Abstract
Human induced pluripotent stem cell (hiPSC)-derived neurons can be exquisitely sensitive to botulinum neurotoxins (BoNTs), exceeding sensitivity of the traditionally used mouse bioassay. In this report, four defined hiPSC-derived neuronal populations including primarily GABAergic, glutamatergic, dopaminergic, and motor neurons were examined for BoNT/A, B, C, D, E, and F sensitivity. The data indicate that sensitivity varies markedly for the BoNTs tested. Motor neurons are significantly more sensitive than other neuron types for all BoNTs except BoNT/D. Examination of SNARE protein levels and BoNT-specific cell surface protein receptors reveal few differences between the cell types except greater expression levels of the receptor protein SV2C and synapsin-IIa in motor neurons. This indicates that differential toxicity of BoNTs for motor neurons compared to other neuronal cell types involves multiple mechanisms.
Keywords: botulinum toxin, botulinum neurotoxin, BoNT, neurons, cell-based assay, iPSC, hiPSC, human, motor-neurons
Introduction:
Stem cell technology, in particular human stem cells, has been transforming the fields of toxicology and pharmacology by providing non-cancerous human specific cell models for research applications and detection of biologic activity of toxins and pharmaceuticals [1–3]. While early studies were limited by controversies surrounding use of embryonic stem cells, discoveries leading to the ability to efficiently re-program adult human cells into human induced pluripotent stem cells (hiPSCs) [4–6] have led to an explosion in progress in this field. While it is still challenging to produce hiPSCs and differentiate them into the desired mature cell types, both hiPSCs and various differentiated hiPSC-derived mature cell types are now commercially available. This enables consistent applications of hiPSc-derived cells for many research purposes. However, this is still a young field and much research needs to be done to validate hiPSCs and hiPSC-derived differentiated cells as replacement of in vivo assays.
Botulinum neurotoxins (BoNTs) are a family of protein neurotoxins that comprise the most potent toxins known to humans and are the causative agent of botulism [7]. Seven serotypes of BoNTs have been described, A – G [8]. BoNTs are 150 kDa dichain proteins consisting of a 50 kDa Light chain (LC) linked via disulfide bond to a 100 kDa Heavy chain (HC) [9]. The HC is functionally and structurally divided into the N-terminal translocation domain (HCN) and the C-terminal receptor binding domain (HCC). BoNTs exert their effect by first associating with the neuronal cell surface via ganglioside and specific cell surface protein interaction of the HCC, leading to endocytosis [10]. In the acidic environment of the endosome, a conformational change leads to the incorporation of the HCN domain into the membrane and translocation of the LC into the cell cytosol [11]. In the cell cytosol, the disulfide bond between LC and HC is reduced [12,13], and the LC is refolded into its enzymatically active conformation and specifically cleaves neuronal SNARE proteins [14,15]. The SNARE cleavage leads to a block in neurotransmitter release. Since BoNTs primarily affect peripheral motor-neurons, blockage of acetylcholine release leads to muscle weakening and flaccid paralysis [16–18]. The apparent preference of BoNTs for motor-neurons has long been recognized based on clinical symptoms and symptoms in animal studies [7]. However, the mechanisms underlying this neuronal subtype preference is unknown, and may involve distribution of the BoNT inside the body, more rapid and enhanced cell entry into motor-neurons than into other neuronal cells, or greater activity inside motor-neurons. Studies using cultured neurons have indicated that BoNTs can enter all types of neuronal cells, cleave their intracellular SNAREs, and block neurotransmitter release [19]. Only a few in vivo studies have examined differential neuronal cell activities of BoNTs. These earlier studies indicate that fluorescently labeled BoNT/A and /B HCCs or radiolabeled BoNT/A or /B appeared to predominantly associate with cholinergic neurons after intestinal intoxication [20–23]. However, these studies did not assess differential cell entry and intracellular activity in different classes of neurons.
Due to their high potency and the severity of the disease botulism, BoNTs are a concern for human and vertebrate health, for food safety, and as potential bio-weapons. However, BoNTs are also widely used as important and unique bio-pharmaceuticals [24,25]. Holotoxin activity determination of BoNTs has traditionally been achieved using the well-established mouse bioassay [26]. In addition to the mouse bioassay, cell-based assays require all steps of cellular intoxication by BoNTs and thereby measure biologic activity of fully functional holotoxins [19]. During the past decade, the use of cell-based assays for BoNTs has increased in basic research and industry applications. Human iPSC-derived neurons are of particular interest as they are human specific models, non-cancerous, and have proven to be highly sensitive [19,27,28]. Now, the availability of high-quality individual classes of hiPSC-derived neurons from research laboratories and commercial companies has provided an opportunity to evaluate the action of BoNTs on individual neuron classes. This study evaluated four different commercially available and quality certified hiPSC-derived neuronal cell types for sensitivity to BoNT/A, B, C, D, E, and F. Our results show that the sensitivity of the isolated neurons and potency of BoNTs varied markedly for the BoNTs tested. Motor Neurons were the most sensitive cell model for all BoNT serotypes tested except BoNT/D, greatly exceeding the sensitivity of the mouse bioassay by up to 160-fold, depending on the serotype.
Materials and Methods:
Biosafety and biosecurity.
The Johnson laboratory and personnel are registered with the Federal Select Agent Program for research involving botulinum neurotoxins (BoNT) and BoNT-producing strains of clostridia. The research program, procedures, documentation, security, and facilities are monitored by the University of Wisconsin-Madison Biosecurity Task Force, the University of Wisconsin-Madison Office of Biological Safety, the University of Wisconsin Select Agent Program, and the Centers for Disease Control and Prevention (CDC) as part of the University of Wisconsin-Madison Select Agent Program. Personnel have undergone suitability assessments and completed rigorous and continuing biosafety training, including biosafety level 3 (BSL3) or BSL2 and select agent practices, before participating in laboratory studies involving BoNTs and neurotoxigenic C. botulinum. All animal experiments have been approved by the University of Wisconsin IACUC.
Botulinum neurotoxins.
BoNTs /A1, /B1, C1, D1, E3, and F1 were purified from C. botulinum strains Hall A-hyper, Okra B, Brazil C, 1873 (D), E 43, and Langeland F as previously described [29–31]. The purity of the toxins was confirmed by spectroscopy and SDS-PAGE as previously published [30], and toxins were sterile filtered using a 0.2 micron filter and stored in 0.01 M sodium phosphate buffer, pH 7 with 40% glycerol at −20°C until use. The specific activity of each BoNT subtype preparation was determined by the intraperitoneal mouse bioassay (MBA) [26,32] and was 5.6 pg/LD50 (A1), 6 pg/LD50 (B1), 160 pg/LD50 (C1), 7.1 pg/LD50 (D1) 61 pg/LD50 (E3), and 72 pg/LD50 (F1).
Neuronal Cell Cultures.
Four different cryopreserved hiPSC-derived neuronal cell models were purchased from Fujifilm Cellular Dynamics. The cells included iCell GABA Neurons (previously described as iCell Neurons [28]), Dopa Neurons, Gluta Neurons, and Motor Neurons. The manufacturer describes the GABA Neurons as >95 % pure population of primarily GABA-ergic neurons, the Dopa Neurons as fully differentiated >80 % pure midbrain dopaminergic neurons, the Gluta Neurons as >90 % pure population of primarily glutamatergic neurons, and the Motor Neurons as fully differentiated >87 % pure population of primarily (>80 %) cholinergic neurons after 14 days in culture. All cells were seeded and fed as recommended by the manufacturer using the media supplied with the cells or, in the case of Gluta Neurons, recommended for the cells. Cells were cultured for 14 days (14 DIV) prior to imaging and to the BoNT assays. For imaging, the cells were grown in a tissue culture treated and coated (as per manufacturer’s instructions for each cell type) 8-well ibidi µ slides for 14 days and stained with CellTracker Green CMFDA. Images were obtained by fluorescent microscopy using an EVOS Auto FL2 scope with the GFP filter.
Cell Based Assay.
For the cell based assay, the four hiPSC-derived neuronal cell models at 14 DIV were exposed to the indicated concentrations of BoNTs in 50 μl of each respective neuronal medium in parallel. To avoid variations in toxin dilutions, BoNTs were first diluted in culture media of GABA Neurons to an about 20-x concentrated working stock common for all cell types, and then further diluted by serial dilution in the respective culture media of each cell type. All samples were tested in triplicate and a negative control without toxin was always included. After a 48 h exposure time, the toxin solution was removed, and cells were lysed in 50 μl of 1× lithium dodecyl sulfate (LDS) sample buffer (Life Technologies). The cell lysates were analyzed by Western blot for SNAP-25 or VAMP2/VAMP1 cleavage as previously described [28,33,34]. Cleaved and uncleaved SNAP-25 bands were quantified by densitometry using a Foto/Analyst FX system and TotalLab Quant software (Fotodyne). For detection of VAMP cleavage, relative levels of VAMP compared to syntaxin (loading control) were analyzed, as the VAMP cleavage products are rapidly degraded in cells. Data plots and EC50 values or estimates were generated in GraphPad PRISM 6 software using a non-linear four parameter curve fit. For the ganglioside pre-treatment assay, cells were exposed for 24 h to their respective culture media containing 100 µg/ml ganglioside GT1b (Sigma) and 75 µg/ml ganglioside GM1 (SIGMA). The toxin exposure assay was then conducted as described above, in parallel in ganglioside pre-treated and not pre-treated cells.
Receptor and SNARE expression analysis.
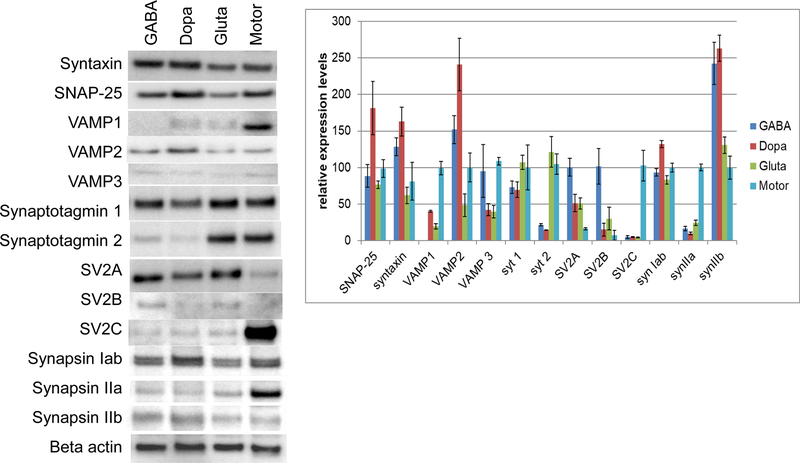
For the receptor expression analysis, cell lysates from each cell type not exposed to BoNT were used. All samples were analyzed in triplicate. Cell lysates were analyzed by Western blot for the expression of SV2A, B, and C isoforms, synaptotagmin I and II, SNAP-25, VAMP1, 2, and 3 isoforms, and syntaxin I using monoclonal antibodies from Synaptic Systems, Göttingen, Germany (SV2A, SV2B, SNAP-25, synaptotagmin I, synaptotagmin II, syntaxin,VAMP2) or Abcam, US (VAMP1, VAMP3, beta actin). Beta-actin was used as a loading control. The SV2C antibody [35] was generously provided by Roger Janz through Synaptic Systems. Western blots were imaged using the PhosphaGlo chemiluminescent substrate (KPL) and a Foto/Analyst FX system. Bands were quantified relative to the loading control (beta actin) by densitometry using TotalLab Quant software. For comparative quantification of expression of each protein in the four cell types, the expression levels of each protein in Motor Neurons was set to 100 %, with the exception of SV2A and B, which was expressed at only barely detectable levels in Motor Neurons and thus was adjusted to 100 % expression in GABA Neurons. Average values and standard deviations were determined and bar graphs generated using Microsoft excel.
Results:
HiPSC-derived neuronal cell classes vary in their sensitivity to BoNTs.
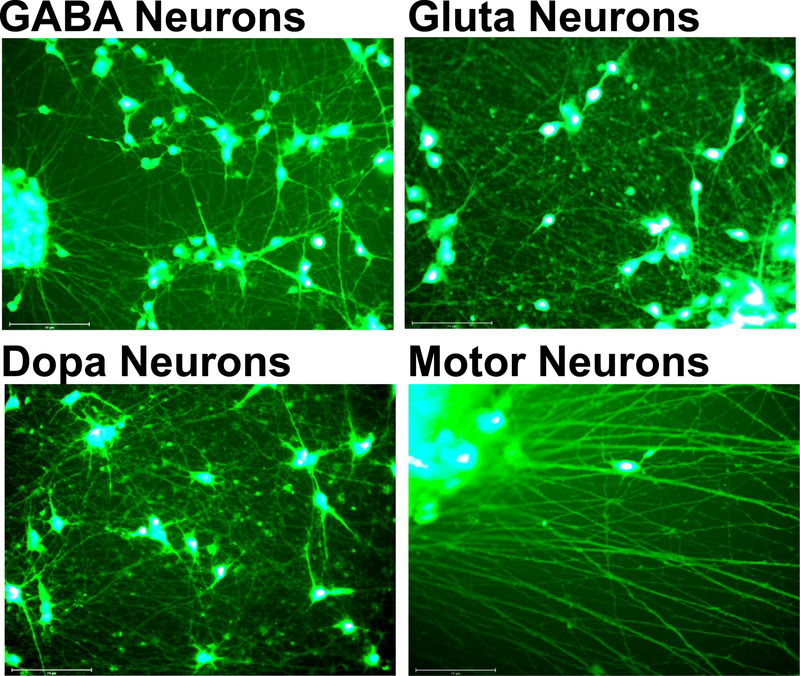
The four hiPSC- derived neuronal cell models, GABA Neuron, Dopa Neurons, Gluta Neurons, and Motor Neurons, had markedly different morphologies after 14 days in vitro (DIV) (Figure 1). In particular, it was apparent that the Motor Neurons displayed longer and more networked neurites than the other cell types and required very gentle media changes to maintain and expose the Motor Neurons to BoNTs without disturbing the extensive neurite network or detaching the cells.
Figure 1.
Human induced pluripotent stem cell (hiPSC)-derived neuronal cell models used in this study at day 14 in culture. Cells were stained with CellTracker Green CMFDA, and images were obained by fluorescent microscopy using an EVOS Aufto FL2 scope with the GFP filter. The size-bar is 75 µm.
Exposure of the four cell models to serial dilutions of BoNT/A, /B, /C, /D, /E, or /F resulted in varying patterns of SNARE cleavage (Figure 2). EC50 values for each BoNT in each cell type were either determined from the dose response curve or estimated based on projected dose response curves of densitometry data of the shown Western blots (Table 1). For all BoNTs tested except for BoNT/D1, motor-neurons were the most sensitive cell model, with estimated EC50 values of 0.006 mouse LD50 U/ well (U/well) for BoNT/A and BoNT/F, 0.008 U/well for BoNT/C, 0.02 U/well for BoNT/E, and 0.1 U/well for BoNT/B. The second most sensitive cell model was GABA Neurons, with estimated EC50 values of 0.2 U for BoNT/A, 0.4 U / well for BoNT/C, 0.9 U/well for BoNT/E, and 1.8 and 1.7 U/well for BoNT/B and/F, respectively. Sensitivity of Dopa Neurons and Gluta Neurons was lower for all tested BoNT types and also markedly varied between the toxins. Sensitivity of Dopa Neurons was ~3–4 U/well for BoNT/A, /C, /E, and /F, whereas it was ~210 U / well for BoNT/B. In contrast, sensitivity of Gluta Neurons was ~ 4 U/well for BoNT/C, ~7 U/well for BoNT/A and /B, and 14 and 32 U/well for BoNT/F and /E, respectively.
Figure 2.
SNARE cleavage Western blots showing cell type and BoNT type specificity. Neuronal cell populations were grown for 14 days, and exposed to BoNT/A, /B, /E, or /F for 48 h in parallel. Cell lysates were analyzed for SNARE cleavage by Western blot using an anti SNAP-25 antibody that recognizes both BoNT/A or /E cleaved and uncleaved SNAP-25 and an anti-VAMP2 antibody which shows only intact uncleaved VAMP2, with syntaxin used as a loading control. All samples were tested in triplicate and one representative Western blot of each is shown. The type of neurons is indicated by name supplied by the manufacturer (Fujifilm CDI).
Table 1 :
Estimated EC50 values
| EC50 [U/well] | BoNT/A1 | BoNT/B1 | BoNT/C1 | BoNT/D1 | BoNT/E3 | BoNT/F1 |
|---|---|---|---|---|---|---|
| GABA Neurons | 0.2 | 1.8 | 0.4 | 2.2 | 0.9 | 1.7 |
| Dopa Neurons | 4.6 | 209 | 4.3 | 2.0 | 3.6 | 3.6 |
| Gluta Neurons | 9.6 | 7.2 | 3.9 | 1.0 | 32 | 14.2 |
| Motor-neurons | 0.006 | 0.1 | 0.008 | 17.3 | 0.02 | 0.006 |
BoNT/D was different from all other BoNT serotypes in that it had a markedly lower potency in Motor Neurons, with an EC50 of ~ 15 U / well. Thus, BoNT/D was almost 200-fold less potent than BoNT/B and ~2,500 fold less potent than BoNT/A and /F in human Motor Neurons. In addition, while all other BoNTs were most potent in Motor Neurons, BoNT/D was least potent in Motor Neurons, with EC50s in the GABA, Dopa, and Gluta Neurons of about 1–3 U /well. Finally, unlike all other BoNTs, BoNT/D was most potent in Gluta Neurons compared to the other neuron classes, with an EC50 of ~ 1 U compared to ~15 U in Motor Neurons and ~ 2 U in GABA and Dopa Neurons.
Expression levels of SNARE proteins and BoNT protein receptors vary amongst the cell models.
In an effort to discern whether SNARE protein expression levels or protein receptor expression levels might be a determining factor in sensitivity of the four cell models, Western blot analysis was used to compare protein levels involved in BoNT toxicity (Figure 3). All cell types expressed a full set of SNARE proteins with only relatively mild variations in levels of SNAP-25, syntaxin, and VAMP2. However, VAMP1 was expressed predominantly in Motor Neurons, with no detectable levels in GABA Neurons and barely detectable levels in Dopa and Gluta Neurons. VAMP3 was detected at very low levels only in all cell types. Synaptotagmin 1 and 2 are the protein receptor for BoNT/B [36–39], whereas SV2 A, B, and C are the protein receptors for BoNT/A, E, and F [10,40–48]. While synaptotagmin 1 was detected at high levels in all four cell types with only minor differences in expression levels, synaptotagmin 2 was detected primarily in Gluta Neurons and Motor Neurons at similar levels and at just detectable levels in GABA and Dopa Neurons. SV2A was detected at similar levels in GABA, Dopa, and Gluta Neurons, and at about 10-fold lower levels in Motor Neurons. SV2B bands were observed just at the detection level by Western blot in all four cell types, while SV2C was expressed at much higher levels in Motor Neurons than in the other cell types. Since the relative patterns of expression levels of protein receptors and SNARE proteins alone cannot explain the differences in BoNT sensitivity of the four cell models analyzed in this study, synapsin I a/b and synapsin II a and b levels were also analyzed as a marker of active synaptic vesicles. Synapsin I a and b were expressed at similar levels in all cell types; however, a markedly stronger band of synapsin IIa was detected in Motor Neurons, whereas the synapsin IIb was increased in GABA and Dopa Neurons.
Figure 3.
SNARE protein and BoNT protein receptor protein levels in the four hiPSC-derived cell models. Neuronal cell populations were grown for 14 days, and cell lysates analyzed for the indicated proteins by Western blot. Beta-actin was used as a loading control. The left panel shows a representative blot of triplicate samples. The graph shows a relative depiction of average and standard deviations of the triplicate samples comparing expression levels of each protein between the four cell populations. Protein bands were quantified by densitometry relative to the beta-actin loading control. Protein levels were then set to 100 % for motor-neurons, except for SV2A and B, which were detected only at very low levels in motor-neurons and were adjusted to 100 % in GABA Neurons.
In order to determine whether ganglioside expression levels may be involved in differential BoNT sensitivity of the various neuronal cell types, cells were either pre-treated or not pre-treated with 100 µg / ml GT1b and 75 µg / ml GM1 for 24 h prior to BoNT/A1 exposure. Polylialylated gangliosides like GT1b have previously beedn shown to be the preferred ganglioside receptor for BoNT/A1 [10,49,50]. While the ganglioside treatment did not affect the morphology of Motor Neurons and Gluta Neurons, the Dopa Neurons and GABA Neurons showed signs of cytotoxicity with cell rounding and partial detachment. BoNT/A1 sensitivity was similar or slightly decreased in all ganglioside pre-treated cells (data not shown), indicating that ganglioside pre-treatment does not increase BoNT sensitivity in any of the tested hiPSC-derived neuronal cell types.
BoNT/D cleaves VAMP2 but not VAMP1 in the hiPSC-derived Motor Neurons.
For the BoNTs cleaving VAMP (B, D, and F), both VAMP2 and VAMP1 cleavage were analyzed in the hiPSC-derived Motor Neurons, which was the only cell model that expressed both isoforms at levels detectable by Western blot. While VAMP1 cleavage was similar to VAMP2 cleavage for BoNT/B and BoNT/F (data not shown), BoNT/D cleaved VAMP2 but not VAMP1 (Fig. 4). Only a small decrease in the VAMP1 band was observed at the highest toxin concentration (13,000 U / well, 1.2 nM), while VAMP2 was cleaved with an EC50 of ~15 U (13 pM) (Fig. 4). Thus, intracellular BoNT/D LC cleaves human VAMP1 over 1,000-fold less efficiently than human VAMP2.
Figure 4.
Western blots showing VAMP1 and VAMP2 cleavage by BoNT/D in human Motor Neurons. Motor Neurons were grown for 14 days, and exposed to BoNT/D for 48 h. The same cell lysates were analyzed for VAMP1 and VAMP2 cleavage by Western blot. Syntaxin was used as a loading standard. All samples were tested in triplicate and one representative Western blot of each is shown.
Discussion:
Botulism is characterized by descending flaccid paralysis, which results from a block in acetylcholine release at the neuromuscular junction of peripheral motor-neurons. This inhibition of neurotransmitter release is due to BoNTs gaining access into the cell cytosol of motor- neurons and the LC intracellular enzymatic cleavage of SNARE proteins that are essential in neurotransmitter release. Based on the symptoms of botulism and after local injection of BoNTs in the clinic, motor-neurons specific cell entry has mainly been assumed. However, it has been recognized that BoNTs can enter all neuron types including brain neurons, sensory neurons, nociceptors, pain fibers, and inhibitory neurons [19,23,51–54]. This recognition of diversity in BoNT on nerves has led to increased pharmacologic use of BoNTs, including somato-sensory disorders and pain [17,24,25,55].
BoNTs comprise a large family of protein toxins, with seven neurotoxic serotypes and several subtypes as well as chimeric toxins [8]. While all BoNTs cause botulism, variation in potency, clinical presentation, duration of action, species specificity, and cell entry properties vary for different BoNT serotypes [7,17,31,56–64]. These variations could be due to differences in LC activity, specific cell association, or entry of the LC into the cell’s cytosol and intracellular trafficking. Relatively little research has been done on differential neuronal cell entry. Fluorescently labeled BoNT/A and /B HCCs or radiolabeled BoNT/A or /B holotoxins have been shown to predominantly associate with cholinergic neurons [20–22] after in vivo intoxication of mice or in vitro in vertebrate tissues [23,52]. Cell based assays have shown wide variability in sensitivity of various cell models, with primary neuronal cell models and stem cell-derived neurons being the most sensitive [19].
This study for the first time compared sensitivities of defined hiPSC-derived neuronal cell populations to BoNTs. The results from these studies showed significantly greater sensitivity of Motor Neurons for all BoNTs tested except for BoNT/D (Figure 2, Table 1), which is consistent with in vivo and in vitro cell association studies for BoNT/A and /B [23,52] [20–22]. Interestingly, there were differences in Motor Neurons potency between the BoNT serotypes tested, with BoNT/A1 and BoNT/F1 being the most potent, followed by BoNT/C1, BoNT/E3, and with BoNT/B1 and BoNT/D being the least potent (Figure 2, Table 1). The relatively lower potency of BoNT/B1 is consistent with previous observations of lower sensitivity of humans to BoNT/B1 due to lower affinity binding to the human synaptotagmin II receptor [36,37]. While the motor-neurons tested here express both synaptotagmin I and II at high levels, synaptotagmin I is also not a high affinity receptor for BoNT/B1 [39]. BoNT/A, /D, /E, and /F all use SV2 isoforms (A, B, C) as their neuronal cell surface protein receptors [10], with data indicating that SV2C is the highest affinity receptor for BoNT/A [40–43,47,48,65], SV2A and SV2B for BoNT/E [46], while the preferred isoforms for BoNT/F and BoNT/D are unknown [45,66,67]. The motor-neurons tested in this study expressed predominantly the SV2C isoform, whereas little SV2C was detected in the other cell models, which appeared to predominantly express SV2A (Figure 3). The greater expression levels of SV2C in motor-neurons is consistent with previous reports of SV2C expression being restricted to evolutionary older brain regions and motor-neurons [35], and might account for the greater sensitivity of BoNT/A and /F in this cell model. However, the greater sensitivity of BoNT/E in these cells is not consistent with previous data suggesting that the SV2A and B isoforms are the predominant protein receptors for BoNT/E [46]. The data presented here raise the possibility that SV2C may be able to substitute as a receptor for BoNT/E. Studies on the binding of BoNT/A and /E HCC fragments indicates parallel binding and cell entry mechanisms into motor-neurons [68].
Our finding that the inhibitory GABA-ergic enriched neuron population was more sensitive than the excitatory glutamatergic enriched population to all BoNTs except BoNT/D differs from earlier findings that indicate that BoNT/A and /E inhibit release from excitatory neurons more efficiently than from GABAergic neurons [69–73]. These observations may be due to low levels or absence of SNAP-25 expression as well as to (SNAP-25 dependent) higher evoked calcium transients in mature GABAergic neurons [71,72,74,75]. Other studies have indicated inhibition of release from GABAergic neurons in the central nervous system after direct delivery of BoNT/A [73], and preferential cell entry and silencing of GABAergic neurons in a mixed human stem cell-derived population containing both GABAergic and glutamatergic neurons [76]. The latter observation may be due to the high SNAP-25 expression levels in the hiPSC-derived GABAergic neurons used in this and our study. Taken together these data indicate that BoNTs can efficiently enter GABAergic inhibitory neurons and that factors other than protein receptor levels affect BoNT sensitivity and inhibition of release in various neuronal cell types. In our study, SNARE protein expression levels were similar in all cell models, with the exception of VAMP1, which was expressed primarily in Motor Neurons and was either not detectable (GABA Neurons) or at the detection limit (Dopa and Gluta Neurons) in the other cell models (Figure 3). Synapsin Ia/b was about equally expressed in all four cell types, indicating the general presence of synaptic vesicles. However, the markedly higher levels of synapsin IIa in motor-neurons, which is the only synapsin isoform shown to rescue depression in synaptic vesicle reserve pools and reported to be responsible for controlling the size of the synaptic vesicle reserve pool [77], may suggest a denser synaptic vesicle pool in motor-neurons. Other factors that affect BoNT sensitivity might include different ganglioside expression profiles, differential glycosylation of cell surface proteins, cell maturity and differentiation, purity of the cell population, synaptic activity, overall cell health, and other factors. Pre-exposure of the four neuronal cell types tested in this study to poly-sialogangliosides GT1b and GM1 for 24 h prior to the BoNT/A1 assay resulted in similar sensitivity, indicating that neuronal cell surface ganglioside levels are not a critical factor in BoNT sensitivity in these cell models. However, while Motor Neurons and Gluta Neurons appeared unaffected by ganglioside exposure, the significant morphological changes of Dopa Neurons and GABA Neurons upon ganglioside exposure indicate cytotoxicity as previously reported for dopaminergic neurons [78,79], which could also affect toxin uptake. Thus, a role of gangliosides in the differential neuronal susceptibility to the various BoNTs cannot be entirely excluded.
An important observation is the differential sensitivities of the four neuronal cell populations for the six BoNT types examined (Figure 2, Table 1). For example, while BoNT/A1 potency in Motor Neurons was ~ 30-fold greater than in GABA Neurons, ~550-fold greater than in Dopa Neurons, and ~1200-fold greater than in Gluta Neurons, potency of BoNT/B1 in Motor Neurons was ~20 fold greater than in GABA Neurons, yet ~2000-fold greater than in Dopa Neurons, and ~ 70-fold greater than in Gluta Neurons. Relative potencies of BoNT/C and BoNT/E in the four neuronal cultures were fairly similar to BoNT/A. Strikingly, BoNT/F1 potency in Motor Neurons was over 280-fold greater than in the other three cell populations. BoNT/D potency in Motor Neurons was lower than in any of the other neuronal cultures and ~2400-fold lower than potency of BoNT/A1, C1, and /F1. Instead, BoNT/D was more potent than the other BoNT serotypes in Gluta Neurons. Together, these data indicate that in addition to cell-specific factors, BoNT specific properties guide the sensitivity of each cell model. This is not surprising considering the structural differences between the BoNT serotypes and further emphasizes serotype specific functional properties of BoNTs that with further research have the potential to lead to novel pharmaceuticals with specific defined properties. Such differences between the many subtypes of BoNTs remain to be elucidated.
BoNT/D has previously been found to cleave human VAMP1 inefficiently, while strongly cleaving human VAMP2 [80–83] and to be poorly effective in humans in inducing muscle paralysis [60]. In this study we confirmed the lower efficiency of VAMP1 cleavage compared to VAMP2 cleavage for the first time within the same neuronal cell model expressing both VAMP1 and VAMP2 (Figure 4). In addition, the less efficient intracellular VAMP2 cleavage also indicates lower cell entry into human neurons compared to other BoNTs, particularly into motor-neurons, which is consistent with a previous report [31]. However, in this report we also found greater potency of BoNT/D in a culture of primarily human glutamatergic neurons compared to other BoNTs. Together these data suggest a unique cell entry pathway by BoNT/D into human neurons.
In summary, the presented data show neuronal cell type-specific and BoNT serotype-specific properties affect sensitivity of the four cell models. The study provides a useful model for determination of the mechanisms affecting BoNT sensitivity for different classes of neurons. The hiPSC-derived Motor Neuron cell model analyzed in this study was by far the most sensitive cell model for all BoNTs examined except BoNT/D; however, the specific mechanisms of the increased sensitivity remains to be shown. These data highlight the importance of considering both the cell source and characteristics and the BoNT type analyzed in cell based studies, and demonstrates the research potential of hiPSC-derived neuronal cell models. Further mechanistic studies determining rate-limiting factors driving sensitivity of BoNTs in defined neuronal cell models, combined with in vivo studies that consider pharmacodynamics, have the potential to dramatically increase our understanding of the biologic properties of the various BoNTs, recombinant derivatives, and novel BoNTs, including mechanisms of botulism and a wider diversity of medical applications.
Acknowledgements:
This work was supported by the National Institute of Allergy and Infectious Diseases R01 AI139306, R56 AI095274. The SV2C antibody [35] was generously provided by Roger Janz through Synaptic Systems. The authors would like to acknowledge the University of Wisconsin Madison Biosecurity Task Force for helping to ensure compliance with Select Agent Regulations.
Abbreviations:
- hiPSC
human induced pluripotent cells
- BoNT
botulinum neurotoxin
- SNARE proteins
Soluble N-ethylmaleimide-sensitive factor attachment protein receptor proteins
- SV2
synaptic vesicle protein
- SNAP-25
synaptosomal nerve-associated protein 25
- VAMP
vesicle associated membrane proteins
References:
- [1].Kim TW, Che JH and Yun JW (2019). Use of stem cells as alternative methods to animal experimentation in predictive toxicology. Regul Toxicol Pharmacol, 105:15–29. [DOI] [PubMed] [Google Scholar]
- [2].Little D, Ketteler R, Gissen P and Devine MJ (2019). Using stem cell-derived neurons in drug screening for neurological diseases. Neurobiol Aging 78, 130–141. [DOI] [PubMed] [Google Scholar]
- [3].Bordoni M, Rey F, Fantini V, Pansarasa O, Di Giulio AM, Carelli S and Cereda C (2018). From Neuronal Differentiation of iPSCs to 3D Neuro-Organoids: Modelling and Therapy of Neurodegenerative Diseases. Int J Mol Sci 19, pii: E3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yu J and Thomson JA (2008). Pluripotent stem cell lines. Genes Dev 22, 1987–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K and Yamanaka S (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872. [DOI] [PubMed] [Google Scholar]
- [6].Yu J et al. (2007). Induced pluripotent stem cell lines derived from human somatic cells. Science (New York, N.Y.) 318, 1917–1920. [DOI] [PubMed] [Google Scholar]
- [7].Johnson EA and Montecucco C (2008) Chapter 11 Botulism. In Handbook of Clinical Neurology (Andrew GE, ed.), pp. 333–368. Elsevier. [DOI] [PubMed] [Google Scholar]
- [8].Peck MW et al. (2017). Historical Perspectives and Guidelines for Botulinum Neurotoxin Subtype Nomenclature. Toxins (Basel) 9, pii: E38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Montal M (2010). Botulinum Neurotoxin: A Marvel of Protein Design. Annual Review of Biochemistry 79, 591–617. [DOI] [PubMed] [Google Scholar]
- [10].Rummel A (2013). Double receptor anchorage of botulinum neurotoxins accounts for their exquisite neurospecificity. Curr Top Microbiol Immunol 364, 61–90. [DOI] [PubMed] [Google Scholar]
- [11].Fischer A et al. (2009). Bimodal modulation of the botulinum neurotoxin protein-conducting channel. Proceedings of the National Academy of Sciences of the United States of America 106, 1330–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pirazzini M, Azarnia Tehran D, Zanetti G, Rossetto O and Montecucco C (2018). Hsp90 and Thioredoxin-Thioredoxin Reductase enable the catalytic activity of Clostridial neurotoxins inside nerve terminals. Toxicon 147, 32–37. [DOI] [PubMed] [Google Scholar]
- [13].Pirazzini M, Azarnia Tehran D, Zanetti G, Lista F, Binz T, Shone CC, Rossetto O and Montecucco C (2015). The thioredoxin reductase--Thioredoxin redox system cleaves the interchain disulphide bond of botulinum neurotoxins on the cytosolic surface of synaptic vesicles. Toxicon 107, 32–6. [DOI] [PubMed] [Google Scholar]
- [14].Schiavo G, Matteoli M and Montecucco C (2000). Neurotoxins affecting neuroexocytosis. Physiological Reviews 80, 717–766. [DOI] [PubMed] [Google Scholar]
- [15].Schiavo G, Rossetto O, Tonello F and Montecucco C (1995). Intracellular targets and metalloprotease activity of tetanus and botulism neurotoxins. Current topics in microbiology and immunology 195, 257–274. [DOI] [PubMed] [Google Scholar]
- [16].Pellett S (2015) Pathogenesis of Clostridium botulinum in Humans. In Human Emerging and Re-emerging Infections (Singh SK, ed.), pp. 821–839. John Wiley & Sons, Inc. [Google Scholar]
- [17].Pirazzini M, Rossetto O, Eleopra R and Montecucco C (2017). Botulinum Neurotoxins: Biology, Pharmacology, and Toxicology. Pharmacol Rev 69, 200–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pantano S and Montecucco C (2014). The blockade of the neurotransmitter release apparatus by botulinum neurotoxins. Cell Mol Life Sci 71, 793–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pellett S (2013). Progress in cell based assays for botulinum neurotoxin detection. Curr Top Microbiol Immunol 364, 257–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fujinaga Y and Popoff MR (2018). Translocation and dissemination of botulinum neurotoxin from the intestinal tract. Toxicon 147, 13–18. [DOI] [PubMed] [Google Scholar]
- [21].Connan C et al. (2016). Translocation and dissemination to target neurons of botulinum neurotoxin type B in the mouse intestinal wall. Cell Microbiol 18, 282–301. [DOI] [PubMed] [Google Scholar]
- [22].Couesnon A, Molgo J, Connan C and Popoff MR (2012). Preferential entry of botulinum neurotoxin A Hc domain through intestinal crypt cells and targeting to cholinergic neurons of the mouse intestine. PLoS Pathog 8, e1002583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Black JD and Dolly JO (1987). Selective location of acceptors for botulinum neurotoxin A in the central and peripheral nervous systems. Neuroscience 23, 767–79. [DOI] [PubMed] [Google Scholar]
- [24].Dressler D (2016). Botulinum toxin drugs: brief history and outlook. J Neural Transm, 123, 277–9. [DOI] [PubMed] [Google Scholar]
- [25].Dressler D (2012) Clinical applications of botulinum toxin. In Curr Opin Microbiol, pp. 325–36, England. [DOI] [PubMed] [Google Scholar]
- [26].Schantz EJ and Kautter DA (1978). Standardized assay for Clostridium botulinum toxins. Journal - Association of Official Analytical Chemists 61, 96–99. [Google Scholar]
- [27].Pellett S et al. (2015). Human Induced Pluripotent Stem Cell Derived Neuronal Cells Cultured on Chemically-Defined Hydrogels for Sensitive In Vitro Detection of Botulinum Neurotoxin. Sci Rep 5, 14566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Whitemarsh RC, Strathman MJ, Chase LG, Stankewicz C, Tepp WH, Johnson EA and Pellett S (2012). Novel application of human neurons derived from induced pluripotent stem cells for highly sensitive botulinum neurotoxin detection. Toxicol Sci 126, 426–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Malizio CJ, Goodnough MC and Johnson EA (2000). Purification of Clostridium botulinum type A neurotoxin. Methods in molecular biology (Clifton, N.J.) 145, 27–39. [DOI] [PubMed] [Google Scholar]
- [30].Prabakaran S, Tepp W and DasGupta BR (2001). Botulinum neurotoxin types B and E: purification, limited proteolysis by endoproteinase Glu-C and pepsin, and comparison of their identified cleaved sites relative to the three-dimensional structure of type A neurotoxin. Toxicon : official journal of the International Society on Toxinology 39, 1515–1531. [DOI] [PubMed] [Google Scholar]
- [31].Pellett S, Tepp WH, Scherf JM, Pier CL and Johnson EA (2015). Activity of botulinum neurotoxin type D (strain 1873) in human neurons. Toxicon 101, 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hatheway CL (1988) Botulism. In Laboratory diagnosis of infectious diseases: principles and practice (Balows A, Hausler WH, Ohashi M and Turano MA, eds), pp. 111–133. Springer-Verlag, New York. [Google Scholar]
- [33].Pellett S, Tepp WH, Toth SI and Johnson EA (2010). Comparison of the primary rat spinal cord cell (RSC) assay and the mouse bioassay for botulinum neurotoxin type A potency determination. Journal of pharmacological and toxicological methods 61, 304–310. [DOI] [PubMed] [Google Scholar]
- [34].Pellett S, Tepp WH, Clancy CM, Borodic GE and Johnson EA (2007). A neuronal cell-based botulinum neurotoxin assay for highly sensitive and specific detection of neutralizing serum antibodies. FEBS letters 581, 4803–4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Janz R and Sudhof TC (1999). SV2C is a synaptic vesicle protein with an unusually restricted localization: anatomy of a synaptic vesicle protein family. Neuroscience 94, 1279–90. [DOI] [PubMed] [Google Scholar]
- [36].Peng L, Berntsson RP, Tepp WH, Pitkin RM, Johnson EA, Stenmark P and Dong M (2012). Botulinum neurotoxin D-C uses synaptotagmin I/II as receptors and human synaptotagmin II is not an effective receptor for type B, D-C, and G toxins. Journal of Cell Science 125, 3233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Strotmeier J, Willjes G, Binz T and Rummel A (2012). Human synaptotagmin-II is not a high affinity receptor for botulinum neurotoxin B and G: increased therapeutic dosage and immunogenicity. FEBS letters 586, 310–313. [DOI] [PubMed] [Google Scholar]
- [38].Rummel A et al. (2007). Identification of the protein receptor binding site of botulinum neurotoxins B and G proves the double-receptor concept. Proceedings of the National Academy of Sciences of the United States of America 104, 359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Dong M, Richards DA, Goodnough MC, Tepp WH, Johnson EA and Chapman ER (2003). Synaptotagmins I and II mediate entry of botulinum neurotoxin B into cells. The Journal of cell biology 162, 1293–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Benoit RM, Scharer MA, Wieser MM, Li X, Frey D and Kammerer RA (2017). Crystal structure of the BoNT/A2 receptor-binding domain in complex with the luminal domain of its neuronal receptor SV2C. Sci Rep 7, 43588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Weisemann J, Stern D, Mahrhold S, Dorner BG and Rummel A (2016). Botulinum Neurotoxin Serotype A Recognizes Its Protein Receptor SV2 by a Different Mechanism than Botulinum Neurotoxin B Synaptotagmin. Toxins (Basel) 8, pii: E 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Yao G et al. (2016). N-linked glycosylation of SV2 is required for binding and uptake of botulinum neurotoxin A. Nat Struct Mol Biol 23, 656–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Benoit RM et al. (2014). Structural basis for recognition of synaptic vesicle protein 2C by botulinum neurotoxin A. Nature 505, 108–11. [DOI] [PubMed] [Google Scholar]
- [44].Strotmeier J, Mahrhold S, Krez N, Janzen C, Lou J, Marks JD, Binz T and Rummel A (2014). Identification of the synaptic vesicle glycoprotein 2 receptor binding site in botulinum neurotoxin A. FEBS Lett 588, 1087–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Fu Z, Chen C, Barbieri JT, Kim JJ and Baldwin MR (2009). Glycosylated SV2 and gangliosides as dual receptors for botulinum neurotoxin serotype F. Biochemistry 48, 5631–5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Dong M, Liu H, Tepp WH, Johnson EA, Janz R and Chapman ER (2008). Glycosylated SV2A and SV2B mediate the entry of botulinum neurotoxin E into neurons. Molecular biology of the cell 19, 5226–5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Dong M, Yeh F, Tepp WH, Dean C, Johnson EA, Janz R and Chapman ER (2006). SV2 is the protein receptor for botulinum neurotoxin A. Science (New York, N.Y.) 312, 592–596. [DOI] [PubMed] [Google Scholar]
- [48].Mahrhold S, Rummel A, Bigalke H, Davletov B and Binz T (2006). The synaptic vesicle protein 2C mediates the uptake of botulinum neurotoxin A into phrenic nerves. FEBS letters 580, 2011–2014. [DOI] [PubMed] [Google Scholar]
- [49].Brunger AT and Rummel A (2009). Receptor and substrate interactions of clostridial neurotoxins. Toxicon : official journal of the International Society on Toxinology 54, 550–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Yowler BC, Kensinger RD and Schengrund CL (2002). Botulinum neurotoxin A activity is dependent upon the presence of specific gangliosides in neuroblastoma cells expressing synaptotagmin I. The Journal of biological chemistry 277, 32815–32819. [DOI] [PubMed] [Google Scholar]
- [51].Aoki KR (2003). Evidence for antinociceptive activity of botulinum toxin type A in pain management. Headache 43 Suppl 1, S9–15. [DOI] [PubMed] [Google Scholar]
- [52].Black JD and Dolly JO (1986). Interaction of 125I-labeled botulinum neurotoxins with nerve terminals. I. Ultrastructural autoradiographic localization and quantitation of distinct membrane acceptors for types A and B on motor nerves. J Cell Biol 103, 521–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Bigalke H, Dreyer F and Bergey G (1985). Botulinum A neurotoxin inhibits non-cholinergic synaptic transmission in mouse spinal cord neurons in culture. Brain research 360, 318–324. [DOI] [PubMed] [Google Scholar]
- [54].Yaksh TL (2011). Spinal toxins can have persistent adverse effects. Pain Med 12, 991–2. [DOI] [PubMed] [Google Scholar]
- [55].Pellett S, Yaksh TL and Ramachandran R (2015). Current status and future directions of botulinum neurotoxins for targeting pain processing. Toxins (Basel) 7, 4519–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Montecucco C and Rasotto MB (2015). On botulinum neurotoxin variability. MBio 6, pii: e02131–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Pellett S, Tepp WH, Lin G and Johnson EA (2018). Substrate cleavage and duration of action of botulinum neurotoxin type FA (“H, HA”). Toxicon 147, 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Pellett S, Tepp WH, Whitemarsh RC, Bradshaw M and Johnson EA (2015). In vivo onset and duration of action varies for botulinum neurotoxin A subtypes 1–5. Toxicon 107, 37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Whitemarsh RC, Tepp WH, Bradshaw M, Lin G, Pier CL, Scherf JM, Johnson EA and Pellett S (2013). Characterization of botulinum neurotoxin A subtypes 1 through 5 by investigation of activities in mice, in neuronal cell cultures, and in vitro. Infect Immun 81, 3894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Eleopra R et al. (2013). Botulinum neurotoxin serotype D is poorly effective in humans: An in vivo electrophysiological study. Clin Neurophysiol 124, 999–1004. [DOI] [PubMed] [Google Scholar]
- [61].Eleopra R, Tugnoli V, Quatrale R, Rossetto O, Montecucco C and Dressler D (2006). Clinical use of non-A botulinum toxins: botulinum toxin type C and botulinum toxin type F. Neurotox Res 9, 127–31. [DOI] [PubMed] [Google Scholar]
- [62].Eleopra R, Tugnoli V, Quatrale R, Rossetto O and Montecucco C (2004). Different types of botulinum toxin in humans. Movement disorders : official journal of the Movement Disorder Society 19 Suppl 8, S53–9. [DOI] [PubMed] [Google Scholar]
- [63].Eleopra R, Tugnoli V, Rossetto O, De Grandis D and Montecucco C (1998). Different time courses of recovery after poisoning with botulinum neurotoxin serotypes A and E in humans. Neuroscience letters 256, 135–138. [DOI] [PubMed] [Google Scholar]
- [64].Eleopra R, Tugnoli V, Rossetto O, Montecucco C and De Grandis D (1997). Botulinum neurotoxin serotype C: a novel effective botulinum toxin therapy in human. Neurosci Lett 224, 91–4. [DOI] [PubMed] [Google Scholar]
- [65].Davies JR, Hackett GS, Liu SM and Acharya KR (2018). High resolution crystal structures of the receptor-binding domain of Clostridium botulinum neurotoxin serotypes A and FA. PeerJ 6, e4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Rummel A et al. (2009). Botulinum neurotoxins C, E and F bind gangliosides via a conserved binding site prior to stimulation-dependent uptake with botulinum neurotoxin F utilising the three isoforms of SV2 as second receptor. J Neurochem 110, 1942–54. [DOI] [PubMed] [Google Scholar]
- [67].Peng L, Tepp WH, Johnson EA and Dong M (2011). Botulinum Neurotoxin D Uses Synaptic Vesicle Protein SV2 and Gangliosides as Receptors. PLoS pathogens 7, e1002008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Restani L, Giribaldi F, Manich M, Bercsenyi K, Menendez G, Rossetto O, Caleo M and Schiavo G (2012). Botulinum neurotoxins a and e undergo retrograde axonal transport in primary motor neurons. PLoS pathogens 8, e1003087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Verderio C et al. (2007). Traffic of botulinum toxins A and E in excitatory and inhibitory neurons. Traffic (Copenhagen, Denmark) 8, 142–153. [DOI] [PubMed] [Google Scholar]
- [70].Matteoli M, Pozzi D, Grumelli C, Condliffe SB, Frassoni C, Harkany T and Verderio C (2009). The synaptic split of SNAP-25: different roles in glutamatergic and GABAergic neurons? Neuroscience 158, 223–30. [DOI] [PubMed] [Google Scholar]
- [71].Verderio C et al. (2004). SNAP-25 modulation of calcium dynamics underlies differences in GABAergic and glutamatergic responsiveness to depolarization. Neuron 41, 599–610. [DOI] [PubMed] [Google Scholar]
- [72].Grumelli C, Corradini I, Matteoli M and Verderio C (2010). Intrinsic calcium dynamics control botulinum toxin A susceptibility in distinct neuronal populations. Cell Calcium 47, 419–24. [DOI] [PubMed] [Google Scholar]
- [73].Bigalke H, Heller I, Bizzini B and Habermann E (1981). Tetanus toxin and botulinum A toxin inhibit release and uptake of various transmitters, as studied with particulate preparations from rat brain and spinal cord. Naunyn Schmiedebergs Arch Pharmacol 316, 244–51. [DOI] [PubMed] [Google Scholar]
- [74].Frassoni C, Inverardi F, Coco S, Ortino B, Grumelli C, Pozzi D, Verderio C and Matteoli M (2005). Analysis of SNAP-25 immunoreactivity in hippocampal inhibitory neurons during development in culture and in situ. Neuroscience 131, 813–23. [DOI] [PubMed] [Google Scholar]
- [75].Garbelli R, Inverardi F, Medici V, Amadeo A, Verderio C, Matteoli M and Frassoni C (2008). Heterogeneous expression of SNAP-25 in rat and human brain. J Comp Neurol 506, 373–86. [DOI] [PubMed] [Google Scholar]
- [76].Beske PH, Scheeler SM, Adler M and McNutt PM (2015). Accelerated intoxication of GABAergic synapses by botulinum neurotoxin A disinhibits stem cell-derived neuron networks prior to network silencing. Front Cell Neurosci 9, 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Gitler D, Cheng Q, Greengard P and Augustine GJ (2008). Synapsin IIa controls the reserve pool of glutamatergic synaptic vesicles. J Neurosci 28, 10835–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Ryu JK et al. (2002). Trisialoganglioside GT1b induces in vivo degeneration of nigral dopaminergic neurons: role of microglia. Glia 38, 15–23. [DOI] [PubMed] [Google Scholar]
- [79].Chung ES et al. (2001). GT1b ganglioside induces death of dopaminergic neurons in rat mesencephalic cultures. Neuroreport 12, 611–4. [DOI] [PubMed] [Google Scholar]
- [80].Yamamoto H et al. (2012). Specificity of botulinum protease for human VAMP family proteins. Microbiol Immunol 56, 245–53. [DOI] [PubMed] [Google Scholar]
- [81].Pellizzari R, Mason S, Shone CC and Montecucco C (1997). The interaction of synaptic vesicle-associated membrane protein/synaptobrevin with botulinum neurotoxins D and F. FEBS Lett 409, 339–42. [DOI] [PubMed] [Google Scholar]
- [82].Yamasaki S et al. (1994). Cleavage of members of the synaptobrevin/VAMP family by types D and F botulinal neurotoxins and tetanus toxin. The Journal of biological chemistry 269, 12764–12772. [PubMed] [Google Scholar]
- [83].Peng L et al. (2014). Widespread sequence variations in VAMP1 across vertebrates suggest a potential selective pressure from botulinum neurotoxins. PLoS Pathog 10, e1004177. [DOI] [PMC free article] [PubMed] [Google Scholar]