ABSTRACT
Coronavirus disease 2019 (COVID-19) has attracted worldwide attention due to its speed of progression and elevated mortality rate. Amid the rush to develop treatments, recent hopes have focused on the anti-malarial drug chloroquine or the derivative hydroxychloroquine. Here, we briefly discuss the evidence for the potential use of these drugs with regard to the current pandemic.
KEYWORDS: Chloroquine, hydroxychloroquine, COVID-19, SARS-CoV-2, clinical trial, autophagy
Coronavirus disease 2019 (COVID-19) is a respiratory disease caused by a new coronavirus, SARS-CoV-2, which differs from SARS-CoV and MERS-CoV in nucleotide sequence [1]. Now, it has caused a worldwide pandemic in 200 countries and territories, becoming a serious public health crisis. Vaccination may be the most beneficial prevention for COVID-19, and 115 candidate vaccines are currently under development [2]. On 16 March 2020, mRNA-1273, the first COVID-19 vaccine candidate from Moderna, entered human clinical testing, but its protective effect remains unknown. In addition, some previously approved medicines may have an emerging role in protecting against a rapidly progressive lethal infection. In particular, recent basic and clinical studies have drawn great attention to the possible benefits of chloroquine or the derivative hydroxychloroquine, an inexpensive anti-malaria drug that has been used for decades, in the treatment of COVID-19 [3,4]. In this comment, we discuss the fact that chloroquine has a dual function in antiviral immunity (Figure 1), which may be critical to consider for decision-making in the treatment of COVID-19.
Figure 1.
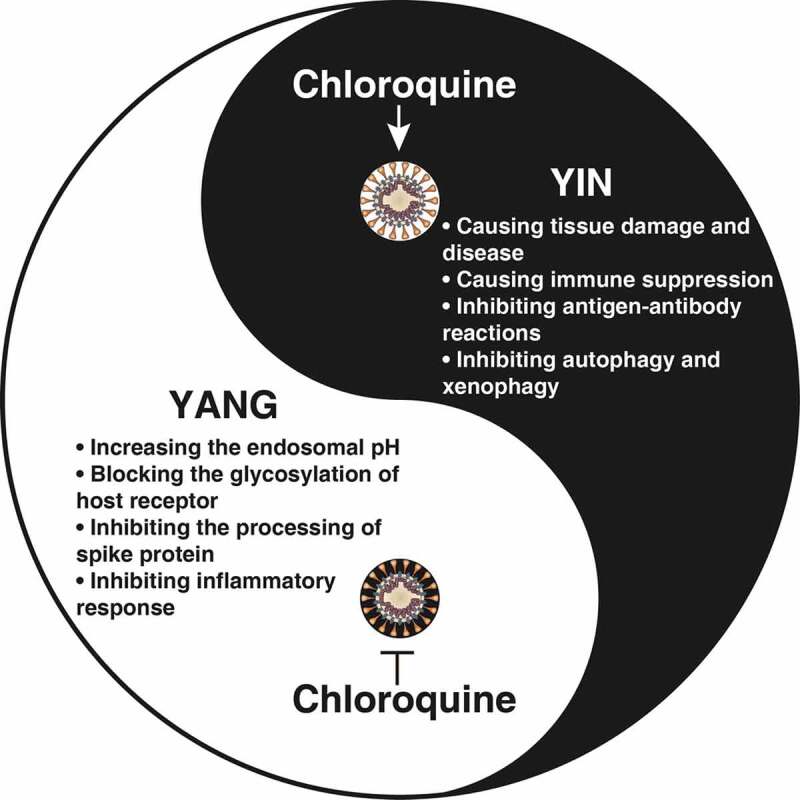
The yin and yang of chloroquine and its analogs in fighting COVID-19
Clinical evidence
Two clinical studies from China and France have shown that chloroquine has an effect on the clinical efficacy and viral clearance of COVID-19, and more clinical trials are ongoing in Italy, England, and the USA. The first clinical trial in China involved 100 patients in 10 hospitals and 6 cities. This study found that using chloroquine phosphate tablets (500 mg, twice daily for 10 days) can reduce viral load and improve lung function and recovery time [5,6]. The second study of 26 patients in France in one hospital also observed that oral hydroxychloroquine sulfate (200 mg, 3 times a day for 10 days), the less toxic derivative of chloroquine, can clear SARS-CoV-2 infection in 3 to 6 days in 75% of patients [7]. The antibiotic azithromycin (500 mg on day 1 followed by 250 mg per day, the next 4 days) enhanced this effect of hydroxychloroquine in 6 patients, although the mechanism is unknown [7]. It is worth noting that both studies have significant limitations, such as small sample sizes, limited follow-up of long-term results, and non-randomized and open-label trials. Currently, there are 142 registered clinical trials involving the use of chloroquine or hydroxychloroquine, either as interventions being tested or as comparators for other drugs. However, no randomized clinical trials have been conducted to support strong clinical recommendations for the use of chloroquine and hydroxychloroquine to treat COVID-19.
Advantages
Chloroquine and its analogs have broad-spectrum antiviral and anti-inflammatory properties and have been used for patients with malaria, rheumatoid arthritis, systemic and discoid lupus erythematosus, scleroderma, pemphigus, lichen planus, polymyositis, sarcoidosis, and porphyria cutanea tarda. The preclinical antiviral activity of chloroquine has been demonstrated in SARS-CoV, MERS-CoV, OC43, enterovirus EV-A71, Zika virus, influenza A H5N1, and SARS-CoV-2 infection [8,9]. Chloroquine can fight coronavirus infection in several different ways: 1) Increasing the endosomal pH. Coronaviruses are enveloped, positive-stranded RNA viruses. After endocytosis, these viruses deliver their genomes into host cells through low-pH endosomes. Chloroquine is a di-protonic weak base. The main mechanism of action of chloroquine with regard to its effect in preventing infection by enveloped viruses is to increase the pH of the endosome, therefore inhibiting the uncoating and replication of these viruses [10]. Chloroquine may also act as a zinc ionophore [11], inhibiting the replication of coronavirus [12]. 2) Blocking the glycosylation of the host receptor. Similar to SARS-CoV, SARS-CoV-2 requires ACE2 (angiotensin I converting enzyme 2) to enter host cells [13]. This receptor is regulated by its glycosylation status [14], and chloroquine blocks terminal glycosylation of ACE2, thus inhibiting SARS-CoV infection [10]. 3) Inhibiting the processing of spike protein. The homotrimers of spike proteins mediate the entry of coronavirus into host cells. Chloroquine can slightly reduce homotrimers of the SARS-CoV spike protein [10]. 4) Inhibiting the inflammatory response. In severe cases, COVID-19 may be complicated by sepsis, which is a life-threatening organ dysfunction due to a host’s dysregulated response to the infection. Chloroquine prevents experimental sepsis and septic shock by reducing the production of cytokines (e.g., TNF, IL1, IL6, and IFNG) and damage-associated molecular patterns (e.g., HMGB1) by interfering with the innate immune pathways (e.g., TLR7, TLR9, and NFKB) of multiple immune cells (e.g., macrophages, monocytes, and neutrophils) [15–17].
Disadvantages
There are several adverse effects of chloroquine and its analogs in antiviral immunity. 1) Causing tissue damage and disease. The most serious complications of chloroquine are cardiomyopathy (e.g., sinus node dysfunction, arrhythmia, and Stokes-Adams syndrome), retinopathy (e.g., macular degeneration), neuromyopathy (e.g., dystonia and dyskinesia), and myopathy (e.g., muscle cramps, stiffness, and spasm) [18]. When using chloroquine to treat COVID-19 patients with these existing conditions or diseases, especially cardiovascular disease, a more comprehensive risk assessment may be required. In Brazil, a preliminary study of COVID-19 patients using chloroquine diphosphate ended early after several patients died due to severe arrhythmias. 2) Causing immune suppression. Viruses have developed many ways to evade immune surveillance and downregulate the host’s immune response. Chloroquine inhibits the proliferation and activation of T cells and induces their apoptosis, which may adversely affect antiviral immunity [19]. 3) Inhibiting antigen-antibody reaction. Antigen-antibody interactions are the basic response in vivo to protect against foreign antigens, including viruses. Chloroquine, as a lysosomotropic agent, also inhibits proteolysis, chemotaxis, phagocytosis, and antigen presentation in various professional antigen-presenting cells (e.g., dendritic cells, B cells, and macrophages) [18]. 4) Inhibiting autophagy and xenophagy. Autophagy is a lysosomal degradation pathway responsible for the removal of intracellular materials, such as superfluous proteins or damaged organelles (e.g., mitochondria), to maintain homeostasis [20,21]. Xenophagy is a selective type of autophagy that is essential for eliminating invading pathogens, including viruses and bacteria [22]. Chloroquine acts as a classic autophagy inhibitor by preventing the fusion of autophagosomes with lysosomes [23]. Therefore, the inhibition of autophagy by chloroquine may lead to cell death and secondary infection.
Considerations
Although chloroquine may be a potential drug for the treatment of COVID-19, randomized controlled trials with large-scale patient populations are needed to ensure its safety and benefits in the treatment of SARS-CoV-2 infection. As mentioned above, chloroquine, along with its beneficial properties, may cause some undesirable effects. Thus, we need to further assess the benefits of chloroquine treatment based on age, clinical manifestations or course of disease. The main considerations for clinical application of chloroquine include: 1) Provide patients with information about common side effects, adverse reactions and potential interactions of the drug. 2) The drug should be used under the guidance of a recognized expert, and self-treatment is not recommended. 3) Perform regular blood tests and electrocardiograms every other day during the medication administration. 4) Ask patients about changes in vision during medication administration. 5) Observe the mental and psychological status of patients during medication administration. Through continuous research to improve our understanding of COVID-19 immunopathology, we can develop effective treatment strategies for this disease. We absolutely need to tackle this COVID-19 challenge globally, but must do so through a rational, scientific and safe approach.
Disclosure statement
No potential conflicts of interest were disclosed.
References
- [1].Tang D, Comish P, Kang R. The hallmarks of COVID-19 disease. PLoS Pathog. 2020;16(5):e1008536. doi: 10.1371/journal.ppat.1008536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Thanh LT, Andreadakis Z, Kumar A, et al. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020.19(5):305–306 [DOI] [PubMed] [Google Scholar]
- [3].Colson P, Rolain JM, Lagier JC, et al. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. 2020;105932. DOI: 10.1016/j.ijantimicag.2020.105932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cortegiani A, Ingoglia G, Ippolito M, et al. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gao J, Tian Z, Yang X.. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72–73. [DOI] [PubMed] [Google Scholar]
- [6].Zhonghua, J., He, H., Xi, H, et al. Multicenter collaboration group of Department of S, Technology of Guangdong Province Health Commission of Guangdong Province for chloroquine in the treatment of novel coronavirus p. [Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:E019. [DOI] [PubMed] [Google Scholar]
- [7].Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 2020105949. DOI: 10.1016/j.ijantimicag.2020.105949 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [8].Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Touret F, de Lamballerie X.. Of chloroquine and COVID-19. Antiviral Res. 2020;177:104762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Xue J, Moyer A, Peng B, et al. Chloroquine is a zinc ionophore. PLoS One. 2014;9:e109180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Te Velthuis AJ, van den Worm SH, Sims AC, et al. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6:e1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chen Y, Guo Y, Pan Y, et al. Structure analysis of the receptor binding of 2019-nCoV. Biochem Biophys Res Commun. 2020;525(1):135–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yang M, Cao L, Xie M, et al. Chloroquine inhibits HMGB1 inflammatory signaling and protects mice from lethal sepsis. Biochem Pharmacol. 2013;86:410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yasuda H, Leelahavanichkul A, Tsunoda S, et al. Chloroquine and inhibition of Toll-like receptor 9 protect from sepsis-induced acute kidney injury. Am J Physiol Renal Physiol. 2008;294:F1050–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Martinson JA, Montoya CJ, Usuga X, et al. Chloroquine modulates HIV-1-induced plasmacytoid dendritic cell alpha interferon: implication for T-cell activation. Antimicrob Agents Chemother. 2010;54:871–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Al-Bari MA. Chloroquine analogues in drug discovery: new directions of uses, mechanisms of actions and toxic manifestations from malaria to multifarious diseases. J Antimicrob Chemother. 2015;70:1608–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Savarino A, Shytaj IL. Chloroquine and beyond: exploring anti-rheumatic drugs to reduce immune hyperactivation in HIV/AIDS. Retrovirology. 2015;12:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Levine B, Kroemer G. Biological functions of autophagy genes: A disease perspective. Cell. 2019;176:11–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sharma V, Verma S, Seranova E, et al. Selective autophagy and xenophagy in infection and disease. Front Cell Dev Biol. 2018;6:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Klionsky DJ, Abdelmohsen K, Abe A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy. 2016;12:1–222. [DOI] [PMC free article] [PubMed] [Google Scholar]


