Abstract
A series of nine methyl sulphones ( 3a –3 i ) starting from the aldehydes ( 1a–1i ) were synthesized in two consecutive steps. In the first step, preparation of allyl alcohols ( 2a–2i ) from their corresponding aldehydes by the reaction of sodium borohydride in methanol at room temperature is reported. Finally, methyl sulphones are synthesized by condensing sodium methyl sulfinates with allyl alcohols in the presence of BF 3 .Et 2 O in acetic acid medium at room temperature for about 2–3 h. The reaction conditions are simple, yields are high (85%–95%), and the products were obtained with good purity. All the synthesized compounds were characterized by their 1 H, 13 C NMR, and mass spectral analysis. All the title compounds were screened for antimicrobial activity. Among the compounds tested, the compound 3f has inhibited both Gram positive and Gram negative bacteria effectively and compound 3i has shown potent antifungal activity. These promising components may help to develop more potent drugs in the near future for the treatment of bacterial and fungal infections.
Keywords: BF 3 .OEt 2, allyl alcohols, methyl sulphones, antibacterial, antifungal
1. Introduction
The alcohol functional group is one of the more important groups for the synthesis of many drugs which are being used widely throughout the world [1–5]. As the alcohol functional group is not a good leaving group, it becomes the main obstacle for producing versatile novel drugs in organic synthesis. The nucleophilic substitution in the alcohol group is very difficult under mild conditions [6–12]. For the replacement of the OH group, one has to convert this alcohol group into a Cl group which is a better leaving group. In the previous studies, it was revealed that the conversion of the OH group into a mesylate group took place [13]. Direct conversion of alcohols into ethers, diaryl alkanes, and sulphonamides was successful [14–16]. So, we have decided to optimize the convenient route for the conversion of alcohols into sulfones under mild conditions. Earlier works revealed that direct conversion of alcohols into sulfones using bronsted acids like formic acids, acetic acid, and HCl [17–20], could be generated from sodium sulfide, sodium sulfinates, sulfonic acids, potassium meta bisulfite, sulfonyl chloride, and arenesulfonyl cyanide [21–27]. Among these reagents, sodium sulfinate is the best reagent due to ease of handling, and from a stability point of view.
Reddy and co-workers [28–29] reported that the reaction between p-toluenesulfonyl cyanide, and allylic alcohols leads to the formation of p-toluenesulfonyl cyanide, in the presence of diisopropylethylamine, later the adduct gets converted into a sulfonyl rearrangement product. Direct substitution of the allylic amine with sodium sulfinates in the presence of boronic acid [30] and the use of FeCl 3 as a catalyst and chlorotrimethylsilane as an additive [31], were also reported. Direct substitution of alcohols in the presence of boron trifluoride etherate with sodium sulfinates was prepared in which dichloromethane as a solvent was used under optimized parameters at 50 °C with 82% yield [32]. Oxidation of the methylthio derivative to the corresponding sulfones using m-CPBA was reported by Pujol et al [33]. Cu-catalyzed aerobic oxidation to synthesize from aryl halides and DMSO is described by Yuan et al. [34]. Fe(OH) 3 -catalyzed synthesis of aryl sulfones using aryl sulfonyl chloride with arenes is also reported [35]. The L-Proline sodium salt/CuI-mediated coupling reaction of aryl halides with sulfinic acids is also documented by Ma and Zhu [36]. Yuan et al. have recently synthesized aryl ethyl sulfones from sodium sulfinate and di- tert- butyl peroxide in the H 2 O medium [37]. Very recently, an eco-friendly approach to the construction of aryl methyl sulfone from SO 2 and methyl reagents is exemplified by Jiang et al. [38]. An excellent review on sulfones was presented very recently by Trost et al. [39]. In view of this and as an extension to our search for novel antimicrobial agents [40–44], the authors herein made an attempt to synthesize the titled sulfones and screen their antimicrobial properties.
2. Present work
We synthesized allyl alcohols, which were derived from respective aldehydes by reduction with sodium borohydride. A total of 9 aryl methyl sulfones were synthesized using BF 3 .OEt 2 as a catalyst and AcOH as a solvent in the present methodology shown in Scheme 1.
Scheme 1.

Synthesis of aryl methylsulfones.
Huang et al. [32] reported on the synthesis of sulfinates using BF 3 .OEt 2 in CH 2 Cl 2 solvent medium optimized at 45–50 °C moderate temperatures via the more favourable SN 1 mechanism through the conversion of sodium p-toluenesulfinate into corresponding nucleophile sulfinic acid, i.e. O-attack. Surprisingly, when acetic acid was used as a solvent, we could observe the formation of sulfones possibly via S-attack following the SN 2 mechanism, thereby indicating the significant role of solvent in product formation. The aim was achieved with various benzyl alcohols and sodium methyl sulfinates. Shorter reaction times and direct isolation of products were the added advantages in using the previous method.
Baidya et al . [45] explained about the thermal stability of the sulfones over the sulfinates. According to their studies, PhSO 2 − reacts with highly stabilized benzhydrylium ions to give sulfone derivatives exclusively, but in the case of highly reactive benzhydrylium ions it gives mixtures of sulfinates Ar 2 CH-OS(O)Ph and sulfones Ar 2 CH-SO 2 Ph; the latter rearranges to the thermodynamically more stable sulfones through an ionization recombination sequence.
In the given Scheme 2, the reaction mechanism was explained schematically. Using acetic acid as a solvent instead of dichloromethane favors reaction at room temperature. Initially, BF 3 .OEt 2 activates the hydroxyl group to become protonated and subsequent elimination of water molecule occurs. As a result carbonium ion formation took place at room temperature itself. The formed carbonium ion was attacked by nucleophile of the sodium methyl sulfinates leading to the formation of sulfinate derivatives. Later, the product rearranged into thermodynamically more stable sulfones.
Scheme 2.

Possible reaction mechanism for conversion of alcohol into sulfones.
We chose the benzyl alcohol, and sodium methyl sulfinates as substrates for optimization of the reaction initially in the presence of BF 3 .OEt 2 .The authors carried out a couple of reactions by changing the concentration of BF 3 .OEt 2 , varying from 0.2 equivalents to 2.0 equivalents. Finally, we could achieve the yields of the target molecule variables from 15 to 92% as shown in Table 1.
Table 1.
Reaction conditions for optimization.
| Entry | BF 3 .Et 2 O | Solvent | T °C | Time (h) | Yield (%) |
|---|---|---|---|---|---|
| 1 | 0.2 | CH 2 Cl 2 | 50 | 3 | 40 |
| 2 | 1.0 | CH 3 COOH | 28 | 3 | 80 |
| 3 | 1.4 | CH 3 COOH | 28 | 3 | 82 |
| 4 | 1.6 | CH 3 COOH | 28 | 3 | 86 |
| 5 | 1.8 | CH 3 COOH | 28 | 3 | 92 |
| 6 | 2.0 | CH 3 COOH | 28 | 3 | 90 |
| 7 | 1.8 | DMSO | 30 | 3 | 25 |
| 8 | 1.8 | THF | 28 | 3 | 20 |
| 9 | 1.8 | CHCl 3 | 28 | 3 | 37 |
| 10 | 1.8 | Cyclohexane | 28 | 3 | 15 |
| 11 | 1.8 | CH 3 NO 2 | 28 | 3 | 39 |
| 12 | 1.8 | C 2 H 5 NO 2 | 28 | 3 | 40 |
| 13 | 1.8 | 1,4-Dioxane | 28 | 3 | 42 |
| 14 | 1.8 | CH 3 CN | 28 | 3 | 25 |
| 15 | 1.8 | DMF | 28 | 3 | 38 |
| 16 | 1.8 | Acetone | 28 | 3 | 30 |
The highest yields of the compound were obtained with 1.8 equivalents of BF 3 .OEt 2 . A couple of reactions were conducted with 1.8 equivalents of BF 3 .OEt 2 at various time periods ranging from 1 to 8 h. During the time period from 1 to 3 h, the yields found increased, and when the time period was prolonged from 3 to 8 h, the yields decreased. This reaction conversion was tremendously effective on the solvent which was used. For this reason, several trial reactions were carried out with both the polar solvents and nonpolar solvents. Lesser yields were reported with the nonpolar solvent cyclohexane (Table 1, entry 10). Next to cyclohexane, polar solvents like DMSO, and THF gave the yields of 25%, and 20% respectively. With the exception of acetic acid other solvents got the yields of the desired product below 50%. The best yields ranging from 80% to 92% (Table 1, entries 2–6) were obtained with the acetic acid. So, finally, we have concluded that the reaction is more favorable with the protic solvents.
The above optimized reaction conditions were verified and or generalized with structurally different types of alcohols. The obtained yields of desired products were mentioned in Table 2, entries 1–9.
The best yield (95%) of the desired molecule was obtained with the nitro alcohol derivatives (3i), under the optimized reaction condition. The lowest yields were obtained with the electron donating groups, which existed in the substrates. Electron withdrawing groups, which were present in the substrate molecules favor the conversion with excellent yields. The alcohol 1a having three donation groups present in the ortho and para position gave less yield (Table 2, entry 1). Due to the presence of orthosteric effect, alcohol derivatives 3a and 3e gave less yields (Table 2, entries 1, 5). The fewer number of donating groups present in the alcohol substrates increase the yields from 85% to 88%. The phenyl ring has a lesser withdrawing effect than the nitro group results and yields almost the highest yields.
Table 2.
Sulfonation of various alcohols with sodium methyl sulfinates.
| Entry | Compound structure | Number | Yield (%) a |
|---|---|---|---|
| 1 |

|
3a | 85 |
| 2 |

|
3b | 88 |
| 3 |

|
3c | 90 |
| 4 |

|
3d | 91 |
| 5 |
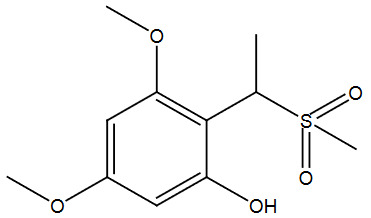
|
3e | 88 |
| 6 |

|
3f | 86 |
| 7 |
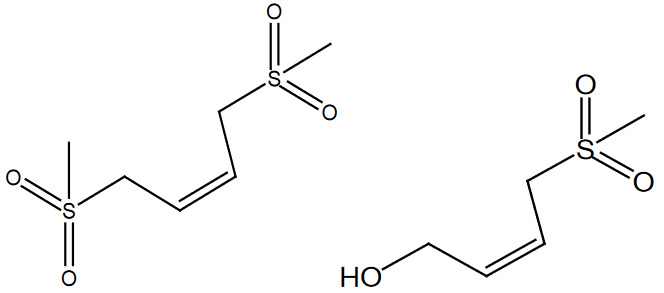
|
3g | 88 |
| 8 |

|
3h | 92 |
| 9 |

|
3i | 95 |
aYields refer to pure products after column chromatography
Reaction conditions: 1a–1i (1.96 μmol), 2 (1.96 μmol), BF 3 .OEt 2 (3.5 mL), Acetic acid (3 mL), at rt for 3h.
3. Biological activity
All the synthesized compounds were screened for antimicrobial activity and results were depicted in Table 3. Among the screened compounds (3a–3i), compound 3f with dimethoxy, hydroxyl benzyl group showed the highest inhibition zone followed by compounds 3a, 3c, and 3h. Further, the compound 3i was found to be effective on fungal strains. The remaining compounds showed moderate activity.
Table 3.
Antimicrobial activity of the synthesized compounds (3a–3i).
| Diameter of zone of inhibition in mm | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound Code | S.aureus (ATCC 25923) | B. Cereus (ATCC | P. Aeruginosa (ATCC 27853) | E.coli (ATCC 35218) | C. Albicans (ATCC 90028) | A. Niger (NCCS 1196) | ||||||||||||
| 50 (mg/mL) | 100 (mg/mL) | 150 (mg/mL) | 50 (mg/mL) | 100 (mg/mL) | 150 (mg/mL) | 50 (mg/mL) | 100 (mg/mL) | 150 (mg/mL) | 50 (mg/mL) | 100 (mg/mL) | 150 (mg/mL) | 50 (mg/mL) | 100 (mg/mL) | 150 (mg/mL) | 50 (mg/mL) | 100 (mg/mL) | 150 (mg/mL) | |
| 3a | - | 11 | 18 | - | - | - | 08 | 17 | 23 | 08 | 12 | 18 | 08 | 12 | 16 | 14 | 16 | 14 |
| 3b | 08 | 12 | 17 | - | - | - | 07 | 15 | 21 | 09 | 13 | 17 | 08 | 13 | 14 | 13 | 15 | 13 |
| 3c | 07 | 12 | 18 | - | - | - | 08 | 16 | 23 | 10 | 12 | 18 | 09 | 13 | 16 | 13 | 15 | 13 |
| 3d | 06 | 13 | 17 | - | - | - | 07 | 13 | 16 | 08 | 12 | 15 | 09 | 14 | 17 | 14 | 15 | 14 |
| 3e | 07 | 14 | 18 | - | - | - | 08 | 16 | 20 | 09 | 13 | 18 | 10 | 14 | 16 | 14 | 16 | 16 |
| 3f | 08 | 16 | 19 | - | - | - | 10 | 17 | 24 | 09 | 14 | 18 | 09 | 16 | 17 | 15 | 17 | 16 |
| 3g | 07 | 14 | 16 | - | - | - | 08 | 12 | 15 | 08 | 14 | 17 | 07 | 14 | 15 | 15 | 15 | 15 |
| 3h | 07 | 13 | 18 | - | - | - | 07 | 18 | 23 | 08 | 15 | 18 | 08 | 16 | 16 | 14 | 15 | 15 |
| 3i | 06 | 14 | 17 | - | - | - | 06 | 12 | 16 | 08 | 14 | 17 | 11 | 14 | 18 | 15 | 17 | 18 |
| Ciproflaxacin(30 mg/disc) | 24 | 18 | 24 | 23 | NA | NA | ||||||||||||
| Fluconazole(25 µg/disc) | NA | NA | NA | NA | 22 | 20 | ||||||||||||
4. Conclusion
This method is a modified method for methyl sulfones and reaction yields of 85% to 95% were obtained. In this method, the solvent acetic acid was used, which is inexpensive when compared with the solvent dichloromethane solvent, and this reaction is carried out at room temperature. We have applied this method for the synthesis of 9 compounds of which 7 are novel (3a–3i).
The compounds bearing dimethoxy, hydroxyl benzyl group have shown prominent antibacterial activity when compared to compounds without these groups. It was also confirmed that the compounds bearing nitro group have shown prominent antifungal activity when compared to other compounds. Further investigation in this area may help to create more potent drugs for the treatment of bacterial and fungal infections.
5. Experimental section
5.1. General preparation of compounds (1a–1i)
NaBH 4 (4.76 μmol) was added to the ethyl alcohol (3 mL) and the reaction mixture was stirred at room temperature for 5 min. Respectively, aldehyde compound (4.76 μmol) was added to the reaction mixture and stirred continuously for 1 h. Reaction mixture completion was confirmed by the TLC. After completion of the reaction, the mixture was quenched with 10% HCl (3 mL) and ethanol was evaporated under reduced pressure. After the complete removal of ethanol, saturated sodium bisulfite (1 × 5 mL) was added. The organic compound was extracted with dichloromethane (20 mL) and water (10 mL). The organic layer was dried over Na 2 SO 4 , filtered, and concentrated under reduced pressure; to give 1a–1i compounds. Yield, 1 H NMR, ESI-MS (M+H) data of all compounds, and CHNS/O u1d57 (Perkin-Elmer 2400, PerkinElmer Inc., Waltham, MA, USA) composition data of each product are given below.
5.1.1. (2,4,6-trimethoxyphenyl)methanol (1a)
Brown solid, yield 91.2%; 1 H NMR (CDCl 3 , 400 MHz): δ 6.12 (s, 2H), 4.69 (s, 2H), 3.81 (s, 6H), 3.80 (s, 3H), 2.16 (s, 1 H,); ESI MS (M+H): m/z 199.01.
5.1.2. 4-(hydroxymethyl)-2-methoxyphenol (1b)
White solid, 1 H NMR (CD 3 OD, 400 MHz): δ 6.95 (s, 1 H), 6.79 (s, 2H), 4.52 (s, 2H), 3.85 (s, 3H); 13 C NMR (CD 3 OD, 100 MHz): δ 147.54, 145.47, 132.86, 119.75, 114.66, 110.79, 63.98, 55.04; ESI MS (M+H): m/z 155.26.
5.1.3. 1,8-dihydroxy-3-(hydroxymethyl)anthracene-9,10-dione (1c)
Pale white solid, 1 H NMR (DMSO-d6, 100 MHz): δ 11.90 (s, 2H), 7.79–7.64 (m, 3H), 7.35–7.24 (m, 2H), 5.57 (t, J = 5.8 Hz, 1 H), 4.57 (d, J = 5.8 Hz, 2H); ESI MS (M+H): m/z 135.19.
5.1.4. 3-phenylprop-2-en-1-ol (1d)
Light yellow solid, 1 H NMR (CDCl 3 , 500 MHz): δ 7.36-7.32 (m, 2H), 7.27–7.24 (m, 2H), 7.23–7.19 (m, 1 H), 6.59 (s, 1 H), 6.55 (s, 1 H), 4.44–4.42 (m, 2H); ESI MS (M+H): m/z 135.19.
5.1.5. 2-(1-hydroxyethyl)-3,5-dimethoxyphenol (1e)
Yellow solid, 1 H NMR (CDCl 3 , 400 MHz): δ 5.85 (s, 1 H), 5.82 (s, 1 H), 4.61 (s, 1 H), 4.0 (s, 1 H), 3.82 (s, 1 H), 3.65 (s, 6H), 1.55 (s, 3H); ESI MS (M+H): m/z 199.06.
5.1.6. 1-(3,4-Dimethoxyphenyl)-1-propanol (1f)
Brown solid, 1 H NMR (CDCl 3 , 400 MHz): δ 7.05 (d, 1 H), 6.94–6.85 (dd, J = 8.1 Hz, 2H), 4.85 (m, 1 H), 3.88 (ds, 6H), 2.45 (bs, OH), 1.84–1.78 (m, 2H), 0.97 (t, J = 7.4 Hz, 3H); ESI MS (M+H): m/z 197.29.
5.1.7. But-2-ene-1,4-diol (1g)
White solid, 1 H NMR (CDCl 3 , 400 MHz): δ 5.80 – 5.78 (m, 2H), 4.25–4.24 (m, 4H); ESI MS (M+H): m/z 89.29.
5.1.8. Diphenylmethanol (1h)
White solid, 1 H NMR (CDCl 3 , 500 MHz): δ 2.37 (bs, 1 H), 5.79 (s, 1 H), 7.25–7.36 (m, 10H); 13 C NMR (CDCl 3 , 125 MHz): δ 143.7, 128.4, 127.5, 126.5, 76.2; ESI MS (M+H): m/z 185.01.
5.1.9. (4-nitrophenyl) methanol (1i)
Light yellow solid, 1 H NMR (CDCl 3 , 300 MHz): δ 8.23 (d, 2H, J = 8.7), 7.54 (d, 2H, J = 8.7), 4.84 (s, 2H); 13 C NMR (CDCl 3 , 75.47 MHz): δ 148.08, 126.99, 123.73, 64.02; ESI MS (M+H): m/z 154.15.
5.2. General preparation of compounds (3a-i)
The respective benzyl alcohol (1.96 μmol) was dissolved in acetic acid (3 mL). BF 3 .OEt 2 (3.5 mL, 3.528 μmol) was added to the reaction mixture at room temperature. Sodium methyl sulfinate (200 mg, 1.96 μmol) was added to the reaction mixture and stirred for 30 min. Reaction mixture completion was confirmed by the TLC. After completion of the reaction, the reaction mixture was quenched with NaHCO 3 solution (10 mL). The organic compound was extracted with dichloromethane (20 mL) and water (10 mL). The organic layer was dried over Na 2 SO 4 , filtered, and concentrated under reduced pressure. The crude material was purified by the silica gel chromatography to give the compounds 3a–3i. Yield, IR, NMR, ESI MS (M+H) data, and CHNS/O u1d5a (Perkin-Elmer 2400) data of each product are given below.
5.2.1. 1,3,5-trimethoxy-2-((methylsulfonyl)methyl)benzene (3a)
Brown solid, yield 85%; IR (u, KBr): 3033 (Aromatic C=C), 2988, 2976 (CH 3 ),1024(SO 2 -) cm -1 ; 1 H NMR (400 MHz, CDCl 3 ): δ 6.16 (s, 2H), 4.38 (s, 2H), 3.85 (s, 6H), 3.82 (s, 3H). 2.77 (s, 3H); 13 C NMR (100 MHz, CDCl 3 ): 162.23, 159.72, 98.86, 91.03, 90.44, 56.04, 55.94, 55.45, 50.26, 40.31; ESI MS: m/z 181 (M-SO 2 Me).CHNS: Anal. calcd. for C 11 H 16 O 5 S; C, 50.75; H, 6.20; S, 12.32. Found: C, 50.61; H, 6.11; S, 12.49.
5.2.2. 2-methoxy-4-((methylsulfonyl)methyl)phenol (3b)
Brown solid, yield 88%; IR (u,KBr): 3329 (phenolic OH),2888, 2785 (CH 3 ), 1020 (SO 2 -) cm -1 ; 1 H NMR (400 MHz, CDCl 3 ) δ 6.70–6.67 (m, 3H), 4.41 (s, 2H), 3.83 (s, 3H), 3.46 (s, 1 H), 2.96 (s, 3H); 13 C NMR 147.84, 147.64, 124.83, 118.45, 117.39, 117.32, 58.97, 56.79, 41.75; ESI MS: m/z 202 (M+-SO 2 ).CHNS: Anal. calcd. for C 9 H 12 O 4 S; C, 49.99; H, 5.59; S, 14.83. Found: C, 49.88; H, 5.43; S, 14.96.
5.2.3. 1,8-dihydroxy-3-((methylsulfonyl)methyl)anthracene-9,10-dione (3c)
Brown solid, yield 90%; IR (u,KBr): 3321 (phenolic OH), 3040 (Aromatic C=C), 1020 (SO 2 -) cm -1 ; 1 H NMR (400 MHz, CDCl 3 ): δ 12.06 (s, 1 H), 12.04 (s, 1 H), 7.85- 7.83 (m, 1 H), 7.78 (s, 1 H), 7.71-7.67 (t , J = 16 Hz, 1 H), 7.32-7.30 (d, 1 H), 7.26 (s, 1 H), 5.19 (s, 2H), 2.19 (s, 3H); 13 C NMR (100 MHz, CDCl 3 ): δ 192.69, 181.48, 170.38, 162.82, 146.52, 137.29, 133.93, 133.60, 124.76, 122.39, 120.15, 118.49, 115.85, 115.32, 64.70, 20.77; ESI MS (M++2H): m/z 333.CHNS: Anal. calcd. for C 16 H 12 O 6 S; C, 57.83; H, 3.64; S, 9.65. Found: C, 57.91; H, 3.49; S, 9.81.
5.2.4. 1-((E)-3-(methylsulfonyl)prop-1-enyl)benzene (3d)
Brown solid, yield 91%; IR (u,KBr): 3033 (Aromatic C=C), 2970, 2965 (CH 3 ), 1024 (SO 2 -) cm -1 ; 1 H NMR (400 MHz, CDCl 3 ): δ 7.31–7.29 (m, 2H), 7.25–7.22 (m, 2H), 7.19– 7.17 (m, 1 H), 6.56–6.53 (m, 1 H), 6.22–6.16 (m, 1 H), 4.09–4.07 (d, J = 8, 2H), 3.00 (s, 3H); 13 C NMR (100 MHz, CDCl 3 ): δ 137.67, 136.33, 129.19, 128.08, 127.08, 122.13, 58.35, 40.25; ESI MS: m/z 118 (M+-SO 2 ).CHNS: Anal. calcd. for C 10 H 12 O 2 S; C, 61.20; H, 6.16; S, 16.34. Found: C, 61.09; H, 6.03; S, 16.47.
5.2.5. 3,5-dimethoxy-2-(1-(methylsulfonyl)ethyl)phenol (3e)
Brown solid, yield 86%; IR (u,KBr): 3041 (Aromatic C=C), 2980, 2889 (CH 3 ), 1024 (SO 2 -) cm -1 ; 1 H NMR (400 MHz, CDCl 3 ): δ 6.20–6.15 (m, 2H), 4.40–4.36 (m, 1 H), 3.82 (s, 3H), 3.80 (s, 3H), 2.97 (s, 3H), 1.79 (s, 3H); 13 C NMR (100 MHz, CDCl 3 ): δ 167.49, 164.44, 162.99, 113.34, 93.61, 91.47, 62.29, 56.79, 56.04, 37.80, 17.52; ESI MS (M+H): m/z 258(M+).CHNS: Anal. calcd. for C 11 H 16 O 5 S; C, 50.75; H, 6.20; S, 12.32. Found: C, 50.60; H, 6.11; S, 12.47.
5.2.6. 1,2-dimethoxy-4-(1-(methylsulfonyl)propyl)benzene (3f)
Brown solid, yield 88%; IR (u,KBr): 3033 (Aromatic C=C), 2988, 2972, 1024 (SO 2 -) cm -1 ; 1 H NMR (400 MHz, CDCl 3 ) δ 6.91 (s, 1 H), 6.84–6.82 (m, 2H), 4.13–4.11 (m, 1 H), 3.82 (s, 6H), 3.03(s, 3H), 2.25–2.20 (m, 2H), 1.04-1.01(s, 3H); 13 C NMR (100 MHz, CDCl 3 ): δ 149.53, 147.02, 135.91, 117.05, 116.41, 113.45, 69.90, 56.78,39.66, 23.95, 11.02; ESI MS (M+H): m/z 215(M+).CHNS: Anal. calcd. for C 12 H 18 O 4 S; C, 55.79; H, 7.02; S, 12.42. Found: C, 55.65; H, 6.89; S, 12.54.
5.2.7. (Z)-1,4-bis(methylsulfonyl)but-2-ene, (Z)-4-(methylsulfonyl)but-2-en-1-ol (3g)
Brown solid, yield 88%; IR (u,KBr): 3323 (-OH), 1020 (SO 2 -) cm -1 ; 1 H NMR (400 MHz, CDCl 3 ): δ 5.83–5.82 (m, 1 H), 5.72–5.70 (m, 3H), 4.67–4.63 (m, 4H), 4.55–4.54 (m, 2H), 2.04 (s, 3H), 2.03 (s, 6H); ESI-MS (M+H): m/z 261 (M+).
5.2.8. (methylsulfonyl)diphenylmethane(3h) [31]
Brown solid, yield 92%; 1 H NMR (400 MHz, CDCl 3 ): δ 7.65–7.57 (m, 4H), 7.43–7.33 (m, 6H), 5.32 (s, 1 H), 2.77 (s, 3H); 13 C NMR (100 MHz, CDCl 3 ): δ 132.80, 129.73, 129.66, 128.50, 74.84, 40.02; ESI MS (M+H): m/z 247. CHNS: Anal. calcd. for C 14 H 14 O 2 S; C, 68.26; H, 5.73; S, 13.02. Found: C, 68.17; H, 5.61; S, 13.16.
5.2.9. 1-((methylsulfonyl)methyl)-4-nitrobenzene (3i) [46]
Brown solid, yield 95%; 1 H NMR (400 MHz, CDCl 3 ) δ8.44 (d, J= 8.8 Hz, 2H), 8.16(d, J= 8.8 Hz, 2H), 3.12 (s, 3H); 13 C NMR (100 MHz, CDCl 3 ): δ 150.9, 145.86, 129.25, 124.36, 44.29; ESI MS (M+H): m/z198.CHNS: Anal. calcd. for C 8 H 9 NO 4 S; C, 44.64; H, 4.21; N, 6.51; S, 14.90. Found: C, 44.51; H, 4.12; N, 6.51; S, 14.99.
5.3. Antibacterial activity [47]
The antibacterial activity of the compounds was determined by means of the disc diffusion method. Cultures of each bacterium ( E.coli, Bacillus cereus, Staphylococcus aureus, and Pseudomonas aeruginosa) were inoculated to the nutrient broth and incubated at 37 °C for 16 h., respective bacterial culture was inoculated in the MHA plate by using the spread plate method. Discs (6 mm in diameter) were impregnated with 25, 50, and 75 µg/ mL concentrations in DMSO solution of the compounds (3a–3i) and placed on the surface of the MHA inoculated with bacteria, which were incubated at 37 °C for 24 h. The inhibition zones were measured with a caliper considering the total diameters. Similarly, each plate carried a blank disc, the disk with DMSO, and ciprofloxacin disc (30 µg/mL) as standard.
5.4. Antifungal activity
The antifungal activity of the compounds was determined by means of the disc diffusion method. Cultures of each fungal ( C.Albicans, and A. niger) were inoculated to the nutrient broth and incubated at 37 °C for 16 h. Respective fungal culture was inoculated in the SDA plate by using the spread plate method. Discs (6 mm in diameter) were impregnated with 25, 50, and 75 µg/ mL concentrations in DMSO solution of the compounds (3a–3i) and placed on the surface of the MHA inoculated with bacteria, which were incubated at 37 °C for 24 h. The inhibition zones were measured with a caliper considering the total diameters. Similarly, each plate carried a blank disc, disc with DMSO, and fluconazole disc (30 µg/mL) as standard.
Acknowledgments
The authors thank Acharya Nagarjuna University, AP-India for constant support and encouragement. Dr. Ravi VARALA thanks Dr. Ch. V. Rajasekhar, Scrips Pharma for his kind support.
References
- Kumar R Van DEEV Recent approaches for C–C bond formation via direct dehydrative coupling strategies. Chemical Society Reviews. 2013;42:1121–1146. doi: 10.1039/c2cs35397k. [DOI] [PubMed] [Google Scholar]
- Katritzky AR Brycki BE The mechanisms of nucleophilic substitution in aliphatic compounds. Chemical Society Reviews. 1990;19:83–105. [Google Scholar]
- Saito T Nishimoto Y Yasuda M Akio B Direct coupling reaction between alcohols and silylcompounds: enhancement of lewisacidity of Me3SiBr using InCl3. Journal of Organic Chemistry. 2006;71:8516–8522. doi: 10.1021/jo061512k. [DOI] [PubMed] [Google Scholar]
- Anlian Z Lingjun L Jianji W Direct nucleophilic substitution reaction of alcohols mediated by a zinc-based ionic liquid. Green Chemistry. 2011;13:1244–1250. [Google Scholar]
- Hang S Liangzhen H Qing L Muhammad IH Jing P Iron-catalysed sequential reaction towards α-aminonitriles from secondary amines, primary alcohols and trimethylsilyl cyanide. Chemical Communications. 2016;52:2776–2779. doi: 10.1039/c5cc10346k. [DOI] [PubMed] [Google Scholar]
- Vanos CM Lambert TH Development of a catalytic platform for nucleophilic substitution: cyclopropenone‐catalyzed chlorodehydration of alcohols. Angewandte Chemie International Edition. 2011;50:12222–12226. doi: 10.1002/anie.201104638. [DOI] [PubMed] [Google Scholar]
- Makoto Y Satoshi Y Yoshiyuki O Akio B Indium-catalyzeddirect chlorination of alcohols using chlorodimethylsilane−benzil as a selective and mild system. Journal of the American Chemical Society. 2004;126:7186–7187. doi: 10.1021/ja048688t. [DOI] [PubMed] [Google Scholar]
- Yasuda M Somyo T Baba A Direct carbon-carbon bond formation from alcohols and active methylenes, alkoxyketones, or indolescatalyzed by indium trichloride. Angewandte Chemie. 2006;118:807–807. doi: 10.1002/anie.200503263. [DOI] [PubMed] [Google Scholar]
- Tao W Ruida M Liu L Zhuang PZ Solvent-free solid acid-catalyzednucleophilic substitution of propargylic alcohols: a green approach for the synthesis of 1,4-diynes. Green Chemistry. 2010;12:1576–1579. [Google Scholar]
- Sundararaju B Achard M Bruneau C Transition metal catalyzed nucleophilicallylic substitution: activation of allylic alcohols via π-allylic species. Chemical Society Reviews. 2012;41:4467–4483. doi: 10.1039/c2cs35024f. [DOI] [PubMed] [Google Scholar]
- Nguyen TV Bekensir A. Aromatic cationactivation: Nucleophilicsubstitution of alcohols and carboxylic acids. Organic Letters. 2014;16:1720–1723. doi: 10.1021/ol5003972. [DOI] [PubMed] [Google Scholar]
- Ohshima T Ipposhi J Nakahara Y Ryozo S Kazushi M Aluminum triflate as a powerful catalyst for direct amination of alcohols, including electron‐withdrawing group‐substituted benzhydrols. Advanced Synthesis and Catalysis. 2012;354:2447–2452. [Google Scholar]
- Murakami T Furusawa K. One-pot synthesis of aryl sulfones from alcohols. Synthesis. 2002;4:479–482. [Google Scholar]
- Li JQ Zhang XH Shen H Qing L Pan J Boron trifluoride diethylether‐catalyzedetherification of alcohols: a metal‐free pathway to diphenylmethylethers. Advanced Synthesis and Catalysis. 2015;357:3115–3120. [Google Scholar]
- Pan J Li J Huang R Zhang X Shen H Metal-free direct N-benzylation of sulfonamides with benzyl alcohols by employing boron trifluoride–diethyl ether complex. Synthesis. 2015;47:1101–1108. [Google Scholar]
- Zhang ST Zhang XH Ling XG Chao He Ruofeng H Superacid BF3–H 2 O promoted benzylation of arenes with benzyl alcohols and acetates initiated by trace water . RSC Advances. 2014;4:30768–30774. [Google Scholar]
- Fischli A Mayer H Simon W Stoller HJ A synthesis of vitamin A according to the sulfone method. Helvetica Chimica Acta. 1976;59:397–405. doi: 10.1002/hlca.19760590208. [DOI] [PubMed] [Google Scholar]
- Jerkeman P Lindberg B Sulphones of lignin models, synthesis and reactions in alkali. Acta Chemica Scandinavica. 1964;18:1477–1482. [Google Scholar]
- Forzelius SE Jerkeman LB Sulphones of some lignin models. Acta Chemica Scandinavica. 1963;17:1470–1471. [Google Scholar]
- Castedo L Delamano J Lopez C Marra BL Synthesis of five-membered heteroarylmethyl p-tolylsulfones from heteroarenemethanols under acidic conditions: Scope and Limitations. Heterocycles. 1994;38:495–502. [Google Scholar]
- Ju Y Kumar D Varma RS Revisiting nucleophilicsubstitution reactions: microwave-assisted synthesis of azides, thiocyanates, and sulfones in an aqueous medium. Journal of Organic Chemistry. 2006;71:6697–6700. doi: 10.1021/jo061114h. [DOI] [PubMed] [Google Scholar]
- Xu YF Liu P Li SL Peipei S. Palladium-catalyzed ortho-sulfonylation of 2-aryloxypyridines and subsequent formation of ortho-sulfonylated phenols. Journal of Organic Chemistry. 2015;80:1269–1274. doi: 10.1021/jo5026095. [DOI] [PubMed] [Google Scholar]
- Razieh F Hamid A Majid M Mona M Nano-rod catalysts: building MOF bottles (MIL-101 family as heterogeneous single-site catalysts) around vanadium oxide ships. Journal of Molecular Catalysis A: Chemical. 2013;374:46–52. [Google Scholar]
- Miles WJ Scott WB Neal PM Robert GB Vincent M. Application of fundamental organometallic chemistry to the development of a gold‐catalyzed synthesis of sulfinate derivatives. Angewandte Chemie International Edition. 2014;53:4404–4407. doi: 10.1002/anie.201400037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felpin FX Landais Y. Practical Pd/C-mediated allylicsubstitution in water. Journal of Organic Chemistry. 2005;70:6441–6446. doi: 10.1021/jo050952t. [DOI] [PubMed] [Google Scholar]
- Crandall JK Pradat C Synthesis of sulfones by phase-transfer alkylation of arenesulfinate salts. Journal of Organic Chemistry. 1985;50:1327–1329. [Google Scholar]
- Zhang D Cui XL Zhang Q Pd-catalyzed direct C–H bond sulfonylation of azobenzenes with arylsulfonyl chlorides. Journal of Organic Chemistry. 2015;80:1517–1522. doi: 10.1021/jo502451k. [DOI] [PubMed] [Google Scholar]
- Reddy LR Hu B Prashad M Kapa P. An unexpected reaction of arenesulfonyl cyanides with allylic alcohols: Preparation of trisubstituted allyl sulfones. Angewandte Chemie. 2009;48:172–174. doi: 10.1002/anie.200803836. [DOI] [PubMed] [Google Scholar]
- Beaulieu GD Wangaand Z David AE A mild and efficient new synthesis of aryl sulfones from boronic acids and sulfinic acid salts. Tetrahedron Letters. 2004;45:3233–3236. [Google Scholar]
- Wu XS Chen Y Li MB Meng-GZ Shi-KT. Journal of the American Chemical Society. 2012;134:14694–14697. doi: 10.1021/ja306407x. [DOI] [PubMed] [Google Scholar]
- Amarnath RM Surendra RP Sreedhar B. Iron(III) chloride-catalyzed direct sulfonylation of alcohols with sodium arenesulfinates. Advanced Synthesis and Catalysis. 2010;352:1861–1869. [Google Scholar]
- Huang M Hu L Shen H Qing L Muhammad IH Sulfination of alcohols with sodium sulfinates promoted by BF3. Green Chemistry. 2016;18:1874–1879. [Google Scholar]
- Navarroa L Rosella G Sanchezc S Boixareud N Porsd K Synthesis and biological properties of aryl methyl sulfones. Bioorganic and Medicinal Chemistry. 2018;26:4113–4126. doi: 10.1016/j.bmc.2018.06.038. [DOI] [PubMed] [Google Scholar]
- Yuan G Zheng J Gao X Li X Huang L Copper-catalyzed aerobic oxidation and cleavage/formation of C–S bond: a novel synthesis of aryl methyl sulfones from aryl halides and DMSO. Chemical Communications. 2012;48:7513–7515. doi: 10.1039/c2cc32964f. [DOI] [PubMed] [Google Scholar]
- Jin T Zhao Y Ma Y Li T. A practical and efficient method for the preparation of aromatic sulfones by the reaction of aryl sulfonyl chlorides with arenes catalysed by Fe(OH) 3 . Indian Journal of Chemsitry Section B. 2005;44B:2183–2185. [Google Scholar]
- Zhu W Synthesis of aryl sulfonesvia l-proline-promoted CuI-catalyzed coupling reaction of aryl halides with sulfinic acid salts. Journal of Organic Chemistry. 2005;70:2696–2700. doi: 10.1021/jo047758b. [DOI] [PubMed] [Google Scholar]
- Lai J Yuan G novel synthesis of aryl methyl sulfones and β-hydroxysulfones from sodium sulfinates and di-tert-butyl peroxide in H 2 O medium . Tetrahedron Letters. 2018;59:524–527. [Google Scholar]
- Wang M Zhao J Jiang X Aryl methyl sulfone construction from eco-friendly inorganic sulfur dioxide and methyl reagents. ChemSusChem. 2019;12:3064–3068. doi: 10.1002/cssc.201802919. [DOI] [PubMed] [Google Scholar]
- Trost BM Sulfones as chemical chameleons: versatile synthetic equivalents of small molecule synthons. Chemistry: A European Journal. 2019;25:11193–11213. doi: 10.1002/chem.201902019. [DOI] [PubMed] [Google Scholar]
- Ravi KG Chandra MK Manideepa I Ramya KP Hari BB -a] phthalazines via Suzuki coupling and evaluation of their anticancer and antimicrobial activity. Synthesis of new analogs of 3-methyl-[1. 2019;8:261–269. [Google Scholar]
- Lourdu RB Vijay K Sivanagi RM Bala MK Hari BB Synthesis, characterization, anticancer and antimicrobial activity studies of novel isomeric 2,4-disubstituted ureide derivatives of pyrimidinopiperidines. Chemistry Select. 2019;4:441–450. [Google Scholar]
- Baby RM Vijaya K Surendranatha RO Murthy SNB Hari BB Synthesis, cytotoxicity and antimicrobial evolution of some new 2-Aryl,5-substituted 1. Chemistry Africa. 2019;2:4–4. [Google Scholar]
- Basavaiah CH Jalaja CH Raghu RM Asha BP Hari BB Synthesis and antimicrobial studies of graphene-silver nanocomposite through a highly environmentally benign reduction methodology. Materials Technology: Advanced Performance Materials. 2018;33:730–736. [Google Scholar]
- Ramesh N Ganagadhara RM Vasu BA Nagababu P Umamaheswara RV Synthesis, antibacterial activity, and docking studies of some novel N-benzylidene-2-(2. Research on Chemical Intermediates. 2017;43:5–5. [Google Scholar]
- Baidya M Kobayashi S Mayr H. Nucleophilicity and nucleofugality of phenylsulfinate (PhSO 2 −): a key to understanding its ambident reactivity . Journal of the American Chemical Society. 2010;132:4796–4805. doi: 10.1021/ja9102056. [DOI] [PubMed] [Google Scholar]
- Xu F Savary K Williams JM Grabowski EJJ Reider PJ Novel synthesis of sulfones from α,α-dibromomethyl aromatics. Tetrahedron Letters. 2003;44:1283–1286. [Google Scholar]
- Collins CH Lyre PM Grange JM Microbiological methods. Butterworths, London: Arnold, 1989;1989 [Google Scholar]


