Abstract
Objectives
At the end of 2019, SARS‐CoV‐2 was identified, the one responsible for the COVID‐19 disease. Between a 5.1% and a 98% of COVID‐19 patients present some form of alteration in their sense of smell. The objective of this study is to determine the diagnostic yield of the smell dysfunction as screening tool for COVID‐19.
Methods
Cross‐sectional, observational, and pro‐elective study was performed in a tertiary care hospital from May 25th to June 30th, 2020. One hundred and thirty‐nine patients were included in the study. Demographic characteristics were collected from anamnesis. A Self‐Perception Questionnaire and psychophysical olfactory test (POT) were applied to all participants. The presence of SARS‐CoV2, was detected by RT‐PCR methods.
Results
51.7% of patients were SARS‐CoV‐2 positive. A sensitivity of 50% was obtained for the self‐perception questionnaire as a screening tool for SARS‐CoV2, with a specificity of 80.59%. The positive predictive value (PPV) was of 73.46%, the negative predictive value (NPV) was of 60%. The POT as a screening tool had a PPV of 82.35%, a NPV of 52.45%, a LR+ of 4.34, a LR‐ 0.84. The combination of anosmia (according to the POT) plus cough and asthenia got an OR of 8.25 for the SARS CoV‐2 infection.
Conclusion
There is a strong association between olfactory dysfunction and COVID‐19. However, it is not really efficient in the screening of SARS‐CoV‐2 infection and thus, they should not be considered as a single diagnostic instrument.
Level of Evidence
4.
Keywords: biomarker, COVID‐19, olfaction, olfactory disorders, olfactory test
A cross‐sectional, observational and pro‐elective study, to determine the diagnostic yield of the questionnaire and the brief smell test as scrutiny instruments for COVID‐19.
1. INTRODUCTION
At the end of 2019, a new type of coronavirus was identified and named SARS‐CoV‐2, the one responsible for the COVID‐19 disease. In the mild to moderate cases, it is characterized by fever, dry cough, headache, asthenia, and odynophagia. 1 From the onset of the COVID‐19 pandemic, there have been multiple reports of patients in the literature with alterations of their sense of smell or taste, secondary to the SARS‐CoV‐2 infection. Most of said reports have been obtained through telephone or online surveys, 2 like the one developed by the American Academy of Otolaryngology‐ Head And Neck Surgery (AAO‐HNS), entitled the COVID‐19 Anosmia Reporting Tool. 3 The first objective smell evaluation was performed using the Connecticut Chemosensory Clinical Research Center Test (CCCRCT) with a population of 72 patients, positive to SARS‐CoV‐2. It obtained a result of 73.6% of patients with chemosensory alterations. 4 Later, a study by Moein et al applied the Persian version of the University of Pennsylvania Smell Identification Test (UPSIT). It compared 60 SARS‐CoV‐2 positive patients against a control group. A prevalence of 98% smell alterations was observed amongst the SARS‐CoV‐2 positive patients. 5 Likewise, Lechien JR et al used a smell identification test, the Sniffin Sticks, and reported that 76% of SARS‐CoV‐2 positive patients presented a smell alteration. 6 Most recently Tong et al, in a meta‐analysis, reported an overall of 52.73% (95% CI, 29.64‐75.23) pooled prevalence of olfactory dysfunction among 1627 COVID‐19 patients, and 43.93% (95% CI, 20.46‐68.95) prevalence of gustatory dysfunction among 1390 COVID‐19 patients. 7
Generally speaking, the information reported so far suggests that between a 5.1% 8 and a 98% 5 of COVID‐19 patients present some form of alteration in their sense of smell or taste; which seems to be a common symptom in the early stages of the diseases, 9 noticeable even before a diagnosis in between 53.1% and 73%, 3 , 10 , 11 ; as a first symptom in between 8.7% 12 and 26.6% 3 , 6 , 13 , 14 ; and it has occasionally been reported as the only symptom in 5.1%. 10 , 15 Recently, Haehner et al published their results of a cross‐sectional study in which a 98.7% specificity and a 22.7% sensitivity were reported in the self‐assessment questionnaire of smell alterations, as a screening method. 16 For all of the above mentioned reasons, some authors 6 , 16 , 17 propose using a smell assessment as a quick, accessible and low‐cost method for detecting COVID‐19 patients.
The objective of the hereby study is to determine the diagnostic yield of the questionnaire and the psychophysical olfactory test as screening instruments for COVID‐19.
2. MATERIALS AND METHODS
A cross‐sectional, observational and pro‐elective study was performed at the Specialties Hospital Centro Médico Nacional Siglo XXI, part of the Mexican Institute of Social Security in Mexico City, Mexico. The study covered a period of time from May 25th to June 30th, 2020; and it included patients who sought a respiratory TRIAGE assessment, due to COVID‐19 suspicion.
The study was carried out in an office next to the respiratory TRIAGE area (medical emergency area for patients with clinical suspicion of COVID‐19), after the emergency medicine doctor's assessment. A team of three otolaryngology residents volunteered to collect the demographic data and medical background; and they also applied the self‐perception questionnaire and the psychophysical olfactory test (POT). It was the same team all throughout the study (Figure 1).
FIGURE 1.
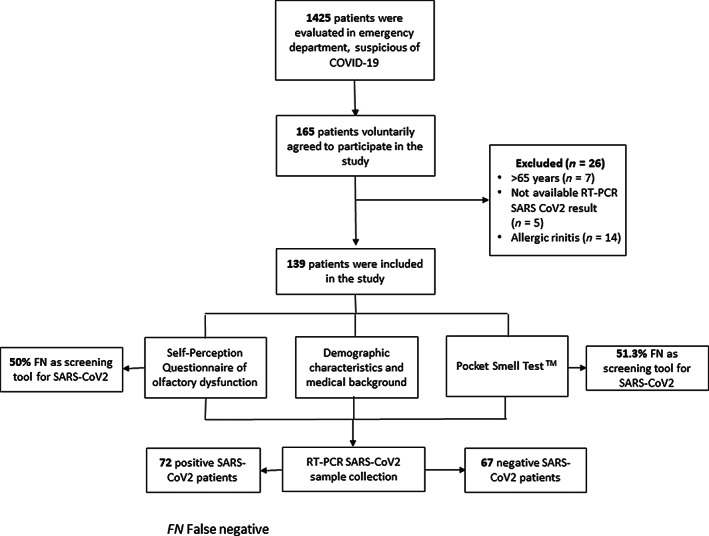
Study flow chart of participants. FN, false negative
The patients had to meet the following inclusion criteria in order to be able to participate: older than 18‐years‐old (of legal age), a nasopharyngeal swab test had to be taken to test for SARS‐CoV‐2, and the patients ought to present a mild to moderate form of the disease, for an ambulatory treatment. They also had to sign their agreement to voluntarily participate in the study.
The exclusion criteria applied did not consider patients older than 65‐years‐old, those with a Parkinson or Alzheimer history, chronic rhinosinusitis, allergic rhinitis; those who did not have a SARS‐CoV‐2 RT‐PCR result; or patients who required hospitalization.
All the patients signed an informed consent form; and the hereby study was approved by the Research and Ethics Committee of the hospital (CI 09015034/CEI 0232017082/R 2020 3601 124).
2.1. Demographic characteristics and medical background
The following information was requested during the interview: age, sex, schooling, surgical background, comorbidities, general symptoms and ENT symptoms (rhinorrhea, dysosmia, nasal obstruction, odynophagia, and dysgeusia). Fever was defined as body temperature greater than 38°C (100.4 °F). The data was entered into an electronic database for further analysis.
2.2. Self‐Perception Questionnaire
The questionnaire enclosed in Appendix A was used. It required the patient to indicate if at the time of the assessment they had suffered loss of smell (yes vs no), to quantify the loss of smell in a visual analog scale (where 0 was no loss of smell and 10 was a total loss of smell). They were also required to state when the loss started (1.—It is my only symptom, 2.—It was the first symptom, before the rest appeared, 3.—It appeared at the same time as the other symptoms, and 4.—It appeared after the rest of the symptoms had appeared).
2.3. Psychophysical olfactory test
The Pocket Smell Test (PST) was used. It contained 3 odors (Mint, Paint Thinner, Peanut). The patient released the smell by scratching the presented odor test strip, after which the participant smelled the odor and tried to identify it. Above each odorant strip, there was a list of four possible responses, and the participant was required to choose one of them. This test is a screening test of gross olfactory dysfunction (that includes hysposmia and anosmia). Normosmia and hyposmia‐anosmia were considered, with a correct identification in 2‐3 and 0‐1 of the smells, respectively. This test was previously validated for the Mexican population by Yañez et al. 18 , 19
2.4. The SARS‐CoV‐2 diagnosis
It was done after the ENT team assessment, by the clinical laboratory staff. A nasopharyngeal swab was taken for each patient in the study; and it was sent to the Central Epidemiology Laboratory of the National Medical Center La Raza for its processing, following the international standards for the transport of infectious substances. The presence of SARS‐CoV‐2 was detected by real‐time RT‐PCR, according to the guidelines certified by the National Institute for Diagnosis and Epidemiological Referral. 20
2.5. Stratified analysis
A stratified analysis was done with the intention of constructing a diagnostic instrument. It included anosmia (detected by the questionnaire and/or the PST), cough and asthenia. Those signs and symptoms were associated to the SARS‐CoV‐2 positive test.
2.6. Statistical analysis
For the data analysis, descriptive and inferential statistics were applied, considering the central and dispersion trend measures. The Chi‐square or the Fisher's exact tests were used for the comparison of frequencies and proportions, according to the value in the boxes. In order to compare the quantitative variables, the Mann‐Whitney U statistical test and/or the Wilcoxon exact test were used; or the T test according to the variables distribution. Sensitivity and specificity were calculated, as well as the likelihood ratios, accuracy and area under the ROC curve (AUC) of each test applied. A Kappa test was used to measure the concordance for agreement between the Self‐Perception Questionnaire of olfactory dysfunction and the PST. A value of P < .05 was considered as statistically significant. A Mantel and Hanzel stratified analysis was used to construct the diagnostic scale. The statistical program used was SPSS version 25.0 (IBM SPSS Statistics for Windows. Armonk, NY: IBM Corp); as well as the Stata SE software, version16 (StataCorp, Texas).
3. RESULTS
3.1. Baseline characteristics
A total of 139 patients were included, out of which 51.7% were SARS‐CoV‐2 positive. Within the SARS‐CoV‐2 positive patients group, 62.5% were female, with an average age of 38.8 years‐old (±10.64). No significant differences were found in the baseline characteristics of the patients (Table 1).
TABLE 1.
Baseline characteristics and clinical history of 139 patients presenting for COVID‐19 suspicion
| Variable | Total (n = 139) | Positive SARS CoV2 patients (n = 72) | Negative SARS CoV2 patients (n = 67) | P value a |
|---|---|---|---|---|
| Age, mean ± SD | 39.02 ± 10.45 | 38.88 ± 10.64 | 39.16 ± 10.32 | .9462 |
| Gender, n (%) | ||||
| Female | 88 (63.31) | 45 (62.5) | 43 (64.18) | .836 |
| Academic level, n (%) | ||||
| Low | 1 (0.72) | 0 | 1 (1.49) | .798 |
| Medium‐high | 138 (99.28) | 72 (100) | 66 (98.51) | .798 |
| Comorbidities, n (%) | ||||
| Nasal surgery | 15 (10.79) | 10 (13.89) | 5 (7.46) | .222 |
| Type 2 diabetes mellitus | 5 (3.60) | 2 (2.78) | 3 (4.48) | .591 |
| Consumption of medications that can alter olfactory function | 4 (2.88) | 1 (1.39) | 3 (4.48) | .276 |
| Hypertension | 15 (10.79) | 7 (9.72) | 8 (11.94) | .674 |
| Asthma | 6 (4.32) | 4 (5.56) | 2 (2.99) | .456 |
| Depression | 2 (1.44) | 1 (1.39) | 1 (1.49) | .959 |
| Hypothyroidism | 6 (4.32) | 2 (2.78) | 4 (5.97) | .355 |
| Migraine | 2 (1.44) | 1 (1.39) | 1 (1.49) | .959 |
| Obstructive sleep apnea syndrome | 1 (0.72) | 0 | 1 (1.49) | .298 |
| Chronic kidney disease | 1 (0.72) | 0 | 1 (1.49) | .298 |
| General symptoms | ||||
| Headache | 81 (58.27) | 42 (58.33) | 39 (58.21) | .988 |
| Abdominal pain | 1 (0.72) | 1 (1.39) | 0 (0) | .333 |
| Cough | 61 (43.88) | 38 (52.78) | 23 (34.33) | .029 |
| Asthenia | 67 (48.20) | 40 (55.55) | 27 (40.30) | .072 |
| Fever | 28 (20.14) | 17 (23.61) | 11 (16.42) | .291 |
| Myalgia | 68 (48.92) | 35 (48.61) | 33 (49.25) | .940 |
| Arthralgia | 52 (37.41) | 31 (43.06) | 21 (31.34) | .154 |
| Conjunctivitis | 22 (15.83) | 13 (18.06) | 9 (13.43) | .456 |
| Diarrhea | 35 (25.18) | 14 (19.44) | 21 (31.34) | .106 |
| ETN symptoms | ||||
| Rhinorrhea | 42 (30.22) | 23 (31.94) | 19 (28.36) | .645 |
| Dysosmia | 3 (2.16) | 3 (4.17) | 0 (0) | .091 |
| Nasal obstruction | 5 (3.6) | 2 (2.78) | 3 (4.48) | .591 |
| Hyposmia‐anosmia | 49 (35.25) | 36 (50) | 13 (19.40) | .000 |
| Odynophagia | 71 (51.08) | 39 (54.17) | 32 (47.76) | .450 |
| Dysgeusia | 53 (38.13) | 38 (52.78) | 15 (22.39) | .000 |
Estimated P value with Wilcoxon test for quantitative variables and estimated P value with Pearson's X 2 test for proportions and frequencies.
3.2. Medical background of the SARS‐CoV‐2 positive patients
Hypertension was present in 9.72%, followed by asthma (5.56%) and type 2 diabetes (2.78%). A 13.89% of the patients reported rhinoplasty/septoplasty. No significant differences were observed in the medical history (Table 1).
3.3. Clinical presentation of SARS‐CoV‐2 positive patients
Headache was reported by 58.33% of the patients, followed by asthenia (55.55%), cough (52.78%) and myalgia (48.61%). Regarding otolaryngology symptoms, odynophagia were the most frequent symptom (54.17%) follower by dysgeusia (52%), hyposmia‐anosmia (50%), rhinorrhea (31.94%), dysosmia (4.17%), and nasal obstruction (2.78%). For the rest of the clinical characteristics see Table 1.
3.3.1. Self‐perception questionnaire of smell disorders in SARS‐CoV‐2 positive patients
Fifty percent (50%) of the patients reported presenting a sense of smell alteration, with a mean score of 7.5 ± 2.54 (P = .043) of the analog visual scale. 44.44% mentioned smell dysfunction appeared after the rest of the symptoms had appeared; 38.9% at the same time as the general symptoms; 13.9% as the first symptom and 2.7% as their only symptom (Table 2).
TABLE 2.
Results of the self‐perception questionnaire of smell disorders of 139 patients presenting for COVID
| Variable | Total (n = 139) | Positive SARS CoV2 patients (n = 72) | Negative SARS CoV2 patients (n = 67) | P value a |
|---|---|---|---|---|
| Do you have loss of smell?, n (%) | ||||
| No | 90 (64.75) | 36 (50) | 54 (80.60) | .000 |
| Yes | 49 (35.25) | 36 (50) | 13 (19.40) | |
| Total (n = 139) | Positive SARS CoV2 patients (n = 72) | Negative SARS CoV2 patients (n = 67) | P value a | |
|---|---|---|---|---|
| Analog visual scale score, mean ± SD | 6.81 ± 2.69 | 7.5 ± 2.54 | 5.53 ± 2.75 | .043 |
| When did your loss of smell begin? | ||||
| 1.‐ It is my only symptom | 3 (6.12) | 1 (2.7) | 2 (15.38) | .001 |
| 2.‐ It was the first symptom, before the rest appeared | 5 (10.20) | 5 (13.9) | 0 (0) | |
| 3.‐ It appeared at the same time as the other symptoms | 20 (40.81) | 14 (38.9) | 6 (46.15) | |
| 4.‐ It appeared after the rest of the symptoms had appeared | 21 (42.85) | 16 (44.44) | 5 (38.46) | |
Estimated P value with Pearson's X 2 test.
A sensitivity of 50% was obtained for the self‐perception questionnaire as a screening instrument for SARS‐CoV2, with a specificity of 80.59%. The positive predictive value (PPV) was of 73.46%, the negative predictive value (NPV) was of 60%, the positive likelihood ratio (LR+) was of 2.57; the negative likelihood ratio (LR‐) of 0.62. The test accuracy was of 64.74% (P = .000) and the AUC was of 0.66 ± 0.04 (IC 95% [0.58‐0.74]) (Table 3).
TABLE 3.
Predictive value of the pocket smell test (PST) and smell disorders self‐perception questionnaire for detection of patients with COVID‐19
| PST | P value a | Questionnaire | P value a | |
|---|---|---|---|---|
| Sensitivity, % | 19.44 | .007 | 50 | .000 |
| Specificity, % | 95.52 | 80.59 | ||
| PPV, % | 82.35 | 73.46 | ||
| NPV, % | 52.45 | 60 | ||
| LR + | 4.34 | 2.57 | ||
| LR − | 0.84 | 0.62 | ||
| Accuracy | 56.11 | 64.74 | ||
| IC 95% | IC 95% | |||
| AUC (±SD) | 0.67 ± 0.05 | 0.57–0.77 | 0.66 ± 0.04 | 0.58–0.74 |
Abbreviations: AUC, area under the ROC curve; LR+, likelihood ratio +; LR−, likelihood ratio −; NPV, negative predictive value; PPV, positive predictive value.
Estimated P value with Pearson X 2 test.
3.3.2. Results of the Pocket Smell Test in SARS‐CoV‐2 positive patients
A 19.44% of the SARS‐CoV‐2 positive patients presented hypsomia‐anosmia, as proven by the pocket smell test; 8.33% of the patients gave 0 smell identifications, 11.11% one identification, 29.1% two identifications and 51.39% three identifications (Table 4).
TABLE 4.
The pocket smell test (PST) results of 139 patients presenting for COVID suspicion
| Variable, n (%) | Total (n = 139) | Positive SARS CoV2 patients (n = 72) | Negative SARS CoV2 patients (n = 67) | P value a |
|---|---|---|---|---|
| Test results | ||||
| Normosmia | 122 (87.77) | 58 (80.56) | 64 (95.52) | .007 |
| Hyposmia‐anosmia | 17 (12.23) | 14 (19.44) | 3 (4.48) | |
| PST score (total correct out of 3) | ||||
| 3 | 93 (66.91) | 37 (51.39) | 56 (83.58) | .001 |
| 2 | 29 (20.86) | 21 (29.1) | 8 (11.94) | |
| 1 | 10 (7.19) | 8 (11.11) | 2 (2.99) | |
| 0 | 7 (5.04) | 6 (8.33) | 1 (1.49) | |
| Odor item identification rates | ||||
| Mint | 122 (87.77) | 58 (80.56) | 64 (95.52) | .007 |
| Peanut | 99 (71.22) | 42 (58.33) | 57 (58.07) | |
| Paint thinner | 118 (84.89) | 54 (75) | 64 (95.52) | |
Estimated P value with Pearson's X 2 test.
The PST as a screening instrument for SARS‐CoV‐2 showed a sensitivity of 19.44%, a specificity of 95.52%, a PPV of 82.35%, a NPV of 52.45%, a LR+ of 4.34, a LR‐ 0.84. The test accuracy was of 56.11% (P = .007) and the AUC was of 0.67 ± 0.05 (IC 95% [0.57‐0.77]) (Table 3).
3.3.3. Concordance between self‐perception questionnaire of smell disorders and the Pocket Smell Test in SARS‐CoV‐2 positive patients
Through the Kappa test a concordance between Self‐perception questionnaire of smell disorders and the PST of 73.38% was observed, for the detection of smell dysfunction, kappa: 0.40, P = .000.
3.3.4. A multiple regression analysis
A multiple logistic regression analysis was performed, including the combination of hyposmia‐anosmia (according to the questionnaire or the PST) plus cough and asthenia, getting an OR of 8.25 (IC 95%[1.00‐67.85], P = .013) for the SARS CoV‐2 infection. See Table 5.
TABLE 5.
Multivariate analysis that included symptomatology of 139 patients presenting for COVID suspicion
| Variable | OR | 95% CI | P value a |
|---|---|---|---|
| Symptom | |||
| Cough | 2.13 | 1.02‐4.49 | .0285 |
| Asthenia | 1.85 | 0.89‐3.84 | .072 |
| Altered smell due to | |||
| Questionnaire | 4.15 | 1.83‐9.68 | .002 |
| PST | 5.14 | 1.32‐29.03 | .007 |
| Combination | |||
| Anosmia‐hyposmia by questionnaire + Cough + Asthenia | 4.86 | 1.54‐15.33 | .002 |
| Anosmia‐hyposmia by PST + Cough + Asthenia | 8.25 | 1.00‐67.85 | .013 |
Multiple logistic regression.
4. DISCUSSION
This study was carried out with the purpose of assessing the performance of the PST and self‐perception of olfactory impairment as a diagnostic instrument for detecting SARS‐CoV‐2.
To our best knowledge, there are no studies with Mexican population to assess this phenomenon. Salmon et al in a prospective multicenter cohort study, which included 1824 patients, found that 40.8% of patients reported alterations in smell and taste, obtaining for smell and taste dysfunction a PPV of 78.5% (95% CI 76.6‐80.3), a sensitivity of 40.8% (95% CI 38.5‐43.0), specificity of 90.3% (95% CI 88.9‐91.6) and a NPV of 63.6% (95% CI 61.4‐65.8) for the diagnosis of SARS‐CoV2 infection. 21 Wee et al 17 obtained a sensitivity of 22.7% (95% CI [20.4‐35.0]), a specificity of 94.8% (95% CI [93.0‐96.3]) for self‐reported smell disorders. Meanwhile, Haehner et al 16 studied 500 patients through a self‐perception questionnaire of smell disorders, obtaining a sensitivity of 65%, a specificity of 90%, a PPV (VPP) of 32% and a 97% of SARS‐CoV‐2 detection. Tostmann et al 22 created a prediction model using a 7 symptom questionnaire, including anosmia, that threw a sensitivity of 91.2%, a specificity of 55.6, and an OR for anosmia as an isolated symptom of 23 (95% CI [8.2‐64.8]). Benezit et al found a sensitivity of 42% (95% CI [27‐58]) and specificity of 95% (95% CI [90‐98]) for the combination of hypogeusia and hiposmia. 23 To the present day, there are no studies to assess the performance of objective smell tests. Our data suggest that an objective psychophysical smell assessment, by applying PST, has a sensitivity of 19.44, a specificity of 95.52, a PPV (VPP) of 82.35, a NPV (VPN) of 52.45, and an accuracy of 56.11 (P = .007). The self‐perception questionnaire has a 50% of sensitivity, and 80.59% of specificity, a PPV (VPP) of 73.46%, a NPV (VPN) of 60% and an accuracy of 64.74 (P = .000), similar to what was reported by Salmon et al, 21 Wee et al 17 and Haehner et al. 16 Smell alteration, reported by Self‐perception questionnaire of smell disorders and by PST in combination with some symptoms (such as cough and asthenia) are useful tools to detect SARS‐CoV2 infection.
A concordance between self‐perception questionnaire of smell disorders and the PST of 73.38% was observed, for the detection of smell dysfunction, kappa: 0.40, P = .000. This lack of concordance may be the consequence of multiple factors such as those reported by Landis et al. The author found an r = −.15 between self‐perception of smell disorders and the psychophysical test. 24 This poor correlation may be due to the fact that the self‐assessment of olfactory sensitivity seems to be strongly influenced by various factors such as: the actual mood, motivation, and motives of the patient.
The low sensitivity of the PST to detect olfactory dysfunction may be the result of the fact that the test consists of 3‐aromas, which limits the ability of the test to discern the presence of slight alterations in smell functions. Another limitation of the test is that it does not assess the threshold or discrimination of odors, it only assesses identification. This test would not have a better performance in evaluating recovery from olfactory dysfunction in patients recovered from SARS‐CoV2 infection due to the limited number of odors that the test constitutes and it would probably be ideal to use the full version consisting of 40 odors.
Regarding anosmia as an isolated symptom, corroborated by the application of PST, an OR of 5.14 (95% CI [1.32‐29.03]) was obtained for the detection of SARS‐CoV‐2 by RT‐PCR, contrasts the reports of Tostmann et al. 22 Finally, in the multiple regression analysis combining the presence of anosmia detected by PST, in association to asthenia and cough; an OR of 8.25 (IC 95%[1.00‐67.85]) was obtained for the detection of SARS‐CoV‐2 by RT‐PCR. Therefore, we consider that smell dysfunction, as an isolated symptom, is not enough to function as a screening test.
Another aspect worth mentioning is that there were no similar results between the self‐perception questionnaire on smell disorders and the results obtained with the PST. In the SARS‐CoV‐2 positive group, 50% reported smell alterations vs a 19.44 proven by the PST. We believe it must be due to the low sensitivity of the smell test to detect hyposmia, since because of its being a pocket test, its strength lies in detecting anosmia.
The presence of 19.4% of patients with olfactory dysfunction, the self‐perception questionnaire of smell disorders, and a negative result for SARS‐CoV2 infection, indicates the limits of RT‐PCR for the detection of SARS‐CoV2. This may be due to situations related to the sampling technique (transport, processing) and the patient's viral load, as reported by Cancella et al. 25
The strength of our study is that it is pro‐elective nature in the data collection, the application of a psychophysical olfactory test, a reasonable sample size, and the bio‐statistical complexity of the study analysis. The weaknesses of the study include: first the sample size. Second the use of a Pocket Smell test of a 3‐item instead of de full version of 40 items and third, the limitation of RT‐PCR for the detection of SARS‐CoV2, in nasopharyngeal swab samples, which has a suboptimal sensitivity (as low as 60%), 26 which leads to a high number of false negatives.
5. CONCLUSION
Both tests, the self‐perception questionnaire of smell disorders and the PST, are useful tools in the detection of SARS‐CoV2 infection, especially in the presence of concomitant symptoms such as cough and asthenia.
Even though there is evidence of a strong association between olfactory alterations (reported by the patient and even by psychophysical olfactory tests) and COVID‐19, they are not really efficient in the screening test of SARS‐CoV‐2 infection due to the low sensitivity and thus, they should not be considered as a single diagnostic instrument. This study proposes the combination of diagnostic instruments (symptomatology scale) to lead to a better screening in the clinical practice of the SARS‐CoV‐2 infection. More studies are needed to assess the diagnostic instruments, and with a larger number of patients.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
ACKNOWLEDGMENT
The present study was carried out with resources from the Mexican Institute of Social Security.
APPENDIX A.
A.‐Do you have loss of smell?
Yes_____ No _____
Please cross the horizontal line with the vertical line at the point you consider appropriate, where 0 is NO LOSS of smell and 10 is TOTAL LOSS of smell.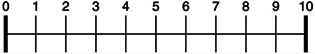
B.‐ When did your loss of smell start?
1. It is my only symptom.
2. It was the first symptom, before the rest appeared.
3. It appeared at the same time as the other symptoms.
4. It appeared after the rest of the symptoms had appeared.
Romero‐Gameros CA, Waizel‐Haiat S, Mendoza‐Zubieta V, et al. Evaluation of predictive value of olfactory dysfunction, as a screening tool for COVID‐19. Laryngoscope Investigative Otolaryngology. 2020;5:983–991. 10.1002/lio2.482
Carlos Alfonso Romero‐Gameros and Salomón Waizel‐Haiat contributed equally to this work. Author order was determined both alphabetically.
BIBLIOGRAPHY
- 1. World Health Organization . Report of the WHO‐China Joint Mission on Coronavirus Disease 2019 (COVID‐19). World Health Organization. https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf. Accessed July 21, 2020.
- 2. Agyeman AA, Lee Chin K, Landersdorfer CB, Liew D, Ofori‐Asenso R. Smell and taste dysfunction in patients With COVID‐19: a systematic review and meta‐analysis. Mayo Clin Proc. 2020;95:1621‐1631. 10.1016/j.mayocp.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaye R, Chang CWD, Kazahaya K, Brereton J, Denneny JC 3rd. COVID‐19 anosmia reporting tool: initial findings. Otolaryngol Head Neck Surg. 2020;163(1):132‐134. 10.1177/0194599820922992. [DOI] [PubMed] [Google Scholar]
- 4. Vaira LA, Deiana G, Fois AG, et al. Objective evaluation of anosmia and ageusia in COVID‐19 patients: single‐center experience on 72 cases. Head Neck. 2020;42(6):1252‐1258. 10.1002/hed.26204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moein ST, Hashemian SM, Mansourafshar B, Khorram‐Tousi A, Tabarsi P, Doty RL. Smell dysfunction: a biomarker for COVID‐19. Int Forum Allergy Rhinol. 2020;10(8):944‐950. 10.1002/alr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lechien JR, Cabaraux P, Chiesa‐Estomba CM, et al. Psychophysical olfactory tests and detection of COVID‐19 in patients with sudden onset olfactory dysfunction: a prospective study. Ear Nose Throat J. 2020;99:579‐583. 10.1177/0145561320929169. [DOI] [PubMed] [Google Scholar]
- 7. Tong JY, Wong A, Zhu D, Fastenberg JH, Tham T. The prevalence of olfactory and gustatory dysfunction in COVID‐19 patients: a systematic review and meta‐analysis. Otolaryngol Head Neck Surg. 2020;163(1):3‐11. 10.1177/0194599820926473. [DOI] [PubMed] [Google Scholar]
- 8. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):1‐9. 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Melley LE, Bress E, Polan E. Hypogeusia as the initial presenting symptom of COVID‐19. BMJ Case Rep. 2020;13(5):e236080 10.1136/bcr-2020-236080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Giacomelli A, Pezzati L, Conti F, et al. Self‐reported olfactory and taste disorders in SARS‐CoV‐2 patients: a cross‐sectional study. Clin Infect Dis. 2020;71(15):889‐890. 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim GU, Kim MJ, Ra SH, et al. Clinical characteristics of asymptomatic and symptomatic patients with mild COVID‐19. Clin Microbiol Infect. 2020;26(7):948.e1‐948.e3. 10.1016/j.cmi.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Speth MM, Singer‐Cornelius T, Oberle M, Gengler I, Brockmeier SJ, Sedaghat AR. Olfactory dysfunction and sinonasal symptomatology in COVID‐19: prevalence, severity, timing, and associated characteristics. Otolaryngol Head Neck Surg. 2020;163(1):114‐120. 10.1177/0194599820929185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lechien JR, Chiesa‐Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild‐to‐moderate forms of the coronavirus disease (COVID‐19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277(8):2251‐2261. 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sayin İ, Yaşar KK, Yazici ZM. Taste and smell impairment in COVID‐19: an AAO‐HNS anosmia reporting tool‐based comparative study. Otolaryngol Head Neck Surg. 2020;163(3):473‐479. 10.1177/0194599820931820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gane SB, Kelly C, Hopkins C. Isolated sudden onset anosmia in COVID‐19 infection. A novel syndrome? Rhinology. 2020;58(3):299‐301. 10.4193/Rhin20.114. [DOI] [PubMed] [Google Scholar]
- 16. Haehner A, Draf J, Dräger S, de With K, Hummel T. Predictive value of sudden olfactory loss in the diagnosis of COVID‐19. ORL J Otorhinolaryngol Relat Spec. 2020;82:1‐6. 10.1159/000509143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wee LE, Chan YFZ, Teo NWY, et al. The role of self‐reported olfactory and gustatory dysfunction as a screening criterion for suspected COVID‐19. Eur Arch Otorhinolaryngol. 2020;277(8):2389‐2390. 10.1007/s00405-020-05999-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yáñez C, Mora N, Nurko B. Prueba corta de olfato a utilizarse como una prueba de diagnóstico confiable. An Med Asoc Med Hosp ABC. 2004;49(2):82‐86. [Google Scholar]
- 19. McCaffrey RJ, Duff K, Solomon GS. Olfactory dysfunction discriminates probable Alzheimer's dementia from major depression: a cross‐validation and extension. J Neuropsychiatry Clin Neurosci. 2000;12(1):29‐33. 10.1176/jnp.12.1.29. [DOI] [PubMed] [Google Scholar]
- 20. Secretaria de salud . Datos Abiertos – Dirección General de Epidemiología. Gobierno de México. https://www.gob.mx/salud/documentos/datos‐abiertos‐152127?idiom=es. Accessed July 21, 2020.
- 21. Salmon Ceron D, Bartier S, Hautefort C, et al. Self‐reported loss of smell without nasal obstruction to identify COVID‐19. The multicenter Coranosmia cohort study. J Infect. 2020;S0163‐4453(20):30463‐30461. 10.1016/j.jinf.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tostmann A, Bradley J, Bousema T, et al. Strong associations and moderate predictive value of early symptoms for SARS‐CoV‐2 test positivity among healthcare workers, The Netherlands. Euro Surveill. 2020;25(16):2000508 10.2807/1560-7917.ES.2020.25.16.2000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bénézit F, Le Turnier P, Declerck C, et al. Utility of hyposmia and hypogeusia for the diagnosis of COVID‐19. Lancet Infect Dis. 2020;20(9):1014‐1015. 10.1016/S1473-3099(20)30297-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Landis BN, Hummel T, Hugentobler M, Giger R, Lacroix JS. Ratings of overall olfactory function. Chem Senses. 2003;28(8):691‐694. 10.1093/chemse/bjg061. [DOI] [PubMed] [Google Scholar]
- 25. Cancella de Abreu M, Choquet C, Petit H, et al. SARS‐CoV‐2 IGM and IGG rapid serologic test for the diagnosis of COVID‐19 in the emergency department. J Infect. 2020;S0163‐4453(20):30513‐30512. 10.1016/j.jinf.2020.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang W, Xu Y, Gao R, et al. Detection of SARS‐CoV‐2 in different types of clinical specimens. JAMA. 2020;323(18):1843‐1844. 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]


