Abstract
Background
Platelet–albumin–bilirubin (PALBI) has been demonstrated to be superior to conventional Child–Pugh (C-P) grade in evaluating liver function and prognosis of HCC patients. However, both thrombocytosis and thrombocytopenia are unfavorable for HCC survival. The aim of this study was to preliminarily investigate the prognostic value of PALBI in HCC patients with thrombocytopenia and excluding thrombocytopenia.
Methods
In this retrospective cohort study, we reviewed 465 cases of HCC patients who underwent radical surgery. PALBI grade was calculated based on preoperative serological examinations. The primary outcomes were overall survival (OS) and recurrence-free survival (RFS), which were assessed by Kaplan–Meier method and Cox regression. The prognostic performances of PALBI and other models were estimated by using the concordance index (C-index).
Results
During a median follow-up time of 28 months, 31.6% (147/465) of patients died and 33.5% (156/465) experienced recurrence. Multivariate analyses revealed that both thrombocytosis and thrombocytopenia were independently associated with poor OS and RFS compared with normal platelet count (PLT) in HCC patients. Stratified analysis further revealed that PALBI was a significant predictor for HCC survival in patients excluding thrombocytopenia but not in patients with thrombocytopenia. In particular, in HCC patients excluding thrombocytopenia, the combination of tumor size with PALBI (C-index = 0.730, 95% CI: 0.674–0.786) may be superior to the classical Barcelona Clinic Liver Cancer (BCLC) and Cancer of Liver Italian Program (CLIP) staging systems in predicting survival.
Conclusion
In conclusion, PALBI grade, in particular the combination with tumor size, is an effective model for discriminating survival in HCC patients excluding thrombocytopenia but not in thrombocytopenic HCC patients.
Keywords: hepatocellular carcinoma, survival, platelet–albumin–bilirubin, platelet count
Introduction
Hepatocellular carcinoma (HCC) is the fourth leading cause of cancer-related deaths worldwide and is responsible for approximately 782,000 deaths each year.1 Chronic hepatitis B and C, alcohol abuse, and non-alcoholic fatty liver disease are the main risk factors for HCC. Accurately evaluating postoperative survival is a crucial strategy in the management of HCC. Liver function abnormality is frequently observed in HCC patients as the background of chronic liver diseases and cirrhosis. It has been suggested that, in addition to tumor burden, the severity of liver function dysfunction influences treatment options and HCC peri-operative outcomes.2,3
Worldwide, Child–Pugh (C-P) classification system is widely considered as an important tool to assess preoperative liver function. The C-P grade incorporates serum total bilirubin (TBIL), albumin (ALB), coagulation function, presence of ascites, and hepatic encephalopathy. However, several disadvantages of C-P grade tend to limit its clinical value. Firstly, ALB and ascites are inter-correlated. Secondly, ascites and encephalopathy are subjective indicators and inter-observer variation is unavoidable. In 2015, Johnson et al introduced a novel and simple model to measure liver function, named the albumin–bilirubin (ALBI) grade,4 which is solely based on laboratory parameters. An increasing number of studies have shown that ALBI provides better discrimination of liver function and higher prognostic value than conventional C-P grade.5,6
Recently, many authors have reported the role of various inflammatory indices in predicting short as well as long-term outcomes in patients with HCC. Platelet–albumin–bilirubin (PALBI), is also widely used to assess liver function and prognosis in HCC.7–11 However, the role of platelets in HCC is quite complicated and is even conflicting. On the one hand, preoperative thrombocytopenia (platelet count (PLT) <100×10⁹/L) is a significant predictor of poor survival and high risk of recurrence in HCC.12,13 On the other hand, thrombocytosis is independently associated with increased tumor burden, high risk of extrahepatic metastasis and poor prognosis in HCC.14,15 Therefore, the prognostic significance of PALBI may depend on the levels of PLT. In this study, we firstly investigated the prognostic values of PALBI in HCC patients with thrombocytopenia and excluding thrombocytopenia.
Methods
Study Population
Between Jan 2008 and June 2018, 465 consecutive patients who underwent hepatectomy for HCC at the First Affiliated Hospital of Bengbu Medical College and the First Affiliated Hospital of Xi’an Jiaotong University were identified and analyzed retrospectively. The inclusion criteria were 1) age from 18 to 80 years; 2) diagnosed as HCC by histological pathology; 3) received radical hepatectomy (with negative surgical margin) for the first time. Exclusion criteria were 1) surgery-related deaths or death within 1 month after surgery; 2) re-hepatectomy; 3) mixed hepatocellular cholangiocarcinoma; 4) extrahepatic metastasis; 5) information was incomplete to calculate PALBI; 6) coexistent hematologic disorders, malnutrition, or other diseases that affect PALBI score. This study was reported according to the statement of Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD)16 and followed the Declaration of Helsinki on medical protocol and ethics.17 It was approved by the Institutional Review Board of Bengbu Medical College and the Institutional Review Board of First Affiliated Hospital of Xi’an Jiaotong University. It was designed as a retrospective study and the results would not affect the clinical care of the patients. Therefore, patient approval and informed consent were waived off by the institutional review boards. We confirmed that the patient data was anonymized and confidential.
Data Collection
Age, gender, tumor number, tumor size, vascular invasion, ascites, liver cirrhosis, pathologic diagnosis, and preoperative serological tests, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), TBIL, ALB, PLT, prothrombin time-international normalized ratio (PT-INR), and alpha-fetoprotein (AFP), were extracted for all patients. We used preoperative data to calculate the scores and grades of ALBI and PALBI according to the following formulas:
ALBI score: 0.66×log10 TBIL (μmol/L)-0.085×ALB (g/L). ALBI grade was defined as: grade 1: ≤–2.60; grade 2: –1.39 to –2.60; grade 3: >–1.39.4
PALBI score: 2.02 × log10TBIL (μmol/L)-0.37 × (log10TBIL)2–0.04 × ALB (g/L) −3.48 × log10PLT (109/L) + 1.01 × (log10PLT).2 PALBI grade was defined as: grade 1: ≤–2.53; grade 2: –2.09 to –2.53; grade 3: >–2.09.7
Follow-Up
After discharge, all patients were regularly followed-up until Dec 2019 or until death. The follow-up contents were enhanced CT/MRI, abdominal ultrasound, AFP, and other serologic tests. Statuses of recurrence and survival were recorded. Recurrent patients received re-hepatectomy, ablation, or transcatheter arterial chemoembolization (TACE), as appropriate.
Statistical Analysis
SPSS version 22.0 was used for data collection and analysis. Continuous variables were expressed as mean value ± standard deviation (SD) or median (interquartile range) according to the normal distribution status. Tests of t, Wilcoxon, or χ2 were performed to compare between groups.
The primary outcomes we analyzed were overall survival (OS) and recurrence-free survival (RFS), which were evaluated by Kaplan–Meier curve and Log rank test. The factors that were statistically significant (P < 0.10) in the univariate analyses entered into the multivariate Cox regression model. The prognostic abilities were compared by using concordance index (C-index, low accuracy: 0.50–0.70; medium accuracy: 0.70–0.90; high accuracy: >0.90).18 It was considered statistically significant provided P value less than 0.05.
Results
Patients’ Characteristics
A total of 465 HCC patients were involved in this study, which consisted of 366 men and 99 women with the mean age 52.9 ± 10.7 years. A total of 418 (89.9%) patients were infected with HBV and 407 (87.5%) patients were C-P grade A. According to ALBI grade, patients could be divided into two groups: ALBI grade 1 (n = 254, 54.6%) and grade 2 (n = 211, 45.4%). Based on PALBI grade, there were 264 (56.8%) patients with grade 1, 146 (31.4%) patients with grade 2, and 55 (11.8%) patients with grade 3.
Associations Between PALBI Grade and Clinicopathologic Feature
Table 1 shows demographic data, serologic tests, tumor characteristics, and tumor stages of patients stratified according to preoperative grade of PALBI. Clinicopathologic features were comparable between PALBI grade 1 and grade 2/3 except for ALT, AST, TBIL, ALB, PLT, ascites, tumor size, C-P and ALBI grades. Specifically, patients with PALBI grade 2/3 had significantly higher levels of ALT, AST, TBIL, PLT, higher risk of ascites, larger tumor size, lower ALB, and higher C-P and ALBI grades.
Table 1.
Basic Characteristics of HCC Patients Stratified According to Levels of the PALBI
| Variables | Overall | PALBI Grade | ||
|---|---|---|---|---|
| 1 (n=264) | 2/3 (n=201) | P value | ||
| Etiology: HBV/others | 418/47 | 234/30 | 184/17 | 0.303 |
| Sex: male/female | 366/99 | 204/60 | 162/39 | 0.386 |
| Age (years) | 52.9±10.7 | 53.1±10.4 | 52.6±11.2 | 0.614 |
| Age: >60/≤60 years | 344/121 | 198/66 | 146/55 | 0.565 |
| ALT (U/L) | 34.4(23.0–59.8) | 29.0(20.0–44.0) | 46.0(26.9–88.0) | <0.001 |
| AST (U/L) | 37.0(26.0–55.0) | 32.5(24.0–44.0) | 43.9(28.0–82.9) | <0.001 |
| TBIL (µmol/L) | 13.4(10.2–18.7) | 11.4(9.0–14.3) | 18.2(13.4–24.9) | <0.001 |
| ALB (g/L) | 39.6(35.6–42.8) | 41.9(39.0–45.0) | 36.2(33.2–39.6) | <0.001 |
| PLT (109/L) | 137(100–197) | 129(97–175) | 152(110–225) | <0.001 |
| AFP (ng/mL) | 62.4(5.1–1051.1) | 104.4(6.3–1210.0) | 38.7(4.7–847.9) | 0.245 |
| AFP: ≥400/<400ng/ml | 151/314 | 87/177 | 64/137 | 0.799 |
| Ascites: yes/no | 65/400 | 28/236 | 37/164 | 0.016 |
| Tumor size (cm) | 5.0(3.5–8.0) | 5.0(3.5–7.5) | 6.0(3.5–9.0) | 0.023 |
| Tumor size: >5/≤5cm | 223/242 | 118/146 | 105/96 | 0.107 |
| Tumor number: multiple/single | 66/399 | 38/226 | 28/173 | 0.887 |
| Vascular invasion: yes/no | 61/404 | 32/232 | 29/172 | 0.465 |
| Cirrhosis: yes/no | 229/236 | 142/122 | 87/114 | 0.025 |
| BCLC: 0+A/B+C | 341/124 | 198/66 | 143/58 | 0.352 |
| CLIP: 0/1-4 | 237/228 | 141/123 | 96/105 | 0.227 |
| C-P grade: A/B | 407/58 | 257/7 | 150/51 | <0.001 |
| ALBI score | −2.64(−2.93 to −2.24) | −2.84(−3.11 to −2.63) | −2.24(−2.52 to −1.96) | <0.001 |
| ALBI grade (1/2) | 254/211 | 209/55 | 45/156 | <0.001 |
Notes: Data were expressed as median (interquartile range), mean value ± SD, or no. Bold font highlights significant results.
Abbreviations: PALBI, platelet–albumin–bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBIL, total bilirubin; ALB, albumin; PLT, platelet count; AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; CLIP, Cancer of the Liver Italian Program; C-P, Child–Pugh; ALBI, albumin–bilirubin.
Prognosis of the Entire Cohort
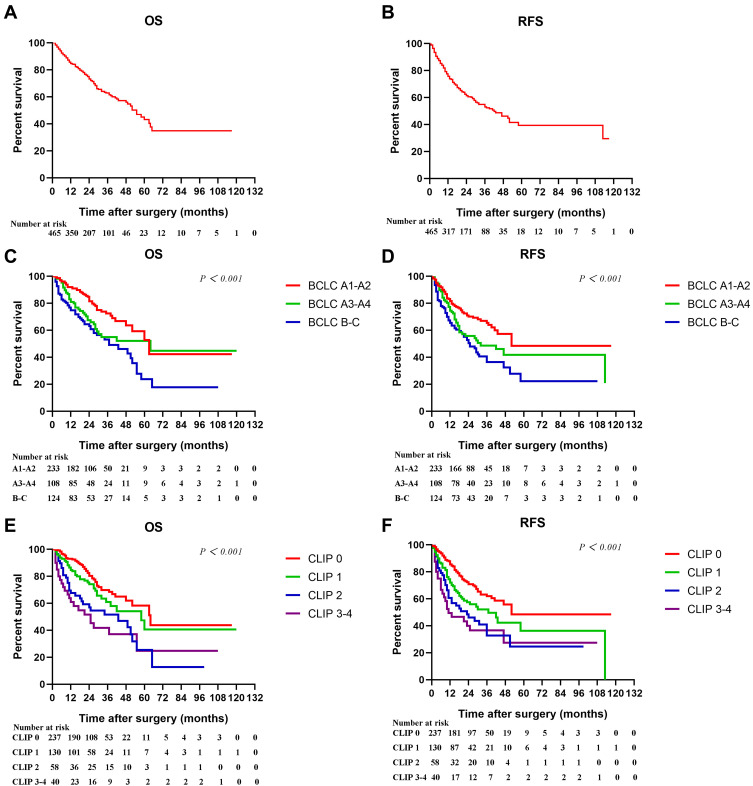
During a median follow-up of 28 months, 156 (33.5%) had postoperative recurrence and 147 (31.6%) patients died. Figure 1A and B showed the Kaplan–Meier cumulative OS and RFS curves of the entire cohort. The 1-, 3-, and 5-year OS and RFS rates were 84.8%, 62.9%, 43.1%, and 75.7%, 53.1%, 39.4%, respectively. Figure 1C–F further showed the Kaplan–Meier curves of patients stratified according to BCLC and CLIP stages. The Log rank tests demonstrated that both of BCLC (Figure 1C and D, both P < 0.05) and CLIP stages (Figure 1E and F, both P < 0.05) were significantly associated with OS and RFS in HCC.
Figure 1.
Kaplan–Meier cumulative overall survival (A) and recurrence-free survival (B) curves of the study population, and overall survival and recurrence-free survival curves of patients stratified according to BCLC (C–D) and CLIP (E–F) staging systems.
Predictors of OS and RFS
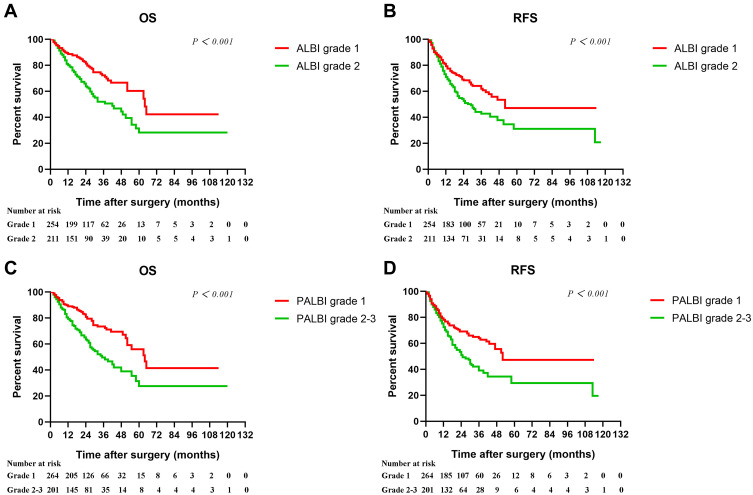
Kaplan–Meier analyses showed that both ALBI (Figure 2A and B) and PALBI (Figure 2C and D) were found to be related to survival. Univariate analyses revealed that AST, TBIL, AFP, tumor size, vascular invasion, BCLC, CLIP, C-P, ALBI, and PALBI were significantly associated with OS and RFS. In addition to the above factors, ALB was a significant predictor of OS while ALT was found to be significantly associated with RFS (Table 2).
Figure 2.
Kaplan–Meier cumulative overall survival and recurrence-free survival curves of patients stratified according to ALBI (A–B) and PALBI (C–D).
Table 2.
Univariate Analysis of Factors Associated with Overall Survival and Recurrence-Free Survival of HCC Patients
| Variables | OS | RFS | ||
|---|---|---|---|---|
| HR(95% CI) | P value | HR(95% CI) | P value | |
| Etiology (HBV/others) | 0.726(0.436–1.206) | 0.218 | 0.816(0.518–1.288) | 0.383 |
| Sex (male/female) | 0.940(0.637–1.387) | 0.755 | 0.942(0.659–1.345) | 0.742 |
| Age (>60/≤60 years) | 1.304(0.919–1.850) | 0.137 | 1.350(0.988–1.845) | 0.059 |
| ALT (>40/≤40 U/L) | 1.256(0.908–1.737) | 0.168 | 1.359(1.018–1.814) | 0.038 |
| AST (>40/≤40U/L) | 1.841(1.327–2.555) | <0.001 | 1.832(1.370–2.449) | <0.001 |
| TBIL (>17/≤17µmol/L) | 1.412(1.011–1.972) | 0.043 | 1.368(1.015–1.845) | 0.040 |
| ALB (<35/≥35g/L) | 1.472(1.031–2.102) | 0.034 | 1.332(0.959–1.849) | 0.087 |
| PLT (<100/100–300×109/L) | 1.472(1.034–2.096) | 0.032 | 1.454(1.059–1.996) | 0.021 |
| PLT (>100/100–300×109/L) | 2.212(1.225–3.994) | 0.008 | 2.181(1.303–3.652) | 0.003 |
| AFP (≥400/<400ng/mL) | 1.689(1.218–2.342) | 0.002 | 1.846(1.379–2.470) | <0.001 |
| Ascites (yes/no) | 1.142(0.743–1.757) | 0.545 | 1.197(0.817–1.752) | 0.356 |
| Tumor size (>5/≤5cm) | 3.013(2.112–4.300) | <0.001 | 2.820(2.075–3.833) | <0.001 |
| Tumor number (multiple/single) | 1.312(0.877–1.962) | 0.186 | 1.315(0.910–1.901) | 0.145 |
| Vascular invasion (yes/no) | 2.167(1.457–3.224) | <0.001 | 1.959(1.350–2.845) | <0.001 |
| Cirrhosis (yes/no) | 0.976(0.698–1.365) | 0.888 | 1.020(0.762–1.365) | 0.894 |
| BCLC (B+C/A) | 1.920(1.379–2.674) | <0.001 | 1.773(1.314–2.394) | <0.001 |
| CLIP (1–4/0) | 1.333(1.179–1.507) | <0.001 | 1.989(1.479–2.675) | <0.001 |
| C-P grade (B/A) | 1.876(1.249–2.818) | 0.002 | 1.618(1.105–2.370) | 0.013 |
| ALBI grade (2/1) | 1.948(1.400–2.710) | <0.001 | 1.584(1.187–2.116) | 0.002 |
| PALBI grade (2–3/1) | 2.074(1.493–2.879) | <0.001 | 1.684(1.261–2.250) | <0.001 |
Note: Bold font highlights significant results.
Abbreviations: OS, overall survival; RFS, recurrence-free survival; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBIL, total bilirubin; ALB, albumin; PLT, platelet count; AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; CLIP, Cancer of the Liver Italian Program; C-P, Child–Pugh; ALBI, albumin–bilirubin; PALBI, platelet–albumin–bilirubin.
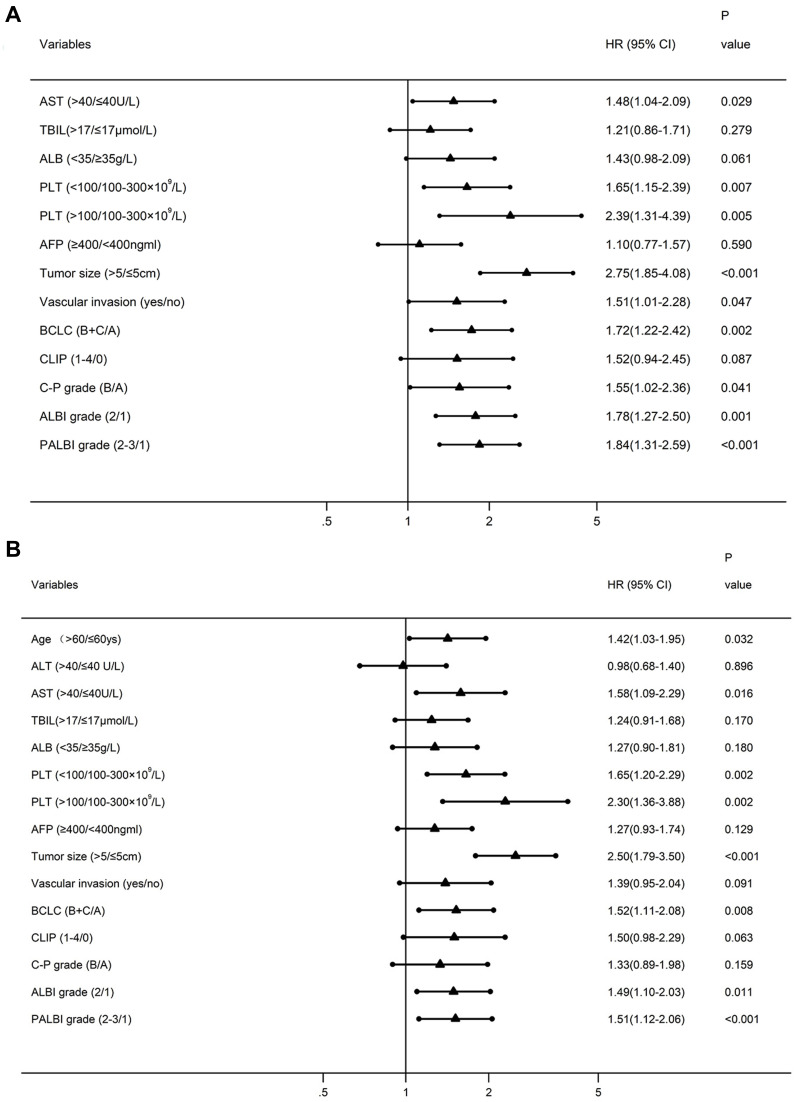
Multivariate analyses were further performed and AFP, tumor size, BCLC, ALBI, and PALBI grades were independent prognostic indicators for both OS and RFS (Figure 3A and B). C-P grade and vascular invasion were independent predictors for OS, but not for RFS. However, CLIP stage was neither independently associated with OS nor with RFS.
Figure 3.
Forest plots based the results of multivariate analysis for overall survival (A) and recurrence-free survival (B).
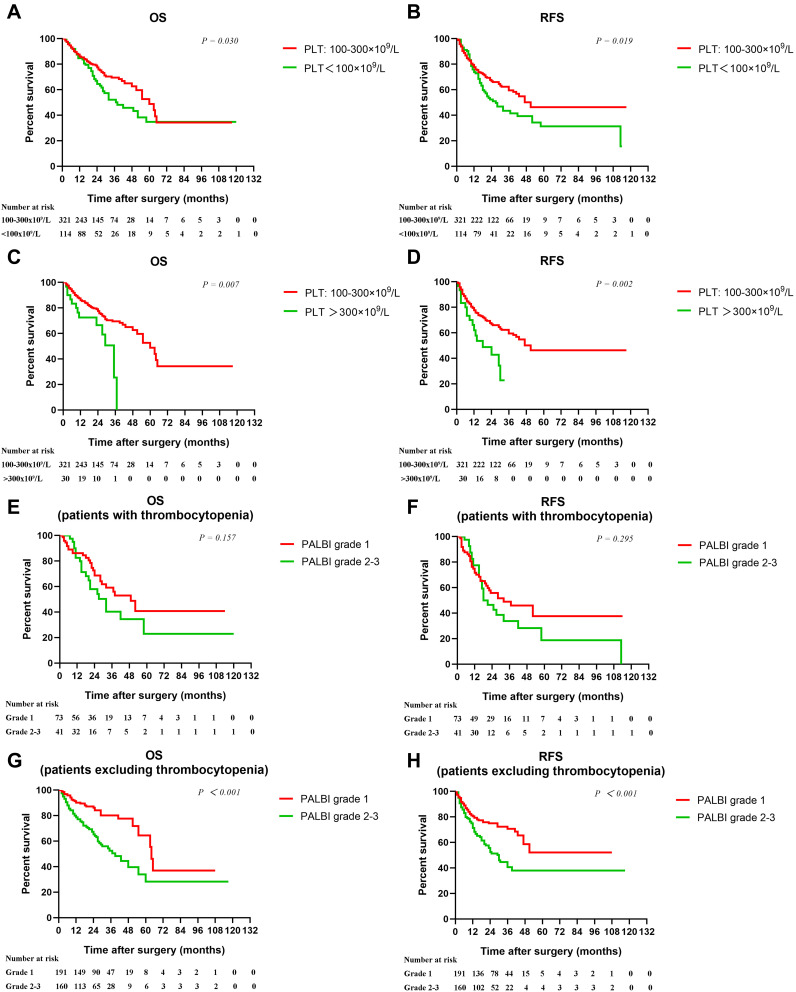
Prognostic Significance of PALBI in Thrombocytopenic HCC Patients or in Patients Excluding Thrombocytopenia
Consistent with previous studies, we found that, compared with normal PLT (100–300×109/L, n=321), thrombocytopenia (n=114) and thrombocytosis (n=30) were both unfavorable factors for survival in HCC (Figure 4A–D). We hypothesize that the inclusion of thrombocytopenic patients may influence the prognostic value of PALBI in HCC. As expected, PALBI was not a significant predictor of OS and RFS in HCC patients with thrombocytopenia (n=114, 24.5%) (Figure 4E and F). However, PALBI was closely related to OS and RFS inpatients excluding thrombocytopenia (n=351, 75.5%) (Figure 4G–H).
Figure 4.
Kaplan–Meier cumulative overall survival and recurrence-free survival curves of patients stratified according to PLT (A–D), PALBI in patients with thrombocytopenia (E–F), and PALBI in patients excluding thrombocytopenia (G–H).
Comparison of Predictive Accuracy for Survival Between the PALBI, ALBI, C-P Grades and BCLC, CLIP Staging Systems
Based on C-index, when all the 465 patients were included, the order of the models in discriminating survival was as follows: BCLC, CLIP, PALBI, ALBI, and C-P (Table 3). In HCC patients with thrombocytopenia, PALBI showed a low predictive value (C-index = 0.570, 95% CI: 0.463–0.678). However, after the exclusion of thrombocytopenic patients, the order was as follows: PALBI, BCLC, ALBI, CLIP, and C-P (Table 4). In our study, among all the prognostic indicators, tumor size was a crucial factor, which was consistent with previous reports.19,20 When combined with tumor size, prognostic value of PALBI (C-index = 0.730, 95% CI: 0.674–0.786) was more superior than PALBI alone (Table 4).
Table 3.
Ranking of Discriminatory Ability of the Prognostic Systems on the Basis of the C-Index in All Patients
| Rank | System | C-Index | 95% CI |
|---|---|---|---|
| 1 | BCLC stage | 0.651 | 0.599–0.704 |
| 2 | CLIP stage | 0.643 | 0.588–0.698 |
| 3 | ALBI score | 0.640 | 0.585–0.694 |
| 4 | PALBI score | 0.631 | 0.576–0.686 |
| 5 | C-P score | 0.585 | 0.529–0.642 |
Abbreviations: BCLC, Barcelona Clinic Liver Cancer; CLIP, Cancer of the Liver Italian Program; ALBI, albumin–bilirubin; PALBI, platelet–albumin–bilirubin; C-P, Child–Pugh.
Table 4.
Ranking of Discriminatory Ability of the Prognostic Systems on the Basis of the C-Index in HCC Patients Excluding Thrombocytopenia
| Rank | System | C-Index | 95% CI |
|---|---|---|---|
| 1 | PALBI + tumor size | 0.730 | 0.674–0.786 |
| 2 | ALBI + tumor size | 0.718 | 0.660–0.776 |
| 3 | C-P + tumor size | 0.701 | 0.642–0.761 |
| 4 | PALBI score | 0.655 | 0.590–0.720 |
| 5 | BCLC stage | 0.648 | 0.585–0.711 |
| 6 | ALBI score | 0.647 | 0.581–0.712 |
| 7 | CLIP stage | 0.645 | 0.578–0.712 |
| 8 | C-P score | 0.578 | 0.510–0.647 |
Abbreviations: PALBI, platelet–albumin–bilirubin; ALBI, albumin–bilirubin; C-P, Child–Pugh; BCLC, Barcelona Clinic Liver Cancer; CLIP, Cancer of the Liver Italian Program.
Stratification of Patients According to the Combination of Tumor Size with PALBI
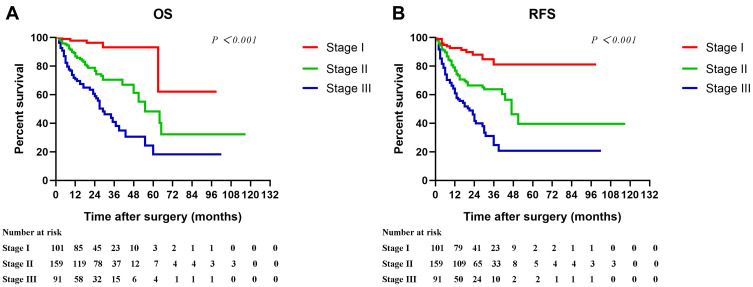
The simple combination of tumor size and PALBI showed medium accuracy (C-index >0.70) in the prediction of survival. Furthermore, based on PALBI and tumor size, we stratified HCC patients excluding thrombocytopenia as Stage I (PALBI grade 1 and tumor size ≤5cm), Stage II (PALBI grade 1 and tumor size >5 cm, or PALBI grade 2–3 and tumor size ≤5cm), and Stage III (PALBI grade 2–3 and tumor size >5cm). Obviously, patients in Stage I had significantly better OS and RFS in comparison with patients in the higher stages (Figure 5A and B, P < 0.001).
Figure 5.
Cumulative overall survival and recurrence-free survival curves of patients stratified according to PALBI and tumor size (A–B, Stage I PALBI grade 1 and tumor size ≤5 cm; Stage II: PALBI grade 1 and tumor size >5 cm, or PALBI grade 2–3 and tumor size ≤5 cm; Stage III: PALBI grade 2–3 and tumor size >5 cm) in HCC patients excluding thrombocytopenia.
Discussion
As most cases originate from chronic liver diseases and cirrhosis, HCC prognosis depends on not only tumor burthen but also liver function impairment.2 For decades, C-P grade has been used as a classic tool for estimating liver function, determining treatment schemes, as well as evaluating prognosis in HCC.19 However, deficiencies of C-P grade have gradually been proposed.21
Recently, Johnson et al established the ALBI grade system,4 which has been demonstrated to be superior to the C-P grade.5,22–25 In addition, another novel liver function grading system PALBI integrates PLT, TBIL, and ALB. According to the PALBI grade, HCC patients could be divided into different groups with clearly different liver function as well as outcomes. Ni et al included 349 consecutive HCC patients and showed that PALBI grade provided a better predictive ability for death than ALBI grade or C-P class.26 In the study by Sonohara et al, PALBI was used as an effective tool for assessing perioperative risks for hepatectomy in HCC.9 Another study enrolling 2038 HCC patients with C-P grade A showed that PALBI grade was a simple, objective and discriminatory model for risk stratification of postoperative liver failure and prognosis.10 In line with the above reports, our findings indicated that PALBI grade could be used as a predictor of survival in HCC. However, PALBI was not a significant predictor of survival in the 36 HCC patients with C-P grade B cirrhosis (not shown, P = 0.948 for OS and P = 0.957 for RFS). In our study, the 1-year OS rate of HCC patients was 84.8%, indicating that approximately 15% of the patients died within 1 year after hepatectomy. This group of patients may get potentially more benefit from the PALBI prediction model. To reduce mortality and improve outcomes, these patients can be recommended for non-hepatectomy approaches, such as ablation, TACE, Y90, or the combination.27
As both thrombocytosis and thrombocytopenia are unfavorable prognostic factors for HCC, the prognostic value of PALBI may be different across different levels of PLT. However, all the previous studies did not take into account the effect of PLT in the significance of PALBI. In our study, we firstly showed that PALBI could be used for evaluating survival in HCC patients excluding thrombocytopenia but not in thrombocytopenic HCC patients. Furthermore, in HCC patients excluding thrombocytopenia, we compared the prognostic performance between ALBI, PALBI, C-P grades and classic HCC stages. Tumor size is an important determinant of outcomes. However, tumor size alone is not considered a contraindication for hepatectomy. Larger tumor is associated with proximity of major vessels, difficulty in surgery, greater blood loss, longer operative time, worse survival, and so forth.19,20,28 In our study, the combination of tumor size with PALBI showed higher performance for survival prediction than C-P, ALBI, BCLC and CLIP staging systems. Patients with lower grade of PALBI and smaller tumor size had significantly better outcomes.
The potential mechanisms enable lower PALBI grade to indicate better survival in HCC are multifaceted. All the components in PALBI, including ALB, TBIL and platelets, are useful indicators of HCC risk and outcomes. Exogenous ALB has been shown to inhabit HCC tumor growth via the modulation of growth-controlling kinases.29 Hypoalbuminemia reflects nutritional risk and is associated with the rapid progress, poor survival, and high risk of recurrence in several types of malignancies, including HCC.30–32 In addition, abnormal level of TBIL is an important reflection of liver injury. Among the prognostic indicators of liver function, serum ALB and TBIL have been demonstrated to be the two most prominent prognostic factors.33
However, platelets act as a double-edged sword role in HCC. On the one hand, platelets are known to stimulate tumor growth of HCC via platelet-derived mediators, such as 5-hydroxytryptamine, vascular endothelial growth factor, and platelet-derived growth factor.34,35 Platelets could also accelerate angiogenesis and metastasis of HCC.34,36 Furthermore, PLT is positively correlated with tumor size, and the risk of vascular invasion and extrahepatic metastases in HCC patients.14,29 On the other hand, due to portal hypertension and several other factors, PLT is generally decreased in cirrhotic and HCC patients. Thrombopoietin promotes liver regeneration and improves liver cirrhosis by increasing the PLT level, indirectly implying that a decrease in PLT would result in a poor prognosis.37 Our recent meta-analyses showed that low preoperative PLT was significantly associated with poor outcomes of HCC.12,13 These may explain that the prognostic value of PALBI varies over different PLT.
The present study has some disadvantages, and further improvement in the future work is required. Firstly, it was designed as a retrospective study with a relatively small sample size; thus, external validation from larger prospective designs is still needed. Secondly, the results were based on HCC patients who underwent radical resection. Therefore, further study is needed to validate the prognostic accuracy of PALBI grade in HCC patients with different treatments. Thirdly, postoperative liver function assessment may be more valuable than that in the pre-operation in predicting HCC outcomes_ENREF_42. However, the majority of patients in our cohort missed the data of dynamic measures due to the retrospective design. Fourthly, the establishment of a nomogram by integrating PALBI, tumor size, and other crucial factors may show better performance in predicting the prognosis of HCC.
Based on PALBI, partial patients with HCC may show poor prognosis after liver resection. Therefore, the choices of therapies are crucial for them to prolong the survival time. For patients with preoperative PALBI grade 2/3, it is urgent to ameliorate the abnormal ALB, TBIL, and/or platelets through pharmacological and nonpharmacological interventions to improve postoperative outcomes. The therapeutic window should take into account that tumour also continues to grow.38 Prehabilitation or rehabilitation can also be recommended to improve post-hepatectomy outcomes.39 In addition, the combination of TACE and ablation offers comparable clinical outcomes but reduces peri-operative-related mortality.27 As patients with liver disfunction had worse outcomes in cases who received liver transplantation or ALPPS,40,41 the two therapies should be used with caution.
Conclusions
PALBI grade, in particular the combination with tumor size, is an effective model for discriminating survival in HCC patients excluding thrombocytopenia but not in thrombocytopenic HCC patients.
Acknowledgment
This work was supported by the Science and Technological Fund of Anhui Province for Outstanding Youth (2008085J37), the First Affiliated Hospital of Bengbu Medical College Science Fund for Distinguished Young Scholars (2019byyfyjq05), the 512 Talent Training Program of Bengbu Medical College (by51201318), the Key Projects of Translational Medicine of Bengbu Medical College (BYTM2019022), and the National College Students’ Innovation and Entrepreneurship Training Program (201910367053).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology. 2019;156(2):477–491.e1. doi: 10.1053/j.gastro.2018.08.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madhavan S, Shelat VG, Soong S-L, et al. Predicting morbidity of liver resection. Langenbecks Arch Surg. 2018;403(3):359–369. doi: 10.1007/s00423-018-1656-3 [DOI] [PubMed] [Google Scholar]
- 4.Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach—the ALBI grade. J Clin Oncol. 2015;33(6):550–558. doi: 10.1200/JCO.2014.57.9151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zou H, Yang X, Li Q-L, et al. A comparative study of albumin-bilirubin score with Child-Pugh Score, model for end-stage liver disease score and indocyanine green R15 in predicting posthepatectomy liver failure for hepatocellular carcinoma patients. Dig Dis. 2018;36(3):236–243. doi: 10.1159/000486590 [DOI] [PubMed] [Google Scholar]
- 6.Peng Y, Wei Q, He Y, et al. ALBI versus Child-Pugh in predicting outcome of patients with HCC: a systematic review. Expert Rev Gastroenterol Hepatol. 2020;14(5):383–400. doi: 10.1080/17474124.2020.1748010 [DOI] [PubMed] [Google Scholar]
- 7.Liu P-H, Hsu C-Y, Hsia C-Y, et al. ALBI and PALBI grade predict survival for HCC across treatment modalities and BCLC stages in the MELD Era. J Gastroenterol Hepatol. 2017;32(4):879–886. doi: 10.1111/jgh.13608 [DOI] [PubMed] [Google Scholar]
- 8.Luo H-M, Zhao S-Z, Li C, Chen L-P. Preoperative platelet-albumin–bilirubin grades predict the prognosis of patients with hepatitis B virus-related hepatocellular carcinoma after liver resection. Medicine. 2018;97(12):e0226. doi: 10.1097/MD.0000000000010226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sonohara F, Yamada S, Tanaka N, et al. Comparison of non-invasive liver reserve and fibrosis models: implications for surgery and prognosis for hepatocellular carcinoma. Hepatol Res. 2019;49(11):1305–1315. doi: 10.1111/hepr.13400 [DOI] [PubMed] [Google Scholar]
- 10.Lu LH, Zhang YF, Mu-Yan C, et al. Platelet-albumin-bilirubin grade: risk stratification of liver failure, prognosis after resection for hepatocellular carcinoma. Dig Liver Dis. 2019;51(10):1430–1437. doi: 10.1016/j.dld.2019.04.006 [DOI] [PubMed] [Google Scholar]
- 11.Shelat VG. Role of inflammatory indices in management of hepatocellular carcinoma—neutrophil to lymphocyte ratio. Ann Transl Med. 2020;8(15):912. doi: 10.21037/atm-2020-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pang Q, Qu K, Zhang J-Y, et al. The prognostic value of platelet count in patients with hepatocellular carcinoma: a systematic review and meta-analysis. Medicine. 2015;94(37):e1431. doi: 10.1097/MD.0000000000001431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pang Q, Qu K, Bi J-B, et al. Thrombocytopenia for prediction of hepatocellular carcinoma recurrence: systematic review and meta-analysis. World J Gastroenterol. 2015;21(25):7895–7906. doi: 10.3748/wjg.v21.i25.7895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu P-H, Hsu C-Y, Su C-W, et al. Thrombocytosis is associated with worse survival in patients with hepatocellular carcinoma. Liver Int. 2020;40(10):2522–2534. doi: 10.1111/liv.14560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee C-H, Lin Y-J, Lin C-C, et al. Pretreatment platelet count early predicts extrahepatic metastasis of human hepatoma. Liver Int. 2015;35(10):2327–2336. doi: 10.1111/liv.12817 [DOI] [PubMed] [Google Scholar]
- 16.Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med. 2015;162(1):55–63. [DOI] [PubMed] [Google Scholar]
- 17.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. [DOI] [PubMed] [Google Scholar]
- 18.Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–387. doi: [DOI] [PubMed] [Google Scholar]
- 19.Galle PR, Forner A, Llovet JM, European Association for the Study of the Liver. Electronic address:: easloffice@easloffice.eu, European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi: 10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 20.Pang Q, Zhou L, Qu K, et al. Validation of inflammation-based prognostic models in patients with hepatitis B-associated hepatocellular carcinoma: a retrospective observational study. Eur J Gastroenterol Hepatol. 2018;30(1):60–70. doi: 10.1097/MEG.0000000000001021 [DOI] [PubMed] [Google Scholar]
- 21.Xavier SA, Vilas-Boas R, Boal Carvalho P, et al. Assessment of prognostic performance of albumin–bilirubin, Child–Pugh, and model for end-stage liver disease scores in patients with liver cirrhosis complicated with acute upper gastrointestinal bleeding. Eur J Gastroenterol Hepatol. 2018;30(6):652–658. doi: 10.1097/MEG.0000000000001087 [DOI] [PubMed] [Google Scholar]
- 22.Chan AWH, Chong CCN, Mo FKF, et al. Applicability of albumin-bilirubin-based Japan integrated staging score in hepatitis B-associated hepatocellular carcinoma. J Gastroenterol Hepatol. 2016;31(10):1766–1772. doi: 10.1111/jgh.13339 [DOI] [PubMed] [Google Scholar]
- 23.Wang Y-Y, Zhong J-H, Su Z-Y, et al. Albumin-bilirubin versus Child-Pugh score as a predictor of outcome after liver resection for hepatocellular carcinoma. Br J Surg. 2016;103(6):725–734. doi: 10.1002/bjs.10095 [DOI] [PubMed] [Google Scholar]
- 24.Chan AWH, Zhong J, Berhane S, et al. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J Hepatol. 2018;69(6):1284–1293. [DOI] [PubMed] [Google Scholar]
- 25.Toyoda H, Lai PB, O’Beirne J, et al. Long-term impact of liver function on curative therapy for hepatocellular carcinoma: application of the ALBI grade. Br J Cancer. 2016;114(7):744–750. doi: 10.1038/bjc.2016.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ni J-Y, Fang Z-T, An C, et al. Comparison of albumin-bilirubin grade, platelet-albumin-bilirubin grade and Child-Turcotte-Pugh class for prediction of survival in patients with large hepatocellular carcinoma after transarterial chemoembolization combined with microwave ablation. Int J Hyperthermia. 2019;36(1):841–853. doi: 10.1080/02656736.2019.1646927 [DOI] [PubMed] [Google Scholar]
- 27.Gui CH, Baey S, R T D, Shelat VG. Trans-arterial chemoembolization + radiofrequency ablation versus surgical resection in hepatocellular carcinoma - A meta-analysis. Eur J Surg Oncol. 2020;46(5):763–771. [DOI] [PubMed] [Google Scholar]
- 28.Shelat VG, Cipriani F, Basseres T, et al. Pure laparoscopic liver resection for large malignant tumors: does size matter? Ann Surg Oncol. 2015;22(4):1288–1293. doi: 10.1245/s10434-014-4107-6 [DOI] [PubMed] [Google Scholar]
- 29.Bagirsakci E, Sahin E, Atabey N, et al. Role of albumin in growth inhibition in hepatocellular carcinoma. Oncology. 2017;93(2):136–142. doi: 10.1159/000471807 [DOI] [PubMed] [Google Scholar]
- 30.Shim JH, Jun MJ, Han S, et al. Prognostic nomograms for prediction of recurrence and survival after curative liver resection for hepatocellular carcinoma. Ann Surg. 2015;261(5):939–946. [DOI] [PubMed] [Google Scholar]
- 31.Almasaudi AS, Dolan RD, Edwards CA, McMillan DC. Hypoalbuminemia reflects nutritional risk, body composition and systemic inflammation and is independently associated with survival in patients with colorectal cancer. Cancers. 2020;12(7):7. doi: 10.3390/cancers12071986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fung J, Cheung K-S, Wong DK-H, et al. Long-term outcomes and predictive scores for hepatocellular carcinoma and hepatitis B surface antigen seroclearance after hepatitis B e-antigen seroclearance. Hepatology. 2018;68(2):462–472. doi: 10.1002/hep.29874 [DOI] [PubMed] [Google Scholar]
- 33.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44(1):217–231. doi: 10.1016/j.jhep.2005.10.013 [DOI] [PubMed] [Google Scholar]
- 34.Carr BI, Cavallini A, D’Alessandro R, et al. Platelet extracts induce growth, migration and invasion in human hepatocellular carcinoma in vitro. BMC Cancer. 2014;14(1):43. doi: 10.1186/1471-2407-14-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zuo X, Chen Z, Cai J, et al. 5-Hydroxytryptamine receptor 1D aggravates hepatocellular carcinoma progression through FoxO6 in AKT-dependent and independent manners. Hepatology. 2019;69(5):2031–2047. doi: 10.1002/hep.30430 [DOI] [PubMed] [Google Scholar]
- 36.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11(2):123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nozaki R, Murata S, Nowatari T, et al. Effects of thrombopoietin on growth of hepatocellular carcinoma: is thrombopoietin therapy for liver disease safe or not? Hepatol Res. 2013;43(6):610–620. doi: 10.1111/hepr.12006 [DOI] [PubMed] [Google Scholar]
- 38.Mohan R, Huey CWT, Junnarkar S, et al. Prehabilitation in elderly patients scheduled for liver resection and protocol for recovery of surgery in elderly. Hepatoma Res. 2020;6(3):13. [Google Scholar]
- 39.Wang B, Shelat VG, Chow JJL, et al. Prehabilitation program improves outcomes of patients undergoing elective liver resection. J Surg Res. 2020;251:119–125. doi: 10.1016/j.jss.2020.01.009 [DOI] [PubMed] [Google Scholar]
- 40.Kornberg A, Witt U, Schernhammer M, et al. The role of preoperative albumin-bilirubin grade for oncological risk stratification in liver transplant patients with hepatocellular carcinoma. J Surg Oncol. 2019;120(7):1126–1136. doi: 10.1002/jso.25721 [DOI] [PubMed] [Google Scholar]
- 41.Kang D, Schadde SE. Hypertrophy and liver function in ALPPS: correlation with morbidity and mortality. Visc Med. 2017;33(6):426–433. doi: 10.1159/000479477 [DOI] [PMC free article] [PubMed] [Google Scholar]