Abstract
Chemotherapy-induced peripheral neuropathic pain (CIPNP) is a serious, undesirable effect of cancer treatment which is particularly difficult to prevent. Berberine and its derivatives have been reported to display robust antioxidant and analgesic effects in rat models of diabetic neuropathic pain and peripheral nerve injury. However, the analgesic role of berberine on oxaliplatin-induced CIPNP remains unknown. The present study aimed to explore the analgesic effect of berberine on CIPNP. Sprague Dawley rats were used to create the CIPNP animal model by oxaliplatin administration. Behavioral tests were performed by von Frey test, acetone drop test, hot plate test, and motor coordination. The protein expression levels of NF-κB p65 and phosphorylated p65 in dorsal root ganglions (DGRs) were detected by western blot analysis. Finally, TNF-α and IL-6 levels in DRGs were measured using specific ELISA kits. The results from the behavioral analysis demonstrated that a single injection of berberine ameliorated the mechanical and cold allodynia and thermal hyperalgesia in the model rats in a dose-dependent manner. Cumulative administration of berberine prevented the mechanical and cold allodynia and thermal hyperalgesia in the development of CIPNP induced by oxaliplatin. This prophylactic effect of berberine was associated with reduced phosphorylation of p65 and with decreased levels of pro-inflammatory cytokines IL-6 and TNF-α. The present study indicated that berberine may have a role in preventing the development of CIPNP and may serve as a therapeutic compound for the treatment of CIPNP.
Keywords: berberine, oxaliplatin, chemotherapy-induced peripheral neuropathic pain, NF-κB, pro-inflammatory cytokines
Introduction
Chemotherapeutic drugs, such as taxanes, vinca alkaloids, platinum analogs, topoisomerase inhibitors and proteasome inhibitors, can cause a series of adverse reactions. The most common and serious side-effect is chemotherapy-induced peripheral neuropathic pain (CIPNP) (1,2), which negatively affects patient quality of life, rendering it a major constraint for therapeutic drug dosage. Oxaliplatin is a platinum derivative that is widely used in first-line colorectal cancer therapy (3). Oxaliplatin can result in acute and chronic distal sensory neuropathy; the former is characterized as acral paresthesia triggered by cold temperatures and the latter is induced by accumulative oxaliplatin (4). Oxaliplatin-induced CIPNP complicates clinical treatment because there is a lack of effective analgesics, therefore, the treatment process may be accompanied by unacceptable side effects (5). There are currently no effective ways to treat or prevent oxaliplatin-induced CIPNP (6), necessitating the development of novel solutions.
Nuclear factor-κB (NF-κB) has a substantial role in regulating inflammation and immune responses (7) and is involved in the nervous system's synaptic plasticity, memory formation, learning, neurotransmission and neuroprotection (8,9). When the NF-κB signaling pathway is activated, NF-κB p65 can bind to the promoters of target genes and enhance their expression (10). Previous research has indicated that NF-κB activation is associated with the pathological pain caused by nerve injury or inflammation (11,12). Recent research has also reported NF-κB to be involved in CIPNP (13) and the establishment and maintenance of acute and chronic pain caused by neuroinflammation or nerve injury (14,15). Furthermore, NF-κB activation was demonstrated to regulate the levels of pro-inflammatory cytokines, including IL-6 and TNF-α, in a neuropathic pain model (16). However, it remains unclear whether oxaliplatin-induced CIPNP is associated with NF-κB pathway activation in dorsal root ganglions (DRGs).
Natural plant products have been shown to effectively treat complex chronic comorbidities (17-19). Berberine, one of the tested compounds, is an isoquinoline alkaloid reportedly purified from herbs and featuring multiple pharmacological effects. Its antimicrobial and antisecretory properties have been used as a treatment to diarrhea and gastroenteritis (20,21). Berberine and its derivatives have also been shown to exhibit potent anti-inflammatory and anticancer properties (22). Additionally, berberine has alleviated allodynia and demonstrated antioxidative effects in models of diabetic neuropathy (23) and peripheral nerve injuries (24). However, there are no relevant reports on berberine's analgesic function on oxaliplatin-induced CIPNP; that is, no research has revealed berberine having a role as an adjuvant during chemotherapy.
The present study explored the effects of single injections and repeated doses of berberine on the induction and prevention of oxaliplatin-induced CIPNP, respectively. To investigate its underlying mechanism, the effects of berberine on oxaliplatin-induced modulation of NF-κB signaling and of pro-inflammatory cytokine secretion were also tested.
Materials and methods
Animals
Sprague Dawley rats (n=88, male, 200-220 g; Laboratory Animal Center of Huazhong University of Science and Technology, Wuhan, China) were housed in a room with 22-24ºC and 12-h light/dark cycle (7:00 am to 7:00 pm). Food and water were freely available. All procedures complied strictly with the Guidelines for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of Huazhong University of Science and Technology (Wuhan, China).
Drug administration and experimental design
Oxaliplatin (Dalian Meilun Biology Technology Co., Ltd.) was dissolved in saline. The rats were injected intraperitoneally (i.p.) with 2.5 mg/kg oxaliplatin for 4 consecutive days, after which a final dose of 10 mg/kg was administered to induce peripheral neuropathic pain. Berberine was used both as a treatment in pre-established oxaliplatin-induced CIPNP and as a prevention during the CIPNP induction process.
Berberine chloride was obtained from Shanghai Shifeng Biotechnology, Ltd. Berberine was dissolved in dimethyl sulfoxide (DMSO) at concentrations of 50, 100 and 200 mg/ml, and diluted in saline (0.9%) for injection. All solutions were filtered using a 0.22-µm membrane filter (Pall Life Sciences) prior to injection. To evaluate the effect of berberine as a treatment in pre-established oxaliplatin-induced CIPNP, a single dose of berberine (5, 10 or 20 mg/kg) was administered i.p. 21 days after the first oxaliplatin injection, according to a previous report (23). Von Frey, acetone drop and hot plate tests were performed 60 min after the injection of berberine. To evaluate the effect of berberine on the prevention of oxaliplatin-induced CIPNP, animals were injected i.p. with berberine or saline every 24 h for 21 consecutive days after the final injection with oxaliplatin. Animals were randomly divided into four groups (n=8): vehicle + saline group, oxaliplatin + saline group, oxaliplatin + berberine (10 mg/kg) group and oxaliplatin + berberine (20 mg/kg) group. Then, tissue samples were collected 24 h after the end of final behavioral tests. All behavioral tests were conducted at 10:00 am of each experiment day by researchers who were blinded to the animal groups.
Mechanical allodynia (von Frey test)
Mechanical allodynia was evaluated by von Frey test. The rats were placed in a plastic chamber (20x17x13 cm) without bottom and with several compartments, and all of them were placed on a 40-cm high wire mesh shelf. At 15 min before the test, the rats were placed in the test box to adapt to the environment. An electronic von Frey instrument (IITC Life Science Inc.) was used to examine the rats' behaviors. The withdrawal threshold was reflected by applying 0-50 g of pressure (with an accuracy of 0.2 g). Below the wire mesh floor, a punctuate stimulus was transmitted through the tip of the von Frey fiber to the middle part of each rats' hind paw for 2 sec. Then the von Frey instrument automatically reads the withdrawal threshold. The rat's sensitivity threshold is considered as the minimum pressure required to make the hind paw produce robust and immediate withdrawal reflex. Movement-related autonomous motions are not considered as withdrawal responses. The stimulus was given to the hind paw every 5 min. The measurement was repeated three times, and the average of three measurements was recorded as the final result.
Cold allodynia (acetone drop test)
According to previous reports (25), cold allodynia was measured by acetone drop tests. In brief, rats were individually placed in plastic chambers which were put on a wire mesh shelf. Then a flat needle of a syringe was used to aspirate some acetone and one drop was gently dropped on the surface of the rat's hind paw. The time of withdrawal/licking reaction was recorded within 40 sec. Acetone was dropped every 10 min during the measurement, and the average of the responses was calculated in each measurement.
Thermal hyperalgesia (hot-plate test)
Thermal hyperalgesia was evaluated by a hot plate analgesic instrument (Bioseb). Rats were placed individually on a hot plate with a constant temperature of 53±1˚C. An electronic timer was used to record the time required for reactions such as jumping or hind paw-licking. To prevent scalding of rat paws, the maximum heating time was set as 30 sec. Three repeat measurements were obtained for each rat, with an interval between each measurement of >10 min, then the average value of each measurement was calculated as the final result. Normal foot-lifting motions were not counted. The researcher performing the behavioral testing was blind to the grouping and administration of rats.
Bodyweight and motor coordination
All rats were weighed weekly on days 0, 7, 14 and 21. A rotarod apparatus (Shanghai Mobile Data Center) was used to evaluate the motor coordination of rats. The rats were trained for 10 min with 10, 20 and 30 rpm on the equipment for 3 consecutive days to let them familiarize with the device, until they could persist for 60 sec with not falling. During the test, the rats were placed with a variable mode of 4-40 rpm and allowed to run until they fell or reached the 5-min cut-off time. The time from the beginning of the test to the fall was recorded.
Tissue collection
After completing the behavioral tests, rats were anesthetized with pentobarbital sodium (50 mg/kg, i.p.; Sigma-Aldrich; Merck KGaA) and sacrificed by cervical dislocation. DRGs from lumbar (L) 4-L6 were dissected and quick-frozen in liquid nitrogen, then stored in -80˚C until further experiments.
Protein preparation and western blot analysis
Samples were homogenized in lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1% sodium deoxycholate, 1% Triton Χ-100, 0.1% SDS, pH 7.4) to extract the protein. The protein preparations were stored at -80˚C. The protein concentration in each sample was determined using a BCA assay. Equal amounts of protein (25 µg) were separated on 10% Tris-Tricine SDS-PAGE and transferred to PVDF membranes, followed by blocking with 5% non-fat milk for 1 h at room temperature. The membranes were then incubated with primary antibodies targeting NF-κB p65 (1:1,000; cat. no. 4764; Cell Signaling Technology, Inc.), phosphorylated (p-) p65 (1:1,000; cat. no. 3033; Cell Signaling Technology, Inc.) and GAPDH (1:5,000; cat. no. ab9485; Abcam) overnight at 4˚C. The next day, after washing, the membranes were incubated with HRP-conjugated goat anti-rabbit polyclonal IgG secondary antibodies (1:2,000; ab6721; Abcam) for half hour at room temperature. Immunoblotting was detected by chemiluminescent substrate and the experimental results were processed with ImageJ software (version 1.51j8, National Institutes of Health).
Enzyme-linked immunosorbent assay (ELISA)
First, ice-cold PBS was used to homogenize DRG samples. Then, the bicinchoninic acid assay was used to determine the protein concentration in the samples. The levels of TNF-α and IL-6 were measured using commercially available rat-specific ELISA kits (IL-6 kit, cat. no. R6000B; TNF-α kit, cat. no. RTA00; R&D Systems, Inc.), following the manufacturer's instructions.
Statistical analysis
Data were presented as the mean ± standard error of the mean. Behavioral and western blotting results were analyzed by single factor or mixed factor designed ANOVA, followed by Dunnett's or simple-effects post hoc tests using SPSS software (version 21.0.0; IBM Corp.). P<0.05 was considered to indicate a statistically significant difference.
Results
Oxaliplatin-induced mechanical allodynia, cold allodynia and thermal hyperalgesia in rats
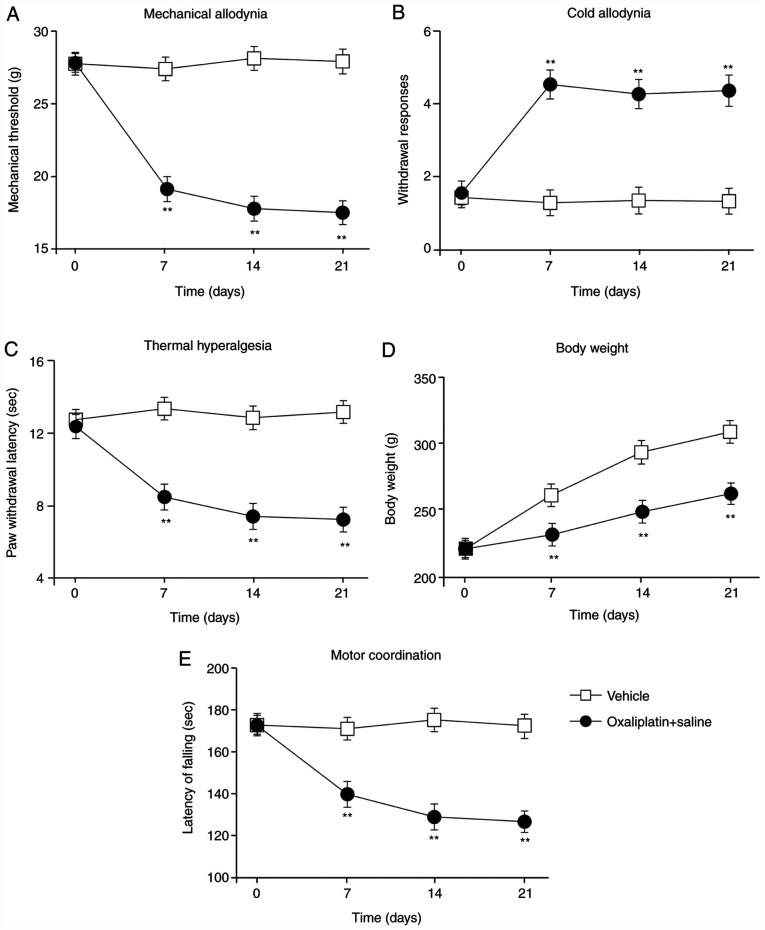
First, the rat model of oxaliplatin-induced CIPNP was generated by injecting oxaliplatin (i.p., 2.5 mg/kg). Before oxaliplatin injection and 1, 2 and 3 weeks post-injection, von Frey hind-paw withdrawal thresholds, withdrawal response times for cold plate tests and hind-paw licking latencies for hot plate tests were assessed (Fig. 1A-C). Compared with vehicle-treated rats, oxaliplatin-treated rats displayed significant mechanical allodynia (Fig. 1A), cold allodynia (Fig. 1B) and thermal hyperalgesia (Fig. 1C) on the 7th day post-injection; these observations were sustained throughout the experiment. Additionally, the weight of the vehicle-treated rats increased during the experiment; although the oxaliplatin-treated rats also gained weight, the effect was less pronounced (Fig. 1D). Finally, motor function was significantly impaired in the oxaliplatin-treated rats compared with the vehicle-treated rats (Fig. 1E). These results indicated that treatment with oxaliplatin resulted in mechanical allodynia, cold allodynia and thermal hyperalgesia. In addition, the impaired weight gain and motor function suggested that the oxaliplatin-induced CIPNP model was successfully established in the present study.
Figure 1.
Establishment of the oxaliplatin-induced peripheral neuropathic pain model. (A) Von Frey, (B) acetone drop and (C) hot plate tests were conducted, and (D) body weight and (E) motor function were measured in rats following either vehicle control or oxaliplatin administration. Measurements were obtained at 0 7, 14 and 21 days post-oxaliplatin injection. Data are presented as mean ± standard error of the mean (n=8 each group). **P<0.01 vs. vehicle group (repeated measures ANOVA followed by Dunnett's test).
Effects of a single injection of berberine on oxaliplatin-induced allodynia
On the 21st day following the first injection of oxaliplatin, a single injection of 5, 10 or 20 mg/kg berberine was administered to the rats to examine the effect of berberine as a treatment on pre-established CIPNP. A previous report (25) reported these relief effects at 60 min after the berberine injection. Fig. 2A demonstrates that, according to a von Frey test, the hind-paw withdrawal thresholds of oxaliplatin-treated rats decreased compared with the vehicle-treated rats. The acetone drop tests showed increased withdrawal response times (Fig. 2B) and the hot plate tests showed decreased hind-paw-licking latency (Fig. 2C) compared with the vehicle-treated rats. However, a single injection of berberine significantly reduced mechanical allodynia (Fig. 2A), cold allodynia (Fig. 2B) and thermal hyperalgesia (Fig. 2C). Although the dose of 5 mg/kg of berberine could effectively relieve cold hyperalgesia and thermal hyperalgesia, it did not have a significant effect on mechanical hyperalgesia; therefore, the doses of 10 and 20 mg/kg of berberine were used for the following experiments.
Figure 2.
Analgesic effects of a single injection of berberine on oxaliplatin-induced CIPNP. Berberine (5, 10 or 20 mg/kg) was injected into CIPNP rats. One hour after the injection of berberine, (A) the Von Frey hind-paw withdrawal threshold, (B) the response times for cold acetone drop tests, and (C) hind-paw-licking latency for hot plate tests were measured. Data are presented as mean ± standard error of the mean (n=8 each group). **P<0.01 vs. vehicle group; #P<0.05 and ##P<0.01 vs. oxaliplatin+saline group (one-way ANOVA followed by Tukey's test). CIPNP, chemotherapy-induced peripheral neuropathic pain.
Effects of repeated doses of berberine on oxaliplatin-induced mechanical allodynia, cold allodynia and thermal hyperalgesia
As shown in Fig. 3, when the rats were repeatedly injected with berberine throughout the CIPNP induction experiment, berberine reversed CIPNP establishment, depending on the dose. The oxaliplatin and berberine-treated rats displayed only partial development of mechanical allodynia and cold allodynia compared with the oxaliplatin-treated rats on all test days (Fig. 3A and B). In hot plate tests, the oxaliplatin and berberine-treated rats displayed a distinct increase in hind paw licking latency compared with the oxaliplatin-treated rats (Fig. 3C). Weight gain measurements produced results similar to those for oxaliplatin-treated rats, with the oxaliplatin and berberine-treated rats not gaining as much weight as the vehicle-treated rats (Fig. 3D). However, motor function in the oxaliplatin and berberine-treated rats was significantly ameliorated compared with the oxaliplatin-treated rats, and similar to the levels of the control vehicle-treated rats (Fig. 3E). These results revealed that berberine dose-dependently prevented the development of CIPNP in rats without affecting motor function.
Figure 3.
Amelioration of oxaliplatin-induced CIPNP by consecutive injections of berberine. During the induction of CIPNP, 10 or 20 mg/kg berberine was injected in the rats. (A) The von Frey hind-paw withdrawal threshold, (B) response times for acetone drop tests, (C) hind-paw-licking latency for hot plate tests, (D) body weight and (E) motor coordination were assessed on 0, 7, 14 and 21 post-oxaliplatin injection. Data are presented as mean ± standard error of the mean (n=8 each group). **P<0.01 vs. vehicle group; #P<0.05 and ##P<0.01 vs. oxaliplatin+saline group (repeated measures ANOVA followed by Dunnett's test). CIPNP, chemotherapy-induced peripheral neuropathic pain.
Effects of repeated injections of berberine on oxaliplatin-induced NF-κB phosphorylation
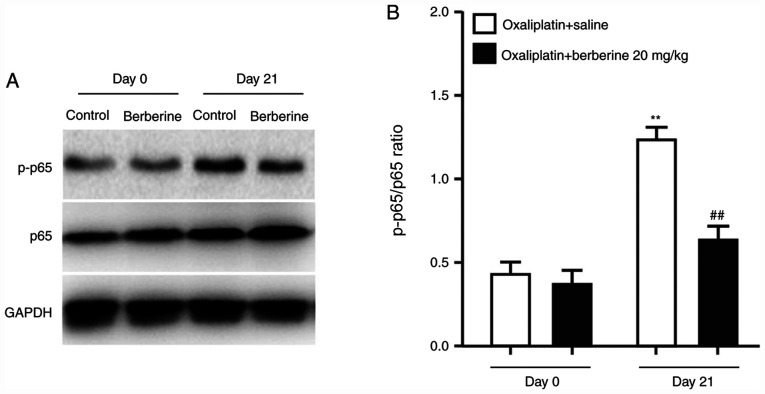
A recent study revealed that the NF-κB signaling pathway is associated with chemotherapy-induced chronic pain (13). In addition, activation of NF-κB in DRGs has been demonstrated to mediate chronic pain caused by inflammatory reactions (14). To understand the mechanism underlying the analgesic effect of berberine, the protein expression levels of NF-κB p65 and the phosphorylated NF-κB p65 were examined in DRGs from the rats administered with oxaliplatin and either saline control or the 20 mg/kg dose of berberine. The western blotting results demonstrated that oxaliplatin treatment significantly induced NF-κB p65 phosphorylation (Fig. 4A and B), while repeated injections of berberine suppressed oxaliplatin-induced NF-κB p65 phosphorylation (Fig. 4A and B). These findings indicated that the analgesic effect of berberine might occur through the regulation of NF-κB phosphorylation in DRGs.
Figure 4.
Phosphorylation of NF-κB p65 in DRGs of CIPNP rats following berberine administration. (A) Western blot analysis results of GAPDH, p65 and p-p65 from DRGs of CIPNP rats after consecutive berberine treatments (20 mg/kg). (B) Quantification of western blotting results as a ratio of p-p65 to total p65 levels. Data are presented as mean ± standard error of the mean (n=8 each group). **P<0.01 vs. vehicle group; ##P<0.01 vs. oxaliplatin+saline group (one-way ANOVA followed by Dunnett's test). DRGs, dorsal root ganglions; CIPNP, chemotherapy-induced peripheral neuropathic pain; p-, phosphorylated.
Effects of repeated injections of berberine on IL-6 and TNF-α levels in rat DRGs
The ELISA method was used to detect the levels of IL-6 and TNF-α in rat DRGs. The results demonstrated that the levels of IL-6 and TNF-α significantly increased in the oxaliplatin-induced CIPNP model compared with the vehicle-treated rats (Fig. 5A and B). However, berberine administration significantly and dose-dependently suppressed these increases in IL-6 and TNF-α levels in the oxaliplatin-induced CIPNP model (Fig. 5A and B).
Figure 5.
Effect of repeated berberine administration on the expression of IL-6 and TNF-α in DRGs. Effect of repeated berberine (10 or 20 mg/kg) injection on the levels of (A) IL-6 (A) and (B) TNF-α in the DRGs of oxaliplatin-induced peripheral neuropathic pain rats. Data are presented as mean ± standard error of the mean (n=8 each group). **P<0.01 vs. vehicle group; ##P<0.01 vs. oxaliplatin+saline group (one-way ANOVA followed by Tukey's test). DRGs, dorsal root ganglions.
Discussion
As many as 80% of patients receiving cytostatic drugs are treated for CIPNP (26). As the number of cancer survivors increases, treating this upsetting side effect has become a top priority. Although several drugs for preventing or treating CIPNP have been considered in experimental research, few have been applied effectively in clinical practice (27). The present study found that repeated doses of berberine during oxaliplatin treatment significantly prevented the severity of CIPNP in rats. This effect was associated with the suppressed phosphorylation of NF-κB p65, and with decreased levels of the cytokines IL-6 and TNF-α, in DRGs. The present results indicate that berberine might have an analgesic role in oxaliplatin-induced CIPNP by preventing NF-κB p65 phosphorylation and pro-inflammatory cytokine release in DRGs.
Berberine has been reported to relieve allodynia and to display antioxidant effects in models of diabetic neuropathy (23) and peripheral nerve injury (24). The present study included behavioral observations to demonstrate that oxaliplatin injection in rats could induce mechanical allodynia, cold allodynia and thermal hyperalgesia. Of note, a single dose of berberine significantly reduced oxaliplatin-induced pain behaviors in the pre-established CIPNP process, while repeated administration acted as a preventative treatment to the development and establishment of oxaliplatin-induced CIPNP. However, berberine did not produce pain behaviors in naive animals (28). The present results suggest that berberine can potentially prevent and treat neuropathic pain, including CIPNP. One of the limitations of the present study was the use of only male animals in the behavioral tests. Further analysis with both male and female animals will be necessary to confirm these conclusions.
Berberine's analgesic mechanism in oxaliplatin-induced CIPNP has not been extensively studied. The main mechanisms of oxaliplatin-induced peripheral neuropathy include ion-channel imbalance, neuronal inflammation, neuronal damage and oxidative stress (26). Accumulating evidence indicates that NF-κB activation and pro-inflammatory cytokines mediate chemotherapy-induced neuropathy and that NF-κB activation is also involved in pathological pain caused by nerve damage or inflammation (11,12). The most well-known functional heterodimer of NF-κB in cells is the p50/p65 complex (29). Given NF-κB p65 requires phosphorylation before binding to specific target genes in the nucleus, NF-κB activation can be demonstrated by an increase in p-p65 expression (30). It has also been reported that berberine prevents the extracellular matrix degradation and apoptosis of human nucleus pulposus cells by inhibiting the NF-κB pathway (31). The present study demonstrated that repeated administration of berberine during the induction of CIPNP significantly inhibited the NF-κB activation in DRGs induced by oxaliplatin. Combined with previous studies, the present results indicate that NF-κB might participate in the mechanism responsible for the analgesic effect of berberine in DRGs.
Previous studies have hypothesized that the spinal cord may be involved in the effect of berberine in CIPNP. For example, it has been reported that berberine reduces spinal cord neuroglia activation in streptozotocin-induced diabetic mice (28). Protein expression levels of inducible nitric oxide synthase and cyclooxygenase-2 in DRG and spinal cord have also been shown to be suppressed by berberine in the context of diabetic neuropathic pain. Levels of IL-6, IL1β and TNF-α, in either the DRG or the spinal cord, have also been shown to be reduced by berberine. These results indicate that the mechanism underlying the effect of berberine might be similar in the DRG and the spinal cord; however, this requires further exploration.
It is known that chemotherapeutic agents, such as paclitaxel, vincristine and oxaliplatin, can induce painful peripheral neuropathy by inducing pro-inflammatory cytokines (32,33). Activation of NF-κB is a critical signaling pathway in the chronic pain context, mediating inflammatory reactions and altering ion channel expression (34). Additionally, NF-κB is a pleiotropic regulator that regulates the expression of many different genes, including genes that transcribe pro-inflammatory cytokines such as IL-6, TNF-α and IL-1β (35). This explains the increased levels of IL-6 and TNF-α in the DRG of rats following oxaliplatin injection observed in the present study. The present study demonstrated that NF-κB p65 phosphorylation and expression of the pro-inflammatory cytokines IL-6 and TNF-α were substantially increased in the DRG of animals with oxaliplatin-induced CIPNP. Furthermore, the present study revealed that these increases were significantly suppressed by berberine, suggesting that berberine might relieve CIPNP through inhibiting NF-κB phosphorylation and the release of pro-inflammatory cytokines. Nonetheless, other mechanisms of berberine that might be involved with CIPNP cannot be excluded.
In conclusion, the present study demonstrated an analgesic effect of berberine both as a treatment on pre-established oxaliplatin-induced CIPNP and as a preventative during the establishment of oxaliplatin-induced CIPNP, by decreasing NF-κB p65 phosphorylation and pro-inflammatory cytokine IL-6 and TNF-α release in DRGs. The present results indicate that berberine may have an analgesic effect on CIPNP, thus further research confirming its effectiveness in a clinical setting is warranted.
Acknowledgements
Not applicable.
Funding
The present work was supported by grants from the Natural Science Foundation of Hubei province (grant no. 2018CκB920).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
QX and WN were responsible for the concept and design of the study. WN, XZ, LH and CK performed experiments and data analysis. QX and WN performed data interpretation, presentation and writing of the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All experimental procedures were performed in compliance with the Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of Huazhong University of Science and Technology (Wuhan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
References
- 1.Park SB, Goldstein D, Krishnan AV, Lin CS, Friedlander ML, Cassidy J, Koltzenburg M, Kiernan MC. Chemotherapy-induced peripheral neurotoxicity: A critical analysis. CA Cancer J Clin. 2013;63:419–437. doi: 10.3322/caac.21204. [DOI] [PubMed] [Google Scholar]
- 2.Argyriou AA, Bruna J, Marmiroli P, Cavaletti G. Chemotherapy-induced peripheral neurotoxicity (CIPN): An update. Crit Rev Oncol Hematol. 2012;82:51–77. doi: 10.1016/j.critrevonc.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 3.André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J, et al. Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) Investigators: Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 4.Cersosimo RJ. Oxaliplatin-associated neuropathy: A review. Ann Pharmacother. 2005;39:128–135. doi: 10.1345/aph.1E319. [DOI] [PubMed] [Google Scholar]
- 5.Farquhar-Smith P. Chemotherapy-induced neuropathic pain. Curr Opin Support Palliat Care. 2011;5:1–7. doi: 10.1097/SPC.0b013e328342f9cc. [DOI] [PubMed] [Google Scholar]
- 6.Brewer JR, Morrison G, Dolan ME, Fleming GF. Chemotherapy-induced peripheral neuropathy: Current status and progress. Gynecol Oncol. 2016;140:176–183. doi: 10.1016/j.ygyno.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 8.Meffert MK, Chang JM, Wiltgen BJ, Fanselow MS, Baltimore D. NF-kappa B functions in synaptic signaling and behavior. Nat Neurosci. 2003;6:1072–1078. doi: 10.1038/nn1110. [DOI] [PubMed] [Google Scholar]
- 9.Kaltschmidt B, Kaltschmidt C. NF-kappaB in the nervous system. Cold Spring Harb Perspect Biol. 2009;1(a001271) doi: 10.1101/cshperspect.a001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wan F, Anderson DE, Barnitz RA, Snow A, Bidere N, Zheng L, Hegde V, Lam LT, Staudt LM, Levens D, et al. Ribosomal protein S3: A KH domain subunit in NF-kappaB complexes that mediates selective gene regulation. Cell. 2007;131:927–939. doi: 10.1016/j.cell.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Zhao LX, Jiang BC, Wu XB, Cao DL, Gao YJ. Ligustilide attenuates inflammatory pain via inhibition of NFκB-mediated chemokines production in spinal astrocytes. Eur J Neurosci. 2014;39:1391–1402. doi: 10.1111/ejn.12502. [DOI] [PubMed] [Google Scholar]
- 12.Chu LW, Chen JY, Wu PC, Wu BN. Atorvastatin prevents neuroinflammation in chronic constriction injury rats through nuclear NFκB downregulation in the dorsal root ganglion and spinal cord. ACS Chem Neurosci. 2015;6:889–898. doi: 10.1021/acschemneuro.5b00032. [DOI] [PubMed] [Google Scholar]
- 13.Huang ZZ, Li D, Ou-Yang HD, Liu CC, Liu XG, Ma C, Wei JY, Liu Y, Xin WJ. Cerebrospinal fluid oxaliplatin contributes to the acute pain induced by systemic administration of oxaliplatin. Anesthesiology. 2016;124:1109–1121. doi: 10.1097/ALN.0000000000001084. [DOI] [PubMed] [Google Scholar]
- 14.Fu ES, Zhang YP, Sagen J, Candiotti KA, Morton PD, Liebl DJ, Bethea JR, Brambilla R. Transgenic inhibition of glial NF-kappaB reduces pain behavior and inflammation after peripheral nerve injury. Pain. 2010;148:509–518. doi: 10.1016/j.pain.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu ES, Zhang YP, Sagen J, Yang ZQ, Bethea JR. Transgenic glial nuclear factor-kappaB inhibition decreases formalin pain in mice. Neuroreport. 2007;18:713–717. doi: 10.1097/WNR.0b013e3280d9e869. [DOI] [PubMed] [Google Scholar]
- 16.Nong X, Lan Y, Picroside II. Picroside II Attenuates CCI-induced neuropathic pain in rats by inhibiting spinal reactive astrocyte-mediated neuroinflammation through the NF-κB pathway. Neurochem Res. 2018;43:1058–1066. doi: 10.1007/s11064-018-2518-7. [DOI] [PubMed] [Google Scholar]
- 17.Tao W, Luo X, Cui B, Liang D, Wang C, Duan Y, Li X, Zhou S, Zhao M, Li Y, et al. Practice of traditional Chinese medicine for psycho-behavioral intervention improves quality of life in cancer patients: A systematic review and meta-analysis. Oncotarget. 2015;6:39725–39739. doi: 10.18632/oncotarget.5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan QL, Guo TM, Liu L, Sun F, Zhang YG. Traditional Chinese medicine for neck pain and low back pain: A systematic review and meta-analysis. PLoS One. 2015;10(e0117146) doi: 10.1371/journal.pone.0117146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Q, Yue N, Liu SB, Wang ZF, Mi WL, Jiang JW, Wu GC, Yu J, Wang YQ, et al. Effects of chronic electroacupuncture on depression- and anxiety-like behaviors in rats with chronic neuropathic pain. Evid Based Complement Alternat Med. 2014;2014(158987) doi: 10.1155/2014/158987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen C, Lu M, Pan Q, Fichna J, Zheng L, Wang K, Yu Z, Li Y, Li K, Song A, et al. Berberine improves intestinal motility and visceral pain in the mouse models mimicking diarrhea-predominant irritable bowel syndrome (IBS-D) symptoms in an opioid-receptor dependent manner. PLoS One. 2015;10(e0145556) doi: 10.1371/journal.pone.0145556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen C, Tao C, Liu Z, Lu M, Pan Q, Zheng L, Li Q, Song Z, Fichna J. A randomized clinical Trial of berberine hydrochloride in patients with diarrhea-predominant irritable bowel syndrome. Phytother Res. 2015;29:1822–1827. doi: 10.1002/ptr.5475. [DOI] [PubMed] [Google Scholar]
- 22.Tan W, Li Y, Chen M, Wang Y. Berberine hydrochloride: Anticancer activity and nanoparticulate delivery system. Int J Nanomedicine. 2011;6:1773–1777. doi: 10.2147/IJN.S22683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim SO, Kim HJ. Berberine ameliorates cold and mechanical allodynia in a rat model of diabetic neuropathy. J Med Food. 2013;16:511–517. doi: 10.1089/jmf.2012.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han AM, Heo H, Kwon YK. Berberine promotes axonal regeneration in injured nerves of the peripheral nervous system. J Med Food. 2012;15:413–417. doi: 10.1089/jmf.2011.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rezaee R, Monemi A, SadeghiBonjar MA, Hashemzaei M. Berberine alleviates paclitaxel-induced neuropathy. J Pharmacopuncture. 2019;22:90–94. doi: 10.3831/KPI.2019.22.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sisignano M, Baron R, Scholich K, Geisslinger G. Mechanism-based treatment for chemotherapy-induced peripheral neuropathic pain. Nat Rev Neurol. 2014;10:694–707. doi: 10.1038/nrneurol.2014.211. [DOI] [PubMed] [Google Scholar]
- 27.Majithia N, Loprinzi CL, Smith TJ. New practical approaches to chemotherapy-induced neuropathic pain: Prevention, assessment, and treatment. Oncology (Williston Park) 2016;30:1020–1029. [PubMed] [Google Scholar]
- 28.Liu M, Gao L, Zhang N. Berberine reduces neuroglia activation and inflammation in streptozotocin-induced diabetic mice. Int J Immunopathol Pharmacol. 2019;33(2058738419866379) doi: 10.1177/2058738419866379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109 (Suppl 1):S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 30.Vermeulen L, De Wilde G, Notebaert S, Vanden Berghe W, Haegeman G. Regulation of the transcriptional activity of the nuclear factor-kappaB p65 subunit. Biochem Pharmacol. 2002;64:963–970. doi: 10.1016/s0006-2952(02)01161-9. [DOI] [PubMed] [Google Scholar]
- 31.Lu L, Hu J, Wu Q, An Y, Cui W, Wang J, Ye Z. Berberine prevents human nucleus pulposus cells from IL 1β induced extracellular matrix degradation and apoptosis by inhibiting the NF κB pathway. Int J Mol Med. 2019;43:1679–1686. doi: 10.3892/ijmm.2019.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoon SY, Oh J. Neuropathic cancer pain: Prevalence, pathophysiology, and management. Korean J Intern Med (Korean Assoc Intern Med) 2018;33:1058–1069. doi: 10.3904/kjim.2018.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carozzi VA, Canta A, Chiorazzi A. Chemotherapy-induced peripheral neuropathy: What do we know about mechanisms? Neurosci Lett. 2015;596:90–107. doi: 10.1016/j.neulet.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed AS, Berg S, Alkass K, Druid H, Hart DA, Svensson CI, Kosek E. NF-κB-associated pain-related neuropeptide expression in patients with degenerative disc disease. Int J Mol Sci. 2019;20(20) doi: 10.3390/ijms20030658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shih RH, Wang CY, Yang CM. NF-kappaB signaling pathways in neurological inflammation: A mini review. Front Mol Neurosci. 2015;8(77) doi: 10.3389/fnmol.2015.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.