Abstract
The study explored factors associated with intention to be screened for Alzheimer’s disease (AD). The study also examined whether self-efficacy mediates the relationship between knowledge about screening and the intention to be screened for AD. A population-based, random-digit dialing survey was performed and 1,043 responses were collected from a sample of nondemented persons (50 years or older) living in urban, suburban, and rural areas in a Midwestern state. The findings showed that participants who were younger and who had higher levels of (a) perceived benefits and barriers, (b) social support, and (c) self-efficacy reported higher levels of intention to be screened for AD. Older adults with positive life orientation reported greater intention to be screened for AD, whereas depressed participants were more likely to report a plan to be screened for AD. Self-efficacy mediated the relationship between knowledge about screening and intention to be screened. Older adults were more likely to report intention to be screened when they had positive attitudes about the screen and believed that they could receive the screen. The intention to be screened for AD could serve public awareness by defining effective ways to assist older adults to seek a cognitive screen.
Keywords: Alzheimer’s disease, health behaviors, intention, screen, self-efficacy
Introduction
Alzheimer’s disease (AD), a progressive neurodegenerative disorder, shows progressive cognitive decline in memory, learning, and language, and other cognitive abilities that interfere with functioning and activities of daily life (Alzheimer’s Association, 2019a; American Psychiatric Association, 2013). It is estimated that 5.6 million older Americans 65 years or older are living with AD in 2019 (Alzheimer’s Association, 2019b) and the number is projected to triple to 13.8 million by 2050.
Although AD is the most common type of neurocognitive disorder (McInnis-Dittrich, 2019), no cure has been identified. Because symptoms associated with AD worsen gradually over time, older adults need intensive care and assistance as the disease progresses, which results in burden for family caregivers (Montgomery, Goren, Kahle-Wrobleski, Nakamura, & Ueda, 2018). Therefore, early screen, diagnosis, and treatment are necessary to delay progression of the disease (Panegyres, Berry, & Burchell, 2016), reduce health care costs and caregiver burden, and improve quality of life (DeKosky, 2003).
Early screening, diagnosis, and treatment
There are many comprehensive AD screening tools available for use; generally, they are of two types; (a) performance tests conducted by clinicians to determine whether cognitive, affective, and/or behavior problems are present in patients (e.g., Mini-Mental State Exam [MMSE], clock drawing test, Montreal Cognitive Assessment [MoCA]); and (b) informant tests, which use an observant collateral source to assess changes in cognition and daily activities (e.g., Informant Questionnaire on Cognitive Decline in the Elderly; Galvin, 2012). These dementia screening tools are most useful at the earliest detectable signs of disease when they reflect pathology and biomarkers changes related to AD (Galvin, 2018). Using biomarkers as an additional measure in a medical setting will aid health care providers in identifying the underlying pathology for the observed symptoms and confirm the presence of AD before AD symptoms emerge (Tolea & Galvin, 2013). Early screening and diagnosis may delay progression of AD, improve clinical outcomes, and keep older adults with AD in the community (Corbyn, 2013; Holsinger, Boustani, Abbot, & Williams, 2011; Maki & Yamaguchi, 2014; Panegyres et al., 2016). Early diagnosis could contribute to comprehensive treatment for patients who experience a mild stage of AD before progressing to advanced levels of AD that could interfere with activities of daily living and quality of life (Borson et al., 2013; Maki & Yamaguchi, 2014).
Early screening and diagnosis could also allow family members to be involved in decisions about care and advanced long-term care and to have access to appropriate social support and resources (e.g., legal arrangements for disease progression; Galvin, 2018; Maki & Yamaguchi, 2014; Relkin, 2000), which may reduce hospitalizations (Lin, Fillit, Cohen, & Neumann, 2013) and lead to improvement in quality of life (Iliffe, Manthorpe, & Eden, 2003; Maki & Yamaguchi, 2014; Montgomery et al., 2018). Although several benefits of early screening and detection have been identified, a significant number of older adults who are referred to be screened for AD or other dementias do not receive screening (Ashford et al., 2007; Boustani, Peterson, Hanson, Harris, & Lohr, 2003). Thus, diagnosis of AD is often undetected or underdiagnosed in the primary care setting (Galvin, 2018; Valcour, Masaki, Curb, & Blanchette, 2000). This may be due to fear associated with a diagnosis of AD or the stigma related to dementia (Corner & Bond, 2004; Holsinger et al., 2011). Older adults may avoid cognitive testing, especially if they are experiencing memory problems (Corner & Bond, 2004; Devlin, MacAskill, & Stead, 2007). In addition, there is concern about adverse effects associated with early screening, including risk of misdiagnosis, emotional impact and stigma, and medical cost and time (Boustani et al., 2003; Bunn et al., 2012). Despite such concerns, early screening serves as an indicator for further clinical assessment, resulting in care cost savings (hospital, nursing homes; Borson et al., 2013), secondary prevention, and planning for long-term care (Bradford, Kunik, Schulz, Williams, & Singh, 2009; Lischka, Mendelsohn, Overend, & Forbes, 2012).
Psychological Factors in Intention to Promote Health Behaviors
In studies of screening for chronic conditions, psychological factors associated with screening were identified (Hay, McCaul, & Magnan, 2006; Hirai et al., 2013). In particular, emotional distress, such as depression and anxiety, that could affect cognitive focus and motivation may be associated with the patient’s willingness to adhere to medical treatments (DiMatteo, Lepper, & Croghan, 2000).
Depressive symptoms are associated with decreased health behaviors and adherence to risk-reducing behaviors for chronic illnesses, including hypertension (Bonnet, Irving, Terra, Nony, Berthezène, & Moulin, 2005) and diabetes (Gharaibeh, Gajewski, Al-smadi, & Boyle, 2016). For example, individuals with depressive symptoms are more likely to fail to use appropriate self-regulatory exercise (i.e., effort to change cognitive, emotional, motivational resources to exercise) and to engage in fewer preventative health behaviors (e.g., reducing exercise or physical activity; Pomp, Lippke, Fleig, & Schwarzer, 2010). Depressed persons displayed less action planning for exercise and nutrition as a form of diabetes self-management (Teal, Haidet, Balasubramanyan, Rodriguez, & Naik, 2012) and increased difficulty in meeting the challenges of barriers to exercise (Vickers, Nies, Patten, Dierkhising, & Smith, 2006).
In addition to depression, older adults have anxiety or fear about being diagnosed with AD (Corner & Bond, 2004; Devlin et al., 2007); however, less is known about how anxiety could affect seeking a cognitive screening (Lundquist, 2012). The study findings show that anxiety can prohibit older adults from seeking cognitive testing (Corner & Bond, 2004). Older adults who are knowledgeable about AD may avoid screening for AD to alleviate their anxiety about the condition (Corner & Bond, 2004; Devlin et al., 2007). It is possible that those with knowledge about AD may consider that an early diagnosis of AD would not benefit them and thus they would be less motivated to be screened (Corner & Bond, 2004; Devlin et al., 2007).
Self-efficacy, as proposed by Bandura (1977, 2006), refers to the belief in the ability to perform activities and duties specific to promoting preventive health (Bandura, 2006). Increased self-efficacy is the most important factor in behavioral change (Glanz, Rimer, & Viswanath, 2008). Individuals with higher levels of self-efficacy are more likely to sustain healthy behaviors and promote health (e.g., physical activity, exercise [Sperber et al., 2014], breast self-examination [Aikman, Doyle-Portillo, Verhaeghen, & Simmons, 2017]) because they often consider obstacles as challenges to overcome (Maibach & Murphy, 1995).
Conceptual Model
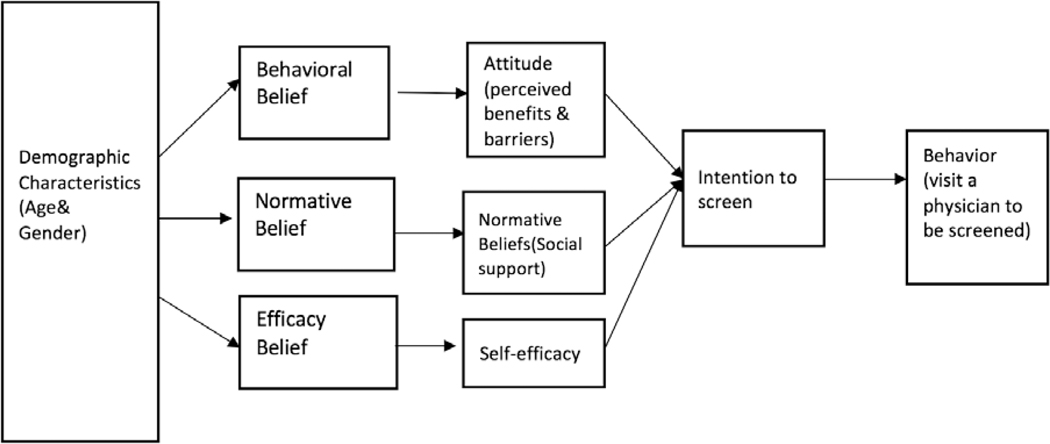
The integrated behavioral model (IBM) developed by Fishbein (Fishbein & Yzer, 2003) was designed to examine factors that predict intention and engagement in health behaviors. IBM integrates the models used in health behavior research (Branscum & Lora, 2017). A modified version of IBM (Figure 1) was used in this study as a conceptual framework to explore nondemented older adults’ intention to be screened for AD. IBM posits that people tend to engage in a health behavior (e.g., visiting a health care provider to screen for AD) based on their determination of the importance of that behavior to their health. According to the modified IBM, if older adults have a strong intention to be screened, it is important to ensure that they have sufficient knowledge about screening to act on this intention and that no serious environmental constraints prevent them from getting the screening (Montano & Kasprzyk, 2008). In this model, the most important determinant is intention based on attitude, perceived norm, and self-efficacy with respect to being screened (Fishbein & Yzer, 2003).
Figure 1.
A modified integrated behavioral model of intention to be screened
In the current study, intention to be screened was predicted by (a) an individual’s attitude toward being screened for AD (i.e., cognitively based belief about positive or negative consequences of health behaviors [Montano, Tshimanga, Hamilton, Gorn, & Kasprzyk, 2018]), (b) perceived norms (i.e., beliefs about whether important referent persons would support them to promote health behaviors) about being screened for AD, and (c) self-efficacy (i.e., perceived ability to overcome obstacles to behavior performance) to be screened for AD (see Figure 1; Bandura, 2006; Fishbein & Yzer, 2003).
In general, positive attitude and perceived norms result in greater perceived control and increase the likelihood of intentions governing changes in behavior (Montano & Kasprzyk, 2008). A person’s perceived norm is determined by individual normative beliefs about whether important referent persons will approve or disapprove of the behavior, weighted by motivation to comply with those referents (Montano & Kasprzyk, 2008). However, it is unclear whether IBM can be applied to intention to be screened for AD. The current study was designed to assess the three constructs associated with intention to be screened for AD. The IBM was selected as the model to reflect the expected complexity related to intention to be screened for AD. Specifically, we explored whether the intention to be screened for AD is primarily a result of attitudinal, normative, or self-efficacy beliefs.
Purpose of the Study
This study expanded on a prior study (Galvin, Fu, Nguyen, Glasheen, & Scharff, 2008), using the data collected from the same participants to address the following three research questions. The previous study (Galvin et al., 2008) explored perceived benefits, knowledge of dementia, preventive behaviors, self-efficacy, and perceived susceptibility based on the Behavioral Model of Health Service Use, associated with intention to be screened for AD.
In the current study, we examined whether the aforementioned three constructors from IBM (attitudes, perceived norms, self-efficacy) were significantly associated with intention to be screened for AD. In addition, we expanded the previous findings by exploring whether self-efficacy exerted a mediation effect in the relationship between knowledge about screening and intention to be screened for AD. In addition, we examined depression, anxiety, and life orientation as potential factors for intention to be screened for AD.
Research Question 1: Based on the IBM, are attitudes toward being screened for AD, perceived norms about being screened, and self-efficacy significantly associated with intention to be screened for AD?
Research Question 2: Are psychological factors (i.e., depression, anxiety, and life orientation) associated with intention to be screened for AD, after controlling for age, gender, family history of AD, education level, and long-term care insurance?
Research Question 3: Does self-efficacy mediate the association between knowledge about screening and intention to be screened for AD?
Materials and Methods
Design
A cross-sectional design was used; each participant participated in a single computer-assisted telephone interview.
Participants
Nondemented participants residing in the St. Louis, Missouri, area were recruited. Inclusion criteria were (a) age 50 years or older, (b) nondemented (a weighted score of ≤ 6 on the Short-Blessed test); (c) speak English; and (d) willing to complete the telephone survey.
Sampling Strategies
A population-based sample was recruited in three Missouri counties: St Louis City (urban), St Louis County (suburban), and Adair County (rural). To recruit eligible participants, random digit dialing (RDD) was used for probability sampling. A commercial software (GeneSys Inc, Washington, PA) was used to generate the telephone numbers. This study examined the health perceptions of an aging population and how these perceptions might relate to decisions to screen for AD.
Study Procedure
This study was approved by the Institutional Review Board of the participating university and met the requirements of the Health Information Portability and Accessibility Act (HIPAA). Potential participants were contacted by the Center for Health Care Quality at the participating university. A computer-assisted telephone interview was conducted. During the telephone contact, the first family member who consented to participate in the project was interviewed. After verbal informed consent was obtained, participants were screened for dementia using a telephone version of the Blessed Test (Kawas, Karagiozis, Resau, Corrada, & Brookmeyer, 1995) before participating in the survey. Upon completion of the study, participants received a $10 gift card to a local grocery store as compensation for their time and effort.
Data Collection
Measure for outcome variable: Intention to be screened for AD
To address the research questions, intention to be screened for AD was assessed as an outcome measure, based on four items that explore intention under various circumstances (Galvin, Scharff, Glasheen, & Fu, 2006; Galvin et al., 2008): (a) plan to be screened for AD at some point in life, (b) plan to be screened for AD in the next year, (c) plan to be screened for AD after the participant reaches a certain age; or (d) plan to be screened for AD in the presence of symptoms for AD. Each item is rated on a 10-point Likert-type scale ranging from strongly disagree (1) to strongly agree (10). The scale has shown moderate internal consistency (Cronbach’s α = .62; Galvin et al., 2006).
Measures for independent variables
Attitude.
As a construct of IBM, attitude toward screening for AD was measured by (a) perceived benefits and (b) perceived barriers. Both scales were developed by Galvin et al. (2006) because attitude was defined as perceived belief about positive (perceived benefits) and negative (perceived barriers) outcomes of being screened for AD. Based on the Health Belief Model (Becker, Maiman, Kirscht, Haefner, & Drachman, 1977), perceived barriers were assessed by seven items (e.g., “burden to others,” afraid to find AD”) and perceived benefits were measured by four items (e.g., “screening test make an early diagnosis”). Each item is rated on a 10-point Likert-type scale from 1 = Strongly Disagree, to 10 = Strongly Agree). Internal consistency for the perceived benefits and perceived barriers to screening measures is moderate (α = .75, α = .70, respectively; Galvin et al., 2006).
Perceived norms.
Perceived norms, as a construct of IBM, were measured by social support belief (Galvin et al., 2006), which includes 4 items scored on a 10-point Likert-type scale from 1 = Strongly Disagree to 10 = Strongly Agree). The items are related to perceived norms (belief about social support received by the participant), such as “my spouse, or other significant others, would support me getting a screening test for memory loss.” Reliability has been reported as α = .44.
Self-efficacy.
Self-efficacy is a construct of IBM and was assessed by the self-efficacy scale for getting a memory test. It includes 7 items (e.g., “I am confident I can get a screening test for memory loss if I have symptoms of memory loss”) with a 10-point Likert-type scale ranging from 1 = Strongly Disagree to 10 = Strongly Agree. The self-efficacy scale assesses participants’ perceived confidence about receiving a screening test for memory loss in general. Reliability was reported as α = .83 (Galvin et al., 2006).
Hospital Anxiety and Depression Scale (HADS).
Depression and anxiety were measured using the HADS (Zigmond & Snaith, 1983) to identify psychological factors associated with intention to be screened for AD. The HADS includes 14 questions, including 7 that measure anxiety (HAD-A) and 7 that measure depression (HAD-D). In this study, depression and anxiety were summarized separately. Each item is rated on a 4-point Likert-type scale (0 to 3). Total scores range from 0 to 21 for the anxiety and depression subscales separately. A sum score of 0 to 7 points is interpreted as normal, 8 to 10 points as mild, and 11 to 21 points as moderate to severe difficulty with regard to each subscale (Zigmond & Snaith, 1983). The internal consistency coefficient (Cronbach’s alpha) was .81 for the HADS and .78 and .71 for the anxiety and depression subscales, respectively (Helvik, Engedal, Skancke, & Selbaek, 2011).
Life orientation.
The Life Orientation Test-Revised (LOT-R) was used to assess dispositional optimism, measured on a continuum on which optimism and pessimism are polar opposites (Scheier & Carver, 1985). The LOT-R is a 10-item measure of optimism versus pessimism: optimism (3 items), pessimism (3 items), and fillers (4 items). Respondents are asked to indicate the degree to which they agree with the items on a 5-point Likert-type scale ranging from 0 = Strongly Disagree to 4 = Strongly Agree. Responses to the negatively worded items are reverse scored and higher scores indicate higher optimism. Scores range from 0 to 12 (Hinz et al., 2017). The subscales and total instrument showed moderate or high reliability scores: optimism (α = .70), pessimism (α = .63 to .74), and total score (α = .66 to .68; Glaesmer et al., 2012; Hinz et al., 2017).
Knowledge about the screening test.
Knowledge about the screening test was measured using a tool developed by Galvin et al. (2006). The 11 items measure the level of knowledge about the screening test (e.g., “I have looked for information about screening tests for memory loss in books, magazines, newspapers, or other sources”). The 10-point Likert-type response scale range from 1 = Strongly Disagree to 10 = Strongly Agree. Internal consistency of the test is moderate (Cronbach’s α = .71; Galvin et al., 2006).
Data Analyses
Data analyses were conducted using SPSS (version 26). Descriptive statistics were performed to calculate frequencies or mean and standard deviation (SD) for demographic variables. An independent-samples t test was conducted to measure the association of participants’ demographic characteristics with their intention to be screened for AD (i.e., gender [1 = male, 2 = female], long-term care insurance [0 = not enrolled, 1 = currently enrolled], family history of AD [0 = no, 1 = yes]).
To address Research Question 1, a linear regression with hierarchical data entry was performed to examine whether three constructs of IBM were associated with intention to be screened for AD in nondemented older adults. Three constructs were included in the regression analysis: attitudes (perceived benefits and perceived barriers), perceived norms (social support belief), and self-efficacy (self-efficacy). Age and gender were entered as Step 1 and perceived barriers and benefits, perceived social support, and self-efficacy were entered as Step 2.
To explore psychological factors associated with intention to be screened for AD (Research Question 2), linear regression with hierarchical data entry was conducted to identify predictors of intention to be screened for AD after controlling for age, gender, family history of AD, education level, and use of long-term care insurance. The controlling variables of age, gender, family history of AD, education level, and long-term care insurance were entered into the regression model as Step 1, followed by independent variables (depression, anxiety, and life orientation) in Step 2.
To test self-efficacy as a mediating effect in the association between knowledge about screening test and intention to be screened for AD (Research Question 3), mediation analyses were completed using PROCESS (Hayes, 2017). The bootstrapping methods (Preacher & Hayes, 2008) were used, generating 5,000 bootstrapping samples. For the analysis, a 95% confidence interval (CI) was applied. The indirect effects of the association between knowledge about screening test and intention to be screened were assessed, as was the 95% bias-corrected confidence interval (CI) for each specific indirect effect. The Preacher and Hayes (2008) PROCESS approach included knowledge about a screening test as the independent variable, self-efficacy as the mediator, intention to be screened for AD as the dependent variable, and age and gender as control variables. A total of specific indirect effects was examined, and self-efficacy was included to mediate the association between knowledge about the screening test and intention to be screened. (Indirect effects in which the 95% CI does not include zero reflect a significant mediation effect.)
Results
Sample characteristics
A total of 1,043 participants were included in the data analyses. Demographic characteristics are presented in Table 1. Two thirds of the participants (66.8%) were female. Participants ranged in age from 50 to 97 years (M = 62.6, SD = 10.2). A majority of the participants were White (82.7%, n = 863) and more than half had attended or completed college (64.6%, n = 673). Approximately 40% (n = 408) lived in an urban area and 23.8% (n = 248) lived in a rural area. In terms of demographic difference in intention to be screened, female participants indicated greater intention to be screened (t = 2.412, p = .016). Less than one third of the participants (26.1%, n = 272) were currently enrolled in long-term care insurance. There was a significant difference in intention to be screened (a) between women (M = 24.5, SD = 12.3) and men (M = 22.5, SD = 11.6), t = −1.983, p = .048, and (b) between participants with long-term care insurance (M = 25.7, SD = 15.0) and those without long-term care insurance (M = 23.2, SD = 10.8), t = 2.097, p = .037 (Table 2). The mean score for intention to be screened for memory loss or AD was 22.8 (SD = 7.02; Max = 40, Min = 6).
Table 1.
Demographic characteristics of the participants (N = 1,043)
| Characteristic and category | n | % |
|---|---|---|
| Age (Mean = 62.6, SD = 10.2, Range = 50–97) | ||
| Gender | ||
| Female | 697 | 66.8 |
| Male | 346 | 33.2 |
| Race | ||
| White | 863 | 82.7 |
| Black or African American | 121 | 11.6 |
| Hispanic | 13 | 1.2 |
| Asian | 6 | 0.6 |
| Native Hawaiian/Pacific Islander | 3 | 0.3 |
| American Indian | 8 | 0.8 |
| Marital status | ||
| Married | 488 | 46.8 |
| Divorced | 211 | 20.2 |
| Widowed | 199 | 19.1 |
| Never married | 104 | 10.0 |
| Separated | 26 | 2.5 |
| Unmarried couple | 15 | 1.4 |
| Highest education level | ||
| Partial college | 278 | 26.7 |
| High school graduate | 269 | 25.8 |
| College graduate | 203 | 19.5 |
| Graduate professional training | 192 | 18.4 |
| Geographic location | ||
| Urban | 408 | 39.1 |
| Suburban | 341 | 32.7 |
| Rural | 248 | 23.8 |
| Household income | ||
| $20,000 to < $25,000 | 615 | 59.0 |
| $15,000 to < $20,000 | 309 | 29.6 |
| $10,000 to < $15,000 | 73 | 7.0 |
| Physical health | ||
| Poor | 57 | 5.5 |
| Fair | 203 | 19.5 |
| Neutral | 58 | 5.6 |
| Good | 492 | 47.2 |
| Excellent | 191 | 18.3 |
| Family history of Alzheimer’s disease | ||
| Yes | 239 | 22.9 |
| No | 741 | 71.0 |
| Health insurance | ||
| Yes | 928 | 89.0 |
| No | 72 | 6.9 |
Table 2.
Difference in gender, family history of Alzheimer’s disease (AD), and long-term insurance in intention to be screened
| Demographic variables | Mean | SD | t | P |
|---|---|---|---|---|
| Gender (n = 667) | ||||
| Male | 22.5 | 11.6 | −1.983 | .048 |
| Female | 24.5 | 12.3. | ||
| Family History of AD (n = 650) | ||||
| Yes | 24.3 | 7.9 | .371. | .710 |
| No | 23.9 | 13.7 | ||
| Long-Term Insurance (n = 666) | ||||
| Yes | 25.7 | 15.0 | 2.097 | .037 |
| No |
Testing three constructors in the theoretical model of IBM
The overall model was significant, n = 700, F(6, 288) = 2.943, p < .0005, accounting for 13.0 % of the variance in the intention to be screened for AD by the set of three constructs (attitudes, perceived norms, and self-efficacy). Regression analysis indicated that age, perceived barriers and benefits (attitude), perceived social support (perceived norm), and self-efficacy were significantly associated with the intention to be screened. Individuals who are younger (β = −.219, p < .0005), higher level of perceived barriers (β = .075, p < .0005) and perceived benefits (β = .244, p < .0005), higher social support (β = .274, p < .0005), and self-efficacy (β = .170, p = .0008) were associated with increased intention to be screened (Table 3).
Table 3.
Linear regression analysis for association between each of three constructs and intention to be screened for Alzheimer’s disease (N = 700)
| Variable | B | SE B | β | t | p |
|---|---|---|---|---|---|
| Model 1. R2 = .748 | |||||
| Age | −0.015 | 0.014 | −.047 | −1.083 | .280 |
| Gender | 14.111 | 0.686 | .899 | 20.571 | .000 |
| Model 2. R2 = .860 | |||||
| Age | −0.069 | 0.011 | −.219 | −6.147 | .000 |
| Gender | 1.173 | 1.026 | .075 | 1.143 | .254 |
| Attitudes | |||||
| Perceived barriers | 0.288 | 0.048 | .374 | 6.007 | .000 |
| Perceived benefits | 0.159 | 0.034 | .244 | 4.685 | .000 |
| Perceived Norms | |||||
| Social support | 0.183 | 0.042 | .274 | 4.375 | .000 |
| Self-Efficacy | |||||
| Self-efficacy | 0.086 | 0.032 | .170 | 2.652 | .008 |
Psychological factors associated with intention to be screened for AD
Preliminary analysis was conducted to ensure no violation of the assumption of normality, linearity, multicollinearity, and homoscedasticity (Cohen, Cohen, West, & Aiken, 2003). In particular, although depression and anxiety are similar concepts, no multicollinearity was identified (r = .253). The overall regression model was significant, n = 700, F(8, 675) = 3.318, p = .001. The set of independent variables (anxiety, depression, and life orientation) accounted for 3.8% of the variance in the intention to be screened.
Regression analysis identified that gender, depression, and life orientation were significantly associated with the intention to be screened. Female participants (β = .080, p = .036), participants who were more depressed (β = .149, p < .0005), and participants with a positive life orientation (β = .120, p = .004) were more likely to indicate intention to be screened for memory issues or AD (Table 4).
Table 4.
Linear regression analysis for intention to be screened (N = 700)
| Variable | B | SE B | β | t | p |
|---|---|---|---|---|---|
| Model 1. R2 = .010 | |||||
| Age | −0.003 | 0.009 | −.011 | −0.289 | .772 |
| Gender | 2.014 | 1.028 | .075 | 1.959 | .051 |
| Family history of AD | −0.705 | 0.543 | −.050 | −1.299 | .194 |
| Education | −0.056 | 0.186 | −.011 | −0.299 | .765 |
| Long-term care insurance | −0.509 | 0.509 | −.039 | −0.999 | .318 |
| Model 2. R2 = .038 | |||||
| Age | −0.001 | 0.009 | −.006 | −0.159 | .915 |
| Gender | 2.151 | 1.025 | .080 | 2.099 | .036 |
| Family history of AD | −0.911 | 0.543 | −.064 | −1.679 | .094 |
| Education | −0.095 | 0.185 | −.019 | −0.512 | .609 |
| Long-term care insurance | −0.599 | 0.508 | −.045 | −1.179 | .239 |
| Anxiety | −0.360 | 0.231 | −.066 | −1.558 | .120 |
| Depression | 0.801 | 0.217 | .149 | 3.696 | .000 |
| Life orientation | 0.313 | 0.108 | .120 | 2.897 | .004 |
Self-efficacy as mediation effect
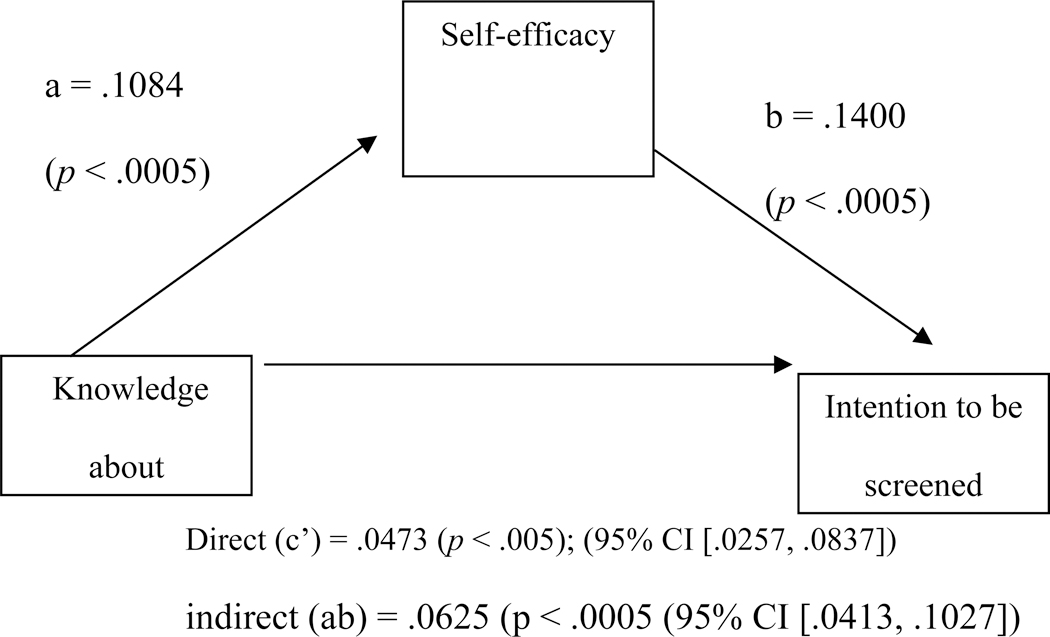
Self-efficacy mediated the association between knowledge about a screening test and intention to be screened for AD (Models on mediation effects are presented in Figure 2). The results from the bootstrapping methods displayed an indirect or mediating effect of self-efficacy (β = .2668, SE = .0148, t = 7.3137, 95% CI = .0793 to .1374, p < .0005) and the total effects were significant (β = .2140, SE = .0108, 95% CI = .0413 to .0837, p < .0005). This led to the conclusion that self-efficacy mediated the positive relationship between knowledge about the screening test and intention to be screened (Figure 2).
Figure 2.
Unstandardized regression coefficient(β) for the association between knowledge about the screening test and intention to be screened as mediated by self-efficacy
Discussion
With regard to the association between participants’ demographic characteristics and their intention to be screened, female participants showed a higher level of intention to be screened, which is consistent with previous studies on screening for disease, in which women were significantly more likely to report screening intention for HIV testing (Liu, 2011) or AD (Tang et al., 2017). It is possible that female participants visited their family physicians and had more opportunities to discuss their health concern more often than did male participants. Participants with long-term care insurance had a higher level of intention to participate in a cognitive screening than those without insurance, probably because long-term care insurance can cover more medical tests and procedures, as well as long-term services, so that insured participants visit health care providers without worrying about medical costs.
The three constructs (i.e., attitudes, perceived norms, and self-efficacy) were significantly related to the intention to be screened for AD, which confirms the modified theoretical IBM. Positive attitude (higher perceived benefits and higher barriers) toward being screened, higher perceived norms, and higher self-efficacy were related to nondemented older adults’ intention to be screened for AD. Among the three constructs, attitude was the strongest predictor associated with intention to be screened for AD, followed by perceived norms. There was a relationship between attitude toward a cognitive screening and intention to be screened, indicating contradictions: Perceived benefits (e.g., Screening tests can make an early diagnosis of memory loss) were associated with increased intention to be screened, while perceived barriers (e.g., I would be more of a burden to others if I were diagnosed earlier with Alzheimer’s disease) were associated with increased intention to be screened. Unlike the dementia study by Werner (2003), higher levels of perceived burden were associated with increased intention to be screened for AD. It appears that older adults are motivated to be screened before symptoms are advanced, which encourages plans for long-term care, including medical power of attorney or living will, and reduces the burden on family caregivers. However, the previous findings are contradictory. In findings on perceived barriers in an Australian study (Phillipson, Magee, Jones, Reis, & Skaldzien, 2015), attitudes associated with fears of stigma or discrimination were significantly linked with intention to delay or avoid seeking help. This is in contrast to several studies in which perceived barriers were negatively associated with intention by older adults with a family history of AD to undergo genetic testing for AD (Werner, 2003).
Older adults are more likely to seek cognitive screening if they have perceived norms (i.e., beliefs about whether important referent persons will support older adults in being screened for AD). Older adults who believe that they will receive social support from family members, significant others, and the family physician are more likely to express intention to be screened for AD. This finding is consistent with previous findings that social support was an important factor associated with increased participation in health promotion (Albrecht & Goldsmith, 2003; Strine, Chapman, Balluz, & Mokdad, 2008) or preventive health behaviors (Harvey & Alexander, 2012), such as a colorectal cancer screening (Honda & Kagawa-Singer, 2006). This finding, in particular, highlights that emotional family support (how much does your family understand the way you feel about things?) or friend support were associated with engaging in colorectal cancer screening.
As psychological risk factors associated with intention to be screened, older adults with positive life orientation reported greater intention to be screened for AD. Those with positive life orientation are more likely to promote a healthful lifestyle and improve motivation toward self-care, which increases intention to be screened for AD (Scheier & Carver, 1992). Positive orientation is the tendency to view life with a positive perspective (Fagnani, Medda, Stazi, Caprara, & Alessandri, 2014), which reflects older adults’ ability to cope with difficulties and life changes in a way that identifies meaningful stimulus for personal growth and development (Eloranta, Arve, Isoaho, Lehtonen, & Viitanen, 2015). While positive life orientation is highly related to intention to be screened, participants with higher depressive symptoms were more likely to report a plan to be screened for AD. It is plausible that depressed older adults show impairment in concentration and are slow in executive function, which is one of the key diagnostic criteria of depression that overlaps with AD (Potter & Steffens, 2007); thus, depressed older adults are likely to be worried about their symptoms and show increased intention to seek a cognitive screening. However, the findings are inconsistent with previous research findings that participants with more depressive symptoms were less likely to be screened for AD (Dale, Hemmerich, Hill, Hougham, & Sachs, 2008).
This study was designed to test the mediation of self-efficacy in the association between older adults’ knowledge about screening and their intention to be screened for AD. To our knowledge, this study is the first attempt to examine self-efficacy as having a mediating role between knowledge about screening and intention to be screened. The findings indicated that older adults with more knowledge about cognitive screening were more likely to have increased self-efficacy, which leads to increased intention to seek screening for AD. Based on the previous study findings (Galvin et al., 2008) that older individuals with a higher level of knowledge about AD symptoms and self-efficacy were significantly more likely to report intention to seek professional help, the current study, using self-efficacy as a mediation effect, indicates that older adults with knowledge about screening will understand the benefits of early screening and diagnosis, which is more likely to improve self-efficacy (i.e., gain confidence to consult with a physician), and thus be more likely to have intention to be screened. However, knowledge about screening for AD is not sufficient to result in action; self-efficacy to seek screening (i.e., confidence in how to get a screening test) will enhance intention to be screened. The findings imply that enhancing self-efficacy should be considered as an integral part in interventions to improve positive health behaviors.
The study results are consistent with the literature on health behavior in chronic conditions such as HIV (Wolf et al., 2007), diabetes self-care (Ishikawa, Takeuchi, & Yano, 2008), cancer screening (Friedman, Puryear, Moore, & Green, 2005), and colorectal cancer screening (Von Wagner, Semmler, Good, & Wardle, 2009). Self-efficacy was reported to mediate the relationship between limited health literacy and adherence to HIV medication (Wolf et al., 2007). Persons with higher self-efficacy are more likely to show intention for be screened for cancer. Participants with lower health literacy scores had lack of knowledge about colorectal screening at the end of the task than those with higher scores (Friedman et al., 2005).
Limitations, strengths, and implications
Intention to be screened could translate clinically to a motivation to discuss cognitive challenges with a health care provider and to accept screening and treatment recommendations (Galvin et al., 2006). Based on the findings in this study health care providers could address issues related to AD individually with patients as they apply, or in a population-based manner through media education or other methods (Galvin et al., 2008).
Limitations
Several limitations in this study are acknowledged. The cross-sectional design limited the ability to infer causality. Future research could use a longitudinal design to examine how older adults increase intention to be screened and eventually promote their health behavior by being screened for AD. Although the study assessed intention to be screened for AD as an outcome variable, the study did not measure whether the participants actually received cognitive testing. All responses on both instruments were based on self-report. In addition, more psychometric testing is required to establish reliability and validity of the instrument measuring intention and other independent variables, although reliability and validity were established in Galvin’s study (Galvin et al., 2006). Participants’ responses may have been biased, and the reported beliefs may not represent actual beliefs.
Strengths
A number of studies have focused on dementia screening and diagnosis and treatment of AD; however, little research has examined the motivation to promote health behaviors in older adults. Unlike other studies using convenience sampling with a relatively small sample size (e.g., Montgomery et al., 2018), the respondents in the current study were a representative sample of gender, race, and geographic location, resulting from use of a random-sample population-based strategy (Galvin et al., 2008). Including both urban and rural areas resulted in equal representation of women and African Americans in the sample. In addition to geographic representation, the study participants were representative of a broad spectrum of socioeconomic status, including occupation and educational levels (Galvin et al., 2008). The sample was assumed to be representative of socioeconomic classes, occupations, and educational levels. Thus, the demographic characteristics were consistent with census demographics for Missouri at the time of the survey (Galvin et al., 2008).
Implications
Increasing participation in screening a health behavior and detecting cognitive impairment is important for preventing increased prevalence of AD. However, effective interventions for increasing screening for cognitive impairment have not been identified (Harada et al., 2017). In a previous study (Lawrence et al., 2003), Screening Day in a community center was offered to nondemented older adults. However, this approach is temporary rather than continuous (Lawrence et al., 2003). Furthermore, little information about cognitive screening and various types about such screening have been provided to older adults in the community (Borson et al., 2013). It is important to help older adults and their family members to understood AD symptoms and to be aware of the need for cognitive screening for AD and other dementias (Borson et al., 2013). Many family caregivers do not understand symptoms related to AD and other dementias or know how to provide care until the family member is diagnosed with AD. Health education programs are often provided at senior centers and congregated facilities to promote health behaviors by older adults. In such programs, educational sessions should be provided to family members as well as to older adults on a regular basis so that the tangible benefits of receiving cognitive testing can be presented, along with support in being evaluated for cognitive disorders (Borson et al., 2013).
Screening is the initial step to discover cognitive impairment, and public awareness is important in bringing about acceptance of screening and in reducing negative perceptions regarding detection of cognitive impairment (Relkin, 2000). Although primary care physicians provide routine physical check-ups, they may not provide cognitive assessments and may not suggest cognitive screening during clinic visits. Health care professionals, including primary care physicians, should strongly recommend cognitive screening to their older patients. Since there is no consensus on how to conduct routine cognitive screening in older adults or how to move forward once results of such testing are obtained, validated methods are necessary to include large-scale, population-based screening for AD or other dementias (Relkin, 2000).
Acknowledgements:
We thank the participants who completed the telephone interview.
Funding: The study was funded by the Alzheimer’s Association [IIRG 03-5578]; National Institute on Aging [R01 AG20764,AGO5681]; National Institute of Neurological Disorders and Stroke [NINDS; R01 NS101483], and the American Federation for Aging Research.
Footnotes
Declaration of interest statement: This manuscript has not been published or submitted simultaneously for publication elsewhere.
References
- Aikman SN, Doyle-Portillo S, Verhaeghen P, & Simmons N (2017). The effect of instruction point of view on self-efficacy for performing breast self-exams. American Journal of Health Education, 48(1), 1–10. [Google Scholar]
- Albrecht TL, & Goldsmith DJ (2003). Social support, social networks, and health In Thompson TL, Dorsey AM, Miller KI, & Parrott R (Eds.), Handbook of health communication (pp. 263–284). Mahwah, NJ: Erlbaum. [Google Scholar]
- Alzheimer’s Association. (2019a). Alzheimer’s disease facts and figures. Retrieved from http://www.alz.org/facts/
- Alzheimer’s Association. (2019b). What is Alzheimer’s disease? Retrieved from https://www.alz.org/alzheimers-dementia/what-is-alzheimers
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: Author. [Google Scholar]
- Ashford JW, Borson S, O’Hara R, Dash P, Frank L, Robert P, … Kraemer HC (2007). Should older adults be screened for dementia? It is important to screen for evidence of dementia! Alzheimer’s & Dementia, 3(2), 75–80. 10.1002/gps.1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura A (1977). Self-efficacy: Toward a unifying theory of behavioral change. Psychological Review, 84, 191–215. [DOI] [PubMed] [Google Scholar]
- Bandura A (2006). Guide for constructing self-efficacy scales In Pajares F, & Urdan T (Eds.), Selfefficacy beliefs of adolescents pp. 307–337. Greenwich, CT: Information Age Publishing. [Google Scholar]
- Becker MH, Maiman LA, Kirscht JP, Haefner DP, & Drachman RH (1977). The Health Belief Model and prediction of dietary compliance: A field experiment. Journal of Health and Social Behavior, 18, 348–366. [PubMed] [Google Scholar]
- Bonnet F, Irving K, Terra JL, Nony P, Berthezène F, & Moulin P (2005). Depressive symptoms are associated with unhealthy lifestyles in hypertensive patients with the metabolic syndrome. Journal of Hypertension, 23(3), 611–617. [DOI] [PubMed] [Google Scholar]
- Borson S, Frank L, Bayley PJ, Boustani M, Dean M, Lin PJ, . . . Stefanacci RG (2013). Improving dementia care: The role of screening and detection of cognitive impairment. Alzheimer’s & Dementia, 9(2), 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boustani M, Peterson B, Hanson L, Harris R, & Lohr KN (2003). Screening for dementia in primary care: A summary of the evidence for the U.S. Preventative Services Task Force. Annals of Internal Medicine, 138, 927–937. [DOI] [PubMed] [Google Scholar]
- Bradford A, Kunik ME, Schulz P, Williams SP, & Singh H (2009). Missed and delayed diagnosis of dementia in primary care: Prevalence and contributing factors. Alzheimer Disease and Associated Disorders, 23, 306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branscum P, & Lora K (2017). Using the integrated behavioral model of prediction to predict maternal monitoring of fruit and vegetable consumption among Hispanic mothers. Family & Community Health, 40(1), 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn F, Goodman C, Sworn K, Rait G, Brayne C, Robinson L, . . . Iliffe S (2012). Psychosocial factors that shape patient and carer experiences of dementia diagnosis and treatment: A systematic review of qualitative studies. PLoS Medicine, 9, e1001331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West S, & Aiken L (2003). Applied multiple regression/correlation analysis for the behavioral sciences (3rd ed.). Mahwah, NJ: Erlbaum. [Google Scholar]
- Corbyn Z (2013). New set of Alzheimer’s trials focus on prevention. The Lancet, 381, 614–615. [DOI] [PubMed] [Google Scholar]
- Corner L, & Bond J (2004). Being at risk of dementia: Fears and anxieties of older adults. Journal of Aging Studies, 18(2), 143–155. [Google Scholar]
- Dale W, Hemmerich J, Hill EK, Hougham GW, & Sachs GA (2008). What correlates with the intention to be tested for mild cognitive impairment (MCI) in healthy older adults? Alzheimer Disease & Associated Disorders, 22(2), 144–152. [DOI] [PubMed] [Google Scholar]
- DeKosky S (2003). Early intervention is key to successful management of Alzheimer disease. Alzheimer Disease & Associated Disorders, 17, S99–S104. [DOI] [PubMed] [Google Scholar]
- Devlin E, MacAskill S, & Stead M (2007). “We’re still the same people”: Developing a mass media campaign to raise awareness and challenge the stigma of dementia. International Journal of Nonprofit and Voluntary Sector Marketing, 12(1), 47–58. [Google Scholar]
- DiMatteo MR, Lepper HS, & Croghan TW (2000). Depression is a risk factor for noncompliance with medical treatment: Meta-analysis of the effects of anxiety and depression on patient adherence. Archives of Internal Medicine, 160(14), 2101–2107. [DOI] [PubMed] [Google Scholar]
- Eloranta S, Arve S, Isoaho H, Lehtonen A, & Viitanen M (2015). Factors connected with positive life orientation at age 70, 80, 85 and 90: The Turku Elderly Study. Scandinavian Journal of Caring Sciences, 29(3), 537–547. [DOI] [PubMed] [Google Scholar]
- Fagnani C, Medda E, Stazi MA, Caprara GV, & Alessandri G (2014). Investigation of age and gender effects on positive orientation in Italian twins. International Journal of Psychology, 49, 453–461. [DOI] [PubMed] [Google Scholar]
- Fishbein M, & Yzer MC (2003). Using theory to design effective health behavior interventions. Communication Theory, 13(2), 164–183. [Google Scholar]
- Friedman LC, Puryear LJ, Moore A, & Green CE (2005). Breast and colorectal cancer screening among low-income women with psychiatric disorders. Psycho-Oncology: Journal of the Psychological, Social and Behavioral Dimensions of Cancer, 14, 786–791. [DOI] [PubMed] [Google Scholar]
- Galvin JE (2012). Optimizing diagnosis and management in mild-to-moderate Alzheimer’s disease. Neurodegenerative Disease Management, 2(3), 291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin JE (2018). Using informant and performance screening methods to detect mild cognitive impairment and dementia. Current Geriatrics Reports, 7(1), 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin JE, Fu Q, Nguyen JT, Glasheen C, & Scharff DP (2008). Psychosocial determinants of intention to screen for Alzheimer’s disease. Alzheimer’s & Dementia, 4, 353–360. doi: 10.1016/j.jalz.2007.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin JE, Scharff DP, Glasheen C, & Fu Q (2006). Development of a population-based questionnaire to explore psychosocial determinants of screening for memory loss and Alzheimer disease. Alzheimer Disease & Associated Disorders, 20(3), 182–191. [DOI] [PubMed] [Google Scholar]
- Gharaibeh B, Gajewski BJ, Al-smadi A, & Boyle DK (2016). The relationships among depression, self-care agency, self-efficacy and diabetes self-care management. Journal of Research in Nursing, 21(2), 110–122. [Google Scholar]
- Glanz K, Rimer BK, & Viswanath K (Eds.). (2008). Health behavior and health education: Theory, research, and practice. New York, NY: Wiley. [Google Scholar]
- Glaesmer H, Rief W, Martin A, Mewes R, Brähler E, Zenger M, & Hinz A (2012). Psychometric properties and population-based norms of the Life Orientation Test Revised (LOT-R). British Journal of Health Psychology, 17, 432–445. [DOI] [PubMed] [Google Scholar]
- Harada K, Lee S, Shimada H, Lee S, Bae S, Anan Y, . . . Suzuki T (2017). Psychological predictors of participation in screening for cognitive impairment among community-dwelling older adults. Geriatrics & Gerontology International, 17(8), 1197–1204. [DOI] [PubMed] [Google Scholar]
- Harvey IS, & Alexander K (2012). Perceived social support and preventive health behavioral outcomes among older women. Journal of Cross-Cultural Gerontology, 27(3), 275–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay JL, McCaul KD, & Magnan RE (2006). Does worry about breast cancer predict screening behaviors? A meta-analysis of the prospective evidence. Preventive Medicine, 42(6), 401–408. [DOI] [PubMed] [Google Scholar]
- Hayes AF (2017). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York, NY: Guilford. [Google Scholar]
- Helvik AS, Engedal K, Skancke RH, & Selbæk G (2011). A psychometric evaluation of the Hospital Anxiety and Depression Scale for the Medically Hospitalized Elderly. Nordic Journal of Psychiatry, 65, 338–344. [DOI] [PubMed] [Google Scholar]
- Hinz A, Sander C, Glaesmer H, Brähler E, Zenger M, Hilbert A, & Kocalevent RD (2017). Optimism and pessimism in the general population: Psychometric properties of the Life Orientation Test (LOT-R). International Journal of Clinical and Health Psychology, 17(2), 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K, Harada K, Seki A, Nagatsuka M, Arai H, Hazama A, . . . Shibuya D (2013). Structural equation modeling for implementation intentions, cancer worry, and stages of mammography adoption. Psychooncology, 22(10), 2339–2346. [DOI] [PubMed] [Google Scholar]
- Holsinger T, Boustani M, Abbot D, & Williams JW (2011). Acceptability of dementia screening in primary care patients. International Journal of Geriatric Psychiatry, 26, 373–379. [DOI] [PubMed] [Google Scholar]
- Honda K, & Kagawa-Singer M (2006). Cognitive mediators linking social support networks to colorectal cancer screening adherence. Journal of Behavioral Medicine, 29, 449–460. [DOI] [PubMed] [Google Scholar]
- Iliffe S, Manthorpe J, & Eden A (2003). Sooner or later? Issues in the early diagnosis of dementia in general practice: A qualitative study. Family Practice, 20, 376–381. [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Takeuchi T, & Yano E (2008). Measuring functional, communicative, and critical health literacy among diabetic patients. Diabetes Care, 31, 874–879. [DOI] [PubMed] [Google Scholar]
- Kawas C, Karagiozis H, Resau L, Corrada M, & Brookmeyer R (1995). Reliability of the Blessed Telephone Information-Memory-Concentration Test. Journal of Geriatric Psychiatry and Neurology, 8, 238–242. [DOI] [PubMed] [Google Scholar]
- Lawrence JM, Davidoff DA, Katt-Lloyd D, Connell A, Berlow YA, & Savoie JA (2003). Is large-scale scale community memory screening feasible? Experience from a regional memory screening day. Journal of the American Geriatrics Society, 51, 1072–1078. [DOI] [PubMed] [Google Scholar]
- Lin PJ, Fillit HM, Cohen JT, & Neumann PJ (2013). Potentially avoidable hospitalizations among Medicare beneficiaries with Alzheimer’s disease and related disorders. Alzheimer’s & Dementia, 9(1), 30–38. [DOI] [PubMed] [Google Scholar]
- Liu L (2011). Gender differentials in factors associated with HIV testing intentions in Kenya: An application of the health belief model. Journal of Public Health and Epidemiology, 3(12), 567–575. [Google Scholar]
- Lischka AR, Mendelsohn M, Overend T, & Forbes D (2012). A systematic review of screening tools for predicting the development of dementia. Canadian Journal on Aging/La Revue Canadienne Du Vieillissement, 31, 295–311. [DOI] [PubMed] [Google Scholar]
- Lundquist TS (2012). Effects of knowledge and anxiety on willingness to screen for Alzheimer’s disease (Unpublished master’s thesis). University of Massachusetts, Amherst. [Google Scholar]
- Maibach E, & Murphy DA (1995). Self-efficacy in health promotion and practice: Conceptualization and measurement. Health Education Research, 10, 37–50. [Google Scholar]
- Maki Y, & Yamaguchi H (2014). Early detection of dementia in the community under a community-based integrated care system. Geriatrics & Gerontology International, 14(2), 2–10. 10.1111/ggi.12259 [DOI] [PubMed] [Google Scholar]
- McInnis-Dittrich K (2019). Social work with older adults: A biopsychosocial approach to assessment and intervention (5th ed.). Boston, MA: Pearson. [Google Scholar]
- Montano DE, & Kasprzyk D (2008). Theory of reasoned action, theory of planned behavior, and the integrated behavioral model In Glanz K, Rimer BK, & Viswanath K (Eds.), Health behavior and education: Theory, research and practice (4th ed., pp. 67–92). San Francisco, CA: Jossey-Bass. [Google Scholar]
- Montano DE, Tshimanga M, Hamilton DT, Gorn G, & Kasprzyk D (2018). Evidence-based identification of key beliefs explaining infant male circumcision motivation among expectant parents in Zimbabwe: Targets for behavior change messaging. AIDS and Behavior, 22, 479–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery W, Goren A, Kahle-Wrobleski K, Nakamura T, & Ueda K (2018). Detection, diagnosis, and treatment of Alzheimer’s disease dementia stratified by severity as reported by caregivers in Japan. Neuropsychiatric Disease and Treatment, 14, 1843–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panegyres PK, Berry R, & Burchell J (2016). Early dementia screening. Diagnostics, 6(1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipson L, Magee C, Jones S, Reis S, & Skaldzien E (2015). Dementia attitudes and help-seeking intentions: An investigation of responses to two scenarios of an experience of the early signs of dementia. Aging & Mental Health, 19, 968–977. [DOI] [PubMed] [Google Scholar]
- Pomp S, Lippke S, Fleig L, & Schwarzer R (2010). Synergistic effects of intention and depression on action control: Longitudinal predictors of exercise after rehabilitation. Mental Health and Physical Activity, 3(2), 78–84. [Google Scholar]
- Potter GG, & Steffens DC (2007). Contribution of depression to cognitive impairment and dementia in older adults. The Neurologist, 13(3), 105–117. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, & Hayes AF (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods, 40, 879–891. [DOI] [PubMed] [Google Scholar]
- Relkin N (2000). Screening and early diagnosis of dementia. American Journal of Managed Care, 6 (22), S1111–S1118. [PubMed] [Google Scholar]
- Scheier MF, & Carver CS (1985). Optimism, coping, and health: Assessment and implications of generalized outcome expectancies. Health Psychology, 4(3), 219–247. [DOI] [PubMed] [Google Scholar]
- Scheier MF, & Carver CS (1992). Effects of optimism on psychological and physical well-being: Theoretical overview and empirical update. Cognitive Therapy and Research, 16(2), 201–228. [Google Scholar]
- Sperber N, Hall KS, Allen K, DeVellis BM, Lewis M, & Callahan LF (2014). The role of symptoms and self-efficacy in predicting physical activity change among older adults with arthritis. Journal of Physical Activity and Health, 11, 528–535. [DOI] [PubMed] [Google Scholar]
- Strine TW, Chapman DP, Balluz L, & Mokdad AH (2008). Health-related quality of life and health behaviors by social and emotional support: Their relevance to psychiatry and medicine. Social Psychiatry and Psychiatric Epidemiology, 43, 151–159. [DOI] [PubMed] [Google Scholar]
- Tang W, Kannaley K, Friedman DB, Edwards VJ, Wilcox S, Levkoff SE, … Belza B (2017). Concern about developing Alzheimer’s disease or dementia and intention to be screened: An analysis of national survey data. Archives of Gerontology and Geriatrics, 71, 43–49. doi: 10.1016/j.archger.2017.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teal CR, Haidet P, Balasubramanyam AS, Rodriguez E, & Naik AD (2012). Measuring the quality of patients’ goals and action plans: Development and validation of a novel tool. BMC Medical Informatics and Decision Making, 12, 152–160. doi: 10.1186/1472-6947-12-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolea MI, & Galvin JE (2013). Current guidelines for dementia screening: Shortcomings and recommended changes. Neurodegenerative Disease Management, 3(6), 565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour VG, Masaki KH, Curb JD, & Blanchette PL (2000). The detection of dementia in the primary care setting. Archives of Internal Medicine, 160, 2964–2968. [DOI] [PubMed] [Google Scholar]
- Vickers KS, Nies MA, Patten CA, Dierkhising R, & Smith SA (2006). Patients with diabetes and depression may need additional support for exercise. American Journal of Health Behavior, 30(4), 353–362. [DOI] [PubMed] [Google Scholar]
- Von Wagner C, Semmler C, Good A, & Wardle J (2009). Health literacy and self-efficacy for participating in colorectal cancer screening: The role of information processing. Patient Education and Counseling, 75, 352–357. [DOI] [PubMed] [Google Scholar]
- Werner P (2003). Factors influencing intentions to seek a cognitive status examination: A study based on the Health Belief Model. International Journal of Geriatric Psychiatry, 18, 787–794. [DOI] [PubMed] [Google Scholar]
- Wolf MS, Davis TC, Osborn CY, Skripkauskas S, Bennett CL, & Makoul G (2007). Literacy, self-efficacy, and HIV medication adherence. Patient Education and Counseling, 65, 253–260. [DOI] [PubMed] [Google Scholar]
- Zigmond AS, & Snaith RP (1983). The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica, 67, 361–370. [DOI] [PubMed] [Google Scholar]