Abstract
Acute hematogenous osteomyelitis (AHO) is a common invasive infection encountered in the pediatric population. In addition to the acute illness, AHO has the potential to create long-term morbidity and functional limitations. While a number of pathogens may cause AHO, Staphylococcus aureus is the most common organism identified. Despite the frequency of this illness, little high-quality data exist to guide providers in the care of these patients. The literature is reviewed regarding the epidemiology, microbiology and management of AHO in children. A framework for empiric therapy is provided drawing from the available literature and published guidelines.
Keywords: osteomyelitis, pediatrics, Staphylococcus aureus, oral antibiotics
Introduction
Osteomyelitis is one of the more common invasive bacterial infections of childhood. Studies from the mid-2000s in the United States suggested that osteomyelitis accounted for at least 6 per 1000 hospital admissions with rising incidence.1 Acute hematogenous osteomyelitis (AHO), the most frequent form of the disease in pediatrics, is often associated with the need for hospitalization and invasive diagnostic and surgical procedures as well as a prolonged course of antimicrobial therapy. Despite the frequency and potential morbidity associated with this infection, there has been a relative paucity of high-quality data available regarding the management of this disease. This review seeks to provide an evidence-based overview of AHO in children with a major focus on clinical management, incorporating the most up to date findings from the literature as well as practice guidelines.
Clinical Forms of Osteomyelitis
Infection can develop in bones through three primary mechanisms:2,3 1) direct inoculation, 2) extension from contiguous foci and 3) as a result of hematogenous dissemination. Direct inoculation osteomyelitis most often occurs through penetrating trauma or open fractures; in such infections, environmental microorganisms are often isolated in addition to skin flora.4 Osteomyelitis may also occur from the extension of infection from sites contiguous or adjacent to bones. This form of osteomyelitis is relatively uncommon in children and most often occurs in those with substantial disabilities (e.g., sacral osteomyelitis in the setting of quadriplegia and decubiti).3 AHO is overwhelmingly the most common form of osteomyelitis in children3,5 and most often affects the metaphysis of long bones.
Epidemiology
While AHO may affect any age group, this disease entity is most commonly seen in school-age children, with the typical age being 7–10 years old.1,6 Boys may be more often affected than girls with an approximately 1.5–2-fold increased rate reported in the literature, although this is not consistently observed.1,6,7 While children with certain underlying conditions (e.g., hemoglobinopathy) may be at increased risk of AHO, the vast majority of patients lack major medical comorbidities.8
Pathogenesis of AHO
While the precise pathogenesis of AHO in children is not completely clear, it is generally believed to be a consequence of the unique anatomy of growing bones combined with transient bacteremia. In the commonly accepted mechanism of pathogenesis as first suggested by Hobo and Trueta,9,10 blood vessels in the growing metaphyses of children form tight hairpin loops which create an area of relative vascular stasis/sluggish flow. These areas of the metaphysis may be susceptible to microhematomas or microthrombi with relatively minor blunt trauma which can, in turn, serve as a nidus for infection. Transient bacteremia may then result in the deposition of microorganisms in the metaphysis. Other investigators have asserted that terminal capillaries in the metaphyses communicate with one another between areas of growing/replicating chondrocytes via anastomoses which in turn contributes to vascular engorgement/stasis.11,12 Regardless of the precise microanatomy and pathophysiology, the replication of microorganisms in this region is responsible for disease in most cases of AHO. The proliferation of microorganisms in the bone with abscess formation may ultimately breach the cortex resulting in elevation of the periosteum and a subperiosteal abscess. These abscesses may in turn rupture through the periosteum into the surrounding soft tissues or into the adjacent joint, particularly in the case of the proximal femur or humerus which have intracapsular metaphyses. Additionally, the growth of abscesses may increase intraosseous pressure to the point at which the vascular supply is compromised resulting in bone necrosis.
In young infants (typically <18 months of age), bridging vessels that traverse the physis may allow for extension of infection into the epiphysis and then the joint space. Notably, contiguous septic arthritis does not always occur as a direct result of extension from the epiphysis/metaphysis, as suggested by the occurrence of this phenomenon in older children as well.13
Clinical Manifestations and Physical Examination Findings
AHO in children typically presents with some combination of fever, pain, swelling, erythema and warmth to the involved area. Patients will commonly have symptoms for 6–8 days prior to presentation, although this varies with the microbial etiology.14,15 Patients also frequently report a history of minor blunt trauma to the affected area. Although AHO may involve any part of the skeleton, the long bones of the lower extremities are among the most frequently affected followed by the pelvis. In one single-center study, the most commonly affected bones were the tibia, fibula, pelvis and femur in descending order.6 The upper extremities are more rarely affected, with infection of the humerus occurring in 10–14% and the radius or ulna in approximately 5% of cases.6,16 When the bones of the lower extremity are involved, children are commonly unable to bear weight or may have a pronounced limp. Infection involving the pelvis may be more subtle and is often associated with a delay in diagnosis. Children with pelvic osteomyelitis frequently (but not consistently) are able to bear weight to some degree but may display a waddling gait as they attempt to shift their weight away from the affected area. Infection involving the vertebrae may be associated with back pain, point tenderness, limited flexion or extension or sometimes change in spinal curvature.
Swelling, erythema, pain and limited function of the infected area are quite common. Patients are frequently moderately to even critically ill17 at the time of presentation. Up to 75% of patients are febrile at the time of admission.6 Care should be taken to perform as thorough a musculoskeletal exam as the child’s condition and level of distress allows; this is important as 5–10% of patients may have multifocal AHO.18 Additionally, contiguous septic arthritis has been reported in up to 35% of cases.6 Importantly, infection caused by less virulent organisms may present with more subtle symptoms and may be more challenging to diagnose.
General Laboratory Evaluation
Hematology studies and measurement of inflammatory indices are commonly performed in the evaluation of serious infection. Serum white blood cell count (WBC) may be normal in children with AHO; the sensitivity of leukocytosis for the diagnosis of AHO is only approximately 35%.19 A complete blood count (CBC) however, may be helpful in evaluating for other causes of bone pain such as leukemia, neuroblastoma or other malignancy. By contrast, C-reactive protein (CRP) is elevated in up to 98% of children with AHO and, while being nonspecific, is a highly sensitive tool.19,20 In general, obtaining a CBC, CRP and erythrocyte sedimentation rate (ESR) is advisable in the initial evaluation of all patients with suspected AHO.21
Microbiology Diagnostic Studies
Efforts to determine the microbiologic cause of AHO should always be undertaken. Identification of a pathogen can allow for targeted antimicrobial therapy and can give a sense of closure to patient families by giving a “name” to the infection. At a minimum, blood cultures of adequate volume should be performed in all patients with suspected AHO. Blood cultures yield a pathogen in 20–46% of patients.1,6,22 Cultures of bone exudates, abscesses or aspirates from contiguous joints or soft tissue collections yield pathogens in 65–82% of specimens;6,23 in nearly half of such patients, surgical specimens serve as the only means of obtaining a microbiologic diagnosis.6 The recovery of certain fastidious organisms (i.e., Kingella kingae) may be enhanced by the inoculation of bone or synovial fluid samples into blood culture bottles and is recommended in young children (<5 years) or when suspicion otherwise exists.24 Notably, anaerobic, fungal and/or mycobacterial cultures obtained from bone or joint specimens have relatively low yield (1–3%)22 in pediatric AHO but should be utilized in those with a history of immunocompromise, atypical symptoms, subacute/chronic disease,5,25 history of penetrating/open trauma4 and/or failure of first-line therapy.3 If such specimens are obtained, care should be taken that they are collected and processed correctly.25 In young infants who acquire infection with pathogens which are also known as common causes of meningitis (e.g., pneumococcus, Group B Streptococcus or Haemophilus influenzae), consideration may need to be given towards performing a lumbar puncture to obtain a CSF culture; meningitis has been described concomitantly, albeit rarely, in such patients.26–28
Beyond traditional culture methods, increasing interest has developed in molecular diagnostics for AHO. A number of multiplex PCR-based panels exist to help identify major AHO pathogens from either bone exudates, purulent collections or synovial fluid; in some cases, these studies are able to identify select antibiotic resistance genes.29 Notably, a number of studies have illustrated that the use of PCR-based assays increases the rates of K. kingae identification by 2–4 fold.30,31 In one series, 89% of cases of K. kingae musculoskeletal infection were diagnosed by molecular methods alone.31
Diagnostic Imaging
Imaging studies are frequently undertaken to help secure the diagnosis of AHO. Plain radiographs have poor ability to identify AHO early in the disease course; however, most experts continue to recommend their use in order to evaluate for alternative causes of musculoskeletal pain (e.g., fracture, bone tumor, etc.).21 Magnetic resonance imaging (MRI) has become the gold standard in the imaging diagnosis of AHO and may be helpful in localizing associated purulent collections. In one study of S. aureus osteomyelitis, MRI detected extraosseous sites of infection in up to 68% of patients.32
Microbial Etiology
A microbiologic etiology is identified in 66–76% of cases of AHO.1,6 The relative frequency of recovery of common organisms in AHO is summarized in Table 1. While AHO may be caused by a variety of microorganisms, the vast majority of cases are a result of S. aureus, which contributes to approximately 60% of all AHO in children.6
Table 1.
Relative Frequency of Organism Identification in Pediatric Acute Hematogenous Osteomyelitis
| Causative Organism | Proportion of Cases (%) |
|---|---|
| Staphylococcus aureus | 43–63 |
| MRSA as a proportion of S. aureus | 0–69.1 |
| Streptococcus pyogenes | 2.5–7 |
| Salmonella spp. | 1–3.8 |
| S. agalactiae | 0.5–5.1 |
| S. pneumoniae | 0–1.9 |
| Kingella kingae | 0–47.4* |
| Other Organisms | 1–3 |
| No Organism Identified | 24–43.7 |
The relative proportion of cases of staphylococcal AHO which are due to methicillin-resistant S. aureus (MRSA) would be expected to vary based on the local community prevalence of MRSA. Cases caused by MRSA tend to be associated with a more severe course including higher and more prolonged fever, a greater elevation of inflammatory markers, large purulent collections, the need for multiple surgical procedures and prolonged length of stay.1,33–35 These findings are largely driven in North America by the predominance of the USA300 pulsotype among community-acquired MRSA. Complications associated with USA300 S. aureus AHO include venous thromboses, septic emboli with/without necrotizing pneumonia and pathologic fractures.36,37 It is unlikely that the negative outcomes associated with MRSA AHO are a result of methicillin-resistance per se, but rather the overall genetic background of the organism.38 The influence of strain type rather than antibiotic resistance on outcome is indirectly supported by observational studies suggesting a more severe clinical course and sequelae of USA300 methicillin-susceptible S. aureus (MSSA) compared to non-USA300 MSSA.39 Importantly, S. aureus AHO as a whole is more often associated with the presence of subperiosteal/intraosseous abscesses and the need for surgical intervention than osteomyelitis due to other etiologies irrespective of antibiotic susceptibility.1,6
Group A Streptococcus (S. pyogenes) is commonly reported as the second most frequently isolated organism in acute musculoskeletal infections in children, occurring in 2–9% of cases.1,6,13 Invasive S. pyogenes infections are frequently associated with regional myositis which may be noted on MRI or CT. S. pyogenes remain universally susceptible to β-lactams which are the drugs of choice. Vancomycin and clindamycin are reasonable substitutes in the situations of β-lactam intolerance or allergy; notably, isolates exhibit in vitro resistance to trimethoprim-sulfamethoxazole (TMP-SMX). Interestingly, in recent studies of skin-and-soft-tissue infections of any etiology (including S. pyogenes), patients treated with TMP-SMX had similar outcomes to those treated with alternative agents.40,41 No data are available regarding the potential use of TMP-SMX for invasive S. pyogenes infection, however.
Non-typhi Salmonella (NTS) spp. are not infrequently identified in children with AHO, accounting for approximately 5% of cases.1,6 Notably, compared to those cases caused by gram-positive pathogens, children with NTS more often have a history of reptile exposure and/or antecedent gastrointestinal symptoms (Table 2).42 Additionally, patients with immunodeficiencies, especially hemoglobinopathies, are at particular risk for invasive salmonellosis; in case series of hemoglobinopathy patients with AHO, Salmonella is recovered with at least equal frequency as S. aureus and in some studies far exceeds the frequency of S. aureus in this population.43–46 Importantly, Salmonella AHO may be associated with long durations of fever, with a median duration of fever after hospital admission of 8.5 days in one study.42
Table 2.
Pathogens to Consider in Unique Populations
| Patient Population | AHO Pathogens |
|---|---|
| Infants < 1 Year of Age | Group B Streptococcus Haemophilus influenzae S. aureus Neisseria gonorrheae Group A Streptococcus Enterobacteriaceae |
| Children 1–5 Years of Age |
Kingella kingae S. aureus S. pneumoniae |
| School Age - Adolescence |
S. aureus Group A Streptococcus |
| Hemoglobinopathy/Asplenia |
Salmonella spp. S. aureus Plesiomonas H. influenzae S. pneumoniae |
| Intravenous Drug Users |
S. aureus Enterobacteriaceae Pseudomonas aeruginosa |
| Animal Exposures |
S. aureus Salmonella spp. Bartonella henselae Brucella spp. |
K. kingae is a pathogen which has been often associated with osteoarticular infections in toddler age children. While more commonly associated with septic arthritis, this pathogen has often been identified in osteomyelitis as well.47 In an Israeli study of osteoarticular infections in children <2 years old, K. kingae was the most frequently identified organism.24 Similarly, in a Swiss study, K. kingae accounted for 87% of confirmed osteoarticular infections in children <4 years old while S. aureus caused 78% in older children.48 K. kingae is frequently associated with a less severe disease presentation than staphylococcal AHO. In a French study comparing K. kingae and S. aureus AHO, K. kingae infections were associated with shorter durations of fever and hospital stay.49 Others have reported that invasive K. kingae infections were associated with normal inflammatory markers in nearly 25% of cases.47 K. kingae has also been noted as a principal cause of hematogenous osteomyelitis of the calcaneus as well as the sternum and may have a predilection for these sites.47,50 While being a notoriously fastidious organism, identification may be aided by inoculation of bone exudates or synovial fluid directly into blood culture bottles51 and/or the utilization of molecular diagnostics.30,31 In many studies in Europe and Israel, K. kingae is the predominant cause of AHO in children, even surpassing S. aureus.48 By contrast, most studies in North America suggest that K. kingae accounts for <5% of cases of AHO overall.6,34 This discrepancy likely reflects a combination of geographic variability in prevalence as well as inconsistent utilization of molecular diagnostics across centers.
The respiratory pathogens Streptococcus pneumoniae and Haemophilus influenzae may rarely cause AHO particularly among those with antecedent respiratory symptoms or those with immunodeficiency or underimmunization.1,6,16 The widespread use of the pneumococcal conjugate vaccines as well as H. influenzae type b vaccine has had a significant impact on these infections.52
Bartonella henselae may rarely cause osteomyelitis as a manifestation of cat scratch disease with a predilection for the axial skeleton.53 Molecular methods may be utilized to detect B. henselae in tissue although serology can also be employed for presumptive diagnosis. Notably, while patients with cat scratch disease typically have cat/kitten exposure, this is not universally the case.54,55
Neonates and young infants represent a unique group for AHO. Group B Streptococcus (GBS, S. agalactiae) is a well-described cause of AHO in neonates and should be high in the differential diagnosis; in some older series, GBS is the most frequent etiology in this age group.28 Gram-negative enterics must also be considered in AHO in neonates, particularly the premature. Notably, however, S. aureus remains a prominent cause of osteoarticular infection in neonates and young infants;56 consideration of these pathogens is important when planning empiric antibiotic coverage.
Management
Supportive Care
Early in the evaluation of children with suspected AHO, attention should be given to the patient’s hemodynamic state, providing prompt fluid resuscitation if indicated. Notably, 5–11% of children with S. aureus AHO require intensive care unit admission.8,18,38 Additionally, analgesics and antipyretics should be administered as needed.
Empiric Antimicrobial Therapy and Specific Agents
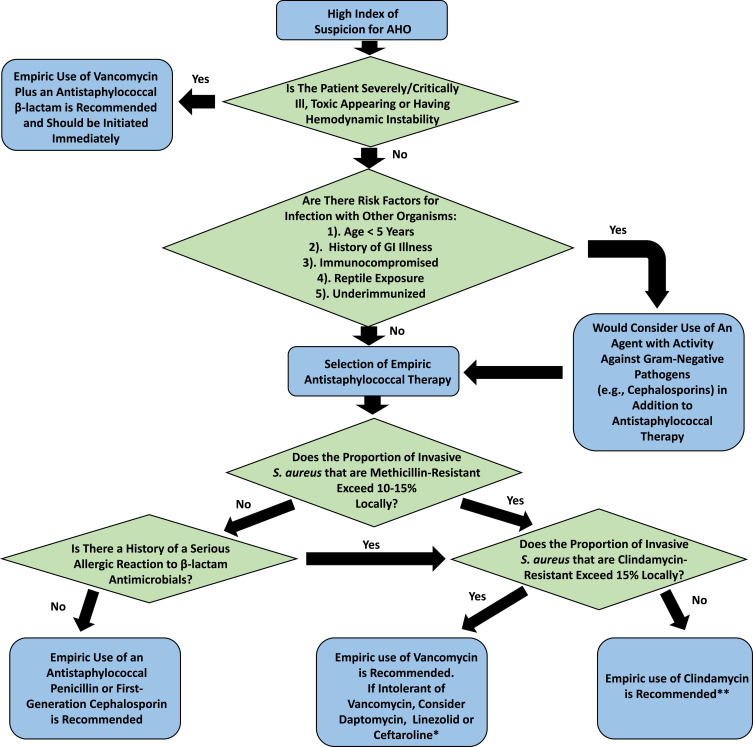
Intravenous antibiotics are typically initiated empirically in patients with known or suspected AHO (Table 3, Figure 1). Given the predominance of S. aureus, empiric parenteral therapy directed at gram-positive pathogens is appropriate in most situations. The European Society for Pediatric Infectious Diseases (ESPID) has published guidelines for the management of osteoarticular infections. In regions with a low prevalence of MRSA, the empiric use of antistaphylococcal penicillins (ASPs, e.g., nafcillin, flucloxacillin) or first-generation cephalosporins (1GCs) is recommended.21 A number of studies have demonstrated that ASPs and 1GCs have similar outcomes in the treatment of invasive MSSA infections.57,58 In a recent study, definitive treatment with a 1GC relative to alternative agents did not increase the likelihood of treatment failure in children with MSSA AHO.8 Cefazolin may have the additional advantage of providing coverage for K. kingae in toddler age children; K. kingae have relatively high MICs to oxacillin (MIC90= 6 µg/mL).59
Table 3.
Potential Antimicrobial Agents for the Management of AHO
| Antimicrobial | Bacteriostatic/Bactericidal | Antimicrobial Spectra* | Adverse Effects | Other Considerations |
|---|---|---|---|---|
| Antistaphylococcal penicillins | Bactericidal | MSSA, Group A Streptococcus | Potential for drug induced neutropenia, nephritis. Oxacillin associated with hepatotoxicity. Phlebitis and/or local discomfort may develop with IV infusion, particularly with nafcillin. | One of the agents of choice for MSSA. Limited activity against K. kingae. Liquid formulations of oral flucloxacillin and dicloxacillin have poor palatability. |
| First-generation cephalosporins | Bactericidal | MSSA, Group A Streptococcus, K. kingae | Potential for drug induced neutropenia, nephritis. | Poor CNS penetration. Some concern exists for inactivation of these drugs in the presence of large inoculum of S. aureus (i.e., cefazolin inoculum effect), although data are limited |
| Vancomycin | Bactericidal | MRSA, MSSA, Group A Streptococcus, Group B Streptococcus | Potential for drug induced neutropenia, thrombocytopenia. Infusion reactions (“Red Man Syndrome”). Nephrotoxicity associated with vancomycin is well described. | Optimal monitoring parameters not established in children. Close monitoring of renal function recommended. Intravenous only administration. |
| Clindamycin | Bacteriostatic | Clindamycin-susceptible MRSA and MSSA, Group A Streptococcus. Some K. kingae strains are susceptible. | Gastrointestinal distress, diarrhea are common. Potential for transaminitis. | May be administered intravenously but has high oral bioavailability. Local rates of clindamycin-resistance among S. aureus should be considered prior to empiric use. Poor CNS penetration. Not recommended if endovascular disease present. Many liquid formulations have poor palatability. |
| Linezolid | Bacteriostatic | S. aureus, Group A Streptococcus, Pneumococcus | Potential for drug induced neutropenia, thrombocytopenia. Prolonged use has been associated with optic neuritis and peripheral neuropathy. Tyramine containing foods should be avoided. | May be administered intravenously but has high oral bioavailability. Close monitoring for adverse events, particularly myelosuppression, is advised. Cost of this agent may be prohibitive |
| Daptomycin | Bactericidal | S. aureus, Group A Streptococcus, Pneumococcus | Potential for transaminitis. Rhabdomyolysis is a reported but rare adverse event | In animal models, inactivated by pulmonary surfactant. Good CNS penetration. Intravenous only administration. Limited data in the setting of pediatric AHO. |
| Trimethoprim-Sulfamethoxazole | Bactericidal | S. aureus, K. kingae, some gram-negative enterics | Drug induced cytopenias, nephritis, rash. May rarely be associated with erythema multiforme/Stevens Johnson | Excellent in vitro activity against S. aureus in most studies. Data are limited in the setting of invasive S. aureus infection particularly AHO. |
| Tetracyclines | Bacteriostatic | S. aureus | Potential for transaminitis. Patients may become very photosensitive and should be advised to wear sun screen while outdoors. Dental staining in young children with prolonged use (relative contraindication in those < 8 years) | Excellent in vitro activity against S. aureus in most studies. Data are limited in setting of invasive S. aureus infection, particularly AHO. |
| Penicillins, Aminopenicillins | Bactericidal | Group A Streptococcus, Group B Streptococcus penicillin-susceptible pneumococci, β-lactamase negative H. influenza | Rash. Drug-induced cytopenias with prolonged use. Nephritis | |
| Third Generation Cephalosporins | Bactericidal | Group A Streptococcus, K. kingae, pneumococci, H. influenzae, many gram-negative enterics (including Salmonella) | Diarrhea. Rash. Drug induced cytopenias, transaminitis, nephritis are possible with prolonged use. | Generally well tolerated class of drugs with agents that can be administered either intravenously or orally. Ceftriaxone with in vitro activity against MSSA, however clinical data in children with invasive MSSA are limited. |
Notes: *The description of antimicrobial spectra is not intended to be comprehensive, but to briefly describe a few relevant AHO pathogens included within the antimicrobial spectra of the agent of interest.
Figure 1.
Suggested Decision Tree for Empiric Therapy Selection in Acute Hematogenous Osteomyelitis. Disclaimer: This is intended as a framework for thought and is no substitute for clinical judgment, obtainment of a thorough patient history and knowledge of local microbiology/epidemiology. *Data are limited regarding the use of ceftaroline and daptomycin in the treatment of osteoarticular infections in children. **Clindamycin is not recommended if patients are critically ill or there is concern for endovascular disease or infection involving the central nervous system.
The need to provide empiric therapy directed at MRSA should be determined based on local prevalence of MRSA as well as individual patient clinical risk factors (e.g., history of prior MRSA infections). The ESPID guidelines recommend empiric coverage for MRSA if the local prevalence of methicillin-resistance among S. aureus exceeds 10–15% (Figure 1).21 Vancomycin is one of the primary agents for treatment of MRSA in children and is recommended for patients with severe disease.21,60 Current guidelines recommend starting vancomycin at doses of 60–80 mg/kg/day divided every 6 hours in children 3 mo–12 years old with serious MRSA infections.61 Notably, depending on the dosing regimen and comorbidities, vancomycin carries with it a non-trivial risk of nephrotoxicity.62–64 Moreover, the appropriate pharmacokinetic/pharmacodynamic parameters for vancomycin monitoring are somewhat controversial in children.61,62,65–67 Close attention to renal function is highly recommended in children receiving vancomycin.
Clindamycin is a well-tolerated alternative to vancomycin which has been demonstrated to be effective in serious S. aureus (and MRSA) musculoskeletal infections including those with bacteremia.66,68,69 The ESPID guidelines recommend the empiric use of clindamycin in regions with a relatively high prevalence of MRSA but with clindamycin resistance in <10–15% of S. aureus provided patients are not severely ill. Notably, however, empiric therapy can be quite challenging in regions with a high prevalence of both methicillin and clindamycin resistance, as occurs in some areas of the US.70 Clindamycin is not recommended for either endovascular infections or when there is the need for penetration into the central nervous system; as such, clindamycin may not be ideal for patients with severe disseminated MRSA infection. Overall, it is important to consider the potential for clindamycin resistance as well as other concomitant sites of infection when selecting empiric therapy in regions with high rates of MRSA.
Other agents to consider for empiric coverage of known or suspected serious MRSA infections include linezolid, daptomycin, and ceftaroline. Linezolid has excellent activity against gram-positive agents and has been used effectively in the setting of AHO in children.71 Notably, however, potential toxicities of this agent (myelosuppression, peripheral neuropathy, optic neuritis, etc.) as well as costs limit its use. A number of case reports and series describe using daptomycin to successfully treat MRSA AHO in children.72,73 In one recent pediatric multicenter randomized controlled trial, there were no statistically significant differences in outcomes between children with AHO treated with daptomycin compared to comparator agents; however, non-inferiority endpoints were not achieved.74 Ceftaroline, an anti-MRSA cephalosporin has the theoretical advantage of providing additional coverage for streptococci, K. kingae and some Enterobacteriaceae. Data on the use of this agent in children with AHO are, however, currently limited to case reports.75
While recognizing that staphylococci and streptococci are the predominant causes of disease, certain clinical situations may necessitate expanding empiric antibiotic coverage (Table 2). In patients with hemoglobinopathies or when Salmonella spp. or H. influenzae are otherwise clinically suspected, antimicrobials active against these pathogens (e.g., third-generation cephalosporins) should be added to typical anti-staphylococcal therapy.21 Children <5 years of age may require coverage for Kingella.21 Other situations which warrant an expansion of antimicrobial coverage include immunocompromised hosts, those with a history of multidrug-resistant pathogens or who have failed first-line therapy.
Questions often arise as to the optimal duration, route and choice of therapy as well as timing of antimicrobial therapy in relation to obtaining deep cultures; such clinical dilemmas will be discussed below.
Duration of Total Antimicrobial Therapy
One of the most often cited studies regarding the appropriate duration of therapy is the 1979 study by Dich et al. These investigators retrospectively reviewed 163 cases of osteomyelitis in North American children. Among children with S. aureus osteomyelitis, those receiving therapy for ≤3 weeks experienced a higher rate of progression to chronic infection (19%) than those who were treated for >3 weeks (2%).16 This formed much of the basis for the commonly recommended duration of therapy of 4–6 weeks in North America. A retrospective study from the United Kingdom, also conducted during the 1970s, revealed a similar rate of treatment failure with <3 weeks of therapy; in the subset treated >3 weeks, those experiencing treatment failure had a higher ESR and/or persistent symptoms compared to those who had treatment success.76 More recently, Peltola conducted a randomized controlled trial of 20 vs 30 days of treatment for AHO in Finland; CRP monitoring was also performed with a CRP < 2.0 mg/dl considered a potential threshold for therapy discontinuation. These investigators found no significant difference in the rates of developing sequelae in the long vs. short therapy arms.14 It is important to note, however, that this study spanned 20 years of enrollment and included a variety of pathogens (including H. influenzae type b) but no cases of infection due to MRSA. Additionally, relatively few patients had disease requiring surgical intervention in this study population. Thus, while very select patients may be able to be successfully treated with ≤20 days of therapy, applying these findings to populations with high rates of MRSA or patients with large disease burdens should be performed cautiously. The current ESPID guidelines recommend a minimum of 3–4 weeks of therapy for AHO.21 The common practice in North America is to treat AHO for at least 4 weeks of total therapy or until inflammatory markers (ESR and CRP) have normalized (or are nearly normal) and symptoms have resolved.3,77 The ESPID guidelines acknowledge that a longer course of therapy may be required for disease caused by MRSA or Salmonella, infection of the pelvis or spinal column, severe/complicated infection or those with slow response to therapy. Regardless of exact duration, prior to considering discontinuation of therapy, virtually all of the patient’s symptoms should have resolved and the CRP nearly normalized.
Oral Step-Down Therapy vs Prolonged Intravenous Therapy
Transition from intravenous antibiotics to oral therapy in AHO was once a controversial clinical issue but has become a much more accepted practice.78 In principle, significantly higher serum concentrations of antibiotics may be achievable with the intravenous route compared to oral administration. However, this is likely unnecessary in the majority of patients. In a large multicenter observational study, transition to oral antibiotic therapy was associated with similar rates of treatment failure compared to prolonged intravenous therapy.79 Additionally, the use of outpatient parenteral antimicrobial therapy (OPAT) rather than discharge on oral antibiotics has been associated with a higher rate of emergency room visits and hospital readmissions, compounding risks intrinsic to having a venous catheter.80–82 Thus, in many situations, the potential risk of adverse events may outweigh the theoretical benefits of prolonged intravenous over oral antibiotics.
Notably, the presence of bacteremia associated with osteomyelitis does not in-and-of-itself justify prolonged parenteral antibiotic therapy. In a single-center retrospective study, patients with S. aureus osteomyelitis and positive blood cultures who were discharged on OPAT were compared to those discharged on oral antibiotics and were found to have similar outcomes. Notably, the transition to oral antibiotics occurred after a median of 7 days of intravenous antibiotics (interquartile range of 5–10 days).66 Similarly, in a sub-analysis of data from a randomized controlled trial in Finland, children with AHO associated with bacteremia were similar to those without bacteremia in terms of time to normalization of ESR and CRP and long-term orthopedic outcomes.83
The optimal duration of intravenous therapy that should be administered prior to transition to oral therapy has not been precisely defined with definitions of early transition ranging from 2 to 14 days in the literature.14,45,66 In practice, the majority of patients can be safely transitioned from intravenous to oral antibiotics once blood cultures have sterilized, they have demonstrated clinical improvement, pain is well controlled and the patient is eating/drinking well and able to tolerate oral medications. Some experts also recommend that CRP should be monitored and demonstrate a downward trend prior to considering oral step-down.84
It should be recognized that situations arise in which the use of prolonged intravenous therapy may be justified. The ESPID guidelines suggest that a longer duration of both total and intravenous therapy may be required for severe multifocal disease, immunocompromised hosts, those of very young age or infections caused by MRSA or Salmonella species,21 albeit there is not robust evidence for such practices. In one small single-center review of osteoarticular infections in children with sickle hemoglobinopathy, Salmonella accounted for 61% (n=14) of cases with many being able to undergo early transition to oral therapy after ≤14 days of intravenous therapy with good outcomes.45 Given the small sample size in this study, caution is nevertheless warranted.
Agents for Oral Therapy
As a general rule, antimicrobial therapy should be tailored toward the susceptibility of the isolated organism. Methicillin-susceptible S. aureus (MSSA) should be treated with an oral antistaphylococcal β-lactam (e.g., dicloxacillin, cephalexin, etc.).21 As stated above, clindamycin may be utilized effectively to treat MRSA isolates susceptible to this agent even in the setting of serious infection and/or bacteremia provided the patient is not critically ill.66 Clindamycin has excellent oral bioavailability and is generally well tolerated in children, although diarrhea is a common side effect. An increased risk of developing Clostridium difficile-associated diarrhea has been reported with this agent, although the absolute risk is relatively low.85 Liquid formulations of clindamycin are often foul tasting which may present challenges for adherence in young children. As stated above, linezolid may be considered in patients with clindamycin-resistant MRSA or those who are intolerant of other therapies; close monitoring for the development of adverse drug events is urged. TMP-SMX has excellent in vitro activity against staphylococci. While there is clinical experience with this agent, little published data exist on the efficacy of TMP-SMX in the setting of pediatric AHO.86 Similarly, tetracyclines also have good in vitro activity against staphylococci but data on their efficacy in children with AHO are lacking.87 Additionally, the typical several week treatment course for AHO effectively restricts the use of tetracyclines to children >8 years old.
K. kingae infection is frequently a concern in young children with AHO even if not confirmed by culture or molecular methods. These isolates are universally resistant to vancomycin and frequently clindamycin but are typically susceptible to β-lactam antimicrobials; K. kingae may produce a β-lactamase in up to 26% of cases.59,88,89 As antimicrobial susceptibility testing cannot frequently be performed in the clinical setting, use of a cephalosporin is often recommended.21 Many strains of K. kingae are also susceptible to TMP-SMX,59,89 potentially making this an attractive alternative to β-lactams.
A number of oral β-lactams are available for the treatment of AHO by other organisms including Salmonella, H. influenza and pneumococcus although the choice of specific agents should be guided by susceptibility testing. Fluoroquinolones have been used successfully as stepdown therapy for Salmonella osteomyelitis90,91 although the emergence of resistance on therapy has been reported.92
Treatment of Culture-Negative AHO
In the event, a pathogen is not definitively identified (culture-negative AHO), therapy is typically directed against gram-positive pathogens unless risk factors for other organisms exist to otherwise guide therapy. Typically, such patients present with milder disease than those in whom a pathogen is identified.93 A number of series have shown that patients with culture-negative AHO can be treated similarly to gram-positive AHO in terms of antibiotic choice, route and duration with a high rate of success (>95%).94,95
Should Antibiotics Be Held Pending Surgical Intervention, Percutaneous Aspiration or Bone Biopsy?
Given the relatively long course of therapy required for AHO and that >50% of patients have negative blood cultures, some experts recommend waiting to start systemic antibiotics until deep cultures (from bone, synovial fluid or adjacent purulent collections) are obtained to maximize culture yield provided patients are clinically stable.96,97 In the setting of osteomyelitis, the use of an active antimicrobial will logically at some point result in sterilization of the bone. That being stated, it is difficult to know to what degree a short period of antibiotic pretreatment may impact culture yield. In one single-center pediatric series, among patients who received antibiotic pretreatment prior to bone biopsy, the duration of antibiotic pretreatment was longer in those with negative cultures than those with positive cultures (mean of 79 vs. 40 hours).23 The extent of disease likely influences the impact of antibiotic pretreatment. Investigators at one center reported that among patients requiring open surgical drainage/debridement, culture yield remained >80% with up to 72 hours of antibiotic pretreatment; by contrast, among the subset of patients undergoing only percutaneous bone biopsy, culture yield declined after only 24 hours of pretreatment.6 Overall, the desire to obtain a definitive microbiologic diagnosis must be carefully balanced with the potential risk for clinical decompensation in a child with an untreated serious infection.
Surgical Intervention
Surgical intervention has the potential for providing specimens for culture and microbiologic diagnosis as well as a therapeutic benefit. Drainage of purulent collections may promote pain relief and facilitate a more rapid response to medical therapy. In general, indications for surgical intervention may include (but are not limited to) the presence of subperiosteal, intraosseous or adjacent soft tissue abscesses or failure to improve with medical therapy alone. Bone biopsy purely for the purpose of obtaining diagnostic specimens, either through open surgical procedures or percutaneous techniques, should also be considered and can be performed relatively safely.6 This may be particularly important in regions with high rates of antibiotic resistance where selection of empiric therapy can be challenging.6
There is emerging data that early surgical intervention in AHO may promote good long-term outcomes. In a single-center study of culture-confirmed S. aureus AHO, early surgical source control (<3 days after presentation to care) was associated with a reduced rate of developing late complications of AHO;18 notably, this study population possessed a higher rate of bone abscesses as well as MRSA than that in other studies which may have biased results. An additional study examining S. aureus AHO found that patients who underwent a planned “second look” operative procedure with irrigation and debridement experienced shorter durations of fever and hospital stay than those who underwent a single surgical procedure with primary closure.98 Of note, as both of these studies focused on culture-confirmed S. aureus disease, it is unclear how big an impact surgical intervention would have if all causes of AHO are considered. Specific surgical techniques utilized may include drilling, corticotomy, incision/drainage of abscesses and curettage; robust clinical data illustrating superiority of one technique over another are lacking.
Additionally, a high index of suspicion must always exist for concomitant infection of the contiguous joint. It is particularly critical to consider adjacent septic arthritis with infection of the bones of either the pelvic or shoulder girdle so that timely arthrocentesis/arthrotomy may be performed in the hip or shoulder to preserve good function.99
It is important to keep in mind, however, that many cases of AHO which are less severe and without purulent collections or contiguous septic joints can be managed without surgical intervention and achieve good outcomes.7 It should be recognized that such patients typically have fewer and smaller abscesses, lower inflammatory markers and are, in general, less ill than their counterparts who undergo combined medical and surgical management, making direct comparisons between treatment groups challenging.18 A multi-disciplinary approach is strongly recommended with close consultation between general pediatricians, infectious diseases specialists and orthopedists in the assessment and management of children with AHO.100
Prognosis
In general, the prognosis for the majority of children with AHO is excellent.14,18 Long-term complications of AHO have been reported in <1–10% of patients.1,8,16,18,37,101 The most common sequelae are recurrence of infection/development of chronic osteomyelitis followed by pathologic fractures.18 It is generally believed that the biggest risk factors for sequelae include inadequate or inappropriate treatment of acute infection.2,3 In one series, the development of chronic infection was associated with delayed source control and prolonged fever as well as infection with certain specific strains of S. aureus;18 many of these patients experienced hospital readmissions and persistent functional limitations. In S. aureus AHO, pathologic fractures have been associated with USA300 S. aureus, large abscesses and the need for multiple surgical debridements.18,37 Other potential complications include growth arrest/limb length discrepancy (particularly with disease of the physis/epiphysis), angular deformity and avascular necrosis, all of which may be associated with significant morbidity. Notably, a small subset of patients may report vague musculoskeletal symptoms or sometimes arthritic symptoms (e.g., morning stiffness, pain/swelling with change in weather, etc.) after musculoskeletal infection,18 although no controlled studies exist regarding these findings. Importantly, while it can be challenging to predict which patients may develop sequelae, the majority of patients have eventual return to normal activities with no long-term consequences.
Conclusion
AHO is a relatively common serious bacterial infection of children. While this disease can be caused by a wide variety of pathogens, S. aureus is the predominant etiology. A multidisciplinary approach including the consideration of combined medical and surgical management should be considered in these patients. Although there is the potential for long-term sequelae, the majority of children with AHO can be transitioned to oral antibiotics once clinically improved and complete their treatment as outpatients. Further studies are needed to help guide the optimal management of these children.
Disclosure
The author reports grants and non-financial support from Allergan and grants from Merck and Nabriva, outside the submitted work; and receives a royalty from UpToDate Online for a review article, and grant funding from the Agency for Healthcare Research and Quality (AHRQ R01HS026896) on work unrelated to osteomyelitis. The author is the local PI on a multicentre clinical trial sponsored by Nabriva Therapeutics unrelated to osteomyelitis. The author is a local-sub-investigator on a multicenter clinical trial sponsored by Merck and an investigator-initiated trial of ceftaroline in osteomyelitis sponsored by Allergan. The author has also received a donation of laboratory materials from Allergan for work unrelated to osteomyelitis. The author reports no other potential conflicts of interest.
References
- 1.Arnold SR, Elias D, Buckingham SC, et al. Changing patterns of acute hematogenous osteomyelitis and septic arthritis: emergence of community-associated methicillin-resistant Staphylococcus aureus. J Pediatr Orthop. 2006;26(6):703–708. doi: 10.1097/01.bpo.0000242431.91489.b4 [DOI] [PubMed] [Google Scholar]
- 2.Guitierrez K. Osteomeylitis In: Long SS, Pickering LK, Prober CG, editors. Principles and Practice of Pediatric Infectious Diseases. New York: Elsevier Saunders; 2012:469–477. [Google Scholar]
- 3.Krogstad P. Osteomyelitis In: Cherry JD, Harrison GJ, Kaplan SL, Steinbach WJ, Hotez PJ, editors. Feigin and Cherry’s Textbook of Pediatric Infectious Diseases. Vol. 1 8 Philadelphia, PA: Elsevier;2019:516–529. [Google Scholar]
- 4.McNeil JC, Vallejo JG, Hulten KG, Kaplan SL. Osteoarticular infections following open or penetrating trauma in children in the post-community-acquired methicillin-resistant Staphylococcus aureus era: the impact of Enterobacter cloacae. Pediatr Infect Dis J. 2018;37(12):1204–1210. doi: 10.1097/INF.0000000000001991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joseph M, Sommer LM, Vallejo JG, McNeil JC The spectrum of chronic osteomyelitis in children. Infectious Diseases Society of America IDWeek Annual Meeting 2020; 2020; Philadelphia, PA (Virtual). [Google Scholar]
- 6.McNeil JC, Forbes AR, Vallejo JG, et al. Role of operative or interventional radiology-guided cultures for osteomyelitis. Pediatrics. 2016;137:e20154616. doi: 10.1542/peds.2015-4616 [DOI] [PubMed] [Google Scholar]
- 7.Peltola H, Unkila-Kallio L, Kallio MJ. Simplified treatment of acute staphylococcal osteomyelitis of childhood. The Finnish Study Group. Pediatrics. 1997;99(6):846–850. doi: 10.1542/peds.99.6.846 [DOI] [PubMed] [Google Scholar]
- 8.McNeil JC, Sommer LM, Boyle M, et al. Cefazolin inoculum effect and methicillin-susceptible Staphylococcus aureus osteoarticular infections in children. Antimicrob Agents Chemother. 2020;64(9):e00703–20. doi: 10.1128/AAC.00703-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trueta J. The three types of acute haematogenous osteomyelitis. J Bone Joint Surg Br. 1959;41–B:671–680. [Google Scholar]
- 10.Hobo T. Zur pathogenese der akuten haematogenen osteomyelitis. Acta Sch Med Univ Kioto. 1921;4:1–29. [Google Scholar]
- 11.Stephen RF, Benson MK, Nade S. Misconceptions about childhood acute osteomyelitis. J Child Orthop. 2012;6(5):353–356. doi: 10.1007/s11832-012-0435-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Speers DJ, Nade SM. Ultrastructural studies of adherence of Staphylococcus aureus in experimental acute hematogenous osteomyelitis. Infect Immun. 1985;49(2):443–446. doi: 10.1128/IAI.49.2.443-446.1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Branson JN, Vallejo JG, Flores AR, et al. The contemporary microbiology and rates of concomitant osteomyelitis in acute septic arthritis. Pediatr Infect Dis J. 2017;36:267–273. doi: 10.1097/INF.0000000000001417 [DOI] [PubMed] [Google Scholar]
- 14.Peltola H, Paakkonen M, Kallio P, Kallio MJ. Osteomyelitis-Septic Arthritis Study G. Short- versus long-term antimicrobial treatment for acute hematogenous osteomyelitis of childhood: prospective, randomized trial on 131 culture-positive cases. Pediatr Infect Dis J. 2010;29(12):1123–1128. doi: 10.1097/INF.0b013e3181f55a89 [DOI] [PubMed] [Google Scholar]
- 15.Calvo C, Nunez E, Camacho M, et al. Epidemiology and management of acute, uncomplicated septic arthritis and Osteomyelitis: Spanish Multicenter Study. Pediatr Infect Dis J. 2016;35(12):1288–1293. doi: 10.1097/INF.0000000000001309 [DOI] [PubMed] [Google Scholar]
- 16.Dich VQ, Nelson JD, Haltalin KC. Osteomyelitis in infants and children. A review of 163 cases. Am J Dis Child. 1975;129(11):1273–1278. doi: 10.1001/archpedi.1975.02120480007004 [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez BE, Martinez-Aguilar G, Hulten KG, et al. Severe Staphylococcal sepsis in adolescents in the era of community-acquired methicillin-resistant Staphylococcus aureus. Pediatrics. 2005;115(3):642–648. doi: 10.1542/peds.2004-2300 [DOI] [PubMed] [Google Scholar]
- 18.McNeil JC, Vallejo JG, Kok EY, Sommer LM, Hulten KG, Kaplan SL. Clinical and microbiologic variables predictive of orthopedic complications following S. aureus acute hematogenous osteoarticular infections in children. Clin Infect Dis. 2019;69(11):1955–1961. doi: 10.1093/cid/ciz109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Unkila-Kallio L, Kallio MJ, Eskola J, Peltola H. Serum C-reactive protein, erythrocyte sedimentation rate, and white blood cell count in acute hematogenous osteomyelitis of children. Pediatrics. 1994;93(1):59–62. [PubMed] [Google Scholar]
- 20.Unkila-Kallio L, Kallio MJ, Peltola H. The usefulness of C-reactive protein levels in the identification of concurrent septic arthritis in children who have acute hematogenous osteomyelitis. A comparison with the usefulness of the erythrocyte sedimentation rate and the white blood-cell count. J Bone Joint Surg Am. 1994;76(6):848–853. doi: 10.2106/00004623-199406000-00008 [DOI] [PubMed] [Google Scholar]
- 21.Saavedra-Lozano J, Falup-Pecurariu O, Faust SN, et al. Bone and joint infections. Pediatr Infect Dis J. 2017;36(8):788–799. doi: 10.1097/INF.0000000000001635 [DOI] [PubMed] [Google Scholar]
- 22.Section J, Gibbons SD, Barton T, Greenberg DE, Jo CH, Copley LA. Microbiological culture methods for pediatric musculoskeletal infection: a guideline for optimal use. J Bone Joint Surg Am. 2015;97(6):441–449. doi: 10.2106/JBJS.N.00477 [DOI] [PubMed] [Google Scholar]
- 23.Zhorne DJ, Altobelli ME, Cruz AT. Impact of antibiotic pretreatment on bone biopsy yield for children with acute hematogenous osteomyelitis. Hosp Pediatr. 2015;5(6):337–341. doi: 10.1542/hpeds.2014-0114 [DOI] [PubMed] [Google Scholar]
- 24.Yagupsky P, Bar-Ziv Y, Howard CB, Dagan R. Epidemiology, etiology, and clinical features of septic arthritis in children younger than 24 months. Arch Pediatr Adolesc Med. 1995;149(5):537–540. doi: 10.1001/archpedi.1995.02170180067010 [DOI] [PubMed] [Google Scholar]
- 25.Brook I. Anaerobic osteomyelitis in children. Pediatr Infect Dis. 1986;5(5):550–556. doi: 10.1097/00006454-198609000-00012 [DOI] [PubMed] [Google Scholar]
- 26.Vallejo JG, McNeil JC, Hulten KG, Sommer LM, Dunn JJ, Kaplan SL. Invasive haemophilus influenzae disease at Texas children’s hospital, 2011 to 2018. Pediatr Infect Dis J. 2019;38(9):900–905. doi: 10.1097/INF.0000000000002383 [DOI] [PubMed] [Google Scholar]
- 27.Rush PJ, Shore A, Inman R, Gold R, Jadavji T, Laski B. Arthritis associated with Haemophilus influenzae meningitis: septic or reactive? J Pediatr. 1986;109(3):412–415. doi: 10.1016/S0022-3476(86)80109-3 [DOI] [PubMed] [Google Scholar]
- 28.Edwards MS, Baker CJ, Wagner ML, Taber LH, Barrett FF. An etiologic shift in infantile osteomyelitis: the emergence of the group B streptococcus. J Pediatr. 1978;93(4):578–583. doi: 10.1016/S0022-3476(78)80891-9 [DOI] [PubMed] [Google Scholar]
- 29.Searns JB, Robinson CC, Wei Q, et al. Validation of a novel molecular diagnostic panel for pediatric musculoskeletal infections: integration of the cepheid xpert MRSA/SA SSTI and laboratory-developed real-time PCR assays for clindamycin resistance genes and kingella kingae detection. J Microbiol Methods. 2019;156:60–67. doi: 10.1016/j.mimet.2018.12.004 [DOI] [PubMed] [Google Scholar]
- 30.Ilharreborde B, Bidet P, Lorrot M, et al. New real-time PCR-based method for Kingella kingae DNA detection: application to samples collected from 89 children with acute arthritis. J Clin Microbiol. 2009;47(6):1837–1841. doi: 10.1128/JCM.00144-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferroni A, Al Khoury H, Dana C, et al. Prospective survey of acute osteoarticular infections in a French paediatric orthopedic surgery unit. Clin Microbiol Infect. 2013;19(9):822–828. doi: 10.1111/clm.12031 [DOI] [PubMed] [Google Scholar]
- 32.Browne LP, Mason EO, Kaplan SL, Cassady CI, Krishnamurthy R, Guillerman RP. Optimal imaging strategy for community-acquired Staphylococcus aureus musculoskeletal infections in children. Pediatr Radiol. 2008;38(8):841–847. doi: 10.1007/s00247-008-0888-8 [DOI] [PubMed] [Google Scholar]
- 33.Bocchini CE, Hulten KG, Mason EO Jr, Gonzalez BE, Hammerman WA, Kaplan SL. Panton-valentine leukocidin genes are associated with enhanced inflammatory response and local disease in acute hematogenous Staphylococcus aureus osteomyelitis in children. Pediatrics. 2006;117(2):433–440. doi: 10.1542/peds.2005-0566 [DOI] [PubMed] [Google Scholar]
- 34.Gafur OA, Copley LA, Hollmig ST, Browne RH, Thornton LA, Crawford SE. The impact of the current epidemiology of pediatric musculoskeletal infection on evaluation and treatment guidelines. J Pediatr Orthop. 2008;28(7):777–785. doi: 10.1097/BPO.0b013e318186eb4b [DOI] [PubMed] [Google Scholar]
- 35.Hawkshead JJ 3rd, Patel NB, Steele RW, Heinrich SD. Comparative severity of pediatric osteomyelitis attributable to methicillin-resistant versus methicillin-sensitive Staphylococcus aureus. J Pediatr Orthop. 2009;29(1):85–90. doi: 10.1097/BPO.0b013e3181901c3a [DOI] [PubMed] [Google Scholar]
- 36.Gonzalez BE, Teruya J, Mahoney DH Jr, et al. Venous thrombosis associated with staphylococcal osteomyelitis in children. Pediatrics. 2006;117(5):1673–1679. doi: 10.1542/peds.2005-2009 [DOI] [PubMed] [Google Scholar]
- 37.Belthur MV, Birchansky SB, Verdugo AA, et al. Pathologic fractures in children with acute Staphylococcus aureus osteomyelitis. J Bone Joint Surg Am. 2012;94(1):34–42. doi: 10.2106/JBJS.J.01915 [DOI] [PubMed] [Google Scholar]
- 38.Kok EY, Vallejo JG, Sommer LM, et al. Association of vancomycin MIC and molecular characteristics with clinical outcomes in methicillin-susceptible Staphylococcus aureus acute hematogenous osteoarticular infections in children. Antimicrob Agents Chemother. 2018;62(5):e00084–18. doi: 10.1128/AAC.00084-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hulten KG, Mason EO, Lamberth LB, Forbes AR, Revell PA, Kaplan SL. Analysis of invasive community-acquired methicillin-susceptible Staphylococcus aureus infections during a period of declining CA-MRSA infections at a large children’s hospital. Pediatr Infect Dis J. 2018;37(3):235–241. doi: 10.1097/INF.0000000000001753 [DOI] [PubMed] [Google Scholar]
- 40.Miller LG, Daum RS, Creech CB, et al. Clindamycin versus trimethoprim-sulfamethoxazole for uncomplicated skin infections. N Engl J Med. 2015;372(12):1093–1103. doi: 10.1056/NEJMoa1403789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bowen AC, Carapetis JR, Currie BJ, Fowler V Jr, Chambers HF, Tong SYC. Sulfamethoxazole-trimethoprim (cotrimoxazole) for skin and soft tissue infections including impetigo, cellulitis, and abscess. Open Forum Infect Dis. 2017;4(4):ofx232. doi: 10.1093/ofid/ofx232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boguniewicz J, Rubiano Landinez A, Kaplan SL, Lamb GS. Comparison of musculoskeletal infections due to nontyphoidal salmonella species and Staphylococcus aureus in immunocompetent children. Pediatr Infect Dis J. 2019;38(10):1020–1024. doi: 10.1097/INF.0000000000002440 [DOI] [PubMed] [Google Scholar]
- 43.Thanni LO. Bacterial osteomyelitis in major sickling haemoglobinopathies: geographic difference in pathogen prevalence. Afr Health Sci. 2006;6(4):236–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burnett MW, Bass JW, Cook BA. Etiology of osteomyelitis complicating sickle cell disease. Pediatrics. 1998;101(2):296–297. doi: 10.1542/peds.101.2.296 [DOI] [PubMed] [Google Scholar]
- 45.Kaplan J, Ikeda S, McNeil JC, Kaplan SL, Vallejo JG. Microbiology of osteoarticular infections in patients with sickle hemoglobinopathies at texas children’s hospital, 2000–2018. Pediatr Infect Dis J. 2019;38(12):1251–1253. doi: 10.1097/INF.0000000000002478 [DOI] [PubMed] [Google Scholar]
- 46.Givner LB, Luddy RE, Schwartz AD. Etiology of osteomyelitis in patients with major sickle hemoglobinopathies. J Pediatr. 1981;99(3):411–413. doi: 10.1016/S0022-3476(81)80330-7 [DOI] [PubMed] [Google Scholar]
- 47.Dubnov-Raz G, Ephros M, Garty BZ, et al. Invasive pediatric kingella kingae infections: a nationwide collaborative study. Pediatr Infect Dis J. 2010;29(7):639–643. doi: 10.1097/INF.0b013e3181d57a6c [DOI] [PubMed] [Google Scholar]
- 48.Juchler C, Spyropoulou V, Wagner N, et al. The contemporary bacteriologic epidemiology of osteoarticular infections in children in Switzerland. J Pediatr. 2018;194:190–196 e191. doi: 10.1016/j.jpeds.2017.11.025 [DOI] [PubMed] [Google Scholar]
- 49.Basmaci R, Lorrot M, Bidet P, et al. Comparison of clinical and biologic features of Kingella kingae and Staphylococcus aureus arthritis at initial evaluation. Pediatr Infect Dis J. 2011;30(10):902–904. doi: 10.1097/INF.0b013e31821fe0f7 [DOI] [PubMed] [Google Scholar]
- 50.Luegmair M, Chaker M, Ploton C, Berard J. Kingella kingae: osteoarticular infections of the sternum in children: a report of six cases. J Child Orthop. 2008;2(6):443–447. doi: 10.1007/s11832-008-0144-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yagupsky P, Dagan R, Howard CW, Einhorn M, Kassis I, Simu A. High prevalence of Kingella kingae in joint fluid from children with septic arthritis revealed by the BACTEC blood culture system. J Clin Microbiol. 1992;30(5):1278–1281. doi: 10.1128/JCM.30.5.1278-1281.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olarte L, Romero J, Barson W, et al. Osteoarticular infections caused by Streptococcus pneumoniae in children in the post-pneumococcal conjugate vaccine era. Pediatr Infect Dis J. 2017;36(12):1201–1204. doi: 10.1097/INF.0000000000001697 [DOI] [PubMed] [Google Scholar]
- 53.Hajjaji N, Hocqueloux L, Kerdraon R, Bret L. Bone infection in cat-scratch disease: a review of the literature. J Infect. 2007;54(5):417–421. doi: 10.1016/j.jinf.2006.10.045 [DOI] [PubMed] [Google Scholar]
- 54.Arisoy ES, Correa AG, Wagner ML, Kaplan SL. Hepatosplenic cat-scratch disease in children: selected clinical features and treatment. Clin Infect Dis. 1999;28(4):778–784. doi: 10.1086/515197 [DOI] [PubMed] [Google Scholar]
- 55.Landes M, Maor Y, Mercer D, et al. Cat scratch disease presenting as fever of unknown origin is a unique clinical syndrome. Clin Infect Dis. 2019. doi: 10.1093/cid/ciz1137 [DOI] [PubMed] [Google Scholar]
- 56.Rubin LG, Shin J, Kaur I, Scheuerman O, Levy I, Long SS. Frequency of multifocal disease and pyogenic arthritis of the hip in infants with osteoarticular infection in three neonatal intensive care units. J Pediatr. 2020;227:157–162. doi: 10.1016/j.jpeds.2020.07.055 [DOI] [PubMed] [Google Scholar]
- 57.Li J, Echevarria KL, Hughes DW, Cadena JA, Bowling JE, Lewis JS 2nd. Comparison of cefazolin versus oxacillin for treatment of complicated bacteremia caused by methicillin-susceptible Staphylococcus aureus. Antimicrob Agents Chemother. 2014;58(9):5117–5124. doi: 10.1128/AAC.02800-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McDanel JS, Perencevich EN, Diekema DJ, et al. Comparative effectiveness of beta-lactams versus vancomycin for treatment of methicillin-susceptible Staphylococcus aureus bloodstream infections among 122 hospitals. Clin Infect Dis. 2015;61(3):361–367. doi: 10.1093/cid/civ308 [DOI] [PubMed] [Google Scholar]
- 59.Yagupsky P. Antibiotic susceptibility of Kingella kingae isolates from children with skeletal system infections. Pediatr Infect Dis J. 2012;31(2):212. doi: 10.1097/INF.0b013e31824041b8 [DOI] [PubMed] [Google Scholar]
- 60.Staphylococcal Infections In: Pickering LK, Baker C, Kimberlin DW, Long SS, editors. Red Book: 2012 Report of the Committee on Infectious Diseases. 29 Elk Grove Village, Il: American Academy of Pediatrics; 2012:653–668. [Google Scholar]
- 61.Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American society of health-system pharmacists, the infectious diseases society of America, the pediatric infectious diseases society, and the society of infectious diseases pharmacists. Am J Health Syst Pharm. 2020;77(11):835–864. [DOI] [PubMed] [Google Scholar]
- 62.McNeil JC, Kok EY, Forbes AR, et al. Healthcare-associated Staphylococcus aureus bacteremia in children: evidence for reverse vancomycin creep and impact of vancomycin trough values on outcome. Pediatr Infect Dis J. 2016;35(3):263–268. doi: 10.1097/INF.0000000000000991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sinclair EA, Yenokyan G, McMunn A, Fadrowski JJ, Milstone AM, Lee CK. Factors associated with acute kidney injury in children receiving vancomycin. Ann Pharmacother. 2014;48(12):1555–1562. doi: 10.1177/1060028014549185 [DOI] [PubMed] [Google Scholar]
- 64.Le J, Ny P, Capparelli E, et al. Pharmacodynamic characteristics of nephrotoxicity associated with vancomycin use in children. J Pediatric Infect Dis Soc. 2015;4(4):e109–e116. doi: 10.1093/jpids/piu110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McNeil JC, Kaplan SL. Vancomycin therapeutic drug monitoring in children: new recommendations, similar challenges. J Pediatr Pharmacol Ther. 2020;25(6):472–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McNeil JC, Kaplan SL, Vallejo JG. The influence of the route of antibiotic administration, methicillin-susceptibility, vancomycin duration and serum trough concentration on outcomes of pediatric Staphylococcus aureus bacteremic osteoarticular infection. Pediatr Infect Dis J. 2017;36(6):572–577. doi: 10.1097/INF.0000000000001503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hahn A, Frenck RW Jr, Allen-Staat M, Zou Y, Vinks AA. Evaluation of target attainment of vancomycin area under the curve in children with methicillin-resistant Staphylococcus aureus bacteremia. Ther Drug Monit. 2015;37(5):619–625. doi: 10.1097/FTD.0000000000000190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martinez-Aguilar G, Avalos-Mishaan A, Hulten K, Hammerman W, Mason EO Jr, Kaplan SL. Community-acquired, methicillin-resistant and methicillin-susceptible Staphylococcus aureus musculoskeletal infections in children. Pediatr Infect Dis J. 2004;23(8):701–706. doi: 10.1097/01.inf.0000133044.79130.2a [DOI] [PubMed] [Google Scholar]
- 69.Kaplan SL, Mason EO Jr, Feigin RD. Clindamycin versus nafcillin or methicillin in the treatment of Staphylococcus aureus osteomyelitis in children. South Med J. 1982;75(2):138–142. doi: 10.1097/00007611-198202000-00005 [DOI] [PubMed] [Google Scholar]
- 70.Al-Zubeidi D, Burnham CA, Hogan PG, Collins R, Hunstad DA, Fritz SA. Molecular epidemiology of recurrent cutaneous methicillin-resistant infections in children. J Pediatric Infect Dis Soc. 2014;3(3):261–264. doi: 10.1093/jpids/pit046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen CJ, Chiu CH, Lin TY, Lee ZL, Yang WE, Huang YC. Experience with linezolid therapy in children with osteoarticular infections. Pediatr Infect Dis J. 2007;26(11):985–988. doi: 10.1097/INF.0b013e31812e62dc [DOI] [PubMed] [Google Scholar]
- 72.Syriopoulou V, Dailiana Z, Dmitriy N, Utili R, Pathan R, Hamed K. Clinical experience with daptomycin for the treatment of gram-positive infections in children and adolescents. Pediatr Infect Dis J. 2016;35(5):511–516. doi: 10.1097/INF.0000000000001076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Erturan G, Holme H, Smith R, Dodds R, Iyer S. Successful use of daptomycin in panton-valentine leucocidin positive Staphylococcus aureus paediatric osteomyelitis. Int J Surg Case Rep. 2012;3(7):238–241. doi: 10.1016/j.ijscr.2012.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bradley JS, Arrieta AC, Digtyar VA, et al. Daptomycin for pediatric gram-positive acute hematogenous osteomyelitis. Pediatr Infect Dis J. 2020;39(9):814–823. [DOI] [PubMed] [Google Scholar]
- 75.Williams AW, Newman PM, Ocheltree S, Beaty R, Hassoun A. Ceftaroline fosamil use in 2 pediatric patients with invasive methicillin-resistant Staphylococcus aureus infections. J Pediatr Pharmacol Ther. 2015;20(6):476–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blockey NJ, Watson JT. Acute osteomyelitis in children. J Bone Joint Surg Br. 1970;52(1):77–87. doi: 10.1302/0301-620X.52B1.77 [DOI] [PubMed] [Google Scholar]
- 77.Krogstad P. Hematogenous osteomyelitis in children: management. Girand HL, McNeil JC In: Baron EL, editor. UpToDate Online. [Web-Based Textbook]. Waltham, MA; 2020. UpToDate. Available from: https://www.uptodate.com/contents/hematogenous-osteomyelitis-in-children-management?search=osteomyelitis%20treatment%20children&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1. Accessed September17, 2020. [Google Scholar]
- 78.McMullan BJ, Andresen D, Blyth CC, et al. Antibiotic duration and timing of the switch from intravenous to oral route for bacterial infections in children: systematic review and guidelines. Lancet Infect Dis. 2016;16(8):e139–e152. doi: 10.1016/S1473-3099(16)30024-X [DOI] [PubMed] [Google Scholar]
- 79.Zaoutis T, Localio AR, Leckerman K, Saddlemire S, Bertoch D, Keren R. Prolonged intravenous therapy versus early transition to oral antimicrobial therapy for acute osteomyelitis in children. Pediatrics. 2009;123(2):636–642. doi: 10.1542/peds.2008-0596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120–128. doi: 10.1001/jamapediatrics.2014.2822 [DOI] [PubMed] [Google Scholar]
- 81.Barrier A, Williams DJ, Connelly M, Creech CB. Frequency of peripherally inserted central catheter complications in children. Pediatr Infect Dis J. 2012;31(5):519–521. doi: 10.1097/INF.0b013e31824571b0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ruebner R, Keren R, Coffin S, Chu J, Horn D, Zaoutis TE. Complications of central venous catheters used for the treatment of acute hematogenous osteomyelitis. Pediatrics. 2006;117(4):1210–1215. doi: 10.1542/peds.2005-1465 [DOI] [PubMed] [Google Scholar]
- 83.Paakkonen M, Kallio PE, Kallio MJ, Peltola H. Does bacteremia associated with bone and joint infections necessitate prolonged parenteral antimicrobial therapy? J Pediatric Infect Dis Soc. 2015;4(2):174–177. doi: 10.1093/jpids/piv009 [DOI] [PubMed] [Google Scholar]
- 84.Arnold JC, Cannavino CR, Ross MK, et al. Acute bacterial osteoarticular infections: eight-year analysis of C-reactive protein for oral step-down therapy. Pediatrics. 2012;130(4):e821–e828. doi: 10.1542/peds.2012-0220 [DOI] [PubMed] [Google Scholar]
- 85.Adams DJ, Eberly MD, Rajnik M, Nylund CM. Risk factors for community-associated clostridium difficile infection in children. J Pediatr. 2017;186:105–109. doi: 10.1016/j.jpeds.2017.03.032 [DOI] [PubMed] [Google Scholar]
- 86.Messina AF, Namtu K, Guild M, Dumois JA, Berman DM. Trimethoprim-sulfamethoxazole therapy for children with acute osteomyelitis. Pediatr Infect Dis J. 2011;30(12):1019–1021. doi: 10.1097/INF.0b013e31822db658 [DOI] [PubMed] [Google Scholar]
- 87.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18–e55. [DOI] [PubMed] [Google Scholar]
- 88.Matuschek E, Ahman J, Kahlmeter G, et al. Antimicrobial susceptibility testing of Kingella kingae with broth microdilution and disk diffusion using EUCAST recommended media. Clin Microbiol Infect. 2017;36(3):396–401. doi: 10.1016/j.cmi.2017.07.019 [DOI] [PubMed] [Google Scholar]
- 89.Yagupsky P, Katz O, Peled N. Antibiotic susceptibility of Kingella kingae isolates from respiratory carriers and patients with invasive infections. J Antimicrob Chemother. 2001;47(2):191–193. doi: 10.1093/jac/47.2.191 [DOI] [PubMed] [Google Scholar]
- 90.AlFawaz T, Alzumar O, AlShahrani D, Alshehri M. Severity of Salmonella infection among sickle cell diseases pediatric patients: description of the infection pattern. Int J Pediatr Adolesc Med. 2019;6(3):115–117. doi: 10.1016/j.ijpam.2019.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kamarulzaman A, Briggs RJ, Fabinyi G, Richards MJ. Skull osteomyelitis due to Salmonella species: two case reports and review. Clin Infect Dis. 1996;22(4):638–641. doi: 10.1093/clinids/22.4.638 [DOI] [PubMed] [Google Scholar]
- 92.Workman MR, Philpott-Howard J, Bragman S, Brito-Babapulle F, Bellingham AJ. Emergence of ciprofloxacin resistance during treatment of Salmonella osteomyelitis in three patients with sickle cell disease. J Infect. 1996;32(1):27–32. doi: 10.1016/S0163-4453(96)80006-5 [DOI] [PubMed] [Google Scholar]
- 93.Williams DJ, Deis JN, Tardy J, Creech CB. Culture-negative osteoarticular infections in the era of community-associated methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J. 2011;30(6):523–525. doi: 10.1097/INF.0b013e318207a7a5 [DOI] [PubMed] [Google Scholar]
- 94.Paakkonen M, Kallio MJ, Kallio PE, Peltola H. Significance of negative cultures in the treatment of acute hematogenous bone and joint infections in children. J Pediatric Infect Dis Soc. 2013;2(2):119–125. doi: 10.1093/jpids/pis108 [DOI] [PubMed] [Google Scholar]
- 95.Floyed RL, Steele RW. Culture-negative osteomyelitis. Pediatr Infect Dis J. 2003;22(8):731–736. doi: 10.1097/01.inf.0000078901.26909.cf [DOI] [PubMed] [Google Scholar]
- 96.Marschall J, Bhavan KP, Olsen MA, Fraser VJ, Wright NM, Warren DK. The impact of prebiopsy antibiotics on pathogen recovery in hematogenous vertebral osteomyelitis. Clin Infect Dis. 2011;52(7):867–872. doi: 10.1093/cid/cir062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Berbari EF, Kanj SS, Kowalski TJ, et al. 2015 Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis. 2015;61(6):e26–e46. doi: 10.1093/cid/civ482 [DOI] [PubMed] [Google Scholar]
- 98.Montgomery NI, Gonzalez E, Kaplan SL, et al. The role of surgery in the management of acute hematogenous osteomyelitis. Abstract 651. American Academy of Pediatrics Meeting; 2018; Vancouver:British Columbia. [Google Scholar]
- 99.Krogstad P. Septic arthritis In: Cherry JD, Harrison GJ, Kaplan SL, Steinbach WJ, Hotez PJ, editors. Feigin and Cherry’s Textbook of Pediatric Infectious Diseases. Vol. 1 7 Philadelphia, PA: Elsevier Saunders;2014:727–734. [Google Scholar]
- 100.Copley LA, Kinsler MA, Gheen T, Shar A, Sun D, Browne R. The impact of evidence-based clinical practice guidelines applied by a multidisciplinary team for the care of children with osteomyelitis. J Bone Joint Surg Am. 2013;95(8):686–693. doi: 10.2106/JBJS.L.00037 [DOI] [PubMed] [Google Scholar]
- 101.Riise OR, Kirkhus E, Handeland KS, et al. Childhood osteomyelitis-incidence and differentiation from other acute onset musculoskeletal features in a population-based study. BMC Pediatr. 2008;8:45. doi: 10.1186/1471-2431-8-45 [DOI] [PMC free article] [PubMed] [Google Scholar]