Abstract
Purpose
A higher creative potential has been reported in narcoleptic patients and linked to lucid dreaming. The aim of the present study was to explore the role of narcolepsy symptoms (presence and severity) in predicting creativity.
Patients and Methods
Sixty-six consecutive type 1 narcolepsy patients (mean age 38.62 ± 17.05, 31 females) took part in this study. Creative achievement in different life domains and creative beliefs were assessed by a self-reported questionnaire and a scale measuring the creative self, respectively; creative performance was measured through a divergent thinking test (generation of alternative original solutions to an open problem).
Results
We found a key effect of hypnagogic hallucinations in modulating creative behaviour. We therefore tested at first whether hypnagogic hallucinations could interact with specific mental states associated with creativity and in particular mind wandering, a factor associated with both creative performance and achievement. Secondly, we verified if hypnagogic hallucinations could influence the definition of creative identity in type 1 narcolepsy patients, which in turn could predict their creative achievement and creative performance. Results showed that spontaneous mind wandering influenced creative achievement through a moderation effect of sleep paralysis and hypnagogic hallucinations. Moreover, sleep paralysis and hypnagogic hallucinations indirectly influenced, through creative identity, both creative achievement and performance (fluency score).
Conclusion
Our results highlight the role of hypnagogic hallucinations in triggering the process of mind wandering which leads to greater creative success. In addition, this symptom affects creative identity in narcolepsy, leading in turn to higher creative success and creative potential of narcoleptic patients.
Keywords: narcolepsy type 1, creativity, hypnagogic hallucinations, daydreaming, mind wandering
Introduction
“I did not sleep but wandered
in that limbo where objects change shape, the mysterious
tracts that separate waking from sleep”
Gustavo Adolfo Bécquer1
Narcolepsy type 1 (NT1) is a chronic neurological disorder characterised by excessive daytime sleepiness (EDS), with rapid access into REM sleep at sleep onset. Naps can occur several times during the day and are frequently characterized by dreaming with recall of the dream content.2–4 NT1 patients also experience clinical phenomena related to wake/REM sleep dissociation, including cataplexy, sleep paralysis, hypnagogic/hypnopompic hallucinations and REM sleep behaviour disorder (ICSD-III).5
Given the features of abnormal REM sleep manifestation in narcolepsy, with privileged access to sleep and dreaming, a recent study assessed whether lucid dreaming (the phenomenon of being aware of dreaming during the dream) could influence creative abilities.6,7 The authors showed that narcolepsy patients have a higher creative potential compared to controls, both in terms of creative profile and of creative achievement. Moreover, narcoleptic patients showed an overall better creative performance in an objective test that evaluated creative abilities, in particular, they scored higher in an integrative task (measuring ideas’ originality) and tended to achieve better scores in a divergent task (measuring the ability to produce multiple ideas) compared to controls. Finally, sleep paralysis, hypnagogic/hypnopompic hallucinations, REM sleep behaviour disorder and lucid dreaming were associated with a more highly creative profile, suggesting that the privileged access to REM sleep could provide a gateway to creativity.
Anecdotally sleep-related hallucinations, fleeting perceptual experiences generally characterized by vivid perceptions occurring at sleep/wake transitions and vice versa, have been related to the creative process in several cases.8–10 The most famous is probably the story of August Kekulè, which describes how the hypnagogic image of a swirling snake biting its tail and this vision allowed him to discover the structure of benzene.11 Although it has long been debated whether sleep-related hallucination is a phenomenon similar to REM or NREM sleep mentation, hypnagogic phenomena are usually characterized by narrative plots and bizarre elements similar to contents of REM sleep.9,12–14
The aim of the present study is to explore creativity mechanisms in NT1 patients, focusing on whether symptom presence, frequency and course influence creative performance and achievements. Moreover, we explored, for the first time in NT1 patients, mind wandering (MW) and daydreaming (DD), two processes that have been associated with creativity.15–17 MW is an uncontrolled (spontaneous) or intentional (deliberate) attentional shift from the ongoing task towards internal thoughts unrelated to the task (memories, prospective thoughts) while DD refers to thoughts immersed in fantasy and mental imaginings.18–21
The study addresses two specific research questions: i) whether hypnagogic hallucinations can influence creative achievement and creative performance through the interaction with specific mental states associated with creativity (and in particular MW); and ii) whether hypnagogic hallucinations can influence the definition of the personality of these patients, investigating their creative self-beliefs, namely the self-beliefs to solve problems requiring thinking and creative functioning, i.e., creative identity. Since creative self-beliefs are one of the most important predictors of creative performance and achievement, we expected that through this influence hypnagogic hallucinations could indirectly predict creative achievement and creative performance of these patients.22
Method
Participants
Sixty-six NT1 patients (mean age 38.62 ± 17.05, 31 females) were consecutively recruited at the Outpatient Clinic of the Center for Narcolepsy of the Department of Biomedical and Neuromotor Sciences of the University of Bologna during routine follow-up evaluations. Inclusion criteria for NT1 patients were age ≥ 18 years and absence of comorbid neurologic and/or psychiatric disorders. All patients fulfilled the current diagnostic criteria for NT1 (International Classification of Sleep Disorders 3rd edition, ICSD-3) presenting mean sleep-onset latency ≤8 min with at least two sleep-onset REM periods (SOREMP) on the Multiple Sleep Latency Test (MSLT), video-documented cataplexy, and reduced/undetectable cerebrospinal fluid hypocretin-1 (CSF hcrt-1) levels.23,24
Mean age at diagnosis was 19.46 ± 12.13 years, mean disease duration was 18.98 ± 10.54 years, and mean duration of education was 12.47 ± 3.41 years (range 5–18 years).
Six patients (9%) were drug naïve/drug free, while 60 patients (91%) were on pharmacological treatment at the time of evaluation (36 (60%) patients on pharmacological monotherapy and 24 (4%) on pharmacological polytherapy). The study was carried out in accordance with the principles of the Declaration of Helsinki and approved by the local ethics committee (Comitato Etico Indipendente di Area Vasta Emilia Centro - CE-AVEC - Prot. N.66930). All participants provided written informed consent.
Materials and Procedure
Questionnaire
All participants filled in questionnaires on sleepiness, NT1 symptoms’ presence and severity, creative achievement, creative identity, MW and DD (Italian versions of all questionnaires have been used).
The Epworth Sleepiness Scale (ESS) was used to assess “trait” sleepiness and the Karolinska Sleepiness Scales (KSS) was used to assess momentary “state” sleepiness.25–27 NT1 symptoms’ presence and severity were assessed with the Narcolepsy Severity Scale (NSS), which provides three separate symptom scores: i) sleep paralysis and hypnagogic hallucinations (SP-H), ii) excessive daytime sleepiness and night-time sleep (EDS-NS) and iii) cataplexy (CATA).28
Creative achievement has been evaluated with the creative activity and accomplishment checklist (CAAC).29–31 The CAAC version used in this study consists of 40 items that cover activities in three domains: artistic, technological, and everyday.32 Participants had to checkmark two responses that best described the frequency with which they performed the activity within their working environment and outside of it, respectively.
The Short Scale of the Creative Self (SSCS) has been used to obtain information on creative self-efficacy (CSE) and creative personal identity (CPI).33
Deliberate and spontaneous mind wandering (MW–D and MW-S, respectively) were assessed with self-report scales in which higher scores indicate a greater tendency to engage in MW.34,35 Finally, daydreaming was assessed with the Daydreaming Frequency Scale (DDFS).18,36,37
Creative Performance
Creative performance has been assessed with the Alternative Uses Task (AUT).38 The AUT is a measure of divergent thinking ability, which has been shown to be a reliable indicator of creative potential.39 During the task, participants are requested to generate as many uncommon/original uses as they can for six common objects (e.g., a book, a chair) randomly presented. Each picture is displayed for three minutes, during which the participant can type responses.
Patients’ responses were evaluated for originality by two independent raters blind to the participants’ status and study aims. Each response received a score rating from 1 (not at all original) to 5 (highly original) (for a detailed description of the task we refer the reader to the paper by Agnoli et al).40 The average originality score and the fluency score (number of responses produced) were considered as an indicator of creative ability or potential.39,41
Procedure
All participants completed a computerized version of the AUT. The task was run on a computer with a 15.4-inch monitor using E-Prime software (Psychology Software Tools Inc., Pittsburgh, PA, United States). At the beginning and at the end of the experimental session participants filled in an electronic version of the KSS; upon completing the AUT participants filled in the questionnaires (ESS, CAAC, SSCS, MW–D and MW–S, DDFS and the NSS). Questionnaires’ order of presentation was randomised to counterbalance order effects. See Supplementary methods for a detailed description of Materials and Procedure.
Statistical Analysis
Paired sample t-test was used to compare differences between KSS score before and after the AUT in order to account for a possible effect of sleepiness on test outcome.
Questionnaires’ internal consistency was calculated by Cronbach’s α coefficient for NSS, CAAC, SSCS, MW–D, MW–S and DDFS.
Interrater reliability for originality in the AUT was calculated and in case of marked discrepancies in ratings (if the score between the two raters differed by 3 or more points on the scale used from 1 to 5), raters reviewed and assigned scores by consensus.
Pearson correlations were used to assess the relationship between indexes of creativity (creative achievement, AUT fluency and AUT originality), questionnaires’ score (SSCS, MW–D and MW–S and DDFS) and clinical data (NSS, ESS, disease duration and age at onset).
To assess whether NT1 symptoms interact with mind wandering (MW) in the prediction of creative achievement and creative performance, we conducted three hierarchical multiple regression analyses including four blocks of variables (Table 1). Variables scores were centred using the sample mean prior to be entered into the regression analysis. In the first step, SP-H was entered; in the second step, MW-S and MW-D were entered; in the third step, the interaction between MW-S and SP-H was entered; in the fourth step, we included the interaction between MW-S and SP-H. In the three hierarchical multiple regression analyses, the criterion variables were, respectively: creative achievement, AUT fluency, and AUT originality. Because the predictors showed important intercorrelations, we controlled for multicollinearity.
Table 1.
Hierarchical Multiple Regression on Creative Achievement and Creative Performance (Fluency and Originality scores)
| Cr. Achievement | Fluency | Originality | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Step 1 | Step 2 | Step 3 | Step 4 | Step 1 | Step 2 | Step 3 | Step 4 | Step 1 | Step 2 | Step 3 | Step 4 | |
| SP-H | 0.19 | 0.18 | 0.25 | 0.24 | 0.24 | 0.23 | 0.26 | 0.27 | 0.17 | 0.12 | 0.17 | 0.16 |
| MW-S | −0.02 | 0.05 | 0.05 | −0.10 | −0.08 | −0.10 | −0.05 | −0.02 | −0.02 | |||
| MW-D | 0.08 | 0.07 | 0.07 | 0.16 | 0.15 | 0.18 | 0.24 | 0.23 | 0.23 | |||
| MW-S × SP-H | −0.38** | −0.36 | −0.14 | −0.34 | −0.24 | −0.21 | ||||||
| MW-D × SP-H | −0.03 | 0.24 | −0.04 | |||||||||
| R2 | 0.02 | −0.01 | 0.12 | 0.10 | 0.04 | 0.02 | 0.02 | 0.02 | 0.01 | 0.02 | 0.06 | 0.04 |
| Δ R2 | 0.04 | 0.00 | 0.13 | 0.00 | 0.06 | 0.01 | 0.02 | 0.02 | 0.03 | 0.04 | 0.06 | 0.00 |
| F | 2.43 | 0.87 | 3.12* | 2.46* | 3.23 | 1.30 | 1.24 | 1.12 | 1.64 | 1.33 | 1.83 | 1.44 |
| ΔF | 2.43 | 0.12 | 9.53* | 0.02 | 3.23 | 0.38 | 1.06 | 1.03 | 1.64 | 1.18 | 3.16 | 0.03 |
| df | 63 | 61 | 60 | 59 | 53 | 51 | 50 | 49 | 53 | 51 | 50 | 49 |
Notes: Step 1: SP-H; Step 2: MW-S and MW-D; Step 3: MW-S × SP-H; Step 4: MW-S × SP-H. Numbers in the first four rows represent standardized regression coefficients; *p<0.05, ** p < 0.01
Abbreviations: SP-H, sleep paralysis and hypnagogic hallucinations; MW-S, mind wandering spontaneous; MW-D, mind wandering deliberate.
To test whether the SP-H symptom could indirectly influence creative achievement and creative performance through its influence on the definition of the creative self we tested an indirect effect model (PROCESS macro for SPSS) on the three indexes of creativity (creative achievement, AUT fluency, and AUT originality);42,43 a Bootstrap with 5.000 samples was used to derive confidence interval for indirect effects. All indirect effect analyses included SP-H and DD as predictor variables and creative personal identity as mediator variable in the relationship between SP-H and the creativity index (creative achievement, fluency, or originality). Because of the possible overlap between the SP-H symptom and the process of daydreaming we controlled for the effect of this latter variable in the analyses.
Data are available from the corresponding author upon reasonable request.
Results
State sleepiness, as measured by the KSS, did not change during the AUT task (KSS pre = 3.27 ± 1.81, post = 3.44 ± 1.89; p = 0.08). The internal consistency of scales ranged between 0.73 (acceptable) and 0.94 (excellent); the interrater reliability calculated for the AUT task was good (Cohen’s κ = 0.66). Demographic, clinical, neurophysiological data and questionnaires scores are reported in Tables 2 and 3.
Table 2.
Demographic, Clinical and Neurophysiological Characteristics of Patients
| Mean ± SD | Range | |
|---|---|---|
| Demographic data | N=66 | |
| Male/female | 31/35 | |
| Age, y | 38.62±17.05 | 18–76 |
| Education, y | 12.47±3.41 | 5–18 |
| Clinical characteristics | ||
| Age at onset, y | 19.46±12.13 | – |
| Disease duration, y | 18.98±10.54 | – |
| BMI | 29.95±5.12 | – |
| BMI category (underweight\normal\overweight\obese) |
4\18\24\20 | |
| Neurophysiological data | ||
| MSLT_sl, min | 4.71±3.35 | – |
| SOREMPs, no. | 3.61±1.35 | |
| Biochemical data | ||
| CSFHcrt-1, pg/mL (n = 66) | 56.15±1 | - |
| HLA DQB1*0602 positivity | 66 | |
| Medication used | ||
| Stimulants | 13 | |
| Sodium oxybate | 23 | |
| Antidepressant | 13 | |
| Stimulants plus sodium oxybate | 11 | |
| No medication | 6 |
Note: HLA DQB1*0602 = positivity for human leukocyte antigen.
Abbreviations: BMI, body mass index; MSLT-sl, mean sleep latency at MSLT; SOREMPs, sleep-onset REM periods at MSLT; CSFHcrt-1, cerebrospinal fluid hypocretin-1.
Table 3.
Questionnaires’ Scores
| Mean ± SD | Range | α | |
|---|---|---|---|
| ESS | 10.69±4.65 | 1–24 | |
| BDI | 8.55±7.90 | 0–33 | |
| Moderate (> 18), n (%) | 5 (7.58%) | ||
| Severe (> 29), n (%) | 3 (4.55%) | ||
| SP-H | 3.41±3.66 | 0–14 | 0.79 |
| EDS-NS | 12.48±6.11 | 0–27 | 0.81 |
| CATA | 5.41±3.95 | 0–13 | 0.73 |
| NSS | 21.30±10.31 | 1–46 | 0.83 |
| CAAC | 1.58±0.34 | 1.00–2.66 | 0.93 |
| Fluency | 26.30±12.23 | 8–55 | |
| Originality | 2.27±0.31 | 1.45–3.05 | |
| CSE | 21.38±4.99 | 8–3 | 0.87 |
| CPI | 17.58±4.68 | 5–25 | 0.87 |
| MW-S | 3.14±1.66 | 1–7 | 0.90 |
| MW-D | 3.53±1.73 | 1–7 | 0.91 |
| DDFS | 15.63±11.22 | 0–48 | 0.94 |
Abbreviations: ESS, Epworth sleepiness scale; BDI, Beck Inventory Scale; SP-H, sleep paralysis and hypnagogic hallucinations; EDS-NS, excessive daytime sleepiness and nighttime sleep; CATA, cataplexy; NSS, narcolepsy severity scale total score; CAAC, creative achievement; CSE, creative self-efficacy; CPI, creative personal identity; MW-S, mind wandering spontaneous; MW-D, mind wandering deliberate; DDFS, daydreaming.
Correlations Analysis
Results of correlation analyses are reported in Table 4. Creative achievement was positively correlated to fluency and originality. Moreover, creative success and fluency in AUT were positively correlated with both indicators of creative identity, CSE and CPI. Originality in AUT was positively correlated with CPI and with factor EDS-NS of the NSS. The two indicators of creative personal identity were correlated positively with the total NSS score and with factor SP-H. CSE correlated positively also with factor CATA of the NSS and CPI correlated positively with the duration of the disease. The mental processes of MW-S and MW-D were both correlated positively with the process of DD and they were both correlated with the total score of the NSS and with factor SP-H and EDS-NS of this scale. Finally, MW-D and DD were both negatively correlated to the age of onset of the disease: more in detail, a lower age of onset of the disorder correlated with a higher level of MW-D and DD.
Table 4.
Descriptive Statistics and Correlations Among the Study Variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CAAC | _ | ||||||||||||||
| 2. | Fluency | 0.439** | _ | |||||||||||||
| 3. | Originality | 0.387** | 0.249 | _ | ||||||||||||
| 4. | CSE | 0.411** | 0.349* | 0.216 | _ | |||||||||||
| 5. | CPI | 0.391** | 0.459** | 0.271* | 0.792** | _ | ||||||||||
| 6. | MW-S | 0.104 | 0.095 | 0.160 | 0.127 | 0.115 | _ | |||||||||
| 7. | MW-D | 0.111 | 0.158 | 0.244 | 0.180 | 0.184 | 0.689** | _ | ||||||||
| 8. | SP-H | 0.160 | 0.221 | 0.178 | 0.309* | 0.253* | 0.409** | 0.278* | _ | |||||||
| 9. | EDS-NS | 0.156 | −.070 | 0.299* | 0.112 | 0.132 | 0.329** | 0.287* | 0.339** | _ | ||||||
| 10. | CATA | 0.058 | 0.017 | −.022 | 0.272* | 0.231 | 0.178 | 0.132 | 0.268* | 0.355** | _ | |||||
| 11. | NSS | 0.172 | 0.040 | 0.232 | 0.280* | 0.256* | 0.408** | 0.319** | 0.658** | 0.848** | 0.688** | _ | ||||
| 12. | DDFS | 0.202 | 0.112 | 0.150 | 0.139 | 0.208 | 0.639** | 0.576** | 0.183 | 0.174 | 0.017 | 0.173 | _ | |||
| 13. | ESS | −.087 | −.031 | 0.119 | −.068 | −.059 | 0.145 | 0.059 | 0.100 | 0.516** | 0.253* | 0.440** | −.080 | _ | ||
| 14. | Disease duration | 0.201 | 0.139 | 0.178 | 0.253 | 0.334* | 0.022 | −.074 | −.100 | 0.136 | −.043 | 0.030 | −.225 | 0.029 | _ | |
| 15. | Age at onset | −.130 | −.063 | 0.064 | −.144 | −.075 | −.158 | −.263* | 0.013 | 0.188 | 0.213 | 0.195 | −.431** | 0.207 | 0.082 | |
| Mean | 1.58 | 26.30 | 2.27 | 21.38 | 17.58 | 3.14 | 3.53 | 3.41 | 12.48 | 5.41 | 21.30 | 15.63 | 10.69 | 18.98 | 19.46 | |
| SD | 0.34 | 12.23 | 0.31 | 4.99 | 4.68 | 1.66 | 1.73 | 3.66 | 6.11 | 3.95 | 10.31 | 11.22 | 4.65 | 10.54 | 12.13 |
Notes: *p<0.05, **p<0.01.
Abbreviations: CAAC, creative achievement; CSE, creative self-efficacy; CPI, creative personal identity; MW-S, mind wandering spontaneous; MW-D, mind wandering deliberate; SP-H, sleep paralysis and hypnagogic hallucinations; EDS-NS, excessive daytime sleepiness and night-time sleep; CATA, cataplexy; NSS, narcolepsy severity scale total score; DDFS, daydreaming; EES, Epworth Sleepiness Scale.
Hierarchical Multiple Regressions
Variance inflation factors showed that the three models were exempt from multicollinearity (variance inflation factors < 10).
In the first hierarchical multiple regression analysis (on creative achievement), neither the introduction of SP-H (step 1) nor the introduction of MW-S and MW-D (step 2) produced significant changes in the regression model. The introduction of the interaction between MW-S and SP-H in step 3 produced instead a significant change in the model. Finally, step 4, introducing the interactions between MW-D and SP-H, did not show any significant increase of the explained variance in the model.
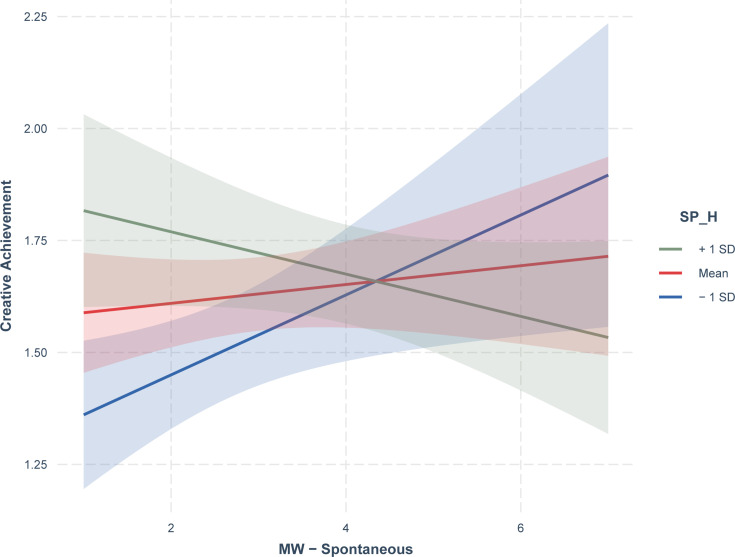
To explain the interaction between MW-S and SP-H observed in the regression model a simple slopes analysis was performed aimed at exploring a possible moderation of SP-H on the relationship between MW-S and creative achievement. This analysis showed that the positive link between MW-S and creative achievement was significant only for low levels of SP-H (ß=0.09, SE=0.04, p=0.02), but not for medium (ß=0.02, SE=0.03, p=0.42) or high levels (ß=−.05, SE=0.03, p=0.13) of this moderator (see Figure 1).
Figure 1.
Moderating effect of sleep paralysis and hypnagogic hallucinations (SP-H) on spontaneous mind wandering (MW-S) in the prediction of creative achievement (CAAC).
Thus, the link between MW-S and creative achievement was moderated by SP-H, revealing that in NT1 patients the association between mind wandering and creative achievement is related to specific levels of this symptom. In particular, the mental state of spontaneous mind wandering appears to influence creative achievement in NT1 only if it is associated with low levels of the SP-H symptom; on the contrary, at medium and high levels of the symptom this association was not observed.
In the second hierarchical multiple regression analysis (on the fluency scores) the main effect of SP-H in step 1 and the main effects of MW-S and MW-D in step 2 were not significant in the prediction of fluency score. The introduction of the interactions between MW-S and SP-H in step 3 and between MW-D and SP-H in step 4 showed no significant effect on the fluency score.
In the third hierarchical multiple regression analysis (on the originality scores), the main effect of SP-H in step 1 and the main effects of MW-S and MW-D in step 2 were not significant in the prediction of originality. The introduction of the interaction between MW-S and SP-H in step 3 and between MW-D and SP-H in step 4 showed no significant effect on the originality score. Results of the hierarchical multiple regression analyses are shown in Table 1.
Indirect Effect Analysis
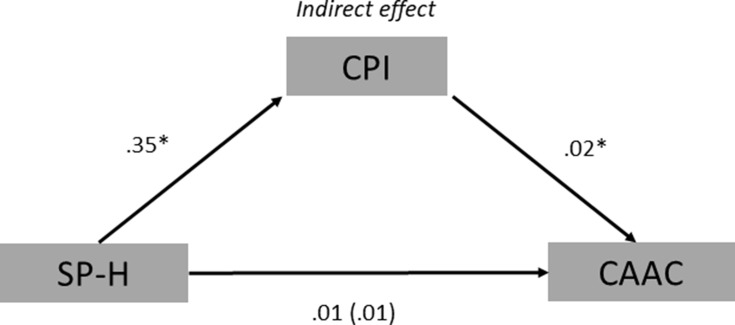
In the first indirect effect analysis creative achievement was included as outcome variable. The model showed that SP-H was positively associated with creative personal identity (ß = 0.35, t = 2.28, p= 0.03), in addition creative personal identity was also positively associated with creative achievement (ß = 0.02, t = 2.60, p= 0.02). While the direct effect of SP-H on creative achievement was not statistically significant (ß = 0.01, t = 0.54, p = 0.59), the indirect effect exerted through creative personal identity emerged as statistically significant (ß= 0.09, SE= 0.05, 95% CI = 0.01, 0.21). DD did not turn out to be significantly associated either with creative achievement (ß = 0.01 t = 1.13, p = 0.26) or to creative personal identity (ß = 0.06 t = 1.28, p = 0.20). Finally, the total effect of SP-H on creative achievement, which included the indirect effect through creative personal identity, was not significant (ß = 0.01, t = 1.26, p= 0.21) (Figure 2). This model revealed that a higher frequency of SP-H does not exert a direct influence on creative achievement, but it does through an indirect effect via creative personal identity, which in turn leads to higher creative achievement. DD instead did not emerge as a significant variable in these relationships.
Figure 2.
Path diagram of the mediation model showing that SP-H was positively associated with CPI, which was also positively associated with CAAC. Unstandardized β regression coefficients are reported (*p < 0.05). The values without parentheses represent the total effect, while the value inside the parentheses represents the direct effect of SP-H on CAAC.
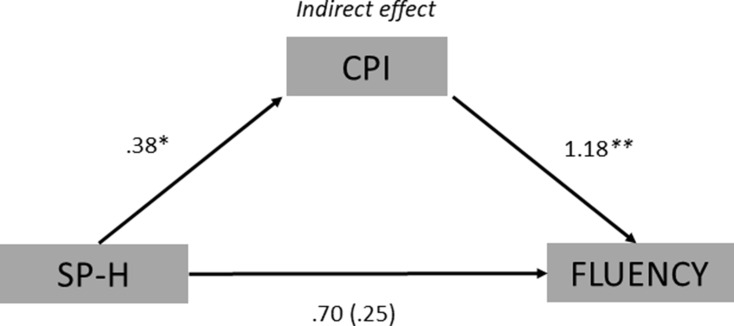
In the second indirect effect analysis fluency in the AUT task was included as outcome variable. The model showed that SP-H was positively associated with creative personal identity (ß = 0.38, t = 2.38, p = 0.02), in addition creative personal identity was positively associated with fluency (ß = 1.18, t = 3.28, p = 0.00). The direct effect of SP-H on fluency was not statistically significant (ß = 0.25, t = 0.56, p = 0.58), while the indirect effect exerted through creative personal identity was statistically significant (ß = 0.13, SE = 0.06, 95% CI = 0.03, 0.26). Again, DD emerged to be not significantly associated either with fluency (ß = −.01, t = −.11, p = 0.91) or with creative personal identity (ß = 0.08, t = 1.53, p = 0.13). Finally, the total effect of SP-H on fluency, which included the indirect effect through creative personal identity, was not significant (ß = 0.69, t = 1.52, p = 0.13). Again, this analysis showed that whereas the frequency of SP-H does not directly influence the ability to produce many alternative ideas (i.e., fluency in the AUT), it indirectly affects this ability via its influence on defining creative personal identity (see Figure 3).
Figure 3.
Path diagram of the mediation model showing that SP-H was positively associated with CPI, which was also positively associated with AUT fluency. Unstandardized β regression coefficients are reported (*p < 0.05, **p < 0.01). The values without parentheses represent the total effect, while the value inside the parentheses represents the direct effect of SP-H on fluency.
In the third indirect effect analysis originality in the AUT task was included as outcome variable. The model showed that while SP-H was positively associated with creative personal identity (ß = 0.38, t = 2.38 p = 0.02), creative personal identity was not associated with originality (ß = 0.02, t = 1.54, p = 0.13). The direct effect of SP-H on originality was not statistically significant (ß = 0.01, t = 0.62, p = 0.54), likewise DD was not significantly associated either with originality (ß = 0.00, t = 0.56, p = 0.58) or with creative personal identity (ß = 0.08, t = 1.53, p = 0.13). This analysis showed that SP-H has not effect (either direct or indirect) on originality.
Discussion
A main finding that emerges from our study is that hypnagogic hallucinations play an important role in predicting NT1 patients’ creative achievement, through its interaction with the mental state of MW.
In line with scientific literature in the field of creativity, our results show a relationship between creative achievement and creative performance in a divergent thinking task.44 Further, a relation has been found among creativity aspects and CSE, which could be considered one of the best predictors of creative achievement and creative performance.45–47
Our study showed that a relationship between narcoleptic symptoms and creativity emerged in the domain of self-perception of creativity. A positive association was found indeed between creative self-efficacy and creative personal identity and the severity of the disease (as measured by the NSS scale), in particular with SP-H factor. A link between the course of the disease and MW-S and MW-D is also supported by the positive correlation between these mental states and the NSS. The correlation is significant especially for the factor SP-H and excessive daytime sleepiness, suggesting that NT1 symptomatology could stimulate the process of MW. Consistent with these results, we also found a negative correlation between the age of onset of the disease and the process of MW and DD: a lower age at the onset of narcolepsy may lead to a greater development of these mental processes.
The hierarchical multiple regression analysis revealed that a greater level of creative success was achieved when MW-S interacted with a low level of SP-H, whereas at medium and high levels of this symptom, no interactive effect emerged. According to studies showing the effect of exposure to external stimuli in affecting the occurrence of MW episodes, also low frequency of hypnagogic hallucinations could thus represent a triggering mechanism for MW in NT1 patients.20,48,49 The result showing that the spontaneous form of MW interacted with hypnagogic hallucinations emerges to be consistent with this explanation. Whereas deliberate MW is a mental state deliberately activated by a person, spontaneous MW is usually triggered by external, involuntary cues. Hypnagogic hallucinations could therefore represent a mechanism that, independently form the patient’s decision, can trigger, if at low levels, the train of thoughts characterizing the mind wandering state.
Whereas SP-H interacts with spontaneous MW tendencies, in predicting creative achievement, no interactive effect emerged in the prediction of creative performance in terms of fluency and originality in the AUT. Even if creative achievement and creative performance are commonly associated constructs, they capture distinct elements of the creative phenomenology. Creative achievement refers to the creative success achieved by a person during her/his lifetime, creative performance as measured in the present study captures instead the divergent thinking ability to generate a multitude of original ideas, a measure of an individual’s creative potential. It is worth highlighting that NT1 patients experience the phenomenon of hypnagogic hallucinations during their life in the course of several years, and they probably turn this symptomatology into an advantage, which can therefore represent a favourable context for achieving in different creative activities.
Furthermore, our indirect effect analysis showed that SP-H exerted an indirect effect through the creative personal identity both on creativity achievement and on the creative performance (in the fluency index but not in originality score in the AUT). SP-H emerged therefore to influence, even if indirectly, the creative behaviour through its effect on the creative identity of the patients. These results suggest that in narcoleptic patients there is an association between self-perception of creativity and hypnagogic phenomena. Intriguingly, we found that creative personal identity was positively associated with the duration of the disease. This suggests that a longer course of disease can affect the beliefs to be creative and consequently to succeed in several creative activities.
Our results further extend the results of a recent study showing a positive association among symptoms (sleep paralysis, hypnagogic hallucinations, lucid dreaming and REM sleep behaviour disorder) and a higher creative profile evaluated in narcoleptic patients.7 In the study of Lacaux et al this greater creative profile highlighted in subjects with narcolepsy has been explained in relation to frequent lucid dreams reported by narcoleptic patients and to their higher ability to recall dreams content compared to controls.6,7,50 In the same way as lucid dreaming has a positive impact on creativity in normal subjects, and in narcoleptic patients, our study showed that also hypnagogic hallucinations may positively predict creativity in narcolepsy, exerting an indirect effect through creative personal identity.7,51
Regarding drug therapy, at the time of the test the majority of patients (91%) were on pharmacological treatment, with more than half of them being treated with sodium oxybate. In literature, it is reported that when on medication a reduction of dream recall frequency can occur and that sodium oxybate may decrease sleep paralysis and hypnagogic hallucinations.50,52,53 Nevertheless, we found similar scores in creative success and creative performance between patients treated and untreated with sodium oxybate, with a positive effect of this drug for sleepiness and cataplexy but not for sleep paralysis and hallucinations (Supplementary material, Table S1).
Some limitations should be acknowledged. First, our study did not include a control group. However, we aimed at addressing the mechanisms underlying creativity in NT1. Second, our sample of patients is not homogeneous as regards the age range and the age at onset of the disease, a variety that however allowed the analysis of the impact of disease duration on creativity features and mechanisms.
To sum up, our study highlights the role of hypnagogic hallucinations in defining both creative success and creative performance of narcoleptic patients by interacting with patients’ mental states leading to higher creativity as well as by influencing their creative identity. On the one hand, this symptom could represent the mechanism that triggers the process of mind wandering and that leads to greater creative success. On the other hand, it can define patients’ creative identity, which in turn can lead to higher creative success and creative potential.
Informing patients about these findings could be useful in order to increase awareness of their creative potential. In this way, patients could learn how to manage some symptoms and turn them into their advantage in everyday life.
Acknowledgments
We are indebted to all the patients participating in this study, most notably the Italian Association of Narcolepsy (AIN onlus) patients. Without their contributions, this study would not have been possible. We also thank Cecilia Baroncini for editing the English text.
Disclosure
Giuseppe Plazzi participated in advisory boards for and received personal fees from UCB, Jazz pharmaceuticals, Bioprojet and Idorsia, outside the submitted work. The authors report no other potential conflicts of interest for this work.
References
- 1.Cohen JM. The Penguin Book of Spanish Verse. Ed. Cohen JM, Penguin UK; 1988. [Google Scholar]
- 2.Vogel G. Studies in psychophysiology of dreams: III. The dream of narcolepsy. Arch Gen Psychiatry. 1960;3(4):421–428. doi: 10.1001/archpsyc.1960.01710040091011 [DOI] [PubMed] [Google Scholar]
- 3.Guilleminault C, Fromherz S. Narcolepsy: diagnosis and management In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4th ed. Elsevier Saunders; 2005:780–790. [Google Scholar]
- 4.D’Atri A, Scarpelli S, Schiappa C, et al. Cortical activation during sleep predicts dream experience in narcolepsy. Ann Clin Transl Neurol. 2019;6(3):445–455. doi: 10.1002/acn3.718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd Darien, IL: American Academy of Sleep Medicine; 2014:ICSD3. doi: 10.1378/chest.14-0970 [DOI] [Google Scholar]
- 6.Dodet P, Chavez M, Leu-Semenescu S, Golmard JL, Arnulf I. Lucid dreaming in narcolepsy. Sleep. 2015;38(3):487–497. doi: 10.5665/sleep.4516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lacaux C, Izabelle C, Santantonio G, et al. Increased creative thinking in narcolepsy. Brain. 2019;142(7):1988–1999. doi: 10.1093/brain/awz137 [DOI] [PubMed] [Google Scholar]
- 8.Maury A. Des hallucinations hypnagogiques, ou des erreurs des sens dans l’état intermédiaire entre la veilleet le sommeil. L. Martinet; 1848. doi: 12148/bpt6k5740964t
- 9.Foulkes D, Vogel G. Mental activity at sleep onset. J Abnorm Psychol. 1965;70(4):231. doi: 10.1037/h0022217 [DOI] [PubMed] [Google Scholar]
- 10.Mavromatis A. Ed. Hypnagogia: The Unique State of Consciousness Between Wakefulness and Sleep. Routledge; 1987. [Google Scholar]
- 11.Mazzarello P. What dreams may come? Nature. 2000;408(6812):523. doi: 10.1038/35046170 [DOI] [PubMed] [Google Scholar]
- 12.Foulkes SPS, Symonds JD. Individual differences in mental activity at sleep onset. J Abnorm Psychol. 1966;71(4):280. doi: 10.1037/h0023581 [DOI] [PubMed] [Google Scholar]
- 13.Wollman MC, Antrobus JS. Cortical arousal and mentation in sleeping and waking subjects. Brain Cogn. 1987;6(3):334–346. doi: 10.1016/0278-2626(87)90130-8 [DOI] [PubMed] [Google Scholar]
- 14.Nielsen TA. A review of mentation in REM and NREM sleep: “covert” REM sleep as a possible reconciliation of two opposing models. Behav Brain Sci. 2000;23(6):851–866. doi: 10.1017/S0140525X0000399X [DOI] [PubMed] [Google Scholar]
- 15.McMillan R, Kaufman SB, Singer JL. Ode to positive constructive daydreaming. Front Psychol. 2013;4:626. doi: 10.3389/fpsyg.2013.00626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agnoli S, Vannucci M, Pelagatti, Corazza GE. Exploring the Link Between Mind Wandering, Mindfulness, and Creativity: A Multidimensional Approach. Creat Res J. 2018;30(1):41–53. doi: 10.1080/10400419.2018.1411423 [DOI] [Google Scholar]
- 17.Zedelius CM, Schooler JW. The richness of inner experience: relating styles of daydreaming to creative processes. Front Psychol. 2016;6:2063. doi: 10.3389/fpsyg.2015.02063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giambra LM. The influence of aging on spontaneous shifts of attention from external stimuli to the contents of consciousness. Exp Gerontol. 1993;28(4–5):485–492. doi: 10.1016/0531-5565(93)90073-M [DOI] [PubMed] [Google Scholar]
- 19.Smallwood J. Distinguishing how from why the mind wanders: a process–occurrence framework for self-generated mental activity. Psychol Bull. 2013;139(3):519. doi: 10.1037/a0030010 [DOI] [PubMed] [Google Scholar]
- 20.Vannucci M, Pelagatti C, Marchetti I. Manipulating cues in mind wandering: verbal cues affect the frequency and the temporal focus of mind wandering. Conscious Cogn. 2017;53:61–69. doi: 10.1016/j.concog.2017.06.004 [DOI] [PubMed] [Google Scholar]
- 21.Seli P, Risko EF, Smilek D, Schacter DL. Mind-wandering with and without intention. Trends Cogn Sci. 2016;20(8):605–617. doi: 10.1016/j.tics.2016.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beghetto RA, Kaufman JC, Baxter J. Answering the unexpected questions: exploring the relationship between students’ creative self-efficacy and teacher ratings of creativity. Psychol Aesthet Creat Arts. 2011;5(4):342. doi: 10.1037/a0022834 [DOI] [Google Scholar]
- 23.Littner MR, Kushida C, Wise M, et al. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005;28(1):113–121. doi: 10.1093/sleep/28.1.113 [DOI] [PubMed] [Google Scholar]
- 24.Vandi S, Pizza F, Antelmi E, et al. A standardized test to document cataplexy. Sleep Med. 2019;53:197–204. doi: 10.1016/j.sleep.2017.08.021 [DOI] [PubMed] [Google Scholar]
- 25.Johns MW. Reliability and factor analysis of the Epworth sleepiness scale. Sleep. 1992;15(4):376–381. doi: 10.1093/sleep/15.4.376 [DOI] [PubMed] [Google Scholar]
- 26.Vignatelli L, Plazzi G, Barbato A, et al. Italian version of the Epworth sleepiness scale: external validity. Neurol Sci. 2003;23(6):295–300. doi: 10.1007/s100720300004 [DOI] [PubMed] [Google Scholar]
- 27.Åkerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52(1–2):29–37. doi: 10.3109/00207459008994241 [DOI] [PubMed] [Google Scholar]
- 28.Dauvilliers Y, Beziat S, Pesenti C, et al. Measurement of narcolepsy symptoms: the narcolepsy severity scale. Neurology. 2017;88(14):1358–1365. doi: 10.1212/WNL.0000000000003787 [DOI] [PubMed] [Google Scholar]
- 29.Milgram RM, Hong E. Creative out-of-school activities in intellectually gifted adolescents as predictors of their life accomplishment in young adults: a longitudinal study. Creat Res J. 1999;12(2):77–87. doi: 10.1207/s15326934crj1202_1 [DOI] [Google Scholar]
- 30.Runco MA, Noble EP, Luptak Y. Agreement between mothers and sons on ratings of creative activity. Educ Psychol Meas. 1990;50(3):673–680. doi: 10.1177/0013164490503025 [DOI] [Google Scholar]
- 31.Agnoli S, Corazza GE, Runco MA. Estimating creativity with a multiple-measurement approach within scientific and artistic domains. Creat Res J. 2016;28(2):171–176. doi: 10.1080/10400419.2016.1162475 [DOI] [Google Scholar]
- 32.Agnoli S, Mastria S, Kirsch C, Corazza GE. Creativity in the advertisement domain: the role of experience on creative achievement. Front Psychol. 2019;10:1899. doi: 10.3389/fpsyg.2019.01899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karwowski M, Lebuda I, Wiśniewska E. Measurement of creative self-efficacy and creative role-identity. High Abil Stud. 2012;22:291. [Google Scholar]
- 34.Carriere JS, Seli P, Smilek D. Wandering in both mind and body: individual differences in mind wandering and inattention predict fidgeting. Can J Exp Psychol. 2013;67(1):19. doi: 10.1037/a0031438 [DOI] [PubMed] [Google Scholar]
- 35.Chiorri C, Vannucci M. Replicability of the psychometric properties of trait-levels measures of spontaneous and deliberate mind wandering. Eur J Psychol Assess. 2019;35(4):459. doi: 10.1027/1015-5759/a000422 [DOI] [Google Scholar]
- 36.Singer JL, Antrobus JS. A factor-analytic study of daydreaming and conceptually-related cognitive and personality variables. Percept Mot Skills. 1963;17(1):187–209. doi: 10.2466/pms.1963.17.1.187 [DOI] [PubMed] [Google Scholar]
- 37.Stawarczyk D, Majerus S, Van der Linden M, D’Argembeau A. Using the daydreaming frequency scale to investigate the relationships between mind-wandering, psychological well-being, and present-moment awareness. Front Psychol. 2012;3:363. doi: 10.3389/fpsyg.2012.00363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guilford JP. Creativity: yesterday, today and tomorrow. J Creat Behav. 1967;1(1):3–14. doi: 10.1002/j.2162-6057.1967.tb00002.x [DOI] [Google Scholar]
- 39.Runco MA, Acar S. Divergent thinking as an indicator of creative potential. Creat Res J. 2012;24(1):66–75. doi: 10.1080/10400419.2012.652929 [DOI] [Google Scholar]
- 40.Agnoli S, Franchin L, Rubaltelli E, Corazza GE. An eye-tracking analysis of irrelevance processing as moderator of openness and creative performance. Creat Res J. 2015;27(2):125–132. doi: 10.1080/10400419.2015.1030304 [DOI] [Google Scholar]
- 41.Runco MA. Divergent Thinking. Norwood, NJ: Ablex Publishing Corporation; 1991. [Google Scholar]
- 42.Hayes AF, Matthes J. Computational procedures for probing interactions in OLS and logistic regression: SPSS and SAS implementations. Behav Res Methods. 2009;41(3):924–936. doi: 10.3758/BRM.41.3.924 [DOI] [PubMed] [Google Scholar]
- 43.Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. Guilford publications; 2017. [Google Scholar]
- 44.Kim KH. Meta-analyses of the relationship of creative achievement to both IQ and divergent thinking test scores. J Creat Behav. 2008;42(2):106–130. doi: 10.1002/j.2162-6057.2008.tb01290.x [DOI] [Google Scholar]
- 45.Beghetto RA. Creative self-efficacy: correlates in middle and secondary students. Creat Res J. 2006;18(4):447–457. doi: 10.1207/s15326934crj1804_4 [DOI] [Google Scholar]
- 46.Jaussi KS, Randel AE, Dionne SD. I am, I think I can, and I do: the role of personal identity, self-efficacy, and cross-application of experiences in creativity at work. Creat Res J. 2007;19(2–3):247–258. doi: 10.1080/10400410701397339 [DOI] [Google Scholar]
- 47.Tierney P, Farmer SM. Creative self-efficacy development and creative performance over time. J Appl Psychol. 2011;96(2):277. doi: 10.1037/a0020952 [DOI] [PubMed] [Google Scholar]
- 48.Maillet D, Seli P, Schacter DL. Mind-wandering and task stimuli: stimulus-dependent thoughts influence performance on memory tasks and are more often past-versus future-oriented. Conscious Cogn. 2017;52:55–67. doi: 10.1016/j.concog.2017.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pelagatti C, Binda P, Vannucci M. Tracking the dynamics of mind wandering: insights from pupillometry. J Cogn. 2018;1:1. doi: 10.5334/joc.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rak M, Beitinger P, Steiger A, Schredl M, Dresler M. Increased lucid dreaming frequency in narcolepsy. Sleep. 2015;38(5):787–792. doi: 10.5665/sleep.4676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zink N, Pietrowsky R. Relationship between lucid dreaming, creativity and dream characteristics. Int J Dream Res. 2013;6(2):98–103. doi: 10.11588/ijodr.2013.2.10640 [DOI] [Google Scholar]
- 52.US Xyrem® Multicenter Study Group. A randomized, double blind, placebo-controlled multicenter trial comparing the effects of three doses of orally administered sodium oxybate with placebo for the treatment of narcolepsy. Sleep. 2002;25(1):42–49. doi: 10.1093/sleep/25.1.42 [DOI] [PubMed] [Google Scholar]
- 53.Thorpy MJ, Bogan RK. Update on the pharmacologic management of narcolepsy: mechanisms of action and clinical implications. Sleep Med. 2020;68:97–109. doi: 10.1016/j.sleep.2019.09.001 [DOI] [PubMed] [Google Scholar]