ABSTRACT
ATG9, the only transmembrane protein in the core macroautophagy/autophagy machinery, is a key player in the early stages of autophagosome formation. Yet, the lack of a high-resolution structure of ATG9 was a major impediment in understanding its three-dimensional organization and function. We recently solved a high-resolution cryoEM structure of the ubiquitously expressed human ATG9A isoform. The structure revealed that ATG9A is a domain-swapped homotrimer with a unique fold, and has an internal network of branched cavities. In cellulo analyses demonstrated the functional importance of the cavity-lining residues. These cavities could serve as conduits for transport of hydrophilic moieties, such as lipid headgroups, across the bilayer. Finally, structure-guided molecular dynamics predicted that ATG9A has membrane-bending properties, which is consistent with its localization to highly curved membranes.
KEYWORDS: ATG9A, autophagosome, cryo-EM, membrane curvature, molecular dynamics, transmembrane protein
After decades of intense research using different model organisms, most of the core components of the autophagy machinery have been identified. Yet, the molecular mechanisms of various stages of autophagy are far from clear. One of the main reasons is that the structures of individual proteins and subcomplexes have only recently begun to be elucidated by crystallography and, increasingly, by cryoelectron microscopy (cryo-EM), providing clues about how these components function together at the molecular level. A case in point is the enigmatic ATG9 protein, the only transmembrane component of the core autophagy machinery. ATG9 and its homologs have long been known to be essential for the formation of the autophagosome, but, without a high-resolution structure, clear hypotheses about its molecular function have remained elusive.
Our four laboratories at the National Institutes of Health (NIH) recently joined forces to begin to address this gap in knowledge using structural biology approaches. We succeeded in obtaining a 2.9-Å resolution structure of the ubiquitous human ATG9A isoform by cryo-EM [1]. The structure revealed that ATG9A adopts a unique fold, assembling into a domain-swapped homotrimer, with each protomer comprising four transmembrane α-helices (α2, α6, α14 and α15) and two α-helices that are only partially embedded in the membrane (α11 and α19). The transmembrane domain is capped by a cytosolic domain, which is mostly α-helical. Trimerization results primarily from each ATG9A protomer inserting its carboxy-terminal transmembrane hairpin (α14 and α15) into a cleft formed by the four membrane helices (α2, α6, α11 and α19) of the adjacent protomer. The resulting wedge-shaped units (derived from domain-swapped protomers) assemble into a trimeric complex (Figure 1), with much of the lipid-exposed protein surface at an ~45° angle relative to the membrane normal. This core membrane-integral region is well-conserved across eukaryotes; in contrast, the extended carboxy-terminal region (amino acids 588 to 839 in human ATG9A) is variant, predicted to be disordered, and not resolved in our cryo-EM reconstructions. We speculate that structures in complex with protein-interacting partners will help resolve this part of the structure of ATG9A.
Figure 1.
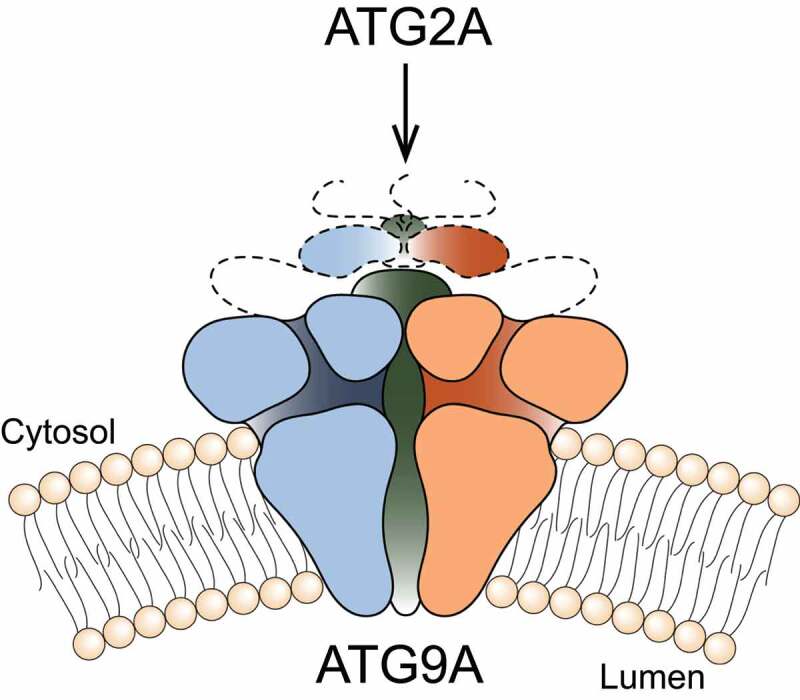
Schematic representation of the three-dimensional structure of human ATG9A. Using a combination of cryo-EM, computational simulations, biochemistry and cell biology approaches, we described in detail the structure and properties of the protein
An important feature of the ATG9A structure is the presence of a branched network of pores through the protein, with outlets facing the cytosol, the outer leaflet lipid head groups, and the lumen. Classification of the cryo-EM imaging revealed two predominant ATG9A conformers (states A and B). The large central pore in the trimeric ATG9A structure is plugged in state A by α-helices αA and αB, which lie toward the carboxy-terminal side of the transmembrane region. Notably, mutation of two amino acids (N265W and R422W) lining one of the pores abolishes the ability of transgenic ATG9A to reverse aberrant LC3B accumulation in an ATG9A knockout cell line, suggesting an essential function of the pore. The dimensions of this pore are sufficient to accommodate phospholipids and may, akin to NPC1 (NPC intracellular cholesterol transporter 1), permit lipid transport from or to a lipid chaperone such as ATG2. Alternatively ATG9 may act as a lipid scramblase, distributing the lipids that arrive through ATG2, among two leaflets of the bilayer. The branched pores may also function as a solvent conduit to relieve osmotic pressures in growing and shrinking ATG9 vesicles.
We and other groups have shown that ATG9A directly interacts with ATG2A and that these interactions are mediated by the ATG9A cytosolic domain (Figure 1). Deletion of this region abrogates both ATG2A co-immunoprecipitation and autophagosome maturation. Because ATG2A has demonstrated lipid transport capability in vitro, the plausible functions of ATG9A are: 1) to facilitate movement of lipids across the bilayer of the growing phagophore – this could be achieved by using the branched pores we see in our structure for moving polar lipid headgroups or by distorting the bilayer, lowering the energetic barrier for transbilayer lipid movement; 2) to act as a membrane-anchored “lasso” to capture and accurately target ATG2A or other lipid chaperones. It is interesting to note that αA and αB, that plug the central pore of the trimer are discernible only in state A, indicating that flexibility of the putative “lasso” could be partly dependent on the conformation of the transmembrane core.
The early stages of autophagy involve a reorganization of intracellular membranes to form the nascent autophagosome. ATG9 is essential for this process, and our structure provides a framework for interrogating its function in bilayer remodeling. Using molecular dynamics simulations based on the cryo-EM data, we found that the architecture of the ATG9A trimer elicits long-ranged membrane curvature (Figure 1), owing to the geometric features and amino-acid make-up of the protein surface exposed to the lipid. The simulations also show that colocalization of multiple ATG9A trimers greatly amplifies this effect and induces macroscopic changes in membrane morphology. This finding corresponds with observations that ATG9 is present in small (30–60 nm) vesicles and thin tubules, and, at least in yeast, localizes to the edges of the growing phagophore. Interestingly, states A and B differ in the tilt-angle of the protomers relative to the membrane normal, implying a tolerance for a range of bilayer curvatures. This leads to the intriguing possibility that ATG9A-induced curvature may put a thumb on the energetic scale and effect lipid diffusion out of the vesicles into another membrane.
Further structural studies of ATG9 proteins will be needed to reveal the mode of interaction with other components of the autophagy machinery, ideally embedded in membrane-mimetic environments that more accurately represent the cell membranes. However, a detailed understanding of the molecular mechanism of ATG9 will necessarily rely on successful use of convergent approaches – biochemical assays in vitro with purified components and cell biological studies honing a deeper understanding of interacting protein partners at different stages of autophagy. Beyond the question of membrane remodeling, computer simulations could be employed to clarify the functional role of the intriguing pores observed within the structure. Ultimately, unraveling the mechanism of ATG9 proteins will bring us one step closer to understanding the process of autophagosome formation, and will serve as a foundation for future studies into the regulation of this essential cellular process by other proteins and small molecules.
Acknowledgments
Work in the authors’ laboratories is supported by the Intramural Research Programs of the National Heart, Lung, and Blood Institute (NHLBI) (J.D.F.G. and J.J.) and of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (J.S.B. project ZIA HD001607 and A.B. project ZIA HD008928), National Institutes of Health (NIH).
Funding Statement
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development [ZIA HD001607 and ZIA HD008928] and by the National Heart, Lung and Blood Institute, National Institutes of Health, USA.
Disclosure statement
The authors declare no competing interests.
Reference
- [1].Guardia CM, Tan X-F, Lian T, et al. Structure of human ATG9A, the only transmembrane protein of the core autophagy machinery. Cell Rep. 2020;31(13):107837. PMID: 32610138. [DOI] [PMC free article] [PubMed] [Google Scholar]


