Abstract
Historical data regarding time to viral rebound following analytical treatment interruption (ATI) have been used to determine therapeutic efficacy in HIV cure trials; however, such data were collected from studies conducted a decade or more ago and included participants receiving older antiretroviral therapy (ART) regimens with infrequent virologic monitoring. We conducted a study of 22 HIV-infected participants receiving modern ART to determine the kinetics of plasma viral rebound following ATI. Our data suggest that modern ART does not alter kinetics of viral rebound when compared to previous regimens and that immunologic interventions may be necessary to achieve ART-free virologic remission.
Clinical Trials Registration ClinicaTrials.gov identifier: NCT03225118.
Keywords: human immunodeficiency virus, analytical treatment interruption, antiretroviral therapy
Durable suppression of human immunodeficiency virus (HIV) is now attainable in the vast majority of infected individuals receiving antiretroviral therapy (ART) [1]. However, complete eradication of the virus by ART alone is currently not feasible due in part to the persistence of HIV reservoirs in the CD4+ T-cell compartment and other cell types [2] and prompt plasma viral rebound following discontinuation of antiretroviral drugs in infected individuals [3]. Considering ART is not curative and poses multiple challenges, including life-long adherence, cumulative toxicities, and economic and logistical obstacles, significant efforts have been made in recent years to achieve antiretroviral drug-free HIV remission in infected individuals [2]. To this end, a variety of therapeutic approaches aimed at achieving sustained virologic remission in the absence of ART are under active investigation. These include: (1) reversal of HIV latency, (2) genetic modification and stem cell transplantation, (3) enhancement of anti-HIV immunity, and (4) passive transfer of broadly neutralizing anti-HIV antibodies [2]. Therapeutic efficacies of such interventional trials are often determined by measurements of the size of persistent HIV reservoirs over time by multiple virologic assays [4]. Although these assays are highly specific and could accurately determine the frequency of HIV-infected cells, it remains unclear if such measurements can predict the outcome of the viral dynamics in infected individuals following interruption of ART in the context of a clinical trial, referred to here as analytical treatment interruption (ATI) [5, 6]. Consequently, ATI has become an integral component for evaluating therapeutic interventions aimed at achieving ART-free HIV remission in infected individuals. HIV cure trials that do not include a placebo arm typically rely on historical data on the time to plasma viral rebound following ATI as a reference to assess the efficacy of therapeutic agents [5]. However, much of such historical data were collected from studies conducted a decade or more ago, prior to the introduction of currently available and highly potent ART regimens, including study participants who were pretreated with mono/dual therapies and/or received therapeutic interventions, were subjected to infrequent monitoring of virologic and immunologic parameters at variable intervals, and reinitiated ART under different criteria [7]. Therefore, it is of great importance to conduct a clinical trial involving frequent measurements of multiple parameters to provide contemporary assessments/reference on the effect of modern ART regimens on plasma viral rebound following ATI for future curative studies. We conducted a single-arm study of 22 participants with HIV who initiated highly effective ART during the chronic phase of infection and were receiving modern drug regimens to determine (1) kinetics of plasma viral rebound following ATI, (2) relationships between baseline virologic and immunologic parameters and timing of viral rebound, and (3) immune correlates of viral decay upon ART resumption.
METHODS
Study Participants
Twenty-two participants with HIV who initiated ART during the chronic phase of infection were studied. All study participants underwent ATI and resumed ART if they met any of the following criteria: (1) plasma viremia > 1000 copies/mL for ≥ 4 weeks, (2) decrease in CD4+ T-cell percentage > 30% or count < 350 cells/µL, (3) HIV-related illness/symptoms, or (4) pregnancy. During the ATI, CD4+ T cells and plasma viremia were monitored every week. Following reinitiation of ART, CD4+ T cells and plasma viremia were measured weekly for the first 4 weeks then monthly thereafter for up to 52 weeks.
Blood and leukapheresed products were collected in accordance with protocols approved by the Institutional Review Board of the National Institute of Allergy and Infectious Diseases, National Institutes of Health and registered on ClinicalTrials.gov, NCT03225118. All subjects provided written informed consent.
Measurements of HIV Reservoirs
The level of cells carrying total HIV DNA was determined by droplet digital polymerase chain reaction (PCR; Bio-Rad Laboratories) using restriction-digested (MscI; New England BioLabs) genomic DNA purified from CD4+ T cells. The PCR reaction was carried out using HIV-specific and RPP30-specific primers and probes. The following primers and probe were used for amplification of HIV LTR: 5′-GRAACCCACTGCTTAAGCCTCAA -3′ (5′ primer) and 5′- TGTTCGGGCGCCACTGCTAGAGA -3′ (3′ primer), and 5′-6FAM-AGTAGTGTGTGCCCGTCTGTT-IABkFQ-3′ (probe). The following primers and probe were used for amplification of RPP30: 5′-GATTTGGACCTGCGAGCG-3′ (5′ primer), 5′-GCGGCTGTCTCCACAAGT-3′ (3′ primer), and 5′-HEX-TTCTGACCTGAAGGCTCTGCGC-IABkFQ-3′ (probe). The level of cell-associated HIV RNA was determined by reverse transcription PCR (RT-PCR). Total RNA was isolated from purified CD4+ T cells using RNeasy Mini kit (Qiagen). cDNA was synthesized by incubating total RNA with qScript XLT cDNA Master Mix (Quanta Biosciences) for 5 minutes at 35°C, 60 minutes at 42°C, and 5 minutes at 85°C. cDNA was subjected to droplet digital PCR (Bio-Rad) using HIV-specific and TATA box binding protein (TBP)-specific primers and probes. The following primers and probe were used for amplification of HIV: 5′- TCTCTAGCAGTGGCGCCCGAACA -3′ (5′ primer), 5′- TCTCCTTCTAGCCTCCGCTAGTC -3′ (3′ primer), and 5′-6FAM- CAAGCCGAGTCCTGCGTCGAGAG -IABkFQ-3′ (probe). The following primers and probe were used for amplification of TBP: 5′- CACGAACCACGGCACTGATT -3′ (5′ primer), 5′- TTTTCTTGCTGCCAGTCTGGAC -3′ (3′ primer), and 5′-HEX- TGTGCACAGGAGCCAAGAGTGAAGA/3-IABkFQ-3′ (probe). HIV RNA copy numbers were normalized per 1 × 106 copies of TBP. The frequency of CD4+ T cells carrying replication-competent HIV was determined using quantitative coculture assays using serially diluted (1 × 106, 200 000, 40 000, 8000, 1600, and 320) and replicates of 5 × 106 CD4+ T cells. The cultures were stimulated with irradiated peripheral blood mononuclear cells (PBMCs) from HIV-negative donors and anti-CD3 antibody. Stimulated, CD8-depleted PBMCs from HIV-negative donors were added to each well followed by periodic addition of fresh media and removal of cells. HIV p24 enzyme-linked immunosorbent assay (ELISA) was performed on the culture supernatants between days 14 and 21. The infectious units per million cells from coculture assays were determined as described [8].
Measurements of Immune Parameters
Frequencies of lymphocyte subsets were measured by flow cytometry using the following fluorophore-conjugated antibodies: CD3 (clone SK7), CD4 (clone SK3), CD8 (clone SK1), CD38 (clone HB7 and HIT2), CD226 (clone DX11), programmed cell death protein 1 (PD-1; clone J105), T-cell immunoglobulin and ITIM domain (TIGIT; MBSA43), and HLA-DR (clone L243). Flow cytometric data were acquired on a BD FACS Canto II flow cytometer with FACSDiva software and analyzed using FlowJo.
Statistical Analysis
Statistical significance in Supplementary Table 1 was determined using the Spearman rank test. Statistical significance in Supplementary Table 2 was determined using the Wilcoxon rank sum test. Due to multiple comparisons (n = 16), P values < .05 were considered to be merely suggestive, <.01 as more convincing, and < .003 (.05/16) as compelling. Survival curves applied to the percent suppression and percent remaining off ART over time were estimated using the Kaplan-Meier method.
RESULTS
The study cohort included 22 participants with HIV who initiated highly effective ART during the chronic phase of infection and were receiving modern drug regimens without history of receiving mono/dual ART or interventional drugs. Clinical features of the study participants are shown in Table 1. At baseline, the vast majority of study participants were receiving integrase strand transfer inhibitor-based regimens (90%) and suppressed plasma viremia below the limits of detection for a median of 7.7 years (range, 2.1–15.9).
Table 1.
Baseline Characteristics of Study Participants
| Characteristic | Value |
|---|---|
| Sex, No. (%) | |
| Male | 20 (90.9) |
| Female | 2 (9.9) |
| Age, y | |
| Median (IQR) | 51 (46–54) |
| Range | 38–61 |
| Race, No. (%) | |
| African American | 9 (40.9) |
| Caucasian | 10 (45.5) |
| Multiple race | 1 (4.5) |
| Unknown | 2 (9.1) |
| Antiretroviral drug regimen at study entry, No. (%) | |
| NNRTI | 1 (4.5) |
| PI | 0 (0) |
| PI/INSTI | 1 (4.5) |
| INSTI | 20 (90.9) |
| Duration of HIV suppression on ART at study entry, y | |
| Median (IQR) | 7.7 (3.1–11.1) |
| Range | 2.08–15.9 |
| CD4+ T-cell count at study entry, cells/mm3 | |
| Median (IQR) | 767 (591–946) |
| Range | 466–1778 |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; INSTI, integrase strand transfer inhibitor; IQR, interquartile range; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
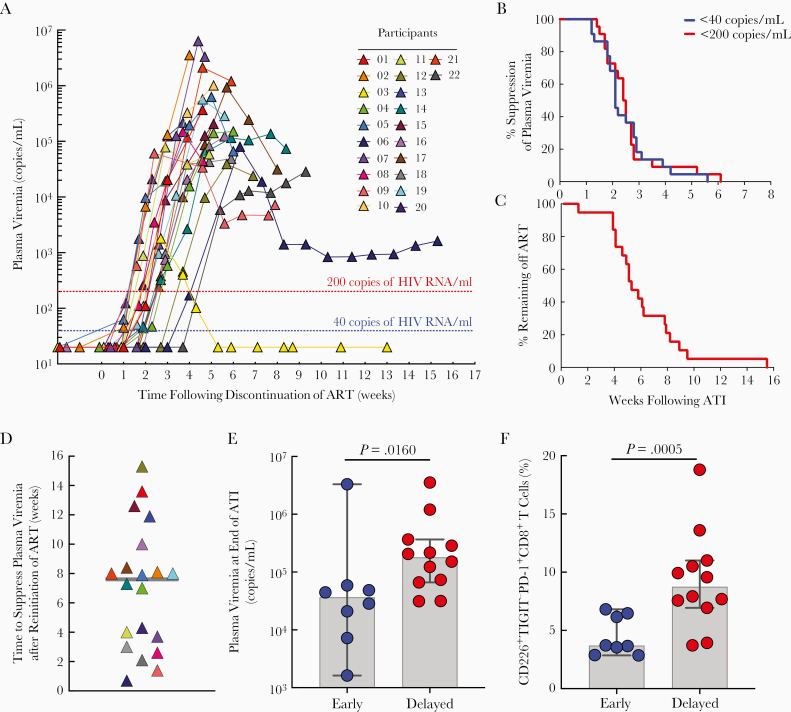
All participants experienced plasma viral rebound following ATI. The median time to plasma viral rebound > 40 and > 200 copies/mL was 1.9 (range, 1.0–5.4) and 2.6 (range, 2.0–5.4) weeks, respectively (Figure 1A and 1B, Supplementary Figure 1, and Supplementary Table 1). The median time to meeting ART restart criteria was 4.9 weeks (range, 1.1–15.3; Figure 1C). Reasons for ART resumption included decreased CD4+ T-cell counts (50%) and sustained plasma viremia (35%); 2 participants resumed ART due to thrombocytopenia (participants 01 and 21). One study participant (03) who spontaneously controlled his plasma viremia reinitiated ART before meeting the restart criteria.
Figure 1.
Virologic and immunologic parameters following analytical treatment interruption (ATI) and reinitiation of antiretroviral therapy (ART) in study participants. A, Kinetics of plasma viral rebound following discontinuation of ART. Study participants remained off ART until they met restart criteria. Plasma viremia was determined by Abbott Real-Time HIV-1 Assay with a detection limit of 40 copies of HIV RNA/mL. B, Kaplan-Meier curve of plasma viral suppression (<40 and < 200 copies/mL) during the treatment interruption phase. C, Kaplan-Meier curve of the percentage of study participants (n = 19) remaining off ART during the treatment interruption phase. D, Time required to reach plasma viremia < 40 copies/mL in the study participants following reinitiation of ART. Grey line represents the median value. E, Association between plasma viremia at the end of ATI phase and time to suppress HIV < 40 copies/mL following reinitiation of ART. F, Association between the percentage of CD226+TIGIT−PD-1+CD8+ T cells at baseline (prior to ATI) and time to suppress the virus < 40 copies/mL following reinitiation of ART. E and F, Blue circles represent study participants whose plasma viremia reached < 40 copies/mL less than or equal to 4 weeks after reinitiation of ART. Red circles represent study participants whose plasma viremia reached < 40 copies/mL greater than 4 weeks after reinitiation of ART. P value was determined using the Mann-Whitney test. Grey bars indicate median values. Black vertical lines represent 95% confidence intervals.
We then evaluated the relationship between baseline virologic and immunologic parameters and time to plasma viral rebound following ATI. Frequencies at baseline of CD4+ T cells carrying total HIV DNA (P = .024) but not cell-associated HIV RNA (P = .883) nor replication-competent virus (P = .136) correlated with time to viral rebound > 200 copies/mL (Supplementary Table 2). Numbers or frequencies at baseline of CD4+, CD38+HLA-DR+CD8+, and CD226+TIGIT−CD8+ T cells correlated with time to plasma viral rebound > 200 copies/mL (P = .037, P = .021, and P = .012, respectively; Supplementary Table 2).
Finally, we evaluated factors that may affect the decay of viremia following reinitiation of ART. When the participants were divided into early (≤ 4 weeks) versus delayed (> 4 weeks) groups based on the time to reach < 40 copies/mL after reinitiation of ART (Figure 1D), the delayed group had higher plasma viremia at the end of the ATI phase (P = .016; Figure 1E) and higher CD226+TIGIT−PD-1+CD8+ T-cell frequencies at baseline, prior to ATI (P = .0005; Figure 1F and Supplementary Table 3), suggesting a potential role for immune exhaustion in the decay rate of antiretroviral drug-mediated plasma viremia.
DISCUSSION
A variety of laboratory-based immunologic and virologic assays are currently being used to assess efficacy of therapeutic strategies aimed at achieving an HIV cure in infected individuals in the absence of ART [2, 4]. Although informative, these laboratory-based assays are not clinically validated to predict the antiviral efficacy of such therapeutic agents nor are they capable of predicting the timing or the extent of plasma viral rebound in HIV-infected individuals following ATI [2]. Considering that essentially all HIV-infected individuals experience plasma viral rebound upon cessation of ART and that plasma viremia remains the only clinically validated virologic marker for progression of HIV disease, it is necessary to incorporate an ATI phase into clinical trials designed to evaluate the efficacy of cure-related therapeutic strategies in humans [5]. In this regard, the time to plasma viral rebound upon discontinuation of ART is considered an important parameter for such clinical trials. Current estimates of the time to viral rebound following ATI are based on data collected over 10–15 years ago, which included infrequent measurements of plasma viremia and inclusion of HIV-infected individuals who had been on first-generation antiretroviral drugs and/or had received mono/dual therapies prior to receiving triple-combination drug regimens [7]. Therefore, we conducted a clinical trial with HIV-infected individuals who had been receiving the state-of-the-art, highly potent combination antiretroviral drugs without histories of receiving mono/dual therapies or immune-based therapeutic agents. In addition, we monitored plasma viremia at a frequency in accordance with current recommendations for conducting ATIs in HIV cure-related clinical trials [5]. Thus, our data provide useful information that can be used in designing future clinical trials aimed at achieving ART-free HIV remission. Given the vast majority of our study participants experienced a rapid viral rebound shortly after ATI, modern antiretroviral drug regimens may not offer additional benefits, such as enhancement of immune-mediated virologic control, beyond their remarkable antiretroviral activities against HIV, which underscores the importance of the development of therapeutic strategies aimed at achieving durable virologic remission in the absence of ART [9, 10]. Although some of the immunologic and virologic parameters we measured at baseline correlated with the time to plasma viral rebound following ATI, these may have been chance findings given the number of comparisons made and the relatively weak statistical significance (.01 < P < .05). Moreover, ATI will likely remain necessary in determining therapeutic efficacy, especially given that most post-ART treatment controllers spontaneously suppress HIV after a rapid but transient burst of plasma viremia [11–13]. Given that a substantial proportion of HIV-infected individuals participating in therapeutic ATI trials would likely need to resume ART, it is of interest to examine factors that influence the decay kinetics of plasma viremia following reinitiation of ART. It has previously been suggested that long-lived HIV reservoirs may contribute to the slow decay of plasma viremia following initiation of ART [14]. We found that high levels of CD226+TIGIT−PD-1+ CD8+ T cells at baseline (prior to ATI) could potentially delay the time to resuppress plasma viremia below the limit of detection (<40 copies/mL) upon reinitiation of ART. It has been shown that TIGIT functions as a coinhibitory receptor and counteracts the costimulatory effect of CD226 on CD8+ T cells by preventing homodimerization of CD226 [15]. It is plausible that exhausted immune cells (such as those expressing PD-1) in the CD226+TIGIT−CD8+ T-cell compartment display impaired effector functions that could delay decay of plasma viremia/viral replication upon reinitiation of ART. Further delineation of immune factors that impact viral dynamics in the settings of ATI and ART resumption may provide guidance for future HIV cure-related trials.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the study volunteers for their participation in this study; and Ms Erika Benko, Dr Colin Kovacs, and the NIAID HIV outpatient clinic staff for their assistance in the execution of this study.
Financial support. This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet 2013; 382:1525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chun TW, Moir S, Fauci AS. HIV reservoirs as obstacles and opportunities for an HIV cure. Nat Immunol 2015; 16:584–9. [DOI] [PubMed] [Google Scholar]
- 3. Davey RT Jr, Bhat N, Yoder C, et al. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci U S A 1999; 96:15109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Siliciano JD, Siliciano RF. Assays to measure latency, reservoirs, and reactivation. Curr Top Microbiol Immunol 2018; 417:23–41. [DOI] [PubMed] [Google Scholar]
- 5. Julg B, Dee L, Ananworanich J, et al. Recommendations for analytical antiretroviral treatment interruptions in HIV research trials—report of a consensus meeting. Lancet HIV 2019; 6:e259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li JZ, Etemad B, Ahmed H, et al. The size of the expressed HIV reservoir predicts timing of viral rebound after treatment interruption. AIDS 2016; 30:343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lau JSY, Smith MZ, Lewin SR, McMahon JH. Clinical trials of antiretroviral treatment interruption in HIV-infected individuals. AIDS 2019; 33:773–91. [DOI] [PubMed] [Google Scholar]
- 8. Clarridge KE, Blazkova J, Einkauf K, et al. Effect of analytical treatment interruption and reinitiation of antiretroviral therapy on HIV reservoirs and immunologic parameters in infected individuals. PLoS Pathog 2018; 14:e1006792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barouch DH, Deeks SG. Immunologic strategies for HIV-1 remission and eradication. Science 2014; 345:169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Collins DR, Gaiha GD, Walker BD. CD8+ T cells in HIV control, cure and prevention [published online ahead of print 12 February 2020]. Nat Rev Immunol doi: 10.1038/s41577-020-0274-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Namazi G, Fajnzylber JM, Aga E, et al. The control of HIV after antiretroviral medication pause (CHAMP) study: posttreatment controllers identified from 14 clinical studies. J Infect Dis 2018; 218:1954–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Colby DJ, Trautmann L, Pinyakorn S, et al. ; RV411 Study Group Rapid HIV RNA rebound after antiretroviral treatment interruption in persons durably suppressed in Fiebig I acute HIV infection. Nat Med 2018; 24:923–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sneller MC, Justement JS, Gittens KR, et al. A randomized controlled safety/efficacy trial of therapeutic vaccination in HIV-infected individuals who initiated antiretroviral therapy early in infection. Sci Transl Med 2017; 9:eaan8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Perelson AS, Essunger P, Cao Y, et al. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature 1997; 387:188–91. [DOI] [PubMed] [Google Scholar]
- 15. Johnston RJ, Comps-Agrar L, Hackney J, et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell 2014; 26:923–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.