Abstract
Mesenchymal stem/stromal cells (MSCs) are multipotent cells that are emerging as the most promising means of allogeneic cell therapy. MSCs have inherent immunomodulatory characteristics, trophic capabilities, high in vitro self-renewal ability, and can be readily engineered to enhance their immune-modulatory functions. MSCs affect the functions of most immune effector cells, via direct contact with immune cells and local microenvironmental factors. Previous studies have confirmed that the immunomodulatory effect of MSCs is mainly communicated via MSC-secreted cytokines; however, apoptotic and metabolically inactivated MSCs have more recently been shown to possess immunomodulatory potential, with regulatory T-cells and monocytes playing a key role. Here we review the immunomodulatory aspects of naïve and engineered MSCs and discuss the strategies of increasing the potential of successfully using MSCs in clinical settings.
Keywords: Mesenchymal stem cells, Immunomodulation, Therapeutics, Engineering
Mesenchymal stem cells
Mesenchymal stem/stromal cells (MSCs) are pluripotent T-cells with self-renewing differentiation capacity and immunomodulatory properties. Their two most attractive features are plasticity (see Glossary) and tropism. They are distinguished from other cell types by expression of cell surface markers including CD73, CD90, and CD105, and lack of expression of CD45, CD34, CD14, CD19, CD11b, or Human Leukocyte Antigen – DR isotype (HLA-DR) [1] and play a central role in tissue repair, apart from anti-tumorigenic, anti-fibrotic, anti-apoptotic, anti-inflammatory, pro-angiogenic, neuroprotective, anti-bacterial and chemo-attractive effects [2, 3]. This unique set of characteristics make MSCs attractive for their therapeutic potential in the fields of regenerative medicine [4], inflammatory disorders [2], and increasingly, cancer therapy [5, 6].
Initially, MSCs were mainly utilized for tissue repair and regeneration [3]. However, they have been used increasingly for diseases that include graft-vs.-host disease (GVHD) and autoimmune diseases such as lupus and Crohn’s disease [2]. In addition, the clinical potential of MSCs has been extended to treat myocardial infarction, stroke, multiple sclerosis, liver cirrhosis, diabetes, lung injuries, and cancer [2, 3]. MSCs have been harvested and expanded from a variety of adult and perinatal tissues such as the bone marrow [7], adipose tissue [7, 8], peripheral blood, fetal tissues [9], dental pulp [8], umbilical cord tissue [7, 8], and placental tissues [7]. Most preclinical studies have been performed with bone marrow-derived MSCs (BM-MSCs); however, adipose tissue-derived MSCs (A-MSCs) and umbilical cord blood-derived MSCs (UC-MSCs) have also received considerable attention in recent years [9].
Immunomodulation of MSCs
MSCs have recently been shown to possess immunomodulatory potential, with interactions with regulatory T-cells (Tregs) and monocytes playing a key role [2, 10]. A number of studies have suggested that A-MSCs exert more potent immunomodulatory effects than BM-MSCs, implying that A-MSCs would be the better alternative for immunomodulatory therapy [11]. UC-MSCs, on the other hand, have been suggested to show minimal risk of initiating an allogeneic immune response when administered in vivo. This, as well as their ease of collection, also make UC-MSCs suitable therapeutic candidates [8]. Below, we will focus on immunomodulatory aspects of naïve and engineered MSCs and synthesize the current understanding of the effects of MSC modulation on immune cells.
Immunomodulatory actions through cell-to-cell contacts
MSCs participate in both innate and adaptive immunity, and their immunomodulatory functions are exerted mainly via interactions with immune cells through cell-to-cell contact and paracrine activity involving T cells, B cells, natural killer (NK) cells, macrophages, monocytes, dendritic cells (DCs) and neutrophils [12] (Figure 1).
Figure 1. Multi-faceted immunomodulatory interactions between MSCs and immune cells.
MSCs exert immunomodulatory functions mainly via interactions with immune cells such as T cells, B cells, natural killer (NK) cells, macrophages, monocytes, dendritic cells (DCs) and neutrophils, through cell-to-cell contacts (blue arrows) and paracrine activity (shown by secretome). MSCs’ secretome bear a number of cytokines, growth factors, and chemokines and the immunomodulatory functions vary depending on the sources of the MSCs, target cells, and the microenvironment. Abbreviations: ICAM-1, intercellular adhesion molecule-1; IDO, indoleamine-pyrrole 2,3-dioxygenase; IFN, interferon; IL, interleukin; PD-L1, programmed-death ligand 1; PD-L2, programmed-death ligand 2; PGE2, prostaglandin E2; TGF- β, transforming growth factor-β; TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth factor.
Cell-to-cell contacts with immune cells of adaptive immunity
In vitro, MSCs have been shown to inhibit naive and memory T-cell responses to communicate with antigen-presenting cells [13] by upregulating intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), which are critical for T-cell activation and leukocyte (WBCs) recruitment to the inflammation site [14]. Furthermore, co-culture of BM-MSCs with activated T-cells has been shown to induce lymphocytes that express interleukin-17A (IL-17A) [15]. MSCs co-cultured with CD4+ T-cells activate the Notch1/ forkhead box P3(FOXP3) pathway and increase the percentage of CD4+CD25 FOXP3+ cells [16]. Furthermore, the knockdown of Galectin-1, a protein abundantly expressed intracellularly and on the cell surface of MSCs, with effects on T lymphocytes and cytokine secretion [17], in MSCs results in loss of immunomodulatory properties and restores the proliferation of CD4+ and CD8+ T-cells [17]. Additionally, BM-MSCs express high levels of Toll-like receptors (TLRs) 3 and 4, which are responsible for nuclear factor kappaB (NF-κB) activity and cytokine production. Expression of Toll-like receptors (TLRs) 3 and 4 in MSCs has been shown to restore efficient T-cell response in case of infection [18]. Human placenta MSCs (PMSCs) have been shown to express high levels of cell adhesion molecule programmed-death ligand 1 (PD-L1) and PD-L2, which can inhibit T-cell proliferation by arresting the cell cycle [19].
In vivo mouse models have also shown immune regulation between MSCs and T-cells. In a syngeneic orthotopic mouse model of ovarian cancer, compact bone-derived MSCs (CB-MSCs) have anti-tumor effects in combination with a fusion protein consisting of anti-mesothelin scFv genetically fused to Mycobacterium tuberculosis heat shock protein 70 (Hsp70) (designated as VIC-008), which is involved in activating CD4+ and CD8+ T-cells and inhibiting Tregs in the tumor microenvironment (TME) [20]. In fetal abortion models, MSCs have been shown to enhance the suppressive regulation of T-cells and macrophages [21]. On the contrary, MSCs primed by activated T cells derived from IFN-γ −/− mouse exhibited dramatically reduced ability to suppress T cell proliferation which supports the cell to cell contact mechanism in the immunosuppressive function of MSCs [22].
In addition to T-cells, MSCs also effect B-cells through cell-to-cell contact in vitro. A-MSCs have been shown to increase the survival of quiescent B-cells via contact-dependent mechanisms and facilitate B-cell differentiation independently of T-cells [23]. Additionally, A-MSCs not only inhibit Caspase 3-mediated apoptosis of B-cells by upregulation of vascular endothelial growth factor (VEGF) [24], but also inhibit proliferation by blocking the cell cycle of B-lymphocytes in the G0/G1 phase mediated by the activation p38 mitogen-activated protein kinase (MAPK) pathways [25].
Cell-to-cell contacts with immune cells of innate immunity
In addition to the adaptive immune system, MSCs also affect the innate immune system through cell-to-cell contact. Tracking studies reveal that infused UC-MSC briefly reside in the lungs and are rapidly phagocytosed by monocytes, which subsequently migrate to other body sites. UC-MSC phagocytosis induces phenotypical and functional changes in monocytes, which in turn modulate cells of the adaptive immune system and thus play a crucial role in mediating, distributing, and transferring the immunomodulatory effect of MSC [26]. Co-culture studies of MSCs with different types of NK cell-lines (KHYG-1 and NK-92) have shown that granule polarization is either suppressed and induced, indicating differential crosstalk between MSCs and cytotoxic NK cells [27]. Additionally, A-MSCs are known to switch activated-M1-like inflammatory macrophages to an M2-like phenotype via prostaglandin E2 (PGE2) [28]. MSCs can also prevent neutrophil death via an ICAM-1-dependent mechanism, to further exert tissue-protective effects [29].
Immunomodulatory actions through paracrine activity
Importantly, MSCs also exert their immunomodulatory properties by secreting multifunctional molecules via paracrine mechanisms [12] (Figure 1). This secretome is a diverse repertoire of multifaceted cytokines, growth factors, and chemokines, which combine to modulate the function of immune and cancer cells. and include transforming growth factor-β1 (TGF- β1), tumor necrosis factor-α (TNF-α), PGE2, IFN-γ, hepatocyte growth factor (HGF), fibroblast growth factor (FGF), indoleamine-pyrrole 2,3-dioxygenase (IDO), and nitric oxide, among many others [30, 31]. These paracrine factors are found encapsulated in cell-secreted extracellular vesicles (EVs), which in turn are usually divided into exosomes, microvesicles (MVs), and apoptotic bodies according to their size and cell origin [30, 32]. Although MSC-derived extracellular vesicles (MSC-EVs) display immunoregulatory functions similar to the parent MSCs[33], the paracrine actions vary depending on the sources of the MSCs, the target cells, and the microenvironment surrounding the cells [34].
Paracrine activity on the adaptive immune system
MSCs have been shown to act on the adaptive immune system, particularly T-cells, via paracrine secretion. MSCs inhibit T helper 17 cell (Th17) differentiation by inducing production of IL-10, PGE2, and inhibiting IL-17, IL-22, and IFN-γ [35]. Co-culture studies of BM-MSCs with T cells have shown that both priming and cell ratio of BM-MSCs with cytokines influence their cytokine profiles and suggest that BM-MSCs modulation on Th17 lymphocytes pathway in a complex manner [15]. However, the mechanisms underlying these interactions between MSCs and Th17-lymphocytes is not fully understood. Previous studies utilizing knockdown of IL-25 in vitro and in vivo have shown that MSCs suppress Th17 responses via regulation of the IL-25/STAT3/PD-L1 axis, [36]. MSCs-secreted IDO induces Tregs, responsible for kidney allograft tolerance [37]. Moreover, co-cultured exosomes derived from BM-MSCs transfected with plasmid encoding shFas and anti-miR-375 and peripheral blood mononuclear cells (PBMCs) suppress immune response in a immunodeficient mouse model by inhibiting the proliferation of peripheral blood mononuclear cells (PBMCs) and enhancing Treg function [38]. Additionally, MSCs secrete PD-1 ligands (including PD-L1 and PD-L2) and exert immunosuppressive effects directly on T-cell behavior by suppressing the activation of CD4+ T-cells [39].
Paracrine activity on the innate immune system
Within the innate immune system MSCs are known to interact with NK cells by inhibiting IL-2-induced NK cell proliferation [40] and inducing cytotoxic activity or cytokine production via secretion of IDO and PGE2 [41]. MSCs have also been shown to enhance the ability of IL-12/IL-18-stimulated NK cells to secrete IFN-γ which has the potential to improve the defense against infections at the site of injury and also influence tissue regeneration [42]. Moreover, MSC-derived IL-6 has been shown to protect neutrophils from apoptosis, thus preserving them in the bone marrow niche [43]. MSC-derived exosomes have been shown to augment neutrophil viability, while MSC-conditioned media (CM) increase neutrophil function, demonstrating that both MSC-derived exosomes and CM are useful for increasing immunity by modulating neutrophils [44]. Lipopolysaccharide (LPS)-stimulated MSCs augment the anti-microbial functions of neutrophils via release of IL-8 and macrophage migration inhibitory factor (MIF), contributing to the resolution of infection and inflammation [45].
MSCs-secreted IL-6 can prevent the differentiation of monocytes towards an anti-inflammatory interleukin-10 producing phenotype [46], whereas MSCs-derived PGE2 empower MSCs to suppress the differentiation of monocytes to mature DCs [47]. MSC-derived EVs have been shown to prevent antigen uptake by immature DCs and attenuate DC maturation that is accompanied by downregulation of markers of mature DCs (CD83, CD38, and CD80) as well as pro-inflammatory cytokines (IL-6 and IL-12p70), and upregulation of anti-inflammatory cytokine TGF-β [48]. Additionally, miR-21–5p, a microRNA found to be enriched in MSC-derived EVs, has been shown to influence DC maturation and function [48]. MSC-EVs also play a critical role in triggering macrophage polarization [49] by enhancing the formation of anti-inflammatory M2 macrophages over M1 like inflammatory macrophages, via downregulation of IL-23 and IL-22 [50]. BM-MSCs activated by LPS or TNF-α reprogram macrophages by releasing PEG2 that acts on the macrophages through the prostaglandin EP2 and EP4 receptors [51]. Human PMSCs have also been shown to transform macrophages from an inflammatory M1 into an anti-inflammatory M2 phenotype, via soluble IL-10, IL-1β, IL-12, and Macrophage inflammatory protein-1 alpha (MIP-1α ) as well as glucocorticoid and progesterone receptors [52].
Preconditioning MSCs to increase their immunomodulatory functions and therapeutic efficacy
Preconditioning of MSCs is known to influence their therapeutic efficacy, hence, modification with diverse factors and a variety of conditions have been explored to manipulate the secretory profiles and enhance the therapeutic efficacy of MSCs [53]. Preconditioning MSCs with hypoxia, oxidative stress, heat shock, starvation, or inflammatory biological agents has been shown to have the potential to increase their survival and potency [53]. The main methods of preconditioning are hypoxia and priming with immunomodulatory factors [32]. Below we discuss these briefly.
Preconditioning with hypoxia
Hypoxia-preconditioning has been shown to have a positive influence on the MSC immune phenotype. Specifically, it increases paracrine and antioxidative effects, particularly the secretion of angiogenic factors as observed in examples of acute kidney injury [54] and bleomycin-induced pulmonary fibrosis [55]. Hypoxia-preconditioning of dental MSCs overexpressing hypoxia-inducible factor 1 α (HIF-1 α) can impair DC differentiation, attract more monocytes, and increase resistance to NK-cell-mediated lysis [56]. Hypoxia-preconditioning of CB-MSCs coupled with pretreatment using proinflammatory cytokines IFN-γ, TNF-α, and IL-17A improves suppression of CD8+ T-cells [57]. Hypoxia-conditioning of MSCs result in higher infiltration of endothelial cells into MSC-containing scaffolds for dermal regeneration [58], positively affecting cell fitness and the transcriptome, potentially improving cellular therapies targeting orthopedic disorders [59] and promoting maintenance of stem-like characteristics with IDO upregulation [60]. Furthermore, MSCs primed with hypoxia and calcium ions (HC-MSC) have been shown to be resistant to passage-dependent senescence mediated via the monocyte chemoattractant protein-1 and p53/p21 cascade and secreted large amounts of pro-angiogenic and immunomodulatory factors, resulting in suppression of T-cell proliferation in vitro. Administration of HC-MSCs significantly ameliorated symptoms of GVHD in a humanized mouse model, resulting in significantly improved survival [61].
Preconditioning by priming with immunomodulatory factors
Another important method of preconditioning is priming with immunomodulatory factors. IFN-γ is a primary factor that can activate the transcription and synthesis of IDO-1, HGF, and TGF-β in MSCs [62]. Single IFN-γ priming induces IDO expression and leads to sustained improvement in the MSC T-cell-suppressive phenotype [63]. MSCs primed by IFN-γ have been shown to have enhanced immunosuppressive capacity and immunosuppressive function via the IFN-γ-JAK-STAT1 pathway [64, 65]. In addition, membrane particles (MP) of MSCs stimulated with IFN-γ can increase mRNA expression of PD-L1 in monocytes and the percentage of anti-inflammatory PDL-1 and CD90 positive monocytes thus demonstrating the potential of MSC derived MPs as a novel cell-free therapy for treatment of immunological disorders [66]. MSCs primed with TNF-α and IFN-γ have been shown to reverse the pro-inflammatory effect of MSCs in palmitate, a potent modulator of MSC immunosuppressive function enriched within type 2 diabetes, and maintain the ability to block T-cell proliferation and cytokine production [62]. More recently, it has been shown that BM-MSCs modified with small heat shock proteins 27 and 20 (sHsp27 and sHsp20) and E7 oncoprotein significantly enhances the E7-specific T-cell responses and suppress tumor growth in mice indicating that MSC-based vaccinations have the potential as a promising approach for immunotherapy and protection against HPV-associated cancers [67].
Engineering MSCs to increase their immunomodulatory functions and therapeutic efficacy
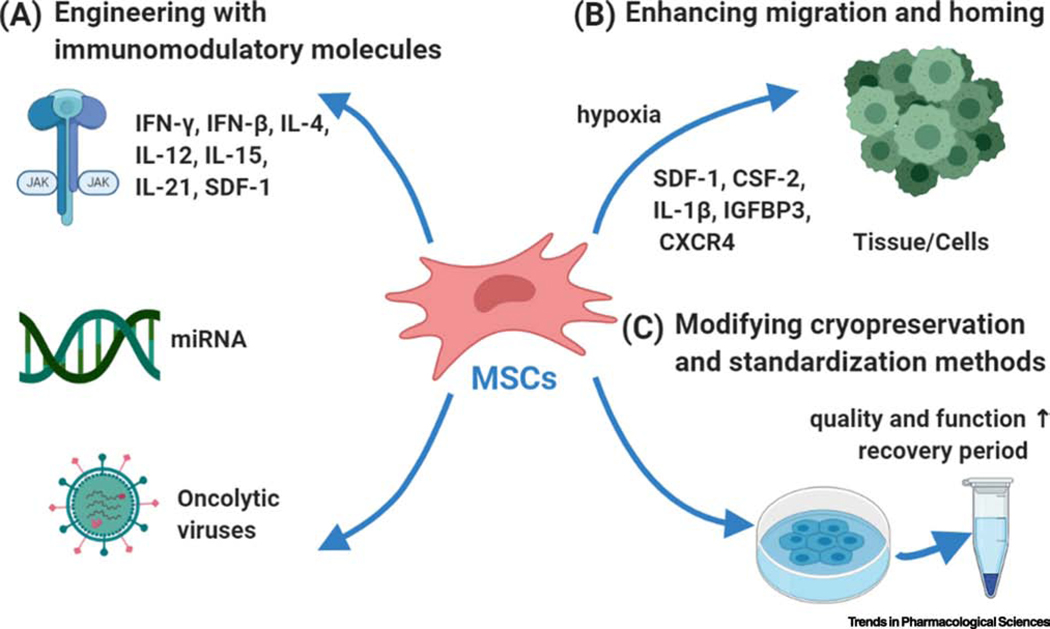
MSCs possess the intrinsic ability to evade the immune response temporarily. In the context of using MSCs for targeted therapy, they are mainly considered gene delivery vehicles for immunomodulatory molecules like IFNs and ILs or engineered to deliver oncolytic viruses (OV) (Figure 2A). Engineering MSCs to express specific immunomodulatory agents contribute to the MSCs’ immunomodulatory capacity and pluripotency, and also enables them to deliver large doses of cancer-targeting biologics with a single dose. Some examples of engineered MSCs expressing diverse immunomodulatory molecules are given in Table 1 and discussed here.
Figure 2. Three strategies to enhance the efficacy of MSC application.
(A) MSCs can be engineered with immunomodulatory molecules like interferons (IFNs), interleukins (ILs) and miRNA or engineered to deliver oncolytic viruses. (B) MSCs can also be primed which can contribute to their efficient homing and migration to the target tissue. (C) Different methods have been modified and standardized to improve the cryopreservation of MSC. Abbreviations: CSF-2, Colony-stimulating factor 2; CXCR4, CXC chemokine receptor 4; IFN, interferon; IL, interleukin; IGFBP3, insulin-like growth factor binding protein 3; miRNA, microRNA, SDF-1, stromal cell-derived factor-1.
Table 1.
Engineered MSCs expressing diverse immunomodulatory molecules
| Engineered agents | Model | Mechanism | Reference |
|---|---|---|---|
| IFN-β | Syngeneic orthotopic Glioblastoma mouse model | Infiltration of CD8 T cells, cell-cycle arrest | [68] |
| IFN-γ | Neuroblastoma murine model | Macrophages polarization | [69] |
| IL-4 | Osteoarthritis rat model | Chondroprotective and anti-inflammatory effects | [70] |
| IL-12 | Renal cell carcinoma, melanoma, breast tumor, and HCC mouse models | Reduction of lymphatic sprouts, apoptosis induction | [71, 72] |
| IL-15 | Pancreatic tumor mouse model | Tumor-specific T-cell immune memory response | [73] |
| IL-21 | B-cell lymphoma mouse model | Induction of effector T and NK cells | [74] |
| SDF-1 | Streptozotocin-induced diabetic rats | Increasing nNOS, VEGF and bFGF | [75] |
| miR-199a | HCC mouse model | mTOR pathway inhibition | [76] |
| Oncolytic virus | Brain metastatic melanoma mouse model | Increasing IFNγ-producing CD8+ tumor-infiltrating T lymphocytes with PD-L1 blockade |
[78] |
| HCC mouse model | Inhibiting HCC cell proliferation | [79] | |
| Lung adenocarcinoma mouse model | TLR9 overexpression and activation of the NF-κB pathway |
[80] |
Abbreviations: IFN, interferon; GVHD, graft-vs.-host disease; IL, interleukin; HCC, hepatocellular carcinoma; NK, natural killer; SDF-1, stromal cell-derived factor-1; nNOS, neuronal nitric oxide synthase; VEGF, vascular endothelial growth factor; bFGF, basic fibroblast growth factor; mTOR, mammalian target of rapamycin.
We have previously shown that encapsulated MSCs engineered to express the cytokine IFN-β increase the survival of mice with highly malignant brain tumor, glioblastoma via promoting selective postsurgical infiltration of CD8 T-cells and inducing cell-cycle arrest in tumor cells (Table 1) [68]. Likewise, recently, engineered MSCs expressing IFN-γ have also been shown to elicit tumor growth reduction and increase survival via polarization of murine macrophages to the pro-inflammatory M1 phenotype in vitro and in vivo [69]. Similarly, MSCs engineered to express IL-4 have been shown to have increased chondroprotective and anti-inflammatory effects in a rat model of osteoarthritis [70], whereas IL-12-engineered MSCs reduce tumor growth and prolong survival in mouse models of renal cell carcinoma, melanoma, breast tumor, and hepatocellular carcino (HCC) via reduction of lymphatic sprouts and induction of apoptosis [71, 72]. Engineered MSCs expressing murine IL-15 significantly inhibit pancreatic cancer growth and prolong the survival of tumor-bearing mice, as well as promoting resistance to pancreatic tumor re-challenge, via induction of tumor-specific T-cell immune memory response [73]. Moreover, MSCs engineered to express IL-21 induce effector T- and NK-cells and prevent the formation of tumor nodules in a mouse model of B-cell lymphoma [74]. Additionally, MSCs engineered to express stromal cell-derived factor-1 (SDF-1) have been shown to result in alleviation of erectile dysfunction in streptozotocin-induced diabetic rats (Table 1) mediated by increasing levels of neuronal nitric oxide synthase (nNOS), VEGF, and basic fibroblast growth factor (bFGF) and lowering levels of the apoptosis factors Bcl2-associated x (Bax) and caspase-3 [75]. Furthermore, exosomes derived from A-MSCs expressing miR-199- have the ability to mediate miR-199a delivery to HCC cells, and effectively sensitize cancer cells to chemotherapeutic agents by targeting the mammalian target of rapamycin (mTOR) pathway [76].
MSCs have also been explored as a potential means of OV delivery, as they can shield the virus from host antiviral immunity and transport viral particles systemically or intratumorally [77]. We discuss some examples where the immunomodulatory function of MSCs have been shown to be enhanced (Table 1). MSCs loaded with oncolytic herpes simplex virus (oHSV) or other OVs have been shown to successfully deliver viral progeny to established glioblastoma tumors and brain-metastatic melanoma, eventually decreasing tumor growth and prolonging mouse survival [77, 78]. Another study reported that MSCs loaded with HCC-targeted OV effectively lyse HCC cells in vitro, ultimately leading to potent tumor growth inhibition [79]. When OV-loaded MSCs were cocultured with allogeneic PBMCs, they induce TLR9 overexpression and activation of the NF-κB pathway, creating a proinflammatory environment and enhancing antitumor efficacy both in vitro and in vivo in lung adenocarcinoma xenograft tumors[80]. Additionally, the combined blockade of MSC-oHSV and PD-L1 increases the number of IFN-γ-producing CD8+ tumor-infiltrating T-cells and further increases the median survival of mice in melanoma brain metastasis models [78, 81].
MSCs mediated immunomodulation in clinical studies and trials
MSC infusion has shown potential efficacy in the treatment of several diseases that resist standard treatment. For example, in a phase I/II clinical trial (EuDRA CT Numberi: 2012-003741-15) involving autologous tumor-specific herpes simplex virus thymidine kinase (HSV-TK), MSCs demonstrated favorable safety and tolerability in patients with gastrointestinal tumors: the median time to progression was 1.8 months, and median overall survival was 15.6 months (Table 1) [82, 83]. However, the study was terminated early due to an insufficient number of patients. Another recent phase I/IIa trial (Clinical Trial Numberii: NCT02351011) using BM-MSCs in patients with osteoarthritis demonstrated that autologous BM-MSCs are safe and elicit significant improvements in pain and synovial inflammation [84]. Similarly, an expanded phase II/III study (Clinical Trial Number: (UMIN-CTR Numberiii: UMIN000006719) demonstrated that BM-MSC infusion improved the overall survival rate in patients with steroid-resistant GVHD (Table 1) [85]. In a phase IIa study of secondary progressive multiple sclerosis, autologous BM-MSCs elicited safe and effective neuroprotection (Clinical Trial Number: NCT00395200) [86]. For patients with moderate-to-severe atopic dermatitis, subcutaneous administration of human UC-MSCs brought about marked improvement of disease features without serious adverse events, and 55% of patients showed a 50% reduction in the Eczema Area and Severity Index (EASI) score (Table 1) [87]. In refractory fistulizing Crohn’s disease, a pilot study therapy with autologous BM-MSCs proved feasible, safe, and beneficial, and sustained complete closure was observed in 7/12 patients [88]. Another important phase III study (Clinical Trial Number: NCT01541579) has assessed the efficacy and safety of expanded A-MSCs (Cx601) for treatment of complex perianal fistulas in patients with Crohn’s disease. Cx601 treatment significantly achieved remission in patients with fewer treatment-related adverse events, suggesting MSCs as an effective strategy in clinical application [89].
Nevertheless, it should be noted that MSCs-based treatment might produce some potential unexpected side effects. For example, recent pre-clinical studies suggest that alteration of the brain microenvironment by acute hypothermia modulates MSC function resulting in a pro-inflammatory environment and increasing long-lasting motor-cognitive deficits in the neonatal hypoxic-ischemic brain [90].
Promising pre-clinical studies that have a potential to increase efficacy of future MSCs trials
Apart from improving immunomodulatory capacity of MSCs (Figure 2A), a number of approaches exist to improve MSC migration or homing mechanisms (Figure 2B). Recent studies have shown that MSC homing is driven by SDF-1-stimulated endothelial cell production of platelet-derived growth factor via activation of platelet-derived growth factor receptor A (PDGFRA)/PI3K/Akt, PDGFRA/MAPK/Grb2, and PDGFRA/JAK2/STAT signaling [91]. Additionally, FGF21 also influences the homing ability of MSCs to injury sites [92] and colony-stimulating factor 2 (CSF-2) has been shown to significantly enhances their differentiation and migratory capacity [93]. IL-1β induces matrix metalloproteinase-1 (MMP-1), which subsequently activates the protease-activated receptor 1 (PAR1 ) and G-protein-coupled signal pathways have been recently shown to promote MSC migration [94]. Interestingly, intranasally administered MSC derived exosomes specifically target and accumulate in the brain in pathological murine models for up to 96 hours, and this long homing response is driven by neuro-inflammatory signals [95]. Hypoxic preconditioning also promotes the migration and survival of MSCs, through increased expression of LincRNA-p21 via the HIF-1α/CXCR4 and CXCR7 pathway [96].
Another way to increase efficacy of MSCs would be towards improving methods of their cryopreservation. Recent studies have indicated the role of cryopreservation on the efficacy and clinical application of MSCs. Specifically, cryopreserved and thawed MSCs have impaired functional properties and stunted immunosuppressive capabilities [97, 98] which have significant implications for therapeutic outcomes [98]. Therefore, MSCs need to be standardized for reliability and robust quality-control purposes, especially in large-scale production of frozen products. A recent study revealed the feasibility of two freezing steps after a cell-culture phase of at least one passage, without affecting basic cell manufacturing parameters or quality attributes of the final frozen and thawed product [98]. A successful phase III clinical trial (Clinical Trial Number: NCT01541579) using expanded A-MSCs (Cx601) to treat patients with Crohn’s disease revealed that a recovery period of at least 24 hours post-cell thawing improved MSC product quality [89]. These MSCs maintained their multipotent differentiation capacity, immunomodulatory function, and anti-inflammatory properties [97]. Therefore, a 24-h acclimation period to reactivate thawed cells is critical to restore their diminished MSC function (Figure 2C).
Conclusions and Future Perspectives
MSCs have emerged as a promising therapeutic strategy because of their tropism to other cell types as well as their immunomodulatory functions. The immunomodulatory ability of MSCs is regulated by different inflammatory cytokines and the interaction between immune cells and MSCs could contribute to regeneration as well as the progression of different inflammatory diseases. The main mechanisms involved in MSC immunomodulation are cell-to-cell contact and paracrine activity primed with cytokines, chemokines, extracellular vesicles, inflammatory stimuli, or co-culture with other cells. Therefore, priming or licensing of MSCs has made cell-free therapy a controllable, manageable, and feasible method. However, there are still several issues that need to be solved (see Outstanding questions). MSCs are very heterogenic and change significantly with inflammatory or anti-inflammatory stimuli. Therefore, it is difficult to understand how MSC variability influences their induced immunomodulatory effects. It is possible that viable MSCs provoke more complex immunomodulatory mechanisms due to their intact secretome. Future work should explore the influence of other factors and/or chronic inflammation on immunomodulation mediated by MSCs. This has the potential to identify new methods of preconditioning to enhance MSC efficacy and minimizing variation in MSC paracrine effectiveness and therapeutic efficacy, especially with regards to clinical translatability.
Outstanding Questions
What external factors can influence immunomodulation mediated by MSCs?
How can we minimize variation in MSC paracrine effectiveness and therapeutic efficacy for clinical translation?
Which new preconditioning techniques can lead to better MSC therapy outcomes and further enhance therapeutic efficacy?
Can MSCs be engineered with multiple immunomodulators that mediate key biological signaling pathways?
How can we efficiently translate promising preclinical studies into clinical settings?
Highlights:
Mesenchymal Stem Cells (MSCs) are multipotent cells that are emerging as the most promising means of allogeneic cell therapy.
MSCs participate in both innate and adaptive immunity, and their immunomodulatory functions are exerted mainly via interactions with immune cells through cell-to-cell contact and paracrine activity.
Engineering MSCs to express specific immunomodulatory agents contributes to the MSCs capacity and pluripotency, and also enables them to deliver large doses of cancer-targeting biologics with a single dose.
MSC administration has shown potential efficacy in the treatment of several diseases that resist standard treatment. However, there are some challenges in efficiently translating MSC-based therapeutics into the clinic.
Efficient homing and migration of MSCs to the target tissue are essential for future development of MSC-based therapies.
In the context of using MSCs for targeted therapy in the clinic, they are mainly considered gene delivery vehicles for immunomodulatory molecules or oncolytic viruses. As engineered MSCs releasing targeted therapeutics are already being used in multiple clinical trials for immune and inflammatory diseases, it is essential to define the targets in the diseased cells/microenvironment in order to develop effective MSC therapies. For example, previous studies have shown that MSC engineered to co- express Epidermal growth factor receptor (EGFR) targeting antigen-specific domain (VHH) and death receptor (DR4/5) ligand(DRL) simultaneously target cell proliferation and death pathways in tumors[99]. Recently MSC engineered to express TandAb, a tetravalent bispecific tandem diabody for CD3 and CD19 have enhanced therapeutic efficacy in a mouse model of B-cell lymphoma as compared to targeting CD3 or CD19 alone[100].
A challenge in the field is the discrepancy between in vitro and in vivo findings in MSC research. For example, in a New Zealand white rabbit osteogenesis model, osteogenic cells differentiated from MSCs (DOC) retained their immunoprivileged and immunomodulatory properties in vitro, but the immunomodulatory properties were lost following transplantation [101]. In a rhesus macaque model, allogeneic vs. autologous MSC transplanted intracranially revealed that allogeneic MSCs, but not autologous MSCs were weakly immunogenic post-transplantation thus negatively impacting durable engraftment levels [102]. These discrepancies signify the importance of careful monitoring of MSC immunogenicity after moving from an in vitro to an in vivo model and further clinical applications and should therefore be considered to ensure a lasting effect. Ultimately, by enhancing immunomodulatory potential, homing mechanisms and improving cryopreservation techniques along with rigorous preclinical and clinical studies, MSC are set to have enormous potential to be used increasingly in clinical settings in the future.
Table 2.
Examples of clinical trials that are currently using MSCs.
| Disease | Origin of MSCs | Treatment routes | Clinical Trial Number | Phase | Therapeutic efficacy | References |
|---|---|---|---|---|---|---|
| Gastrointestinal adenocarcinoma | Autologous BM-MSCs |
Intravenous infusion | EudraCT numberi: 2012-003741-15 | I/II | Favorable safety and tolerability, median overall survival is 15.6 months | [82, 83] |
| Osteoarthritis | Autologous BM-MSCs |
Intralesional injection | Clincial Trial Numberii: NCT02351011 | I/II | Safe, improvements in pain and symptoms and reduced synovial inflammation | [84] |
| GVHD | Allogenous BM-MSCs |
Infusion | UMIN-CTR Numberiii: UMIN000006719 | II/III | Improve the overall survival rate | [85] |
| Multiple sclerosis | Autologous BM-MSCs |
Intravenous infusion | NCT00395200 | IIa | Safe, effective neuroprotection | [86] |
| Atopic dermatitis | Human UC-MSCs |
Subcutaneously | NCT01927705 | I/II | A marked improvement of features without serious adverse events | [87] |
| Crohn’s disease | Allogeneic A-MSCs |
Intralesional injection | NCT01541579 | III | Increasing remission with less treatment-related adverse events | [89] |
Abbreviations: BM-MSCs, bone marrow-derived MSCs; A-MSCs, adipose tissue-derived MSCs; UC-MSCs, umbilical cord blood-derived MSCs; GVHD, graft-vs.-host disease.
ACKNOWLEDGEMENTS
This work was supported by NIH grants R01-NS107857 (K.S.)
GLOSSARY
- Adaptive immunity
A subset of the immune system that is activated by exposure to pathogens and acts on the threat using an immunological memory to enhance its effect. Cells of the adaptive immune system include B- and T-cells.
- Allogeneic
Tissues or cells that are from individuals of the same species, but which are genetically dissimilar and hence immunologically incompatible.
- Dendritic cells (DCs)
antigen-presenting cells in the mammalian immune system. They act as messengers between the innate and the adaptive immune systems.
- Eczema Area and Severity Index (EASI) score
A clinical scoring system that is used to measure the extent and severity of actopic eczema
- Granule polarization
A prelude to the release of cytotoxic contents in response to target-cell binding.
- Innate immunity
A subset of the immune system that is activated by the presence of antigens and their chemical properties, using nonspecific defense mechanisms, meaning anything that is identified as foreign or non-self is a target. Cells of the innate immune system include NK cells, macrophages, mast cells, neutrophils, and dendritic cells.
- Lymphocyte
Subtype of white blood cells that include natural killer (NK) cells, T cells and B cells.
- M1 macrophages
Polarized macrophages that encourage inflammation.
- M2 macrophages
Polarized macrophages that decrease inflammation and encourage tissue repair.
- Macrophage polarization
Refers to how macrophages have been activated at a given point in space and time in response to the signals from their microenvironment.
- Membrane particles (MP)
Particles of the membrane of a cell ranging from 63 to 700 nm
- Memory T-cells
a subset of infection- and cancer-fighting T cells. They can reproduce to mount a faster and stronger immune response and recognize foreign invaders, such as bacteria or viruses, as well as cancer cells.
- Monocytes
the largest type of white blood cells that can differentiate into macrophages and myeloid lineage dendritic cells.
- Naïve T-cells
Primary cellular effectors in the adaptive immune system, playing critical functions in antigen specificity.
- Natural killer cells
A type of cytotoxic lymphocyte critical to the innate immune system, providing rapid responses to virus-infected cells and tumor cells.
- Neutrophils
The most abundant type white blood cells forming an essential part of the innate immune system.
- Plasticity
The capacity to differentiate into other cell types in response to different stimuli.
- Regulatory T cells (Tregs)
Formerly known as suppressor T cells, are a subpopulation of T cells that modulate the immune system to downregulate induction and proliferation of effector T cells.
- Secretome
The set of proteins expressed by an organism and secreted into the extracellular space, including cytokines, growth factors, extracellular matrix proteins, regulators and shed receptors.
- T helper 17 cells
a subset of pro-inflammatory T helper cells defined by production of interleukin 17 (IL-17).
- Toll- like receptors (TLR)
Toll-like receptors sense invading pathogens or endogenous damage signals and initiate the innate and adaptive immune response. TLR 3 and 4 are responsible for nuclear factor kappaB (NF-κB) activity and cytokine production and expressed highly in BM-MSCs.
- Tropism
The ability to migrate to damaged or diseased tissues or cells.
Footnotes
DISCLAIMER STATEMENT
K.S. owns equity in and is a member of the Board of Directors of AMASA Therapeutics, a company developing stem cell-based therapies for cancer. K.S.’s interests were reviewed and are managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies. The other authors declare that they have no competing interests.
RESOURCES
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Dominici M. et al. (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8 (4), 315–317. [DOI] [PubMed] [Google Scholar]
- 2.Galipeau J. and Sensébé L. (2018) Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell stem cell 22 (6), 824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Timaner M. et al. , The multifaceted role of mesenchymal stem cells in cancer, Seminars in cancer biology, Elsevier, 2019. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y. et al. (2008) Mesenchymal stem cells: A promising candidate in regenerative medicine. The International Journal of Biochemistry & Cell Biology 40 (5), 815–820. [DOI] [PubMed] [Google Scholar]
- 5.Dai L-J et al. (2011) Potential implications of mesenchymal stem cells in cancer therapy. Cancer Letters 305 (1), 8–20. [DOI] [PubMed] [Google Scholar]
- 6.Shah K. (2012) Mesenchymal stem cells engineered for cancer therapy. Advanced Drug Delivery Reviews 64 (8), 739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heo JS et al. (2016) Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue. International journal of molecular medicine 37 (1), 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim J-H et al. (2018) Comparison of immunological characteristics of mesenchymal stem cells from the periodontal ligament, umbilical cord, and adipose tissue. Stem cells international 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah K. (2016) Stem cell-based therapies for tumors in the brain: Are we there yet? Neuro-oncology 18 (8), 1066–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiss ARR and Dahlke MH (2019) Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of Action of Living, Apoptotic, and Dead MSCs. Frontiers in Immunology 10 (1191). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melief SM et al. (2013) Multipotent stromal cells skew monocytes towards an anti-inflammatory interleukin-10-producing phenotype by production of interleukin-6. Haematologica 98 (6), 888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Y. et al. (2019) The immunomodulatory functions of mesenchymal stromal/stem cells mediated via paracrine activity. Journal of clinical medicine 8 (7), 1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krampera M. et al. (2003) Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood, The Journal of the American Society of Hematology 101 (9), 3722–3729. [DOI] [PubMed] [Google Scholar]
- 14.Ren G. et al. (2010) Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. The Journal of Immunology 184 (5), 2321–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Najar M. et al. (2019) Immunological modulation following bone marrow-derived mesenchymal stromal cells and Th17 lymphocyte co-cultures. Inflammation Research 68 (3), 203–213. [DOI] [PubMed] [Google Scholar]
- 16.Del Papa B. et al. (2013) Notch1 modulates mesenchymal stem cells mediated regulatory T‐cell induction. European journal of immunology 43 (1), 182–187. [DOI] [PubMed] [Google Scholar]
- 17.Gieseke F. et al. (2010) Human multipotent mesenchymal stromal cells use galectin-1 to inhibit immune effector cells. Blood, The Journal of the American Society of Hematology 116 (19), 3770–3779. [DOI] [PubMed] [Google Scholar]
- 18.Liotta F. et al. (2008) Toll‐like receptors 3 and 4 are expressed by human bone marrow‐derived mesenchymal stem cells and can inhibit their T‐cell modulatory activity by impairing Notch signaling. Stem cells 26 (1), 279–289. [DOI] [PubMed] [Google Scholar]
- 19.Wang G. et al. (2013) Expression and biological function of programmed death ligands in human placenta mesenchymal stem cells. Cell biology international 37 (2), 137–148. [DOI] [PubMed] [Google Scholar]
- 20.Zeng Y. et al. (2019) CD90low MSCs modulate intratumoral immunity to confer antitumor activity in a mouse model of ovarian cancer. Oncotarget 10 (43), 4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y. et al. (2019) Cell–cell contact with proinflammatory macrophages enhances the immunotherapeutic effect of mesenchymal stem cells in two abortion models. Cellular & molecular immunology, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheng H. et al. (2008) A critical role of IFNγ in priming MSC-mediated suppression of T cell proliferation through up-regulation of B7-H1. Cell research 18 (8), 846–857. [DOI] [PubMed] [Google Scholar]
- 23.Franquesa M. et al. (2015) Human adipose tissue‐derived mesenchymal stem cells abrogate plasmablast formation and induce regulatory B cells independently of T helper cells. Stem cells 33 (3), 880–891. [DOI] [PubMed] [Google Scholar]
- 24.Healy ME et al. (2015) Mesenchymal stromal cells protect against caspase 3-mediated apoptosis of CD19+ peripheral B cells through contact-dependent upregulation of VEGF. Stem cells and development 24 (20), 2391–2402. [DOI] [PubMed] [Google Scholar]
- 25.Tabera S. et al. (2008) The effect of mesenchymal stem cells on the viability, proliferation and differentiation of B-lymphocytes. haematologica 93 (9), 1301–1309. [DOI] [PubMed] [Google Scholar]
- 26.de Witte SF et al. (2018) Immunomodulation by therapeutic mesenchymal stromal cells (MSC) is triggered through phagocytosis of MSC by monocytic cells. Stem Cells 36 (4), 602–615. [DOI] [PubMed] [Google Scholar]
- 27.Hu C-HD et al. (2019) Differential immunomodulatory effects of human bone marrow-derived mesenchymal stromal cells on natural killer cells. Stem cells and development 28 (14), 933–943. [DOI] [PubMed] [Google Scholar]
- 28.Manferdini C. et al. (2017) Adipose stromal cells mediated switching of the pro-inflammatory profile of M1-like macrophages is facilitated by PGE2: in vitro evaluation. Osteoarthritis and cartilage 25 (7), 1161–1171. [DOI] [PubMed] [Google Scholar]
- 29.Jiang D. et al. (2016) Suppression of neutrophil‐mediated tissue damage—A novel skill of mesenchymal stem cells. Stem Cells 34 (9), 2393–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li N. and Hua J. (2017) Interactions between mesenchymal stem cells and the immune system. Cellular and Molecular Life Sciences 74 (13), 2345–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.J Salgado A. et al. (2010) Adipose tissue derived stem cells secretome: soluble factors and their roles in regenerative medicine. Current stem cell research & therapy 5 (2), 103–110. [DOI] [PubMed] [Google Scholar]
- 32.Ferreira JR et al. (2018) Mesenchymal stromal cell secretome: influencing therapeutic potential by cellular pre-conditioning. Frontiers in immunology 9, 2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mardpour S. et al. (2019) Interaction between mesenchymal stromal cell‐derived extracellular vesicles and immune cells by distinct protein content. Journal of cellular physiology 234 (6), 8249–8258. [DOI] [PubMed] [Google Scholar]
- 34.Madrigal M. et al. (2014) A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. Journal of translational medicine 12 (1), 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghannam S. et al. (2010) Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. The Journal of Immunology 185 (1), 302–312. [DOI] [PubMed] [Google Scholar]
- 36.Wang W-B et al. (2015) Interleukin-25 mediates transcriptional control of PD-L1 via STAT3 in multipotent human mesenchymal stromal cells (hMSCs) to suppress Th17 responses. Stem Cell Reports 5 (3), 392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ge W. et al. (2010) Regulatory T-cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2, 3-dioxygenase expression. Transplantation 90 (12), 1312–1320. [DOI] [PubMed] [Google Scholar]
- 38.Wen D. et al. (2016) Mesenchymal stem cell and derived exosome as small RNA carrier and Immunomodulator to improve islet transplantation. Journal of Controlled Release 238, 166–175. [DOI] [PubMed] [Google Scholar]
- 39.Davies LC et al. (2017) Mesenchymal stromal cell secretion of programmed death‐1 ligands regulates T cell mediated immunosuppression. Stem Cells 35 (3), 766–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spaggiari GM et al. (2006) Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood 107 (4), 1484–1490. [DOI] [PubMed] [Google Scholar]
- 41.Spaggiari GM et al. (2008) Mesenchymal stem cells inhibit natural killer–cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2, 3-dioxygenase and prostaglandin E2. Blood, The Journal of the American Society of Hematology 111 (3), 1327–1333. [DOI] [PubMed] [Google Scholar]
- 42.Thomas H. et al. (2014) Interaction with mesenchymal stem cells provokes natural killer cells for enhanced IL-12/IL-18-induced interferon-gamma secretion. Mediators of inflammation 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raffaghello L. et al. (2008) Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem cells 26 (1), 151–162. [DOI] [PubMed] [Google Scholar]
- 44.Mahmoudi M. et al. (2019) Comparison of the effects of adipose tissue mesenchymal stromal cell-derived exosomes with conditioned media on neutrophil function and apoptosis. International immunopharmacology 74, 105689. [DOI] [PubMed] [Google Scholar]
- 45.Brandau S. et al. (2014) Mesenchymal stem cells augment the anti-bacterial activity of neutrophil granulocytes. PLoS One 9 (9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Melief SM et al. (2013) Adipose tissue‐derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow‐derived counterparts. Stem cells translational medicine 2 (6), 455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spaggiari GM et al. (2009) MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood, The Journal of the American Society of Hematology 113 (26), 6576–6583. [DOI] [PubMed] [Google Scholar]
- 48.Reis M. et al. (2018) Mesenchymal stromal cell-derived extracellular vesicles attenuate dendritic cell maturation and function. Frontiers in immunology 9, 2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sicco CL et al. (2017) Mesenchymal stem cell‐derived extracellular vesicles as mediators of anti‐inflammatory effects: Endorsement of macrophage polarization. Stem cells translational medicine 6 (3), 1018–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hyvärinen K. et al. (2018) Mesenchymal stromal cells and their extracellular vesicles enhance the anti-inflammatory phenotype of regulatory macrophages by downregulating the production of interleukin (IL)-23 and IL-22. Frontiers in immunology 9, 771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Németh K. et al. (2009) Bone marrow stromal cells attenuate sepsis via prostaglandin E 2–dependent reprogramming of host macrophages to increase their interleukin-10 production. Nature medicine 15 (1), 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abumaree M. et al. (2013) Human placental mesenchymal stem cells (pMSCs) play a role as immune suppressive cells by shifting macrophage differentiation from inflammatory M1 to anti-inflammatory M2 macrophages. Stem Cell Reviews and Reports 9 (5), 620–641. [DOI] [PubMed] [Google Scholar]
- 53.Silva LH et al. (2018) Strategies to improve the therapeutic effects of mesenchymal stromal cells in respiratory diseases. Stem cell research & therapy 9 (1), 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang W. et al. (2014) Hypoxia-pretreated human MSCs attenuate acute kidney injury through enhanced angiogenic and antioxidative capacities. BioMed research international 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lan Y-W et al. (2015) Hypoxia-preconditioned mesenchymal stem cells attenuate bleomycin-induced pulmonary fibrosis. Stem cell research & therapy 6 (1), 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martinez VG et al. (2017) Overexpression of hypoxia-inducible factor 1 alpha improves immunomodulation by dental mesenchymal stem cells. Stem cell research & therapy 8 (1), 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sivanathan KN et al. (2017) Immunodepletion and hypoxia preconditioning of mouse compact bone cells as a novel protocol to isolate highly immunosuppressive mesenchymal stem cells. Stem cells and development 26 (7), 512–527. [DOI] [PubMed] [Google Scholar]
- 58.Fierro FA et al. (2015) Hypoxic pre-conditioning increases the infiltration of endothelial cells into scaffolds for dermal regeneration pre-seeded with mesenchymal stem cells. Frontiers in cell and developmental biology 3, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Elabd C. et al. (2018) Comparing atmospheric and hypoxic cultured mesenchymal stem cell transcriptome: implication for stem cell therapies targeting intervertebral discs. Journal of translational medicine 16 (1), 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kadle RL et al. (2018) Microenvironmental cues enhance mesenchymal stem cell-mediated immunomodulation and regulatory T-cell expansion. PloS one 13 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim Y. et al. (2018) Small hypoxia-primed mesenchymal stem cells attenuate graft-versus-host disease. Leukemia 32 (12), 2672–2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boland L. et al. (2018) IFN-γ and TNF-α pre-licensing protects mesenchymal stromal cells from the pro-inflammatory effects of palmitate. Molecular Therapy 26 (3), 860–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zimmermann JA et al. (2017) Enhanced immunosuppression of T cells by sustained presentation of bioactive interferon‐γ within three‐dimensional mesenchymal stem cell constructs. Stem cells translational medicine 6 (1), 223–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klinker MW et al. (2017) Morphological features of IFN-γ–stimulated mesenchymal stromal cells predict overall immunosuppressive capacity. Proceedings of the National Academy of Sciences 114 (13), E2598–E2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim DS et al. (2018) Enhanced immunosuppressive properties of human mesenchymal stem cells primed by interferon-γ. EBioMedicine 28, 261–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gonçalves F.d.C. et al. (2017) Membrane particles generated from mesenchymal stromal cells modulate immune responses by selective targeting of pro-inflammatory monocytes. Scientific reports 7 (1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bolhassani A. et al. (2019) Modified DCs and MSCs with HPV E7 antigen and small Hsps: Which one is the most potent strategy for eradication of tumors? Molecular immunology 108, 102–110. [DOI] [PubMed] [Google Scholar]
- 68.Choi SH et al. (2017) Tumor resection recruits effector T cells and boosts therapeutic efficacy of encapsulated stem cells expressing IFNβ in glioblastomas. Clinical Cancer Research 23 (22), 7047–7058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Relation T. et al. (2018) Intratumoral delivery of interferonγ‐secreting mesenchymal stromal cells repolarizes tumor‐associated macrophages and suppresses neuroblastoma proliferation in vivo. Stem Cells 36 (6), 915–924. [DOI] [PubMed] [Google Scholar]
- 70.Song SY et al. (2020) Interleukin‐4 Gene Transfection and Spheroid Formation Potentiate Therapeutic Efficacy of Mesenchymal Stem Cells for Osteoarthritis. Advanced Healthcare Materials, 1901612. [DOI] [PubMed] [Google Scholar]
- 71.Gao P. et al. (2010) Therapeutic potential of human mesenchymal stem cells producing IL-12 in a mouse xenograft model of renal cell carcinoma. Cancer letters 290 (2), 157–166. [DOI] [PubMed] [Google Scholar]
- 72.Chen X. et al. (2008) A tumor-selective biotherapy with prolonged impact on established metastases based on cytokine gene-engineered MSCs. Molecular Therapy 16 (4), 749–756. [DOI] [PubMed] [Google Scholar]
- 73.Jing W. et al. (2014) Human Umbilical Cord Blood–Derived Mesenchymal Stem Cells Producing IL15 Eradicate Established Pancreatic Tumor in Syngeneic Mice. Molecular cancer therapeutics 13 (8), 2127–2137. [DOI] [PubMed] [Google Scholar]
- 74.Kim N. et al. (2015) IL-21-expressing mesenchymal stem cells prevent lethal B-cell lymphoma through efficient delivery of IL-21, which redirects the immune system to target the tumor. Stem cells and development 24 (23), 2808–2821. [DOI] [PubMed] [Google Scholar]
- 75.Jeon SH et al. (2018) Engineered mesenchymal stem cells expressing stromal cell-derived factor-1 improve erectile dysfunction in streptozotocin-induced diabetic rats. International journal of molecular sciences 19 (12), 3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lou G. et al. (2020) MiR-199a-modified exosomes from adipose tissue-derived mesenchymal stem cells improve hepatocellular carcinoma chemosensitivity through mTOR pathway. Journal of Experimental & Clinical Cancer Research 39 (1), 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kavari SL and Shah K. (2019) Concise Review: Engineered Stem Cells Targeting Multiple Cell Surface Receptors in Tumors. STEM CELLS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Du W. et al. (2017) Stem cell-released oncolytic herpes simplex virus has therapeutic efficacy in brain metastatic melanomas. Proceedings of the National Academy of Sciences 114 (30), E6157–E6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yoon A-R et al. (2019) Mesenchymal Stem Cell–Mediated Delivery of an Oncolytic Adenovirus Enhances Antitumor Efficacy in Hepatocellular Carcinoma. Cancer research 79 (17), 4503–4514. [DOI] [PubMed] [Google Scholar]
- 80.Moreno R. et al. (2019) Enhanced Antitumor Efficacy of Oncolytic Adenovirus–loaded Menstrual Blood–derived Mesenchymal Stem Cells in Combination with Peripheral Blood Mononuclear Cells. Molecular cancer therapeutics 18 (1), 127–138. [DOI] [PubMed] [Google Scholar]
- 81.Kaczorowski A. et al. (2016) Delivery of improved oncolytic adenoviruses by mesenchymal stromal cells for elimination of tumorigenic pancreatic cancer cells. Oncotarget 7 (8), 9046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Niess H. et al. (2015) Treatment of advanced gastrointestinal tumors with genetically modified autologous mesenchymal stromal cells (TREAT-ME1): study protocol of a phase I/II clinical trial. BMC cancer 15 (1), 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.von Einem JC et al. (2019) Treatment of advanced gastrointestinal cancer with genetically modified autologous mesenchymal stem cells: Results from the phase 1/2 TREAT‐ME‐1 trial. International journal of cancer 145 (6), 1538–1546. [DOI] [PubMed] [Google Scholar]
- 84.Chahal J. et al. (2019) Bone marrow mesenchymal stromal cell treatment in patients with osteoarthritis results in overall improvement in pain and symptoms and reduces synovial inflammation. Stem cells translational medicine 8 (8), 746–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Muroi K. et al. (2016) Bone marrow-derived mesenchymal stem cells (JR-031) for steroid-refractory grade III or IV acute graft-versus-host disease: a phase II/III study. International journal of hematology 103 (2), 243–250. [DOI] [PubMed] [Google Scholar]
- 86.Connick P. et al. (2012) Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study. The Lancet Neurology 11 (2), 150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim HS et al. (2017) Clinical trial of human umbilical cord blood‐derived stem cells for the treatment of moderate‐to‐severe atopic dermatitis: Phase I/IIa studies. Stem Cells 35 (1), 248–255. [DOI] [PubMed] [Google Scholar]
- 88.Ciccocioppo R. et al. (2011) Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn’s disease. Gut 60 (6), 788–798. [DOI] [PubMed] [Google Scholar]
- 89.Panés J. et al. (2016) Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial. The Lancet 388 (10051), 1281–1290. [DOI] [PubMed] [Google Scholar]
- 90.Herz J. et al. (2018) Interaction between hypothermia and delayed mesenchymal stem cell therapy in neonatal hypoxic-ischemic brain injury. Brain, behavior, and immunity 70, 118–130. [DOI] [PubMed] [Google Scholar]
- 91.Popielarczyk TL et al. (2019) Human Bone Marrow-Derived Mesenchymal Stem Cells Home via the PI3K-Akt, MAPK, and Jak/Stat Signaling Pathways in Response to Platelet-Derived Growth Factor. Stem cells and development 28 (17), 1191–1202. [DOI] [PubMed] [Google Scholar]
- 92.Shahror RA et al. (2019) Enhanced homing of mesenchymal stem cells overexpressing fibroblast growth factor 21 to injury site in a mouse model of traumatic brain injury. International journal of molecular sciences 20 (11), 2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Park S-R et al. (2019) A novel endogenous damage signal, CSF-2, activates multiple beneficial functions of adipose tissue-derived mesenchymal stem cells. Molecular Therapy 27 (6), 1087–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen M-S et al. (2018) IL-1β-Induced matrix metalloprotease-1 promotes mesenchymal stem cell migration via PAR1 and G-protein-coupled signaling pathway. Stem cells international 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Perets N. et al. (2019) Golden exosomes selectively target brain pathologies in neurodegenerative and neurodevelopmental disorders. Nano letters 19 (6), 3422–3431. [DOI] [PubMed] [Google Scholar]
- 96.Meng S-S et al. (2018) LincRNA-p21 promotes mesenchymal stem cell migration capacity and survival through hypoxic preconditioning. Stem cell research & therapy 9 (1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Antebi B. et al. (2019) Cryopreserved mesenchymal stem cells regain functional potency following a 24-h acclimation period. Journal of translational medicine 17 (1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Oja S. et al. (2019) The Utilization of Freezing Steps in Mesenchymal Stromal Cell (MSC) Manufacturing: Potential Impact on Quality and Cell Functionality Attributes. Frontiers in immunology 10, 1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhu Y. et al. (2017) Bi-specific molecule against EGFR and death receptors simultaneously targets proliferation and death pathways in tumors. Sci Rep 7 (1), 2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang X. et al. (2017) Mesenchymal stromal cells as vehicles of tetravalent bispecific Tandab (CD3/CD19) for the treatment of B cell lymphoma combined with IDO pathway inhibitor d-1-methyl-tryptophan. Journal of Hematology & Oncology 10 (1), 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu H. et al. (2006) The Immunogenicity and Immunomodulatory Function of Osteogenic Cells Differentiated from Mesenchymal Stem Cells. The Journal of Immunology 176 (5), 2864–2871. [DOI] [PubMed] [Google Scholar]
- 102.Isakova IA et al. (2014) Allo-Reactivity of Mesenchymal Stem Cells in Rhesus Macaques Is Dose and Haplotype Dependent and Limits Durable Cell Engraftment In Vivo. PLOS ONE 9 (1), e87238. [DOI] [PMC free article] [PubMed] [Google Scholar]