Abstract
Reactive oxygen species induce vascular dysfunction and hypertension by directly interacting with nitric oxide (NO) which leads to NO inactivation. In addition to a decrease in NO bioavailability, there is evidence that oxidative stress can also modulate NO signaling during hypertension. Here, we investigated the effect of oxidative stress on NO signaling molecules cGMP-dependent protein kinase (PKG) and vasodilator-stimulated phosphoprotein (VASP) which are known to mediate vasodilatory actions of NO. Male Sprague Dawley (SD) rats were provided with tap water (control), 30 mM L-buthionine sulfoximine (BSO, a pro-oxidant), 1mM tempol (T, an antioxidant) and BSO + T for 3 wks. BSO-treated rats exhibited high blood pressure and oxidative stress. Incubation of mesenteric arterial rings with NO donors caused concentration-dependent relaxation in control rats. However, the response to NO donors was significantly lower in BSO-treated rats with a marked decrease in pD2. In control rats, NO donors activated mesenteric PKG, increased VASP phosphorylation and its interaction with transient receptor potential channels 4 (TRPC4) and inhibited store-operated Ca2+ influx. NO failed to activate these signaling molecules in mesenteric arteries from BSO-treated rats. Supplementation of BSO-treated rats with tempol reduced oxidative stress and blood pressure and normalized the NO signaling. These data suggest that oxidative stress can reduce NO-mediated PKG activation and VASP-TRPC4 interaction which leads to failure of NO to reduce Ca2+ influx in smooth muscle cells. The increase in intracellular Ca2+ contributes to sustained vasoconstriction and subsequent hypertension. Antioxidant supplementation decreases oxidative stress, normalizes NO signaling and reduces blood pressure.
Introduction
Vascular endothelium releases a variety of relaxing factors including nitric oxide (NO) which regulate acute and long-term vascular tone. An abnormal increase in smooth muscle tone, due to endothelial dysfunction and alterations in the production or action of these factors, has been implicated in the pathogenesis of several cardiovascular disease states, including arterial hypertension (5, 8, 27). NO induces vasorelaxation by increasing tissue levels of the secondary messenger cGMP via activation of soluble guanylyl cyclase (sGC)(5). The accumulation of cGMP activates two isoforms of cGMP-dependent protein kinase (PKG) of which PKG1 is predominant in vascular smooth muscle cells. PKG mediates NO/cGMP-induced vasorelaxation, via phosphorylation of proteins that affect myosin light chains and intracellular Ca2+ levels (5, 10, 15, 16, 25). Studies with PKG1-deficient mice demonstrated a complete disruption of the NO/cGMP signaling in the vascular smooth muscle indicating a critical role of PKG1 in NO-mediated vasorelaxation (20).
Ca2+ influx in smooth muscle cells regulates contraction and vascular tone. In these cells, the predominant Ca2+ entry pathway is the store-operated calcium channels (SOC), a branch of TRPC (transient receptor potential [TRP] superfamily of cation channels), in which Ca2+ entry is governed by the Ca2+ content of intracellular Ca2+ stores (19). A release of Ca2+ from intracellular stores causes store depletion, which then activates Ca2+ entry from extracellular space. This Ca2+ entry, which has been termed the store-operated Ca2+ entry, may be the basis by which these cells maintain elevated intracellular Ca2+ and replenish their intracellular Ca2+ stores in response to agonist stimulation and maintain vascular tone (19). Several studies have shown that cGMP regulates Ca2+ signaling and SOC activity in a negative manner (13, 26, 30). Elevated cGMP level attenuated the store-operated Ca2+ entry and that this inhibitory effect is mediated by a PKG-dependent mechanism (13). Although, these results suggest that cGMP and PKG-mediated vasorelaxation could involve the regulation of the store-operated Ca2+ entry in vascular smooth muscle cells, the involvement and the knowledge about molecules involved proximal to PKG1 are still elusive. However, there is evidence that vasodilator-stimulated phosphoprotein (VASP) is a substrate for PKG1 and therefore could modulate NO effects in smooth muscle cells (11, 18). VASP, abundantly expressed in the vasculature, plays an important role in the regulation of cytoskeleton dynamics and cell migration (7). There are two major phosphorylation sites on VASP. Serine 157 (Ser157) is PKA specific, whereas serine 239 (Ser239) is specific for PKG. Phosphorylation of VASP at Ser239 by PKG causes filament retraction in vascular smooth muscle, in part, by capping actin filaments (7, 11). Interestingly, VASP can also associate with SOC/TRPC and thus could regulate Ca2+ modulation and contraction in smooth muscle cells (28).
Reduced vasodilation in response to endothelium-dependent vasodilators such as acetylcholine is a hallmark of endothelial dysfunction and development of hypertension (8, 27). The mechanisms underlying endothelial dysfunction are likely to be multifactorial but could be due to NO degradation or defective NO signaling caused, at least in part, by oxidative stress (8, 27). Previously we have shown that rats treated with L-buthionine sulfoximine (BSO), a glutathione synthesis inhibitor, exhibited oxidative stress and increased blood pressure (3). The blood vessels from these rats showed decreased response to acetylcholine and sodium nitroprusside (SNP) (3). The reduced response to acetylcholine in hypertensive animals has been extensively studied and could be explained by a decreased bioavailability of NO, partly due to its inactivation by oxidative stress. However, the diminished response to SNP remains unknown. Therefore, the present studies were carried out in endothelium-denuded mesenteric tissue to unravel the mechanisms for oxidative stress-mediated attenuation of NO signaling (independent of NO bioavailability) in smooth muscles which could be an important contributor to the development of hypertension. Since PKG is a primary determinant of NO-mediated vasorelaxation, the present study was designed to investigate the effect of oxidative stress on PKG signaling. SD rats were treated with BSO for 3 weeks and endothelial denuded blood vessels were used to study the impairment in NO/PKG/VASP pathway in the setting of hypertension.
Materials and Methods
Male Sprague-Dawley rats (200 to 250 g) were obtained from Harlan (Indianapolis, Indiana) and divided into 4 experimental groups: V, animals maintained on tap water (vehicle); BSO, animals provided with 30 mM L-buthionine sulfoximine (Sigma, St. Louis MO); T, animals provided with 1 mM tempol (Sigma); BSO+T, animals receiving BSO plus tempol. BSO induces oxidative stress by inhibiting glutamate cysteine ligase, a key enzyme for glutathione synthesis. Both BSO and tempol (an antioxidant) were provided in drinking water for 3 weeks. Conscious BP of rats was measured by radio telemetry (DSI, Minneapolis, MN) as detailed before (12). Briefly, anesthesia was induced by 5% isoflurane and maintained at 2–2.5% isoflurane throughout the surgery. Abdominal aorta was exposed by making a midline abdominal incision and an implantable radio transmitter was inserted into the abdominal aorta and secured with tissue adhesive (Vetbond from 3M St Paul, MN). The body of the transmitter was sutured to the abdominal musculature and animals were allowed to recover for 7–10 days prior to the recording of blood pressure. Blood pressure was measured throughout the treatment and daily recordings of individual rats were averaged.
For tissue preparations, a midline abdominal incision was made, and the mesenteric artery was removed and immediately placed in Krebs-Henseleit buffer (KHB, in mM: NaCl 118.4, KCl 4.7, CaCl2 2.5, KH2PO4 1.2, MgSO4 1.2, NaHCO3 25.0, and glucose 10.0, pH 7.4) (3). The mesenteric artery was cleaned of adherent tissue and endothelium was mechanically removed by gentle rubbing with moistened cotton and cut into rings. Each ring was fixed under a resting tension of 0.6 g in a 10 mL organ bath filled with KHB (37°C) and the rings were allowed to equilibrate for 90 minutes before the start of the experiments. Isometric tension change was measured with a digital force isometric transducer (Harvard Apparatus; Holliston, MA) connected to a data acquisition system (AD Instruments; Colorado Springs, CO). Experiments were performed in accordance with NIH guidelines and protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Houston.
Protein was determined by using a bicinchoninic acid kit (Peirce; Rockford, IL). Mesenteric tissue malondialdehyde was determined by measuring the malondialdehyde level in butanol extracts of cell lysate by the method of Mihara and Uchiyama (17). Plasma 8-Isoprostane was determined by a commercially available immunoassay kit (Cayman; Ann Arbor, MI). cGMP was measured as previously described (3) using the RIA kit from Calbiochem (La Jolla, CA) and results were expressed as picomoles cGMP per milligram tissue protein. Nitrotyrosine was determined by using a chemiluminescence assay kit from Millipore (Cat #: 17–376).
Vascular Relaxation Studies
To determine endothelium-independent vasorelaxation, endothelium-denuded mesenteric rings were pre-constricted with phenylephrine, and cumulative concentration-response curves to hydralazine (1nM to 100 μM) and NO donors diethylenetriamine NONOate (DETA NONOate 100 pM to 1 μM), SNP (100 pM to 1 μM) and nitroglycerin (NG, 100 pM to 1 μM), were obtained. Endothelium denudation was confirmed by the disappearance of 1 μM acetylcholine-induced relaxation response (3). Mesenteric rings were also pre-incubated with methylene blue (20 μM), a guanylyl cyclase inhibitor, before exposure to hydralazine to determine the involvement of cGMP in hydralazine-mediated vasorelaxation.
PKG activity assay
PKG activity was determined by the method described by Fiscus and Murad (9). Briefly, the mesenteric tissue was incubated with 1 μM DETA NONOate or 50 μM cGMP analog 8-(4-chlorophenylthio)guanosine 3′,5′-cyclic monophosphate (8p-CPT-cGMP) for 20 min and treated with lysis buffer (13). The lysates were centrifuged at 8000 × g at 4 °C for 30 s, and the supernatant fractions were used for kinase assay. Kinase activity was determined by measuring the amount of 32P transferred from [γ−32P]ATP to histone H2b by adding 7 μl of supernatant fraction of tissue homogenates to 35 μl of reaction mixture containing 15 mM K2PO4, pH 7.0, 7 mM magnesium acetate, 0.5 mg/ml histone H2b, 20 mM NaF, 0.25 mM isobutylmethylxanthine, 30 μM ATP with [γ−32P]ATP and 0.3 μM KT5720 (a highly specific inhibitor of cAMP kinase). The reaction was stopped by transferring 35 μl of the final reaction mixture to Whatman No. 3MM chromatography paper squares. We also used PKG assay kit Cyclex® (Cyclex; Woburn, MA) and the results were comparable to the above-mentioned method.
Western blotting
Isolated mesenteric tissue was incubated with DETA NONOate or 8p-CPT-cGMP for 20 min and homogenized in lysis buffer containing 0.25 mM of sucrose, 50 mM of dithiothreitol, 3 mM of HEPES (pH 7.9), 0.5 mM of EGTA, 0.4 mM of phenylmethanesulfonyl fluoride, Complete®-protease inhibitor mixture (Roche; Indianapolis, IN), and 1% Triton X-100. Samples were centrifuged and supernatants were mixed with an equal volume of Laemmli buffer and subjected to western blotting for expression of sGC, PKG, VASP and VASP phosphorylation (P-VASP) at serine 239 with following antibodies: primary rabbit anti sGC-α1 (1:5,000), sGC-β1 (1:6,000), anti-PKG-Iα/β (1:500), and VASP (1:10,000), monoclonal anti-P-VASP at serine 239 (1:500) (22, 28). The coimmunoprecipitation was performed as described previously (2). Briefly, the cell lysates were incubated with mouse monoclonal P-Ser239-VASP IgG overnight at 4°C. The immunoprecipitate was pulled-down with protein G agarose beads, incubated with 2× lamellae buffer and subjected to western blotting for TRPC4. Immunoprecipitate was probed with polyclonal goat TRPC4 IgG (1 μg/ml). Regular Western blot analysis for TRPC4 was performed as a positive control. Antibodies were obtained from following sources, sGC and TRPC4 (Santa Cruz Biotech, Santa Cruz, CA), sGC (Sigma), PKG (Calbiochem) and P-VASP (Upstate; Charlottesville, VA).
Ca2+ measurement
Mesenteric tissue was incubated for 1 h in the dark at room temperature with 10 μM membrane-permeant Fura 2-AM and 0.02% Pluronic F-127 in normal physiological saline solution (N-PSS) that contained 140 mM NaCl, 1 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 10 mM glucose, and 5 mM Hepes, pH 7.4.(13) Tissue was pretreated with 10 μM cyclopiazonic acid (CPA) for 40 min and washed in and maintained briefly in a Ca2+ free medium (0Ca2+-PSS). CPA selectively inhibits endoplasmic reticulum Ca2+ ATPase and prevents Ca2+ uptake and thus stimulates Ca2+ entry by receptor-independent mechanisms. Ca2+ influx was initiated by replacing 0Ca2+-PSS with N-PSS, which contained 1 mM CaCl2. In some experiments tissue was pretreated with 50 μM 8p-CPT-cGMP or 1 μM KT5823 (PKG inhibitors), or 3 mM Ni2+ (a Ca2+ entry blocker) for 5 min. The tissue was illuminated at 340/380 nm and the fluorescence signal (500/520 nM) was recorded by Bio Tek spectrofluorometer (Winooski, VT).
Statistical analysis
Differences between means were evaluated using 1-way, 2-way or repeated measures ANOVA followed by post-hoc Newman-Keuls multiple test. P<0.05 was considered statistically significant.
Results
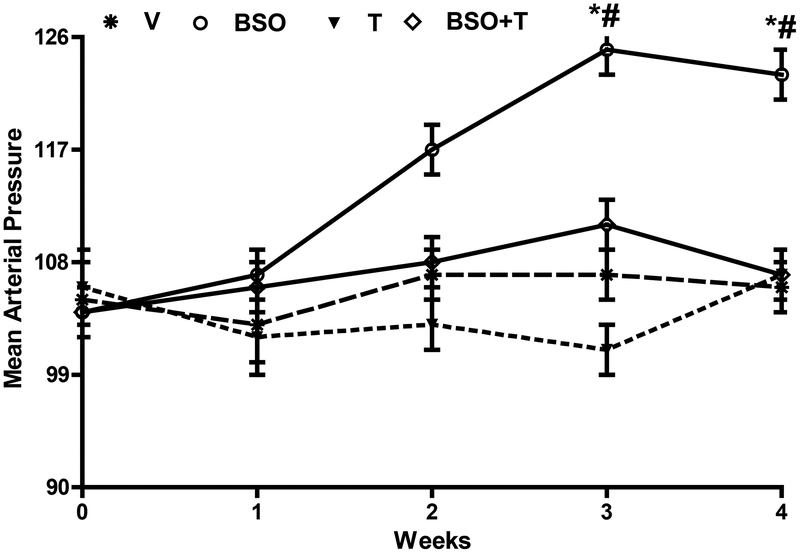
Animals consumed an equal amount of food and water (data not shown) and gained similar body weight (g, V—365.31 ± 20.11, BSO—358.12 ± 22.9, T—376.31 ± 16.22, BSO+T—361.30 ± 18.16). Animals treated with BSO exhibited high blood pressure (figure 1) and oxidative stress as evidenced by increased plasma 8-isoprostane (pg/ml, V—145.25 ± 15.21, BSO—209.13 ± 16.33*, T—132.33 ± 12.09#, BSO+T—155.81± 16.88#; *P<0.05 vs. V and #P<0.05 vs. BSO) and mesenteric lipid peroxidation (malondialdehyde, nmol /mg protein, V—0.15 ± 0.02, BSO—0.43 ± 0.04*, T—0.12 ± 0.03#, BSO+T—0.18 ± 0.04#; *P<0.05 vs. V and #P<0.05 vs. BSO) and protein nitrosylation (counts per sec/mg protein, C—75265.11 ± 563.31, BSO—131713.72 ± 689.32*, T—70265.65 ± 840.88#, BSO+T—82301.79 ± 978.32#; *P<0.05 vs. V and #P<0.05 vs. BSO). Supplementation of antioxidant tempol to BSO-treated rats normalized blood pressure (figure 1) and oxidative stress. In the absence of BSO, tempol showed no effect on blood pressure or oxidative stress.
Figure 1: Effect of BSO and tempol treatment on BP in rats.
SD rats were treated with BSO and/or tempol for three weeks and conscious blood pressure was measured continuously. *P<0.05 vs. week 0 of BSO group by using repeated measures ANOVA and #P<0.05 vs. V at corresponding time points using 1-way ANOVA followed by post hoc Newman-Keuls multiple test.
Endothelium-independent vasorelaxation
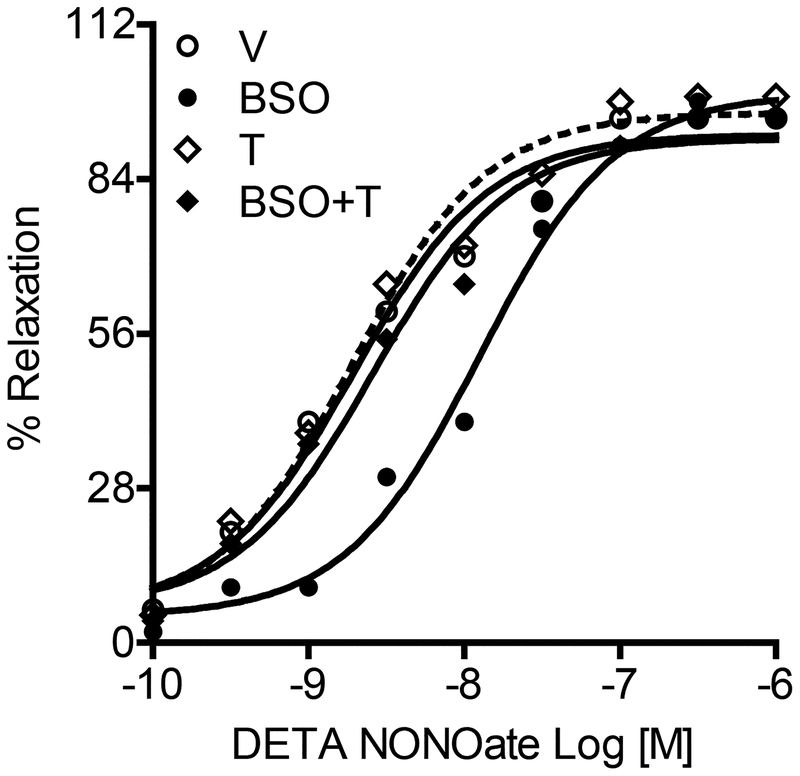
To determine the effect of oxidative stress on NO signaling in smooth muscles rather than its bioavailability or endothelial generation we used denuded mesenteric arteries. Denuded mesenteric artery rings were pre-constricted with phenylephrine followed by incubation with varying concentrations of DETA NONOate. Mesenteric vasoconstriction in response to KCl and phenylephrine was similar in all animals (data not shown). Mesenteric artery rings from vehicle-treated rats showed dose-dependent relaxation in response to DETA NONOate (figure 2). However, the response to DETA NONOate was significantly attenuated in blood vessels from BSO-treated rats especially at lower drug concentrations causing a decrease in pD2 (pD2 is a negative logarithm to the base 10 of EC50 (half maximum effective concentration) and is also referred to as pEC50); vehicle—8.76 ± 0.13; BSO—7.42 ± 0.08*; T—8.80 ± 0.12†; BSO+T—8.62 ± 0.13†, *P < 0.05 vs. V, †P < 0.05 vs. BSO, using one-way ANOVA followed by post-hoc Newman-Keuls multiple comparison test. Denuded mesenteric arteries from BSO-treated rats also showed a decreased response to two other NO donors SNP and NG (table 1). Tempol normalized the drug response in BSO-treated rats, whereas vasorelaxation was similar in tempol and vehicle-treated rats (figure 2, table 1). Interestingly, the vasodilatory response to non-NO dilator hydralazine was similar in all experimental groups (table 1). In addition, the hydralazine-induced vasorelaxation was similar and insensitive to methylene blue (guanylyl cyclase inhibitor) in endothelial intact and denuded mesenteric arteries from vehicle (pD2, with endothelium—4.41 ± 0.16, without endothelium—4.19 ± 0.19 and in presence of methylene blue—4.32 ± 0.13) or other groups (data not shown). Also, hydralazine failed to increase cGMP (pmol/mg protein) accumulation in mesenteric rings from vehicle (basal—4.51 ± 0.16 and hydralazine—4.61 ± 0.21) or BSO and tempol groups (data not shown).
Figure 2. Endothelium independent vasorelaxation in response to Nitric oxide donor.
Diethylenetriamine NONOate (DETA NONOate)-induced relaxation in mesenteric rings from vehicle (V), L-buthionine sulfoximine (BSO), tempol (T) and BSO + T -treated rats. A single experiment is shown, representative of 6–8 individual experiments performed in triplicate.
Table 1.
Effect of oxidative stress on sodium nitroprusside (SNP), nitroglycerin (NG) and hydralazine on vasodilatory response in mesenteric arteries
| Parameters | V | BSO | T | BSO+T |
|---|---|---|---|---|
| SNP, NG and hydralazine –mediated vasodilatation (pD2) | ||||
| SNP | 8.51 ± 0.16 | 7.11 ± 0.16* | 8.71 ± 0.20# | 8.21 ± 0.13# |
| NG | 7.62 ± 0.09 | 6.15 ± 0.08* | 7.82 ± 0.10# | 7.32 ± 0.17# |
| Hydralazine | 4.63 ± 0.20 | 4.52 ± 0.15 | 4.73 ± 0.19 | 4.65 ± 0.18 |
| SNP and NG –mediated PKG stimulation (pmol P/mg protein/min) | ||||
| Basal | 3.92 ± 0.21 | 3.52 ± 0.15 | 4.20 ± 0.14 | 3.7 ± 0.10 |
| SNP | 6.81± 0.61† | 3.9 ± 0.14 | 7.4 ± 0.17† | 6.5 ± 0.13† |
| NG | 7.11 ± 0.51† | 3.8 ± 0.16 | 6.9 ± 0.15† | 6.4 ± 0.14† |
Animals (n=6–8 rats) were provided with tap water (V), 30mM L-buthionine sulfoximine (BSO), 1mM tempol (T) or BSO plus T for 3 weeks.
P<0.05 vs. V,
P<0.05 vs. BSO and
P<0.05 vs. basal, using 1-way ANOVA followed by posthoc Newman-Keuls multiple comparison test.
PKG activation and expression
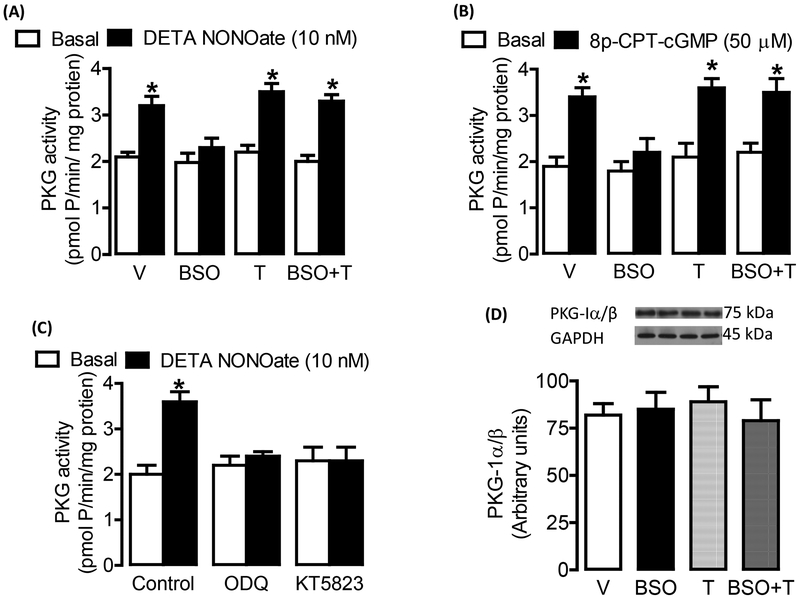
Since PKG activation is primarily responsible for NO-mediated smooth muscle relaxation, we first measured PKG stimulation in response to various NO donors. We measured the PKG activity as histone phosphorylation. As shown in figure 3, incubation of mesenteric vessels with DETA NONOate (figure 3A) or 50 μM 8-para-chlorophenylthio-cGMP (8p-CPT-cGMP; a PKG activator) (figure 3B) increased PKG activity in vehicle-treated rats. However, in blood vessels from BSO-treated rats, both DETA NONOate and 8p-CPT-cGMP failed to activate PKG (figure 3 A,B). Also, SNP and NG failed to activate PKG in BSO-treated rats (table 1). Tempol restored the DETA NONOate and 8p-CPT-cGMP (figure 3A, B) as well as SNP and NG (table 1) induced PKG activation in BSO-treated rats. Preincubation of mesenteric tissue with 1 μM KT5823 (a PKG inhibitor) and 20 μM ODQ (a sGC inhibitor) blocked the DETA NONOate mediated PKG activation in vehicle-treated rats (figure 3C). Similar effects of these inhibitors were seen on PKG activation in blood vessels from tempol and BSO plus tempol -treated rats (data not shown). The expression of PKG-I α/β was similar in all experimental groups (figure 3D).
Figure 3. cGMP-dependent protein kinase (PKG) activation in response to NO donor.
PKG activation in response to A, diethylenetriamine NONOate (DETA NONOate) and B, 8-para-chlorophenylthio-cGMP (8p-CPT-cGMP) in mesenteric vessels from vehicle (V), L-buthionine sulfoximine (BSO), tempol (T) and BSO + T -treated rats. C, PKG activation in response to DETA NONOate in presence and absence of sGC inhibitor ODQ (20 μM) and PKG inhibitor KT5823 (1 μM) in mesenteric tissue from vehicle treated rats. D, PKG-1α/β protein expression in mesenteric tissue homogenates. Bars represent mean ± SE from 6–8 rats performed in triplicate. *P<0.05 vs. respective basal, using 2-way ANOVA followed by post hoc Newman-Keuls multiple test.
VASP expression and Phosphorylation
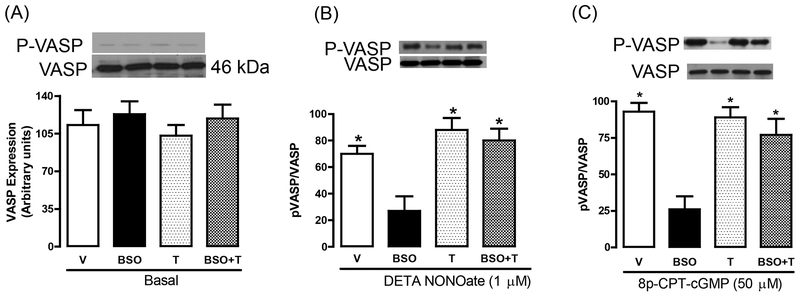
VASP has been recognized as a substrate for PKG-I and the phosphorylation of VASP (P-VASP) at serine-239 is considered as an indicator of PKG activation. First, we measured the basal protein expression of VASP protein and its serine-239 phosphorylation. As shown in figure 4A, the expression of VASP was similar in all experimental groups. We could not find appreciable serine-239 phosphorylation under basal conditions in any experimental group (figure 4A). Incubation of mesenteric tissue from vehicle-treated rats with DETA NONOate or 8p-CPT-cGMP caused a robust increase in VASP serine-239 phosphorylation without affecting VASP protein expression (figure 4B, C). However, DETA NONOate or 8p-CPT-cGMP failed to phosphorylate VASP in blood vessels from BSO-treated rats (figure 4B, C). Tempol restored the DETA NONOate and 8p-CPT-cGMP -mediated VASP phosphorylation in BSO-treated rats (figure 4B, C). Animals treated with tempol alone showed results similar to vehicle-treated rats (figure 4B, C).
Figure 4. Vasodilator-stimulated phosphoprotein (VASP) expression and phosphorylation in response to NO donor.
A, representative western blots for VASP serine-239 (P-VASP, upper band) and VASP protein (lower band) under basal conditions in mesenteric vessels from vehicle (V), L-buthionine sulfoximine (BSO), tempol (T) and BSO + T -treated rats. Mesenteric vessels were incubated with DETA NONOate (B) or 8p-CPT-cGMP (C) and immunoblotted for P-VASP and VASP. The bands shown are representative western blots. Bars represent mean ± SE from 6–8 rats performed in triplicate. *P<0.05 vs. V and #P<0.05 vs. BSO, using 1-way ANOVA followed by post hoc Newman-Keuls multiple test.
VASP and TRPC4 interaction
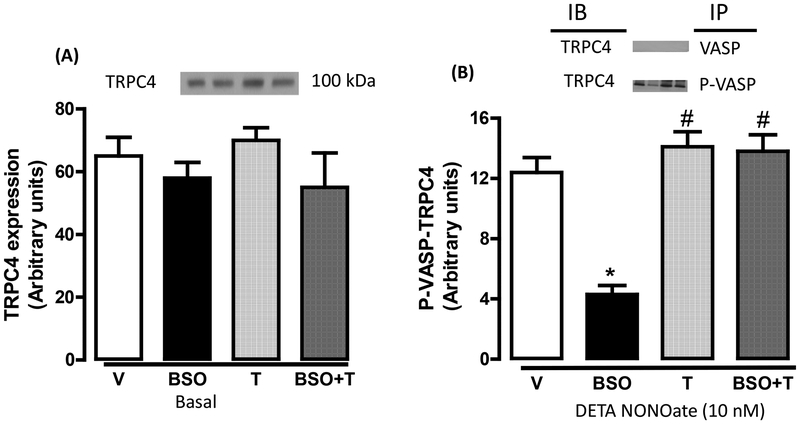
It is reported that phosphorylated VASP can interact with TRPC4 and modulate SOC dependent cellular Ca2+ mobilization. In here, as seen with basal VASP expression (figure 5A), treatment of rats with BSO or tempol had no effect on basal TRPC4 protein expression (figure 5A). Also, immunoprecipitation of VASP showed no association between VASP and TRPC4 in any experimental group (figure 5B, top panel). However, incubation of vessels with DETA NONOate caused phosphorylation and colocalization of VASP with TRPC4 in vehicle-treated rats (figure 5B). DETA NONOate failed to induce such colocalization of these proteins in BSO-treated rats (figure 5B). Tempol restored the phosphorylation of VASP (figure 4 A and B) and its coimmunoprecipitation with TRPC4 in BSO-treated rats (figure 5B). We could not detect phosphorylation of TRPC4 under basal conditions or after incubation of blood vessels with DETA NONOate in any group (data not shown).
Figure 5. Vasodilator-stimulated phosphoprotein (VASP)—transient receptor potential channel 4 (TRPC4) interaction in response to NO donor.
A, TRPC4 expression under basal conditions in mesenteric vessels from vehicle (V), L-buthionine sulfoximine (BSO), tempol (T) and BSO + T -treated rats. B, mesenteric vessels were incubated with diethylenetriamine NONOate (DETA NONOate) followed by immunoprecipitation by VASP and phosphorylated VASP (P-VASP) antibodies. The immunoprecipitates were immunoblotted for TRPC4. Bars represent mean ± SE from 6–8 rats performed in triplicate. *P<0.05 vs. V and #P<0.05 vs. BSO, using 1-way ANOVA followed by post hoc Newman-Keuls multiple test.
Calcium mobilization
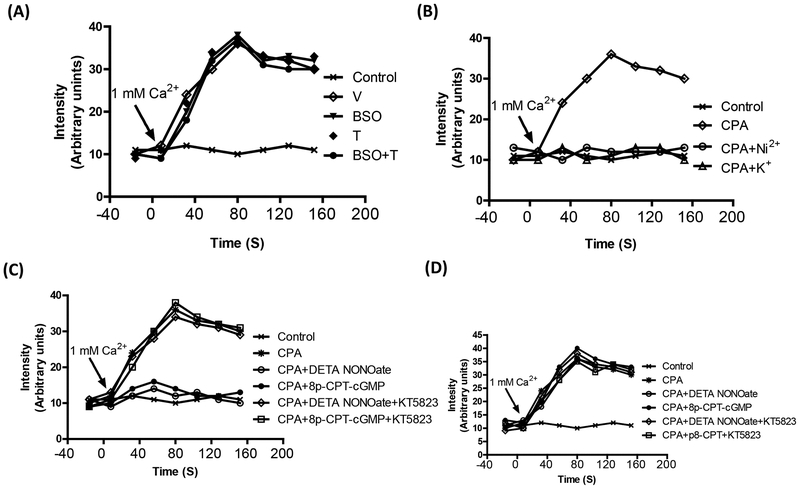
Most of the vasoactive compounds affect vascular tone by modulating intracellular Ca2+. SOC is an important regulator of cellular Ca2+ and its function could be modulated by VASP. To determine the effect oxidative stress on NO-mediated Ca2+ sequestration via PKG—VASP/TRPC4—SOC pathway, the mesenteric tissue was incubated with 10 μM cyclopiazonic acid (CPA) in Ca2+ free buffer (0Ca2+-PSS) for 30 min to induce the Ca2+ store depletion. CPA selectively inhibits endoplasmic reticulum Ca2+ ATPase and prevents Ca2+ uptake and thus stimulates Ca2+ entry by receptor-independent mechanisms. Tissue incubated in Ca2+ free buffer without CPA served as control. The influx of Ca2+ was initiated by changing the buffer from Ca2+ free to Ca2+ containing solution. The change in extracellular buffer showed no effect on the Ca2+ influx in control tissue (figure 6A). Since the Ca2+ influx in absence of CPA was similar in blood vessels from all the animals, we selected the vessels from vehicle-treated rats as a control. In cells pretreated with CPA, there was a robust increase in Ca2+ influx when the extracellular buffer was changed from Ca2+ free buffer to solution containing Ca2+ (figure 6A). To ascertain that CPA-induced increase in intracellular Ca2+ is due to the Ca2+ influx, we used blockers of Ca2+ entry in blood vessels from vehicle-treated rats. As shown in figure 6B, Ni (2 mM), which competes with the Ca2+ binding site, blocked the increase in intracellular Ca2+. We also used 80 mM K+ for membrane depolarization. As shown in figure 6B, membrane depolarization also blocked the Ca2+ influx in CPA treated cells.
Figure 6. Cyclopiazonic acid (CPA)-induced increase in intracellular Ca2+.
A, Mesenteric vessels, from vehicle (V), L-buthionine sulfoximine (BSO), tempol (T) and BSO + T -treated rats, was incubated with 10 μM CPA in Ca2+ free buffer followed by change to 1mM Ca2+ buffer. Ca2+ influx was monitored by Fluo2/AM fluorescence. B, Ca2+ influx in mesenteric vessel from vehicle-treated rats incubated with CPA in the presence and absence of Ni2+ (3 mM) which competes with Ca and blocks its entry and 80 mM K+ for membrane depolarization. C, Ca2+ influx in mesenteric vessels from vehicle-treated rats incubated with CPA in the presence and absence diethylenetriamine NONOate (DETA NONOate), 8-para-chlorophenylthio-cGMP (8p-CPT-cGMP) or KT5823 (1 μM, a PKG inhibitor). D, Ca2+ influx in mesenteric vessel from BSO-treated rats incubated with CPA in the presence and absence DETA NONOate, 8p-CPT-cGMP or KT5823. A single experiment is shown, representative of 6–8 individual experiments performed in triplicate.
To investigate the involvement of NO signaling in the inhibition of store-operated Ca2+ influx, the tissue was pretreated with DETA NONOate or 8p-CPT-cGMP followed by incubation of tissue with CPA in Ca2+ free buffer. Preincubation of vessel tissue with DETA NONOate or 8p-CPT-cGMP blocked the CPA-induced increase in the Ca2+ entry in vessels from vehicle-treated (figure 6C). A similar effect of DETA NONOate or 8p-CPT-cGMP was seen in blood vessels from tempol and BSO plus tempol-treated rats (data not shown). However, DETA NONOate or 8p-CPT-cGMP failed to block the CPA-induced Ca2+ influx in blood vessels from BSO-treated rats (figure 6D). The inhibitory effect of DETA NONOate or 8p-CPT-cGMP was abolished by PKG inhibitor KT5823 in the vehicle (figure 6C), tempol and BSO plus tempol treated rats (data not shown).
Discussion
The present study demonstrates that oxidative stress impairs vascular NO-PKG-VASP signaling which contributes to an increase in blood pressure and these defects are reversed by antioxidant supplementation. Sprague Dawley rats treated with BSO for 3 weeks exhibited oxidative stress and high blood pressure. Blood vessels from these rats showed decreased vasorelaxation in response to NO donors. Also NO donors or 8p-CPT-cGMP failed to activate PKG, phosphorylate VASP serine-239, elicit P-VASP-TRPC4 association and inhibit store-operated Ca2+ mobilization. Supplementation of BSO-treated rats with tempol reduced oxidative stress and blood pressure and restored the NO signaling.
Oxidative stress has been implicated in the development of hypertension; however, the pathways mediating this phenomenon are still controversial (6, 14, 29). However, the cellular and functional elements contributing to high blood pressure during oxidative stress seem to involve endothelial dysfunction (6, 14, 29). The endothelium and vascular smooth muscle cells interact in a complex manner to modulate vascular tone with NO playing an important role. Most of the reports studying endothelial dysfunction during oxidative stress have mainly focused on NO bioavailability and its inactivation due to superoxide-mediated conversion to peroxynitrite (6, 14, 29). However, we and others have shown that oxidative stress can also have a detrimental effect on NO signaling distal to the availability of NO (3, 22). Since NO is predominantly generated in endothelium and its vasodilatory effect is mostly confined to smooth muscles we used endothelium denuded mesenteric arteries to determine function and signaling of NO. Herein we show that in BSO-treated rats which exhibit oxidative stress and high blood pressure, there was reduced vasorelaxation in response to NO donors. The decrease in response was more pronounced at lower concentrations of NO donors with a significant decrease in pD2. These effects were reversed by antioxidant tempol suggesting that, in addition to a reduction in NO bioavailability in endothelium, oxidative stress can directly impair NO-mediated vasorelaxation in smooth muscles. The defect seems to be specific to NO as the vasodilatory response to non-NO dilator like hydralazine is not affected by BSO.
It is widely recognized that PKG facilitates NO-mediated vasodilation and its activation is a useful indicator for functional NO signaling. To investigate the effect of oxidative stress on PKG we measured its enzymatic activity in response NO donors and 8p-CPT-cGMP. We found that NO donors or 8p-CPT-cGMP failed to activate PKG in BSO-treated rats. The inability of NO donors to activate PKG could be due to reduced sGC activity or increased phosphodiesterase activity. However, the failure of 8p-CPT-cGMP to activate PKG is important as it suggests that oxidative stress can directly impair NO-mediated PKG stimulation without changing its basal protein expression or enzymatic activity. It has been reported that reactive oxygen species can impair NO-induced vasodilation by directly affecting sGC stimulation (23). Our data show that oxidative stress can further render vasodilator therapy with NO donors less effective by impairing the ability of cGMP to stimulate PKG. This is supported by the finding that tempol can normalize the 8p-CPT-cGMP-mediated activation of PKG in BSO-treated rats. We also studied serine-239 phosphorylation of VASP in response to DETA NONOate and 8p-CPT-cGMP. VASP is a physiological substrate of PKG and is also considered as a downstream molecule of NO signaling (18). We found that oxidative stress abolished PKG-mediated VASP serine-239 phosphorylation which was reverted by tempol. The decrease in phosphorylation cannot be attributed to reduced VASP content as protein expression was similar in BSO and vehicle-treated rats. These data reinforce our hypothesis that oxidative stress can modulate the PKG activation in response to NO and antioxidants can normalize the NO signaling.
Depletion of endoplasmic reticulum Ca2+ stores activates store-operated Ca2+ channels (SOC), a branch of TRPC. PKG stimulation regulates a variety of ion channels including Ca2+-activated K channels and TRPC (24, 26, 30). There are potential PKG sites on TRPC4, however, incubation of blood vessels with DETA NONOate did not phosphorylate TRPC4 or modulate its protein expression suggesting the involvement of an intermediate protein. There is evidence that phosphorylated VASP can interact with TRPC4 and modulate intracellular Ca2+. In the present study we also found that DETA NONOate increased the association of TRPC4 and phosphorylated VASP in vehicle-treated rats. However, DETA NONOate failed to cause VASP-239 phosphorylation or increase P-VASP-TRPC4 association in BSO-treated rats. This data shows that in normotensive animals NO-mediated PKG can regulate SOC via VASP-TRPC4 association. On the other, oxidative stress can reduce the NO-dependent PKG activation and abolish subsequent SOC regulation by blocking the P-VASP-TRPC4 association. An antioxidant can ameliorate the oxidative stress and restore SOC regulation via P-VASP-TRPC interaction.
In vascular smooth muscle cells, PKG is stimulated by NO-cGMP which leads to relaxation (1, 4, 21). Although the exact mechanisms are still controversial it is likely that a decrease in intracellular Ca2+ by PKG activation plays a role in smooth muscle relaxation (1, 4, 21). Therefore, we wanted to determine if oxidative stress could lead to failure of NO donors in inhibiting store-operated Ca2+ entry into smooth muscles due to defective NO-PKG signaling. Our data shows that while DETA NONOate was able to inhibit the store-operated Ca2+ entry into smooth cells from vehicle-treated rats this effect was abolished by oxidative stress in BSO-treated rats. However, antioxidant tempol which restored the NO-PKG signaling was able to reverse the inhibition of store-operated Ca2+ entry in response to DETA NONOate. Taken together, these data show that oxidative stress can prevent the decrease in intracellular Ca2+ in response to NO and lead to sustained vasoconstriction.
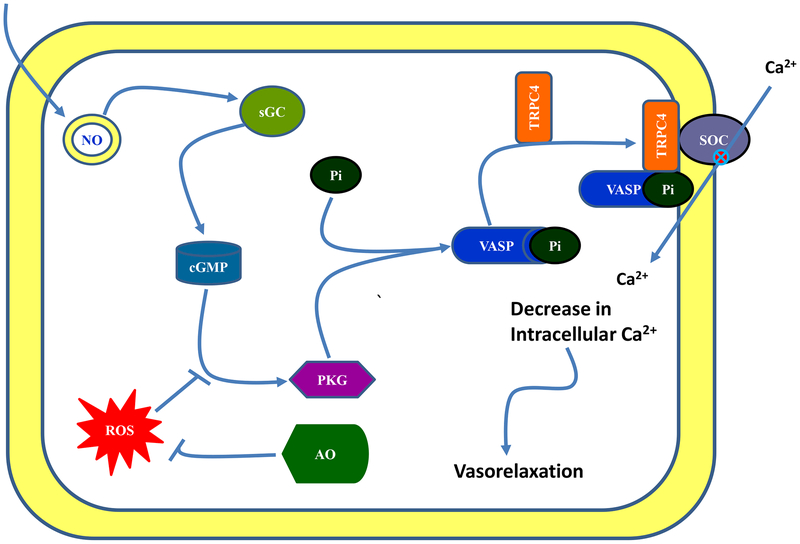
Our conclusion is that in normotensive animals NO activates PKG which phosphorylates VASP at serine 239. The phosphorylated VASP interacts with TRPC4 and regulates store-operated Ca2+ channel function and reduces Ca2+ influx in smooth muscle cells which leads to vasorelaxation. During oxidative stress, NO is unable to inhibit the SOC mainly due to its failure to activate PKG-VASP/TRPC signaling which leads to increased in Ca2+ influx and contributes to vasoconstriction. Antioxidant supplementation reduces the oxidative stress and restores NO signaling thereby reducing the blood pressure (figure 7).
Figure 7. Schematic presentation of nitric oxide signaling in mesenteric artery smooth muscle.
Signaling pathway (→), decrease (–) and inhibition (⊗). NO—nitric oxide, sGC—soluble guanylyl cyclase, PKG—protein kinase G, VASP—Vasodilator-stimulated phosphoprotein, TRPC—transient receptor potential channel 4 (TRPC4), ROS—reactive oxygen species and AO—antioxidant.
Perspective
Endothelial dysfunction, an important contributor to hypertension, is widely seen as a failure of blood vessels to relax in response to NO-dependent vasodilatory compounds such as acetylcholine. Mechanistically, endothelial dysfunction is defined as a failure of endothelium to increase NO levels which could be due nitric oxide synthase uncoupling or NO inactivation by reactive oxygen species. The present study provides strong evidence that oxidative stress can directly impact NO signaling which could be a major independent risk factor for the development of hypertension as it relates NO system. Taken together, our data suggest that impaired NO system function could be a combination of decreased endothelium NO production, increased NO degradation/inactivation, and defective NO signaling in smooth muscle cells. Therefore, future studies should focus on all of these phenomena to better understand the mechanism(s) of hypertension.
Acknowledgements
None
Grants
This work was supported by The American Heart Association, National Center—Scientist Development Grant [0835428N] and NIH-NIDDK (DK098509).
Footnotes
Disclosure
None
References
- 1.Ay B, Iyanoye A, Sieck GC, Prakash YS, and Pabelick CM. Cyclic nucleotide regulation of store-operated Ca2+ influx in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 290: L278–283, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Banday AA, Fazili FR, and Lokhandwala MF. Oxidative stress causes renal dopamine D1 receptor dysfunction and hypertension via mechanisms that involve nuclear factor-kappaB and protein kinase C. J Am Soc Nephrol 18: 1446–1457, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Banday AA, Muhammad AB, Fazili FR, and Lokhandwala M. Mechanisms of oxidative stress-induced increase in salt sensitivity and development of hypertension in Sprague-Dawley rats. Hypertension 49: 664–671, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Blatter LA, and Wier WG. Nitric oxide decreases [Ca2+]i in vascular smooth muscle by inhibition of the calcium current. Cell Calcium 15: 122–131, 1994. [DOI] [PubMed] [Google Scholar]
- 5.Bryan NS, Bian K, and Murad F. Discovery of the nitric oxide signaling pathway and targets for drug development. Front Biosci 14: 1–18, 2009. [DOI] [PubMed] [Google Scholar]
- 6.Ceriello A Possible role of oxidative stress in the pathogenesis of hypertension. Diabetes Care 31 Suppl 2: S181–184, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Chen L, Daum G, Chitaley K, Coats SA, Bowen-Pope DF, Eigenthaler M, Thumati NR, Walter U, and Clowes AW. Vasodilator-stimulated phosphoprotein regulates proliferation and growth inhibition by nitric oxide in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 24: 1403–1408, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferroni P, Basili S, Paoletti V, and Davi G. Endothelial dysfunction and oxidative stress in arterial hypertension. Nutr Metab Cardiovasc Dis 16: 222–233, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Fiscus RR, and Murad F. cGMP-dependent protein kinase activation in intact tissues. Methods Enzymol 159: 150–159, 1988. [DOI] [PubMed] [Google Scholar]
- 10.Gill RM, Braz JC, Jin N, Etgen GJ, and Shen W. Restoration of impaired endothelium-dependent coronary vasodilation in failing heart: role of eNOS phosphorylation and CGMP/cGK-I signaling. Am J Physiol Heart Circ Physiol 292: H2782–2790, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Ibarra-Alvarado C, Galle J, Melichar VO, Mameghani A, and Schmidt HH. Phosphorylation of blood vessel vasodilator-stimulated phosphoprotein at serine 239 as a functional biochemical marker of endothelial nitric oxide/cyclic GMP signaling. Mol Pharmacol 61: 312–319, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Javkhedkar AA, Lokhandwala MF, and Banday AA. Defective nitric oxide production impairs angiotensin II-induced Na-K-ATPase regulation in spontaneously hypertensive rats. Am J Physiol Renal Physiol 302: F47–51, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Kwan HY, Huang Y, and Yao X. Store-operated calcium entry in vascular endothelial cells is inhibited by cGMP via a protein kinase G-dependent mechanism. J Biol Chem 275: 6758–6763, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Li H, and Forstermann U. Nitric oxide in the pathogenesis of vascular disease. J Pathol 190: 244–254, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Lincoln TM, and Cornwell TL. Intracellular cyclic GMP receptor proteins. FASEB J 7: 328–338, 1993. [DOI] [PubMed] [Google Scholar]
- 16.Lohmann SM, Vaandrager AB, Smolenski A, Walter U, and De Jonge HR. Distinct and specific functions of cGMP-dependent protein kinases. Trends Biochem Sci 22: 307–312, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Mihara M, and Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 86: 271–278, 1978. [DOI] [PubMed] [Google Scholar]
- 18.Oelze M, Mollnau H, Hoffmann N, Warnholtz A, Bodenschatz M, Smolenski A, Walter U, Skatchkov M, Meinertz T, and Munzel T. Vasodilator-stimulated phosphoprotein serine 239 phosphorylation as a sensitive monitor of defective nitric oxide/cGMP signaling and endothelial dysfunction. Circ Res 87: 999–1005, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Parekh AB, and Putney JW Jr. Store-operated calcium channels. Physiol Rev 85: 757–810, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Pfeifer A, Klatt P, Massberg S, Ny L, Sausbier M, Hirneiss C, Wang GX, Korth M, Aszodi A, Andersson KE, Krombach F, Mayerhofer A, Ruth P, Fassler R, and Hofmann F. Defective smooth muscle regulation in cGMP kinase I-deficient mice. EMBO J 17: 3045–3051, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prakash YS, Iyanoye A, Ay B, Sieck GC, and Pabelick CM. Store-operated Ca2+ influx in airway smooth muscle: Interactions between volatile anesthetic and cyclic nucleotide effects. Anesthesiology 105: 976–983, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Russo I, Del Mese P, Doronzo G, Mattiello L, Viretto M, Bosia A, Anfossi G, and Trovati M. Resistance to the nitric oxide/cyclic guanosine 5’-monophosphate/protein kinase G pathway in vascular smooth muscle cells from the obese Zucker rat, a classical animal model of insulin resistance: role of oxidative stress. Endocrinology 149: 1480–1489, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Stasch JP, Schmidt PM, Nedvetsky PI, Nedvetskaya TY, H SA, Meurer S, Deile M, Taye A, Knorr A, Lapp H, Muller H, Turgay Y, Rothkegel C, Tersteegen A, Kemp-Harper B, Muller-Esterl W, and Schmidt HH. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J Clin Invest 116: 2552–2561, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stockand JD, and Sansom SC. Mechanism of activation by cGMP-dependent protein kinase of large Ca(2+)-activated K+ channels in mesangial cells. Am J Physiol 271: C1669–1677, 1996. [DOI] [PubMed] [Google Scholar]
- 25.Surks HK, Mochizuki N, Kasai Y, Georgescu SP, Tang KM, Ito M, Lincoln TM, and Mendelsohn ME. Regulation of myosin phosphatase by a specific interaction with cGMP- dependent protein kinase Ialpha. Science 286: 1583–1587, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Swayze RD, and Braun AP. A catalytically inactive mutant of type I cGMP-dependent protein kinase prevents enhancement of large conductance, calcium-sensitive K+ channels by sodium nitroprusside and cGMP. J Biol Chem 276: 19729–19737, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Torok J Participation of nitric oxide in different models of experimental hypertension. Physiol Res 57: 813–825, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Pluznick JL, Settles DC, and Sansom SC. Association of VASP with TRPC4 in PKG-mediated inhibition of the store-operated calcium response in mesangial cells. Am J Physiol Renal Physiol 293: F1768–1776, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Ward NC, and Croft KD. Hypertension and oxidative stress. Clin Exp Pharmacol Physiol 33: 872–876, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Zhou XB, Schlossmann J, Hofmann F, Ruth P, and Korth M. Regulation of stably expressed and native BK channels from human myometrium by cGMP- and cAMP-dependent protein kinase. Pflugers Arch 436: 725–734, 1998. [DOI] [PubMed] [Google Scholar]