design
The mice were randomly divided into three groups (n=10) following adaptive feeding for 1 week as follows: i) The control group (control); ii) 3% DSS-induced group (DSS) and iii) 3% DSS-induced + 5-AIQ group (5-AIQ). Apart from those in the control group, mice were exposed to 3% DSS for 7 days to develop symptoms of acute experimental colitis (25). Aside from the mice in the 5-AIQ group, an intraperitoneal injection of physiological saline was administered to the remaining mice, and 5-AIQ (1.5 mg/kg) dissolved in water was injected intraperitoneally into mice in the 5-AIQ group for 7 days (26). During the experimental period, the food and water intake and the disease activity index (DAI), including body weight, stool consistency and stool occult blood, were evaluated each day for each animal (Table I).
Table I.
Disease activity index score.
| Score | Weight loss | Stool consistency | Bloody stool |
|---|---|---|---|
| 0 | None | Normal | None |
| 1 | 1-5% | Paste stools | Occult blood |
| 2 | 6-10% | Loose stools | Bleeding |
| 3 | >10% | Diarrhea | Gross bleeding |
Histopathological assessment
Mice were sacrificed by cervical dislocation on the 8th day of colitis induction. Colorectal and ileocecal tissue sections (thickness, 4 µm) were obtained from the mice and the length of the colon was measured. Part of the colon was fixed in 4% paraformaldehyde at 4˚C for 24 h and embedded in paraffin, followed by hematoxylin and eosin staining for 97 min at room temperature and observed under a light microscope, (magnification, x100 and x200). Intestinal inflammation was assessed in a blinded manner and the histological score was evaluated as described in Table II.
Table II.
Histological score.
| Score | Percent of tissue damage | Extent of tissue damage | Degree of inflammation | Extent of crypt damage |
|---|---|---|---|---|
| 0 | None | None | None | None |
| 1 | ≤25% | Mucosa | Slight | Basal 1/3 |
| 2 | ≤50% | Mucosa and submucosa | Moderate | Basal 2/3 |
| 3 | ≤75% | Beyond the submucosa | Severe | Only the surface epithelium was intact |
| 4 | 100% | - | - | The entire crypt and epithelium were lost |
Flow cytometry
The spleen of the mice was aseptically isolated, filtered with a nylon mesh, and centrifuged for 10 min at 4˚C at 1,500 x g to obtain a single-cell suspension. Cells from the single-cell suspension were seeded into 96-well plates (1-3x106 lymphocytes/well) and stimulated for 7 h using Leukocyte Activation Cocktail (BD Biosciences) in an incubator. The cells were then collected, stained, fixed and permeabilized strictly according to the instructions provided with the kit. Flow cytometry antibodies, including anti-CD3 FITC, anti-CD4 and anti-CD25 phycoerythrin, were then added to each tube in turn, followed by mixing and incubation for 35 min at room temperature. The cells were centrifuged at 1,500 x g for 3 min at 4˚C and the supernatants were discarded. Fixation/Permeabilization working solution (1 ml; eBioscience; Thermo Fisher Scientific, Inc.) was added to each sample before incubation for 30 min in the dark at room temperature. Subsequently, permeabilization buffer (2 ml; eBioscience; Thermo Fisher Scientific, Inc.) was added to each sample before centrifuging again at 400 x g for 5 min at 4˚C. Intracellular cytokine antibodies anti-Foxp3 and anti-IL-17A allophycocyanin were added. The solutions were well mixed and incubated for 30 min in the dark at room temperature. The proportions of Treg and Th17 cells were analyzed by flow cytometer (BD Biosciences) and FlowJo 7.0 (FlowJo LLC) software was used to analyze data.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) for mRNA expression analysis
First, total RNA was isolated from colon samples using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.). After isolation of RNA, total RNA was reverse transcribed into cDNA using a First Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.) with the following temperature protocol: 25˚C for 5 min, 42˚C for 60 min, 70˚C for 5 min and 4˚C for 10 min. Following RT, the target gene was amplified, and RT-qPCR was performed on the ABI 7500 Real-time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.) using SYBR-Green PCR Master Mix (Thermo Fisher Scientific, Inc.) under the following thermocycling conditions: Initial denaturation at 95˚C for 10 min, followed by 40 cycles of 95˚C for 30 sec and annealing/extension at 60˚C for 30 sec. β-actin served as the endogenous control. Expressions were analyzed using the 2-ΔΔCq method (27). The sequences of the primers used are listed in Table III.
Table III.
PCR primers.
| Name | Primer sequences |
|---|---|
| β-actin | F: 5'-CACGATGGAGGGGCCGGACTCATC-3' |
| R: 5'-TAAAGACCTCTATGCCAACACAGT-3' | |
| IL-1β | F: 5'-TCAGGCAGGCAGTATCACTC-3' |
| R: 5'-AGCTCATATGGGTCCGACAG-3' | |
| TNF-α | F: 5'-ACCCTCACACTCACAAACCA-3' |
| R: 5'-GGCAGAGAGGAGGTTGACTT-3' | |
| IL-17 | F: 5'-GAAGGCCCTCAGACTACCTC-3' |
| R: 5'-CAGCATCTTCTCGACCCTGA-3' | |
| IL-10 | F: 5'-GCTGGACAACATACTGCTAACCG-3' |
| R: 5'-CACAGGGGAGAAATCGATGACAG-3' | |
| RORγt | F: 5'-CCTGGGCTCCTCGCCTGACC-3' |
| R: 5'-TCTCTCTGCCCTCAGCCTTGCC-3' | |
| Foxp3 | F: 5'-GAGAAGCTGAGTGCCATGCA-3' |
| R: 5'-GCCACAGATGAAGCCTTGGT-3' | |
| IL-6 | F: 5'-GTTGCCTTCTTGGGACTGAT-3' |
| R: 5'-ATTAAGCCTCCGACTTGTGA-3' | |
| TGF-β1 | F: 5'-GCTGAGCGCTTTTCTGATCCT-3' |
| R: 5'-GAGTGTGCTGCAGGTAGACA-3' |
RORγt, retinoic acid-related orphan receptor; Foxp3, forkhead box protein P3.
Western blot analysis
Protein for western blot analysis was extracted from colonic tissue using RIPA Pierce™ buffer (Thermo Fisher Scientific, Inc.) supplemented with protease inhibitor at a final 1X concentration (Halt™ Phosphatase Inhibitor Cocktail; Thermo Fisher Scientific, Inc.). Protein concentrations were measured using a BCA protein assay kit (Thermo Fisher Scientific, Inc.). A total of 20 µg protein/lane were separated by 10% SDS-PAGE and electrophoresis was performed for 1.5 h prior to protein transfer onto PVDF membranes (EMD Millipore). Subsequently, membranes were blocked with TBS-0.1%-Tween-20 (TBS-T) containing 5% skim milk for 2 h at room temperature. The membranes were then incubated with the following primary antibodies at 4˚C overnight: Anti-NF-κB p65 (cat. no. 10745-1-AP; 1:2,000), anti-STAT3 (cat. no. 10253-2-AP; 1:1,000), anti-PARP (cat. no. 66520-1-IG; 1:1,000), anti-Foxp3 (cat. no. 22173-1-AP; 1:1,000; all, ProteinTech Group, Inc.), anti-phosphorylated (p)-STAT3 (cat. no. AF3293; 1:500; Affinity Biosciences, Inc.), anti-phosphorylated (p)-NF-κB p65 (cat. no. 3033; 1:1,000; Cell Signaling Technology, Inc.), anti-RORγt (cat. no. bs-23110; 1:1,000), anti-IκB-α (cat. no. bs-1287; 1:1,000; all from BIOSS), anti-GAPDH (cat. no. AB-P-R001; 1:1,000; Hangzhou Goodhere Biotech Co., Ltd.). After washing five times with TBS-T for 5 min each, membranes were further immunoblotted with an horseradish peroxidase-conjugated AffiniPure goat anti-rabbit IgG (cat. no. BA1054; 1:50,000; Boster Biological Technology) secondary antibodies for 2 h at 37˚C. Membranes were then washed in TBS-T and signals were detected using BandScan v5.0 software (Glyko Biomedical Ltd.).
Statistical analysis
Data are presented as the mean ± SD and analyzed using SPSS 20.0 software (IBM Corp.). One-way ANOVA followed by the Tukey-Kramer test was used for comparisons between groups. P<0.05 were considered to indicate a statistically significant difference.
Results
Protective effects of 5-AIQ against DSS-induced colitis
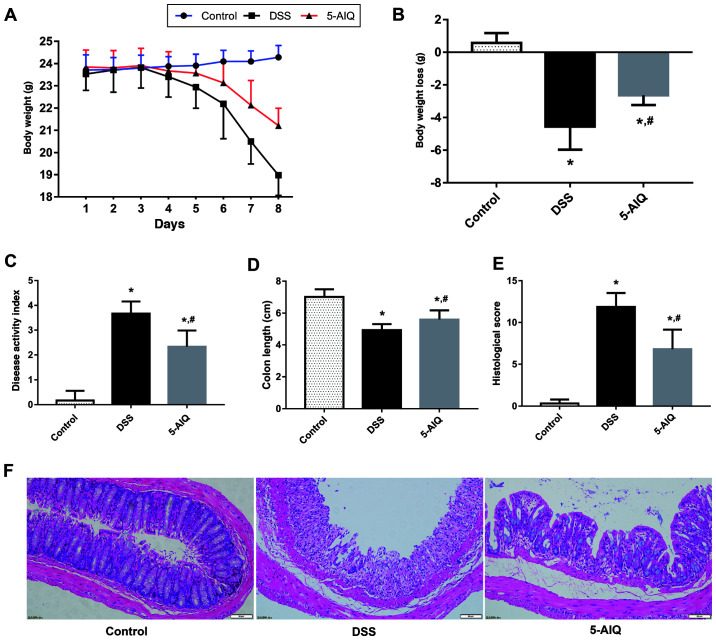
The DAI score of mice with DSS-induced UC was significantly higher compared with the control group. Mice with DSS-induced UC that receiving 5-AIQ treatment exhibited significantly lower body weight loss and DAI scores compared with untreated mice with DSS-induced UC (Fig. 1A-C). Moreover, the colon length is a useful index to reflect the severity of inflammation. The colon length of the mice in the DSS group was significantly lower compared with controls and this reduction was alleviated by the administration of 5-AIQ (Fig. 1D).
Figure 1.
Effects of 5-AIQ on DSS-induced colitis in mice. (A) Body weight, (B) body weight loss, (C) disease activity index score, (D) colon length, (E) histological scores and (F) hematoxylin and eosin staining images (magnification, x200). The results are presented as the mean ± SD (n=10). *P<0.05 vs. control; #P<0.05 vs. DSS group. 5-AIQ, 5-aminoisoquinolinone; DSS, dextran sodium sulfate.
5-AIQ attenuates histological damage in mice with DSS-induced colitis
Colonic mucosal epithelial cells in the control group exhibited an intact structure, normal and neatly arranged lamina propria glands and normal crypts. By contrast, the mucosa of the mice in the DSS group exhibited evident acute inflammatory reaction, which was characterized by the infiltration of neutrophils and lymphocytes edema, erosion and ulcers. However, the damage to the colonic mucosa was alleviated following 5-AIQ treatment (Fig. 1F). Treatment with 5-AIQ significantly lowered the histological score compared with the DSS group (Fig. 1E).
5-AIQ ameliorates the inflammatory response in mice DSS-induced colitis
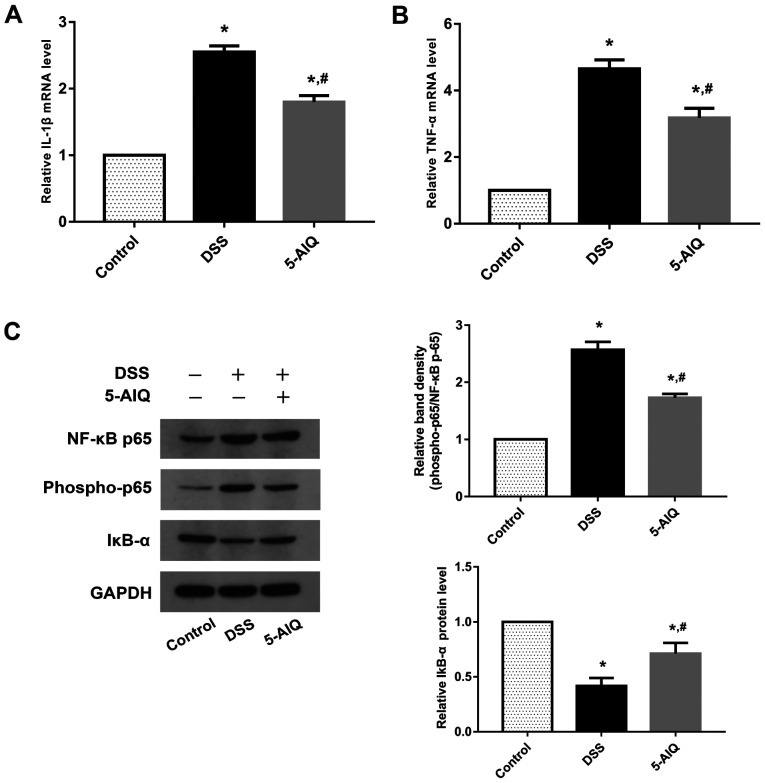
To evaluate the protective effects of 5-AIQ, RT-qPCR analysis of pro-inflammatory cytokines, such as IL-1 and TNF-α, was performed. Mice exposed to DSS exhibited significantly higher levels of TNF-α and IL-1β compared with controls. By contrast, 5-AIQ treatment significantly attenuated the expression of these cytokines (Fig. 2A and B). The levels of NF-κB p65, phosphorylated (p)-NF-κB p65 and IκB-α was further investigated, as NF-κB p65 regulates the production of pro-inflammatory cytokines. Western blot analysis demonstrated that 5-AIQ inhibited p-NF-κB p65 expression and suppressed the degradation of IκB-α. Treatment with 5-AIQ inhibited p-NF-κB p65/NF-κB p65 ratios compared with the DSS group (Fig. 2C).
Figure 2.
Expression of IL-1β, TNF-α, IκB-α, NF-κB p65 and phospho-NF-κB p65 in colonic tissues. The mRNA levels of (A) IL-1β and (B) TNF-α were measured by reverse transcription-quantitative PCR. (C) The expression levels of IκB-α, NF-κB p65 and phospho-NF-κB p65 were measured by western blot analysis. GAPDH was used as an internal control for grayscale analyses. The results are presented as the mean ± SD (n=10). *P<0.05 vs. control; #P<0.05 vs. DSS group. 5-AIQ, 5-aminoisoquinolinone; DSS, dextran sodium sulfate; phospho, phosphorylated.
5-AIQ inhibits Th17 cell production in mice with DSS-induced colitis
The percentage of Th17 cells in the spleen was significantly elevated in mice exposed to DSS compared with normal control mice. Notably, 5-AIQ significantly decreased the proportion of Th17 cells (Fig. 3A and B). Moreover, IL-17A expression was decreased in mice with DSS-induced UC treated with 5-AIQ (Fig. 3C). Subsequently, RORγt expression was examined at both the mRNA and protein levels. It was found that 5-AIQ significantly reduced the expression of RORγt compared with the DSS group (Fig. 3D and E).
Figure 3.
Detection of the frequency of Th17 and Treg cells in the spleen. (A) Flow cytometry and (B) mean averages of the frequency of Th17 cells in the spleen. (C) RT-qPCR analysis of IL-17 A expression. (D) Western blot and (E) RT-qPCR analysis of RORγt expression. (F) Flow cytometry and (G) mean averages of the frequency of Treg cells in the spleen. (H) RT-qPCR analysis of IL-10 expression. (I) Western blot and (J) RT-qPCR analysis of Foxp3 expression. GAPDH was used as an internal control for grayscale analyses. The results are presented as the mean ± SD (n=10). *P<0.05 vs. control; #P<0.05 vs. DSS group. 5-AIQ, 5-aminoisoquinolinone; DSS, dextran sodium sulfate; RT-qPCR. reverse transcription-quantitative PCR; Foxp3, forkhead box protein P3; PE, phycoerythrin; Treg, regulatory T cells; Th17, T helper 17 cells.
5-AIQ promotes Treg development in mice with DSS-induced colitis
The percentage of activated Tregs in the spleen was significantly increased following 5-AIQ treatment compared with mice with DSS-induced colitis (Fig. 3F and G). Additionally, IL-10 levels were increased in mice with DSS-induced colitis treated with 5-AIQ (Fig. 3H). Furthermore, compared with the normal mice, Foxp3 expression was also elevated in mice with DSS-induced colitis. As shown by the results of RT-qPCR and western blot analysis, 5-AIQ significantly upregulated the levels of Foxp3 (Fig. 3I and J).
Effects of 5-AIQ on STAT3 and PARP/NF-κB pathway activation in the colon
The ratios of p-STAT3/STAT3 were significantly upregulated in mice with DSS-induced colitis. Following 5-AIQ treatment, these ratios were significantly reduced compared with the DSS group (Fig. 4A). Furthermore, 5-AIQ significantly prevented the activation of PARP-1 (Fig. 4A). Thus, these results indicated that 5-AIQ can downregulate the STAT3 and PARP/NF-κB pathway in mice with colitis. Moreover, following 5-AIQ treatment, the expression of IL-6 was significantly reduced compared with mice with DSS-induced colitis (Fig. 4B). Additionally, the expression of TGF-β1 was significantly upregulated in the mice with DSS-induced colitis treated with 5-AIQ (Fig. 4C).
Figure 4.
Expression of STAT3, phospho-STAT3, PARP-1, IL-6 and TGF-β1 in colonic tissues. (A) The protein levels of STAT3, phospho-STAT3 and PARP-1 were measured by western blot analysis. GAPDH was used as an internal control for grayscale analyses. (B and C) The mRNA levels of IL-6 and TGF-β1 were measured by RT-qPCR. The results are presented as the means ± SD (n=10). *P<0.05 vs. control; #P<0.05 vs. DSS group.5-AIQ, 5-aminoisoquinolinone; DSS, dextran sodium sulfate.
Discussion
PARP is a type of ribozyme closely associated with DNA damage repair and gene transcription (28). PARP-1, the most abundant isoform, plays a key role in inflammatory pathways, promoting inflammatory responses through the stimulation of pro-inflammatory signal transduction pathways (15). Thus, the association between PARP-1 and inflammatory responses has been extensively investigated. Several studies have demonstrated that PARP-1 physically interacts with NF-κB, one of the main pro-inflammatory transcription factors, leading to the activation of inflammatory signaling (29). Recently, research conducted on mice revealed PARP-1 inhibitors exerted protective effects against several inflammatory disorders (26,30,31). In addition, the number of Tregs increased in multiple organs of PARP-1-deficient mice (21). Larmonier et al (32) demonstrated that the transcriptional reprogramming of the intestines of PARP-1 knockout mice exerted protective effects against experimental colitis. Moreover, the protective effects of 5-AIQ on various types of inflammation (20,33-35) have attracted wide attention (23).
5-AIQ has been reported to exert a protective effect against carrageenan-induced lung inflammation and rheumatoid arthritis (26,35). In a previous study, during severe acute pancreatitis-associated lung injury, 5-AIQ was shown to inhibit the activity of PARP-1, reduce NF-κB signaling levels and decrease the levels of downstream inflammatory factors, such as IL-1β and IL-6 to attenuate injury (36). Since 5-AIQ exerts a number of important pharmacological effects and has therapeutic benefits, its effects in experimental colitis in mice warrant further investigation. In the present study, the occult blood test in mice with colitis began to yield positive results at 2 to 3 days, and blood in the stool began to appear on the 3rd to 4th day, which gradually became more severe. In addition, evident anal ulceration in mice with DSS-induced colitis was observed. However, 5-AIQ reversed these effects, and the DAI score was significantly lower following treatment with 5-AIQ. Simultaneously, 5-AIQ intervention reduced weight loss, maintained the colon length and attenuated histological damage to the colon tissue. Thus, several lines of observations support the pharmacological action of 5-AIQ in experimental colitis.
NF-κB is a key regulatory point in downstream inflammatory cytokines activated by PARP-1(29). It is generally known that the abnormal activation of intestinal inflammatory cytokines is an important mechanism of the pathogenesis of UC, and the imbalance in the secretion of these cytokines lies in the abnormal activation of NF-κB, which regulates their gene transcription (37). In addition, NF-κB is highly expressed in the intestinal mucosa in UC and is significant for disease evaluation and judgement of treatment effects (38). Therefore, NF-κB activation is one of the key factors involved in the development of UC. To elucidate the molecular mechanisms of 5-AIQ in improving the inflammatory pathology of UC, the present study evaluated the effects of 5-AIQ on the expression of key molecules (IκB-α and NF-κB p65) in the NF-κB signaling pathway. IκB/NF-κB is one of the most classic signaling pathways, with IκB being an inhibitory protein. Activated NF-κB can translocate to the nucleus, where it can activate or inhibit the transcription of various target genes, such as IL-1β, IL-6 and TNF-α (39). Subsequently, the inflammatory process can be amplified and sustained, thereby damaging the intestinal mucosa, ultimately leading to the occurrence of UC (39,40). Therefore, the levels of IL-1β, TNF-α, IκB-α, NF-κB p65, and p-NF-κB p65 are worthy of observation. Following intervention with 5-AIQ, the present study observed an increase in the expression of IκB-α in the colon, while the levels of the other aforementioned parameters were all decreased. Based on these findings, not only does 5-AIQ treatment reduce the recruitment of pro-inflammatory factors to the colon and the production of pro-inflammatory mediators, but it also results in a reduction in overall inflammation and colonic injury.
An abnormal intestinal mucosal immune system is a key factor in the pathogenesis of UC (41). Moreover, the activation of effector T cells is the starting point for intestinal mucosal immunity and subsequent inflammation (42,43). Following inflammation, naïve T cells differentiate into various subsets, such as Th17 and Treg cells (44). A study suggested that the imbalance of these cells is essential for the pathogenesis of UC (45). The transcription factor Foxp3 expressed by Tregs acts decisively in maintaining Treg cell maturation and controlling inflammatory processes (46). Furthermore, the transcription factor RORγt controls the development and function of Th17 cells (46,47). Th17 cells in patients with UC are mainly concentrated in the lamina propria of the colon and secrete IL-17, which mediates the local infiltration of inflammatory cells, resulting in intestinal mucosal tissue damage (7). A study indicated that zinc deficiency activates the IL-23/Th17 axis, aggravating experimental colitis in mice (48). In addition, in a model of colitis, the transplantation of defined microbial flora has been shown to restore the balance of Th17/Tregs (49). Moreover, studies have confirmed that both Compound Sophorae Decoction and Rhubarb Peony Decoction exert protective effects against DSS-induced colitis in mice, and the mechanisms are related to the regulation of the Th17/Treg balance (50,51). Therefore, maintaining the Treg/Th17 balance may provide a treatment strategy for DSS-induced UC. In the present study, the results revealed that intervention with 5-AIQ reduced the production of Th17 cells and upregulated the proportion of Tregs. In addition, the imbalance between the two was restored. Furthermore, the results revealed that 5-AIQ inhibited the expression of RORγt, which led to a significant reduction in IL-17 secretion. Compared with mice with DSS-induced colitis, 5-AIQ upregulated the expression of IL-10 by increasing Foxp3 production.
The critical role of IL-6/STAT3 and NF-κB signaling has been well-established in recent years and is considered as a primary target in the treatment of colonic inflammation (52). The IL-6/STAT3 pathway exerts potent anti-apoptotic effects on T cells in colonic inflammation (53). It has been demonstrated that the cytokine TGF-β1 exerts anti-inflammatory effects, and a high concentration of this cytokine can result in Treg cell differentiation (54). However, when naïve T cells are exposed to a high concentration of IL-6 and a low concentration of TGF-β1, the specific transcription factor RORγt of Th17 cells is activated via the STAT3 pathway, and naïve T cells then differentiate into Th17 cells (55). A recent study indicated that the maintenance of Th17 cells requires a continuous IL-6 signal, which is significant for the treatment of Th17-mediated diseases (12). It was also demonstrated that STAT3 and NF-κB cooperatively regulate the expression of several gene products (56). In addition, the PARP-1/NF-κB interaction contributes to the development of inflammation (29). It is worth noting that the lack of PARP-1 inhibits the activation of NF-κB and leads to the suppression of innate immunity (57). In the T cell immune response, PARP-1 controls the immunosuppressive function of Tregs by destabilizing Foxp3(58). In the present study, it was found that the levels of PARP-1 and phosphorylated STAT3 decreased following intervention with 5-AIQ. Therefore, the present study further quantified indicators, such as IL-6 and TGF-β1. Of particular interest is that the concentration of IL-6 decreased in mice with DSS-induced colitis treated with 5-AIQ. 5-AIQ upregulated the expression of TGF-β1 by inhibiting PARP-1 and NF-κB production, compared with the DSS group.
In conclusion, the present study indicated that 5-AIQ exerts a pharmacologically protective effect against acute experimental colitis in mice, and the mechanisms are related to regulating the balance between Th17 and Tregs, as well as inhibition of PARP-1/NF-κB and STAT3 signaling. Therefore, 5-AIQ, an inhibitor of PARP-1, may prove to be a novel therapeutic agent for UC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed in the current study are available from the corresponding author on reasonable request.
Authors' contributions
LS and SSW designed the experiments, and interpreted and analyzed the data. SP, MXT and HML performed the experiments and statistical analysis. SP drafted and revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All experimental procedures were approved by the Ethics Committee at the Renmin Hospital of Wuhan University (approval no. 20190330).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389:1756–1770. doi: 10.1016/S0140-6736(16)32126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology. 2017;152:313–321.e2. doi: 10.1053/j.gastro.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 3.de Souza HSP, Fiocchi C, Iliopoulos D. The IBD interactome: An integrated view of aetiology, pathogenesis and therapy. Nat Rev Gastroenterol Hepatol. 2017;14:739–749. doi: 10.1038/nrgastro.2017.110. [DOI] [PubMed] [Google Scholar]
- 4.Torres J, Colombel JF. Genetics and phenotypes in inflammatory bowel disease. Lancet. 2016;387:98–100. doi: 10.1016/S0140-6736(15)00464-X. [DOI] [PubMed] [Google Scholar]
- 5.Silva FA, Rodrigues BL, Ayrizono ML, Leal RF. The immunological basis of inflammatory bowel disease. Gastroenterol Res Pract. 2016;2016(2097274) doi: 10.1155/2016/2097274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fasching P, Stradner M, Graninger W, Dejaco C, Fessler J. Therapeutic potential of targeting the Th17/Treg axis in autoimmune disorders. Molecules. 2017;22(134) doi: 10.3390/molecules22010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ueno A, Jeffery L, Kobayashi T, Hibi T, Ghosh S, Jijon H. Th17 plasticity and its relevance to inflammatory bowel disease. J Autoimmun. 2018;87:38–49. doi: 10.1016/j.jaut.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Yamada A, Arakaki R, Saito M, Tsunematsu T, Kudo Y, Ishimaru N. Role of regulatory T cell in the pathogenesis of inflammatory bowel disease. World J Gastroenterol. 2016;22:2195–2205. doi: 10.3748/wjg.v22.i7.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L, Zhang Y, Zhong W, Di C, Lin X, Xia Z. Heme oxygenase-1 ameliorates dextran sulfate sodium-induced acute murine colitis by regulating Th17/Treg cell balance. J Biol Chem. 2014;289:26847–26858. doi: 10.1074/jbc.M114.590554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao J, Wei C, Wang JY, Zhang R, Li YX, Wang LS. Effect of resveratrol on Treg/Th17 signaling and ulcerative colitis treatment in mice. World J Gastroenterol. 2015;21:6572–6581. doi: 10.3748/wjg.v21.i21.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salas A, Hernandez-Rocha C, Duijvestein M, Faubion W, McGovern D, Vermeire S, Vetrano S, Vande Casteele N. JAK-STAT pathway targeting for the treatment of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17:323–337. doi: 10.1038/s41575-020-0273-0. [DOI] [PubMed] [Google Scholar]
- 12.Harbour SN, DiToro DF, Witte SJ, Zindl CL, Gao M, Schoeb TR, Jones GW, Jones SA, Hatton RD, Weaver CT. TH17 cells require ongoing classic IL-6 receptor signaling to retain transcriptional and functional identity. Sci Immunol. 2020;5(eaaw2262) doi: 10.1126/sciimmunol.aaw2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Britton GJ, Contijoch EJ, Mogno I, Vennaro OH, Llewellyn SR, Ng R, Li Z, Mortha A, Merad M, Das A, et al. Microbiotas from humans with inflammatory bowel disease alter the balance of gut Th17 and RORγt+ regulatory T cells and exacerbate colitis in mice. Immunity. 2019;50:212–224.e4. doi: 10.1016/j.immuni.2018.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gong Y, Lin Y, Zhao N, He X, Lu A, Wei W, Jiang M. The Th17/Treg immune imbalance in ulcerative colitis disease in a Chinese han population. Mediators Inflamm. 2016;2016(7089137) doi: 10.1155/2016/7089137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupte R, Liu Z, Kraus WL. PARPs and ADP-ribosylation: Recent advances linking molecular functions to biological outcomes. Genes Dev. 2017;31:101–126. doi: 10.1101/gad.291518.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosado MM, Bennici E, Novelli F, Pioli C. Beyond DNA repair, the immunological role of PARP-1 and its siblings. Immunology. 2013;139:428–437. doi: 10.1111/imm.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abd Elmageed ZY, Naura AS, Errami Y, Zerfaoui M. The poly(ADP-ribose) polymerases (PARPs): New roles in intracellular transport. Cell Signal. 2012;24:1–8. doi: 10.1016/j.cellsig.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 18.Ba X, Garg NJ. Signaling mechanism of poly(ADP-ribose) polymerase-1 (PARP-1) in inflammatory diseases. Am J Pathol. 2011;178:946–955. doi: 10.1016/j.ajpath.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sodhi RK, Singh N, Jaggi AS. Poly(ADP-ribose) polymerase-1 (PARP-1) and its therapeutic implications. Vascul Pharmacol. 2010;53:77–87. doi: 10.1016/j.vph.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Quesada A, O'Valle F, Montoro-Molina S, Gómez-Morales M, Caba-Molina M, González JF, de Gracia MC, Osuna A, Vargas F, Wangensteen R. 5-aminoisoquinoline improves renal function and fibrosis during recovery phase of cisplatin-induced acute kidney injury in rats. Biosci Rep. 2018;38(BSR20171313) doi: 10.1042/BSR20171313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fehr AR, Singh SA, Kerr CM, Mukai S, Higashi H, Aikawa M. The impact of PARPs and ADP-ribosylation on inflammation and host-pathogen interactions. Genes Dev. 2020;34:341–359. doi: 10.1101/gad.334425.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo X, Nie J, Wang S, Chen Z, Chen W, Li D, Hu H, Li B. Poly(ADP-ribosyl)ation of FOXP3 protein mediated by PARP-1 protein regulates the function of regulatory T cells. J Biol Chem. 2015;290:28675–28682. doi: 10.1074/jbc.M115.661611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Threadgill MD. 5-Aminoisoquinolin-1-one (5-AIQ), a water-soluble inhibitor of the poly(ADP-Ribose)polymerases (PARPs) Curr Med Chem. 2015;22:3807–3829. doi: 10.2174/0929867322666151002110602. [DOI] [PubMed] [Google Scholar]
- 24.Brady PN, Goel A, Johnson MA. Poly(ADP-Ribose) polymerases in host-pathogen interactions, inflammation, and immunity. Microbiol Mol Biol Rev. 2019;83:e00038–18. doi: 10.1128/MMBR.00038-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wirtz S, Popp V, Kindermann M, Gerlach K, Weigmann B, Fichtner-Feigl S, Neurath MF. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat Protoc. 2017;12:1295–1309. doi: 10.1038/nprot.2017.044. [DOI] [PubMed] [Google Scholar]
- 26.Ahmad SF, Zoheir KM, Bakheet SA, Ashour AE, Attia SM. Poly(ADP-ribose) polymerase-1 inhibitor modulates T regulatory and IL-17 cells in the prevention of adjuvant induced arthritis in mice model. Cytokine. 2014;68:76–85. doi: 10.1016/j.cyto.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Gibson BA, Kraus WL. New insights into the molecular and cellular functions of poly(ADP-ribose) and PARPs. Nat Rev Mol Cell Biol. 2012;13:411–424. doi: 10.1038/nrm3376. [DOI] [PubMed] [Google Scholar]
- 29.Hassa PO, Hottiger MO. The functional role of poly(ADP-ribose)polymerase 1 as novel coactivator of NF-kappaB in inflammatory disorders. Cell Mol Life Sci. 2002;59:1534–1553. doi: 10.1007/s00018-002-8527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niyazoglu M, Baykara O, Koc A, Aydoğdu P, Onaran I, Dellal FD, Tasan E, Sultuybek GK. Association of PARP-1, NF-κB, NF-κBIA and IL-6, IL-1β and TNF-α with graves disease and graves ophthalmopathy. Gene. 2014;547:226–232. doi: 10.1016/j.gene.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 31.Dharwal V, Naura AS. PARP-1 inhibition ameliorates elastase induced lung inflammation and emphysema in mice. Biochem Pharmacol. 2018;150:24–34. doi: 10.1016/j.bcp.2018.01.027. [DOI] [PubMed] [Google Scholar]
- 32.Larmonier CB, Shehab KW, Laubitz D, Jamwal DR, Ghishan FK, Kiela PR. Transcriptional reprogramming and resistance to colonic mucosal injury in poly(ADP-ribose) polymerase 1 (PARP1)-deficient mice. J Biol Chem. 2016;291:8918–8930. doi: 10.1074/jbc.M116.714386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cuzzocrea S, McDonald MC, Mazzon E, Dugo L, Serraino I, Threadgill M, Caputi AP, Thiemermann C. Effects of 5-aminoisoquinolinone, a water-soluble, potent inhibitor of the activity of poly(ADP-ribose) polymerase, in a rodent model of lung injury. Biochem Pharmacol. 2002;63:293–304. doi: 10.1016/s0006-2952(01)00864-4. [DOI] [PubMed] [Google Scholar]
- 34.Di Paola R, Genovese T, Caputi AP, Threadgill M, Thiemermann C, Cuzzocrea S. Beneficial effects of 5-aminoisoquinolinone, a novel, potent, water-soluble, inhibitor of poly(ADP-ribose) polymerase, in a rat model of splanchnic artery occlusion and reperfusion. Eur J Pharmacol. 2004;492:203–210. doi: 10.1016/j.ejphar.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 35.Ahmad SF, Zoheir KM, Ansari MA, Korashy HM, Bakheet SA, Ashour AE, Al-Shabanah OA, Al-harbi MM, Attia SM. The role of poly(ADP-ribose) polymerase-1 inhibitor in carrageenan-induced lung inflammation in mice. Mol Immunol. 2015;63:394–405. doi: 10.1016/j.molimm.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 36.Yang B, Guo WY, Yu J, Zhao KL, Shi Q, Zuo T, Wang WX. Expression of PARP/NF-κB and intervention effect of 5-AIQ/PDTC in SAP rats with adrenal damage. Zhonghua Yi Xue Za Zhi. 2013;93:3063–3067. (In Chinese) [PubMed] [Google Scholar]
- 37.Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329–342. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- 38.Fonseca-Camarillo G, Yamamoto-Furusho JK. Immunoregulatory pathways involved in inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:2188–2193. doi: 10.1097/MIB.0000000000000477. [DOI] [PubMed] [Google Scholar]
- 39.Hayden MS, Ghosh S. NF-kappaB in immunobiology. Cell Res. 2011;21:223–244. doi: 10.1038/cr.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atreya I, Atreya R, Neurath MF. NF-kappaB in inflammatory bowel disease. J Intern Med. 2008;263:591–596. doi: 10.1111/j.1365-2796.2008.01953.x. [DOI] [PubMed] [Google Scholar]
- 41.de Souza HS, Fiocchi C. Immunopathogenesis of IBD: Current state of the art. Nat Rev Gastroenterol Hepatol. 2016;13:13–27. doi: 10.1038/nrgastro.2015.186. [DOI] [PubMed] [Google Scholar]
- 42.Wallace KL, Zheng LB, Kanazawa Y, Shih DQ. Immunopathology of inflammatory bowel disease. World J Gastroenterol. 2014;20:6–21. doi: 10.3748/wjg.v20.i1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Wijk F, Cheroutre H. Intestinal T cells: Facing the mucosal immune dilemma with synergy and diversity. Semin Immunol. 2009;21:130–138. doi: 10.1016/j.smim.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zenewicz LA, Antov A, Flavell RA. CD4 T-cell differentiation and inflammatory bowel disease. Trends Mol Med. 2009;15:199–207. doi: 10.1016/j.molmed.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 45.Liu TC, Stappenbeck TS. Genetics and pathogenesis of inflammatory bowel disease. Annu Rev Pathol. 2016;11:127–148. doi: 10.1146/annurev-pathol-012615-044152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang BH, Hagemann S, Mamareli P, Lauer U, Hoffmann U, Beckstette M, Föhse L, Prinz I, Pezoldt J, Suerbaum S, et al. Foxp3(+) T cells expressing RORγt represent a stable regulatory T-cell effector lineage with enhanced suppressive capacity during intestinal inflammation. Mucosal Immunol. 2016;9:444–457. doi: 10.1038/mi.2015.74. [DOI] [PubMed] [Google Scholar]
- 47.Ohnmacht C, Park JH, Cording S, Wing JB, Atarashi K, Obata Y, Gaboriau-Routhiau V, Marques R, Dulauroy S, Fedoseeva M, et al. Mucosal immunology. The microbiota regulates type 2 immunity through RORγt+ T cells. Science. 2015;349:989–993. doi: 10.1126/science.aac4263. [DOI] [PubMed] [Google Scholar]
- 48.Higashimura Y, Takagi T, Naito Y, Uchiyama K, Mizushima K, Tanaka M, Hamaguchi M, Itoh Y. Zinc deficiency activates the IL-23/Th17 axis to aggravate experimental colitis in mice. J Crohns Colitis. 2020;14:856–866. doi: 10.1093/ecco-jcc/jjz193. [DOI] [PubMed] [Google Scholar]
- 49.Britton GJ, Contijoch EJ, Spindler MP, Aggarwala V, Dogan B, Bongers G, San Mateo L, Baltus A, Das A, Gevers D, et al. Defined microbiota transplant restores Th17/RORγt+ regulatory T cell balance in mice colonized with inflammatory bowel disease microbiotas. Proc Natl Acad Sci USA. 2020;117:21536–21545. doi: 10.1073/pnas.1922189117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu M, Duan XY, Chen QY, Fan H, Hong ZC, Deng SJ, Nan Z, Wu H, Dong YL, Liu YJ, Zhou CZ. Effect of compound sophorae decoction on dextran sodium sulfate (DSS)-induced colitis in mice by regulating Th17/Treg cell balance. Biomed Pharmacother. 2019;109:2396–2408. doi: 10.1016/j.biopha.2018.11.087. [DOI] [PubMed] [Google Scholar]
- 51.Luo S, Wen R, Wang Q, Zhao Z, Nong F, Fu Y, Huang S, Chen J, Zhou L, Luo X. Rhubarb peony decoction ameliorates ulcerative colitis in mice by regulating gut microbiota to restoring Th17/Treg balance. J Ethnopharmacol. 2019;231:39–49. doi: 10.1016/j.jep.2018.08.033. [DOI] [PubMed] [Google Scholar]
- 52.Serrano C, Galán S, Rubio JF, Candelario-Martínez A, Montes-Gómez AE, Chánez-Paredes S, Cedillo-Barrón L, Schnoor M, Meraz-Ríos MA, Villegas-Sepúlveda N, et al. Compartmentalized response of IL-6/STAT3 signaling in the colonic mucosa mediates colitis development. J Immunol. 2019;202:1239–1249. doi: 10.4049/jimmunol.1801060. [DOI] [PubMed] [Google Scholar]
- 53.Coskun M, Salem M, Pedersen J, Nielsen OH. Involvement of JAK/STAT signaling in the pathogenesis of inflammatory bowel disease. Pharmacol Res. 2013;76:1–8. doi: 10.1016/j.phrs.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 54.Hadaschik EN, Enk AH. TGF-β1-induced regulatory T cells. Hum Immunol. 2015;76:561–564. doi: 10.1016/j.humimm.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 55.Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, Ramos HL, Wei L, Davidson TS, Bouladoux N, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-β signalling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martincuks A, Andryka K, Küster A, Schmitz-Van de Leur H, Komorowski M, Müller-Newen G. Nuclear translocation of STAT3 and NF-κB are independent of each other but NF-κB supports expression and activation of STAT3. Cell Signal. 2017;32:36–47. doi: 10.1016/j.cellsig.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 57.Pazzaglia S, Pioli C. Multifaceted Role of PARP-1 in DNA repair and inflammation: Pathological and therapeutic implications in cancer and non-cancer diseases. Cells. 2019;9(41) doi: 10.3390/cells9010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang P, Maruyama T, Konkel JE, Abbatiello B, Zamarron B, Wang ZQ, Chen W. PARP-1 controls immunosuppressive function of regulatory T cells by destabilizing Foxp3. PLoS One. 2013;8(e71590) doi: 10.1371/journal.pone.0071590. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed in the current study are available from the corresponding author on reasonable request.