Graphical abstract
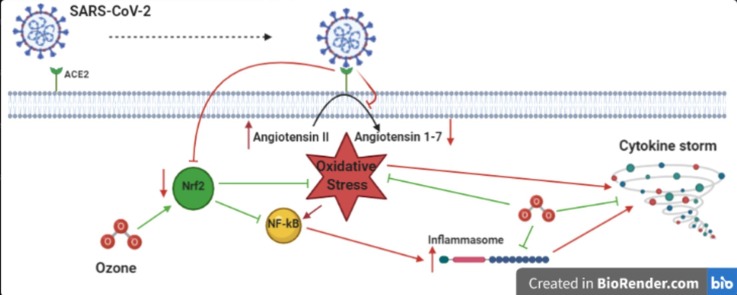
A scheme revealing angiotensin-converting enzyme 2 (ACE2) receptor-mediated COVID-19 following SARS-CoV-2 infection together with the mechanism of Ozone (O3).
Keywords: COVID-19, Pneumonia, ARDS, ALI, Oxidative stress, Ozone (O3) the
Abstract
Severe forms of COVID-19 can evolve into pneumonia, featured by acute respiratory failure due to acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). In viral diseases, the replication of viruses is seemingly stimulated by an imbalance between pro-oxidant and antioxidant activity as well as by the deprivation of antioxidant mechanisms. In COVID-19 pneumonia, oxidative stress also appears to be highly detrimental to lung tissues. Although inhaling ozone (O3) gas has been shown to be toxic to the lungs, recent evidence suggests that its administration via appropriate routes and at small doses can paradoxically induce an adaptive reaction capable of decreasing the endogenous oxidative stress. Ozone therapy is recommended to counter the disruptive effects of severe COVID-19 on lung tissues, especially if administered in early stages of the disease, thereby preventing the progression to ARDS.
1. Introduction
Coronavirus infectious disease 2019 (Covid-19), caused by Severe Acute Respiratory Syndrome Coronavirus type 2 (SARS-CoV-2), rapidly spread worldwide to become a pandemic on March 11, 2020 [1], [2], [3].
Seven coronavirus strains discovered thus far can cause infectious disease in humans. Whilst strains 229E, HKU1, OC43 and NL63 cause mild respiratory diseases, often presenting with common cold symptoms, the other three types can determine severe infectious diseases and include:
-
•
The Severe Acute Respiratory Syndrome Coronavirus type 1 (SARS-CoV-1), which was associated with an outbreak in Hong Kong and elsewhere during 2002–2003 [4], [5];
-
•
The Middle East Respiratory Syndrome Coronavirus (MERS-CoV), first appeared in 2012 and still circulating among certain animals such as camels, mainly in the Middle-East [6]; and
-
•
SARS-CoV-2.
There are high similarities between the latter three human coronaviruses, with SARS-CoV-2 sharing 51.8% and 79% nucleotide homology with MERS-CoV and SARS-CoV-1 [7].
The clinical pattern of COVID-19 varies extensively from mild/moderate (81%) to severe (14%) or critical (5%) [9], [10], [11]. Among 2634 hospitalized patients with confirmed COVID-19 in New York City, Long Island and Westchester County from March 1 to April 4, 2020, 14.2% needed admission to intensive care units (ICUs), with invasive mechanical ventilation required in 12.2% of them [12]. Despite a mortality rate of approximately 2.3% - considerably lower than MERS-CoV (35%) - the base reproductive number (Ro) of SARS-CoV- 2 has been estimated to fall between 2 and 3, similar to SARS-CoV-1 (Ro = 1.95) but much higher than MERS-CoV (Ro = 0.5). SARS-CoV-2 is therefore more contagious as compared with MERS-CoV [12], [13], [14], especially since asymptomatic/pre-symptomatic COVID-19 patients can shed high loads of virus in the surrounding environment [10]. In a recent meta-analysis on 28 high/moderate quality studies including cohorts or studies testing individuals irrespective of their COVID-19 symptoms, or case series with tracking report of asymptomatic patients, 8.7% study subjects were found to be COVID-19 positive. The percentage of asymptomatic in the latter metanalysis was 20% to 75% among COVID-19 confirmed cases [8].
In a viewpoint just published in JAMA, Kim et al., urgently called for new outpatients’ therapies which, combined with an effective vaccine, could significantly contribute to end this ongoing COVID-19 pandemic [15]. Whilst some drugs (especially corticosteroids) are currently used against severe COVID-19, therapeutic remedies for initial/moderate COVID-19 pneumonia are still missing. Treatments effective in early stage COVID-19 pneumonia could have a significantly impact on patients’ prognosis, reduction of hospital admissions, prevention of long-term sequelae and containment of the communicability window of COVID-19, hence reducing the respective risk of infection [15]. Leading candidates for COVID-19 treatment examined by Kim et al. included emerging antivirals, immunomodulatory drugs and antibody-based immunotherapy, with ozone (O3) being neglected [15].
Ozone is a triatomic unstable gas composed of 3 oxygen (O2) molecules featured by a 1 h half time, rapidly reverting to O2 at ambient temperature [16]. Ozone has potent oxidizing activity and already proved effective cidal effect against bacteria, fungi and viruses [17], [18], [19], including SARS-CoV-1 [20], through oxidation of double bonds [16].
For its immunomodulatory and anti-inflammatory properties Ozone has also recently been suggested as potential, inexpensive and easily available adjuvant therapy against CODVI-19, especially in mild to moderate pneumonia, to prevent the progression to critical disease [21], [22].
In this study we conducted a scoping review of the evidence on the potential application of ozone (O3) to treat/prevent the severe forms of COVID-19.
2. Methods
2.1. Searching strategy
PubMed, Scopus, Google Scholar, Web of Science and Cochrane library were searched using the following keywords: “COVID-19 Infection AND oxidative stress”; “SARS-CoV-2 AND oxidative stress”; “Infectious disease AND oxidative stress”; “Inflammation AND oxidative stress”; “Viral disease AND oxidative stress”; “Pneumonia AND oxidative stress”; “Ozone (therapy) AND Oxidative Stress”; “Ozone (therapy) AND pneumonia”; “Ozone (therapy) AND Viral Disease”; “Ozone (therapy) AND COVID-19”; “ozone therapy AND SARS-CoV-2”; “Ozone (therapy) AND Inflammation”; “Ozone (therapy) AND acute lung injury (ALI)”; “Ozone (therapy) and Acute Respiratory Distress Syndrome (ARDS)”; “Ozone (therapy) and ARDS”; “Ozone (therapy) AND Severe Acute Respiratory Syndrome”; “Ozone (therapy) AND SARS”; “Ozone (therapy) AND cytokines”; “Angiotensin-Converting Enzyme-2 (ACE2) receptor AND Oxidative stress”. Retrieved items were screen by title and abstract. Only articles in English were considered; dissertations, conference abstracts and duplicate publications were discarded.
3. Discussion
3.1. Viral diseases and oxidative stress
In viral diseases, the replication of viruses is seemingly influenced by an imbalance between pro-oxidant and antioxidant activity as well as by the deprivation of antioxidant mechanisms [23]. In an experimental animal model, SARS-CoV-1 infection was found to be linked to elevated reactive oxygen species (ROS) levels and disruption of antioxidant defences [24]. Hypoxia, that can be caused by viral sepsis, produces ROS such as superoxide radicals [25], [26], [27], [28]. Increased oxidative stress is severely damaging for the lung, causing acute respiratory failure sustained by ALI and ARDS, featured by considerably high mortality and morbidity [29], [30]. ALI/ARDS also characterize patients affected by severe/critical COVID-19, especially those referred to ICUs, where multiple factors such as hypoxemia, inflammation and mechanical ventilation with high fractions of inhaled O2 magnify oxidant generation [31], [32]. Elevated High Sensitivity C-Reactive Protein (hsCRP), an indicator of inflammation and oxidative stress, has been found in 93% of patients affected by COVID-19 pneumonia [33].
3.2. Renin-Angiotensin-Aldosterone System (RAAS) and oxidative stress
RAAS seems to be involved in the pathogenesis of severe ALI. SARS-CoV-1 is capable of binding to the Angiotensin-Converting Enzyme-2 (ACE2) through its spike protein (Fig. 1 ), downregulating its expression, which would have a physiological protective effect against ALI [34]. Likewise, SARS-CoV-2 also exploits the ACE2 receptor for cell internalization [35].
Fig. 1.
Possible mechanisms by which ozone therapy can reduce oxidative stress and disease severity in COVID-19 patients. Green lines denote activating effects and red lines denote inhibiting effects. NF-kB, Nuclear Factor kappa-light-chain-enhancer of activated B cells; Nrf2, Nuclear factor erythroid 2-related factor 2; RAAS, Renin Angiotensin Aldosterone System. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The carboxypeptidase ACE2 is a crucial element of RAAS for the control of blood pressure [36], [37]. It seems that Angiotensin-Converting Enzyme (ACE) and ACE2 antagonize with each other [37]. Angiotensin I (AT1) and angiotensin II (AT2) are converted by ACE2 into the inactive molecule angiotensin 1–9 and angiotensin 1–7, respectively [38]. Angiotensin 1–7 has anti-proliferative and vasodilatory effects and reduces the oxidative stress [39]. As mentioned above, some critically ill patients with COVID-19 develop ALI and ARDS, which lead to pulmonary oedema and lung failure [40], [41]. In the pathogenesis of ALI, ACE upregulates AT2, which in turn causes severe lung injury through binding with the AT2 subtype 1a receptor [34]. AT2 has potent vasoconstrictor effects and induces oxidative stress [42] predominantly through activation of NADPH oxidase, one of the most prominent producers of superoxide radical [43]. The serum level of AT2 is reported to be considerably elevated in COVID-19 patients and exhibits a positive linear correlation with viral load and lung injury [44]. By contrast, increasing levels of ACE2 and AT2 receptors had a protective effect in vitro against lung injury induced by SARS-CoV-1, MERS-CoV and SARS-CoV-2 [34], [44], [45], [46].
3.3. Inflammation and oxidative stress
In severe forms of COVID-19 a phenomenon known as ‘cytokines storm’ can be observed [40]. The increased levels of cytokines such as Monocyte Chemotactic Protein 1 (MCP1), IFN-γ-inducible protein 10, IFN-γ, IL-1β, IL-6 and IL-18, which has been found in lymphoid tissues, blood and lungs of COVID-19 patients, point toward an increased activity of the inflammasome [47], [48], [49]. The inflammasome, a protein complex of the cytosol, is one of the first components of the host innate immunity, involved in anti-viral responses by mediating the secretion of pro-inflammatory cytokines [50]. Rather than directly recognizing pathogenic elements, NLRP3 (NOD-, LRR- and pyrin domain-containing protein 3) the inflammasome appears to detect pathogenic-induced oxidative stress [51]. Nonetheless, it seems that SARS-CoV-1 directly encodes some of the known activators of NLRP3 inflammasome such as the envelope (E) protein, ORF8b, and ORF3a, which share respectively 95%, 40%, and 72% amino acid sequence with their counterpart molecules in SARS-CoV-2 [52], [53]. Significantly increased levels of NLRP3 inflammasome in leukocytes of affected lung areas have recently been reported in fatal COVID-19 pneumonia [54].
Similar to SARS-CoV-2, an excessive release of proinflammatory cytokines has been reported for SARS-CoV-1 [55], [56]. A number of COVID-19 patients not presenting ARDS show signs of extrapulmonary tissue damage (e.g. elevated creatinine and liver enzymes), possibly due to pro-inflammatory cytokine storm [57].
The generation of ROS-dependent respiratory burst is one of the mechanisms used by activated phagocytic cells such as neutrophils to suppress microbes during inflammation processes [58]. However, dysregulated interactions between ROS and inflammation may be linked to the pathogenesis of cytokine storm caused by COVID-19 (Fig. 1). While inflammation enhances ROS levels, increased levels of ROS in turn can boost inflammation, thereby creating a vicious circle [59]. The hyper-inflammatory state sustained by phagocytes likely explain the diffuse alveolar lesions with potential emphysema and even pneumothorax observed in critical COVID-19 pneumonia. On the other hand, it is hypothesized that ROS is implicated in activating the NLRP3 inflammasome [60], [61], [62].
3.4. COVID-19 risk factors and oxidative stress
The risk of ARDS and related COVID-19 mortality increases with patients’ age [63], which is associated with both cumulative damage caused by oxidative stress and reduced antioxidant activity [64], [65]. Results of a study on gene expression of type II pneumocytes revealed that the most downregulated gene in the elderly subjects is that encoding the superoxide dismutase 3 (SOD3). Genes encoding other molecules with antioxidant activity were also found to be downregulated in this population [66].
Oxidative stress and ROS are also key factors involved in pathological processes such as diabetes [67], hypertension [68], Chronic Obstructive Pulmonary Disease [69], obesity [70], [71], cancer [72], [73], [74], AIDS [75] and cardiovascular disease [76], [77]. Comorbidities, which increase linearly with age, in turn, enhances the risk of severe COVID-19 [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80].
3.5. COVID-19 and oxidative stress
A few investigations assessed the induction of oxidative stress due to COVID-19. A recent study reported increased serum levels of sNox2-dp, a NADPH oxidase activation marker in COVID-19 patients in comparison with healthy individuals [81]. Furthermore, higher serum levels of sNox2-dp have been reported among ICU patients as compared to non-ICU patients [81].
Cellular ROS were considerably increased in human promonocyte cells expressing SARS-CoV-1 3CLpro (viral 3-chymotrypsin-like cysteine protease) [82]. There is 99.02% homology between sequences of SARS-CoV-2 3CLpro and SARS-CoV-1 3CLpro [83], which further strengthens the argument that SARS-CoV-2 can cause oxidative stress.
Another remarkable finding is that serum albumin, which is considered a major component of serum antioxidant defence [84], is considerably decreased in patients suffering from COVID-19 [85], pointing towards a disruption of redox balance in these patients. Therefore, oxidative stress may be implicated in the pathogenesis of COVID-19 pneumonia (Fig. 1).
3.6. Ozone therapy and oxidative stress
Although the inhalation of O3 gas is very toxic for the lungs [86], recent evidence on O3 biochemical activity has shown that its administration via appropriate routes and at small doses can paradoxically be involved in induction of an adaptive reaction capable of decreasing the endogenous oxidative stress [87], [88], [89], [90]. There is a growing consensus that an accurately adjusted oxidative stress has therefore the ability to boost the antioxidant activities.
Various experimental studies assessed the antioxidative effects of ozone therapy (Table 1 ), mostly in rats with ischemia-reperfusion injury (IRI) because oxidative stress largely contributes to IRI [91], [92]. Hepatic [93], [94], renal [95], [96], intestinal [97], cochlear [98], retinal [99] and testicular [100] tissues among others have been investigated so far. According to these studies, ozone therapy has a protective role against IRI by shifting the redox balance towards the antioxidant activity.
Table 1.
Experimental animal studies on antioxidative effects of ozone therapy; CAT = catalase; GSH = glutathione; GSH-Px = glutathione peroxidase; IRI = Ischemia-Reperfusion Injury; SOD = superoxide dismutase; TAC = Total Antioxidant Capacity.
| Authors | Year | Sample size | Investigated conditions/tissues | Outcome | Reference |
|---|---|---|---|---|---|
| Peralta C et al. | 1999 | N = 18 | Hepatic IRI | Increase in SOD and preservation of GSH level | [94] |
| Ajamieh H et al. | 2004 | N = 60 | Hepatic IRI | Increase in SOD activity | [93] |
| Gonzalez R et al. | 2004 | N = 48 | Cisplatin-induced acute nephrotoxicity | Increase in GSH, SOD, CAT, and GSH-Px | [95] |
| Onal O et al. | 2015 | N = 28 | Intestinal IRI | Increase in SOD, GSH-Px, CAT and TAC | [97] |
| Kurtoglu T et al. | 2015 | N = 32 | Contrast-induced nephropathy | increase in renal antioxidant activity | [96] |
| Naserzadeh P et al. | 2017 | N = 40 | Brain and cochlear IRI | Increase in enzymatic and non-enzymatic antioxidants | [98] |
| Kal A et al. | 2017 | N = 14 | Retinal IRI | Increase in SOD, GSH-Px and TAC | [99] |
| Naserzadeh P et al. | 2019 | N = 40 | Testicular IRI | Increase in antioxidant capacity | [100] |
To date, the antioxidative effects of systemic ozone therapy have been studied (Table 2 ), both on healthy volunteers [101], [102], [103] and patients with different clinical conditions such as rheumatoid arthritis (RA) [104], advanced non-small cell lung cancer [105], coronary artery disease [106], myocardial infarction [107], heart failure [108], multiple sclerosis [109], multi-drug resistance TB [110], diabetes [111], [112], knee osteoarthritis [113], cancer patients under palliative care [114], in addition to endothelial [115] and HeLa cells [116]. According to these studies, ozone therapy significantly increases the level of FRAP (Ferric Reducing Ability of Plasma), an indicator of total antioxidant capacity, as well as antioxidants (e.g., superoxide dismutase, glutathione peroxidase, glutathione, glutathione S-transferase, etc.). Furthermore, ozone therapy determines a decrease in the levels of oxidative stress markers, including peroxidation potential, total hydroperoxides, malondialdehyde, nitric oxide (NO) and advanced oxidation protein products (AOPP).
Table 2.
Experimental Human clinical studies on the antioxidative effects of Ozone therapy. AOPP = advanced oxidation protein products; BAP = biological antioxidant potential; CAT = catalase; CRP,C-reactive protein; FRAP = ferric reducing ability of plasma; FiO2 = Fraction of inspired oxygen; G6PD = glucose 6 phosphate dehydrogenase; GGT = glutamyl transferase; GSH = glutathione; GSH-Px = glutathione peroxide; MDR-TB = multidrug resistance tuberculosis; MDA = malondialdehyde; NO = nitric oxide; PaO2 = Partial pressure of oxygen; PP = peroxidation potential; ROM = reactive oxygen metabolites; SOD = superoxide dismutase; TH = total hydroperoxides.
| Authors | Year | Sample size | Investigated conditions/tissues | Outcome | Reference |
|---|---|---|---|---|---|
| Hernandez F et al. | 1995 | N = 22 | Myocardial Infarction | Increase in GSH-Px and G6PD | [107] |
| Martinez-Sanchez G et al. | 2005 | N = 101 | Diabetic foot | Activation of SOD and normalization of organic peroxides | [112] |
| Inal M et al. | 2011 | N = 11 | Healthy subjects | Increase in SOD and CAT and decrease in MDA | [102] |
| Emma BJ et al. | 2012 | N = 40 | Non-small cell lung cancer | Decrease in dROM and increase in BAP | [105] |
| Martinez-Sanchez et al. | 2012 | N = 53 | Coronary Artery Disease | Increase in GSH and FRAP and decrease in PP, AOPP and MDA | [106] |
| Re L et al. | 2014 | N = 6 | Healthy subjects | Increased activities of SOD and CAT | [101] |
| Fernandez OSL | 2016 | N = 40 | Rheumatoid Arthritis | Increase in SOD, CAT, GSH and decrease in MDA, NO, AOPP | [104] |
| Buyuklu M et al. | 2017 | N = 40 | Heart Failure | Increase in SOD, CAT, GSH, GSH-Px and decrease in NO, MDA | [108] |
| Delgado-Roche L et al. | 2017 | N = 28 | Multiple Sclerosis | Increase in GSH and decrease of oxidative damage on proteins and lipids | [109] |
| Totolici IP et al. | 2017 | N = 10 | Cancer patients receiving palliative care | Increase in SOD and GSH-Px | [114] |
| Shah MA et al. | 2018 | N = 12 | Type II Diabetes | Decrease in CRP and biomarkers of lipid and protein oxidation | [111] |
| Loprete F et al. | 2019 | N = 45 | Healthy subjects and with various diseases | Decrease in total oxidizing capacity and increase in antioxidant response | [103] |
| Shah MA et al. | 2019 | N = 7 | MDR-TB | Increase in SOD | [110] |
| Fernandez OSL et al. | 2020 | N = 40 | Knee osteoarthritis | Increase in GGT, CAT, GSH and decrease in MDA, TH | [113] |
| Franzini M et al | 2020 | N = 50 | Patients undergoing ICU hospitalization for COVID-19 | A notable decline of inflammatory and thromboembolic markers (CRP, IL-6, D-dimer) and improvement in the respiratory and gas exchange markers | [139] |
| Tascini C et al | 2020 | N = 60 | In patients affected by mild to moderate COVID-19 pneumonia | Lower PaO2/FiO2 and SpO2/FiO2 ratio and lower lymphocytes count. | [145], [146] |
Systemic O3 can be administered by different routes, such as major auto-hemotherapy, minor auto-hemotherapy and rectal insufflation, among others [117]. At therapeutic doses and with appropriate dose intervals, O3 administration regulates multiple biochemical mechanisms mostly via the activation of secondary messengers [118].
O3 therapy stimulates the expression and activity of Nuclear erythroid 2-related factor 2 (Nrf2) [119]. It is argued that low dose ozone is capable of exerting anti-inflammatory and antioxidant activities by means of activating Nrf2, which contributes substantially to the effectiveness of O3-O2 treatments [120], [121], [122]. In a study on multiple sclerosis patients, rectal insufflation with O3 increased Nrf2 phosphorylation in mononuclear cells, improved the activity of antioxidant enzymes and reduced pro-inflammatory cytokines [109].
Nrf2 is defined as an important modulator of cytoprotective protein driven by the antioxidant response element, and Nrf2 pathway activation significantly prevents the oxidative stress determined by injuring cells and tissues [122]. Increasing the transcription of antioxidant enzymes (e.g. biliverdin reductase, heme oxygenase-1, peroxiredoxin 1, peroxiredoxin 6, glutathione peroxidase 2, glutathione peroxidase 4, and glutathione reductase, thioredoxin-1, etc.) is the mechanism by which Nrf2 prevents the oxidative stress [123], [124], [125], [126]. A study on biopsy specimens of COVID-19 patients found that the gene expression pathway of Nrf2 was suppressed [127].
Homeostatic control of ROS, accomplished by Nrf-2, can break the vicious circle of ROS and inflammation. In addition, Nrf2 reduces the generation of pro-inflammatory cytokines such as IL1β and IL-6 through prevention of RNA polymerase II transcriptional activity, which further suppresses the inflammatory response [128]. Furthermore, Nrf2 regulates gene expression in activated macrophages through two-way interactions with Nuclear Factor kappa-light-chain-enhancer of activated B cells (NF-kB) transcription factor. Nrf2 performs regulated self-transcription [129], and decreases NF-kB transcriptional activity [130].
NF- kB activation increases the generation of pro-inflammatory cytokines such as IL8, TNFα, IL6, IL1β, IFNγ, as well as proinflammatory enzymes like inducible Nitric Oxide Synthase and cyclooxygenase-2 [131]. In an animal model of ALI caused by SARS-CoV-1 infection, the generation of oxidized low-density lipoprotein (OxLDL) enhanced the innate human immune activity through Toll-like receptor 4 (TLR4)/NF-kB signalling pathway and subsequent excessive production of IL-6 by alveolar macrophages [132]. The fact that antioxidants such as vitamin E, green tea polyphenols, L-cysteine, thiols and N-acetylcysteine (NAC) can block the activating effects of almost all stimuli on NF-κB further confirms the possible role that ROS play in NF-κB activation [133], [134].
Ozone therapy decreases the level of NLRP3 inflammasome either directly or via Nrf2 activation/ROS reduction/NF-kB inhibition pathway [112]. Decreasing levels of ROS or inhibition of NF-κB prevent components of the NLRP3 inflammasome protein from being assembled, thus subsequently reducing its activity [135], [136], [137], [138].
3.7. Ozone therapy and COVID-19
A mixture of oxygen-ozone (O2-O3) infusion therapy has proven beneficial for COVID-19 patients admitted to forced non-invasive ventilation, contributing to restore their O2 saturation in a relatively short time [139]. To date, a few investigations have assessed the effects of ozone therapy in patients suffering from COVID-19. A study on 50 ICU patients with ARDS caused by COVID-19 reported clinical improvement sustained by increased O2 saturation and PaO2/FiO2 ratio following systemic ozone therapy [139]. In addition, thromboembolic and inflammatory markers such as D-dimer, IL-6, CRP were significantly reduced in these patients. Similar findings were reported in other clinical studies [137], [138], [139], [140], [141], [142], [143], [144], [145], [146]. Although O2-O3 autohemotherapy is regarded very safe - having a complication rate as low as 0.7/100,000 – and cost-effective, it needs to be delivered using proper devices and adapted to different phenotypes of COVID-19 patients [139], [147], [148].
The Italian Society of Ozone and Oxygen therapy (SIOOT) recently issued a clinical protocol, approved by the Italian National Institute of Health (ISS, Italian acronym), for the management of COVID-19 patients by O2-O3 auto-hemotherapy. The latter protocol stratifies COVID-19 patients into 5 phenotype classes, each corresponding to a different therapeutic approach, with phenotypes 1, 2, 3 (early stages COVID-19 infection) being more responsive to O2-O3 therapy (Table 3 ). Homogenous O2-O3 mixtures need to be produced with a precise and easily adjustable concentration, using devices made of ozone-resistant materials. O2 saturation of COVID-19 patients treated by O2-O3 therapy needs to be monitored on a daily basis, whereas laboratory tests (CRP, fasting glucose, ALT, creatinine, leukocytes, LDH, pro-calcitonin, L-6, among others) can be weekly checked [139], [149].
Table 3.
Six different phenotypes to various therapeutic protocol.
| Phenotype class | Clinical pattern | Therapeutic management | |
|---|---|---|---|
| 1 |
Fever With/without respiratory symptoms Negative chest X ray Normal pO2 |
Discharge | |
| 2–3 MAHT per week for 2–3 weeks (40–50 mg/150–200 cc ozone in 150/200 cc blood) | |||
| Ozone oil (RINOZONE) nasal spray 2/day | |||
| Ambient air sanitation (using AirKing) | |||
| 2 |
Fever GGO (at chest X ray) OR low pO2 |
Admission and follow up | |
| 3 MAHT per week for 3 weeks (40–50 mg/200 cc ozone in 200cc blood) | |||
| Rinozone spray (ozonized oil) 2/3 times per day | |||
| Hyper-ozonized water | to drink (2 glasses/8h) | ||
| mouth and eye rinses | |||
| Ambient air sanitation (using AirKing) | |||
| 3 |
Fever Multiple GGO (at chest X ray) Low pO2 |
Sub-intensive care needed | |
| O2 therapy (15 L/m) | |||
| 4 MAHT per week for 3 weeks (40–50 mg/150–200 cc ozone in 150/200 cc blood) | |||
| Rectal insufflation with ozone (20–30 mg/100 cc) | |||
| Ozone oil (RINOZONE) nasal spray 2–3/day | |||
| Hyper-ozonized water | to drink (2 glasses/8h) | ||
| mouth and eye rinses | |||
| Ambient air sanitation (using AirKing) | |||
| 4 | Pre-ARDS | CPAP | |
| 1st week: 1 MACHT/day for 7 days a week (40–50 mg/200 cc ozone in 200 cc blood) | |||
| 2nd week: 4 MACHT/week (40–50 mg/200 cc ozone in 200 cc blood) | |||
| 3rd week: 3 MACHT/week (40–50 mg/200 cc ozone in 200 cc blood) | |||
| Rectal insufflation with ozone (20 mg/100 cc) | |||
| Ozone oil (RINOZONE) nasal spray 2–3/day | |||
| Hyper-ozonized water | to drink (2 glasses/8h) | ||
| mouth and eye rinses | |||
| Ambient air sanitation (using AirKing) | |||
| 5 |
ARDS Very low pO2 (up to 35-40 mmHg) Pulmonary Interstitial syndrome |
CPAP attempt (in case of WET interstitial syndrome) | |
| Intubation (in case of DRY Interstitial syndrome) | |||
| 1 MAHT/day for 5 days/week (40–50 mg/200 cc ozone in 200cc blood) | |||
| Rectal insufflation (20 mg/100 cc ozone) for 4 weeks | |||
| Ozone oil (RINOZONE) nasal spray 2–3/day | |||
| Hyper-ozonized water | to drink (2 glasses/8h) | ||
| mouth and eye rinses | |||
| Ambient air sanitation (using AirKing) | |||
MAHT: Major Auto-Hemo Therapy
CPAP: Continuous Positive Airway Pressure
The mechanisms of O2-O3 therapy against COVID-19 is still unknown, but the activation of Nrf2 induced by ozone appears to suppress the production of pro-inflammatory cytokines, hence modulating the hyper-coagulate state associated with severe forms of COVID-19 [128], [139]. Furthermore, O2-O3 seems to be capable to directly inactivate coronaviruses spike envelope proteins - abundant of cysteine and tryptophan amino acids - thereby interfering with the binding of SARS-CoV-2 with ACE2 cell receptor [150]. The binding of SARS-CoV-2 with the ACE2 receptor may also be prevented by the inhibition of the palmytoilation of the spike envelope mediated by nitric oxide signalling pathways, also enhanced by O2-O3 [151], [152].
4. Conclusions
Ozone therapy could be a potential resource to modulate the patient immune response against SARS-CoV-2, contributing to contain the cellular oxidative stress of COVID-19 pneumonia and breaking the vicious cycle of cytokine storm observed in severe forms of the disease. Ozone therapy may also be a useful complementary treatment to be considered in patients suffering from early stage COVID-19 pneumonia, to prevent the progression to life-threatening disease.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Sharma A., Tiwari S., Deb M.K., Marty J.L. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): a global pandemic and treatment strategies. Int. J. Antimicrob. Agents. 2020;56(2) doi: 10.1016/j.ijantimicag.2020.106054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chauhan S. Comprehensive review of coronavirus disease 2019 (COVID-19) Biomed. J. 2020;43(4):334–340. doi: 10.1016/j.bj.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acter T., Uddin N., Das J., Akhter A., Choudhury T.R., Kim S. Evolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as coronavirus disease 2019 (COVID-19) pandemic: A global health emergency. Sci. Total Environ. 2020;730 doi: 10.1016/j.scitotenv.2020.138996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhong N.S., Zheng B.J., Li Y.M., et al. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People's Republic of China. Lancet. 2003;362(9393):1353–1358. doi: 10.1016/S0140-6736(03)14630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drosten C., Günther S., Preiser W., et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348(20):1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 6.A. Pavli, S. Tsiodras, H.C. Maltezou, Middle East respiratory syndrome coronavirus (MERS-CoV): prevention in travelers, Travel Med. Infect. Dis. 2014;12(6 Pt A):602–608. [DOI] [PMC free article] [PubMed]
- 7.Ren L.-L., Wang Y.-M., Wu Z.-Q., et al. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin. Med. J. (Engl.) 2020;133(9):1015–1024. doi: 10.1097/CM9.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yanes-Lane M., Winters N., Fregonese F., Bastos M., Perlman-Arrow S., Campbell J.R., Menzies D. Proportion of asymptomatic infection among COVID-19 positive persons and their transmission potential: A systematic review and meta-analysis. PLoS ONE. 2020;15(11) doi: 10.1371/journal.pone.0241536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cegolon L., Pichierri J., Mastrangelo G., Cinquetti S., Sotgiu G., Bellizzi S., Pichierri G. Hypothesis to explain the severe form of COVID-19 in Northern Italy. BMJ Glob. Health. 2020;5(6) doi: 10.1136/bmjgh-2020-002564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cegolon L., Javanbakht M., Mastrangelo G. Nasal disinfection for the prevention and control of COVID-19: A scoping review on potential chemo-preventive agents. Int. J. Hyg. Environ. Health. 2020;230 doi: 10.1016/j.ijheh.2020.113605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Z., McGoogan J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 12.Richardson S., Hirsch J.S., Narasimhan M., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV). Available from: https://www.who.int/health-topics/middle-east-respiratory-syndrome-coronavirus-mers#tab=tab_1 (last accessed on 18th November 2020).
- 14.Cegolon L. Investigating hypothiocyanite against SARS-CoV-2. Int. J. Hyg. Environ. Health. 2020 Jun;227 doi: 10.1016/j.ijheh.2020.113520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim PS, Read SW, Fauci AS. Therapy for Early COVID-19: A Critical Need. JAMA. 2020 Nov 11. doi: 10.1001/jama.2020.22813. Epub ahead of print. [DOI] [PubMed]
- 16.Gavazza A., Marchegiani A., Rossi G., Franzini M., Spaterna A., Mangiaterra S., Cerquetella M. Ozone Therapy as a Possible Option in COVID-19 Management. Front. Public Health. 2020;8:417. doi: 10.3389/fpubh.2020.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hudson J.B., Sharma M., Vimalanathan S. Development of a practical method for using ozone gas as a virus decontaminating agent. Ozone Sci. Eng. 2009;31:216–223. [Google Scholar]
- 18.Tseng C., Li C. Inactivation of surface viruses by gaseous ozone. J. Environ. Health. 2008;70:56–63. [PubMed] [Google Scholar]
- 19.Li C.S., Wang Y.C. Surface germicidal effects of ozone for microorganisms. AIHA J. 2003;64:533–537. doi: 10.1202/559.1. [DOI] [PubMed] [Google Scholar]
- 20.Zhou M. Ozone: a powerful weapon to combat COVID-19 out-break. 2020. http://www.china.org.cn/opinion/2020-02/26/con-tent_75747237_4.htm (last accessed on 20th November 2020).
- 21.Rowen R.J., Robins H. A plausible “penny” costing effective treatment for corona virus - ozone therapy. J. Infect. Dis. Epidemiol. 2020;6:113. [Google Scholar]
- 22.Hernández A., Papadakos P.J., Torres A., González D.A., Vives M., Ferrando C., Baeza J. Two known therapies could be useful as adjuvant therapy in critical patients infected by COVID-19. Rev. Esp. Anestesiol. Reanim. 2020;67(5):245–252. doi: 10.1016/j.redar.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khomich O.A., Kochetkov S.N., Bartosch B., Ivanov A.V. Redox Biology of Respiratory Viral Infections. Viruses. 2018 Jul 26;10(8):392. doi: 10.3390/v10080392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van den Brand J.M., Haagmans B.L., van Riel D., Osterhaus A.D., Kuiken T. The pathology and pathogenesis of experimental severe acute respiratory syndrome and influenza in animal models. J. Comp. Pathol. 2014;151(1):83–112. doi: 10.1016/j.jcpa.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mantzarlis K., Tsolaki V., Zakynthinos E. Role of Oxidative Stress and Mitochondrial Dysfunction in Sepsis and Potential Therapies. Oxid. Med. Cell Longev. 2017;2017:5985209. doi: 10.1155/2017/5985209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fink M.P. Bench-to-bedside review: Cytopathic hypoxia. Crit. Care. 2002;6(6):491–499. doi: 10.1186/cc1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeda K., Shimada Y., Amano M., Sakai T., Okada T., Yoshiya I. Plasma lipid peroxides and alpha-tocopherol in critically ill patients. Crit. Care Med. 1984;12(11):957–959. doi: 10.1097/00003246-198411000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Ademowo O.S., Dias H.K.I., Burton D.G.A., Griffiths H.R. Lipid (per) oxidation in mitochondria: an emerging target in the ageing process? Biogerontol. 2017;18(6):859–879. doi: 10.1007/s10522-017-9710-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hecker L. Mechanisms and consequences of oxidative stress in lung disease: therapeutic implications for an aging populace. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018;314(4):L642–L653. doi: 10.1152/ajplung.00275.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan X., Fu X., Jia Y., et al. Nrf2/Keap1/ARE signaling mediated an antioxidative protection of human placental mesenchymal stem cells of fetal origin in alveolar epithelial cells. Oxid. Med. Cell Longev. 2019;2019:2654910. doi: 10.1155/2019/2654910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tamura D.Y., Moore E.E., Partrick D.A., Johnson J.L., Offner P.J., Silliman C.C.J.S. Acute hypoxemia in humans enhances the neutrophil inflammatory response. Shock. 2002;17(4):269–273. doi: 10.1097/00024382-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Sarma J.V., Ward P.A. Oxidants and redox signaling in acute lung injury. Compr. Physiol. 2011;1(3):1365–1381. doi: 10.1002/cphy.c100068. [DOI] [PubMed] [Google Scholar]
- 33.Chen L., Liu H., Liu W., et al. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Chinese J. Tuberculosis Respirat. Dis. 2020;43:E005. doi: 10.3760/cma.j.issn.1001-0939.2020.0005. [DOI] [PubMed] [Google Scholar]
- 34.Imai Y., Kuba K., Rao S., et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nat. 2005;436(7047):112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou P., Yang X.-L., Wang X.-G., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nat. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rice G.I., Thomas D.A., Grant P.J., Turner A.J., Hooper N.M. Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem. J. 2004;383(Pt 1):45–51. doi: 10.1042/BJ20040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Danilczyk U., Eriksson U., Crackower M.A., Penninger J.M. A story of two ACEs. J. Mol. Med. 2003;81(4):227–234. doi: 10.1007/s00109-003-0419-x. [DOI] [PubMed] [Google Scholar]
- 38.Lely A., Hamming I., van Goor H., Navis G.J. Renal ACE2 expression in human kidney disease. J. Pathol. 2004;204(5):587–593. doi: 10.1002/path.1670. [DOI] [PubMed] [Google Scholar]
- 39.De Farias Lelis D., de Freitas D.F., Machado A.S., Crespo T.S., Santos S.H.S. Angiotensin-(1–7), Adipokines and Inflammation. Metabolism: Clin. Exp. 2019;95:36–45. doi: 10.1016/j.metabol.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 40.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu Z., Shi L., Wang Y., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nehme A., Zouein F.A., Deris Zayeri Z. Zibara KJJocd, disease (2019) An update on the tissue renin angiotensin system and its role in physiology and pathology. J. Cardiovasc. Dev. Dis. 2019;6(2):14. doi: 10.3390/jcdd6020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rajagopalan S., Kurz S., Münzel T., et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J. Clin. Invest. 1996;97(8):1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y., Yang Y., Zhang C., et al. (2020) Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020;63(3):364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuba K., Imai Y., Rao S., et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat. Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wösten-van Asperen R.M., Lutter R., Specht P.A., et al. Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin-(1–7) or an angiotensin II receptor antagonist. J. Pathol. 2011;225(4):618–627. doi: 10.1002/path.2987. [DOI] [PubMed] [Google Scholar]
- 47.Huang K.J., Su I.J., Theron M., et al. An interferon-γ-related cytokine storm in SARS patients. J. Med. Virol. 2005;75(2):185–194. doi: 10.1002/jmv.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang Y., Xu J., Zhou C., et al. Characterization of cytokine/chemokine profiles of severe acute respiratory syndrome. Am. J. Respir. Crit. Care Med. 2005;171(8):850–857. doi: 10.1164/rccm.200407-857OC. [DOI] [PubMed] [Google Scholar]
- 49.Triantafilou K., Triantafilou M. Ion flux in the lung: virus-induced inflammasome activation. Trends Microbiol. 2014;22(10):580–588. doi: 10.1016/j.tim.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martínez-Sánchez G., Schwartz A., Donna V. Potential Cytoprotective Activity of Ozone Therapy in SARS-CoV-2/COVID-19. Antioxidants. 2020;9:389. doi: 10.3390/antiox9050389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martinon F. Detection of immune danger signals by NALP3. J. Leukoc. Biol. 2008;83(3):507–511. doi: 10.1189/jlb.0607362. [DOI] [PubMed] [Google Scholar]
- 52.Shi C.-S., Nabar N.R., Huang N.-N., Kehrl J.H. SARS-Coronavirus Open Reading Frame-8b triggers intracellular stress pathways and activates NLRP3 inflammasomes. Cell Death Discovery. 2019;5(1):1–12. doi: 10.1038/s41420-019-0181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fung S.Y., Yuen K.S., Ye Z.W., Chan C.P., Jin D.Y. A tug-of-war between severe acute respiratory syndrome coronavirus 2 and host antiviral defence: lessons from other pathogenic viruses. Emerg. Microbes Infect. 2020;9(1):558–570. doi: 10.1080/22221751.2020.1736644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.S. Toldo, R. Bussani, V. Nuzzi et al., Inflammasome formation in the lungs of patients with fatal COVID-19, Inflamm. Res. 2020 Oct 20;1-4. doi: 10.1007/s00011-020-01413-2. Online ahead of print. [DOI] [PMC free article] [PubMed]
- 55.Parsons P.E., Eisner M.D., Thompson B.T., et al. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit. Care Med. 2005;33(1):1–6. doi: 10.1097/01.ccm.0000149854.61192.dc. [DOI] [PubMed] [Google Scholar]
- 56.Pedersen S.F., Ho Y.C. SARS-CoV-2: a storm is raging. J. Clin. Invest. 2020;130(5):2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ye Q., Wang B., Mao J. The pathogenesis and treatment of theCytokine Storm'in COVID-19. J. Infect. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bogdan C., Röllinghoff M., Diefenbach A. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr. Opin. Immunol. 2000;12(1):64–76. doi: 10.1016/s0952-7915(99)00052-7. [DOI] [PubMed] [Google Scholar]
- 59.Joseph J., Ametepe E.S., Haribabu N., et al. Inhibition of ROS and upregulation of inflammatory cytokines by FoxO3a promotes survival against Salmonella typhimurium. Nat. Commun. 2016;7(1):1–14. doi: 10.1038/ncomms12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martinon F., Mayor A., Tschopp J. The inflammasomes: guardians of the body. Ann. Rev. Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 61.Mason R.J. Pathogenesis of COVID-19 from a cell biology perspective. In. Eur. Respiratory Soc. 2020;55(4) doi: 10.1183/13993003.00607-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun R., Liu H. Wang XJKJoR (2020) Mediastinal emphysema, giant bulla, and pneumothorax developed during the course of COVID-19 pneumonia. Korean J. Radiol. 2020;21(5):541–544. doi: 10.3348/kjr.2020.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu C., Chen X., Cai Y., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Internal Med. 2020;180(7) doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gil del Valle L., Gravier Hernández R., Delgado Roche L., León Fernández O.S. Oxidative stress in the aging process: fundamental aspects and new insights. Oxidative Stress: Diagnostics, Prevention, and Therapy Volume 2. ACS Publications. 2015:177–219. [Google Scholar]
- 65.Davies K.J. The oxygen paradox, oxidative stress, and ageing. Arch. Biochem. Biophys. 2016;595:28–32. doi: 10.1016/j.abb.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abouhashem A.S., Singh K., Azzazy H.M., Sen C.K.J.A., Signaling R. Is Low Alveolar Type II Cell SOD3 in the Lungs of Elderly Linked to the Observed Severity of COVID-19? Antioxid. Redox Signal. 2020;33(2):59–65. doi: 10.1089/ars.2020.8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Muhammad S., Bierhaus A., Schwaninger M. Reactive oxygen species in diabetes-induced vascular damage, stroke, and Alzheimer's disease. J. Alzheimers Dis. 2009;16(4):775–785. doi: 10.3233/JAD-2009-0982. [DOI] [PubMed] [Google Scholar]
- 68.Droge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82(1):47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 69.Kirkham P.A., Barnes P.J. Oxidative stress in COPD. Chest. 2013;144(1):266–273. doi: 10.1378/chest.12-2664. [DOI] [PubMed] [Google Scholar]
- 70.Atabek M.E., Vatansev H., Erkul I. Oxidative stress in childhood obesity. J. Pediatr. Endocrinol. Metab. 2004;17:1063–1068. doi: 10.1515/jpem.2004.17.8.1063. [DOI] [PubMed] [Google Scholar]
- 71.Furukawa S., Fujita T., Shimabukuro M., et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 2004;114(12):1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lau A.T., Wang Y., Chiu J.F. Reactive oxygen species: current knowledge and applications in cancer research and therapeutic. J. Cell. Biochem. 2008;104(2):657–667. doi: 10.1002/jcb.21655. [DOI] [PubMed] [Google Scholar]
- 73.Renschler M.F. The emerging role of reactive oxygen species in cancer therapy. Eur. J. Cancer. 2004;40(13):1934–1940. doi: 10.1016/j.ejca.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 74.Weinberg F., Chandel N.S. Reactive oxygen species-dependent signaling regulates cancer. Cell. Mol. Life Sci. 2009;66(23):3663–3673. doi: 10.1007/s00018-009-0099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Papadopulos-Eleopulos E. Reappraisal of AIDS—is the oxidation induced by the risk factors the primary cause? Med. Hypotheses. 1988;25:151–162. doi: 10.1016/0306-9877(88)90053-9. [DOI] [PubMed] [Google Scholar]
- 76.Touyz R.M. Reactive oxygen species and angiotensin II signaling in vascular cells: implications in cardiovascular disease. Braz. J. Med. Biol. Res. 2004;37(8):1263–1273. doi: 10.1590/s0100-879x2004000800018. [DOI] [PubMed] [Google Scholar]
- 77.Yoshizumi M., Tsuchiya K., Tamaki T. Signal transduction of reactive oxygen species and mitogen-activated protein kinases in cardiovascular. J. Med. Inves. 2001;48(1–2):11–24. [PubMed] [Google Scholar]
- 78.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jordan R.E., Adab P., Cheng K. Covid-19: risk factors for severe disease and death. BMJ. 2020;368 doi: 10.1136/bmj.m1198. [DOI] [PubMed] [Google Scholar]
- 80.Lighter J., Phillips M., Hochman S., et al. Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission. Clin. Infect. Dis. 2020;71(15):896–897. doi: 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Violi F., Oliva A., Cangemi R., et al. Nox2 activation in Covid-19. Redox Biol. 2020;36 doi: 10.1016/j.redox.2020.101655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lin C.-W., Lin K.-H., Hsieh T.-H., Shiu S.-Y., Li J.-Y.J.F.I., Microbiology M. Severe acute respiratory syndrome coronavirus 3C-like protease-induced apoptosis. FEMS Immunol. Med. Microbiol. 2006;46(3):375–380. doi: 10.1111/j.1574-695X.2006.00045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ul Qamar M.T., Alqahtani S.M., Alamri M.A., Chen L.L. Structural basis of SARS-CoV-2 3CLpro and anti-COVID-19 drug discovery from medicinal plants. J. Pharm. Anal. 2020 Aug;10(4):313–319. doi: 10.1016/j.jpha.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roche M., Rondeau P., Singh N.R., Tarnus E., Bourdon E. The antioxidant properties of serum albumin. FEBS Lett. 2008;582(13):1783–1787. doi: 10.1016/j.febslet.2008.04.057. [DOI] [PubMed] [Google Scholar]
- 85.Violi F., Ceccarelli G., Cangemi R., et al. Hypoalbuminemia, Coagulopathy and Vascular Disease in Covid-19. Circ. Res. 2020;127(3):400–401. doi: 10.1161/CIRCRESAHA.120.317173. [DOI] [PubMed] [Google Scholar]
- 86.Hazucha M.J., Bates D.V., Bromberg P.A. Mechanism of action of ozone on the human lung. J. Appl. Physiol. 1989;67(4):1535–1541. doi: 10.1152/jappl.1989.67.4.1535. [DOI] [PubMed] [Google Scholar]
- 87.Bocci V., Borrelli E., Travagli V., Zanardi I. The ozone paradox: ozone is a strong oxidant as well as a medical drug. Med. Res. Rev. 2009;29(4):646–682. doi: 10.1002/med.20150. [DOI] [PubMed] [Google Scholar]
- 88.Bocci V., Zanardi I., Huijberts M.S., Travagli V. Diabetes and chronic oxidative stress. A perspective based on the possible usefulness of ozone therapy. Diabetes Metab. Syndr. 2011;5(1):45–49. doi: 10.1016/j.dsx.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 89.Bocci V. Is it true that ozone is always toxic? The end of a dogma. Toxicol. Appl. Pharmacol. 2006;216(3):493–504. doi: 10.1016/j.taap.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 90.Bocci V. The case for oxygen-ozonetherapy. Br. J. Biomed. Sci. 2007;64(1):44–49. doi: 10.1080/09674845.2007.11732755. [DOI] [PubMed] [Google Scholar]
- 91.Laubach V.E., Sharma A.K. Mechanisms of lung ischemia-reperfusion injury. Curr. Opin. Organ Transplant. 2016;21(3):246–252. doi: 10.1097/MOT.0000000000000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ferrari R.S., Andrade C.F. Oxidative stress and lung ischemia-reperfusion injury. Oxid. Med. Cell Longev. 2015;2015 doi: 10.1155/2015/590987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ajamieh H., Menéndez S., Martínez-Sánchez G., et al. Effects of ozone oxidative preconditioning on nitric oxide generation and cellular redox balance in a rat model of hepatic ischaemia–reperfusion. Liver Int. 2004;24(1):55–62. doi: 10.1111/j.1478-3231.2004.00885.x. [DOI] [PubMed] [Google Scholar]
- 94.Peralta C., Leon O., Xaus C., et al. Protective effect of ozone treatment on the injury associated with hepatic ischemia-reperfusion: antioxidant-prooxidant balance. Free Radic. Res. 1999;31(3):191–196. doi: 10.1080/10715769900300741. [DOI] [PubMed] [Google Scholar]
- 95.González R., Borrego A., Zamora Z., et al. Reversion by ozone treatment of acute nephrotoxicity induced by cisplatin in rats. Mediators Inflamm. 2004;13(5–6):307–312. doi: 10.1155/S0962935104000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kurtoglu T., Durmaz S., Akgullu C., et al. Ozone preconditioning attenuates contrast-induced nephropathy in rats. J. Surg. Res. 2015;195(2):604–611. doi: 10.1016/j.jss.2015.01.041. [DOI] [PubMed] [Google Scholar]
- 97.Onal O., Yetisir F., Sarer A., et al. Prophylactic ozone administration reduces intestinal mucosa injury induced by intestinal ischemia-reperfusion in the rat. Mediators Inflamm. 2015;2015 doi: 10.1155/2015/792016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nasezadeh P., Shahi F., Fridoni M., Seydi E., Izadi M. Salimi AJFRR (2017) Moderate O3/O2 therapy enhances enzymatic and non-enzymatic antioxidant in brain and cochlear that protects noise-induced hearing loss. Free Radic. Res. 2017;51(9–10):828–837. doi: 10.1080/10715762.2017.1381695. [DOI] [PubMed] [Google Scholar]
- 99.Kal A., Kal O., Akillioglu I., et al. The protective effect of prophylactic ozone administration against retinal ischemia-reperfusion injury. Cutan. Ocul. Toxicol. 2017;36(1):39–47. doi: 10.3109/15569527.2016.1156120. [DOI] [PubMed] [Google Scholar]
- 100.P. Naserzadeh, Z. Jamali, H. Choobineh, M. Izadi, A. Salimi, Induced Mild Oxidative Stress by Ozone/Oxygen Therapy Enhances Therapy Antioxidant Capacities and Protects Testicular Ischemia/Reperfusion Injury in Rats. Res Square. Preprint. Posted 15 Sep, 2019. doi: 10.21203/rs.2.14446/v1.
- 101.Re L., Martínez-Sánchez G., Bordicchia M., et al. Is ozone pre-conditioning effect linked to Nrf2/EpRE activation pathway in vivo? A preliminary result. Eur. J. Pharmacol. 2014;742:158–162. doi: 10.1016/j.ejphar.2014.08.029. [DOI] [PubMed] [Google Scholar]
- 102.Inal M., Dokumacioglu A., Özcelik E., Ucar O. The effects of ozone therapy and coenzyme Q 10 combination on oxidative stress markers in healthy subjects. Ir. J. Med. Sci. 2011;180(3):703–707. doi: 10.1007/s11845-011-0675-7. [DOI] [PubMed] [Google Scholar]
- 103.Loprete F, Vaiano F, Valdenassi L. Outpatient evaluation of oxidative stress in subjects undergoing systemic oxygen-ozone therapy. Ozone Therapy 4(1). https://doi.org/10.4081/ozone.2019.8175.
- 104.Fernández O.S.L., Viebahn-Haensler R., Cabreja G.L., Espinosa I.S., Matos Y.H., Roche L.D., Santos B.T., Oru G.T., Polo Vega J.C. Medical ozone increases methotrexate clinical response and improves cellular redox balance in patients with rheumatoid arthritis. Eur. J. Pharmacol. 2016;789:313–318. doi: 10.1016/j.ejphar.2016.07.031. [DOI] [PubMed] [Google Scholar]
- 105.Emma B. Treatment of advanced non-small-cell lung cancer with oxygen ozone therapy and mistletoe: an integrative approach. EurJIntegrative Med. 2012;4:130. [Google Scholar]
- 106.Martínez-Sánchez G., Delgado-Roche L., Díaz-Batista A., Pérez-Davison G., Re L. Effects of ozone therapy on haemostatic and oxidative stress index in coronary artery disease. Eur. J. Pharmacol. 2012;691(1–3):156–162. doi: 10.1016/j.ejphar.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 107.Hernández F., Menéndez S., Wong R. Decrease of blood cholesterol and stimulation of antioxidative response in cardiopathy patients treated with endovenous ozone therapy. Free Radic. Biol. Med. 1995;19(1):115–119. doi: 10.1016/0891-5849(94)00201-t. [DOI] [PubMed] [Google Scholar]
- 108.Buyuklu M., Kandemir F.M., Set T., et al. Beneficial effects of ozone therapy on oxidative stress, cardiac functions and clinical findings in patients with heart failure reduced ejection fraction. Cardiovasc. Toxicol. 2017;17(4):426–433. doi: 10.1007/s12012-017-9400-8. [DOI] [PubMed] [Google Scholar]
- 109.Delgado-Roche L., Riera-Romo M., Mesta F., et al. Medical ozone promotes Nrf2 phosphorylation reducing oxidative stress and pro-inflammatory cytokines in multiple sclerosis patients. Eur. J. Pharmacol. 2017;811:148–154. doi: 10.1016/j.ejphar.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 110.Shah MA, Anande LK, Powar A, Captain J, Mk Nair PJMEJoR, Studies H. The Role of Medical Ozone in Improving Antioxidant Status in Multiple Drug-Resistant Tuberculosis Patients: A Quasi-experimental Study. Middle East J. Rehabil. Health Stud. 2019; 6(4):e97125.
- 111.M.A. Shah, Ozone Therapy in oxidative stress disorders and evaluation of C- reactive proteins [abstract], Proceedings of the 5Th WFOT Meeting; 2016 Nov 18-20; Mumbai, India, J. Ozone Ther. 2018;2(2). doi: 10.7203/jo3t.2.2.2018.11148.
- 112.Martinez-Sanchez G., Al-Dalain S.M., Menendez S., et al. Therapeutic efficacy of ozone in patients with diabetic foot. Eur. J. Pharmacol. 2005;523(1–3):151–161. doi: 10.1016/j.ejphar.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 113.Fernandez O.S.L., Oru G.T., Vega J.C.P., et al. Ozone+ Arthroscopy: Improved Redox Status, Function and Surgical Outcome in Knee Osteoarthritis Patients. Int. J. Innov. Surg. 2020;3(1):1011. [Google Scholar]
- 114.Totolici I.P., Pascu A.M., Poroch V., Mosoiu D. The impact of ozone therapy on antioxidant status and quality of life in palliative care-exploratory study. Rev. Chim. 2017;68(10):2416–2421. [Google Scholar]
- 115.Pecorelli A., Bocci V., Acquaviva A., et al. NRF2 activation is involved in ozonated human serum upregulation of HO-1 in endothelial cells. Toxicol. Appl. Pharmacol. 2013;267(1):30–40. doi: 10.1016/j.taap.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 116.Galiè M., Costanzo M., Nodari A., et al. Mild ozonisation activates antioxidant cell response by the Keap1/Nrf2 dependent pathway. Free Radic. Biol. Med. 2018;124:114–121. doi: 10.1016/j.freeradbiomed.2018.05.093. [DOI] [PubMed] [Google Scholar]
- 117.Natural Holistic Health Care Ozone And UNB Therapy. Available from https://www.naturalholistic.com/ozone-and-unb-therapy (accessed 21 July 2020).
- 118.Bocci V.A., Zanardi I., Travagli V. Ozone acting on human blood yields a hormetic dose-response relationship. J. Transl. Med. 2011;9:66. doi: 10.1186/1479-5876-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang Z., Zhang A., Meng W., Wang T., Li D., Liu Z., Liu H. Ozone protects the rat lung from ischemia-reperfusion injury by attenuating NLRP3-mediated inflammation, enhancing Nrf2 antioxidant activity and inhibiting apoptosis. Eur. J. Pharmacol. 2018;835:82–93. doi: 10.1016/j.ejphar.2018.07.059. [DOI] [PubMed] [Google Scholar]
- 120.Sagai M., Bocci V. Mechanisms of Action Involved in Ozone Therapy: Is healing induced via a mild oxidative stress? Med. Gas Res. 2011;1:29. doi: 10.1186/2045-9912-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bocci V. How a calculated oxidative stress can yield multiple therapeutic effects. Free Radic. Res. 2012;46(9):1068–1075. doi: 10.3109/10715762.2012.693609. [DOI] [PubMed] [Google Scholar]
- 122.Bocci V., Valacchi G. Nrf2 activation as target to implement therapeutic treatments. Front. Chem. 2015;3:4. doi: 10.3389/fchem.2015.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hayes J.D., Dinkova-Kostova A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014;39(4):199–218. doi: 10.1016/j.tibs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 124.Tanito M., Agbaga M.P., Anderson R.E. Upregulation of thioredoxin system via Nrf2-antioxidant responsive element pathway in adaptive-retinal neuroprotection in vivo and in vitro. Free Radic. Biol. Med. 2007;42(12):1838–1850. doi: 10.1016/j.freeradbiomed.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 125.MacLeod A.K., McMahon M., Plummer S.M., Higgins L.G., Penning T.M., Igarashi K., Hayes J.D. Characterization of the cancer chemopreventive NRF2-dependent gene battery in human keratinocytes: demonstration that the KEAP1-NRF2 pathway, and not the BACH1-NRF2 pathway, controls cytoprotection against electrophiles as well as redox-cycling compounds. Carcinogenesis. 2009;30(9):1571–1580. doi: 10.1093/carcin/bgp176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Agyeman A.S., Chaerkady R., Shaw P.G., Davidson N.E., Visvanathan K., Pandey A., Kensler T.W. Transcriptomic and proteomic profiling of KEAP1 disrupted and sulforaphane-treated human breast epithelial cells reveals common expression profiles. Breast Cancer Res. Treat. 2012;132(1):175–187. doi: 10.1007/s10549-011-1536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Olagnier D., Farahani E., Thyrsted J., et al. SARS-CoV2-mediated suppression of NRF2-signaling reveals potent antiviral and anti-inflammatory activity of 4-octyl-itaconate and dimethyl fumarate. Nat. Commun. 2020;11(1):1–12. doi: 10.1038/s41467-020-18764-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kobayashi E.H., Suzuki T., Funayama R., et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016;7(1):1–14. doi: 10.1038/ncomms11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rushworth S.A., Zaitseva L., Murray M.Y., Shah N.M., Bowles K.M., MacEwan D.J. The high Nrf2 expression in human acute myeloid leukemia is driven by NF-κB and underlies its chemo-resistance. Blood. 2012;120(26):5188–5198. doi: 10.1182/blood-2012-04-422121. [DOI] [PubMed] [Google Scholar]
- 130.Thimmulappa R.K., Lee H., Rangasamy T., Reddy S.P., Yamamoto M., Kensler T.W., Biswal S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J. Clin. Invest. 2006;116(4):984–995. doi: 10.1172/JCI25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ahmed S.M., Luo L., Namani A., Wang X.J., Tang X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta, Mol. Basis Dis. 2017;1863(2):585–597. doi: 10.1016/j.bbadis.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 132.Imai Y., Kuba K., Neely G.G., et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133(2):235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nomura M., Ma W., Chen N., Bode A.M., Dong Z. Inhibition of 12-O-tetradecanoylphorbol-13-acetate-induced NF-kappaB activation by tea polyphenols, (-)-epigallocatechin gallate and theaflavins. Carcinogenesis. 2000;21(10):1885–1890. doi: 10.1093/carcin/21.10.1885. [DOI] [PubMed] [Google Scholar]
- 134.Schulze-Osthoff K., Bauer M.K., Vogt M., Wesselborg S. Oxidative stress and signal transduction. Int. J. Vitam. Nutr. Res. 1997;67(5):336–342. [PubMed] [Google Scholar]
- 135.Guo H., Callaway J.B., Ting J.P. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat. Med. 2015;21(7):677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Minutoli L., Antonuccio P., Irrera N., et al. NLRP3 inflammasome involvement in the organ damage and impaired spermatogenesis induced by testicular ischemia and reperfusion in mice. J. Pharmacol. Exp. Ther. 2015;355(3):370–380. doi: 10.1124/jpet.115.226936. [DOI] [PubMed] [Google Scholar]
- 137.Yin N., Peng Z., Li B., Xia J., Wang Z., Yuan J., Fang L., Lu X. Isoflurane attenuates lipopolysaccharide-induced acute lung injury by inhibiting ROS-mediated NLRP3 inflammasome activation. Am. J. Transl. Res. 2016;8(5):2033–2046. [PMC free article] [PubMed] [Google Scholar]
- 138.Dong W., Yang R., Yang J., Yang J., Ding J., Wu H., Zhang J. Resveratrol pretreatment protects rat hearts from ischemia/reperfusion injury partly via a NALP3 inflammasome pathway. Int. J. Clin. Exp. Pathol. 2015;8(8):8731–8741. [PMC free article] [PubMed] [Google Scholar]
- 139.Franzini M., Valdenassi L., Ricevuti G., Chirumbolo S., Depfenhart M., Bertossi D., Tirelli U. Oxygen-ozone (O2–O3) immunoceutical therapy for patients with COVID-19. Preliminary evidence reported. Int. Immunopharmacol. 2020;88 doi: 10.1016/j.intimp.2020.106879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.A. Hernandez, M. Vinals, A. Pablos et al., Ozone therapy for patients with SARS-COV-2 pneumonia: a single-center prospective cohort study, 2020. Preprint, medRxiv, doi: https://doi.org/10.1101/2020.06.03.20117994.
- 141.Fernández-Cuadros M.E., Albaladejo-Florín M.J., Álava-Rabasa S., et al. Effect of Rectal Ozone (O 3) in Severe COVID-19 Pneumonia: Preliminary Results. SN Comprehensive Clin. Med. 2020;2(9):1328–1336. doi: 10.1007/s42399-020-00374-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hernández A., Viñals M., Isidoro T., Vilás F. Potential Role of Oxygen-Ozone Therapy in Treatment of COVID-19 Pneumonia. Am. J. Case Rep. 2020;21 doi: 10.12659/AJCR.925849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Z. Zheng, M. Dong, K. Hu, A preliminary evaluation on the efficacy of ozone therapy in the treatment of COVID-19, J. Med. Virol. 2020 May 21:10.1002/jmv.26040. doi: 10.1002/jmv.26040. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 144.Wu J., Tan C., Yu H., et al. Case Report: Recovery of One Icu-Acquired Covid-19 Patient Via Ozonated Autohemotherapy. SSRN Electron. J. 2020 [Google Scholar]
- 145.Wu J., Tan C.S., Yu H., et al. Recovery of Four COVID-19 Patients via Ozonated Autohemotherapy. Innovation (N Y). 2020;1(3) doi: 10.1016/j.xinn.2020.100060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.C. Tascini, G. Sermann, A. Pagotto et al., Blood ozonization in patients with mild to moderate COVID-19 pneumonia: a single centre experience, Intern. Emerg. Med. 2020 Nov 1:1–7. doi: 10.1007/s11739-020-02542-6. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 147.Valdenassi L., Franzini M., Ricevuti G., Rinaldi L., Galoforo A.C., Tirelli U. Potential mechanisms by which the oxygen-ozone (O2–O3) therapy could contribute to the treatment against the coronavirus COVID-19. Eur. Rev. Med. Pharmacol. Sci. 2020;24(8):4059–4061. doi: 10.26355/eurrev_202004_20976. [DOI] [PubMed] [Google Scholar]
- 148.Simonetti V., Quagliariello V., Franzini M., Iaffaioli R.V., Maurea N., Valdenassi L. Ozone Exerts Cytoprotective and Anti-Inflammatory Effects in Cardiomyocytes and Skin Fibroblasts after Incubation with Doxorubicin. Evid. Based Complement Alternat. Med. 2019;2019:2169103. doi: 10.1155/2019/2169103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Italian Society of Oxygen Ozone Therapy (SIOOT). A different therapeutic protocol for each of the five different phenotypes. Available from: (last accessed on 30th November 2020).
- 150.J.M. Zhang, Y. Penninger, N. Li, A.S. Zhong, Slutsky, Angiotensin-converting en- zyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target, Intensive Care Med. 2020;46 (4):586–590. [DOI] [PMC free article] [PubMed]
- 151.Akerström S., Gunalan V., Keng C.T., Tan Y.J., Mirazimi A. Dual effect of nitric oxide on SARS-CoV replication: viral RNA production and palmitoylation of the Sprotein are affected. Virology. 2009;395(1):1–9. doi: 10.1016/j.virol.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Robba C., Robba C., Battaglini D., Ball L., Patroniti L.N., Loconte M., Brunetti I., Vena A., Giacobbe D., Bassetti M., Rocco P.R.M., Pelosi P. Distinct phenotypes require distinct respiratory management strategies in severe COVID-19. Respir. Physiol. Neurobiol. 2020;279 doi: 10.1016/j.resp.2020.103455. [DOI] [PMC free article] [PubMed] [Google Scholar]