Abstract
Ischemic diseases, especially in the heart and the brain, have become a serious threat to human health. Growth factor and cell therapy are emerging as promising therapeutic strategies; however, their retention and sustainable functions in the injured tissue are limited. Self-assembling peptide (SAP)-based hydrogels, mimicking the extracellular matrix, are therefore introduced to encapsulate and controllably release cells, cell-derived exosomes or growth factors, thus promoting angiogenesis and tissue recovery after ischemia. We will summarize the classification, composition and structure of SAPs, and the influencing factors for SAP gelation. Moreover, we will describe the functionalized SAPs, and the combinatorial therapy of cells, exosomes or growth factors with functionalized SAPs for angiogenic process as well as its advantage in immunogenicity and injectability. Finally, an outlook on future directions and challenges is provided.
Keywords: self‐assembling peptide, hydrogel, angiogenesis, survival, retention
Introduction
Ischemic diseases in various tissues, such as heart, brain, limb and skin, have severely threatened the life quality and health conditions of human beings, and brought huge economic burdens worldwide. Therapeutic strategies focus on the acceleration of tissue repair by promoting new vessel formation, the process of which consists of stimulation of endothelial cells (ECs) by angiogenic factors; degradation of extracellular matrix (ECM) via extracellular proteinases such as matrix metalloproteinases (MMPs); capillary sprouting and EC migration via integrins; and vessel maturation mediated by growth factors.1–3 Currently, stem cells and growth factors are proven to be effective in promoting angiogenesis and tissue recovery after ischemia despite the low retention rate.4,5 In addition, ECM plays an important role in the angiogenic process through sequestering growth factors and mediating signal transduction.6 It is imperative to replenish a huge amount of ECM that is seriously destructed in ischemia. Facing these obstacles, biomaterials are specifically designed to promote angiogenesis, mimicking the natural features of ECM on the one hand, and encapsulating and sustainably releasing the bioactive agents on the other.7,8
Hydrogels are widely used in the therapeutics of ischemic diseases experimentally and clinically.7,9 Different hydrogelators can form hydrogels, including natural biological molecules (chitosan, hyaluronic acid, collagen, fibroin or acellular ECM), synthetic molecules (polyethylene glycol, polyacrylamide, polymethacrylamide) and supramolecular hydrogelators (self-assembling peptide, nucleobases or monosaccharide).10,11 Self-assembling peptide (SAP) hydrogels, as a supramolecular hydrogelator, have attracted much attention for their bioactivity, biocompatibility, biodegradability and tunable mechanical stability.12–14 SAPs, as a tailor-made material, can be designed with angiogenic bioactivity aiming to enhance neovascularization.15–17 More importantly, the structurally changeable properties of SAPs make them compatible to encapsulate cells, exosomes and growth factors.18–20 In our review, we summarize the classification, composition and structure, and the influencing factors for SAPs. Moreover, we will describe the functionalized SAPs, the combinatorial therapy of cells, exosomes or growth factors with functionalized SAPs and the advantage of using SAPs for angiogenic process as well as its advantage in immunogenicity and injectability.
Self-Assembling Peptide Hydrogels
With building blocks including the natural amino acids and/or hydrophobic fatty acid chain, self-assembling peptides, as one of the supramolecular hydrogelators, can be self-organized into ordered nanofibers and further into well-ordered scaffolds in water. Since their discovery, diverse classes of short SAPs have been invented with a wide range of applications, including 3D tissue cell culture, reparative and regenerative medicine, tissue engineering and slow drug release.21 We will elaborate the physical features of SAPs, which lays the foundation of SAP bioactivity, biocompatibility and biodegradability.
Classification of Self-Assembled Peptides
Depending on different chemical or biological modifications, SAPs can be categorized into native peptides, peptides with terminal acetylation (or formylation) and amidation, peptides modified with an alkyl chain, peptide derivatives containing an aromatic group (fluorene-based, naphthalene-based22 and pyrene-based23) and peptide derivatives containing a photosensitive group. Chemical modification of peptides, such as terminal acetylation and amidation, or attaching an alkyl chain, could change the solubility and stability of SAPs. Self-complementary ionic peptides are peptides with N-terminal acetylation (or formylation) and C-terminal amidation; peptide amphiphiles are hydrogels modified by an alkyl chain. Many native peptides consist of different numbers of amino acids, from dipeptide and tripeptide to hexadecapeptide. The length of the peptides is correlated with the strength of intermolecular interactions. Currently, the longest synthesized peptide consists of 30 amino acids (CKQLEDKIEELLSKAACKQLEDKIEELLSK).24 In our review, we focus on two classes of SAPs, peptide amphiphiles and self-complementary ionic peptides, which are broadly used in angiogenesis research.25
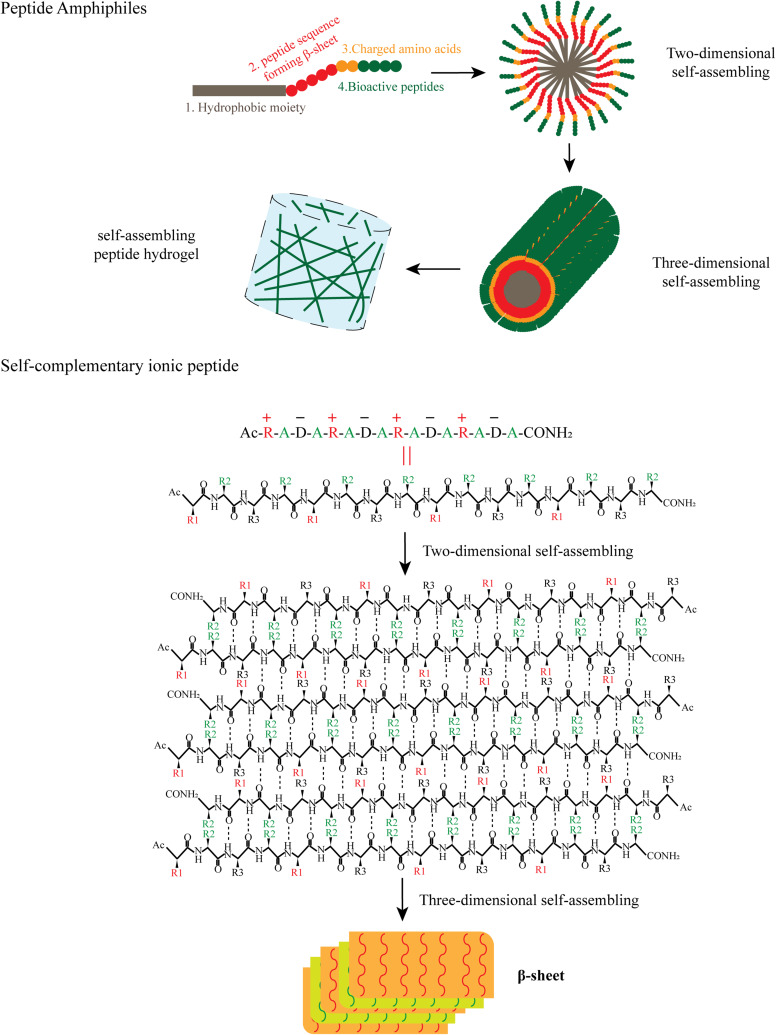
Peptide amphiphiles (PA) and self-complementary ionic peptides are the major classes of self-assembling peptides, sharing many similarities in their composition and structure.26 Peptide amphiphiles can be designed with four domains as shown in Figure 1. The first domain is a hydrophobic moiety, which is usually an unbranched alkyl group, such as palmitic acid. Occasionally, fluorenylmethoxycarbonyl (Fmoc) and 2-(naphthalen-2-yl) acetic acid are used as the alternative hydrophobic moiety. The second domain is the β-sheet-forming peptide sequence, which is the central part for nanostructure morphology and can be adjusted to tune the mechanical properties and gelation kinetics. The third domain often consists of one to three charged amino acids to improve aqueous solubility of the PA hydrogel. The fourth domain typically introduces a bioactive signaling peptide, such as the cell-adhesive peptide arginine-glycine-aspartic acid-serine (RGDS). In some instances, PA hydrogels are absent of the third and fourth domains, conferring them enhanced gelling properties as pro-gelators. The four PA domains play different roles in the gelation process (Figure 1). The first domain forms an internal hydrophobic core, while the fourth domain forms an external active group, with the second and third domains adjusting the stability of the hydrophobic core and the solubility of the peptide in aqueous solution, which is then extended to form a 3D nanofiber in water. Subsequently, the nanofibers become longer and interact with the H2O to form into hydrogel through the modification of salt, temperature, Gibbs free energy etc.
Figure 1.
Self-assembling of peptide amphiphiles and RADA16-I. Peptide amphiphiles, hydrophobic moiety including palmitic acid, Fmoc and 2-(naphthalen-2-yl) acetic acid can react with the N terminal of peptide to form the peptide amphiphiles. Peptide amphiphiles self-assemble into nanofibers and hydrogels through hydrophobic interaction and hydrogen bonding. Repeats of alternating ionic hydrophilic and hydrophobic amino acids are distributed in RADA-I. RADA-I self-assembles to form β-sheets and nanofibers, with the hydrophobic alanine sandwiched inside and hydrophilic residues on the outside. R, arginine; A, alanine; D, aspartate; R1, R2, R3, the side chains of arginine, alanine and aspartate.
Self-complementary ionic peptides are another common type of self-assembling peptide, and consist of 50% charged residues.27 These peptides are periodic repeats of alternating ionic hydrophilic and hydrophobic amino acids, forming a β-sheet with two distinct polar and non-polar surfaces. The polar surface of the β-sheet consists of both positive and negative amide acids, while the non-polar surface is composed of hydrophobic amide acids.28,29 The hydrophobic side forms a double sheet on the inside of the fiber to keep away from water, and the hydrophilic side faces outwards interacting with water molecules. For example, RADA16-I (Ac-RADARADARADARADA-NH2) can form stable β-sheet structures in water and spontaneously assemble into nanofiber scaffolds with the aid of hydrophobic interaction and hydrogen bonding (Figure 1). Short bioactive signaling peptides can be added to the middle or the end of self-complementary ionic peptides to emphasize the application related with their respective bioactivities; thus synthesized peptides are called as multidomain peptides (MDP).30
Ultrashort peptides (< 8 amino acids) are more appealing in biomedical applications owing to their easy synthesis, relatively low sensitivity to enzymatic digestion and high biocompatibility.25 Normally, hydrogen bonding, π–π, van der Waals, electrostatic and hydrophobic interactions are responsible for the self-assembly of ultrashort peptides and also long peptides (> 8 amino acids).25 Ultrashort peptides self-assemble into hydrogels depending on their amphiphilicity and/or self-complementarity. Heptapeptide, EDLIIKG and MNFGAFS, and its derived peptide, DLII and NFGAF, can form self-supportive translucent hydrogels with a clear secondary structure change from α-helix to β-sheet during the self-assembly process.31
Multiple Factors Influence Gelation of the Self-Assembling Peptides
The gelation process of SAPs can be affected by two types of factors, including the basic chemical features of the peptides and the environmental factors. We summarize all the factors that influence the gelling ability, degradation rate and mechanical properties of SAPs (Table 1), providing an insight on designing personalized hydrogels. The basic chemical features of the peptides greatly depend on the peptide design itself. The composition and arrangement of amino acids determine the hydrophobicity and solubility of the peptides. Peptides with high hydrophobicity could easily form nanofibers with superior mechanical strength.32 While high percentage of hydrophobic residues, alanine (Ala), valine (Val), isoleucine (Ile), leucine (Leu), tyrosine (Tyr), phenylalanine (Phe), tryptophan (Trp) decreases the solubility of the peptide, charged amine acids are therefore introduced at the end of the SAP to improve its solubility.33, Switching the position of I and L in the hydrophobic tail of an ultrashort peptide (changing LIVAGKC into ILVAGKC) resulted in a gel that demanded a higher peptide concentration for gelation and became less stiff.34 The length of the peptide is another determinant factor for gelation. The intermolecular interactions and physical cross-linking are enhanced for elongated peptides, especially for self-complementary ionic peptides.35 Conformational change or modification of amino acids and peptides is the most common way to increase the stability of the hydrogels. Natural L-amino acids could be replaced by synthetic D-amino acid counterparts, and the resultant D-peptide-based hydrogels proved to be more stable in vivo since D-form peptide bonds resist natural L-enzyme degradation. Capping the N-terminal of SAPs by an acetyl or the C-terminal by an amide, or both ends, could also enhance the self-organization into hydrogels in water.36
Table 1.
Summary of Influencing Factors for SAP Gelation
| Influencing Factors | Mechanisms | References |
|---|---|---|
| Peptide-related factors | ||
| Peptide Hydrophobicity | Peptide with high hydrophobicity is difficult to dissolve, but easier to gelate. | (Banwell et al, 2009)32 |
| Composition of Amino Acids | Peptides with high ratio of hydrophobic residues form hydrogels with better mechanical properties. | (Lutolf et al, 2003)33 |
| Peptide Concentration | Higher concentration benefits gelling. | (Hauser and Zhang, 201021; Du et al, 201512) |
| Peptide Length | Longer peptide benefits gelling. | (Fletcher et)35 |
| Amino Acid Chirality | D-peptide-based hydrogel is more stable than natural L-amino acid-based peptide. | (Schutz et al, 2015)12 |
| Peptides with Capped N- and C-Terminals | Peptides with capped N- and C-Terminals benefit gelling. | (Solaro, 2010)36 |
| Environment-related factors | ||
| Salt | Salt changes the ionic strength, thus inducing noncovalent interactions among peptides. | (Ozbas et al, 200438; Feng et al, 201237) |
| Temperature | Heating and cooling achieve highly ordered hydrogel, especially entropy-driven assembled hydrogel. | Du et al, 201512 |
| Sonication | Ultrasound breaks self-locked intramolecular hydrogen bonds or π stacking, and interlocked structures between peptide and water molecule are formed. | (Yokoi et al, 200541; Pappas et al, 201540) |
| pH | pH can affect protonation/deprotonation of basic or acidic groups in peptide. | (Hutchinson etal, 201942; Lopez-Silva etal, 2019)30 |
| Photochemical | Photo affects the gelling ability inhibited by photo-reactive groups. | (Collier et al, 2001)43 |
| Enzyme | Enzyme cleaves the enzyme-sensitive peptide to remove peptide-inhibited gelation and accelerates the degradation of SAP hydrogel. | (Lian et al, 2016)44 |
Various environmental factors can stimulate the hydrogelation of the SAPs. The gelation process starts when Gibbs free energy turns negative under the stimulation of either physical or chemical methods. Modulating the ionic strength, changing the temperature and applying ultrasound are commonly used methods.12 Changing the ionic strength by adding polyvalent cations or polyvalent anions, such as Ca2+, Mg2+, or PO43-, can induce the gelation of SAPs.37,38 For example, addition of PO43− to the Ac-KKSLSLSLSLSLSLKK-NH2 solution increased the length of the nanofibers and the storage modulus.12 In another example, PELELELELELEP peptide, containing only negatively charged residues, formed hydrogels with an almost 10-fold increase of storage modulus in the presence of calcium ion.12, In addition, temperature obviously affects the structure and mechanical properties of hydrogels. It was reported that peptide nanofibers assemble faster and more strongly at 25°C than at 5°C.39 Application of sonication is another way to stimulate gelation. The disarranged nanofibers could be disrupted by sonication, and form arranged and longer nanofibers energetically favorable and further gelate.40,41, A pH change could also stimulate the gelation by changing the protonation/deprotonation of basic or acidic groups.30,42 The gelation of smart hydrogel, with the photo-reactive or enzyme-sensitive peptide introduced inside, could be stimulated by treatment with photochemicals or enzymes.43,44 Thus, the designable and adjustable mechanical properties make the self-assembling peptide a better alternative in tissue engineering and angiogenesis.
Self-Assembling Peptides in Angiogenesis
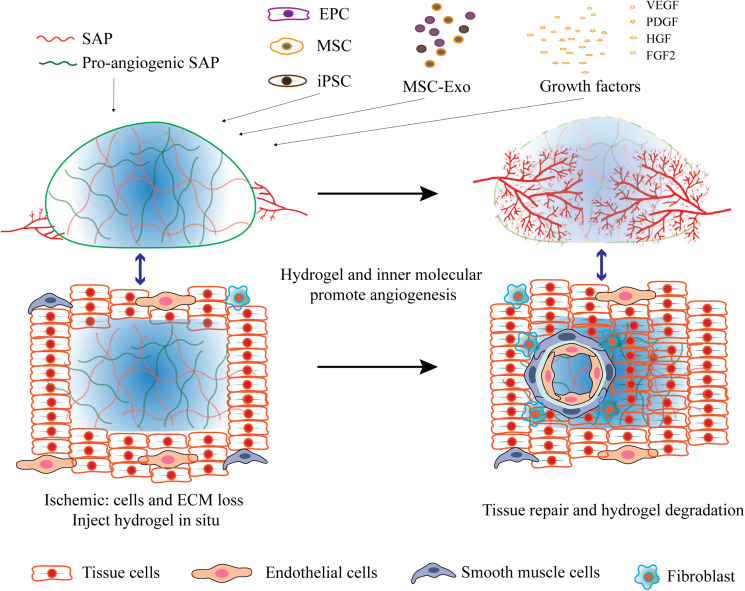
Angiogenesis is a complicated process involving several chronological steps. Angiogenic factors, MMPs and MMP-induced ECM degradation play important roles in EC migration and vessel maturation. Molecular manipulation of SAPs could mimic the biological feature of ECM and facilitate these steps: integration of MMP-sensitive motifs increases the biodegradability of SAPs; employment of cell-adhesive ligands facilitates EC adhesion and migration; introduction of growth factor-mimicking peptides initiates angiogenesis and accelerates vessel maturation. Another well-established approach is the encapsulation of cells, cell-derived exosomes or growth factors by SAP hydrogels (Figure 2), which sustains the constant release of cell secretome in situ to promote angiogenesis.
Figure 2.
Self-assembling peptides in angiogenesis. Hydrogels formed by pro-angiogenic SAPs, or combined with pro-angiogenic cells, exosomes and growth factors promote angiogenesis.
Molecular Modification of Self-Assembling Peptides
A dominant feature of SAPs is the ability to append functional domains, such as MMP-sensitive peptides, cell affinity peptides and proangiogensis peptides, onto their termini without disrupting the assembly properties of the original peptide (Table 2). In this way, SAPs appended with functional domains can provide scaffolds mimicking the native ECM structure and enhancing specific cellular responses as well (Figure 2).
Table 2.
Summary of Pro-Angiogenic Modifications in SAP Hydrogels
| Peptide Sequences | Pro-Angiogenic Modifications | Applications | References |
|---|---|---|---|
| RADA16-I and RADA16-I -SVVYGLR (10:1) | SVVYGLR | Facilitate angiogenesis and neurogenesis at the brain injury site | (Wang et al, 2017)77 |
| RADA16-II andRADA16-II-substance P (200:1) | Substance P | Promote angiogenesis | (Im et al, 2018)63 |
| RADA-I-GPRGDSGYRGDS or RADA-I-KLTWQELYQLKYKGI | PRGDSGYRGDS, KLTWQELYQLKYKGI | Improve angiogenesis in chicken embryo chorioallantoic membrane | (Liu et al, 2012)49 |
| KKSLSLSLSLSLSLKK | Highly vascularized after subcutaneous injection | Du et al, 201512 | |
| KSLSLSLRGSLSLSLKGRGDS or KSLSLSLRGSLSLSLKGKLTWQELYQLKYKGI | LRG, RGDS, KLTWQELYQLKYKGI | Promote tissue regeneration in ischemic tissue disease | (Kumar et al, 2015)16 |
| KSLSLSLRGSLSLSLK–G–KLTWQELYQLKYKGI | LRG, KLTWQELYQLKYKGI | Promote recovery from traumatic brain injury | (Ma et al, 2020)15 |
Abbreviations: SVVYGLR, high affinity for integrin α9β1 and α4β1; PRGDSGYRGDS, cell adhesion peptide; KLTWQELYQLKYKGI, pro-angiogenic sequence; LRG, MMP2-sensitive peptide.
MMPs are multifunctional enzymes capable of cleaving the ECM components including collagens, laminin, fibronectin and many others. The gelatinase MMP2 can recognize and cleave sequences at a defined motif with three prioritized amino acids, with the first amino acid hydrophobic, the second amino acid either hydrophobic or basic and a small residue (alanine, glycine or serine) at the third position.45 LRG in KSLSLSLRGSLSLSLKG, LIG in GTAGLIGQ and KLDLPVGLIGKLDL, and PVG in KLDLPVGLIGKLDL are in line with the requirement of the prioritized amino acid motif and are introduced in many cases as the MMP2-sensitive sequence. For instance, appending the MMP-sensitive sequence, like LIG or LRG, to the middle of SAPs can accelerate the degradation of hydrogels facilitating the cell migration.46
Cell-adhesive ligand Arg-Gly-Asp-Ser (RGDS) is identified as the key sequence in the ECM proteins including fibronectin, vitronectin and laminin. RGDS is sufficient and indispensable for cell membrane binding by interacting with membrane protein integrins.47 Cell affinity peptides containing the RGD motif conjugated with SAPs can strengthen the binding of peptides to endothelial cells, therefore enhancing capillary sprouting as ECM is degraded. For example, RGDS-modified hydrogels could greatly promote cell adhesion and survival.19 In addition, the functional motif PRGDSGYRGDS, containing two similar RGD motif sequences PRGDS and YRGDS, has been shown to distinctly promote the survival, proliferation, migration and morphological differentiation of human umbilical vein endothelial cells (HUVECs).48,49
Pro-angiogenesis peptides, such as QHREDGS, SVVYGLR and KLTWQELYQLKYKGI, can also conjugate with SAPs, promoting growth and migration of ECs via transferring of pro-angiogenic signals. For example, the QHREDGS sequence, as the integrin-binding site in angiopoietin-1, could enhance EC survival, tube formation and cell barrier functionality.50,51 QHREDGS-RADA16-I hydrogel was reported to promote angiogenesis, thereby reducing scar size and restoring cardiac function after MI.50 Another reported sequence, SVVYGLR, derived from osteopontin, could also stimulate angiogenesis in vitro and in vivo.52 The SVVYGLR motif conjugated to the terminal of RADA16 increased the adhesion, migration and tube formation of ECs.53 Notably, VEGF-mimicking peptide KLTWQELYQLKYKGI-modified RADA16 scaffolds could increase HUVEC survival and attachment when compared to pure RADA16 and induce morphologies similar to Matrigel and collagen.49 GHRPS, belonging to the group of growth hormone-releasing peptides, was also reported to activate pro-survival pathways such as PI-3K/AKT1, increase angiogenesis and reduce inflammation by inhibiting the NF-κB pathway.54 The last example discussed here is multidomain peptides. A single SAP could be functionalized with multiple peptide domains, like C16-GTAGLIGQ-RGDS, KSLSLSLRGSLSLSLKGRGDS, or KSLSLSLRGSLSLSLKG-KLTWQELYQLKYKGI, conferring on them enhanced angiogenic capabilities.8
Encapsulation of Cells and Exosomes by Self-Assembling Peptide Hydrogels
The crucial roles of various cell types in angiogenesis under different ischemic conditions are well established.8 Endothelial progenitor cells (EPCs) improve local angiogenesis through direct contribution to neovascularization and releasing paracrine factors.55,56 Mesenchymal stem cells (MSCs) can also improve neovascularization in a paracrine stimulation of angiogenesis and regulation of angiogenic molecules.57 Induced pluripotent stem cells (iPSCs) showed substantial beneficial effects on the angiogenic process through differentiation into endothelial cells or mesenchymal stem cells. Exosomes, as important secretes vesicles, exhibited similar therapeutic effects compared to their derived cells.58–61 Importantly, the exosomes do not trigger immune responses in the host, avoiding the risk of rejection in stem cell transplantation. However, both cells and exosomes only demonstrate moderate angiogenesis in vivo due to the low retention rate in ischemic tissue and the high clearance rate caused by activated systemic immune response.4,5 Currently, SAP hydrogels have attracted much attention since they could encapsulate and protect the cells from a harsh microenvironment, significantly improving the cell viability and the retention time in vivo, and ensuring the continuous and steady release of cells and their derivatives (Figure 2).
Among the SAPs for cell encapsulation, the most widely studied are self-complementary ionic peptides RADA16-I and RADA16-II (Table 3). RADA16-I hydrogel-encapsulated microvascular cells promoted angiogenesis in spinal cord injury by reducing inflammation and glial scar formation.17 Combinatorial therapy with adipose-derived stem cells (ADSCs) and RADA16-II hydrogel demonstrated a three-fold increase in survival and retention of transplanted cells, a 54.25 ± 4.42% increase in the ejection fraction, and more established vascular networks than did administration of ADSC alone.62 Moreover, RADA16 is easily functionalized using biologically active epitopes. The functionalized RADA16 scaffolds encapsulating BMSCs demonstrated beneficial effects in both myocardial infarction and skin defect.50,53,63 For instance, BMSCs encapsulated in QHREDGS-modified RADA16-I hydrogel effectively reduced cell apoptosis and improved cardiac function in the post-MI heart by activating the miR-21-related signaling pathway.50
Table 3.
Summary of Cell, Exosomes and Growth Factors Encapsulated by SAP Hydrogels
| Peptide Sequences | Pro-Angiogenic Modification | Pro-Angiogenic Factors | Application | References |
|---|---|---|---|---|
| Cells encapsulated in hydrogel | ||||
| RADA16-I | Microvascular cells | Promote repair of spinal cord injury | (Tran et al, 2020)17 | |
| RADA16-I and QHREDGS-modified RADA16-I (1:1) | QHREDGS | Rat BMSCs | Improve angiogenesis and cardiac function after MI | (Cai et al, 2019)50 |
| RADA16-I -SVVYGLR | SVVYGLR | Rat BMSCs | Promote angiogenesis and cardiac repair after MI | (Gao et al, 2017)53 |
| RADA16-II | ADSCs | Promote angiogenesis and preserve cardiac function in MI | (Kim et al, 201762 | |
| RADA16-II and RADA16-II-substance P (200:1) | Substance P | Human dermal fibroblasts | Promote angiogenesis and recovery of skin defect | (Im et al, 2018)63 |
| C16-GTAGLIGQ-RGDS | LIG, RGDS | CMs from mESCs | Promote cardiac repair | (Ban et al, 2014)64 |
| C16-GTAGLIGQ-RGDS | LIG, RGDS | hPSC-CDH5+ cells | Promote vascular regeneration in hindlimb ischemia | (Lee et al, 2017)19 |
| Exosomes encapsulated in hydrogel | ||||
| Ac-KLDLPVGLIGKLDL-CONH2 | LIG | Exosomes from mouse BMSCs | Decrease chronic renal fibrosis in I/R mice | (Zhou et al, 2019)20 |
| NapFF and C16-GTAGLIGQ-GG-GHRPS | LIG, GHRPS | Exosomes from human UMSCs | Promote cardiac repair after MI | (Han et al, 2019)66 |
| Proteins/Peptides encapsulated in hydrogel | ||||
| Attaching the LRKKLGKA to RADA16-I | LRKKLGKA | VEGF | Improve cardiac function after MI | (Guo et al, 2012)18 |
| RADA16-I-GGQQLK or RADA16-I-GGLRKKLGKA | LRK | VEGF and HGF | Promote angiogenesis in minimally invasive surgery, ischemic tissue disorders and chronic wound healing | (Huang et al, 2019)69 |
| M-RADA16-II (H2N-RARADADARARADADA-OH) | Notch ligand Jagged-1 mimics | Improve cardiac function after MI | (Boopathy et al, 2015)67 | |
| KSLSLSLRGSLSLSLKGRGDS | LRG, RGDS | TGFβ1, FGF2, VEGF | Promote regeneration of endodontics | (Galler et al, 2012)68 |
Notes: In this Table, green color labels bioactive peptides, and red color labels MMP2-sensitive peptides.
Abbreviations: RADA16-I, AcN-RADARADARADARADA-CONH2; RADA16-II, AcN-RARADADARARADADA-CONH2, C16, palmitic acid; QHREDGS, prosurvival peptide; SVVYGLR, high affinity for integrin; Substance P, an 11-amino acid neuropeptide extensively found in nervous systems, RPKPQQFFGLM; RGDS, high cell adhesion peptide; LRG and LIG, MMP2-sensitive peptide; LRKKLGKA, heparin-binding; LRK, affinity to proteoglycan heparan sulfate; Notch ligand Jagged-1 mimic, H2N-CDDYYYGFGCNKFCRPR-OH; GHRPS, prosurvival peptide; BMSCs, bone marrow-derived mesenchymal stem cell; ADSC, adipose-derived stromal cells; UMSCs, umbilical cord mesenchymal stem cell; CMs, cardiomyocytes; mESCs, mouse embryonic stem cells; hPSC-CDH5+ cells, human pluripotent stem cell-derived endothelial cells with CDH5+ expression; EV, extracellular vesicle; TGFβ1, transforming growth factor beta 1; FGF2, basic fibroblast growth factor; VEGF, vascular endothelial growth factor; MI, myocardial infarction; HI, ischemic hindlimbs.
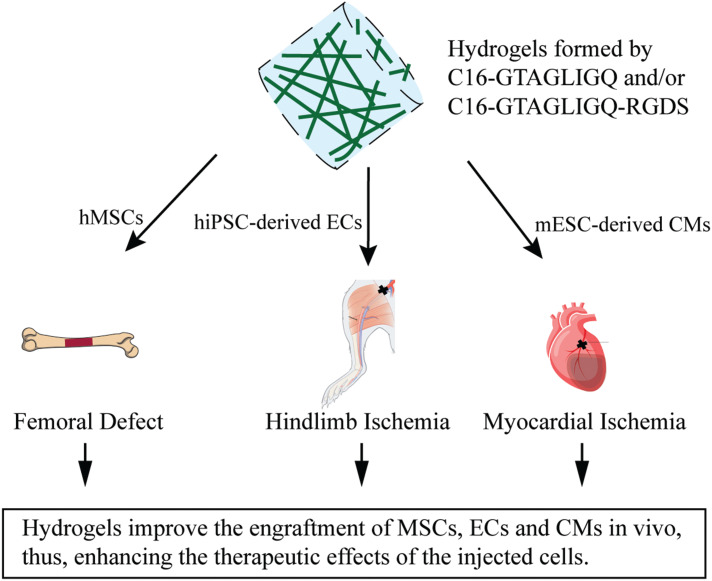
Peptide amphiphile hydrogels are also used to encapsulate and protect transplanted cells (Table 3). Notably, mESCs-derived cardiomyocytes encapsulated in C16-GTAGLIGQ-RGDS demonstrated approximately 3-fold higher engraftment in hearts, and sustained better cardiac function after MI for 12 weeks (Figure 3).64 C16-GTAGLIGQ-RGDS hydrogel could also protect hiPSC-derived ECs under oxidative stress and improve structural neovascularization for up to 10 months (Figure 3).19 In addition, C16-GTAGLIGQ-RGDS hydrogel was also reported to induce the osteogenic differentiation of engrafted hMSCs (Figure 3).65 As a carrier, PA hydrogels have also been used to deliver exosomes. NapFF and PA-GHRPS hydrogels were able to effectively deliver exosomes and sustain the stable release of exosomes, thus promoting angiogenesis in MI hearts.66
Figure 3.
Hydrogels improved the engraftment of hMSCs, ECs or CMs and enhanced their therapeutic effects in ischemia.
Abbreviations: C16, palmitic acid; hMSCs, human mesenchymal stem cells; ECs, endothelial cells; mESCs, mouse embryonic stem cells; CMs, cardiomyocytes.
Encapsulation of Growth Factors by Self-Assembling Peptide Hydrogels
The loss of extracellular matrix and growth factors in an ischemic region makes the adjacent cells lose cell–cell contact and changes the microenvironment. A SAP hydrogel could act as a scaffold for stable release of growth factors. Owing to their tunable physical properties, hydrogels can provide spatial and temporal control over the release of various growth factors, such as VEGF, VEGF165, VEGF121, FGF, HGF, HIF and PDGF (Figure 2). VEGF and HGF delivered by functionalized RADA16-I, RADA16-II or KSLSLSLRGSLSLSLKGRGDS could enhance angiogenesis and functional recovery in ischemic diseases (Table 3).67–69 For example, a LRKKLGKA-conjugated RADA16 hydrogel provided the constant release of VEGF for up to one month, thus greatly improving cardiac function after MI.18 Interestingly, the SAP hydrogel with two sequences of RADA16-GGQQLK (QLK) and RADA16-GGLRKKLGKA (LRK) can be crosslinked and strengthened by transglutaminase to prolong the degradation rate of VEGF and HGF.69 An ultrashort peptide, naphthyl group-linked Phe–Phe dipeptide (NapFF), being introduced with RGD domain and followed by encapsulation with VEGF, greatly improved cell adhesion and cell growth and triggered angiogenesis in vivo when subcutaneously injected in mice.70 It was also reported that when insulin-like growth factor-I (IGF-1)-derived peptide, GYGSSSRRAPQT, was introduced into the ultrashort peptide NapFF, the β-sheet structure of the combined peptide efficiently activated the IGF-1 downstream pathway and significantly promoted angiogenesis.71
Advantages and Application of SAP Hydrogels in Angiogenesis
Self-assembling peptides possess designable immunogenicity and minimally invasive injectability, which make SAP-based hydrogel a better candidate in biomedical application. Amino acid composition, peptide modification as well as surface charge are the most important factors for the immunogenicity of SAPs. Self-assembling peptide OVA-Q11 caused strong T-cell-dependent antibody responses in mice. However, when OVA-Q11 was conjugated to non-antigenic peptides RGD, the immune responses were substantially decreased. Another study also showed that a peptide amphiphile hydrogel induced no inflammation or autoimmune response after modification with IKVAV in mice spinal cord injury.72,73 Change of surface charge provides a strategy to switch off potentially problematic immunogenicity into nonimmunological application.74 For instance, negative surface charge of fibrillized SAP completely abolished T-cell responses in mice. Anionic aspartic acid and glutamic acid-based SAP surface provokes a low inflammatory response, cationic lysine-based SAP surface elicits a mild inflammatory response, while cationic arginine-based SAP surface provokes a stronger inflammatory response.29 The immunogenicity of self-assembling peptide hydrogel is adjustable according to different applications such as vaccine adjuvant and angiogenesis. Naphthalene peptide Nap-GFFY has been used as the adjuvant to induce CD8+ T-cell response. Moreover, Nap-GDFDFDY, formed by D-type amino acids, is able to induce a stronger CD8+ T-cell response compared with.75 Nap-GFFpY-OMe (naphthylacetic acid-modified phosphorylated tetra-peptide of GFFpY with C-terminal methyl ester group) has been used to co-assemble with HIV DNA molecules and enhance both humoral and cellular immune response against HIV.76 When D-tetrapeptide (GDFDFDY)-based supramolecular hydrogelators are linked with different hydrophobic domains, such as flurbiprofen (Fbp), carprofen (Car), naproxen (Npx), Fbp- and Car-gels, they exhibit excellent tumor elimination properties in both protective and therapeutic immune assays.77
A better understanding of the chemical and physical properties of SAPs is imperative for developing the delivery method of hydrogels. Hydrogen bonding and hydrophobic interactions are the main driving forces for SAP gelation. Peptides can easily associate and disassociate based on temperature and pH. Therefore peptide fibers are able to easily break and re-form.12 This equilibrium allows the preformed hydrogels to shear thin and shear recover readily, such that preformed hydrogels can be injected by application of shear stress (during injection) and quickly self-heal after removal of shear. Besides, some injectable liquid SAPs can form into hydrogels upon stimulation by salt ions in the physiological microenvironment of the target tissue. Interestingly, smart hydrogel has been designed to adapt to complex repair processes. For instance, adding aniline into ultrashort peptide Fmoc-FF can make the hydrogel conductive,78 which supports cardiomyocyte organization into a spontaneously contracting system.78 A multiphase transitioning peptide hydrogel, with the sequence of Ac-VKVKVKGKVDPPTKXEVKVKV−NH2 (X stands for photocaged MNI-glutamic acid), was reported to have been injected into the lumen of vessels to facilitate suturing. The multiphase transitioning peptide forms solid gel in a syringe and can be delivered to the lumen of collapsed vessels to distend the vessel by shear-thin force, and the space between two vessels to approximate the vessel ends. After exposing the small vessel to light, the hydrogel network in the lumen will be disrupted, leading to gel-sol phase transition, gel removal and blood flow resumption.79 In a word, the injectability of SAP hydrogels makes them the superior therapeutic scaffolds with minimal invasiveness and applicable in a variety of clinical applications.8,12,16,80
Currently, many peptide-based hydrogels such as Puramatrix (RADA16-I) are commercially available and used for 3D cell culture to support stem cells in the repair of tissue injury.81 Some hydrogels have been proved in preclinical use; for example, direct myocardial injection of 1% SAP (AcN-RARADADARARADADA-CNH2) with PDGF-BB decreased infarct size after ischemia/reperfusion through activating Akt phosphorylation in cardiomyocytes.82 Moreover, some hydrogels are in clinical trial. For instance, P11-4 (Ac-QQRFEWEFEQQ-NH2), a synthetic α-peptide that can be self-assembled into β-sheet amyloids with a hydrogel appearance at low pH, is being used in biomimetic mineralization, enamel regeneration and oral care agent.83
Summary and Perspective
In conclusion, SAPs hold great promise owing to their bioactivity, biocompatibility and biodegradability. Their tunable physical properties make them injectable, functional and compatible for encapsulation and controlled release of cells, exosomes and growth factors. Smart hydrogel, which is responsive to the in vivo environment through internal or external stimuli, might represent a future direction. Besides, it is promising to design a complex scaffold system that is suitable for heterogeneous cell populations, and able to dynamically change the composition and physical properties. Furthermore, a molecularly sophisticated SAP hydrogel has been developed to direct migration of stem cells to damaged areas, but the desired homing and in situ assembly still need to be explored. Overall, self-assembling peptide-based hydrogel has a bright future to serve as an effective treatment for a broad range of ischemic diseases. Since the research on the self-assembling peptide hydrogel in angiogenesis is in its infancy, many challenges need to be overcome. The main challenges are how to match the mechanical properties of hydrogel to the natural ECM, and how to precisely control degradation speed of the hydrogel and release speed of the internal molecules or cells. There is still a long way to transform the self-assembling peptide-based hydrogels into clinical applications.
Funding Statement
This review was supported by National Natural Science Foundation of China (No.8197020412) to QM, Scientific Research Projects of Jiangsu Province Department of Health (H2018007) to QM, Wu Jieping Medical Foundation Basic Medical Scientific Research Project (No. 320.6750.15153) to CR, Pre-research fund project of the second affiliated hospital of Soochow university (SDFEYBS1912) to ZZ.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest for this work and declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9(6):653–660. doi: 10.1038/nm0603-653 [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Fundamental concepts of the angiogenic process. Curr Mol Med. 2003;3(7):643–651. doi: 10.2174/1566524033479465 [DOI] [PubMed] [Google Scholar]
- 3.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146(6):873–887. doi: 10.1016/j.cell.2011.08.039 [DOI] [PubMed] [Google Scholar]
- 4.Imai T, Takahashi Y, Nishikawa M, et al. Macrophage-dependent clearance of systemically administered B16BL6-derived exosomes from the blood circulation in mice. J Extracell Vesicles. 2015;4(1):26238. doi: 10.3402/jev.v4.26238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi Y, Nishikawa M, Shinotsuka H, et al. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J Biotechnol. 2013;165(2):77–84. doi: 10.1016/j.jbiotec.2013.03.013 [DOI] [PubMed] [Google Scholar]
- 6.Davis GE, Senger DR. Extracellular matrix mediates a molecular balance between vascular morphogenesis and regression. Curr Opin Hematol. 2008;15(3):197–203. doi: 10.1097/MOH.0b013e3282fcc321 [DOI] [PubMed] [Google Scholar]
- 7.Briquez PS, Clegg LE, Martino MM, Mac Gabhann F, Hubbell JA. Design principles for therapeutic angiogenic materials. Nat Rev Mater. 2016;1(15006). doi: 10.1038/natrevmats.2015.6 [DOI] [Google Scholar]
- 8.Rufaihah AJ, Seliktar D. Hydrogels for therapeutic cardiovascular angiogenesis. Adv Drug Deliv Rev. 2016;96:31–39. doi: 10.1016/j.addr.2015.07.003 [DOI] [PubMed] [Google Scholar]
- 9.Liberski A, Latif N, Raynaud C, Bollensdorff C, Yacoub M. Alginate for cardiac regeneration: from seaweed to clinical trials. Glob Cardiol Sci Pract. 2016;2016(1):e201604. doi: 10.21542/gcsp.2016.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliva N, Conde J, Wang K, Artzi N. Designing hydrogels for on-demand therapy. Acc Chem Res. 2017;50(4):669–679. doi: 10.1021/acs.accounts.6b00536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang YS, Khademhosseini A. Advances in engineering hydrogels. Science. 2017;356(6337):eaaf3627. doi: 10.1126/science.aaf3627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du X, Zhou J, Shi J, Xu B. Supramolecular hydrogelators and hydrogels: from soft matter to molecular biomaterials. Chem Rev. 2015;115(24):13165–13307. doi: 10.1021/acs.chemrev.5b00299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koutsopoulos S. Self-assembling peptide nanofiber hydrogels in tissue engineering and regenerative medicine: progress, design guidelines, and applications. J Biomed Mater Res A. 2016;104(4):1002–1016. doi: 10.1002/jbm.a.35638 [DOI] [PubMed] [Google Scholar]
- 14.Matson JB, Zha RH, Stupp SI. Peptide self-assembly for crafting functional biological materials. Curr Opin Solid State Mater Sci. 2011;15(6):225–235. doi: 10.1016/j.cossms.2011.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma X, Agas A, Siddiqui Z, et al. Angiogenic peptide hydrogels for treatment of traumatic brain injury. Bioact Mater. 2020;5(1):124–132. doi: 10.1016/j.bioactmat.2020.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarkar B, Nguyen PK, Gao W, Dondapati A, Siddiqui Z, Kumar VA. Angiogenic self-assembling peptide scaffolds for functional tissue regeneration. Biomacromolecules. 2018;19(9):3597–3611. doi: 10.1021/acs.biomac.8b01137 [DOI] [PubMed] [Google Scholar]
- 17.Tran KA, Partyka PP, Jin Y, Bouyer J, Fischer I, Galie PA. Vascularization of self-assembled peptide scaffolds for spinal cord injury repair. Acta Biomater. 2020;104:76–84. doi: 10.1016/j.actbio.2019.12.033 [DOI] [PubMed] [Google Scholar]
- 18.Guo H-D, Cui G-H, Yang -J-J, et al. Sustained delivery of VEGF from designer self-assembling peptides improves cardiac function after myocardial infarction. Biochem Biophys Res Commun. 2012;424(1):105–111. doi: 10.1016/j.bbrc.2012.06.080 [DOI] [PubMed] [Google Scholar]
- 19.Lee SJ, Sohn YD, Andukuri A, et al. Enhanced therapeutic and long-term dynamic vascularization effects of human pluripotent stem cell-derived endothelial cells encapsulated in a nanomatrix gel. Circulation. 2017;136(20):1939–1954. doi: 10.1161/CIRCULATIONAHA.116.026329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Y, Liu S, Zhao M, et al. Injectable extracellular vesicle-released self-assembling peptide nanofiber hydrogel as an enhanced cell-free therapy for tissue regeneration. J Control Release. 2019;316:93–104. doi: 10.1016/j.jconrel.2019.11.003 [DOI] [PubMed] [Google Scholar]
- 21.Hauser CA, Zhang S. Designer self-assembling peptide nanofiber biological materials. Chem Soc Rev. 2010;39(8):2780–2790. doi: 10.1039/b921448h [DOI] [PubMed] [Google Scholar]
- 22.Muro-Small ML, Chen J, McNeil AJ. Dissolution parameters reveal role of structure and solvent in molecular gelation. Langmuir. 2011;27(21):13248–13253. doi: 10.1021/la202702r [DOI] [PubMed] [Google Scholar]
- 23.Mukherjee S, Kar T, Das PK. Pyrene-based fluorescent supramolecular hydrogel: scaffold for energy transfer. Research support, Non-U.S. Gov’t.. Chem Asian J. 2014;9(10):2798–2805. doi: 10.1002/asia.201402358 [DOI] [PubMed] [Google Scholar]
- 24.Wagner DEP, Ali CL, Nybakken WM, Crawford GE;. EDS, development of peptide nanofilaments and nanoropes as smart materials. Proc Natl Acad Sci. 2005;102:12656–12661. doi: 10.1073/pnas.0505871102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yadav N, Chauhan MK, Chauhan VS. Short to ultrashort peptide-based hydrogels as a platform for biomedical applications. Review. Biomater Sci. 2020;8(1):84–100. doi: 10.1039/C9BM01304K [DOI] [PubMed] [Google Scholar]
- 26.Eskandari S, Guerin T, Toth I, Stephenson RJ. Recent advances in self-assembled peptides: implications for targeted drug delivery and vaccine engineering. Adv Drug Deliv Rev. 2017;110–111:169–187. doi: 10.1016/j.addr.2016.06.013 [DOI] [PubMed] [Google Scholar]
- 27.Xiumei Wang AH, Zhang S. Designer functionalized self-assembling peptide nanofiber scaffolds for growth, migration, and tubulogenesis of human umbilical vein endothelial cells. Soft Matter. 2008;4:2388–2395.2395. doi: 10.1039/B807155A [DOI] [Google Scholar]
- 28.Nagai Y, Unsworth LD, Koutsopoulos S, Zhang S. Slow release of molecules in self-assembling peptide nanofiber scaffold. J Control Release. 2006;115(1):18–25. doi: 10.1016/j.jconrel.2006.06.031 [DOI] [PubMed] [Google Scholar]
- 29.Zhang H, Park J, Jiang Y, Woodrow KA. Rational design of charged peptides that self-assemble into robust nanofibers as immune-functional scaffolds. Acta Biomater. 2017;55:183–193. doi: 10.1016/j.actbio.2017.03.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopez-Silva TL, Leach DG, Li IC, Wang X, Hartgerink JD. Self-assembling multidomain peptides: design and characterization of neutral peptide-based materials with ph and ionic strength independent self-assembly. ACS Biomater Sci Eng. 2019;5(2):977–985. doi: 10.1021/acsbiomaterials.8b01348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saini A, Chauhan VS. Self-assembling properties of peptides derived from TDP-43 C-terminal fragment. Research support, Non-U S Gov’t. Langmuir. 2014;30(13):3845–3856. doi: 10.1021/la404710w [DOI] [PubMed] [Google Scholar]
- 32.Banwell EF, Abelardo ES, Adams DJ, et al. Rational design and application of responsive alpha-helical peptide hydrogels. Nat Mater. 2009;8(7):596–600. doi: 10.1038/nmat2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lutolf MP, Lauer-Fields JL, Schmoekel HG, et al. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics. Proc Natl Acad Sci U S A. 2003;100(9):5413–5418. doi: 10.1073/pnas.0737381100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seow WY, Salgado G, Lane EB, Hauser CA. Transparent crosslinked ultrashort peptide hydrogel dressing with high shape-fidelity accelerates healing of full-thickness excision wounds. Research support, Non-U S Gov’t. Sci Rep. 2016;6(32670). doi: 10.1038/srep32670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fletcher NL, Paquet N, Dickinson EL, Dexter AF. Bioproduction of highly charged designer peptide surfactants via a chemically cleavable coiled-coil heteroconcatemer. Biotechnol Bioeng. 2015;112(2):242–251. doi: 10.1002/bit.25446 [DOI] [PubMed] [Google Scholar]
- 36.Desii ADFCRDSMRTR, Chiellini F, Di Stefano R, Tiné MR, Solaro R. Hydrogel scaffolds by self-assembly of a complementary ionic tetrapeptide. J Polym Sci A Polym Chem. 2010;48(4):986–990. doi: 10.1002/pola.23841 [DOI] [Google Scholar]
- 37.Feng Y, Taraban M, Yu YB. The effect of ionic strength on the mechanical, structural and transport properties of peptide hydrogels. Soft Matter. 2012;8(46):11723–11731. doi: 10.1039/C2SM26572A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ozbas B, Rajagopal K, Schneider JP, Pochan DJ. Semiflexible chain networks formed via self-assembly of β -hairpin molecules. Phys Rev Lett. 2004;93(26):268106. doi: 10.1103/PhysRevLett.93.268106 [DOI] [PubMed] [Google Scholar]
- 39.Ramachandran S, Taraban MB, Trewhella J, Gryczynski I, Gryczynski Z, Yu YB. Effect of temperature during assembly on the structure and mechanical properties of peptide-based materials. Biomacromolecules. 2010;11(6):1502–1506. doi: 10.1021/bm100138m [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pappas CG, Frederix PWJM, Mutasa T, et al. Alignment of nanostructured tripeptide gels by directional ultrasonication. Chem Commun (Camb). 2015;51(40):8465–8468. doi: 10.1039/c5cc02049b [DOI] [PubMed] [Google Scholar]
- 41.Yokoi H, Kinoshita T, Zhang S. Dynamic reassembly of peptide RADA16 nanofiber scaffold. Proc Natl Acad Sci U S A. 2005;102(24):8414–8419. doi: 10.1073/pnas.0407843102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hutchinson JA, Hamley IW, Edwards-Gayle CJC, et al. Melanin production by tyrosinase activity on a tyrosine-rich peptide fragment and pH-dependent self-assembly of its lipidated analogue. Org Biomol Chem. 2019;17(18):4543–4553. doi: 10.1039/c9ob00550a [DOI] [PubMed] [Google Scholar]
- 43.Collier JH, Hu BH, Ruberti JW, et al. Thermally and photochemically triggered self-assembly of peptide hydrogels. J Am Chem Soc. 2001;123(38):9463–9464. doi: 10.1021/ja011535a [DOI] [PubMed] [Google Scholar]
- 44.Lian M, Chen X, Lu Y, Yang W. Self-assembled peptide hydrogel as a smart biointerface for enzyme-based electrochemical biosensing and cell monitoring. ACS Appl Mater Interfaces. 2016;8(38):25036–25042. doi: 10.1021/acsami.6b05409 [DOI] [PubMed] [Google Scholar]
- 45.Turk BE, Huang LL, Piro ET, Cantley LC. Determination of protease cleavage site motifs using mixture-based oriented peptide libraries. Nat Biotechnol. 2001;19(7):661–667. doi: 10.1038/90273 [DOI] [PubMed] [Google Scholar]
- 46.Galler KM, Aulisa L, Regan KR, D’Souza RN, Hartgerink JD. Self-assembling multidomain peptide hydrogels: designed susceptibility to enzymatic cleavage allows enhanced cell migration and spreading. J Am Chem Soc. 2010;132(9):3217–3223. doi: 10.1021/ja910481t [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Agthoven JF, Xiong JP, Alonso JL, et al. Structural basis for pure antagonism of integrin alphaVbeta3 by a high-affinity form of fibronectin. Nat Struct Mol Biol. 2014;21(4):383–388. doi: 10.1038/nsmb.2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horii A, Wang X, Gelain F, Zhang S, Isalan M. Biological designer self-assembling peptide nanofiber scaffolds significantly enhance osteoblast proliferation, differentiation and 3-D migration. PLoS One. 2007;2(2):e190. doi: 10.1371/journal.pone.0000190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu X, Wang X, Horii A, et al. In vivo studies on angiogenic activity of two designer self-assembling peptide scaffold hydrogels in the chicken embryo chorioallantoic membrane. Nanoscale. 2012;4(8):2720–2727. doi: 10.1039/c2nr00001f [DOI] [PubMed] [Google Scholar]
- 50.Cai H, Wu F-Y, Wang Q-L, et al. Self-assembling peptide modified with QHREDGS as a novel delivery system for mesenchymal stem cell transplantation after myocardial infarction. FASEB J. 2019;33(7):8306–8320. doi: 10.1096/fj.201801768RR [DOI] [PubMed] [Google Scholar]
- 51.Miklas JW, Dallabrida SM, Reis LA, Ismail N, Rupnick M, Radisic M. QHREDGS enhances tube formation, metabolism and survival of endothelial cells in collagen-chitosan hydrogels. PLoS One. 2013;8(8):e72956. doi: 10.1371/journal.pone.0072956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hamada Y, Yuki K, Okazaki M, et al. Osteopontin-derived peptide SVVYGLR induces angiogenesis in vivo. Dent Mater J. 2004;23(4):650–655. doi: 10.4012/dmj.23.650 [DOI] [PubMed] [Google Scholar]
- 53.Gao XR, Xu HJ, Wang LF, Liu CB, Yu F. Mesenchymal stem cell transplantation carried in SVVYGLR modified self-assembling peptide promoted cardiac repair and angiogenesis after myocardial infarction. Biochem Biophys Res Commun. 2017;491(1):112–118. doi: 10.1016/j.bbrc.2017.07.056 [DOI] [PubMed] [Google Scholar]
- 54.Berlanga-Acosta J, Abreu-Cruz A, Herrera DGB, et al. Synthetic Growth Hormone-Releasing Peptides (GHRPs): a historical appraisal of the evidences supporting their cytoprotective effects. Clin Med Insights Cardiol. 2017;11:1179546817694558. doi: 10.1177/1179546817694558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang J, Ii M, Kamei N, et al. CD34+ cells represent highly functional endothelial progenitor cells in murine bone marrow. PLoS One. 2011;6(5):e20219. doi: 10.1371/journal.pone.0020219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.You J, Sun J, Ma T, et al. Curcumin induces therapeutic angiogenesis in a diabetic mouse hindlimb ischemia model via modulating the function of endothelial progenitor cells. Stem Cell Res Ther. 2017;8(1):182. doi: 10.1186/s13287-017-0636-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kasper G, Dankert N, Tuischer J, et al. Mesenchymal stem cells regulate angiogenesis according to their mechanical environment. Research support, Non-U.S. Gov’t. Stem Cells. 2007;25(4):903–910. doi: 10.1634/stemcells.2006-0432 [DOI] [PubMed] [Google Scholar]
- 58.Khan M, Nickoloff E, Abramova T, et al. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ Res. 2015;117(1):52–64. doi: 10.1161/circresaha.117.305990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu L, Jin X, Hu CF, Li R, Zhou Z, Shen CX. Exosomes derived from mesenchymal stem cells rescue myocardial ischaemia/reperfusion injury by inducing cardiomyocyte autophagy via AMPK and Akt pathways. Cell Physiol Biochem. 2017;43(1):52–68. doi: 10.1159/000480317 [DOI] [PubMed] [Google Scholar]
- 60.Radosinska J, Bartekova M. Therapeutic potential of hematopoietic stem cell-derived exosomes in cardiovascular disease. Adv Exp Med Biol. 2017;998:221–235. doi: 10.1007/978-981-10-4397-0_15 [DOI] [PubMed] [Google Scholar]
- 61.Sun J, Zhang Z, Ma T, et al. Endothelial progenitor cell-derived exosomes, loaded with miR-126, promoted deep vein thrombosis resolution and recanalization. Stem Cell Res Ther. 2018;9(1):223. doi: 10.1186/s13287-018-0952-8 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 62.Kim JH, Park Y, Jung Y, Kim SH, Kim SH. Combinatorial therapy with three-dimensionally cultured adipose-derived stromal cells and self-assembling peptides to enhance angiogenesis and preserve cardiac function in infarcted hearts. J Tissue Eng Regen Med. 2017;11(10):2816–2827. doi: 10.1002/term.2181 [DOI] [PubMed] [Google Scholar]
- 63.Im H, Kim SH, Kim SH, Jung Y. Skin regeneration with a scaffold of predefined shape and bioactive peptide hydrogels. Tissue Eng Part A. 2018;24(19–20):1518–1530. doi: 10.1089/ten.TEA.2017.0489 [DOI] [PubMed] [Google Scholar]
- 64.Ban K, Park HJ, Kim S, et al. Cell therapy with embryonic stem cell-derived cardiomyocytes encapsulated in injectable nanomatrix gel enhances cell engraftment and promotes cardiac repair. ACS Nano. 2014;8(10):10815–10825. doi: 10.1021/nn504617g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anderson JM, Kushwaha M, Tambralli A, Bellis SL, Camata RP, Jun HW. Osteogenic differentiation of human mesenchymal stem cells directed by extracellular matrix-mimicking ligands in a biomimetic self-assembled peptide amphiphile nanomatrix. Research support, N I H, extramural research support, non-U S Gov’t. Biomacromolecules. 2009;10(10):2935–2944. doi: 10.1021/bm9007452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Han C, Zhou J, Liang C, et al. Human umbilical cord mesenchymal stem cell derived exosomes encapsulated in functional peptide hydrogels promote cardiac repair. Biomater Sci. 2019;7(7):2920–2933. doi: 10.1039/c9bm00101h [DOI] [PubMed] [Google Scholar]
- 67.Boopathy AV, Martinez MD, Smith AW, Brown ME, Garcia AJ, Davis ME. Intramyocardial delivery of notch ligand-containing hydrogels improves cardiac function and angiogenesis following infarction. Tissue Eng Part A. 2015;21(17–18):2315–2322. doi: 10.1089/ten.TEA.2014.0622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Galler KM, Hartgerink JD, Cavender AC, Schmalz G, D’Souza RN. A customized self-assembling peptide hydrogel for dental pulp tissue engineering. Tissue Eng Part A. 2012;18(1–2):176–184. doi: 10.1089/ten.TEA.2011.0222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang LC, Wang HC, Chen LH, et al. Bioinspired self-assembling peptide hydrogel with proteoglycan-assisted growth factor delivery for therapeutic angiogenesis. Theranostics. 2019;9(23):7072–7087. doi: 10.7150/thno.35803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cheng B, Yan Y, Qi J, et al. Cooperative assembly of a peptide gelator and silk fibroin afford an injectable hydrogel for tissue engineering. ACS Appl Mater Interfaces. 2018;10(15):12474–12484. doi: 10.1021/acsami.8b01725 [DOI] [PubMed] [Google Scholar]
- 71.Shang Y, Zhi D, Feng G, et al. Supramolecular nanofibers with superior bioactivity to insulin-like growth factor-I. Research support, Non-U S Gov’t. Nano Lett. 2019;19(3):1560–1569. doi: 10.1021/acs.nanolett.8b04406 [DOI] [PubMed] [Google Scholar]
- 72.Cui H, Webber MJ, Stupp SI. Self-assembly of peptide amphiphiles: from molecules to nanostructures to biomaterials. Research support, N.I.H., extramural research support, U.S. Gov’t, Non-P.H.S. Review. Biopolymers. 2010;94(1):1–18. doi: 10.1002/bip.21328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tysseling-Mattiace VM, Sahni V, Niece KL, et al. Self-assembling nanofibers inhibit glial scar formation and promote axon elongation after spinal cord injury. Research support, N.I.H., extramural research support, Non-U.S. Gov’t. J Neurosci. 2008;28(14):3814–3823. doi: 10.1523/JNEUROSCI.0143-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wen Y, Waltman A, Han H, Collier JH. Switching the immunogenicity of peptide assemblies using surface properties. ACS Nano. 2016;10(10):9274–9286. doi: 10.1021/acsnano.6b03409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Luo Z, Wu Q, Yang C, et al. A powerful CD8(+) T-cell stimulating D-tetra-peptide hydrogel as a very promising vaccine adjuvant. Adv Mater. 2017;29(5):14. doi: 10.1002/adma.201601776 [DOI] [PubMed] [Google Scholar]
- 76.Tian Y, Wang H, Liu Y, et al. A peptide-based nanofibrous hydrogel as a promising DNA nanovector for optimizing the efficacy of HIV vaccine. Research support, Non-U S Gov’t. Nano Lett. 2014;14(3):1439–1445. doi: 10.1021/nl404560v [DOI] [PubMed] [Google Scholar]
- 77.Wang Z, Liang C, Shi F, et al. Cancer vaccines using supramolecular hydrogels of NSAID-modified peptides as adjuvants abolish tumorigenesis. Nanoscale. 2017;9(37):14058–14064. doi: 10.1039/C7NR04990K [DOI] [PubMed] [Google Scholar]
- 78.Chakraborty P, Guterman T, Adadi N, et al. A self-healing, all-organic, conducting, composite peptide hydrogel as pressure sensor and electrogenic cell soft substrate. Research support, Non-U S Gov’t. ACS Nano. 2019;13(1):163–175. doi: 10.1021/acsnano.8b05067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smith DJ, Brat GA, Medina SH, et al. A multiphase transitioning peptide hydrogel for suturing ultrasmall vessels. Research support, N I H, Intramural. Nat Nanotechnol. 2016;11(1):95–102. doi: 10.1038/nnano.2015.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Matson JB, Stupp SI. Self-assembling peptide scaffolds for regenerative medicine. Chem Commun (Camb). 2012;48(1):26–33. doi: 10.1039/c1cc15551b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aligholi H. P4: puraMatrix supports neural stem cells to repair brain injury. Neurosci J Shefaye Khatam. 2017;4(S2):26. [Google Scholar]
- 82.Hsieh PC, Davis ME, Gannon J, MacGillivray C, Lee RT. Controlled delivery of PDGF-BB for myocardial protection using injectable self-assembling peptide nanofibers. Research support, N I H, extramural research support, Non-U S Gov’t. J Clin Invest. 2006;116(1):237–248. doi: 10.1172/JCI25878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jablonski-Momeni A, Korbmacher-Steiner H, Heinzel-Gutenbrunner M, Jablonski B, Jaquet W, Bottenberg P. Randomised in situ clinical trial investigating self-assembling peptide matrix P11-4 in the prevention of artificial caries lesions. randomized controlled trial research support, Non-U S Gov’t. Sci Rep. 2019;9(1):018–36536. [DOI] [PMC free article] [PubMed] [Google Scholar]