ABSTRACT
MicroRNAs (miRNAs) are post-transcriptional regulators of gene expression which act by guiding AGO (argonaute) proteins to target RNA transcripts in the RNA-induced silencing complex (RISC). This macromolecular complex includes multiple additional components (e.g., TNRC6A) that allow for interaction with enzymes mediating inhibition of translation or RNA decay. However, miRNAs also reside in low-molecular weight complexes without being engaged in target repression, and their function in this context is largely unknown. Our recent findings show that endothelial cells exposed to protective high-shear stress or MTORC inhibition activate the macroautophagy/autophagy machinery to sustain viability by promoting differential trafficking of MIR126 strands and by enabling unconventional features of MIR126-5p. Whereas MIR126-3p is degraded upon autophagy activation, MIR126-5p interacts with the RNA-binding protein MEX3A to form a ternary complex with AGO2. This complex forms on the autophagosomal surface and facilitates its nuclear localization. Once in the nucleus, MIR126-5p dissociates from AGO2 and establishes aptamer-like interactions with the effector CASP3 (caspase 3). The binding to MIR126-5p prevents dimerization and proper active site formation of CASP3, thus inhibiting proteolytic activity and limiting apoptosis. Disrupting this pathway in vivo by genetic deletion of Mex3a or by specific deficiency of endothelial autophagy aggravates endothelial apoptosis and exacerbates the progression of atherosclerosis. The direct inhibition of CASP3 by MIR126-5p reveals a non-canonical mechanism by which miRNAs can modulate protein function and mediate the autophagy-apoptosis crosstalk.
KEYWORDS: microRNA, Atherosclerosis, Autophagy, MEX3A, miR-126-5p, Noncanonical miRNA functions, Endothelial cells
Since the pioneering studies in C. elegans, the mechanism of miRNA functions is explained by a well-established paradigm where miRNA nucleotides 2–7 (so-called, “seed sequence”) recognize specific sites within the 3ʹ UTRs of target RNA transcripts by Watson-Crick pairing, promote their recruitment in the RISC and ultimately their decay or translational inhibition. In mammals, efficient miRNA-mediated regulation of gene expression requires multiple components, such as TNRC6A (trinucleotide repeat containing adaptor 6A), the CCR4-NOT complex, and PABPC (poly(A) binding protein cytoplasmic), which are found in high molecular mass (>2 MDa) RISCs in the cytoplasm. Nonetheless, miRNAs are also withheld into low-molecular mass (<200 kDa) complexes with AGO2, which also localize in other subcellular compartments (e.g., nucleus) and whose assembly is regulated by the MTOR (mechanistic target of rapamycin kinase) complex 1 (MTORC1). Although these complexes prevail in most of the adult tissues, it is largely unknown whether they may enable interactions of hosted miRNAs with components not involved in the canonic RISC functions.
In this context, our recent study [1] describes an unexpected role of the autophagy machinery in ruling intracellular homeostasis and functions of MIR126, the most highly expressed miRNA in endothelial cells (ECs). Contrary to most miRNAs, both strands of MIR126 (MIR126-3p and MIR126-5p) are functional to promote anti-atherosclerotic features. We found that exposure to protective shear stress, overexpression of KLF2 (Krüppel like factor 2), which coordinates most of the transcriptional programs evoked by shear stress, or inhibition of MTORC1 by rapamycin activate the autophagic flux in ECs, privilege the assembly of low-molecular weight RISCs, and differentially affect MIR126 strands. While MIR126-3p undergoes autolysosomal degradation reverted by treatment with bafilomycin A1, MIR126-5p is enriched in the nucleus. The nuclear shuttling of MIR126-5p required an intact autophagy machinery, as shown by knockdown experiments for ATG5 and ATG7, and the RNA-binding protein MEX3A. Little was known about the biological function of MEX3A, a member of the MEX3 family which interacts with AGO2 and shuttles between the nucleus and cytoplasm. We combined molecular dynamic simulation with biophysical and structural experiments to show that MEX3A directly interacts with MIR126-5p through its two K-homology (KH) domains. The binding with MIR126-5p promotes structural rearrangements which may contribute to the preferential affinity for MIR126-5p (compared to MIR126-3p) and allows further interaction with AGO2 to form a ternary complex. Detailed subcellular localization analyses revealed that the complex assembles on the extralumenal surface of autophagosomes, thus presenting MIR126-5p for nuclear internalization while preserving it from degradation (Figure 1A,B). These results reveal a regulatory role of the autophagy machinery and MEX3A in the processes of selective intracellular miRNA trafficking.
Figure 1.
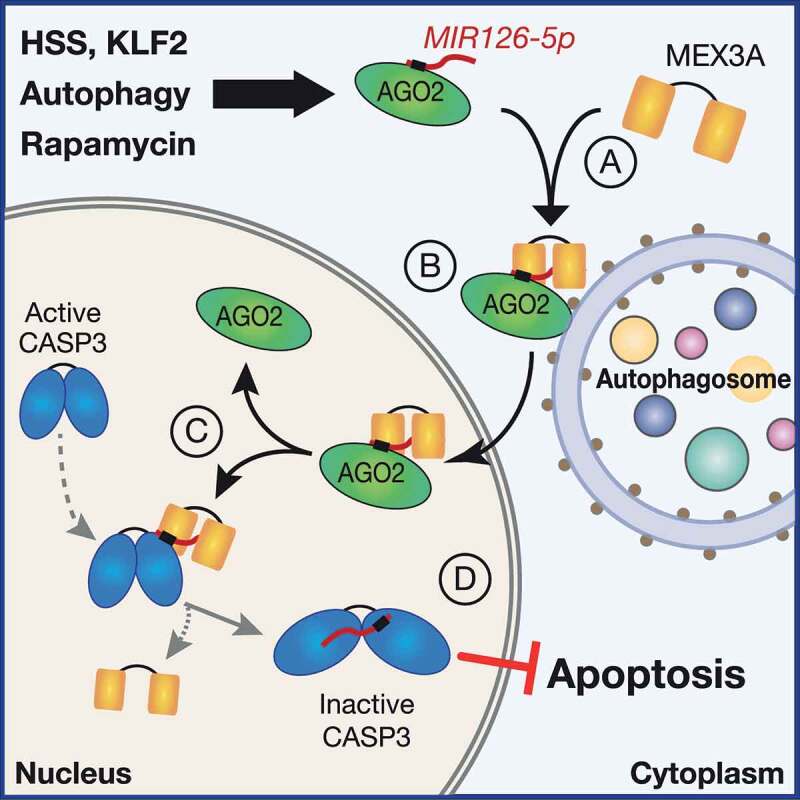
Summary of the MEX3A-guided nuclear MIR126-5p pathway. Atheroprotective high shear stress (HSS), activation of KLF2 (Krüppel like factor 2), and MTOR-inhibition by rapamycin favors the assembly of a ternary complex of MIR126-5p with MEX3A and AGO2 (A). This complex localizes on the extralumenal surface of autophagosome, which preserves it from degradation and leads to its nuclear transfer (B). In the nucleus, MIR126-5p dissociates from AGO2 and MEX3A and becomes available for binding the effector caspase, CASP3, which can be transferred to the nucleus when activated (C). The direct interaction inhibits CASP3 catalytic activity, thus reducing its function as the terminal effector of apoptotic cell death (D). The reduced apoptotic rate preserves endothelial cell viability to protect against atherosclerosis. The direct interference with protein activity expands the range of post-transcriptional regulation by miRNAs and highlights an anti-apoptotic mechanism for protecting integrity of the endothelium in health and disease
We then questioned the biological relevance of MEX3A-guided nuclear MIR126-5p. Interestingly, we found that a significant share of nuclear MIR126-5p dissociates from AGO2 and MEX3A to be available for binding with additional interactors. In particular, we identify the effector CASP3 as a binding partner of nuclear MIR126-5p (Figure 1C,D). This finding was corroborated by multiple lines of evidence (e.g., RNA-immunoprecipitation, confocal microscopy), and in vitro experiments (e.g., nuclear magnetic resonance, surface plasmon resonance) confirmed a direct interaction which did not require any additional adaptor. The unexpected nature of the finding granted us the task additional work for dissecting its significance. Molecular dynamic simulations implied a mode of interaction in which MIR126-5p preferentially binds to CASP3 monomers, engaging in interactions that involve the substrate-binding pocket and the heterodimer interface of CASP3. Consistently, experimental validation by surface plasmon resonance revealed that MIR126-5p could disrupt dimerization of CASP3 subunits. Dimerization is crucial for the activity of all caspases, as the four loops forming the active sites are provided by both dimer subunits; thus, we hypothesized a possible functional role. We found that MIR126-5p decreases substrate cleavage by recombinant CASP3 in a cell-free assay. Additionally, in silico computations predicted that the seed sequence of MIR126-5p may bind the substrate-binding pocket. Interestingly, mutational changes of the seed sequences impair binding and the inhibitory effect on CASP3. Although the structural basis requires future investigations, our data suggest that MIR126-5p may affect CASP3 activity by a dual mechanism: disrupting dimerization and preventing substrate binding. The biological relevance of this mechanism was proved in vitro in ECs and in vivo in transgenic animal models. The pathway for nuclear MIR126-5p enrichment was blocked with two genetic approaches: deletion of Mex3a and blockage of the endothelial autophagy machinery (obtained in Chd5-Cre+ atg5fl/fl animals). These models revealed a higher endothelial apoptosis rate that culminated in enhanced development of atherosclerosis, likely due to defective endothelial integrity.
In conclusion, the inhibition of CASP3 by nuclear MIR126-5p epitomizes a novel non-canonical miRNA function, modulating protein activity independent of canonical mRNA silencing and translational repression. This mechanism contributes to the inhibitory crosstalk between apoptotic and autophagic pathways, with profound implications in the equilibrium between cell death and survival, and ultimately in organism homeostasis and disease.
Funding Statement
The work of the authors is supported by Deutsche Forschungsgemeinschaft (DFG): [TRR267-A2 to C.W. and M.S.; INST409/150-1 FUGG to R.T.A.M. and C.W.; SFB1123-A1/A10 to C.W. and J.D.; SFB1123-A2 to P.v.H.; SFB1123-Z1 to R.T.A.M.; SFB1123-A5 to E.L.; STE-1053/5-1 to S.S.; SFB1035 and GRK1721 to M.S.]; and by the European Research Council: [ERC AdG°692511 to C.W. and ERC CoG°683145 to T.C].
Disclosure statement
No potential conflict of interest was reported by the authors.
Reference
- [1].Santovito D, Egea V, Bidzhekov K, et al. Noncanonical inhibition of caspase-3 by a nuclear microRNA confers endothelial protection by autophagy in atherosclerosis. Sci Transl Med. 2020;12:eaaz2294. [DOI] [PubMed] [Google Scholar]


