Abstract
BACKGROUND & AIMS:
We investigated the effects of inducing deep remission in patients with early Crohn’s disease (CD).
METHODS:
We collected follow-up data from 122 patients (mean age, 31.2 ± 11.3 y) with early, moderate to severe CD (median duration, 0.2 years; interquartile range, 0.1–0.5) who participated in the Effect of Tight Control Management on CD (CALM) study, at 31 sites, representing 50% of the original CALM patient population. Fifty percent of patients (n = 61) were randomly assigned to a tight control strategy (increased therapy based on fecal level of calprotectin, serum level of C-reactive protein, and symptoms), and 50% were assigned to conventional management. We categorized patients as those who were vs were not in deep remission (CD endoscopic index of severity scores below 4, with no deep ulcerations or steroid treatment, for 8 or more weeks) at the end of the follow-up period (median, 3.02 years; range, 0.05–6.26 years). The primary outcome was a composite of major adverse outcomes that indicate CD progression during the follow-up period: new internal fistulas or abscesses, strictures, perianal fistulas or abscesses, or hospitalization or surgery for CD. Kaplan-Meier and penalized Cox regression with bootstrapping were used to compare composite rates between patients who achieved or did not achieve remission at the end of the follow-up period.
RESULTS:
Major adverse outcomes were reported for 34 patients (27.9%) during the follow-up period. Significantly fewer patients in deep remission at the end of the CALM study had major adverse outcomes during the follow-up period (P = .01). When we adjusted for potential confounders, deep remission (adjusted hazard ratio, 0.19; 95% confidence interval, 0.07–0.31) was significantly associated with a lower risk of major adverse outcome.
CONCLUSIONS:
In an analysis of follow-up data from the CALM study, we associated induction of deep remission in early, moderate to severe CD with decreased risk of disease progression over a median time of 3 years, regardless of tight control or conventional management strategy.
Keywords: Adalimumab, CDEIS, IBD, Inflammatory Bowel Diseases
Graphical Abstract
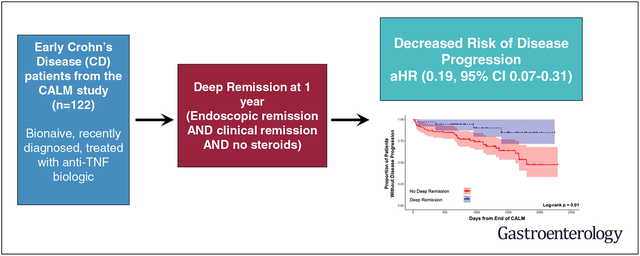
Crohn’s disease (CD) is a chronic, progressive condition of the gastrointestinal tract that, over time, leads to the accumulation of bowel damage and complications such as strictures, abscesses, and fistulae.1 Despite advances in medical therapy for CD, most notably the introduction of biologic agents, many patients still have disease progression and require surgery and hospitalization.2–5 In other immune-mediated diseases, such as rheumatoid arthritis, disease modification studies have shown that early effective therapy can improve long-term patient outcome.6,7 In an effort to similarly improve the natural history of CD, the treatment paradigm has recently shifted to emphasize early intervention, treat to target, and tight control (TC) approaches. Early intervention with the use of antitumor necrosis factor biologics (anti-TNF) in patients with moderate to severe CD with shorter disease duration has been associated with higher clinical response and remission rates in post hoc analyses of clinical trials.8 In addition, the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) program provided an expert consensus on preferred treatment strategies and emphasized the importance of treating to target in CD, with the target being deep remission, defined as resolution of symptoms and objective resolution of inflammation on endoscopy.9
The Effect of Tight Control Management on CD (CALM) study recently showed that a TC approach to patient management, in which therapy is escalated based on objective markers of inflammation (fecal calprotectin and C-reactive protein) in addition to symptoms, is an effective strategy to achieve the targets of endoscopic and deep remission.10 Early intervention, treat to target, and TC treatment strategies have been adopted in other diseases, such as rheumatoid arthritis. However, only recently are these strategies gaining acceptance in the management of CD. Although CALM showed that a TC approach improved endoscopic and deep remission in patients with early CD, there are currently no data on the impact of reaching these remission targets on long-term disease progression.
The CALM study population is unique in that it includes patients with CD who were all, early in their disease course, treated with anti-TNF, with prospective assessment of endoscopic activity using a validated score. This offered the opportunity to better understand the long-term impact of reaching preferred treatment targets in patients with early CD. We therefore collected follow-up data on CALM participants with the aim of describing the impact of achieving early deep and endoscopic remission on long-term disease progression in patients with CD.
Methods
Study Design
We conducted a retrospective cohort observational study according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. All patients from the initial CALM study were eligible, regardless of randomization arm. Inclusion criteria for CALM (NCT01235689) have been described previously.10 Briefly, CALM was a multicenter, randomized, open-label, active-controlled, 48-week, phase 3 trial to assess tight control (TC) versus conventional management (CM) algorithms in adult patients with moderate to severe CD. Patients in the TC arm had medical therapy escalation in response to clinical symptoms (assessed by the Crohn’s disease activity index [CDAI]) or elevation in fecal calprotectin or C-reactive protein, whereas patients in the CM arm had therapy escalated in response to symptoms only (CDAI). After ≤8 weeks of prednisone induction therapy and mandated taper, patients were randomly assigned to the TC or CM groups in a 1:1 ratio, stratified by site, smoking status, and weight. If failure criteria were met at scheduled study visits, treatment was escalated stepwise from no treatment, to adalimumab 160/80 mg at weeks 0/2 followed by 40 mg every other week, to adalimumab 40 mg every week, to adalimumab 40 mg every week plus 2.5 mg/kg azathioprine per day. Patients who did not meet a failure criterion continued to receive the same treatment option. Starting at weeks 23 and 35 after randomization, patients receiving weekly adalimumab could de-escalate to the previous treatment option if failure criteria were not met. Ileocolonoscopies were performed at study screening and at 48 weeks after randomization and were assessed by the site investigator for Crohn’s Disease Endoscopic Index of Severity (CDEIS). The primary endpoint in CALM was CDEIS of <4 and no deep ulcers at 48 weeks after randomization.
To evaluate long-term outcomes, the original CALM study sites were contacted and invited to participate in the present study. Sites were sent a standardized electronic questionnaire to collect data on CALM participants through medical record review. Data collected included whether the patient was lost to follow, total duration of follow-up, if the patient was still receiving the same therapy from CALM at the end of follow-up (and any changes in therapy and reason for change), and occurrence of any CD-related complication. These included any new CD-related hospitalization (primary reason for admission was CD-related); new CD-related surgery; and any new CD complication, defined as any new internal fistula/abscess, intestinal stricture (obstructive symptoms plus evidence of bowel narrowing on imaging and/or endoscopy), or perianal disease (any new perianal abscess, need for seton, or diversion surgery). Each participating site obtained any necessary local institutional review board approvals. All data were centralized and reviewed for accuracy and consistency by 2 investigators (RCU and CY).
Outcomes and Variables
The primary outcome was a composite of major adverse outcomes reflecting disease progression: new internal fistula/abscess, stricture, perianal fistula/abscess, CD hospitalization, or CD surgery since end of CALM. Secondary outcomes included the individual components of the composite primary outcome and proportion of patients still receiving adalimumab therapy at the end of follow-up.
Patients were then stratified by level of remission obtained at the end of CALM to compare long-term outcomes. The primary variable of interest for this stratification was deep remission (defined as CDAI of <150, CDEIS of <4 with no deep ulcerations, and no steroids for ≥8 weeks) at the end of CALM, given this is the recommended STRIDE target for CD. Other definitions of remission that were examined included endoscopic remission (defined as the primary endpoint of CALM, CDEIS of <4 with no deep ulcers), clinical remission (defined as a CDAI of <150), CDEIS of 0, CDEIS of <4, CDEIS of <4 in every segment, no deep ulcerations in every segment, and CDEIS decrease of >5 points. These variables were defined according to the original trial; therefore, patients who were early terminators in CALM and did not complete the study were considered as not being in remission. In addition, baseline (at the time of the start of CALM randomization) age, sex, disease duration, race, disease location (ileal, ileocolonic, or colonic), disease behavior (inflammatory, stricturing, or penetrating), history of CD surgery, smoking status, fecal calprotectin, CDEIS score, CDAI score, and randomization arm (TC or CM) were recorded. The impact of original randomization arm during the CALM study (TC or CM) on the primary outcome (progression after the end of CALM) was also examined. In addition, we conducted a sensitivity analysis looking at the primary and secondary outcomes restricted to only those patients who completed the CALM trial (excluding patients considered not in remission because they did not complete the study).
Statistical Analysis
AbbVie, Inc, provided access to the primary data from the original clinical trial; however, the study investigators did all analyses independently. Continuous variables were described using means (±standard deviation) or medians (±interquartile range [IQR]), and categorical variables were described using proportions. Comparison of parametric continuous variables was performed by using the 2-sample t test. Nonparametric continuous variables were compared by using Wilcoxon’s rank-sum test. The chi-squared or Fisher’s exact test was used to compare categorical variables. For univariate analyses, Kaplan-Meier and Cox regressions were used to compare rates of CD complications (composite primary outcome) between patients who achieved or did not achieve different definitions of remission at the end of CALM. Patient follow-up was censored at the date of either the first occurrence of an adverse event or at the date of last office visit for those with no event (right censored). No violations of the proportional hazards assumption were observed. For multivariate analyses, we performed Cox regression, adjusting for confounders including CALM treatment arm, age, sex, disease duration, baseline C-reactive protein level, baseline calprotectin level, disease location, smoking, prior surgery, and history of structuring disease. For both univariate and multivariate models, the 95% confidence intervals (CIs) of hazard ratios (HRs) were estimated via bootstrapping. Specifically, 1000 bootstrap iterations were considered, with 80% of samples randomly selected at each iteration. CIs were computed across bootstrap iterations, with the median of adjusted HR (aHR) across iterations considered as the estimated value. To further evaluate the marginal effect of deep remission on our primary outcome, we also derived the 95% confidence intervals of standardized survival curves across 1000 bootstrap iterations. For each bootstrap iteration, the averaged survival curves for patients in the 2 arms were computed across all combinations of other covariates included in the multivariate model. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC) and R CRAN (R Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 122 patients who participated in CALM from 31 different sites had data available since the end of the study and were included in the present long-term follow-up study. This represented 50% (122/244) of the original CALM population (Figure 1). The remainder of the original CALM population was not included either because of lack of follow-up data or inability of study site to participate. Baseline characteristics from the start of the CALM study of patients included in the current study are provided in Table 1. The median follow-up duration since the end of the CALM study was 3.02 years. Half of the patients in the current study were originally in the TC arm of CALM. At the start of the CALM trial, patients included in the current long-term follow-up study had short disease duration (median, 0.2 years), moderate to severe disease by CDAI and CDEIS, and low rates of prior complications (stricture or CD-related surgery), consistent with the overall CALM trial patient population. There were no significant differences in baseline characteristics in patients with follow-up data and those lost to follow-up, with the exception of a slightly higher CDEIS score in patients lost to follow-up (14.6 vs 12.9; P = .04) (Supplementary Table 1). Median time to last follow-up in those in deep remission at the end of CALM compared to those not in deep remission was not significantly different (1126 vs 1075 d; P = .28).
Figure 1.
Flow chart of the study cohort creation. There were 32 patients in deep remission and 56 patients not in deep remission at the end of the CALM study who did not have any events during follow up (right-censored). The median follow-up was similar between these 2 groups (deep remission: 1274 days; IQR, 748–1688 vs no deep remission: 1219 days; IQR, 885–1665; P = .97).
Table 1.
Baseline patient characteristics from start of the CALM study
| Baseline patient characteristics | Values |
|---|---|
| Age, y, mean (SD) | 31.2 (11.3) |
| Disease duration, y, median (IQR) | 0.2 (0.1–0.5) |
| Female sex, n (%) | 72 (59) |
| Follow-up time since end of CALM, y, median (range) | 3.02 (0.05–6.26) |
| Randomized to TC arm, n (%) | 61 (50) |
| White race, n (%) | 119 (97.5) |
| Disease location, n (%) | |
| Ileal (L1) | 19 (15.5) |
| Ileocolonic (L2) | 67 (55) |
| Colonic (L3) | 36 (29.5) |
| Prior CD surgery, n (%) | 7 (5.7) |
| History stricturing behavior (B2), n (%) | 12 (9.8) |
| Baseline CDEIS score, mean (SD) | 12.9 (5.9) |
| Baseline CDAI score, mean (SD) | 268.5 (55.3) |
SD, standard deviation.
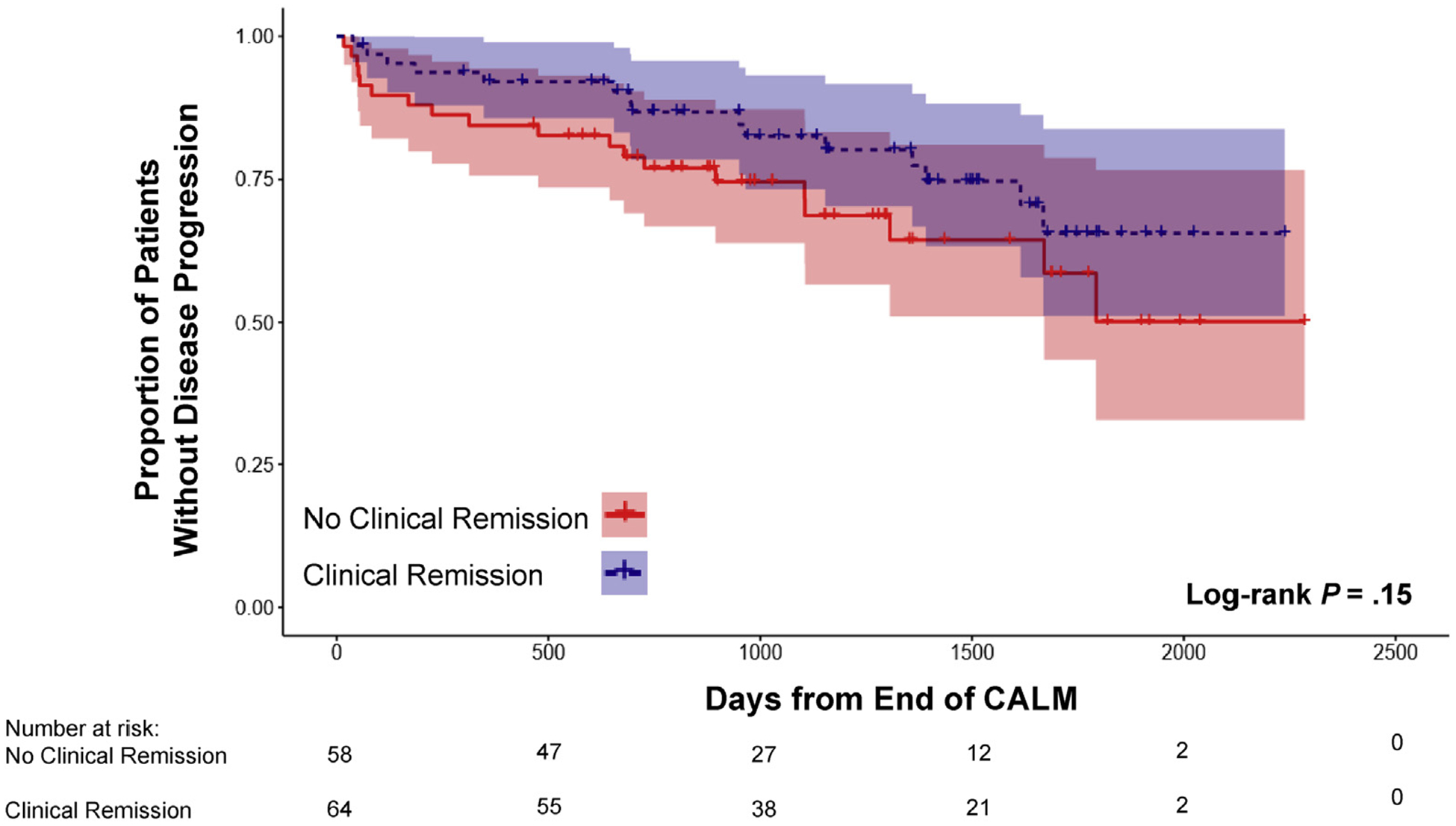
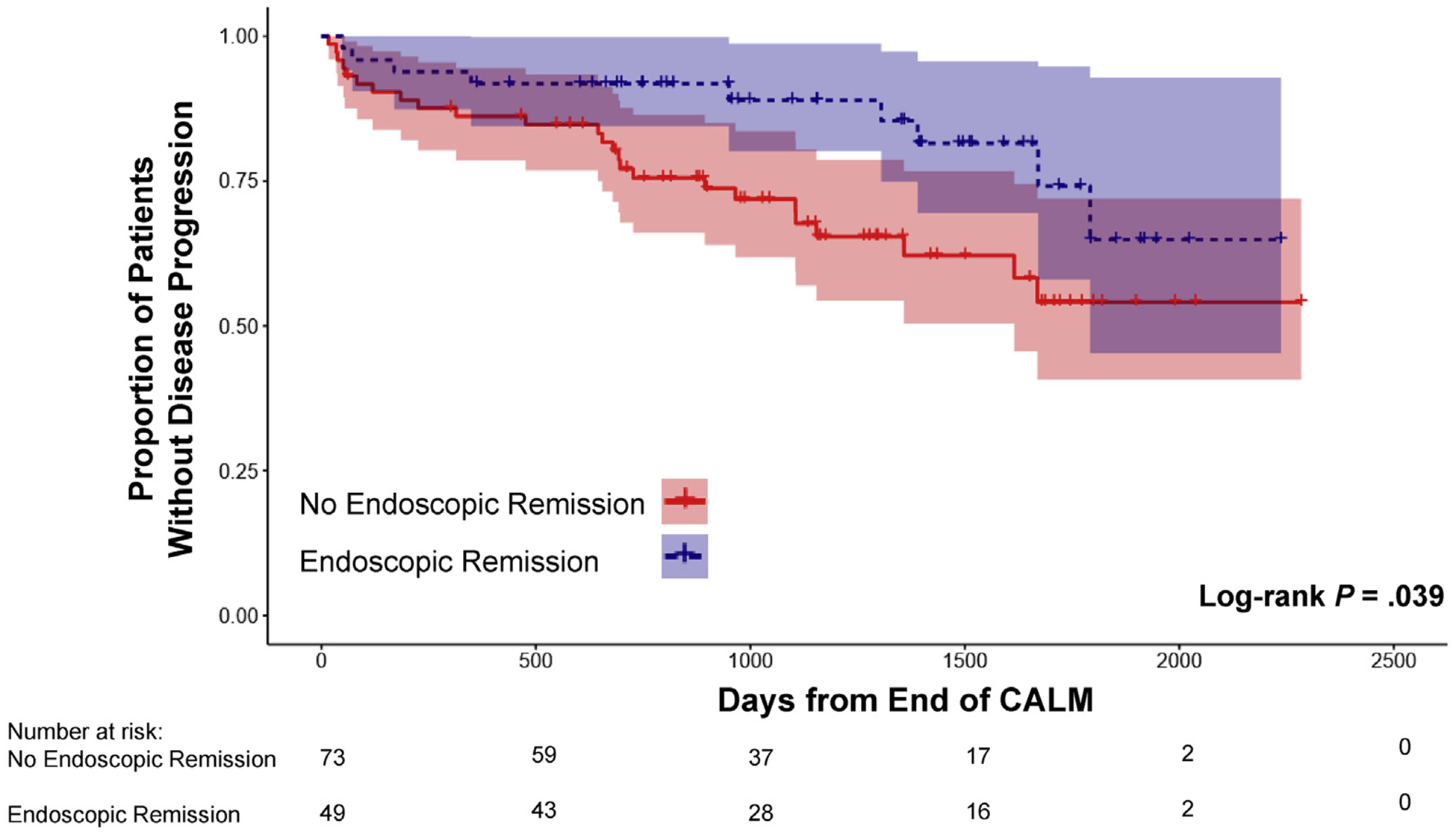
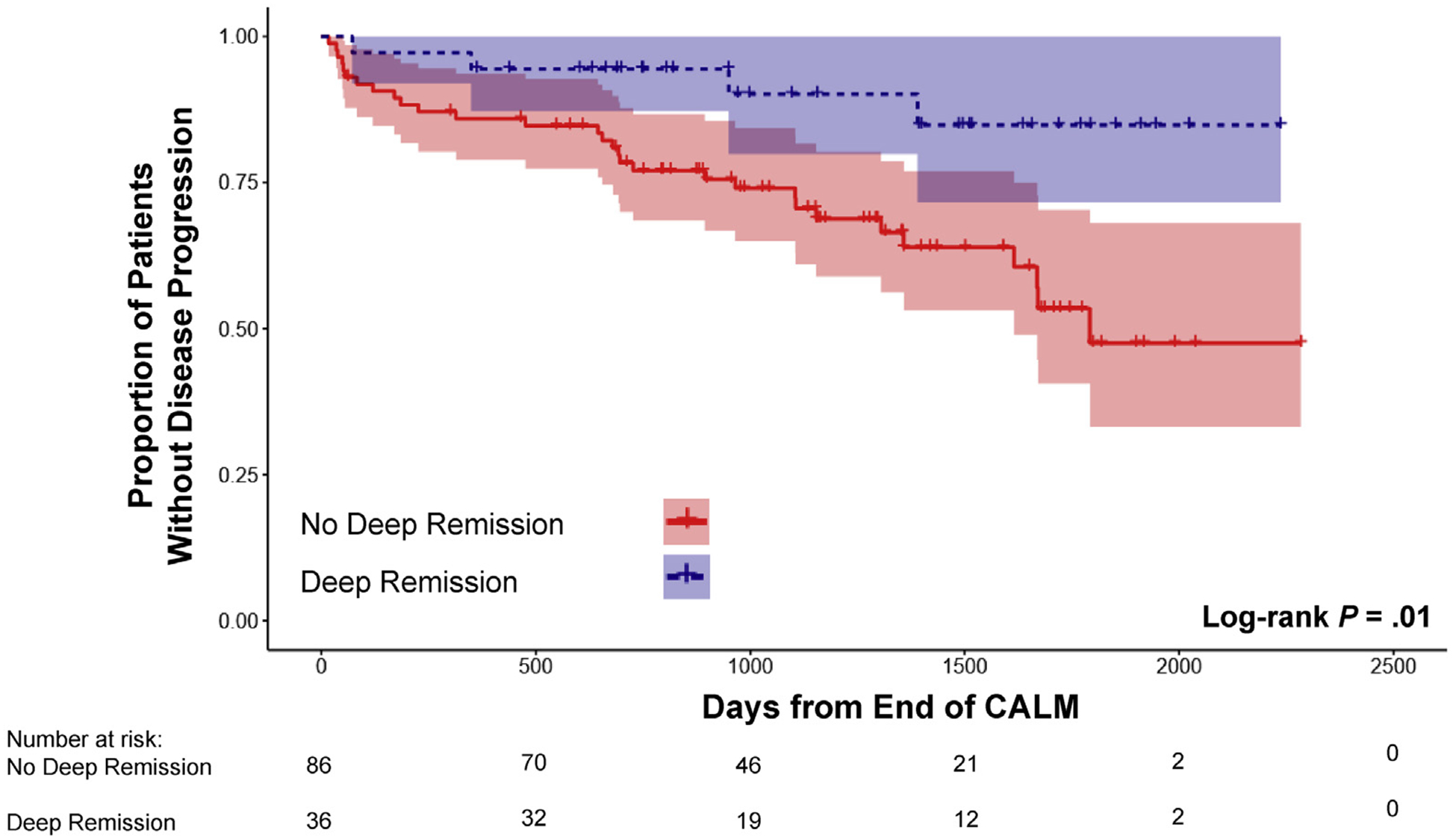
The primary outcome of disease progression occurred in 34 patients (27.9%) since the end of the CALM study. In univariate Kaplan-Meier analysis, the risk of disease progression during follow-up was not significantly different when comparing patients in clinical remission (n = 64) at the end of the CALM study with those who were not (Figure 2). In contrast, patients in endoscopic (n = 49) and deep remission (n = 36) at the end of the CALM study had a significantly lower risk of disease progression compared to those not in remission (Figures 3 and 4). The impact of different definitions of remission on disease progression is described in Table 2. On univariate Cox regression analysis, deep remission (HR, 0.28; 95% CI, 0.10–0.80) was associated with significantly decreased risk of CD disease progression. Endoscopic and clinical remission were also associated with decreased progression but with more modest HRs. Alternative definitions of endoscopic response and remission, which were secondary outcomes in the original CALM study, were not significantly associated with decreased risk of disease progression, but all had HR trending toward protection (Supplementary Table 2). Achieving deep remission or endoscopic remission was associated with or trended toward a decreased risk of each component of the composite primary outcome of disease progression (when considering only components with at least 10 events) (Supplementary Table 3). In addition, the impact of deep remission on the primary outcome was still significant based on standardized survival curves (Supplementary Figure 1). Of note, patients in the TC arm during the CALM study did not have a significantly decreased risk of disease progression after the end of the study compared to those in the CM arm (HR, 0.89; 95% CI, 0.45–1.75) (Supplementary Figure 2).
Figure 2.
Kaplan-Meier estimates of CD disease progression based on clinical remission at the end of the CALM study with 95% CIs. Disease progression was defined as any major adverse outcome: composite of new internal fistula/abscess, stricture, perianal fistula/abscess, CD hospitalization, or CD surgery since end of the CALM study.
Figure 3.
Kaplan-Meier estimates of CD disease progression based on endoscopic remission at the end of the CALM study with 95% CIs. Disease progression was defined as any major adverse outcome: composite of new internal fistula/abscess, stricture, perianal fistula/abscess, CD hospitalization, or CD surgery since end of the CALM study.
Figure 4.
Kaplan-Meier estimates of CD disease progression based on deep remission at the end of the CALM study with 95% CIs. Disease progression was defined as any major adverse outcome: composite of new internal fistula/abscess, stricture, perianal fistula/abscess, CD hospitalization, or CD surgery since end of the CALM study.
Table 2.
Association of Remission Definitions at the End of the CALM Study With Disease Progression Based on Univariate Analysis
| Variable | HR (95% CI) |
|---|---|
| Deep remission | 0.23 (0.09–0.32) |
| Endoscopic remission | 0.46 (0.31–0.60) |
| Clinical remission | 0.64 (0.34–0.61) |
We next performed multivariate analysis, controlling for potential confounders. In multivariate models, deep remission (aHR, 0.19; 95% CI, 0.07–0.31) was significantly associated with a decreased risk of progression (Table 3). Again, deep remission had the most marked decrease in risk. Both endoscopic and clinical remission were significantly associated with a decreased risk of CD disease progression after adjusting for confounders. Baseline patient characteristic associations with disease progression in the multivariate deep remission model are provided in Supplementary Table 4. Baseline variables that were significant in the multivariate deep remission model were disease duration (aHR, 0.69; 95% CI, 0.53–0.81), stricturing disease behavior (aHR, 2.18; 95% CI, 1.22–3.78), and prior history of surgery (aHR, 3.83; 95% CI, 1.50–8.16). We then also conducted sensitivity analyses, removing patients who were considered not in remission because they discontinued the original study early. This resulted in a subcohort of 101 patients who completed CALM. In univariate analyses, our results were consistent, with our primary analysis with deep remission (HR, 0.26; 95% CI, 0.09–0.73) significantly associated with decreased risk of CD disease progression. Our results also held in multivariate analysis in this subcohort. On adjusted analyses, deep remission (aHR, 0.14; 95% CI, 0.04–0.47) was still protective against CD progression. For an additional sensitivity analysis, we adjusted for study site region (Western Europe, Eastern Europe, or other region) in a multivariate model that also included all baseline variables of interest and observed that deep remission was still significantly associated with decreased risk of disease progression (aHR, 0.21; 95% CI, 0.08–0.34).
Table 3.
Association of Remission Definitions at the End of the CALM Study With Disease Progression Based on Multivariate Penalized Cox Regression
| Variable | aHR (95% CI) |
|---|---|
| Deep remission | 0.19 (0.08–0.31) |
| Endoscopic remission | 0.41 (0.24–0.60) |
| Clinical remission | 0.40 (0.26–0.57) |
NOTE. Multivariate cox models adjusted for CALM treatment arm, age, sex, disease duration, baseline C-reactive protein, baseline calprotectin, disease location, smoking, prior surgery, and history of stricturing disease.
Last, we examined the impact of deep remission on treatment changes and reasons for stopping treatment after the end of the CALM study. We observed that patients achieving deep remission were more likely to still be receiving adalimumab at the end of follow-up. Among patients in deep remission at the end of CALM, 61.1% were still receiving adalimumab at last follow-up compared to 34.9% of those not in deep remission (P = 001). After the end of CALM, patients in deep remission, compared to those not in deep remission, had lower rates of stopping adalimumab because of loss of response (7% vs 45%) or adverse effects (14% vs 25%). Among patients in deep remission, the most common reasons for stopping adalimumab were cost or logistical reasons (36%) and patient preference (29%). Overall, 43% of patients receiving azathioprine at the end of CALM who achieved deep remission (n = 7) were still taking this medication at the end of follow-up compared to 25% of patients receiving azathioprine at the end of CALM who were not in deep remission (n = 24). Patients in deep remission who stopped azathioprine did so because of preference (50%) or as part of a de-escalation strategy (50%). In comparison, the majority of patients not in deep remission receiving azathioprine at the end of CALM stopped because of effects (56%). When patients started a new therapy after CALM, the most commonly used first-line medication was infliximab (49%), followed by vedolizumab (18%), azathioprine (16%), ustekinumab (9%), and clinical trial agent (8%). During follow-up, patients in deep remission were started on a lower mean number of new therapies compared to those not in deep remission (0.27 vs 0.75; P = .01).
Discussion
In this long-term follow-up study of patients with CD from CALM, we observed that achieving deep remission in patients with early CD was significantly associated with a decreased risk of disease progression. Deep remission was associated with an 81% decrease in risk of adverse outcomes over a median of 3 years. Our findings provide evidence supporting the concept that early deep remission in CD can lead to disease modification with a significant decrease in long-term complications.
Prior studies have shown that patients with CD have improved clinical response and remission rates with anti-TNF when used earlier in the disease course. Post hoc analyses of clinical trials of anti-TNF agents, including infliximab, adalimumab, and certolizumab, have consistently observed higher clinical response rates in patients with CD with a disease duration of less than 2 years.11–13 In addition, observational studies have shown improved outcomes with earlier introduction of immunosuppression. A health claims study from the United States found that a top-down approach (with earlier introduction of biologics) was associated with lower rates of steroid use and surgery.14 In a cohort study from Switzerland, early use of immunosuppression (within 2 years of diagnosis) was associated with decreased rates of strictures over time.15 A similar impact of early intervention has been seen in pediatrics and with a large prospective cohort study showing that the use of anti-TNF within the 3 months of diagnosis resulted in higher rates of corticosteroid-free remission at 1 year.16 However, these studies mostly reported shorter-term outcomes and did not examine the long-term impact of achieving the target of early deep remission.
The STRIDE program recommended deep remission (endoscopic and clinical remission) as the preferred treatment target for CD.9 However, to date, data on the impact of achieving deep remission in CD are relatively limited. Prior analyses have focused on 1-year outcomes or earlier timepoints for endoscopic remission. A meta-analysis of 10 studies found that mucosal healing was significantly associated with long-term clinical remission (odds ratio, 2.80; 95% CI, 1.91–4.10).17 Pooling 3 studies that included data on long-term surgery outcomes found that mucosal healing had a nonsignificant trend toward a higher rate of being surgery-free (odds ratio, 2.22; 95% CI, 0.86–5.69). These prior studies tended to include patients with varying disease duration, had differing definitions of endoscopic remission, and had a shorter length of follow-up than the current study. We observed the strongest impact on decreased risk of CD disease complications with deep remission. Endoscopic remission was also significantly associated with decreased risk of disease progression but to a lower effect size than deep remission. One possible explanation is that patients in endoscopic but not deep remission may be more likely to have active inflammation more proximal to the reach of a colonoscope (eg, upper tract disease) and/or deeper transmural inflammation detectable only on cross-sectional imaging. It is also important to note that although the other definitions of endoscopic response and remission studied did not reach statistical significance, they all had a trend toward also improving rates of CD disease progression. This may be due to lower power to detect more modest, but significant, effect sizes for these other remission definitions. Future larger studies should determine the exact degree of endoscopic response that is necessary to improve long-term outcomes. Of note, clinical remission was associated with a decreased risk of disease progression on multivariate analysis. It is possible that after adjusting for confounders in a clinical trial population that required endoscopic activity for entry, clinical remission may more closely correlate with harder outcomes of deep or endoscopic remission. In addition, it is important to note that we did not observe that being in the TC arm during the initial CALM study significantly improved long-term outcomes. Although the treatment strategy of TC will achieve deep and endoscopic remission more frequently than CM, the disease-monitoring strategy alone did not alter outcomes after the CALM trial. Achieving the target of deep remission is what has the more lasting impact, and future trials should focus on strategies and therapies that further improve early remission rates.
Our study had several strengths, including having multiple centers from different countries that were able to provide long-term follow-up data reflective of CD disease course. In addition, endoscopic disease activity was assessed by using a standardized validated endoscopy score (CDEIS). Finally, this patient cohort is the ideal cohort in which to evaluate the long-term impact of achieving early remission in CD because patients were recently diagnosed, immunosuppressant naive, and treated with a specific treatment protocol with early introduction of an anti-TNF agent. We also acknowledge that our study had several limitations. Follow-up data were collected retrospectively through medical chart review and so were limited to what was available in the patient record. Follow-up information since the end of CALM was not available in roughly half of patients from the original CALM study, which may introduce bias. However, there were no significant differences in the baseline patient characteristics at the start of the CALM study for patients included in this long-term follow-up study compared to those who were lost to follow-up aside from marginally higher CDEIS scores in patients with no follow-up. In addition, remission rates at the end of CALM in the current long-term study population are very similar to those of the overall original CALM study population: 29.5% of patients in the current study were in deep remission compared to 29.9% in the original CALM study, and 40% of patients were in endoscopic remission in the long-term cohort compared to 38.1% of the overall CALM population. Another potential limitation is that some of the outcomes of interest may have been present during the CALM trial but were undiagnosed (eg, internal fistula or stricture). However, CALM patients underwent stricture screening for study entry, so, although possible, it is unlikely that these patients had an undiagnosed CD complication. Although the treatment after the end of CALM was at the discretion of the treating physician and subject to variation, achieving the target of deep remission after 1 year of treatment still appears to have a lasting impact.
In summary, we observed that achieving deep remission in patients with early CD can significantly decrease the risk of long-term disease progression. Our data validate the current recommended CD treatment strategies of early therapeutic intervention, TC, and treat to target by highlighting the impact of early deep remission on long-term disease modification.
Supplementary Material
WHAT YOU NEED TO KNOW.
BACKGROUND AND CONTEXT
It is not clear whether induction of deep remission during the early stages of Crohn’s disease (CD) prevents disease progression.
NEW FINDINGS
In an analysis of data from patients who participated in the effect of tight control management on CD (CALM) study, we associated induction of deep remission in early-stage, moderate to severe CD with a decreased risk of disease progression over a median time of 3 years.
LIMITATIONS
This was a retrospective analysis of follow-up data from a large clinical trial.
IMPACT
Remission should be induced early in the disease course in patients with CD, because it prevents disease progression.
Acknowledgments
The authors thank Nadia Arab for assistance with collecting data and all patients who have participated to the study.
https://clinicaltrials.gov/ct2/show/NCT01235689
Funding
The CALM clinical trial was funded by AbbVie, Inc. The long-term follow-up study design, data collection, and analyses were done without funding, independent of AbbVie, Inc. Ryan C. Ungaro is supported by a Career Development Award from the Crohn’s and Colitis Foundation and a National Institutes of Health K23 Career Development Award (K23KD111995-01A1).
Abbreviations used in this paper:
- aHR
adjusted hazard ratio
- anti-TNF
anti-tumor necrosis factor biologics
- CALM
Effect of Tight Control Management on Crohn’s Disease
- CD
Crohn’s disease
- CDAI
Crohn’s disease activity index
- CDEIS
Crohn’s Disease Endoscopic Index of Severity
- CI
confidence interval
- CM
conventional management
- HR
hazard ratio
- IQR
interquartile range
- STRIDE
Selecting Therapeutics Targets in Inflammatory Bowel Disease
- TC
tight control
Footnotes
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at https://doi.org/10.1053/j.gastro.2020.03.039.
Conflicts of interest
These authors disclose the following: Ryan C. Ungaro has served as an advisory board member or consultant for Eli Lilly, Janssen, Pfizer, and Takeda and has received research grants from AbbVie, Boehringer Ingelheim, and Pfizer. Peter Bossuyt has received financial support for research from AbbVie, Mundipharma, Pfizer, Janssen, and Mylan; lecture fees from AbbVie, Takeda, Pfizer, and Janssen; and advisory board fees from AbbVie, Takeda, Hospira, Janssen, MSD, Mundipharma, Roche, Pfizer, Sandoz, and Pentax. Filip J. Baert has received research grants from AbbVie, Chiesi, Ipsen, MSD, Roche and speakers and consultancy fees from AbbVie, Falk, Ferring, Janssen, Mundipharma, MSD, Pfizer, Takeda, and Vifor. Thomas Vinasek has served as advisory member for Hospira, Pfizer, and Takeda and has received lecture fees from Takeda. Remo Panaccione has received consultant fees from AbbVie, ActoGeniX, AGI Therapeutics, Alba Therapeutics Albireo, Alfa Wasserman, Amgen, AMPharma BV, Anaphore, Aptalis, Astellas, Athersys, Atlantic Healthcare, BioBalance, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Celek, Cellerix, Cerimon, ChemoCentryx, CoMentis, Cosmo Technologies, Coronado Biosciences, Cytokine PharmaSciences, Eagle, Eisai Medical Research, Elan, enGene, Eli Lilly, EnteroMedics, Exagen Diagnostics, Ferring, Flexion Therapeutics, Funxional Therapeutics, Genentech, Genzyme, Gilead, Given Imaging, GlaxoSmithKline, Human Genome Sciences, Ironwood, Janssen, KaloBios, Lexicon, Lycera, Meda, Merck & Co, Merck Research Laboratories, Merck Serono, Millennium, Nisshin Kyorin, Novo Nordisk, NPS Pharmaceuticals, Optimer, Orexigen, PDL Biopharma, Pfizer, Procter and Gamble, Prometheus Laboratories, ProTAB, Purgenesis Technologies, Receptos, Relypsa, Salient, Salix, Santarus, Shire Pharmaceuticals, Sigmoid Pharma, Sirtris (a GlaxoSmithKline company), SLA Pharma (UK), Targacept, Teva, Therakos, Tillotts, TxCell SA, UCB Pharma, Vascular Biogenics, and Viamet. Walter Reinisch has been a speaker for Abbott Laboratories, AbbVie, Aesca, Aptalis, Astellas, Centocor, Celltrion, Danone Austria, Elan, Falk Pharma GmbH, Ferring, Immundiagnostik, Mitsubishi Tanabe Pharma Corporation, MSD, Otsuka, PDL, Pharmacosmos, PLS Education, Schering-Plough, Shire, Takeda, Therakos, Vifor, and Yakult; has been a consultant for Abbott Laboratories, AbbVie, Aesca, Amgen, AM Pharma, Astellas, AstraZeneca, Avaxia, Roland Berger GmbH, Bioclinica, Biogen IDEC, Boehringer Ingelheim, Bristol Myers Squibb, Cellerix, ChemoCentryx, Celgene, Centocor, Celltrion, Covance, Danone Austria, Elan, Ernest & Young, Falk Pharma GmbH, Ferring, Galapagos, Genentech, Gilead, Grünenthal, ICON, Index Pharma, Inova, Janssen, Johnson & Johnson, Kyowa Hakko Kirin Pharma, Lipid Therapeutics, Mallinckrodt, MedImmune, Millennium, Mitsubishi Tanabe Pharma Corporation, MSD, Nestlé, Novartis, Ocera, Otsuka, Parexel, PDL, Pharmacosmos, Pfizer, Procter & Gamble, Prometheus, Robarts Clinical Trial, Sandoz, Schering-Plough, Second Genome, SetPoint Medical, Sigmoid, Takeda, Therakos, TiGenix, UCB, Vifor, Zealand, Zyngenia, and 4SC; has been an advisory board member for Abbott Laboratories, AbbVie, Aesca, Amgen, AM Pharma, Astellas, AstraZeneca, Avaxia, Biogen IDEC, Boehringer Ingelheim, Bristol Myers Squibb, Cellerix, ChemoCentryx, Celgene, Centocor, Celltrion, Danone Austria, Elan, Ferring, Galapagos, Genentech, Grünenthal, Inova, Janssen, Johnson & Johnson, Kyowa Hakko Kirin Pharma, Lipid Therapeutics, MedImmune, Millennium, Mitsubishi Tanabe Pharma Corporation, MSD, Nestlé, Novartis, Ocera, Otsuka, PDL, Pharmacosmos, Pfizer, Procter & Gamble, Prometheus, Sandoz, Schering-Plough, Second Genome, SetPoint Medical, Takeda, Therakos, TiGenix, UCB, Zealand, Zyngenia, and 4SC; and has received research funding from Abbott Laboratories, AbbVie, Aesca, Centocor, Falk Pharma GmbH, Immundiagnostik, and MSD. Alessandro Armuzzi has received consultant fees from AbbVie, Allergan, Amgen, Biogen, Bristol Myers Squibb, Celgene, Celltrion, Eli Lilly, Ferring, Hospira, Janssen, MSD, Mundipharma, Mylan, Pfizer, Roche, Samsung Bioepis, Sandoz, Sofar, Takeda; has received lecture fees from AbbVie, Amgen, AstraZeneca, Chiesi, Ferring, Hospira, Janssen, Medtronic, MSD, Mitsubishi Tanabe, Mundipharma, Nikkiso, Otsuka, Pfizer, Samsung Bioepis, Takeda, TiGenix, Zambon and has received research grants from MSD, Takeda, Pfizer. Simon P. Travis has received grants/research support from AbbVie, Buhlmann, Celgene, International Organization for the study of Inflammatory Bowel Diseases, Janssen, Lilly, Takeda, UCB, Vifor, and Norman Collisson Foundation; has received consulting fees from Abacus, AbbVie, Actial, ai4gi, Alcimed, Allergan, Amgen, Arena, Asahi, Astellas, Atlantic, AstraZeneca, Barco, Biocare, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Buhlmann, Calcico, Celgene, Celsius, Cellerix, Cerimon, ChemoCentryx, Cisbio, Coronado, Cosmo, Ducentis, Dynavax, Elan, Enterome, Falk, Ferring, FPRT Bio, Giuliani SpA, Genentech, Genzyme, Glenmark, Grunenthal, GlaxoSmithKline, GW Pharmaceuticals, Immunocore, Immunometabolism, Indigo, Janssen, Lexicon, Lilly, Medarex, Merck, MSD, Netbiotix, Neovacs, Novartis, Novo Nordisk, NPS Pharmaceuticals, Ocera, Ptima, Otsuka, Palau, Pentax, Pfizer, Philips, Procter & Gamble, Pronota, Proximagen, Resolute, Receptos, Robarts, Roche, Sandoz, Santarus, Sensyne, Shire, Sigmoid Pharma, SynDermix, Synthon, Takeda, Theravance, TiGenix, Tillotts, Topivert, TxCell, UCB, Vertex, VHsquared, Vifor, Warner Chilcott, and Zeria; and has received speaker fees from AbbVie, Amgen, Biogen, Falk, Ferring, GlaxoSmithKline, Janssen, Shire, Takeda, and Zeria. Xavier Hébuterne has served an advisory board member or consultant for AbbVie, Arkopharma, Astellas, Janssen, Nutricia, Pfizer, and Takeda; has received speaker’s fees from AbbVie, ARARD, Arkopharma, Baxter, Bristol Myers Squibb, Ferring, Janssen, MSD, Nutricia, Pfizer, Tillotts, Sanofi-Aventis, and Takeda; has undertaken clinical research activities with AbbVie, Abivax, Alfasigma, Arena, Celgene, Gilead, Eli Lilly, Enterome, Janssen, InDex Pharmaceuticals, Pfizer, Roche, Salix, Takeda, and Theravance. Gerhard Rogler has consulted to AbbVie, Augurix, Bristol Myers Squibb, Boehringer, Calypso, Celgene, Dr Falk Pharma, Ferring, Fisher, Genentech, Gilead, Janssen, MSD, Novartis, Pfizer, Phadia, Roche, UCB, Takeda, Tillotts, Vifor, Vital Solutions, and Zeller; has received educational grants and research grants from AbbVie, Ardeypharm, Augurix, Calypso, Dr Falk Pharma, Flamentera, MSD, Novartis, Pfizer, Roche, Takeda, Tillotts, UCB, and Zeller; has served as an advisory board member or consultant for AbbVie, Boehringer Ingelheim, Sandoz, Ferring Pharmaceuticals, Janssen, Pfizer, and Biocodex Polska; and has been a speaker for AbbVie, Ferring, Astellas, Takeda, Gilead, Genentech, Alvogen, Biocodex Polska, and Walmark. Marc Ferrante has received speaker fees and/or consultant fees from AbbVie, MSD, Ferring, Takeda, Janssen, Pfizer, Boehringer Ingelheim, Biogen, Tillotts, Celgene, and Gilead. Mathurin Fumery has received research grants from Janssen, Pfizer, Takeda; has received consultancy fees from AbbVie, Boehringer Ingelheim, Ferring, Janssen, Mitsubishi Tanabe, MSD, and Pfizer; and has received speakers fees from AbbVie, Boehringer Ingelheim, Chiesi, Dr Falk Pharma, Ferring, Janssen, Lamepro, Mitsubishi Tanabe, MSD, Pfizer, Tramedico, Tillotts, and Zeria. Silvio Danese reports personal fees from AbbVie, Actelion Pharmaceuticals, Alfa Wasserman, AstraZeneca, Cellerix, Cosmo Pharmaceuticals, Ferring Pharmaceuticals, Genentech, Grunenthal, Johnson & Johnson, Millennium Pharmaceuticals, Merck & Co, Novo Nordisk, Nycomed, Pfizer, Pharmacosmos, Schering-Plough, Salix, Takeda, UCB Pharma, and Vifor. Grazyna Rydzewska has received speaker’s honoraria from AstraZeneca, AbbVie, Dr Falk Pharma, Janssen, MSD, Pfizer, Phadia, Takeda, Tillotts, UCB, Vifor, and Zeller. Benjamin Pariente has served as an advisory board member or consultant for AbbVie, Lilly, Ferring, Janssen, Pfizer, and Takeda; has served as a speaker for AbbVie, Ferring, Takeda, Theradiag, MSD, and Janssen; and has received research grants from AbbVie and Janssen. Erik Hertervig has served on advisory boards for AbbVie, Pfizer, Takeda, and Janssen and has received lecture fees from Takeda. Laurent Peyrin-Biroulet has served as a consultant, advisory board member, or speaker for Tillotts, Celltrion, Allergan, Biogen, MSD, Genentech, Index Pharmaceuticals, Ferring, Roche, Arena, Sterna, Gilead, Nestlé, Boehringer Ingelheim, Sandoz, Celgene, Enterome, Pfizer, Samsung, AbbVie, Takeda, Pharmacosmos, Janssen, Hikma, Alma, Amgen. David Laharie has received board and lecture fees from AbbVie, Biogaran, Biogen, Ferring, HAC Pharma, Janssen, MSD, Novartis, Pfizer, Prometheus, Roche, Takeda, Theradiag, Tillotts. Jonas Halfvarson has served as an advisory board member or consultant for AbbVie, Celgene, Celltrion, Ferring, Hospira, Janssen, Meda, Medivir, MSD, Pfizer, Prometheus Laboratories, Sandoz, Shire, Takeda, Tillotts Pharma, and Vifor Pharma and has received grant support from Janssen, MSD, and Takeda. James W. Butler and Joel Petersson are employees of AbbVie, Inc. Jean-Frederic Colombel has served as an advisory board member or consultant for AbbVie, Amgen, Boehringer Ingelheim, Arena Pharmaceuticals, Celgene Corporation, Celltrion, Enterome, Eli Lilly, Ferring Pharmaceuticals, Genentech, Janssen and Janssen, Medimmune, Merck & Co., Nextbiotix, Novartis Pharmaceuticals Corporation, Otsuka Pharmaceutical Development & Commercialization, Inc, Pfizer, Protagonist, Second Genome, Gilead, Seres Therapeutics, Shire, Takeda, Theradiag; has served as a speaker for AbbVie, Ferring, Takeda, and Celgene Corporation; holds stock options for Intestinal Biotech Development and Genefit; and research grants from AbbVie, Takeda, Janssen. The remaining authors disclose no conflicts.
References
- 1.Torres J, Mehandru S, Colombel J-F, et al. Crohn’s disease. Lancet 2017;389(10080):1741–1755. [DOI] [PubMed] [Google Scholar]
- 2.Cosnes J, Bourrier A, Nion-Larmurier I, et al. Factors affecting outcomes in Crohn’s disease over 15 years. Gut 2012;61:1140–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malarcher CA, Wheaton AG, Liu Y, et al. Hospitalizations for Crohn’s disease — United States, 2003–2013. MMWR Morb Mortal Wkly Rep 2017;66:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma C, Moran GW, Benchimol EI, et al. Surgical rates for Crohn’s disease are decreasing: a population-based time trend analysis and validation study. Am J Gastroenterol 2017;112:1840–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burisch J, Kiudelis G, Kupcinskas L, et al. Natural disease course of Crohn’s disease during the first 5 years after diagnosis in a European population-based inception cohort: an Epi-IBD study. Gut 2019;68:423–433. [DOI] [PubMed] [Google Scholar]
- 6.Markusse IM, Akdemir G, Dirven L, et al. Long-term outcomes of patients with recent-onset rheumatoid arthritis after 10 years of tight controlled treatment: a randomized trial. Ann Intern Med 2016;164:523–531. [DOI] [PubMed] [Google Scholar]
- 7.Choy EHS, Smith CM, Farewell V, et al. Factorial randomised controlled trial of glucocorticoids and combination disease modifying drugs in early rheumatoid arthritis. Ann Rheum Dis 2008;67:656–663. [DOI] [PubMed] [Google Scholar]
- 8.Berg DR, Colombel J-F, Ungaro R. The role of early biologic therapy in inflammatory bowel disease. Inflamm Bowel Dis 2019;25:1896–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol 2015;110:1324–1338. [DOI] [PubMed] [Google Scholar]
- 10.Colombel J-F, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet 2018;390(10114):2779–2789. [DOI] [PubMed] [Google Scholar]
- 11.Colombel JF, Reinisch W, Mantzaris GJ, et al. Randomised clinical trial: deep remission in biologic and immunomodulator naïve patients with Crohn’s disease -a SONIC post hoc analysis. Aliment Pharmacol Ther 2015;41:734–746. [DOI] [PubMed] [Google Scholar]
- 12.Panaccione R, Löfberg R, Rutgeerts P, et al. Efficacy and safety of adalimumab by disease duration: analysis of pooled data from Crohn’s disease studies. J Crohns Colitis 2019;13:725–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schreiber S, Colombel J-F, Bloomfield R, et al. Increased response and remission rates in short-duration Crohn’s disease with subcutaneous certolizumab pegol: an analysis of PRECiSE 2 randomized maintenance trial data. Am J Gastroenterol 2010;105:1574–1582. [DOI] [PubMed] [Google Scholar]
- 14.Rubin DT, Uluscu O, Sederman R. Response to biologic therapy in Crohn’s disease is improved with early treatment: an analysis of health claims data. Inflamm Bowel Dis 2012;18:2225–2231. [DOI] [PubMed] [Google Scholar]
- 15.Safroneeva E, Vavricka SR, Fournier N, et al. Impact of the early use of immunomodulators or TNF antagonists on bowel damage and surgery in Crohn’s disease. Aliment Pharmacol Ther 2015;42:977–989. [DOI] [PubMed] [Google Scholar]
- 16.Walters TD, Kim M-O, Denson LA, et al. Increased effectiveness of early therapy with anti-tumor necrosis factor-a vs an immunomodulator in children with Crohn’s disease. Gastroenterology 2014;146:383–391. [DOI] [PubMed] [Google Scholar]
- 17.Shah SC, Colombel JF, Sands BE, et al. Systematic review with meta-analysis: mucosal healing is associated with improved long-term outcomes in Crohn’s disease. Aliment Pharmacol Ther 2016;43:317–333. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


