Abstract
Background
Prostate cancer is the most common cancer in men and second leading cause of cancer-related deaths. Changes in screening guidelines, adoption of active surveillance (AS), and implementation of high-cost technologies have changed treatment costs. Traditional cost-effectiveness studies rely on clinical trial protocols unlikely to capture actual practice behavior, and existing studies use data predating new technologies. Real-world evidence reflecting these changes is lacking.
Objective
To assess real-world costs of first-line prostate cancer management.
Design, setting, and participants
We used clinical electronic health records for 2008–2018 linked with the California Cancer Registry and the Medicare Fee Schedule to assess costs over 24 or 60 mo following diagnosis. We identified surgery or radiation treatments with structured methods, while we used both structured data and natural language processing to identify AS.
Outcome measurements and statistical analysis
Our results are risk-stratified calculated cost per day (CCPD) for first-line management, which are independent of treatment duration. We used the Kruskal-Wallis test to compare unadjusted CCPD while analysis of covariance log-linear models adjusted estimates for age and Charlson comorbidity.
Results and limitations
In 3433 patients, surgery (54.6%) was more common than radiation (22.3%) or AS (23.0%). Two years following diagnosis, AS ($2.97/d) was cheaper than surgery ($5.67/d) or radiation ($9.34/d) in favorable disease, while surgery ($7.17/d) was cheaper than radiation ($16.34/d) for unfavorable disease. At 5 yr, AS ($2.71/d) remained slightly cheaper than surgery ($2.87/d) and radiation ($4.36/d) in favorable disease, while for unfavorable disease surgery ($4.15/d) remained cheaper than radiation ($10.32/d). Study limitations include information derived from a single healthcare system and costs based on benchmark Medicare estimates rather than actual payment exchanges.
Patient summary
Active surveillance was cheaper than surgery (−47.6%) and radiation (−68.2%) at 2 yr for favorable-risk disease, while savings diminished by 5 yr (−5.6% and −37.8%, respectively). Surgery cost less than radiation for unfavorable risk for both intervals (−56.1% and −59.8%, respectively).
Keywords: Prostate cancer treatment cost, Active surveillance, High value care, Medicare Fee Schedule, Electronic health records, Calculated cost per day
Take Home Message
We found that Active Surveillance costs less than surgery (−47.6%) and radiation (−68.2%) for favorable-risk cancer by 2 yr, although savings decreased by 5 yr (−5.6% and −37.8%, respectively). Surgery was less costly than radiation for unfavorable-risk disease at both 2 yr (−56.1%) and 5 yr (−59.8%).
1. Introduction
Prostate cancer is the most commonly diagnosed cancer in men and second leading cause of cancer-related deaths, with 192 000 new diagnoses and 33 000 deaths expected in 2020 [1]; yet the majority of cases are detected by screening, are slow growing, and will not become clinically evident during the patient's lifetime [2]. Active surveillance (AS) of low-risk cancers, which defers aggressive treatment until disease progression, is an increasingly followed management strategy [3]. This strategy aims to lower cost [4], [5] and decrease treatment-related morbidity without impacting survival [6]. However, changes in the risk composition of the patient population due to changing screening guidelines [7], [8], [9] and recent incorporation of expensive new technologies such as routine multiparametric magnetic resonance imaging (mpMRI) into surveillance regimens have likely increased cost [10], [11], [12].
Previous cost-effectiveness studies of management strategies for localized prostate cancer usually favor AS with follow-up durations under 10 yr, while those with longer follow-up support aggressive surgical or radiation treatment [4], [5], [6], [13], [14], [15]. However, these studies have important limitations. They rely on simulation of theoretical patients in which cost estimates reflect only services included in clinical trial protocols, which greatly differ from routine clinical care. Many studies assume that all patients’ decisions conform to those of the average patient, do not account for patient comorbidities, and do not account for deviation from standard care [16], [17], [18], [19], [20]. This is especially relevant in AS, which lacks consistent long-term protocols. Furthermore, existing literature relies on data collected before the dissemination of new high-cost technologies [10], [11], [12]. Given differences in patient populations and clinical outcomes between randomized trials and real-world data, these assumptions underlying previous studies are concerning.
The USA addressed the need to incorporate evidence from real-world data under the 21st Century Cures Act [21]. Specifically, clinical assertions should include evidence from routinely captured clinical data, including electronic health records (EHRs) [21]. In parallel, insurance companies are increasingly demanding proof of real-world effectiveness of treatments to support reimbursement decisions [9]. However, secondary use of EHRs is challenging. The data are noisy, and require repurposing of billing codes and use of artificial intelligence to process multimodal data, including clinical notes [22], [23]. For example, AS, which does not have a designated billing code, is difficult to identify reliably. In addition, it is challenging to obtain useful, reliable cost data that can be shared easily; closely guarded negotiated payments between hospitals and payors are proprietary, actual costs vary between payers, patients can change insurance coverage, and treatments have different densities of utilization and charges over time.
Understanding rising costs in routine care is therefore pertinent yet challenging, particularly in prostate cancer where incorporation of new high-cost technologies is coupled with an extended treatment course [24]; furthermore, patients are increasingly sharing in the burden of these rising costs [25], [26]. In this study using real-world data, we characterize initial management costs of prostate cancer at 2 and 5 yr following initial diagnosis. We leverage an existing cost-of-care methodology [27] and the US Medicare Fee Schedule [28] to produce risk-stratified estimates of average calculated cost per day (CCPD). This framework provides increased transparency in healthcare spending, which can facilitate innovation, targeted reform, and shared decision-making.
2. Patients and methods
2.1. Data sources and study cohort
We used a clinical data warehouse (CDW) that integrates patient-level clinical data from EHRs reflecting clinical care at a tertiary academic medical center and associated network practice sites [29]. The CDW included free-text clinical notes, Common Procedural Terminology (CPT) codes, and curated patient-specific tumor characteristics and treatment information from the California Cancer Registry [29]. We studied men aged 35–89 yr diagnosed during 2009–2018 with localized (nonmetastatic) prostate cancer seeking cancer care at our center (more than one urology or oncology office visit, pursued first-line management within 24 mo of diagnosis; Fig. 1). We identified surgery and radiation therapy from CPT codes, and AS with either structured methods or natural language processing of free clinical text (Supplementary material).
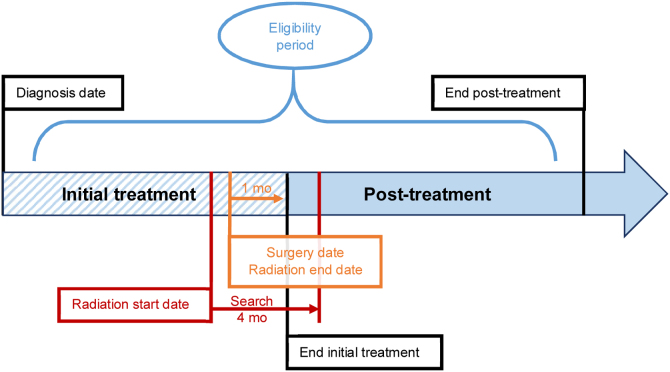
Fig. 1.
Cohort selection. CDW = clinical data warehouse; CPT = Common Procedural Terminology. aDiagnosis date identified as the first diagnostic code within the electronic health record (ICD9:185, ICD10:C61). bDefinitive treatments identified in the CDW by structured methods. cActive surveillance identified by structured data and natural language processing (Supplementary material). dEligible clinical encounters included unique dates compiled from encounters or CPT codes associated with eligible clinical specialty (urology, oncology, primary care), treatment dates, or prostate-specific antigen laboratory dates. eLast follow-up determined at the last eligible clinical encounter (refer to footnote “d”). fConcurrent malignancies identified by ICD codes for the top 10 cancers by US incidence other than prostate [1] (Supplementary material).
We excluded 788 (15.9%) patients with fewer than two encounters (urology, oncology, and primary care) or <6 mo of follow-up to remove second-opinion cases. We limited the study to primary prostate cancers by excluding 395 (9.4%) patients with pre-existing malignancies (Supplementary material). We excluded 355 (9.4%) patients lacking both Gleason grade group and stage (either clinical T or summary stage), as they could not be assigned cancer risk (Fig. 1).
Patients were classified as “unfavorable” if they had either stage ≥3 or Gleason grade group ≥3 disease and were otherwise classified to have a “favorable” risk. Age was calculated at diagnosis. Charlson Comorbidity Index at diagnosis was determined using active diagnoses in the patient's EHR over the past year. This study received approval from Stanford University's Institutional Review Board.
2.2. Outcomes
For each patient, all CPT codes were gathered with year of service and assigned a “cost” for each service, drug, or procedure by matching with the US Centers for Medicare and Medicaid Services (CMS) Medicare Fee Schedule and incorporating facility payments from CMS under the inpatient and relevant payment systems [27], [28], adjusted to 2017-US$ via the US Bureau of Economic Analysis GDP Implicit Price Deflator [30]. Receipt of mpMRI was determined through data mining of radiological reports.
For primary analyses, an episode of care was defined from the date of diagnosis to the last follow-up or the maximum study interval (24 or 60 mo), whichever is earlier. Calculations over 60 mo were restricted to patients with follow-up ≥4 yr. For secondary analyses, the episode of care was split at 30 d following initial therapy into “initial treatment” and “post-treatment surveillance” periods (Fig. 2). A time period of 1 mo after treatment was chosen to capture immediate complications and postoperative care. For each patient, all billing codes within the episode of care were collected, assigned costs, summed, and then divided by the episode's duration to yield CCPD. All billing codes within the given interval were used to capture most potential complications, rather than making a priori assumptions on relevant services since the analysis is comparative and one cannot be certain if indirect events such as a pneumonia were or were not related to the cancer or treatment. We expressed time in days instead of months or years to enable a realistic comparison between management strategies that differ in distribution of services over time.
Fig. 2.
Defining episodes of care for cost calculations. We conducted primary cost calculations for each individual patient by tabulating Common Procedural Terminology (CPT©) codes in the eligibility period defined as either the first 24 mo or 60 mo (Table 2) after the diagnosis date, using the earlier of the maximum eligibility period or duration from diagnosis to last follow-up as the time period for determining the calculated cost per day. Secondary analysis separately assessed the initial treatment and post-treatment component periods for patients receiving definitive management with surgery or radiation (24 mo: Table 2; 60 mo: Supplementary Table 4). As active surveillance (AS) has no distinction between initial treatment and post-treatment components, given that patients forgo definitive treatment in favor of carefully monitoring for disease progression, we assessed only AS in the primary analysis over the entire eligibility period. We defined the initial treatment period as the time from the diagnosis date to 1 mo after either the date of surgery or the date of last radiation treatment, while the post-treatment period comprised the remainder of the entire eligibility period. We chose the time period of 1 mo after treatment to capture immediate treatment complications and postoperative care. We determined the last radiation treatment code by searching the 4 mo following treatment start date; the period of 4 mo was chosen to ensure that codes were associated with initial and not subsequent treatment.
2.3. Statistical analysis
We separately compared men with favorable-risk and those with unfavorable-risk disease by treatment type. We plotted the density of healthcare encounters over time as the number of unique dates with at least one CPT code normalized by the number of uncensored patients in monthly bins. We assessed unadjusted CCPD using the Kruskal-Wallis test given a right-skewed cost distribution and implemented an analysis of covariance model with log-linear transform to provide the estimated mean CCPD adjusted for age and Charlson comorbidity. SQL was used for database extraction and calculation of CCPD within the CDW. Statistical analyses were performed using R version 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Study cohort and characteristics
A total of 3433 men were included in the study (Fig. 1), with a mean age of 65 yr, generally low comorbidities (78.1% Charlson 0), and predominantly (67.8%) favorable-risk disease; the sample comprised mostly non-Hispanic white individuals insured through Medicare or privately (Table 1). Surgery (54.6%) was most common, followed by radiation (22.3%) and then AS (23.0%). Compared with AS, surgery patients were younger with fewer comorbidities, privately insured, and non-Hispanic white. Radiation patients also had fewer comorbidities than AS patients but were older, insured through Medicare, and included more racial/ethnic minorities, with the greatest difference seen in black men (Table 1 and Supplementary Table 1). In comparison with surgery, radiation patients were more likely to have unfavorable disease (Table 1).
Table 1.
| Overall | AS | SUR | RAD | |
|---|---|---|---|---|
| Patients, n (% total) | 3433 | 791 (23.0) | 1875 (54.6) | 767 (22.3) |
| Age, mean (SD) | 64.6 (7.8) | 64.8 (7.7) | 62.9 (7.3) | 68.5 (7.7) |
| Charlson comorbidity, n (%) | ||||
| 0 | 2681 (78.1) | 535 (67.6) | 1565 (83.5) | 581 (75.7) |
| 1 | 102 (3.0) | 28 (3.5) | 48 (2.6) | 26 (3.4) |
| 2 | 511 (14.9) | 184 (23.3) | 218 (11.6) | 109 (14.2) |
| 3 | 71 (2.1) | 22 (2.8) | 24 (1.3) | 25 (3.3) |
| ≥4 | 68 (2.0) | 22 (2.8) | 20 (1.1) | 26 (3.4) |
| Race/ethnicity, n (%)b | ||||
| Non-Hispanic white | 2478 (72.2) | 572 (72.3) | 1388 (74.0) | 518 (67.5) |
| Asian | 428 (12.5) | 95 (12.0) | 218 (11.6) | 115 (15.0) |
| Black | 152 (4.4) | 28 (3.5) | 79 (4.2) | 45 (5.9) |
| Hispanic/Latino | 285 (8.3) | 51 (6.4) | 163 (8.7) | 71 (9.3) |
| Other/unknown | 90 (2.6) | 45 (5.7) | 27 (1.4) | 18 (2.3) |
| Insurance, n (%) | ||||
| Medicare | 1800 (52.4) | 454 (57.4) | 860 (45.9) | 486 (63.4) |
| Medicaid | 101 (2.9) | 25 (3.2) | 48 (2.6) | 28 (3.7) |
| Private | 1331 (38.8) | 279 (35.3) | 901 (48.1) | 151 (19.7) |
| Self-pay | 78 (2.3) | 19 (2.4) | 32 (1.7) | 27 (3.5) |
| Unknown | 123 (3.6) | 14 (1.8) | 34 (1.8) | 75 (9.8) |
| Unfavorable disease = 0/1, n (%)c | 2327/1106 (67.8/32.2) | 791/0 (100.0/0.0) | 1175/700 (62.7/37.3) | 361/406 (47.1/52.9) |
| Gleason grade group, n (%)c | ||||
| 1 | 1045 (30.4) | 589 (74.5) | 338 (18.0) | 118 (15.4) |
| 2 | 1273 (37.1) | 161 (20.4) | 869 (46.3) | 243 (31.7) |
| 3 | 536 (15.6) | 0 (0.0) | 388 (20.7) | 148 (19.3) |
| 4 | 228 (6.6) | 0 (0.0) | 104 (5.5) | 124 (16.2) |
| 5 | 274 (8.0) | 0 (0.0) | 163 (8.7) | 111 (14.5) |
| Unknown | 77 (2.2) | 41 (5.2) | 13 (0.7) | 23 (3.0) |
| Stage, n (%)c | ||||
| 1 | 1939 (56.5) | 561 (70.9) | 1059 (56.5) | 319 (41.6) |
| 2 | 929 (27.1) | 163 (20.6) | 519 (27.7) | 247 (32.2) |
| 3 | 184 (5.4) | 0 (0.0) | 106 (5.7) | 78 (10.2) |
| 4 | 57 (1.7) | 0 (0.0) | 40 (2.1) | 17 (2.2) |
| Unknown | 324 (9.4) | 67 (8.5) | 151 (8.1) | 106 (13.8) |
AS = active surveillance; RAD = radiation therapy; SD = standard deviation; SUR = surgery.
Patients were diagnosed with prostate cancer during 2009–2018.
Full demographics by race/ethnicity are given in Supplementary Table 1.
Unfavorable is defined as either stage ≥ 3 or Gleason grade group ≥ 3; patients missing both variables were unable to be assigned and excluded from analysis cohort.
All were significant at p < 0.001.
3.2. Healthcare system interactions
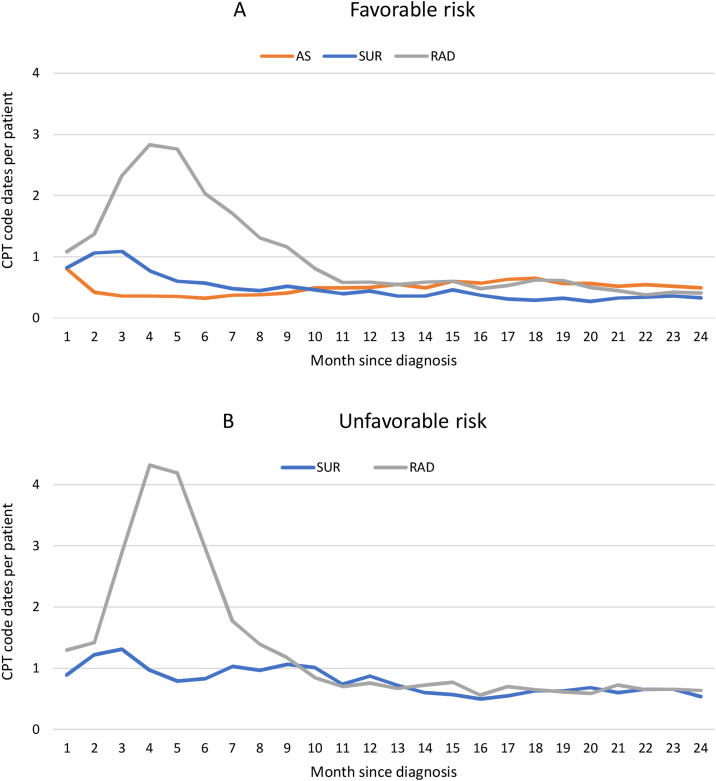
Favorable-risk patients had fewer encounters than their unfavorable counterparts. Over 2 yr, radiation had the most visits (18) followed by surgery (eight) and AS (eight), and unfavorable-risk patients undergoing radiation had more activity (43) than surgery patients (10). Differences were most evident in the first 6 mo and initial treatment period. When followed for 5 yr, AS had more (22) visits than surgery (12) but fewer than radiation (40; Fig. 3 and Table 2).
Fig. 3.
Density of healthcare encounters by first-line management strategy over time. Patients were diagnosed with (A) favorable- and (B) unfavorable-risk prostate cancer during 2009–2018. Unfavorable is defined as either stage ≥ 3 or Gleason grade group ≥ 3. Encounters are tabulated as unique dates with a CPT code normalized to total uncensored patients per monthly bin. AS = active surveillance; CPT = Common Procedural Terminology; RAD = radiation therapy; SUR = surgery.
Table 2.
Calculated cost per day (CCPD) by first-line management strategy a
| Favorable risk |
Unfavorable risk |
||||||
|---|---|---|---|---|---|---|---|
| AS | SUR | RAD | p value | SUR | RAD | p value | |
| Total 24-mo period | |||||||
| Patients, n (%) | 791 (23.0) | 1175 (34.2) | 361 (10.5) | 700 (20.4) | 406 (11.8) | ||
| Healthcare encounters, median (IQR) | 8 (4, 15) | 8 (5, 14) | 18 (6, 46) | <0.001 | 10 (5, 26) | 43 (16, 55) | <0.001 |
| Unadjusted CCPD, $/d (IQR) | 3.06 (1.55, 6.88) | 6.52 (4.32, 9.05) | 13.16 (4.09, 32.73) | <0.001 | 7.72 (4.59, 15.67) | 24.10 (11.72, 51.15) | <0.001 |
| Adjusted CCPD, $/d (95% CI) | 2.97 (2.73, 3.23) | 5.67 (5.29, 6.08) | 9.34 (8.26, 10.58) | <0.001 | 7.17 (6.48, 7.94) | 16.34 (14.26, 18.72) | <0.001 |
| Initial treatment component of 24 mo period | |||||||
| Patients, n | 1073 | 314 | 582 | 368 | |||
| Healthcare encounters, median (IQR) | 6 (3, 7) | 8 (2, 38) | <0.001 | 5 (2, 7) | 34 (6, 46) | <0.001 | |
| Unadjusted CCPD, $/d (IQR) | 35.05 (20.37, 48.68) | 47.44 (25.31, 93.75) | <0.001 | 37.91 (18.65, 53.34) | 79.96 (41.63, 174.70) | <0.001 | |
| Adjusted CCPD, $/d (95% CI) | 27.68 (25.92, 29.57) | 34.69 (30.64, 39.28) | 0.002 | 27.30 (24.75, 30.11) | 61.32 (54.07, 69.54) | <0.001 | |
| Post-treatment surveillance component of 24-mo period | |||||||
| Patients, n | 788 | 291 | 515 | 347 | |||
| Healthcare encounters, median (IQR) | 3 (0, 7) | 4 (1, 10) | <0.001 | 5 (0, 17) | 6 (1, 14) | 0.17 | |
| Unadjusted CCPD, $/d (IQR) | 1.08 (0.49, 3.64) | 1.26 (0.56, 3.02) | 0.66 | 2.96 (0.69, 18.90) | 1.91 (0.83, 4.74) | 0.001 | |
| Adjusted CCPD, $/d (95% CI) | 1.45 (1.30, 1.61) | 1.30 (1.09, 1.55) | 0.30 | 3.24 (2.79, 3.75) | 2.06 (1.71, 2.47) | <0.001 | |
| Total 60-mo period | |||||||
| Patients, n | 365 (22.9) | 591 (37.1) | 160 (10.1) | 306 (19.2) | 169 (10.6) | ||
| Healthcare encounters, median (IQR) | 22 (12, 46) | 12 (6, 26) | 40 (12, 70) | <0.001 | 22 (7, 60) | 66 (44, 86) | <0.001 |
| Unadjusted CCPD, $/d (IQR) | 3.38 (1.51, 6.19) | 2.96 (1.89, 4.34) | 6.09 (1.55, 22.48) | <0.001 | 3.88 (2.07, 10.83) | 19.71 (7.86, 26.10) | <0.001 |
| Adjusted CCPD, $/d (95% CI) | 2.71 (2.39, 3.06) | 2.87 (2.60, 3.16) | 4.36 (3.62, 5.26) | <0.001 | 4.15 (3.57, 4.83) | 10.32 (8.38, 12.71) | <0.001 |
AS = active surveillance; CCPD = calculated cost per day; CI = confidence interval; IQR = interquartile range; RAD = radiation therapy; SUR = surgery.
Patients were diagnosed with prostate cancer during 2009–2018. Unfavorable defined as either stage ≥3 or Gleason grade group ≥3. CCPD values were obtained from Medicare Fee Schedule [28] and presented in 2017 USD/d [30]. Episodes of care (Fig. 2) include the total period over the first 24 mo or 60 mo from diagnosis (primary analysis), and for patients receiving definitive management with SUR or RAD, additional subdivisions of initial treatment and post-treatment component periods of the 24 mo since diagnosis (see Supplementary Table 4 for 60-mo equivalents). Unadjusted CCPD was assessed by the Kruskal-Wallis test by ranks. Adjusted CCPD accounts for age and comorbidity via analysis of covariance log-linear models.
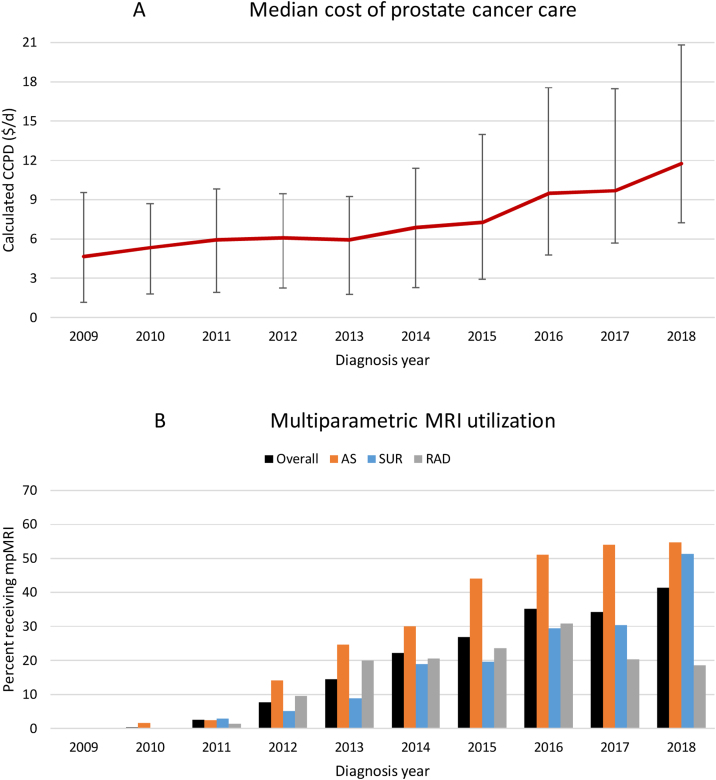
3.3. Costs of treatment
The median cost of care increased over the study period, with CCPD particularly increasing around 2013, which coincided with increasing utilization of mpMRI across all treatments, especially AS (Fig. 4). For favorable-risk patients, those undergoing AS had the lowest unadjusted CCPD ($3.06/d) over the first 2 yr after diagnosis, followed by surgery ($6.52/d) and radiation ($13.16/d; p < 0.001). After adjusting for age and Charlson comorbidity, AS retained the lowest CCPD ($2.97/d) compared with surgery ($5.67/d) or radiation ($9.34/d; p < 0.001). Surgery had significantly lower unadjusted ($35.05/d vs $47.44/d, p < 0.001) and adjusted ($27.68/d vs 34.69/d, p = 0.002) CCPD compared with radiation in the initial treatment period, but no significant difference was observed in the post-treatment surveillance period either before ($1.08/d vs $1.26/d, p = 0.7) or after ($1.45/d vs $1.30/d, p = 0.3) adjustment (Table 2). Assessing over 5 yr, unadjusted CCPD was lower for surgery ($2.96/d) than for AS ($3.38/d) or radiation ($6.09/d; p < 0.001), but after adjustment AS remained slightly cheaper ($2.71/d) than surgery ($2.87/d) or radiation ($4.36/d; p < 0.001; Table 2).
Fig. 4.
Increasing calculated cost per day (CCPD) and multiparametric MRI utilization. Patients were diagnosed with prostate cancer during 2009–2018. (A) Unadjusted CCPD calculated over 24 mo following the diagnosis date using Medicare Fee Schedule [28], presented in 2017 USD/d [30], with median and error bars representing 25–75th percentile. (B) Multiparametric MRI utilization is given as the percentage of patients with imaging within the first 24 mo following diagnosis. AS = active surveillance; CCPD = calculated cost per day; MRI = magnetic resonance imaging; RAD = radiation therapy; SUR = surgery.
For patients with unfavorable disease, CCPD was significantly lower for surgery than for radiation before ($7.72/d vs $24.10/d, p < 0.001) and after ($7.17/d vs $16.34/d, p < 0.001) adjusting for age and Charlson comorbidity over the first 2 yr, and surgery remained cheaper over 5 yr before ($3.88/d vs $19.71/d, p < 0.001) and after ($4.15/d vs $10.32/d, p < 0.001) adjustment. This difference was largest in the initial treatment period with surgery being significantly cheaper before ($37.91/d vs $79.96/d, p < 0.001) and after ($27.30/d vs $61.32/d) adjusting for age and comorbidity, while surgery was slightly more expensive than radiation for post-treatment surveillance before ($2.96/d vs $1.91/d, p = 0.001) and after ($3.24/d vs $2.06/d, p < 0.001) adjustment (Table 2).
4. Discussion
We developed a framework for comparing cost of care for localized prostate cancer using data from a real-world setting. We found that AS was the least costly strategy over the first 2 yr of management of favorable-risk tumors, providing savings of 47.6% and 68.2% compared with surgery and radiation, respectively, while savings were much smaller by 5 yr at 5.6% and 37.8%, respectively. At both 2 and 5 yr, surgery was cheaper than radiation in both favorable (39.3% and 34.2%, respectively) and unfavorable (56.1% and 59.8%, respectively) risk. Diminishing savings with AS at longer time intervals likely represents continued costs of surveillance as well as reclassification and treatment of some AS patients compared with predominantly one-time definitive treatments for patients with favorable risk. The introduction of expensive technologies such as mpMRI appears to coincide with increasing costs of care, although for now AS remains a cheaper strategy despite increasing utilization. Given the differences in age and comorbidity between surgery and radiation, it is reassuring that these relationships remained consistent before and after adjusting CCPD. We additionally provide new data on the distribution of healthcare system interactions over time, demonstrating a concentration of services in the initial treatment period with radiation therapy involving the greatest intensity of visits. Our findings support the view that AS can be a preferable treatment for favorable-risk localized prostate cancer, providing both higher quality of life and up-front cost savings, although these savings appear to diminish over more extended timeframes.
Our findings leverage real-world actual practice data that include new high-cost technologies to provide cost estimates for prostate cancer management in routine clinical care. Our study has the strength that it reflects the realities of actual clinical care that may deviate from guidelines or clinical trial protocols that typically underpin the assumptions used to design traditional cost-effectiveness modeling studies. Further, our approach is transparent and generalizable, and can easily be implemented in any claims or EHR-based data ecosystem where codes reflecting services can be linked to a fee schedule; in our US study, we link CPT codes with the Medicare Fee Schedule and hospital facility payment systems, but these could be substituted for studies in other delivery systems. The cost estimates we attribute to AS, surgery, and radiation and initial up-front savings provided by AS that diminish with extended follow-up are in line with prior reviews [12] and simulation studies [5], [6], [13], [14] that did not consider new high-cost technologies such as mpMRI in their design. Interestingly, one recent simulation that considered the role of mpMRI in AS, as compared with traditional transrectal ultrasound-guided biopsy, found that mpMRI was cost effective only at lengthy 5-yr surveillance intervals at Medicare rates with substantial sensitivity to price, being no longer cost effective at private charge [31]. Given that all these studies use simulated data extrapolated from clinical trials, they all make assumptions regarding which services and charges to assign to each treatment pathway, resulting in an unclear picture whether high-cost technology is impacting the potential cost savings of AS. Our estimates derive from actual practice without reliance on such assumptions, and we demonstrate initial savings with AS; however, prostate cancer has a protracted course, and we found savings substantially diminished with assessment over 5 compared with 2-yr intervals. Further work will be needed to determine whether these cost relationships hold or reverse with even more distant time horizons, as suggested by modeling studies.
Few studies have attempted to use real-world data to assess costs in localized prostate cancer, with most limited in scope to smaller cohorts using Spanish [4] or German [15] data with limited applicability to the US setting. The sole US study was limited to a Medicare-derived population that was generally older than 75 yr and was therefore ineligible for AS rather than for watchful waiting [32]. While these reports found cost savings with forms of delayed treatment, none included recent data after the introduction of mpMRI, which experienced a rapid 486% increase in utilization between 2013 and 2015 according to one study [10]. While some work has explored real-world costs of new treatments within radiotherapy [12], [24], similar comparative estimates between different management strategies such as AS, surgery, and radiation are absent. Given the need for more current real-world cost data, particularly in the USA, our study sought to fill this gap. We have provided comparative estimates demonstrating that AS continues to deliver up-front savings despite recent changes in the clinical landscape, although these diminish as follow-up increases. CCPD will be a useful tool for future work aimed to explore the drivers of increasing cost of care.
4.1. Limitations
Information was derived from a single healthcare system, and patterns in regional practices or local population attributes may limit generalizability to other settings; therefore, CCPD must be validated further in other healthcare networks. As it is challenging to maintain extended patient follow-up in real-world data, we were able to assess CCPD through 5 yr only, necessitating further work to assess longer time frames. Although the network contains an academic hospital, a community hospital, and a specialty care alliance, patient activity outside the network may not be captured, leading to an underestimation of costs. Medicare reimbursements are typically less than those from private payors, which would further underestimate the costs. An assessment of actual costs from payment exchanges would require access to closely guarded proprietary accounting data. However, CCPD benchmarks as proxy for cost adequately suited our purposes to obtain comparative measures, especially given that private insurance companies use Medicare prices as a benchmark for setting their own prices [33], and we anticipate that CCPD's shortcomings are likely distributed uniformly and therefore impact relative comparisons minimally. We believe that such comparable benchmarks are more useful for understanding the trends in healthcare costs by focusing on trends in delivery of services rather than on intricacies of constantly changing negotiated rates that generate heterogeneous payment exchanges that vary within institutions among payors and patient plans. Future work will need to address these limitations by assessing costs in other systems, determining areas driving rising costs, and understanding relationships between costs and clinical outcomes.
5. Conclusions
AS is a viable management strategy that can be encouraged to optimize the quality of life in select patients with favorable-risk disease; using real-world data, we found that initial costs may be reduced with AS, although these savings may not hold for patients followed over extended periods. Generally, definitive treatment with surgery appears less costly than radiation in both favorable and unfavorable disease.
There is a lack of high-quality real-world cost data despite the fact that the widespread presence of EHR data ecosystems, which we demonstrate, can be harnessed to obtain comparable cost benchmarks such as CCPD that avoid traditional challenges to cost transparency. These methods are widely generalizable for application to other areas of clinical care and, for example, have also been applied to assess breast cancer survivorship care [27], another application with treatment options varying in duration and intensity. It is essential to supplement traditional modeling and decision analysis with the understanding of real-world costs of new technologies and management strategies to inform recommendations and identify opportunities to promote high-value care. Therefore, further resources should be devoted to harnessing EHR data for these purposes.
Author contributions: Tina Hernandez-Boussard had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Magnani, Baker, Brooks, Blayney, Hernandez-Boussard.
Acquisition of data: Magnani, Bievre, Hernandez-Boussard.
Analysis and interpretation of data: Magnani, Bievre, Baker, Brooks, Blayney, Hernandez-Boussard.
Drafting of the manuscript: Magnani.
Critical revision of the manuscript for important intellectual content: Magnani, Bievre, Baker, Brooks, Blayney, Hernandez-Boussard.
Statistical analysis: Magnani.
Obtaining funding: Hernandez-Boussard.
Administrative, technical, or material support: Hernandez-Boussard.
Supervision: Hernandez-Boussard.
Other: None
Financial disclosures: Tina Hernandez-Boussard certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award number R01CA183962. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Christopher J. Magnani was supported by the Stanford University MedScholars program. These organizations were not involved in management of data, analysis, or preparation of the manuscript.
Acknowledgments: We would like to acknowledge Tina Seto, MS, for her assistance with managing the CDW and linkage of the Medicare Fee Schedule, as well as Selen Bozkurt, PhD, for her assistance with conceptualizing the statistical approach.
As these data contain patient identifiers, data sharing is not permitted under the constraints of the Institutional Review Board. However, the statistical code will be considered if the proposed use aligns with public good purposes, does not conflict with other requests, contingent on approval from the local ethics committee. Requests can be addressed to the corresponding author.
Associate Editor: Guillaume Ploussard
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:10.1016/j.euros.2020.11.004.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Bell K.J.L., Del Mar C., Wright G., Dickinson J., Glasziou P. Prevalence of incidental prostate cancer: a systematic review of autopsy studies. Int J Cancer. 2015;137:1749–1757. doi: 10.1002/ijc.29538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiner A.B., Patel S.G., Etzioni R., Eggener S.E. National trends in the management of low and intermediate risk prostate cancer in the United States. J Urol. 2015;193:95–102. doi: 10.1016/j.juro.2014.07.111. [DOI] [PubMed] [Google Scholar]
- 4.Pozo C., Hernández V., Capitán C. A comprehensive analysis of cost of an active surveillance cohort compared to radical prostatectomy as primary treatment for prostate cancer. World J Urol. 2019;37:1297–1303. doi: 10.1007/s00345-018-2500-7. [DOI] [PubMed] [Google Scholar]
- 5.Keegan K.A., Dall’Era M.A., Durbin-Johnson B., Evans C.P. Active surveillance for prostate cancer compared with immediate treatment: an economic analysis. Cancer. 2012;118:3512–3518. doi: 10.1002/cncr.26688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koerber F., Waidelich R., Stollenwerk B., Rogowski W. The cost-utility of open prostatectomy compared with active surveillance in early localised prostate cancer. BMC Health Serv Res. 2014;14:163. doi: 10.1186/1472-6963-14-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roach M., Thomas K. Overview of randomized controlled treatment trials for clinically localized prostate cancer: implications for active surveillance and the United States preventative task force report on screening? J Natl Cancer Inst Monographs. 2012;2012:221–229. doi: 10.1093/jncimonographs/lgs039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grossman D.C., Curry S.J., Owens D.K. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1901–1913. doi: 10.1001/jama.2018.3710. [DOI] [PubMed] [Google Scholar]
- 9.Magnani C.J., Li K., Seto T. PSA testing use and prostate cancer diagnostic stage after the 2012 U.S. Preventive Services Task Force guideline changes. J Natl Compr Canc Netw. 2019;17:795–803. doi: 10.6004/jnccn.2018.7274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oberlin D.T., Casalino D.D., Miller F.H., Meeks J.J. Dramatic increase in the utilization of multiparametric magnetic resonance imaging for detection and management of prostate cancer. Abdom Radiol. 2017;42:1255–1258. doi: 10.1007/s00261-016-0975-5. [DOI] [PubMed] [Google Scholar]
- 11.Fam M.M., Yabes J.G., Macleod L.C. Increasing utilization of multiparametric magnetic resonance imaging in prostate cancer active surveillance. Urology. 2019;130:99–105. doi: 10.1016/j.urology.2019.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schroeck F.R., Jacobs B.L., Bhayani S.B., Nguyen P.L., Penson D., Hu J. Cost of new technologies in prostate cancer treatment: systematic review of costs and cost effectiveness of robotic-assisted laparoscopic prostatectomy, intensity-modulated radiotherapy, and proton beam therapy. Eur Urol. 2017;72:712–735. doi: 10.1016/j.eururo.2017.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma V., Wymer K.M., Borah B.J. Cost-effectiveness of active surveillance, radical prostatectomy and external beam radiotherapy for localized prostate cancer: an analysis of the ProtecT trial. J Urol. 2019;202:964–972. doi: 10.1097/JU.0000000000000345. [DOI] [PubMed] [Google Scholar]
- 14.Hayes J.H., Ollendorf D.A., Pearson S.D. Observation versus initial treatment for men with localized, low-risk prostate cancer: a cost-effectiveness analysis. Ann Intern Med. 2013;158:853–860. doi: 10.7326/0003-4819-158-12-201306180-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brandes A., Koerber F., Schwarzkopf L., Hunger M., Waidelich R., Rogowski W. Cost-effectiveness of radical prostatectomy, radiation therapy and active surveillance for the treatment of localized prostate cancer—a claims data analysis. Value Health. 2014;17:A636–A637. doi: 10.1016/j.jval.2014.08.2287. [DOI] [PubMed] [Google Scholar]
- 16.Smyth B., Haber A., Trongtrakul K. Representativeness of randomized clinical trial cohorts in end-stage kidney disease: a meta-analysis. JAMA Intern Med. 2019;179:1316–1324. doi: 10.1001/jamainternmed.2019.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chari A., Romanus D., Palumbo A. Randomized clinical trial representativeness and outcomes in real-world patients: comparison of 6 hallmark randomized clinical trials of relapsed/refractory multiple myeloma. Clin Lymphoma Myeloma Leuk. 2020;20 doi: 10.1016/j.clml.2019.09.625. 8–17.e16. [DOI] [PubMed] [Google Scholar]
- 18.Fortin M., Dionne J., Pinho G., Gignac J., Almirall J., Lapointe L. Randomized controlled trials: do they have external validity for patients with multiple comorbidities? Ann Fam Med. 2006;4:104–108. doi: 10.1370/afm.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kennedy-Martin T., Curtis S., Faries D., Robinson S., Johnston J. A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results. Trials. 2015;16:495. doi: 10.1186/s13063-015-1023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dall’Era M.A., Albertsen P.C., Bangma C. Active surveillance for prostate cancer: a systematic review of the literature. Eur Urol. 2012;62:976–983. doi: 10.1016/j.eururo.2012.05.072. [DOI] [PubMed] [Google Scholar]
- 21.Public Law 114-255: 21st Century Cures Act. https://www.congress.gov/114/plaws/publ255/PLAW-114publ255.pdf.
- 22.Hersh W.R., Weiner M.G., Embi P.J. Caveats for the use of operational electronic health record data in comparative effectiveness research. Medical Care. 2013;51:S30. doi: 10.1097/MLR.0b013e31829b1dbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernandez-Boussard T., Blayney D.W., Brooks J.D. Leveraging digital data to inform and improve quality cancer care. Cancer Epidemiol Biomarkers Prev. 2020;29:816–822. doi: 10.1158/1055-9965.EPI-19-0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobs B.L., Zhang Y., Skolarus T.A., Hollenbeck B.K. Growth of high-cost intensity-modulated radiotherapy for prostate cancer raises concerns about overuse. Health Aff (Millwood) 2012;31:750–759. doi: 10.1377/hlthaff.2011.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imber B.S., Varghese M., Ehdaie B., Gorovets D. Financial toxicity associated with treatment of localized prostate cancer. Nat Rev Urol. 2020;17:28–40. doi: 10.1038/s41585-019-0258-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Claxton G., Rae M., Long M., Damico A., Whitmore H. Health benefits in 2018: modest growth in premiums, higher worker contributions at firms with more low-wage workers. Health Aff (Millwood) 2018;37:1892–1900. doi: 10.1377/hlthaff.2018.1001. [DOI] [PubMed] [Google Scholar]
- 27.Blayney D.W., Lindquist C., Seto T., Hoang N.M., Kurian A.W. Computing the cost of care per day of breast cancer survivor care. JCO. 2018;36(7_suppl):10. [Google Scholar]
- 28.U.S. Centers for Medicare & Medicaid Services. Fee Schedules. PFS National Payment Amount File 2017. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Relative-Value-Files.
- 29.Seneviratne M.G., Seto T., Blayney D.W., Brooks J.D., Hernandez-Boussard T. Architecture and implementation of a clinical research data warehouse for prostate cancer. EGEMS (Wash DC) 2018;6:13. doi: 10.5334/egems.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.U.S. Bureau of Economic Analysis. National Income and Product Accounts. Table 1.1.9. Implicit price deflators for gross domestic product. https://apps.bea.gov/iTable/iTable.cfm?reqid=19&step=2#reqid=19&step=2&isuri=1&1921=survey.
- 31.Sathianathen N.J., Konety B.R., Alarid-Escudero F., Lawrentschuk N., Bolton D.M., Kuntz K.M. Cost-effectiveness analysis of active surveillance strategies for men with low-risk prostate cancer. Eur Urol. 2019;75:910–917. doi: 10.1016/j.eururo.2018.10.055. [DOI] [PubMed] [Google Scholar]
- 32.Trogdon J.G., Falchook A.D., Basak R., Carpenter W.R., Chen R.C. Total Medicare costs associated with diagnosis and treatment of prostate cancer in elderly men. JAMA Oncol. 2019;5:60–66. doi: 10.1001/jamaoncol.2018.3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clemens J., Gottlieb J.D. In the shadow of a giant: Medicare's influence on private physician payments. J Polit Econ. 2017;125:1–39. doi: 10.1086/689772. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.