Abstract
Objectives
To determine the prevalence of olfactory and taste dysfunction (OD; TD) among COVID‐19 positive health care workers (HCWs), their associated risk factors and prognosis.
Methods
Between May and June 2020, a longitudinal multicenter study was conducted on symptomatic COVID‐19 PCR confirmed HCWs (COVID‐19 positive) in London and Padua.
Results
Hundred and fourteen COVID‐19 positive HCWs were surveyed with a response rate of 70.6% over a median follow‐up period of 52 days. UK prevalence of OD and TD was 73.1% and 69.2%, respectively. There was a male to female ratio of 1:3 with 81.6% being white, 43.7% being nurses/health care assistants (HCAs), and 39.3% being doctors. In addition, 53.2% of them worked on COVID‐19 wards. Complete recovery was reported in 31.8% for OD and 47.1% for TD with a 52 days follow‐up. The job role of doctors and nurses negatively influenced smell (P = .04 and P = .02) and taste recovery (P = .02 and P = .01). Ethnicity (being white) showed to positively influence only taste recovery (P = .04). Sex (being female) negatively influenced OD and TD recovery only in Paduan HCWs (P = .02 and P = .011, respectively). Working on a COVID‐19 ward did not influence prognosis.
Conclusions
The prevalence of OD and TD was considerably higher in HCWs. The prognosis for OD and TD recovery was worse for nurses/HCAs and doctors but working on a COVID‐19 ward did not influence prognosis. Sixty‐eight percent of surveyed HCWs at 52 days continued to experience OD or TD requiring additional future medical management capacity.
Level of Evidence
4.
Keywords: COVID‐19, olfactory dysfunction, rhinology, smell, survey, taste, taste dysfunction
XXX.
1. INTRODUCTION
Health care workers (HCWs) have been identified as a high‐risk group to acquire COVID‐19. 1 In Europe, HCWs account for 10.7% (Italy) to 30.5% [United Kingdom (UK)] of the total number of COVID‐19 positive cases. 2 , 3 A different figure was released by the International Council of Nurses based on data acquired from 30 countries reporting that, on average, 6% of all confirmed cases of COVID‐19 were among HCWs. 4 Similarly, an Indian questionnaire‐based survey found that only 1.8% (20/1113) of the HCWs tested were positive for the virus. 5 The specific job role of COVID‐19 HCWs is also potentially relevant with a higher prevalence in doctors (43.9%) and nurses/health care assistants (HCAs) (41%). 6 Particularly, otolaryngologists and intensive care/anesthetists have demonstrated a higher risk of contracting COVID‐19 owing to their higher viral load exposure. 7
The World Health Organization has included “loss of smell” and “taste” among the less common symptoms of COVID‐19 infection. 8 Nonetheless, the estimated prevalence of olfactory and taste dysfunction (OD, TD) among COVID‐19 subjects in the general population is as high as 38.5% and 30.4%, respectively. 9 Because of the work‐related risks, HCWs are exposed daily to higher viral load which may lead to a different expression of the chemosensory disorders, both in terms of prevalence, severity and/or recovery rate. In a survey conducted by the American Academy of Otolaryngology‐Head and Neck Surgery, 1/3 of COVID‐19 positive patients with anosmia were HCWs. 10 Moreover, Lan et al found that anosmia/ageusia was reported by 15.7% (13/83) of COVID‐19 positive HCWs in the United States. 11 In a more recent American study a higher percentage of positive HCWs reported anosmia or ageusia, respectively 51% (26/51) and 53% (27/51). 12
The true prevalence in Europe remains unknown. According to available data between 14.4% (20/139) and 79% (77/97) of the adult COVID‐19 positive patients reporting OD and TD were HCWs. 1 , 6 , 13 A very recent Belgian study found that almost 40% (62/156) of positive HCWs self‐reported loss of sense of smell/taste 13 while a Danish study conducted on a bigger sample found that loss of sense of smell or taste was reported by 32.4% (377/1163) of the tested positive HCWs. 14 Smaller European case series (less than 6 subjects) on OD and TD among HCWs are also available but inconclusive. 15 , 16 , 17 In the UK the prevalence of OD and TD among COVID‐19 positive HCWs is unknown. Moreover, the risk factors and prognosis for OD and TD among HCWs are mostly unknown.
The aim of this study is to determine the prevalence of OD and TD among COVID‐19 positive HCWs in the UK and ascertain risk factors and prognosis in two European hospitals [London (UK) and Padua (Italy)] which have been significantly affected by COVID‐19.
2. MATERIALS AND METHODS
This study was conducted in accordance with the 1996 Helsinki Declaration and approved by the research ethic committee (IRAS project ID: 156511), the UCL joint research office and the Padua Otolaryngology Section's in‐house ethical committee. All respondents were invited to take part in this survey via email which included a study information pack and consent form with a cooling off period.
2.1. Setting of the survey
Between May 26 and June 10, 2020 an international multicenter survey on sense of smell and taste dysfunction in mild‐to‐moderate symptomatic COVID‐19 positive HCWs, defined as home‐managed subjects with symptoms that did not require an intensive care or other hospital admissions, was conducted at the Whittington Hospital (London, UK) and the Hospital of Padua (Padua, Italy). The survey questionnaire was validated locally and nationally by both ENT and infection clinicians as well as patient advocates to ensure clarity and to exclude ambiguity. In the UK, the survey was performed via Survey Monkey (San Mateo, California) and emailed to all COVID‐19 positive HCWs. The questionnaire was translated into Italian and equally validated and administered by hand in Padua.
Inclusion criteria were age >18 years old, laboratory confirmation of SARS‐CoV2 infection (by reverse transcription polymerase chain reaction [RT‐PCR]), good comprehension of the language used in the questionnaire and absence of any clinical impairment to complete the questionnaire. Participants with a past history of OD and/or TD or those admitted to hospital at the moment of the survey were excluded from the study. Informed consent was obtained from each participant before starting any study‐related procedure.
2.2. Population and data collection
The recipients of this survey were mild‐to‐moderate symptomatic HCWs who tested positive by RT‐PCR for SARS‐CoV2 and were working at their own hospital during COVID‐19 pandemic. Participants were selected using the databases of the Microbiology Laboratory in London and the Infectious Disease Department in Padua. Data were collected anonymously mainly on olfactory and gustatory disorders presentation, type of onset and recovery status, while the presence of other systemic symptoms has not been investigated. Demographic data including age, sex, ethnicity, job role and department of origin were also collected for all the participants to investigate any potential influence.
2.3. Statistical analysis
Quantitative variables were summarized using median and interquartile range (P25‐P75) while qualitative variables were described with frequency and percentage. Missing values (ie, people who did not answer the question) were not considered in the calculation of percentages (valid percent). However, unanswered items have been reported in the tables.
Survival analysis was implemented to study recovery time from onset of both sense of smell and taste. Participants that had not recovered at the date of questionnaire administration were considered as censored, with censor time described as the number of days since the onset of the symptom up to the day of the questionnaire. Survival curves have been estimated with Kaplan‐Meier estimator, log‐rank tests have been performed to compare subpopulations and Cox proportional hazard model has been fitted to model the joint effect of all available variables on the recovery time. The best model has been chosen by stepwise selection based on Akaike Information Criterion (AIC). Likelihood ratio tests have been used to test comparisons between means and proportions, and Pearson chi‐square test with Yates correction to compare categorical variables.
3. RESULTS
3.1. Response rate
One‐hundred and fifty‐five HCWs, 119 from London and 36 from Padua received the questionnaire. The different method of questionnaire administration led to a different response rate of 70.6% (84/119) in London and of 100% (36/36) in Padua.
3.2. Population characteristics
After further analysis, we excluded two participants who did not accept the consent form and four participants who did not answer any question, leading to a final population of 114 HCWs who completed the survey. The total population was composed of 28 men and 86 women (male to female ratio approximately of 1:3), ranging from 23 to 65 years, with a median age of 38 years. Most of the HCWs were white (62; 81.6%), worked on COVID‐19 wards (59; 53.2%) and were either nurses/HCA (43.7%) or doctors (39.3%). A significant difference in the composition of participants at the two hospitals was observed according to ethnicity (P < .00001) and department of origin (P = .00035), whereas they were similar in terms of age (P = .72), sex ratio (P = 1) and job role (P = .067). Detailed characteristics of the population at each institution are reported in Table 1.
TABLE 1.
Detailed characteristics of the populations
| Combined (n = 114) | London (n = 78) | Padua (n = 36) | Difference between London and Padua (P‐value) | |
|---|---|---|---|---|
| Age, median [P25‐P75], year | 38 [29.5‐48] | 39 [32‐47] | 39 [27.5‐52] | P = .72 |
| Sex, No (%) | ||||
| Female | 86 (75.4%) | 59 (75.6%) | 27 (75.0%) | P = 1 |
| Male | 28 (24.6%) | 19 (24.4%) | 9 (25.0%) | |
| Ethnicity, No (%) a | ||||
| White | 62 (81.6%) | 26 (65.0%) | 36 (100.0%) | |
| Asian | 12 (15.8%) | 12 (30.0%) | 0 (0.0%) | |
| Black/African/Caribbean | 1 (1.3%) | 1 (2.5%) | 0 (0.0%) | P < .00001 * |
| Mixed/multiple ethnic groups | 1 (1.3%) | 1 (2.5%) | 0 (0.0%) | |
| Missing | 38 | 38 | 0 | |
| Role, No (%) a | ||||
| Nurse/HCA | 49 (43.7%) | 32 (41.0%) | 17 (50.0%) | |
| Doctor | 44 (39.3%) | 29 (37.2%) | 15 (44.1%) | P = .067 |
| Allied health professional | 16 (14.3%) | 14 (18.0%) | 2 (5.9%) | |
| Non‐clinical role | 3 (2.7%) | 3 (3.8%) | 0 (0.0%) | |
| Missing | 2 | 0 | 2 | |
| Department of origin, No (%) a | ||||
| COVID‐19 ward | 59 (53.2%) | 51 (65.4%) | 8 (24.2%) | |
| Non‐COVID‐19 ward | 47 (42.3%) | 24 (30.8%) | 23 (69.7%) | |
| Office/laboratory | 5 (4.5%) | 3 (3.8%) | 2 (6.1%) | P = .00035 * |
| Missing | 3 | 0 | 3 | |
| Type of disfunction reported, No (%) | ||||
| Olfactory dysfunction | NA | 57 (73.1%) | NA | NA |
| Taste dysfunction | 54 (69.2%) | |||
| Both | 48 (61.5%) | |||
| OD characteristics, No (%) a | ||||
| First symptom | 19 (21.6%) | 10 (18.9%) | 9 (25.7%) | P = .62 |
| Only symptom b | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ‐ |
| Onset | ||||
| Sudden | 69 (78.4%) | 42 (79.2%) | 27 (77.1%) | P = 1 |
| Progressive | 19 (21.6%) | 11 (20.8%) | 8 (22.9%) | |
| Missing | 5 | 4 | 1 | |
| TD characteristics, No (%) a | ||||
| First symptom | 14 (16.1%) | 6 (11.3%) | 8 (23.5%) | P = .23 |
| Only symptom | 1 (1.1%) | 1 (1.9%) | 0 (0.0%) | ‐ |
| Onset | ||||
| Sudden | 65 (74.7%) | 42 (79.2%) | 23 (67.6%) | P = .34 |
| Progressive | 22 (25.3%) | 11 (20.8%) | 11 (32.4%) | |
| Missing | 7 | 5 | 2 | |
| Dysgeusia c | 30 (65.2%) | 9 (39.1%) | 21 (91.3%) | P = .002 * |
Note: NA: Not Applicable. Not possible because prevalence not performed in Padua. P‐values indicate differences in the distribution between the two Institutions.
Valid percent, not including missing values.
Please note that in seven subjects (7.5%) olfactory dysfunction was associated to taste dysfunction alone.
Dysgeusia has been calculated only considering those subjects who reported hypogeusia or ageusia at the moment of questionnaire administration (n = 46).
Significant P‐values in bold. Level of significance P < .05.
3.3. Olfactory and taste subjective dysfunction characteristics
The prevalence of reported olfactory and taste alteration was 73.1% and 69.2%, respectively, in London HCWs. Prevalence was not obtained in the Paduan population due to the fact that the questionnaire was administered only to HCWs with a reported smell impairment.
In the total study population, among the 93 HCWs who experienced OD, this was the first symptom in 19 participants (21.6%), but none of them reported this to be the only COVID‐19 related symptom. Additionally, only 8 of those who reported OD as a first symptom (8/19) also complained of nasal obstruction. In 7 participants (7.5%) it was associated with TD and these were the only symptoms experienced during their COVID‐19 illness. The onset of OD was reported to be sudden by 69 participants (78.4%), while it was progressive in 19 of them (21.6%).
Similarly, among the 94 HCWs who had TD during their illness this was the first symptom in 14 of them (16.1%) and was the only one experienced in 1 participant (1.1%). Deterioration of sense of taste was described as sudden by 65 HCWs (74.7%) and as progressive by 22 of them (25.3%). Dysgeusia (distortion of sense of taste) was reported by 30 participants (65.2%). Apart from dysgeusia which was significantly more prevalent among Paduans (P = .002), no differences were observed in terms of presentation (first symptom, only symptom and type of onset) between the two Hospitals. Detailed characteristics of smell and taste dysfunction among HCWs according to Institution are reported in Table 1.
3.4. Prognosis of smell and taste dysfunction
At 52 days follow‐up, 28 HCWs (31.8%) reported that OD had completely recovered while the majority of them (49; 55.7%) reported that their sense of smell had improved but was still lower than before (hyposmia). It was still absent (anosmia) in 11 participants (12.5%) (Table 2). None of the subjects had started any specific treatment for the OD.
TABLE 2.
Time for sense of smell recovery in the three subgroups of subjects who experienced olfactory dysfunction
| Sense of smell | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | Time to OD onset | Time for recovery to start a | Time to questionnaire administration b | |||||||||
| Median [P25‐P75], days | Median [P25‐P75], days | Median [P25‐P75], days | ||||||||||
| Combined | London | Padua | Combined | London | Padua | Combined | London | Padua | Combined | London | Padua | |
| Recovered | 28 (31.8%) | 22 (41.5%) | 6 (17.1%) | 4 [2–5] | 4 [2‐5] | 2.5 [1.3‐8.3] | 10 [7‐14.5] | 12 [7‐14.8] | 8 [6‐17] | 52.5 [47.3‐62.8] | 53 [49.8‐63.8] | 24 [13.8‐65.3] |
| Still hyposmia | 49 (55.7%) | 28 (52.8%) | 21 (60.0%) | 4 [2‐6] | 4.5 [3‐6.8] | 3 [2–5] | 20 [10‐30] | 21 [15.5‐40] | 10 [7.5‐22.5] | 51 [35‐62] | 54 [50‐64.3] | 35 [22‐62.5] |
| Not recovered | 11 (12.5%) | 3 (5.7%) | 8 (22.9%) | 3 [1‐5.75] | 3 [1‐8.5] | 3 [1‐5] | NA | NA | NA | 41 [24.5‐54.5] | 56 [53‐66] | 34.5 [12.3‐45.5] |
Note: NA: Not Applicable. Not possible to calculate considering that sense of smell in these subjects had not started to recover.
Abbreviation: OD, olfactory dysfunction.
Time for the recovery to begin after first symptom onset.
Interval of time between first symptom onset and questionnaire administration.
With regards to sense of taste, 41 HCWs (47.1%) reported that TD had completely recovered at the time of the questionnaire administration. Thirty‐eight participants (43.7%), still reported a lower sense of taste (hypogeusia) while it was still absent (ageusia) in 8 participants (9.2%) (Table 3). No significant differences were noted between the two institutions. The median time for the recovery start as well as the median time to questionnaire administration for both smell and taste are reported in Tables 2 and 3.
TABLE 3.
Time for sense of taste recovery in the three subgroups of subjects who experienced taste dysfunction
| Sense of taste | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | Time to OD onset | Time for recovery to start a | Time to questionnaire administration b | |||||||||
| Median [P25‐P75], days | Median [P25‐P75], days | Median [P25‐P75], days | ||||||||||
| Combined | London | Padua | Combined | London | Padua | Combined | London | Padua | Combined | London | Padua | |
| Recovered | 41 (47.1%) | 30 (56.6%) | 11 (32.4%) | 4 [2–5] | 4 [2‐5.3] | 2 [1.5‐6] | 10 [8‐18] | 12.5 [7.3‐15] | 10 [9‐20] | 53 [47‐63] | 53.5 [48.8‐65.3] | 47 [24‐63] |
| Still hypogeusia | 38 (43.7%) | 20 (37.7%) | 18 (52.9%) | 4.5 [2‐6] | 5 [2.8‐7] | 3 [2‐5] | 15 [10‐30] | 20 [11‐30.5] | 14 [9.3‐20] | 51.5 [34‐62] | 53 [50‐61.5] | 34.5 [28.8‐64] |
| Not recovered | 8 (9.2%) | 3 (5.7%) | 5 (14.7%) | 3 [2–5] | 3 [2‐4.5] | 3 [2‐4.8] | NA | NA | NA | 33 [10.3‐52.8] | 54 [49‐62] | 11 [9‐33] |
Note: NA: Not Applicable. Not possible to calculate considering that sense of taste in these subjects had not started to recover.
Abbreviation: TD, taste dysfunction.
Time for the recovery to begin after first symptom onset.
Interval of time between first symptom onset and questionnaire administration.
3.5. Influence of available variables on OD and TD prognosis
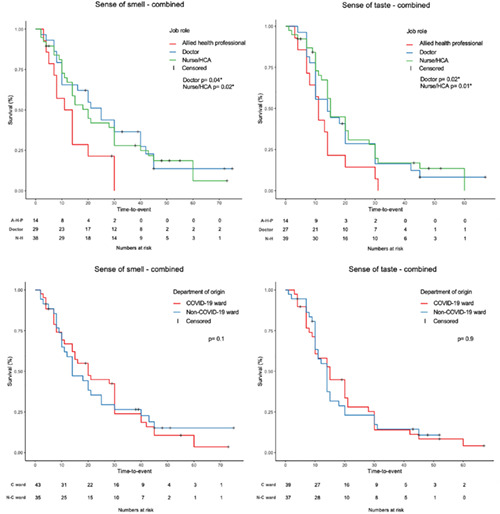
Considering the whole population, certain job roles negatively influenced the time to recovery both for sense of smell (doctor P = .04; nurse/HCA P = .02) and taste (doctor P = .02; nurse/HCA P = .01) (Figure 1; Table 4) In addition, following multiple regression analysis, ethnicity (being white) was shown to positively influence sense of taste recovery time (P = .036) but not for sense of smell (P = .5) (Table 4). Conversely, no influence on smell and taste recovery was observed when considering age, sex, department of origin, presentation as first symptom or only symptom and type of onset (sudden or progressive) (Figure 1; Table 4).
FIGURE 1.
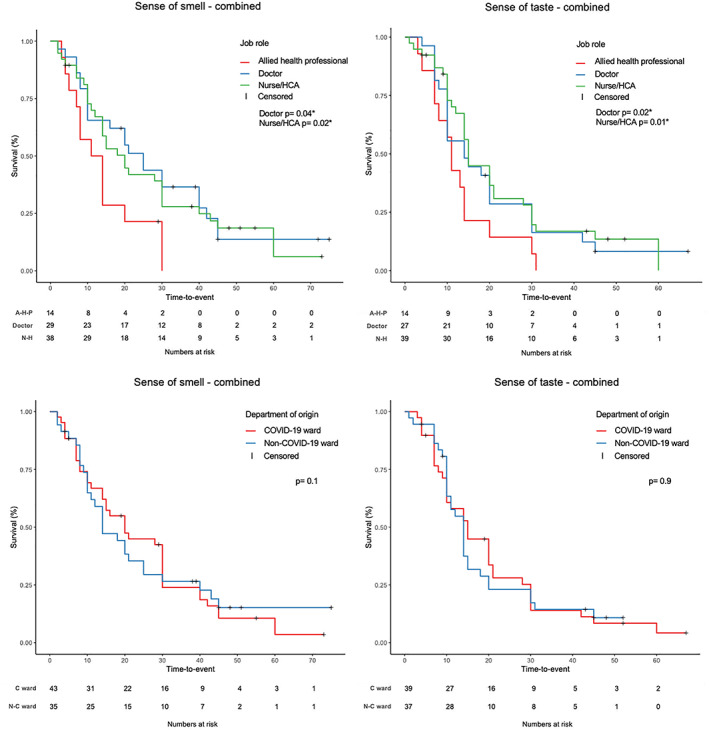
Kaplan‐Meier survival curves for smell and taste recovery time according to job role (upper left and right) and to department of origin (lower left and right). AHP, allied health professional; C ward, COVID‐19 ward; HCA, health care assistants; N‐C ward: Non‐COVID‐19 ward; NH: nurse/health care assistants. *Significant P‐values. Level of significance P < .05
TABLE 4.
Influence of available variables on recovery rate in the population of health care workers who experienced smell and/or taste dysfunction
| Sense of smell prognosis | Sense of taste prognosis | |||||
|---|---|---|---|---|---|---|
| Combined | London | Padua | Combined | London | Padua | |
| Age | P = .9 | P = .5 | P = .6 | P = .1 | P = .4 | P = .3 |
| Sex | P = .9 | P = .06 | P = .02 * | P = .9 | P = .3 |
P = .1 (P = .011 * at multiple regression) |
| Ethnicity | P = .5 | P = .2 |
P = .09 (P = .036 * at multiple regression) |
P = .06 (P = .022 * at multiple regression) |
||
| Role |
P = .04 * (Doctor) P = .02 * (Nurse/HCA) |
P = .0566 (Doctor) P = .002 * (Nurse/HCA) |
P = .8 (Doctor) P = .6 (Nurse/HCA) |
P = .02 * (Doctor) P = .01 * (Nurse/HCA) |
P = .204 (Doctor) P = .02 * (Nurse/HCA) |
P = .391 (Doctor) P = .733 (Nurse/HCA) |
| Department | P = .1 | P = .9 | P = .2 | P = .9 | P = 1 | P = .7 |
| First Symptom | P = .6 | P = .9 | P = .7 | P = .6 | P = .7 | P = .9 |
| Only Symptom | P = .9 | P = .9 | P = 1 | P = .4 | P = .2 | P = .9 |
| Type of onset | P = .9 | P = .8 | P = .8 | P = .2 | P = .4 | P = .4 |
Abbreviation: HCA, health care assistants.
Significant P‐values in bold. Level of significance P < .05.
Analyzing the results from the two hospitals individually, the prognosis of OD among Paduans was negatively influenced by female sex (P = .02). Following multiple regression analysis, female sex was shown to negatively influence TD recovery as well. Conversely, in London, job role (Nurse/HCA) negatively influenced OD and TD (P = .002 and P = .02, respectively). Following multiple regression analysis, ethnicity (being white) was also shown to positively influence sense of taste recovery time (P = .022) (Table 4).
4. DISCUSSION
To the best of our knowledge, our study represents the first multicentric European survey evaluating olfactory and taste dysfunction on COVID‐19 positive HCWs with a response rate higher than 70%.
In the UK, the prevalence of both OD and TD among our COVID‐19 positive HCWs was 73.1% and 69.2%, respectively. These rates are significantly higher than those found within the general population (38.5% and 30.4%, respectively, according to a recent meta‐analysis) 9 and equally considerably higher when compared to HCW prevalence rates in the United States 11 , 12 or in other European countries. 13 , 14 In addition, a higher rate of dysgeusia was particularly highlighted in our European cohort which had not been previously described. The higher prevalence rates of both olfactory and taste disturbance are unexpected when compared to current published data within the general population. One proposed explanation is that HCWs are more prone to OD and TD because they could be exposed to a higher Sars‐Cov‐2 viral load within their place of work. 6 An alternative explanation for the higher prevalence rates among HCWs in our study is a consequence of the higher sensitivity of our survey whereby milder cases of OD and TD are being captured. In addition, all our HCWs were assessed from the time of their diagnosis and then studied longitudinally over a median of 52 days from COVID‐19 symptom onset. This enabled us to evaluate the whole of their COVID‐19 journey and not just at a single point in time and avoids missing OD and TD before it even started.
Previous studies conducted in Europe on OD and TD among COVID‐19 HCWs 6 , 13 , 14 have focused more on general COVID‐19 symptoms with only rudimentary smell and taste dysfunction evaluation and/or not exploring patients with very mild symptoms which may explain why their prevalence rates are lower than ours. Patients with milder OD have been shown to be unaware of their symptoms and therefore less likely to report a problem. 18 A responder bias also needs to be considered. It is possible that those with OD and TD were more likely to respond to the questionnaire; however, this is unlikely given that our response rate was over 70%.
The presentation of OD and TD, in terms of smell and taste onset (sudden/progressive and first/only), in our population of HCWs seemed to be similar to that seen in the general population. In our study OD and TD occurred suddenly (78.4% and 74.7%, respectively) at a median time of 4 days which is similar to the general population. 19 , 20 , 21 Similarly, smell and taste impairment presented as the first symptom in 21.6% and 16.1% respectively, in line with previous surveys on the general population. 10 , 21 , 22 None of our respondents described loss of sense of smell as an isolated symptom, but in seven participants (7.5%) OD and TD were their only COVID‐19 symptoms. This percentage is similar to another Italian study. 22 Conversely, a previous survey on 2428 subjects with new‐onset anosmia showed that 17% reported OD as an isolated symptom 23 ; however, this finding was not confirmed by our results.
Our results also showed a higher prevalence of OD and TD among COVID‐19 positive doctors and nurses/HCA as compared to other HCWs, which reflects previous Italian findings. 2 , 6 More importantly, we observed that HCW's job role negatively influenced prognosis and their time to recovery both for sense of smell (doctor P = .04; nurse/HCA P = .02) and taste (doctor P = .02; nurse/HCA P = .01) (Figure 1, Table 4) with implications to change future behavior to mitigate this risk.
Notably, we did not observe that the department of work influenced prognosis of OD (P = .1) and TD (P = .9) (Figure 1) which potentially confirms the effectiveness of preventive measures in higher risk departments. In support of our results, Wang et al also found that the majority of the infected HCWs in Wuhan had worked on the general wards (77.5%), with a lower prevalence in the emergency department (17.5%) and ICU (5%). 24
According to our findings, ethnicity appears to affect prognosis. We demonstrated that prognosis was significantly more favorable in white HCWs but only for TD (P = .036) (Table 4) and this novel finding has not been previously described in COVID‐19 patients. However, it corroborates what had been reported by Doty, prior to the COVID‐19 pandemic, that being an ethnic minority represents a risk factor for OD. 25 Overall, white and Asian subjects were the most widely affected group among our HCW population which is similar to previous reports showing OD and TD being three times more common in Caucasians compared to East Asians. 9
In our population 75.4% of the HCWs who experienced OD and/or TD were female with a median age of 38 years which confirms previous findings that COVID‐19 related OD disproportionately affects the younger generation 13 , 26 , 27 , 28 and the female sex. 28 We also demonstrated that female HCWs in the Paduan population showed a worse prognosis for OD and TD. However, this finding was not confirmed when considering the total study population and therefore it could be related to a bias in the composition of the Paduan sample. Age did not demonstrate an influence on smell or taste recovery time.
The true prognosis of OD and TD among COVID‐19 HCWs is not known because the follow‐up time to date has been too short to draw reasonable conclusions. We have found that the sense of smell and taste started to improve spontaneously on average after a median time of 15 days from onset of symptoms. (Figure 2 left; Tables 2 and 3) We observed a bimodal trend in recovery for sense of taste with two identifiable peaks roughly at 15 and 30 days (Figure 2 left—yellow area); conversely, this was not evident for smell recovery where at least another peak at 22 days was recognized (Figure 2 left—green area). Early spontaneous recovery of sense of smell may indicate a conductive cause for COVID‐19 OD, as it has been reported that olfactory disorders may last 3 to 4 weeks after clinical onset, or longer, in case of damage of the olfactory epithelium (ie, support cell, stem cell, and perivascular cell of the olfactory epithelium). 29 Moreover, the infection of basal cells could block or slow down sensory cell turnover which normally lasts 28 to 30 days, 30 justifying the longer recovery period observed in some subjects. In this regard, it has been reported that in non‐COVID‐19 post‐viral anosmia more than 80% of the patients may experience a subjective improvement of OD after a follow‐up period of 1 year whereas only 30% would experience a spontaneous recovery in the same period of time. 31
FIGURE 2.
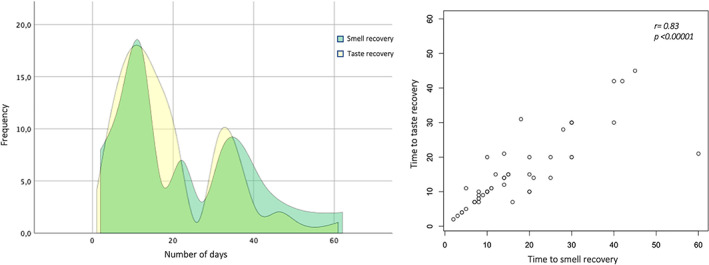
Frequency polygon (left) and scatter plot (right) showing time to smell and taste recovery
Additionally, it must be noted that smell and taste recovery correlated each other in our population (r = 0.83; P < .00001) confirming that TD is caused by an impairment of the retronasal olfaction, rather than impaired gustation itself (Figure 2 right). 32
Complete recovery was reported within a median time of 52 days in 31.8% (OD) and 47.1% (TD) of HCWs. Unfortunately, OD and TD was still present in 68.2% and 52.9% of our respective subjects. (Tables 2 and 3) The complete resolution of smell or taste recovery rates observed in our COVID‐19 HCWs population (31.8% for OD and 47.1% for TD) are averagely higher when compared to those previously reported in both the general population (11.5% at a follow up ranging from 1 to 4 weeks 27 ) and the HCWs populations (12.5% at a median time of 12 days 1 or 13% at 10 days 10 ). However, a 48.7% rate of complete resolution was reported by Boscolo‐Rizzo et al at a follow‐up of 4 weeks. 33 Therefore, the high recovery rates observed in our population could be explained by our larger sample size and longer follow‐up period over 52 days.
Considering the huge number of people infected in this pandemic and the significant proportion with long‐lasting OD and TD (up to 70%), there will be a need for additional capacity to offer treatment for smell and taste impairment in the post‐COVID‐19 recovery phase. As a consequence of increased media coverage, the number of patients coming to otolaryngology clinics is also expected to be higher than normal. In addition to current available therapies for OD, 32 there is a need to embrace new therapies which explore the regeneration of damaged neurons. 34 , 35
4.1. Strengths and limitations of the study
To our knowledge, this is the first multi‐site European study to evaluate risk and prognosis of OD and TD among COVID‐19 positive HCWs.
A study limitation was the inability to calculate OD and TD prevalence in the Paduan population due to the fact that questionnaire was only administered to HCWs with smell impairment. A possible bias in the composition of the samples in terms of sex and ethnicity, may have influenced our findings. Additionally, HCWs have been included within previous studies of the general population. This may have inflated the rates of OD and TD observed in these studies and thus distorted our comparison between HCWs, and all other members of the general population.
Finally, as most of the currently available studies on COVID‐19, OD and TD diagnosis was based on self‐reported symptoms which can have added a potential bias considering the low correlation between objective and self‐rating olfactory loss. 36 However, even if it were possible that subjects not reporting smell or taste dysfunction may have a degree of impairment, it is also true that those complaining of smell and/or taste loss more than likely will have an impairment in the chemosensory function. In this regard, our results may have underestimated the real prevalence of OD and TD among HCWs. Validated olfactory and gustatory tests should be encouraged in future studies as soon as the condition will allow it.
5. CONCLUSION
This study is the first to demonstrate that the UK prevalence of OD and TD among COVID‐19 positive HCWs was, respectively, 73.1% and 69.2% which is unexpectedly high when compared to previous published results in the general and HCW populations. This study has demonstrated that nurses/HCAs and doctors have a worse prognosis in OD and TD recovery. Interestingly, working on a COVID‐19 ward did not influence prognosis confirming preventive measures are effective. Ethnicity (being white) positively influenced only taste recovery. Importantly, up to 68% of the surveyed HCWs continued to experience OD or TD after 52 days and this will require an increase in treatment capacity if spontaneous improvement does not occur in medium to long term.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ETHICS STATEMENT
Informed consent was obtained from all individual participants included in the study.
ACKNOWLEDGMENTS
We thank Graeme Muir for his assistance with the set‐up of the questionnaire.
Andrews PJ, Pendolino AL, Ottaviano G, et al. Olfactory and taste dysfunction among mild‐to‐moderate symptomatic COVID‐19 positive health care workers: An international survey. Laryngoscope Investigative Otolaryngology. 2020;5:1019–1028. 10.1002/lio2.507
Peter J. Andrews and Alfonso Luca Pendolino contributed equally and share the first authorship.
REFERENCES
- 1. Renaud M, Leon A, Trau G, et al. Acute smell and taste loss in outpatients: all infected with SARS‐CoV‐2? Rhinology. 2020;58(4):406‐409. [DOI] [PubMed] [Google Scholar]
- 2. Italian National Institute of Health (ISS) . COVID‐19 integrated surveillance: key national data. COVID‐19 Epidemic; 2020. National Update. (in Italian). https://www.epicentro.iss.it/coronavirus/sars-cov-2-sorveglianza-dati. Accessed July 3, 2020.
- 3. https://www.cebm.net/covid-19/covid-19-how-many-healthcare-workers-are-infected/. Accessed July 3, 2020.
- 4. https://www.euronews.com/2020/05/06/at-least-90-000-healthcare-workers-infected-by-covid-19-says-nursing-group. Accessed July 3, 2020.
- 5. Jha S, Soni A, Siddiqui S, et al. Prevalence of flu‐like symptoms and COVID‐19 in healthcare workers from India. J Assoc Physicians India. 2020;68:27‐29. [PubMed] [Google Scholar]
- 6. Lombardi A, Consonni D, Carugno M, et al. Characteristics of 1573 healthcare workers who underwent nasopharyngeal swab testing for SARS‐CoV‐2 in Milan, Lombardy, Italy. Clin Microbiol Infect. 2020;S1198‐743X:30354‐2 (Published online ahead of print, June 20, 2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vukkadala N, Qian ZJ, Holsinger FC, Patel ZM, Rosenthal E. COVID‐19 and the otolaryngologist: preliminary evidence‐based review. Laryngoscope. 2020. 10.1002/lary.28672 (Published online ahead of print, March 26, 2020). [DOI] [PubMed] [Google Scholar]
- 8. https://www.who.int/health-topics/coronavirus#tab=tab_3. Accessed July 3, 2020.
- 9. von Bartheld CS, Hagen MM, Butowt R. Prevalence of chemosensory dysfunction in COVID‐19 patients: a systematic review and meta‐analysis reveals significant ethnic differences. Preprint. medRxiv; 2020. 10.1101/2020.06.15.20132134 [DOI] [PMC free article] [PubMed]
- 10. Kaye RCC, Kazahaya K, Brereton J, Denneny JC III. COVID‐19 anosmia reporting tool: initial findings. Otolaryngol Head Neck Surg. 2020;163:132‐134. [DOI] [PubMed] [Google Scholar]
- 11. Lan FY, Filler R, Mathew S, et al. COVID‐19 symptoms predictive of healthcare workers' SARS‐CoV‐2 PCR results. PLoS One. 2020;15:e0235460 10.1371/journal.pone.0235460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kempker RR, Kempker JA, Peters M, et al. Loss of smell and taste among healthcare personnel screened for coronavirus 2019. Clin Infect Dis. 2020;ciaa877 10.1093/cid/ciaa877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Van Loon N, Verbrugghe M, Cartuyvels R, Ramaekers D. Diagnosis of COVID‐19 based on symptomatic analysis of hospital healthcare workers in Belgium: observational study in a large Belgian tertiary care center during early COVID‐19 outbreak. J Occup Environ Med. 2020. 10.1097/JOM.0000000000002015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iversen K, Bundgaard H, Hasselbalch RB, Kristensen JH, et al. Risk of COVID‐19 in health‐care workers in Denmark: an observational cohort study. Lancet Infect Dis. 2020;S1473‐3099(20):30589‐30582. 10.1016/S1473-3099(20)30589-2 (Epub ahead of print. Erratum in: Lancet Infect Dis. 2020 October; 20(10):e250). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ollarves‐Carrero MF, Rodriguez‐Morales AG, Bonilla‐Aldana DK, Rodriguez‐Morales AJ. Anosmia in a healthcare worker with COVID‐19 in Madrid. Spain Travel Med Infect Dis. 2020;35:101666 10.1016/j.tmaid.2020.101666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lechner M, Chandrasekharan D, Jumani K, et al. Anosmia as a presenting symptom of SARS‐CoV‐2 infection in healthcare workers—a systematic review of the literature, case series, and recommendations for clinical assessment and management. Rhinology. 2020;58:394‐399. [DOI] [PubMed] [Google Scholar]
- 17. Ottaviano G, Carecchio M, Scarpa B, et al. Olfactory and rhinological evaluations in SARS‐CoV‐2 patients complaining of olfactory loss. Rhinology. 2020;58:400‐401. [DOI] [PubMed] [Google Scholar]
- 18. Moein ST, Hashemian SMR, Mansourafshar B, Khorram‐Tousi A, Tabarsi P, Doty RL. Smell dysfunction: a biomarker for COVID‐19. Int Forum Allergy Rhinol. 2020;10:944‐950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gane SB, Kelly C, Hopkins C. Isolated sudden onset anosmia in COVID‐19 infection. A novel syndrome? Rhinology. 2020;58:299‐301. [DOI] [PubMed] [Google Scholar]
- 20. Haehner A, Draf J, Dräger S, de With K, Hummel T. Predictive value of sudden olfactory loss in the diagnosis of COVID‐19. ORL J Otorhinolaryngol Relat Spec. 2020;82:175‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Speth MM, Singer‐Cornelius T, Obere M, Gengler I, Brockmeier SJ, Sedaghat AR. Olfactory dysfunction and Sinonasal symptomatology in COVID‐19: prevalence, severity, timing, and associated characteristics. Otolaryngol Head Neck Surg. 2020;163:114‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Spinato G, Fabbris C, Polesel J, et al. Alterations in smell or taste in mildly symptomatic outpatients With SARS‐CoV‐2 infection. Jama. 2020;323:2089‐2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hopkins C, Surda P, Kumar N. Presentation of new onset anosmia during the COVID‐19 pandemic. Rhinology. 2020;58:295‐298. [DOI] [PubMed] [Google Scholar]
- 24. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients With 2019 novel coronavirus‐infected pneumonia in Wuhan, China. Jama. 2020;323:1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Doty RL. Epidemiology of smell and taste dysfunction. Handb Clin Neurol. 2019;164:3‐13. [DOI] [PubMed] [Google Scholar]
- 26. Lechien JR, Chiesa‐Estomba CM, Place S, et al. Clinical and epidemiological characteristics of 1420 European patients with mild‐to‐moderate coronavirus disease 2019. J Intern Med. 2020;288:335‐344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hopkins C, Surda P, Whitehead E, Kumar BN. Early recovery following new onset anosmia during the COVID‐19 pandemic—an observational cohort study. J Otolaryngol Head Neck Surg. 2020;49:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee Y, Min P, Lee S, Kim SW. Prevalence and duration of acute loss of smell or taste in COVID‐19 patients. J Korean Med Sci. 2020;35:e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brann DH, Tsukahara T, Weinreb C, et al. Non‐neuronal expression of SARS‐CoV‐2 entry genes in the olfactory system suggests mechanisms underlying COVID‐19‐associated anosmia. BioRxiv. 2020;6:eabc5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Graziadei PPC, Monti Graziadei GA. Neurogenesis and neuron regeneration in the olfactory system of mammals. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J Neurocytol. 1979;8:1‐18. [DOI] [PubMed] [Google Scholar]
- 31. Lee DY, Lee WH, Wee JH, Kim JW. Prognosis of postviral olfactory loss: follow‐up study for longer than one year. Am J Rhinol Allergy. 2014;28(5):419‐422. [DOI] [PubMed] [Google Scholar]
- 32. Whitcroft KL, Hummel T. Olfactory dysfunction in COVID‐19: diagnosis and management. Jama. 2020;323:2512‐2514. [DOI] [PubMed] [Google Scholar]
- 33. Boscolo‐Rizzo P, Borsetto D, Fabbris C, et al. Evolution of altered sense of smell or taste in patients With mildly symptomatic COVID‐19. JAMA Otolaryngol Head Neck Surg. 2020;146:729 10.1001/jamaoto.2020.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smith KE, Whitcroft K, Law S, Andrews P, Choi D, Jagger DJ. Olfactory ensheathing cells from the nasal mucosa and olfactory bulb have distinct membrane properties. J Neurosci Res. 2020;98:888‐901. [DOI] [PubMed] [Google Scholar]
- 35. Hamilton NJI, Hynds RE, Gowers KHC, et al. Using a three‐dimensional collagen matrix to deliver respiratory progenitor cells to Decellularized trachea in vivo. Tissue Eng Part C Methods. 2019;25:93‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Soter A, Kim J, Jackman A, Tourbier I, Kaul A, Doty RL. Accuracy of self‐report in detecting taste dysfunction. Laryngoscope. 2008;118(4):611‐617. [DOI] [PubMed] [Google Scholar]


